Abstract
Objective
To determine the effect of a soluble human tumor necrosis factor alpha (TNF-α) receptor blocker (Etanercept) on an inducible olfactory inflammation (IOI) mouse model
Study Design
An in vivo study using a transgenic mouse model
Setting
Research laboratory
Subjects and Methods
To study the impact of chronic inflammation on the olfactory system, a transgenic mouse model of chronic rhinosinusitis (CRS)-associated olfactory loss was utilized (IOI mouse), expressing TNF-α in a temporally-controlled fashion specifically within the olfactory epithelium. In one group of mice (n=4), Etanercept was injected intraperitoneally (100 µg/dose, 3 times/week) concurrent with a 2-week period of TNF-α expression. A second group of mice (n=2) underwent induction of TNF-α expression for 8 weeks, with Etanercept treatment administered during the final 2 weeks of inflammation. Olfactory function was assayed by elecro-olfactogram (EOG), and olfactory tissue was processed for histology and immunohistochemical staining. Each group was compared with equal number of control group.
Results
Compared to non-treated IOI mice, Etanercept -treated IOI mice showed significantly improved EOG responses after 2 weeks (p<0.001). After 8 weeks of induced inflammation, there was massive loss of olfactory epithelium and no EOG response in non-treated IOI mice. However, in Etanercept - treated mice, regeneration of olfactory epithelium was observed.
Conclusion
Concomitant administration of Etanercept in IOI mice results in interruption of TNF-α-induced olfactory loss and induction of neuroepithelial regeneration. This demonstrates that Etanercept has potential utility as a tool for elucidating the role of TNF-α in other olfactory inflammation models.
Keywords: Olfactory loss, rhinosinusitis, TNF-α, transgenic model, inflammation
Introduction
Impaired sense of smell is a common problem in patients with chronic rhinosinusitis (CRS). About 69 percent of CRS patients suffer from olfactory dysfunction,1 and CRS is a major cause of olfactory loss.2 Among the several symptoms related to CRS, olfactory loss is often overlooked, as compared to symptoms such as nasal obstruction or purulent rhinorrhea; however, it has a critically negative impact on quality of life.3,4 Despite its clinical significance, the mechanism of olfactory loss associated with CRS is incompletely understood due to difficulties in researching human olfactory loss, such as the relative inaccessibility of the olfactory cleft and the infeasibility of repeatedly obtaining large amounts of olfactory tissue. Olfactory loss is proposed to have conductive and sensorineural components. Conductive olfactory loss arises from mechanical blockage of odorant delivery from the outer environment to the olfactory epithelium,5 whereas sensorineural olfactory loss is caused by functional or microstructural damage to the olfactory neuroepithelium (OE). Inflammatory cytokines released from infiltrating inflammatory cells are suspected to contribute to neuroepithelial damage.6
To investigate the effects of inflammation on the OE in vivo, our group developed a transgenic mouse model of inducible olfactory inflammation (IOI) in which tissue-specific production of tumor necrosis factor alpha (TNF-α) can be temporally controlled.7 The production of TNF-α by sustentacular cells in the OE is initiated and maintained by administration of doxycycline.7 Continuous local production of TNF-α within the OE results in progressive inflammation that mimics histologic features of CRS-associated olfactory loss.8 TNF-α is a potent inflammatory cytokine with various roles in multiple organ systems that is associated with diverse inflammatory diseases, including CRS.9 Histologically, the olfactory epithelium of CRS patients is characterized by an inflammatory cell infiltrate10 producing inflammatory cytokines and chemokines, such as TNF-α, RANTES, eotaxin, and interleukin-13.9,11 Via the IOI mouse model, we have shown that TNF-α-induced inflammation causes loss of electrical odorant responses, thinning of the OE, and loss of mature receptor neurons.12 Expression of TNF-α induces a cascade of cytokines within the OE of the IOI mouse. The precise role of TNF-α in the complex cytokine milieu present in human CRS remains unclear. Mechanisms of inflammation-induced olfactory dysfunction can be studied in the IOI mouse model by exogenous expression of other inflammatory cytokines, as well as via the use of cytokine inhibitors and genetic ablation to dissect the contribution of specific mediators and molecular inflammatory pathways.
Etanercept (Enbrel®) is a fusion protein produced by DNA recombination. It is comprised of a combination of human soluble TNF receptor 2 and the Fc portion of immunoglobulin G1. It functions as a decoy receptor that inhibits TNF-α by competitive binding.13 In this study, we administered Etanercept to IOI mice to investigate whether this agent could block TNF-α-induced inflammation, as assessed by electrophysiologic and morphological analysis.
Materials and Methods
Inducible Olfactory Inflammation (IOI) mice
The creation of the IOI mouse line has been described previously.7 Briefly, the reverse tetracycline transactivator (rtTA) gene was knocked into the olfactory-specific cyp2g1 coding region, generating a cyp2g1-rtTA strain. This line was crossed with a line containing the TNF-α gene under the control of a tetracycline-responsive element (TNF-α) to generate the IOI mouse. Doxycycline (DOX) was used to induce TNF-α expression in adult mice between the ages of 6–8 weeks old. IOI mice were induced to express TNF-α in the olfactory epithelium for 2 weeks in a short-term group, and for 8 weeks in a long-term group. The animal protocol was approved by Johns Hopkins University Institutional Animal Care and Use Committee (IACUC).
Administration of Etanercept
Etanercept (Amgen, Thousand Oaks, CA) was delivered to IOI mice via intraperitoneal injection at a dose of 100 µg/mouse. In the short-term treatment group (2-week group), administration began 3 days before DOX treatment and continued during a 2-week course of DOX treatment with a frequency of 3 times/week. Doxycycline treatment for 2 weeks was sufficient to induce olfactory dysfunction as per EOG without histological change. An equivalent volume of normal saline was administered to the wild type control group on the same administration schedule. In the long-term treatment group (8-week group), IOI mice were treated with DOX for 6 weeks to induce epithelial loss of mice, as treatment for 6 weeks was sufficient to induce epithelial loss in our previous study.7 After a 6-week course of DOX treatment, Etanercept was administered for 2 weeks, during which doxycycline administration was maintained. The total duration of DOX treatment was therefore 8 weeks. Figure 1 shows a diagram of the study design for the 2-week inflammation experiments.
Figure 1.
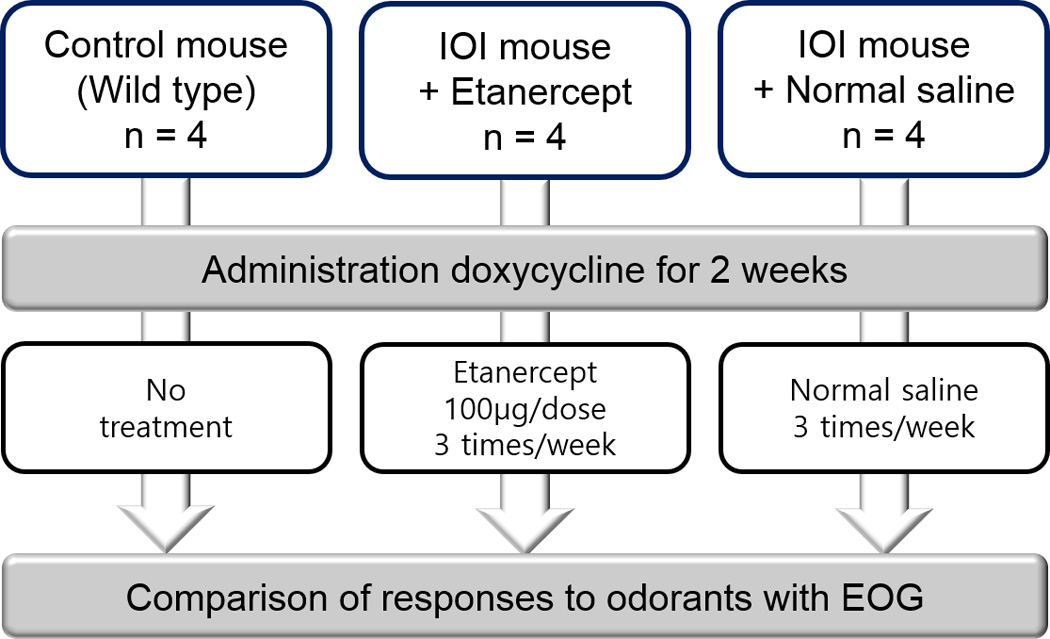
Diagram of study design in 2-week Etanercept treatment group; IOI, inducible olfactory inflammation; EOG, electro-olfactogram
Electro-olfactogram (EOG)
The medial surface of the olfactory turbinate was prepared for recording after mice were euthanized via CO2 according to the IACUC guidelines. Odorant solutions (Aldrich, St. Louis, MO) were prepared in dimethyl sulfoxide (DMSO) and diluted with water to the appropriate working concentration just before EOG recording. Test odorants for air delivery were prepared at a liquid concentration of 10−3 M [final DMSO concentration of 0.2% (v/v)] and diluted to concentrations of 10−4 and 10−5 M. Responses to DMSO diluent alone were measured. Odorant stimulation was delivered in the vapor phase as a 100 ms pulse by injection into a continuous stream of humidified air heated to 36°C. The odorant stimulus pathway was cleaned by air between each stimulus presentation with a minimum interval of 1 min between two adjacent stimuli. Mucosal surface potentials were measured with 2 electrodes, placed on either olfactory turbinate IIb or turbinate III, to acquire simultaneous recordings. Data was analyzed with Clampfit (Axon Instruments, Union City, CA, USA). Peak amplitudes were determined with reference to the prepulse baseline.
Histological analyses
In bisected heads, one nasal cavity was used for EOG and the other was processed for histological analysis. Tissue was fixed and decalcified by immersion in TBD2 solution (Shandon, Pittsburgh, PA, USA) for 24 hours, then embedded in paraffin. Six-µm sections were obtained and collected on glass slides for hematoxylin and eosin staining. For frozen section analysis, the tissue was infiltrated with tissue-freezing medium (TFM; Triangle Biomedical Science, Inc., Durham, NC, USA) after dehydration with sucrose, and frozen on dry ice in a plastic mold. Sections of mouse olfactory tissue in OCT were cut on a cryostat (thickness of 10 µm), placed on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA), and dried at 4°C overnight. Slides were stored at −80°C for future staining.
Immumfluorescence technique
We employed immunofluorescence staining to assess cellular markers and define structural changes to the olfactory epithelium in wild type control, short-term treatment, and long-term treatment groups. Cryostat sections were washed in phosphate-buffered saline (PBS) and blocked for 1 hour in PBS containing 10% normal goat serum. They were subsequently incubated overnight at 4°C in 5% normal serum containing primary antibodies to olfactory marker protein (OMP) (1:250; Abcam, Cambridge, MA, USA) and neural cell adhesion molecules (NCAMs) (1:250; Millipore, Billerica, MA, USA). An antigen retrieval step was performed prior to washing: slides were microwaved at 1000 mW power for 10 minutes in 0.01 M citrate buffer, pH 6.0. Primary antibodies were detected using secondary antibodies fluorescently tagged to Alexa 488 or Alexa 594 (Alexa-Fluor; Invitrogen, Carlsbad, CA, USA). If necessary, samples were counterstained with the nuclear stain, 4', 6-diamidino-2-phenylindole (DAPI) (Vector Labs, Burlingame, CA, USA). Z-stack images were obtained using an LSM510 confocal microscope (Carl Zeiss Micro Imaging, Thornwood, NY, USA).
Quantification of TNF-α expression
Bisected heads were immersed in 250 µl PBS for 1 hour in an ice bucket prior to histological analysis. The PBS was centrifuged briefly and the supernatant fluid was kept at −80°C for use in an enzyme-linked immunosorbent assay (ELISA). TNF-α was quantified using an ELISA kit according to the manufacturer’s (R&D Systems, Minneapolis, MN, USA) instructions.
Data analysis and statistical tools
The results of the EOG were compared using a Mann-Whitney U test for each group after Bonferroni correction. A value of P < .05 was considered statistically significant. Statistical analyses were performed using SPSS software (Version 12.0, Statistical Package for Social Science, Chicago, IL, USA) and Prism Graphpad 6.0 (La Jolla, CA, USA).
Results
Etanercept treatment blocks TNF-α induced loss of electrical odorant responses
After 2 weeks of doxycycline administration to IOI mice, TNF-α was highly expressed (>100 pg/ml) and detectable in nasal lavage fluid of all IOI mice, irrespective of treatment with Etanercept. In wild type control mice, TNF-α was undetectable in the lavage fluid by ELISA (<5 pg/ml). Treatment with Etanercept did not affect the production of TNF-α by sustentacular cells in the IOI mice. At the 2-week time point, the olfactory neuroepithelium remained intact and grossly normal in appearance (Figure 2).
Figure 2.
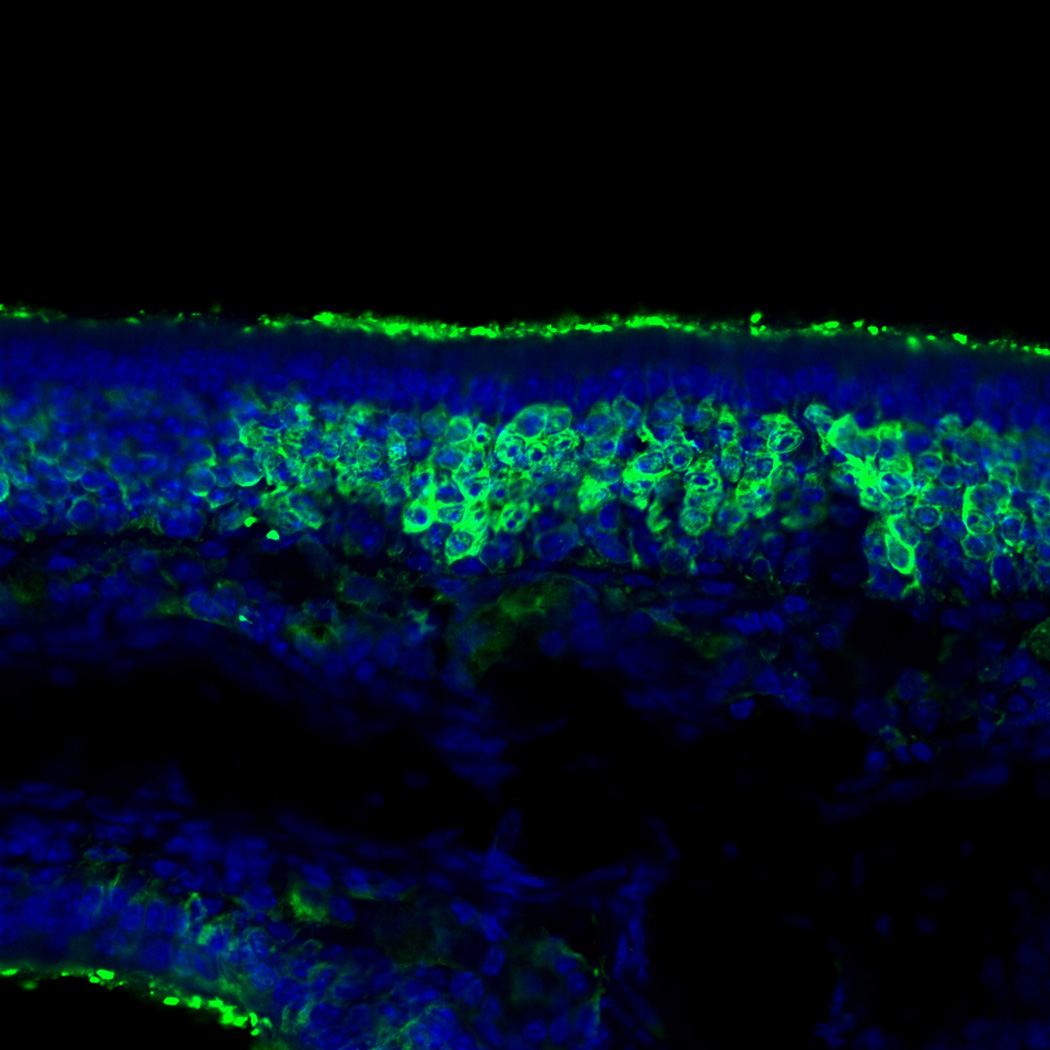
Olfactory marker protein (OMP) immunohistochemistry in the short-term treatment group. The staining of OMP remained intact and grossly normal in appearance. Blue staining is the nuclear marker, DAPI (250×).
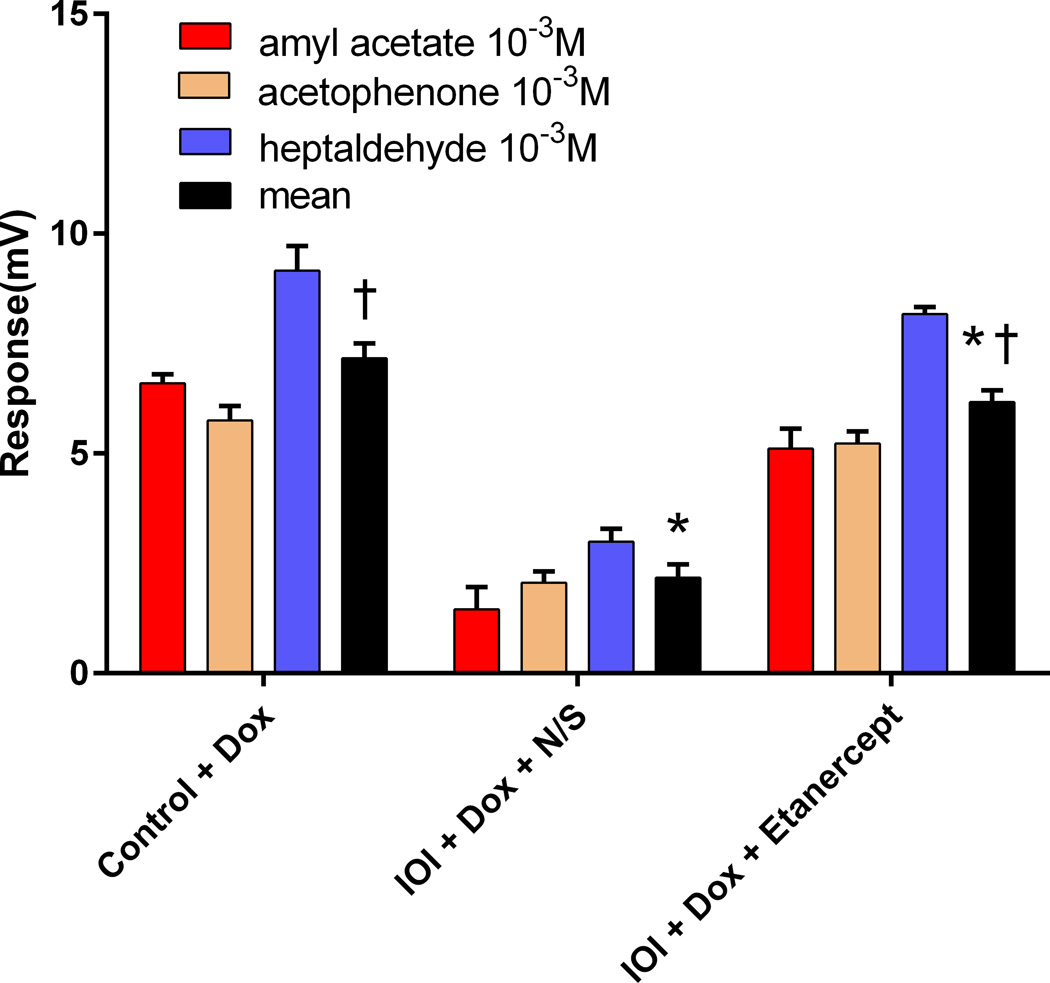
The effect of Etanercept on sensory function was assessed by EOG recording. After 2 weeks of doxycycline administration, odorant responses in IOI mice without Etanercept were significantly reduced in comparison with Etanercept treated mice (p<0.001). However, mice treated with Etanercept showed grossly normal odorant responses that were not statistically significantly different from control mouse (p=0.077) (Figure 3).
Figure 3.
Quantitative assessments of electro-olfactogram responses of three different groups. The data represent average responses from 4 independent recordings. * p<0.001. † p=0.077. Error bar represents SEM. Dox, Doxycycline; IOI, inducible olfactory inflammation; N/S, normal saline.
Etanercept reverses loss of olfactory neuroepithelium despite ongoing TNF-α-induced inflammation
After 6 weeks of doxycycline administration to IOI mice, the OE was significantly thinned, and there was a considerable loss of olfactory receptor neurons (Figure 4A). In the subepithelium, the diameters of the axon bundles were significantly reduced (Figure 4B). Following treatment with a 2-week course of Etanercept after 6 weeks of induced inflammation in IOI mice, the thickness of the olfactory neuroepithelium was recovered in most regions, despite continuous administration of doxycycline for 2 weeks (Figure 5). However, EOG responses to odorants were not restored (data not shown).
Figure 4.
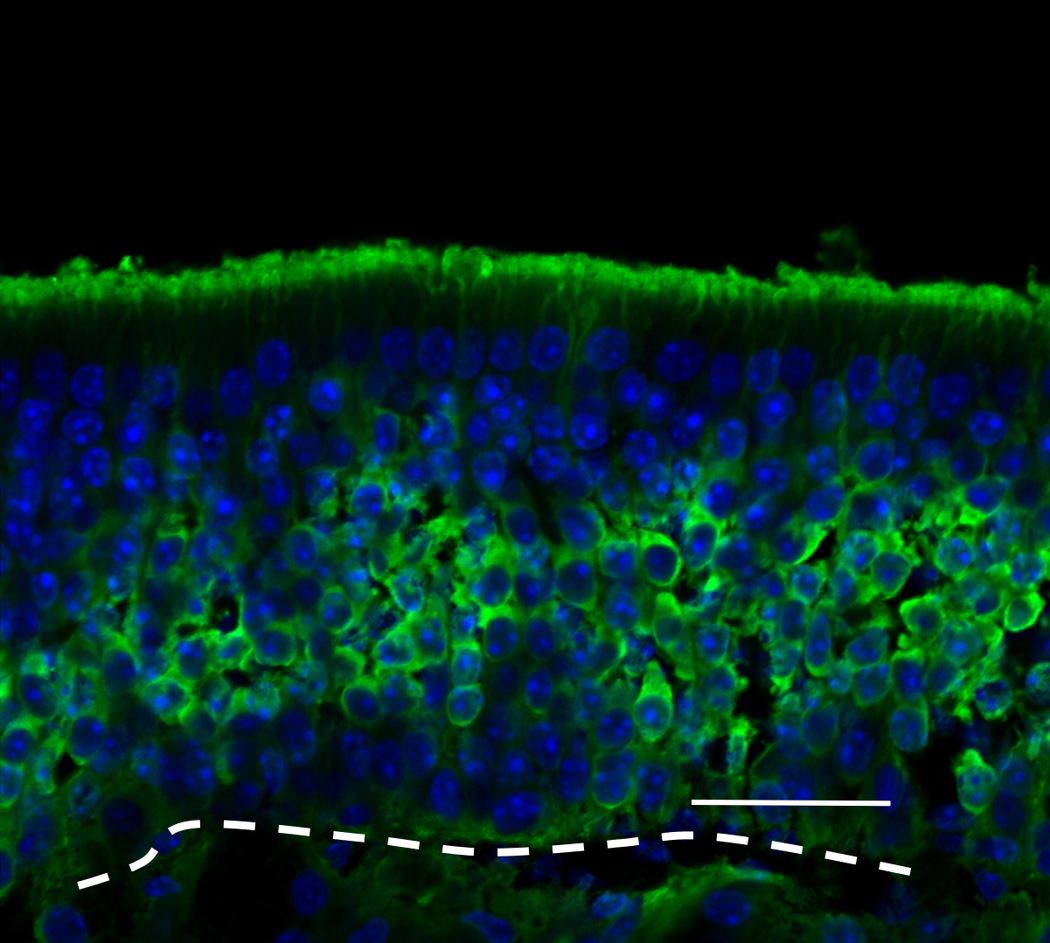
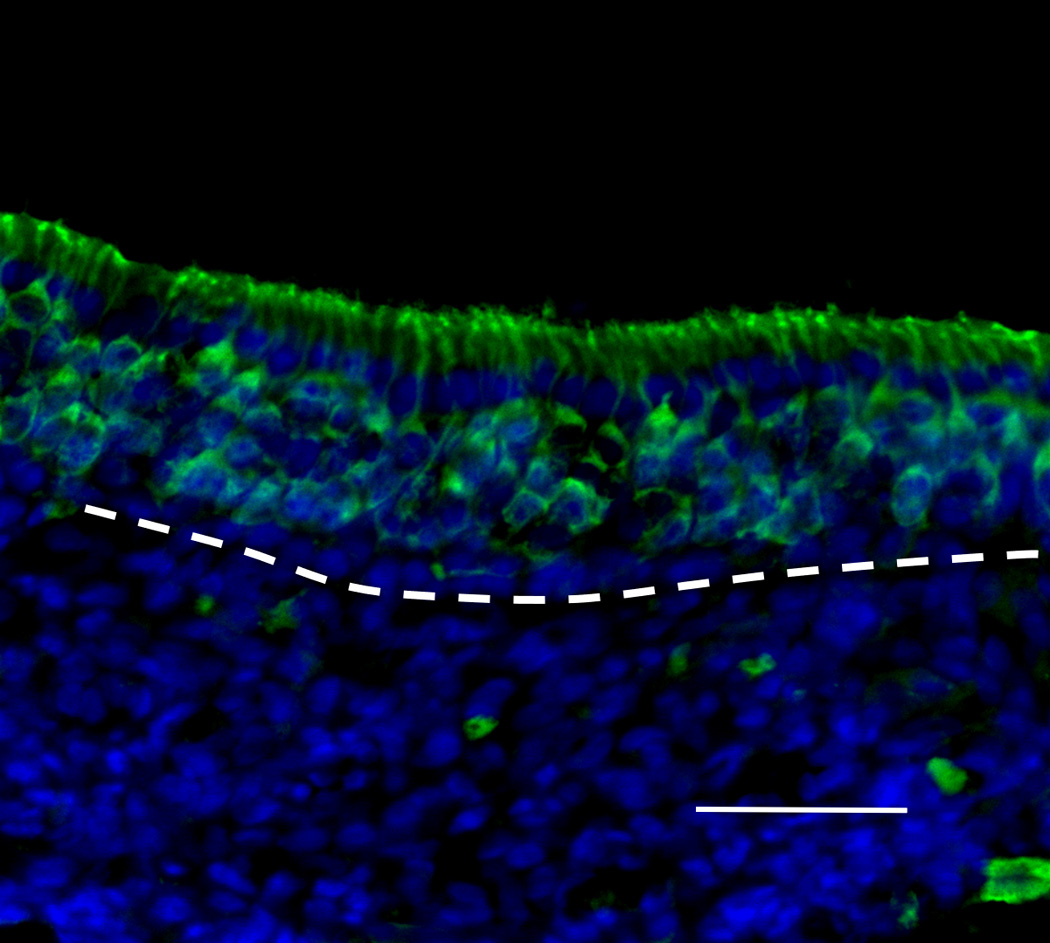
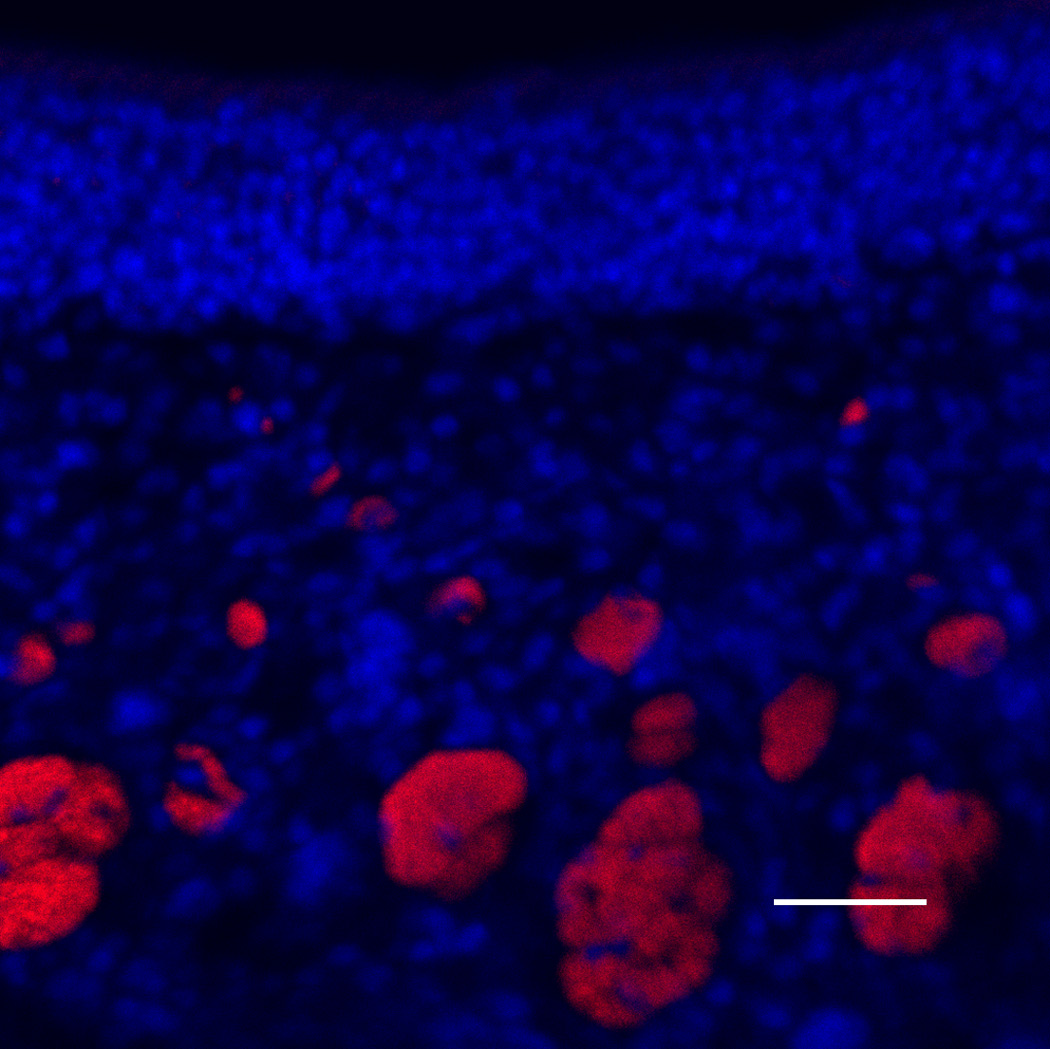
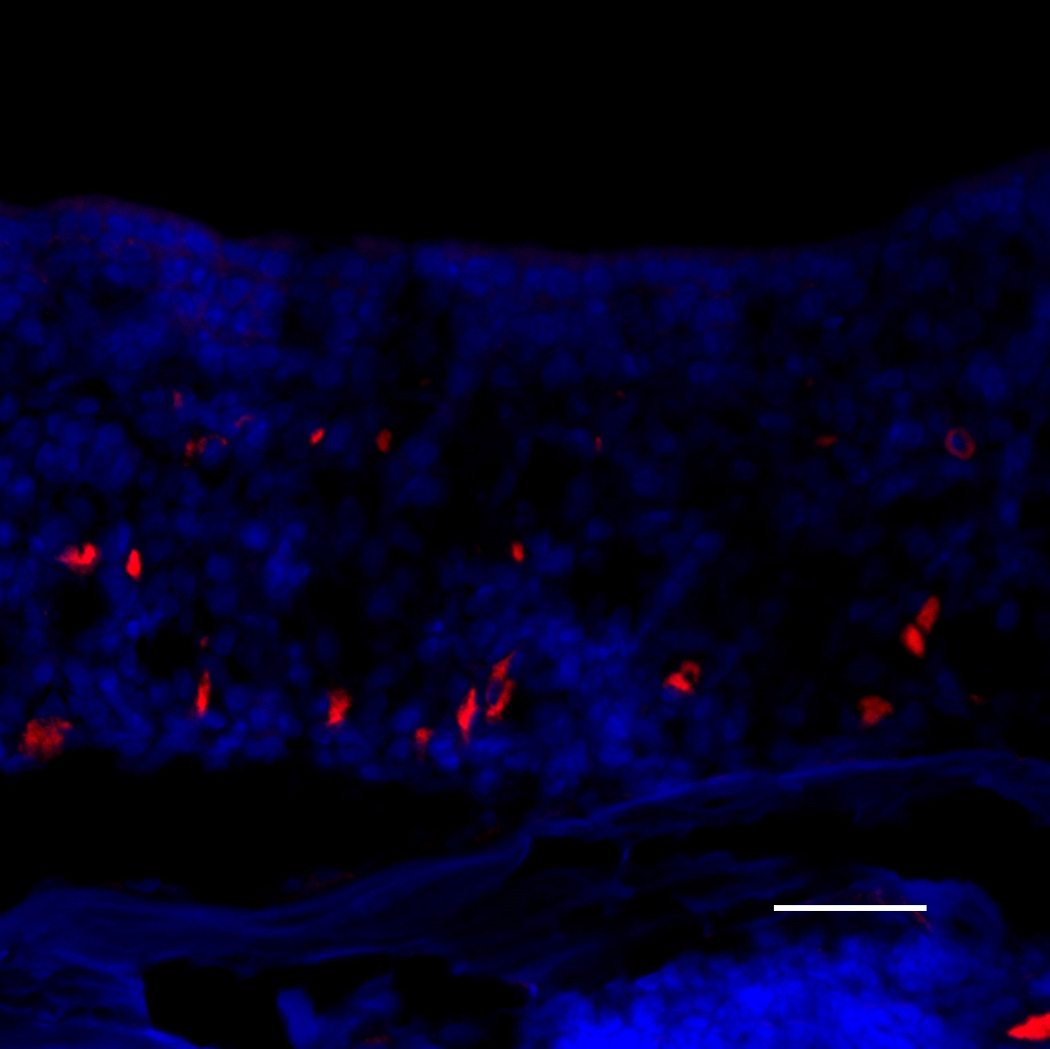
(A) Olfactory marker protein (OMP) immunohistochemistry of wild type control mice. Green staining indicates OMP, and blue staining is the nuclear marker, DAPI (250×). The white broken line indicates the basement membrane.; (B) OMP staining of IOI mice after administration of doxycycline for 6 weeks. The olfactory epithelium was significantly thinned, and considerable loss of olfactory receptor neurons was observed; (C) Neural cell adhesion molecule (NCAM) immunohistochemistry of wild type control mice. Red staining is NCAM (250×). Scale bar = 25µm; (D) NCAM staining of 6-week IOI mice. The diameter of the subepithelial axon bundle was significantly reduced.
Figure 5.
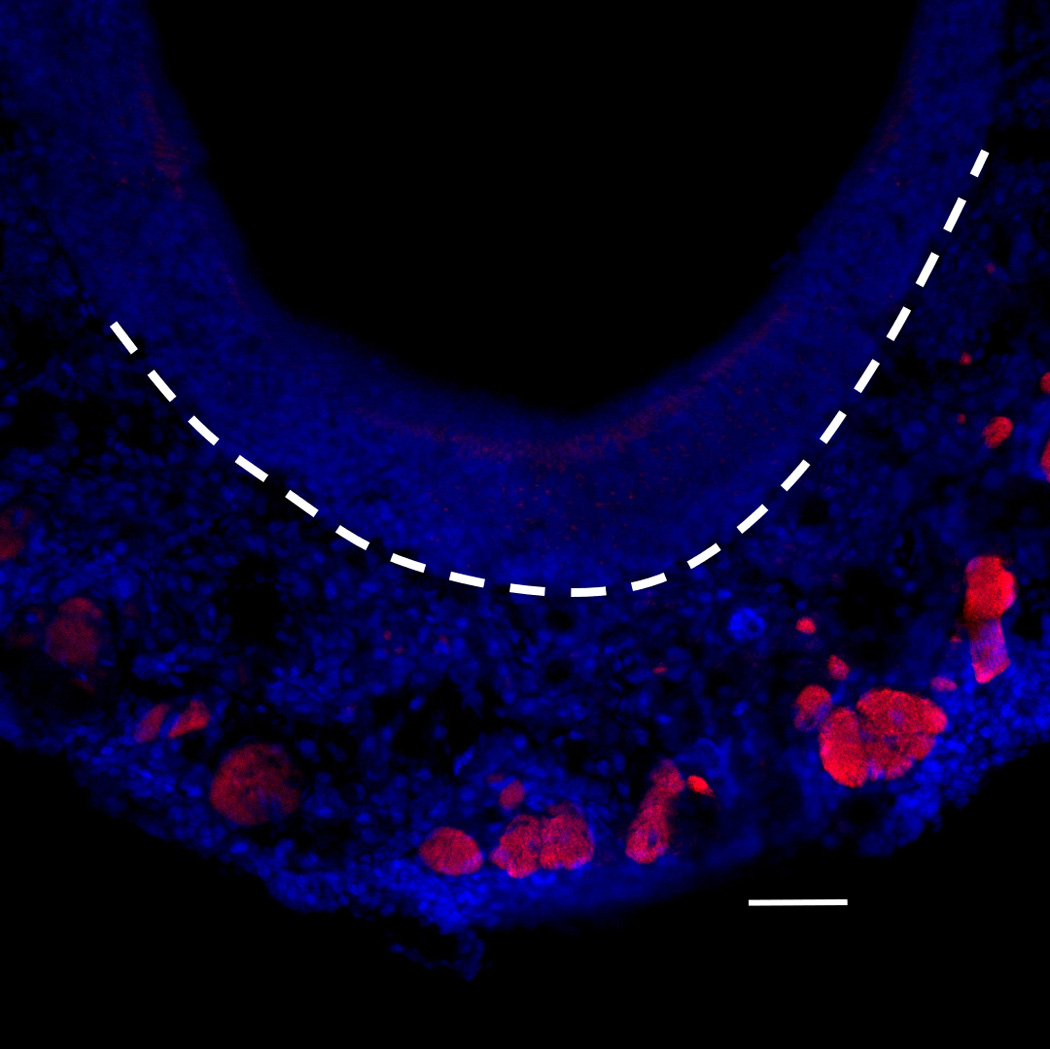
Histologic findings in IOI mice after administration of doxycycline for 8 weeks and treatment with Etanercept during the last 2 weeks thereof. Neural cell adhesion molecule (NCAM) immunohistochemistry. The diameter of the subepithelial axon bundle was significantly thickened compared with Figure 4(D). Red staining indicates NCAM and blue staining is the nuclear marker, DAPI (250×). White broken line indicates the basement membrane. Scale bar = 25µm.
Discussion
In this study we demonstrated that TNF-α-induced inflammation of the OE in the IOI transgenic mouse model could be blocked by systemic treatment with a TNF-α inhibitor. While administration of Etanercept concurrent with TNF-α induction successfully blocked the development of olfactory loss, addition of Etanercept after 6 weeks of untreated inflammation reversed only the histologic changes but not the decrease in odorant electrical responses. Based on these results, we hypothesize that functional olfactory loss precedes structural loss, and structural recovery of olfactory epithelium precedes functional recovery.
These findings provide insight into the role of TNF-α in the IOI mouse model, and support the feasibility of the use of Etanercept to dissect the role of TNF-α in other of experimentally-induced olfactory inflammation. In CRS, multiple cytokines are present, and are likely to be involved in the development of sensorineural olfactory loss.14 The IOI mouse model provides an opportunity to explore the effects of specific cytokines on the function of the olfactory neuroepithelium in vivo. The results of this study do not imply that Etanercept would be an effective therapy for human CRS-associated olfactory loss. Rather, our results demonstrate that this agent can be utilized as an olfactory research tool to pharmacologically block TNF-α pathways that might contribute to olfactory dysfunction in the setting of inflammation.
Etanercept is a soluble TNF-α binding protein with a long half-life (70–132 hours). It was developed to treat autoimmune diseases such as rheumatoid arthritis and ankylosing spondylitis.13,15,16 Its pharmacological action is effected by binding to TNF-α, thus inhibiting the biological activity of that potent inflammatory cytokine. Although Etanercept was designed to target human TNF-α-related autoimmune inflammatory disease, there have been several studies in which Etanercept was used to successfully control inflammation in various mouse disease models. Etanercept effectively reduced inflammation and provided protection against acute encephalitis in a Japanese encephalitis-infected mouse model.17 Etanercept treatment led to thinner epithelial and basement membrane layers and lower goblet and mast cell numbers than in untreated asthmatic mice.18 In addition, this agent decreased blood pressure and protected kidney function in a mouse model of systemic lupus erythematosus.19
Much remains unknown about the pathophysiology of CRS-associated olfactory loss, due in part to the relative inaccessibility of the human olfactory cleft and the limitations inherent in obtaining repeated tissue samples. The development of the IOI transgenic mouse model greatly facilitates research on cellular and molecular pathways that would be nearly impossible to explore in human subjects. The present study is the first to apply Etanercept in the study of inflammatory olfactory loss. The known modes of toxicity of Etanercept include systemic infection and sepsis, but we found no adverse effects in mice at a dose of 100 µg/dose (3 times/week). We believe that this dosage, treatment schedule, and delivery route are optimal for the effective blocking of TNF-α. Our study design was limited to examining the effects of Etanercept only on the early (2 weeks) and late stages (8 weeks) stages of inflammation in the TNF-α-expressing IOI mouse. Future studies will involve analysis of the effects of TNF-α inhibition at additional time points, and in IOI mice with induced expression of other cytokines in the OE.
Conclusion
Concomitant administration of Etanercept to IOI mice results in interruption of TNF-α-induced olfactory loss and partial recovery of the olfactory epithelium. This demonstrates that the human soluble TNF receptor blocker, Etanercept, can block the TNF-α-induced inflammatory process in an IOI mouse model, and thus has potential to be a useful tool for elucidating the role of TNF-α in other olfactory inflammation models.
Acknowledgments
Sponsorships: None.
Funding source: R01DC09026 (A.P.L)
Footnotes
Author Contributions
Yong Gi Jung: acquisition of data, study design, statistical analysis, drafting, presentation at an annual academy meeting, final approval, accountability for all aspects of the work
Andrew P. Lane: analysis or interpretation of data, revising the work, final approval, accountability for all aspects of the work
Disclosures
Competing interests: None.
References
- 1.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 2.Mann NM, Lafreniere D. Anosmia and nasal sinus disease. Otolaryngol Clin North Am. 2004;37:289–300. vi. doi: 10.1016/S0030-6665(03)00157-9. [DOI] [PubMed] [Google Scholar]
- 3.Jung YG, Lee JS, Park GC. Does post-infectious olfactory loss affect mood more severely than chronic sinusitis with olfactory loss? Laryngoscope. 2014;124:2456–2460. doi: 10.1002/lary.24691. [DOI] [PubMed] [Google Scholar]
- 4.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125:116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 5.Raviv JR, Kern RC. Chronic sinusitis and olfactory dysfunction. Otolaryngol Clin North Am. 2004;37:1143–1157. v–vi. doi: 10.1016/j.otc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000;110:1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010;30:2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan B, May LA, Lane AP. The role of TNF-alpha in inflammatory olfactory loss. Laryngoscope. 2011;121:2481–2486. doi: 10.1002/lary.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000;14:367–373. doi: 10.2500/105065800779954329. [DOI] [PubMed] [Google Scholar]
- 10.Kern RC, Conley DB, Haines GK, 3rd, Robinson AM. Pathology of the olfactory mucosa: implications for the treatment of olfactory dysfunction. Laryngoscope. 2004;114:279–285. doi: 10.1097/00005537-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Kuehnemund M, Ismail C, Brieger J, Schaefer D, Mann WJ. Untreated chronic rhinosinusitis: a comparison of symptoms and mediator profiles. Laryngoscope. 2004;114:561–565. doi: 10.1097/00005537-200403000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. 2010;24:192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott LJ. Etanercept: a review of its use in autoimmune inflammatory diseases. Drugs. 2014;74:1379–1410. doi: 10.1007/s40265-014-0258-9. [DOI] [PubMed] [Google Scholar]
- 14.Pozharskaya T, Lane AP. Interferon gamma causes olfactory dysfunction without concomitant neuroepithelial damage. Int Forum Allergy Rhinol. 2013;3:861–865. doi: 10.1002/alr.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdaca G, Gulli R, Spano F, Mandich P, Puppo F. Pharmacogenetics and future therapeutic scenarios: what affects the prediction of response to treatment with etanercept? Drug Dev Res. 2014;75(Suppl 1):S7–S10. doi: 10.1002/ddr.21185. [DOI] [PubMed] [Google Scholar]
- 16.Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res. 2014;60:277–288. doi: 10.1007/s12026-014-8565-5. [DOI] [PubMed] [Google Scholar]
- 17.Ye J, Jiang R, Cui M, et al. Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis. 2014;210:875–889. doi: 10.1093/infdis/jiu179. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz O, Karaman M, Bagriyanik HA, et al. Comparison of TNF antagonism by etanercept and dexamethasone on airway epithelium and remodeling in an experimental model of asthma. Int Immunopharmacol. 2013;17:768–773. doi: 10.1016/j.intimp.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Venegas-Pont M, Manigrasso MB, Grifoni SC, et al. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]