Abstract
Recent years have seen major breakthroughs in genome-engineering systems, such as transposon-mediated gene delivery systems and CRISPR-Cas9-mediated genome-editing tools. In these systems, transient expression of auxiliary genes is responsible for permanent genomic modification. For both systems, it would be valuable to select for cells that are likely to undergo stable genome modification. Importantly, in particular for clinical applications of genome-engineered cell products, it will also be of importance to remove those cells that, due to random vector integration, display an unwanted stable expression of the auxiliary gene. Here, we develop a traceless selection system that on the one hand allows efficient enrichment of modified cells, and on the other hand can be used to select against cells that retain expression of the auxiliary gene. The value of this system to produce highly enriched-auxiliary gene-free cell products is demonstrated.
Keywords: deletion, gene insertion, modification
Introduction
Over the past few years, a number of novel tools have been developed that greatly facilitate genome engineering, both for research purposes, and for the creation of genome-modified cell therapeutics. Two remarkable examples of this are the molecularly evolved transposon systems1,2 and the CRISPR system.3,4,5 Transposon-based gene delivery systems are nonviral systems for gene delivery that are based on the transfection of two plasmids, of which the first encodes the transposase that mediates the integration into the host genome of the transposon sequence that is present within the second. Likewise, the CRISPR system is based on two elements, the Cas9 gene product and the single-guiding RNA (sgRNA) that are together required for genome modification. In this case, the sequence of the sgRNA guides the Cas9 enzyme to a specific spot in the genome where it induces a DNA double strand break. This system can be used to destroy a target open reading frame, exploiting the nonhomologous end joining machinery, or to induce defined genomic alterations, by homology-mediated DNA repair.6
Although used for different purposes, in both these systems transient enzymatic activity is required to produce a stable genomic modification. Furthermore, in both cases, continuous expression of this auxiliary gene product is undesirable, because of the possibility of further genomic alterations, an issue that appears of particular importance for the generation of genome-engineered cell therapeutics.7 One potential strategy to avoid this issue is the use of RNA encoding the auxiliary factor, but in many settings, the use of DNA-encoded auxiliary factors is more convenient in terms of practicality and costs. Furthermore, as the efficiency of both CRISPR and transposon genome modification platforms is variable and can be low in particular in primary cells, we set out to develop a selection system that allows the enrichment of cells undergoing transposase-mediated gene transfer or Cas9-mediated genome editing, and that at the same time avoids the safety risks associated with DNA-encoded transposase or Cas9.
To achieve this, we modified the plasmids encoding either the molecularly enhanced Sleeping Beauty (SB100X) transposase or the Cas9 gene, by the addition of different selection markers. We show that such introduction of selection markers into vectors that encode these auxiliary genes can be used to efficiently select for cells that subsequently undergo stable genome engineering. Furthermore, we show that the same markers can be used to select against cells harboring unwanted subsequent integration of the auxiliary gene. Among the marker systems utilized, the truncated version of the epidermal growth factor receptor (trEGFR)8 is of particular interest for clinical applications, as reagents required for cell enrichment are available in clinical grade, and as depletion of cells with prolonged auxiliary gene expression may not only be achieved in vitro but also in vivo, by application of the EGFR targeting antibody cetuximab. We believe that the method described here can greatly facilitate the clinical application of plasmid-based genome-engineering systems.
Results
Design of a traceless cell selection system
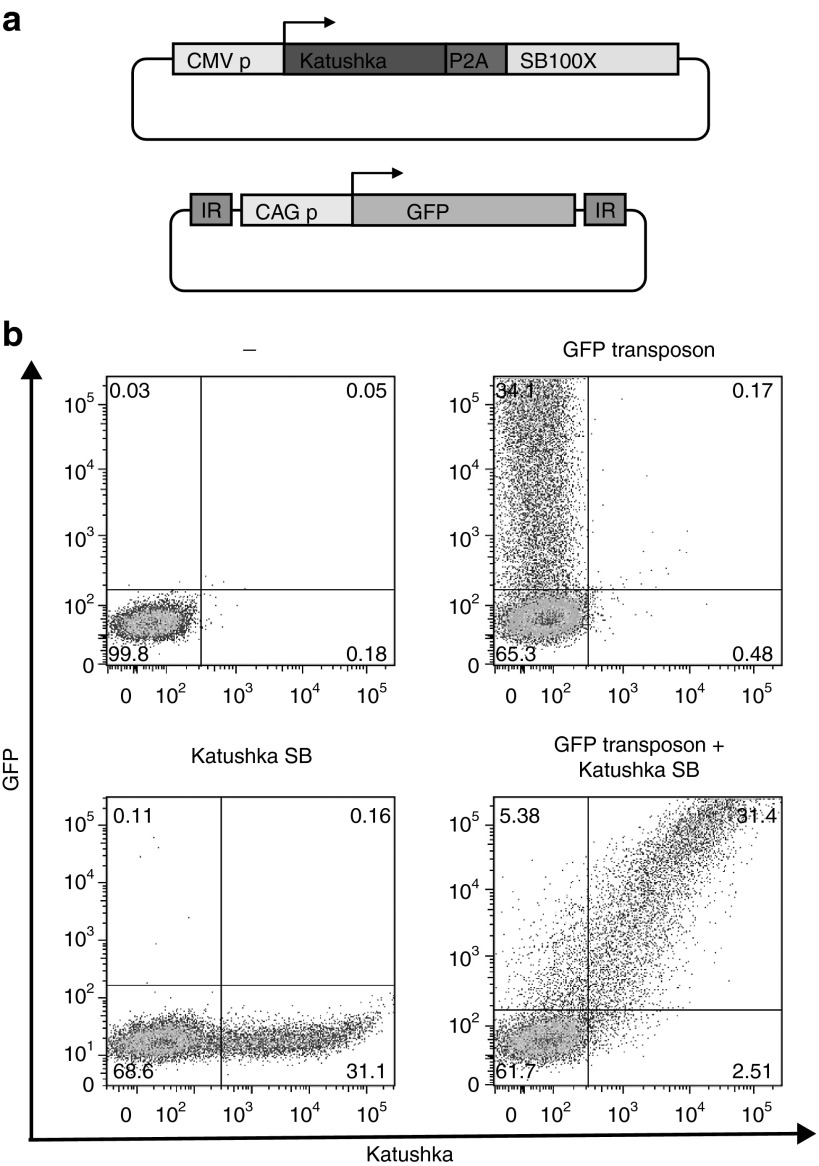
With the aim to develop a “traceless” selection system to, shortly after transfection, enrich cells that are likely to subsequently undergo stable gene modification, we first evaluated the kinetic of expression of two genes expressed from two independent plasmids when simultaneously transfected. To this purpose, we cotransfected a plasmid encoding the Katushka red fluorescent protein plus SB100X transposase (“KPS”), together with a GFP encoding transposon plasmid (Figure 1a) into peripheral blood mononuclear cells (PBMCs) and evaluated fluorochrome expression after 24 hours. Notably, the vast majority of cells either expressed neither of the two fluorochromes or both. Furthermore, flurochrome expression levels in the small fraction of cells that expressed only a single fluorochrome was low (Figure 1b). Thus, as shown long ago for calcium phosphate based transfection, in which DNA aggregates are taken up, also upon electroporation most cells that are successfully transfected receive multiple plasmids. These results suggested that efficient selection of cells that can undergo stable gene modification may be feasible by introduction of a selection marker solely with the plasmid encoding the auxiliary gene product.
Figure 1.
Two vector electroporation dynamics in human peripheral blood mononuclear cells (PBMCs). (a) Vector design. CMV p: immediate-early cytomegalovirus promoter; P2A: porcine teschovirus-1 2A element; SB100X: molecularly evolved sleeping beauty transposase; IR: SB transposon inverted repeat; GFP, enhanced green fluorescent protein. (b) Flow cytometric analysis of human PBMCs transfected with KPS, GFP encoding transposon, or both, 24 hours after transfection. Note that when both vectors are cotransfected (bottom right plot) the large majority of cells either express none or both fluorochromes.
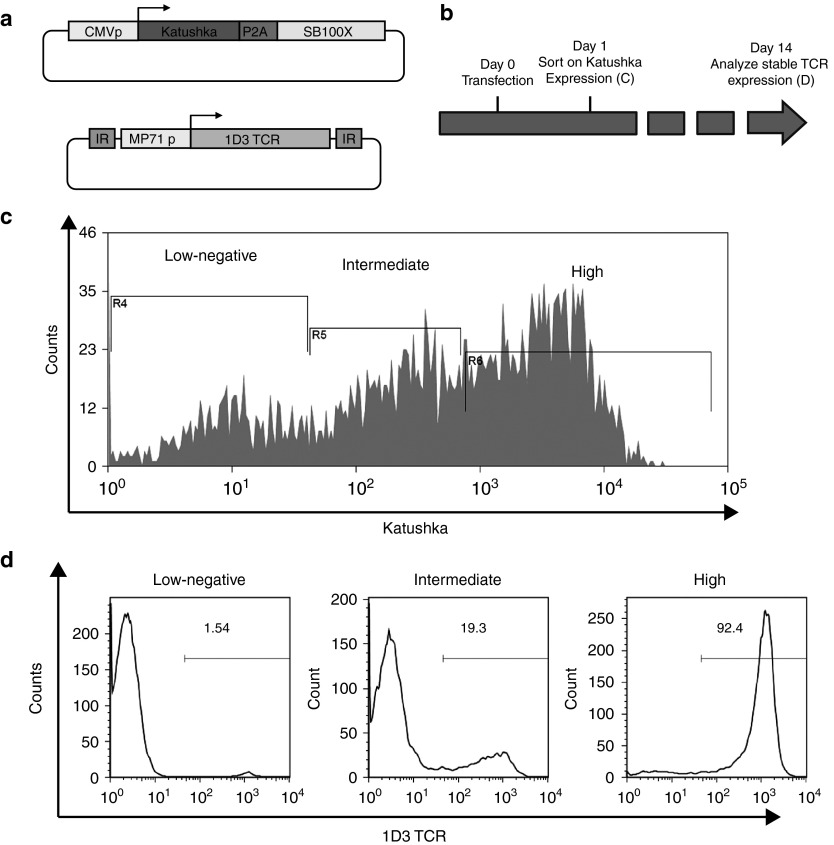
To test this possibility, human PBMCs were cotransfected with the KPS plasmid and a transposon plasmid encoding the 1D3 T cell receptor (TCR; Figure 2a). Twenty-four hours after transfection, cells were sorted into populations that displayed either absent-low, intermediate, or bright Katushka expression (Figure 2b,c). Following cell sorting, cells were stimulated with anti-CD3/CD28 beads and cultured for 14 days, and the percentage of 1D3 TCR-positive cells in the different fractions was analyzed by MHC multimer staining and flow cytometry. Only a minor fraction of cells with absent-low Katushka expression shortly after transfection showed expression of the 1D3 TCR at a later time point. Furthermore, 1D3 TCR expression was also only observed in a small percentage of cells with intermediate Katushka expression shortly after transfection. In contrast, stable gene modification was observed in a very high fraction (>90%) of cells that displayed high initial Katushka expression (Figure 2c). These data establish the feasibility of cell enrichment on the basis of auxiliary gene expression, thereby allowing the development of traceless selection systems.
Figure 2.
Early enrichment of cells undergoing stable SB transposition. (a) Vectors design. MP71 p: hybrid promoter MP71; 1D3: 1D3 TCR. (b) Schematic experimental time line. Peripheral blood mononuclear cells (PBMCs) were thawed 24 hours prior transfection, transfected and sorted by FACS 24 hours after transfection. Stable gene modification in the indicated fractions was assessed 14 days after transfection. (c) Sorting strategy. Indicated cell populations with either low-negative, intermediate, or high expression levels of Katushka were isolated and cultured in order to analyze stable gene modification efficiency. (d) Stable gene modification efficiency of the indicated cell populations, as established 14 days after transfection. Only cells isolated on the basis of high expression of Katushka show a very high fraction of cells stably expressing the TCR.
Efficient selection of transposon and CRISPRs gene-modified cells on the basis of transient puromycin resistance
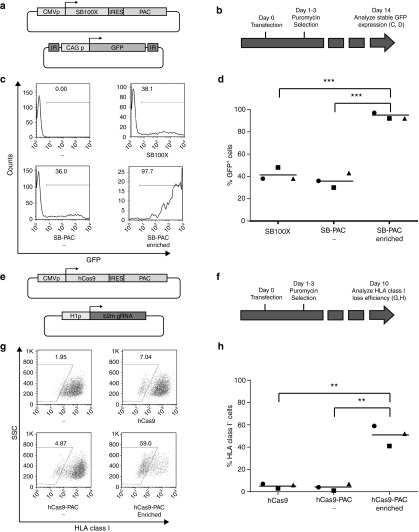
Given these results, we aimed to develop a traceless system that allows the efficient selection of cells undergoing genome modifications. Of the available drug resistance systems, puromycin resistance is particularly attractive for this purpose, as puromycin-mediated cell death occurs within days, thereby allowing the selection of cells that carry the auxiliary plasmid episomally for only a limited amount of time. To this purpose, we generated a vector encoding the SB100X transposase plus the puromycin N-acetyl-transferase (PAC) gene (SB100X-IRES-PAC, Figure 3a). Subsequently, HeLa cells were transfected with a GFP encoding transposon plasmid, together with either the SB100X-IRES-PAC plasmid or the parental SB100X plasmid. Twenty-four hours after transfection, parts of the cells transfected with the SB100X-IRES-PAC plasmid were treated with puromycin for a 48-hour time period (Figure 3b). Notably, this brief period of puromycin selection shortly after transfection led to a marked increase in the frequency of cells showing stable transposon-mediated gene integration. Specifically, at day 14 of culture, 94% of the puromycin-treated cells expressed GFP, whereas GFP expression was only observed in 41 and 36% of the cells transfected with either SB100X or SB100X-IRES-PAC (average of three experiments, SB100X-IRES-PAC enriched versus SB100X-IRES-PAC and SB100X-IRES-PAC enriched versus SB100X: both P < 0.001; SB100X versus SB100X-IRES-PAC: not significant, Figure 3c,d).
Figure 3.
Drug selection based enrichment of SB and hCas9 gene-modified cells. (a) Vectors used to evaluate the effect of puromycin selection on stable SB100X transposition. CAG p, chicken β-actin promoter with CMV enhancer; IRES, hepatitis C virus internal ribosome entry site; PAC, puromycin N-acetyl-transferase cassette. (b) Schematic experimental time line. HeLa cells were transfected and, after 24 hours, cultured in the presence of puromycin for 48 hours. 14 days after transfection, stable transposition was assessed by flow cytometry. (c) Flow cytometric analysis of the effect of early puromycin selection on stable GFP transposition. Puromycin-selected cells (bottom right plot) become almost uniformly GFP positive. (d) Summary of three independent SB gene transfer enrichment experiments. In all cases, early puromycin selection leads to very high stable GFP expression (***P < 0.001). (e) Vectors used to evaluate the effect of puromycin selection on hCas9-mediated genome editing. hCas9, humanized Cas9; H1p, DNA polymerase III promoter H1; b2m sgRNA, β2M-specific guiding RNA. (f) Schematic experimental time line. HeLa cells were transfected and, after 24 hours, cultured in the presence of puromycin for 48 hours. Fourteen days after transfection, stable HLA class I loss was evaluated by flow cytometry. (g) Flow cytometric analysis of the effect of early puromycin selection on stable HLA class I loss. Puromycin-treated cells (bottom right plot) are highly enriched for HLA class I-negative cells. (h) Summary of three independent hCas9 genome editing enrichment experiments. In all cases, early puromycin selection greatly increases the percentage of cells with stable HLA class I loss (**P value < 0.01).
To evaluate whether the same selection system can also be utilized to enhance the efficiency of CRISPR-mediated genome editing, we first generated a set of guiding RNAs (sgRNAs) for the β2 microglobulin (β2m) gene that is required for cell surface HLA class I expression. HeLa cells were transfected with hCas9 plus sgRNA, and loss of HLA class I expression was evaluated after 5 days. The highest frequency of HLA class I loss that was achieved with this set of sgRNAs was 8.4% (data not shown), indicating that selection of cells that are likely to undergo genome editing could be of value. To evaluate this, we generated a plasmid encoding hCas9 and PAC in an IRES-linked configuration (Figure 3e). We subsequently transfected HeLa cells with the β2m sgRNA encoding plasmid, together with either the hCas9 plasmid or the hCas9-IRES-PAC plasmid. Twenty-four hours after transfection, cells transfected with the hCas9-IRES-PAC plasmid were either left untreated or exposed to puromycin for 48 hours (Figure 3f). Analysis of HLA class I expression after 10 days of culture demonstrated that puromycin-treated cells contained very high frequencies of cells negative for HLA class I (average of 51%), as compared to cells transfected with the same plasmid system that were not exposed to puromycin (average of 4%), and to cells modified with the standard hCas9 (average of 5%) (average of three experiments, hCas9-IRES-PAC enriched versus hCas9-IRES-PAC, and hCas9-IRES-PAC enriched versus hCas9: both P < 0.01; hCas9 versus hCas9-IRES-PAC: not significant, Figure 3g,h). Genome editing was confirmed by analysis of the genomic area targeted by the sgRNA using the Tracking of Indels by Decomposition (TIDE) algorithm, and by sgRNA requirement (Supplementary Figures S1a and S2).9 Collectively, these data demonstrate that the introduction of fluorescent or drug resistance markers within auxiliary plasmids allows the efficient selection of stably modified cells in a simple, fast and traceless manner, both in the context of transposon-mediated gene transfer and in the context of CRISPR-hCas9-mediated genome editing.
Efficient selection of transposon gene-modified cells on the basis of transient trEGFR expression
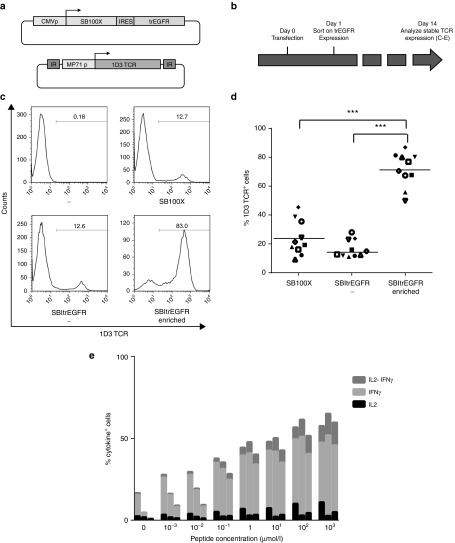
In order to exploit this concept in a clinically applicable format, we generated a vector that encodes the truncated EGFR receptor8 plus the SB100X transposase in an IRES-linked configuration (SB-IRES-trEGFR). This design offers the advantage that trEGFR expression is modest relative to that of SB100X, ensuring that selection of cells on the basis of trEGFR yields a cell population that expresses high SB100X levels. To test the possibility of enrichment of cells that are likely to undergo stable gene modification on the basis of transient trEGFR expression, PBMCs were electroporated with the 1D3 transposon and SB-IRES-trEGFR vectors, and after 24 hours, trEGFR-expressing cells were isolated by magnetic bead sorting (Figure 4a,b). As a first control, a fraction of the transfected cells was left unsorted and cultured in parallel. As a second control, cells were transfected with the 1D3 transposon in combination with the nonmodified SB100X vector. Analysis of 1D3 TCR expression 14 days after transfection demonstrated that cell populations isolated on the basis of trEGFR expression showed a very high level of stable TCR gene modification (Figure 4c,d; 71.7 ± 11.7%), with a high recovery of the TCR-modified cell population (51.6 ± 18.7%). By comparison, frequency of cells showing stable 1D3 TCR expression was 18.7 ± 10.8% for cells modified with the same plasmid system that had not undergone cell enrichment, and 24.0 ± 12.0% for cells transfected with the standard SB100x transposon system (SB-IRES-trEGFR enriched versus SB-IRES-trEGFR, and SB-IRES-trEGFR enriched versus SB100X: both P < 0.0001; SB100X versus SB-IRES-trEGFR: not significant, Figure 4c,d). Furthermore, this increase in the percentage of 1D3 TCR expressing T cells was accompanied by a quantitatively similar increase in the number of gene-modified cells capable of recognizing MART-I26-35-positive target cells, as shown by cytokine secretion assays (Figure 4e).
Figure 4.
Enrichment of SB gene-modified primary T cells on the basis of transient trEGFR expression. (a) Vectors used. trEGFR: truncated epithelial growth factor receptor. (b) Schematic experimental time line. PBMCs were thawed 24 hours prior to electroporation. Twenty-four hours after transfection, trEGFR cells were magnetically sorted. T cells were cultured for 14 days, and 1D3 TCR stable expression and functionality were evaluated by flow cytometry. (c) Flow cytometric analysis of the effect of early trEGFR selection on stable 1D3 transposition. (d) Summary of ten independent SB gene transfer enrichment experiments, using cells from independent donors. In all cases, early enrichment greatly increases the percentage of stably modified cells (***P < 0.001). (e) For each peptide concentration, the percentage of cytokine secreting T cells of TCR redirected T cells is shown. Bars represent SB redirected (left), SBItrEGFR redirected (central), and SBItrEGFR redirected and selected (right). Data were normalized for the percentage 1D3 TCR+ cells.
Efficient selection of CRISPRs genome-edited cells on the basis of episomal expression of truncated EGFR.
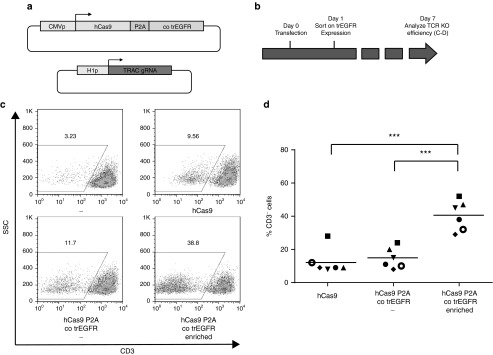
In order to evaluate the feasibility of enrichment of cells undergoing hCas9 genome modification on the basis of transient trEGFR expression, we first developed an optimal sgRNA specific for the TCR-α constant chain (TRAC) by screening a series of TRAC sgRNAs in Jurkat T cells (data not shown). In addition, a vector encoding the codon optimized truncated EGFR receptor plus hCas9 gene in a P2A-linked configuration (hCas9 P2A cotrEGFR) was developed. Subsequently, activated PBMCs were transfected with hCas9 P2A cotrEGFR and the TRAC sgRNA, and 24 hours after electroporation, trEGFR-expressing cells were isolated by magnetic bead sorting or left untreated (Figure 5a,b). As a second control, cells were transfected with TRAC sgRNA and the standard hCas9 plasmid. Cell populations isolated on the basis of trEGFR expression shortly after transfection showed a high frequency of CD3-negative cells at day 7 after transfection (40.5 ± 9.0%, Figure 5c,d). As a comparison, the frequency of CD3-negative cells was approximately threefold lower in both control conditions (14.7 ± 6.3% for hCas9P2AtrEGFR-transfected cells that were not subjected to enrichment, and 12.5 ± 7.7% for cells transfected with the standard hCas9 system; hCas9 P2A cotrEGFR enriched versus hCas9 P2A cotrEGFR, and hCas9 P2A cotrEGFR enriched versus SB100X: both P < 0.001, hCas9 versus hCas9 P2A cotrEGFR: not significant, Figure 5c,d). Cas9-mediated genome editing was again confirmed by sgRNA dependency (Supplementary Figure S1b) and analysis of the genomic area targeted by the sgRNA (Supplementary Figure S3) of both the unselected and the enriched fraction for two donors. Additionally, we performed the same type of analysis on the top three predicted off target sites (Supplementary Table S1 and Supplementary Figure S4). As compared to the background signal seen in untransfected samples, evidence was obtained for off-target editing of one of these sites in one sample, but this was not increased relative to cells transfected with a control Cas9 vector (Supplementary Figure S4).
Figure 5.
Enrichment of hCas9 genome-edited primary T cells on the basis of transient trEGFR expression. (a) Vectors used. cotrEGFR, codon-optimized truncated epithelial growth factor receptor; TRAC sgRNA, T-cell receptor-α constant-specific guiding RNA. (b) Schematic experimental time line. Peripheral blood mononuclear cells were thawed and activated 2–3 days prior to electroporation. Twenty-four hours after transfection, trEGFR cells were magnetically sorted and cultured. Seven days after transfection, efficiency of stable gene inactivation was evaluated by flow cytometry. (c) Flow cytometric analysis of the effect of early cotrEGFR selection on the efficiency of stable TCR-α gene inactivation. (d) Summary of six independent hCas9 genome-editing enrichment experiments, involving cells from different donors. (***P < 0.001).
Efficient counter-selection of cells harboring stable integration of the auxiliary plasmid
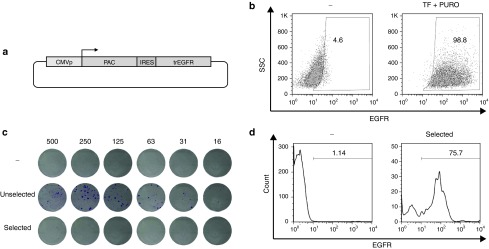
A clear advantage of the introduction of selectable surface markers with an auxiliary vector is that it not only allows the positive selection of transfected cells early after gene modification, but also the removal of cells that inadvertently show continuous expression of the auxiliary gene at later time points. In order to evaluate whether cells that continue to express the auxiliary gene after random plasmid integration also show continued expression of the selection marker, we generated a plasmid encoding the puromycin resistance gene product and the trEGFR linked by an IRES element (PAC-IRES-trEGFR, Figure 6a). Jurkat T cells were then transfected with PAC-IRES-trEGFR and 4 days after transfection puromycin was added to select for cells that had undergone random integration of the auxiliary vector. Following a 30-day culture in the presence of puromycin, cells were analyzed for EGFR expression. This analysis showed a near homogenous (>98%) EGFR positivity (Figure 6b). As this vector design recapitulates the design of enrichment vectors (see Figures 4a and 5a), these data indicate that those cells that would retain expression of transposase or hCas9 after random auxiliary plasmid integration would also retain expression of trEGFR, thereby allowing their subsequent depletion.
Figure 6.
Counter-selection of cells harboring unwanted auxiliary vector integration. (a) Vector used. (b) Flow cytometric analysis of untransfected Jurkat cells and transfected Jurkat cells that were exposed to puromycin selection. Cells resistant to puromycin are almost uniformly positive for EGFR. (c) B16 colony formation by the indicated cell populations. Top: untransfected cells; middle: transfected cells; bottom: transfected cells that were subjected to negative selection. Numbers indicate input cell numbers times 10–3. Note that counter-selection against EGFR expression leads to the formation of only few puromycin-resistant colonies. (d) EGFR expression on untransfected cells (left) and transfected and puromycin selected cells (right). The majority of cells surviving puromycin treatment retain EGFR expression.
To test the efficiency of such depletion, B16 murine melanoma cells (that are negative for cetuximab staining, data not shown) were transfected with PAC-IRES-trEGFR. After 30 days of culture, cells were either magnetically depleted of EGFR-positive cells or left untreated. Subsequently, serial dilutions (5 × 105–15.625 × 103) of depleted, nondepleted, or untransfected cells were plated and cultured in presence of puromycin and, after 10 days, stained with crystal violet. Cells depleted from the EGFR+ fraction formed drastically fewer colonies than the nondepleted control (131 versus 4 colonies for 5 × 105 cells, 82 versus 1 colonies for 2.5 × 105 cells, 65 versus 0 for 1.25 × 105 cells, 38 versus 0 for 62.5 × 104 cells, 19 versus 0 for 31.25 × 104 cells, and 10 versus 0 for 15.625 × 104 cells (Figure 6c), showing the feasibility of counter-selection of cells harboring unwanted expression of the auxiliary gene. The few cells that did grow out from the EGFR-depleted cell population upon puromycin treatment remained largely EGFR positive, indicating that they potentially may still be targeted by repeated cetuximab exposure (Figure 6d). Since transposase- or Cas9-mediated double-strand DNA breaks that may facilitate aberrant integration were absent in the above setting, we subsequently performed experiments in which, together with PAC-IRES-trEGFR, a GFP encoding transposon and the SB-IRES-trEGFR were cotransfected (Supplementary Figure S5a). Transfected cells were either left unsorted or magnetically sorted for absence or presence of EGFR expression. After 14 days of culture, cells were either magnetically depleted of EGFR-positive cells or left untreated. Serial dilutions (5 × 105–15.625 × 103) of cells from all conditions were plated and cultured for 10 days in presence of puromycin (Supplementary Figure S5b). Importantly, we still were able to counter select 86% of those cells harboring a stable episomal integration (total of 186 versus 25 colonies), showing that the counter-selection is feasible also in a context where double-strand break is induced.
Discussion
Recent years have seen a rapid development in technologies that can be utilized for genome engineering. Two examples of those recently introduced technologies are the molecularly enhanced Sleeping Beauty transposase,1 that allows nonviral gene transfer with high efficiency and the Cas9-mediated genome editing system, that allows genome editing in a straightforward way.3,4,5 Both these systems rely on the expression of an auxiliary gene whose activity is required only temporarily for stable genome modification, and for which continued activity is undesirable in clinical use. In this work, we show that, by selecting for cells expressing a selection marker that is coexpressed with the auxiliary gene required for genome modification (i.e., the transposase or the hCas9), it is possible to obtain a cell product that is (i) highly enriched for genome-modified cells, and (ii) depleted of cells with continued auxiliary gene expression.
To our knowledge, this report provides the first strategy that allows the traceless enrichment and counter-selection of gene-modified cells in a single system. With respect to the potential enrichment of genome-edited cells, Kim and colleagues10,11 have described vectors suitable for enrichment of TALEN and CRISPR genome-edited cells, based on an out-of-frame selection marker downstream of a TALEN or CRISPR recognized site. In this set up, the generation of indels after genome editing of the plasmid has a certain probability to re-establish the correct frame, thereby permitting cell selection. Although not evaluated in primary cells, data obtained in transformed cell systems demonstrate the feasibility of this approach. However, it does not allow the counter-selection of cells that inadvertently integrate the auxiliary gene. Ran and colleagues6 have described vectors in which the sgRNA and the hCas9 are linked to a puromycin selection marker, but this system can not be used for counter-selection. Furthermore, the selection—counter-selection strategy we describe in this report is easily translatable to clinical application, as all the necessary reagents are available in clinical grade.
While we have here focused on the use of the truncated EGFR as a clinically useful selection/counter-selection system for the creation of transposon or hCas9 gene-modified cells, it is evident that the same approach may be used for other recently developed marker systems, such CD19 (refs. 12,13), CD20 (ref. 14), or RQR8 (ref. 15). By the same token, while we here demonstrate enrichment of both SB gene-modified cells and hCas9 genome-edited cells, it is apparent that the same strategy can be applied to other transposon gene transfer systems2 and to other tools for genome editing, such as zinc finger nucleases16 or TALENs.17 As a side note, in all these cases, the “traceless” nature of the positive selection system avoids the potential deletion of the gene-modified T cells in vivo as a consequence of inadvertent immune recognition, as has for instance been observed for the HSV TK suicide switch.18
Materials and methods
Vectors. The pT2-CAGGS-GFP transposon vector, encoding GFP under control of the chicken albumin promoter, and the SB100X transposase vector, encoding the hyperactive SB100X transposase under control of the cytomegalovirus (CMV) promoter were generously provided by Z. Izsvak (Max Delbrück Institute, Berlin, Germany) and have been described previously.1 The transposon vector encoding the codon-optimized 1D3 T-cell receptor specific for the melanocyte differentiation antigen MART-1 (ref. 19), under control of the MP71 (ref. 20) promotor was generated by cloning the corresponding expression cassette21 between the EcoRI and NotI sites of pT2-HB.22 To generate the SB P2A Katushka plasmid, the Katushka encoding sequence was amplified by polymerase chain reaction (PCR) with a forward primer containing the P2A sequence and cloned into the ApaI site of the SB100X plasmid. To generate the SB100X IRES PAC plasmid that encodes the PAC enzyme, an IRES PAC cassette was amplified by PCR and cloned into the ApaI site of the SB100X plasmid. To generate the SB100X IRES trEGFR plasmid that encodes the truncated EGFR, an IRES trEGFR cassette was generated and cloned into the ApaI site of the SB100X transposase vector. The hCas9 vector (Addgene plasmid 41815) has been described previously.5 The hCas9 IRES PAC plasmid was generated by cloning a PCR amplified IRES PAC fragment into the PmeI site of the hCas9 plasmid. To create the hCas9 P2A codon optimized trEGFR (cotrEGFR) expression cassette, a gene string fragment (Invitrogen, Carlsbad, CA) containing part of hCas9, the P2A element, and the cotrEGFR was cloned between the AscI and PmeI sites of the hCas9 plasmid. The PAC IRES trEGFR plasmid was generated by replacement of the SB100X coding sequence within the SB100X IRES trEGFR vector with the PAC-coding sequence. Guiding RNA (sgRNA) plasmids were ordered as geneblocks (Integrated DNA Technology, Coralville, IA), containing an H1 promoter, the target RNA, and the sgRNA scaffold, and directly cloned into the topo 2.1 vector (Invitrogen). The genomic sequences targeted by the sgRNAs are CGTGAGTAAACCTGAATCTT (β2m) and CTCGACCAGCTTGACATCAC (TRAC)
Cell culture, transfection, and enrichment. All cell lines were maintained in RPMI (GIBCO, Invitrogen), in the presence of 10% fetal calf serum (FCS; Sigma-Aldrich, St Louis, MO). Tranfection of HeLa and B16 cells was performed using Fugene 6 (Promega, Madison, WI). Adherent cells were seeded 24 hours before transfection into a six-well plate at 1*105 cells/ well, and were transfected using 5 μl of Fugene 6 (Promega) and either 0.5 μg of each plasmid for experiments using hCas9, or 1 μg of plasmid for experiments using PAC IRES trEGFR. Puromycin selection was initiated 24 hours after transfection, for a total of 48 hours at a concentration of 3 μg ml−1.
Jurkat cells were transfected using the Amaxa Nucleofector 4D system (Lonza, Basel, Switzerland), according to the manufacturer's protocol. Briefly, cells were washed once with PBS/0.5% BSA, and for each transfection reaction, 106 cells were resuspended in 100 µl nucleofection reagent SE and transferred into a cuvette with 5 μg of plasmid DNA. For transfection, program CL-120 was used. PBMCs, derived from buffy coats from anonymous healthy donors (Sanquin, Amsterdam, The Netherlands) were isolated by Ficoll-Isopaque density centrifugation and cryopreserved in liquid nitrogen.
For DNA electroporation, cells were transfected using the Amaxa Nucleofector 4D system (Lonza), according to the manufacturer's protocol. For transposon experiments, PBMCs were thawed 24 hours prior to nucleofection and cultured in RPMI supplemented with 10% human serum, 50 IU ml−1 IL-2 (Novartis, Basel, Switzerland), and 10 ng ml−1 IL-15 (Peprotech, Rocky Hill, NJ). On the day of transfection, cells were harvested and washed once with phosphate-buffered saline/0.5% bovine serum albumin. For each transfection reaction, 8 × 106 cells were resuspended in 100 µl nucleofection reagent P3 and transferred into a cuvette together with 5 µg of transposase and 10 µg of transposon vector DNA.
For hCas9 electroporation, cells were activated using Phytohemagglutinin (2 μg ml−1, Biochrom AG, Berlin, Germany). 48–72 hours after activation, cells were harvested and washed once with PBS/0.5% BSA. For each transfection reaction, 5 × 106 cells were resuspended in 100 µl nucleofection reagent P3 and transferred into a cuvette together with 4 μg of hCas9 and 4 μg of sgRNA plasmid DNA. For all experiments involving PBMCs, program EO-115 was used. Following transfection, prewarmed medium was added to the cells, and cells were transferred into a 24-well plate. One day after transfection, trEGFR-expressing cells were purified on an MS column (Miltenyi Biotec, Bergisch Gladbach, Germany) with biotinylated cetuximab and Strep-Tactin microbeads (IBA GmbH, Göttingen, Germany), following the manufacturer's instructions. In SB100X transposon experiments, T cells were activated using anti-CD3/CD28 Human T-Activator beads (1 bead/cell, Invitrogen) following selection, and maintained in culture as described above.
Cytokine secretion assay. T2 cells were pulsed with MART-I26-35 peptide for 1–2 hours at 37 °C. Subsequently, 0.5 × 106 TCR-modified PBMCs were incubated with 0.5 × 106 peptide-pulsed T2 cells in RPMI containing 10% FCS and 1 μl ml−1 Golgiplug (BD Biosciences, San Jose, CA). After a 4- to 5-hour incubation at 37 °C, cells were washed and stained with FITC-labeled anti-CD3- and PerCP-Cy5-labeled anti-CD8, and analyzed for IFN-γ and IL-2 production by intracellular cytokine staining (all antibodies from BD Biosciences).
Statistical analysis and calculation of cell enrichment. Enrichment yield was calculated as (n cells pos fraction × TF efficiency pos fraction)/(n cells presorting × TF efficiency unsorted). All statistical comparisons were performed using a two-sided student's t-test.
Analysis of genome editing. To assess the efficiency of genome editing, genomic DNA was extracted and PCR reactions were performed using Phusion HF polymerase (New England Biolabs) with primer pairs spanning over the sgRNA target site (Supplementary Table S1). PCR products were purified (Illustra GFX PCR DNA purification kit, GE LifeSciences) and Sanger-sequenced. Each sequence chromatogram was analyzed with the online TIDE9 software (available at http://tide.nki.nl). Off target sites were predicted by using the CRISPR design tool23 (available at http://crispr.mit.edu).
SUPPLEMENTARY MATERIAL Figure S1. sgRNA dependency of genome editing. Figure S2. Analysis of genome editing in HeLa cells. Figure S3. Analysis of genome editing in PBMCs. Figure S4. Analysis of off-target genome editing in PBMCs. Figure S5. Counter-selection of cells harbouring unwanted auxiliary vector integrations generated in presence of transposase activity. Table S1. gRNA target sites and PCR primer pairs spanning the target sites.
Acknowledgments
We would like to thank S. Riddell and M. Jensen (University of Washington, USA) for sharing the trEGFR vector system, and Z. Izsvák (Max Delbrück Center for Molecular Medicine, Germany) for sharing the SB100X vector system. This work was supported by the EU FP7 ITN ATTACK and by the EU FP7 project SUPERSIST. R.M. designed, performed, analyzed and interpreted experiments, and wrote the paper. A.H. designed, performed, analyzed, and interpreted experiments. R.G.-E. designed, performed, analyzed, and interpreted experiments. T.N.S supervised the project, designed and interpreted experiments, and wrote the paper. The authors declare no competing financial interests.
Supplementary Material
sgRNA dependency of genome editing.
Analysis of genome editing in HeLa cells.
Analysis of genome editing in PBMCs.
Analysis of off-target genome editing in PBMCs.
Counter-selection of cells harbouring unwanted auxiliary vector integrations generated in presence of transposase activity.
gRNA target sites and PCR primer pairs spanning the target sites.
References
- Mátés, L, Chuah, MK, Belay, E, Jerchow, B, Manoj, N, Acosta-Sanchez, A et al. (2009). Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet 41: 753–761. [DOI] [PubMed] [Google Scholar]
- Yusa, K, Zhou, L, Li, MA, Bradley, A and Craig, NL (2011). A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci USA 108: 1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong, L, Ran, FA, Cox, D, Lin, S, Barretto, R, Habib, N et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek, M, East, A, Cheng, A, Lin, S, Ma, E and Doudna, J (2013). RNA-programmed genome editing in human cells. Elife 2: e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali, P, Yang, L, Esvelt, KM, Aach, J, Guell, M, DiCarlo, JE et al. (2013). RNA-guided human genome engineering via Cas9. Science 339: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, FA, Hsu, PD, Wright, J, Agarwala, V, Scott, DA and Zhang, F (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, PB, Largaespada, DA and Cooper, LJ (2010). A transposon and transposase system for human application. Mol Ther 18: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X, Chang, WC, Wong, CW, Colcher, D, Sherman, M, Ostberg, JR et al. (2011). A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118: 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman, EK, Chen, T, Amendola, M and van Steensel, B (2014). Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42: e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H, Um, E, Cho, SR, Jung, C, Kim, H and Kim, JS (2011). Surrogate reporters for enrichment of cells with nuclease-induced mutations. Nat Methods 8: 941–943. [DOI] [PubMed] [Google Scholar]
- Ramakrishna, S, Cho, SW, Kim, S, Song, M, Gopalappa, R, Kim, JS et al. (2014). Surrogate reporter-based enrichment of cells containing RNA-guided Cas9 nuclease-induced mutations. Nat Commun 5: 3378. [DOI] [PubMed] [Google Scholar]
- Tey, SK, Dotti, G, Rooney, CM, Heslop, HE and Brenner, MK (2007). Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant 13: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi, A, Tey, SK, Dotti, G, Fujita, Y, Kennedy-Nasser, A, Martinez, C et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365: 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini, M, Manganini, M, Borleri, G, Bonamino, M, Imberti, L, Biondi, A et al. (2004). Characterization of CD20-transduced T lymphocytes as an alternative suicide gene therapy approach for the treatment of graft-versus-host disease. Hum Gene Ther 15: 63–76. [DOI] [PubMed] [Google Scholar]
- Philip, B, Kokalaki, E, Mekkaoui, L, Thomas, S, Straathof, K, Flutter, B et al. (2014). A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124: 1277–1287. [DOI] [PubMed] [Google Scholar]
- Urnov, FD, Miller, JC, Lee, YL, Beausejour, CM, Rock, JM, Augustus, S et al. (2005). Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435: 646–651. [DOI] [PubMed] [Google Scholar]
- Christian, M, Cermak, T, Doyle, EL, Schmidt, C, Zhang, F, Hummel, A et al. (2010). Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini, C, Ferrari, G, Verzeletti, S, Servida, P, Zappone, E, Ruggieri, L et al. (1997). HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276: 1719–1724. [DOI] [PubMed] [Google Scholar]
- Jorritsma, A, Gomez-Eerland, R, Dokter, M, van de Kasteele, W, Zoet, YM, Doxiadis, II et al. (2007). Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood 110: 3564–3572. [DOI] [PubMed] [Google Scholar]
- Engels, B, Cam, H, Schüler, T, Indraccolo, S, Gladow, M, Baum, C et al. (2003). Retroviral vectors for high-level transgene expression in T lymphocytes. Hum Gene Ther 14: 1155–1168. [DOI] [PubMed] [Google Scholar]
- Bendle, GM, Linnemann, C, Hooijkaas, AI, Bies, L, de Witte, MA, Jorritsma, A et al. (2010). Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 16: 565–70, 1p following 570. [DOI] [PubMed] [Google Scholar]
- Cui, Z, Geurts, AM, Liu, G, Kaufman, CD and Hackett, PB (2002). Structure-function analysis of the inverted terminal repeats of the sleeping beauty transposon. J Mol Biol 318: 1221–1235. [DOI] [PubMed] [Google Scholar]
- Hsu, PD, Scott, DA, Weinstein, JA, Ran, FA, Konermann, S, Agarwala, V et al. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sgRNA dependency of genome editing.
Analysis of genome editing in HeLa cells.
Analysis of genome editing in PBMCs.
Analysis of off-target genome editing in PBMCs.
Counter-selection of cells harbouring unwanted auxiliary vector integrations generated in presence of transposase activity.
gRNA target sites and PCR primer pairs spanning the target sites.