Abstract
Corneal neovascularization (CNV) is a sight-threatening condition that is encountered in various inflammatory settings including chemical injury. We recently confirmed that angiopoietin-like protein 2 (ANGPTL2) is a potent angiogenic and proinflammatory factor in the cornea, and we have produced a single-stranded proline-modified short hairpin anti-ANGPTL2 RNA interference molecule that is carried in a lipid nanoparticle (ANGPTL2 Li-pshRNA) for topical application. In this study, we have further examined the topical delivery and anti-ANGPTL2 activity of this molecule and have found that fluorescence-labeled ANGPTL2 Li-pshRNA eye drops can penetrate all layers of the cornea and that ANGPTL2 mRNA expression was dramatically inhibited in both epithelium and stroma at 12 and 24 hours after administration. We also examined the inhibitory effect of ANGPTL2 Li-pshRNA on CNV in a mouse chemical injury model and found that the area of angiogenesis was significantly decreased in corneas treated with ANGPTL2 Li-pshRNA eye drops compared to controls. Together, these findings indicate that this modified RNA interference agent is clinically viable in a topical formulation for use against CNV.
Keywords: angiogenesis, cornea, lipid nanoparticles, topical RNAi eye drops
Introduction
Corneal neovascularization (CNV) is a sight-threatening condition that is encountered in various inflammatory settings including chemical injury. CNV can lead to corneal scarring, edema, and lipid deposition that may compromise visual function and decrease the success rate of subsequent penetrating keratoplasty.1 Topical corticosteroids have been the mainstay for suppressing CNV.2 However, the inhibitory effects of corticosteroids are limited and may be accompanied by adverse effects including delayed wound healing, infection, posterior subcapsular cataracts, and elevation of intraocular pressure. As an alternative, antibody-based anti-vascular endothelial growth factor molecular targeting therapy has recently become available for the treatment of CNV.3,4 However, this treatment also has several drawbacks, including high costs and questionable efficacy in terms of vessel regression and repair of epithelial defects.5
RNA interference (RNAi) occurs when small interfering RNA (siRNA) molecules, in association with a nucleolytic cytoplasmic protein complex, mediate sequence-specific degeneration of target RNA molecules. Theoretically, this mechanism can silence the expression of virtually any gene in a sequence-directed manner with high specificity. The potential clinical applications of this phenomenon are now being explored.
Angiopoietin-like protein 2 (ANGPTL2) is a member of the angiopoietin-like protein family and is essential for various physiologic activities, including lipid metabolism, angiogenesis, and inflammation.6,7 Kanda et al. have reported that ANGPTL2 in the ocular tissue was related to acute inflammation in the retina in an endotoxin-induced uveitis model via activation of an NF-κB signaling pathway, and they indicated that ANGPTL2 could be a therapeutic target in acute inflammatory disease.8 In addition, we have previously found that ANGPTL2 is a potent proangiogenic factor in mouse corneal inflammation and that subconjunctival injection of ANGPTL2 siRNA in mice dramatically suppressed local expression of ANGPTL2 mRNA and reduced the extent of CNV.9 This finding represents a new approach to the treatment of angiogenesis-associated corneal diseases. However, it is not ideal to perform repeated subconjunctival injections in the clinical setting, and a common problem with RNAi agents has been the lack of an effective in vivo drug delivery system. Moreover, because siRNAs are double stranded, they might act as ligands for Toll-like receptor 3,10 which may induce innate immune reactions.
To address these problems, we generated a single-stranded RNAi that can be delivered in a lipid nanoparticle for use as a topical anti-ANGPTL2 agent in the treatment of ocular diseases. In this study, we have examined its efficacy in terms of drug delivery and inhibition of CNV in a mouse alkali-burn model.
Results
Safety of proline-modified short hairpin RNA-loaded lipid nanoparticles in vitro
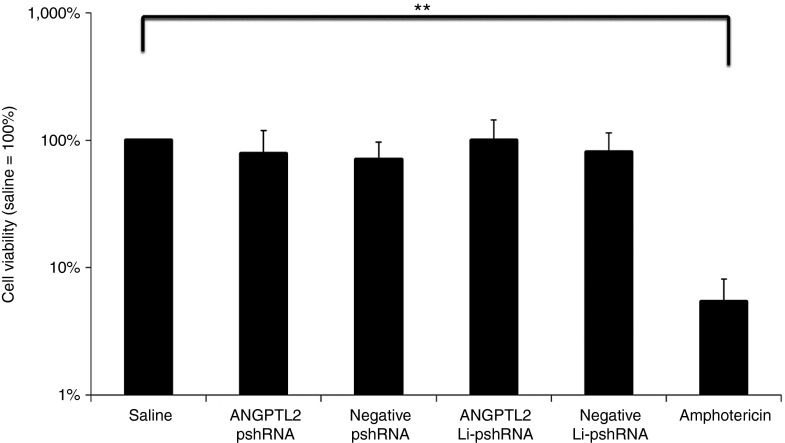
Cell viability was examined using simian virus 40-immortalized human corneal epithelial cells. There was no significant difference in posttreatment viability among five groups (control group, negative proline-modified short hairpin RNA (pshRNA) group, ANGPTL2 pshRNA group, negative pshRNA wrapped with liposome (Li-pshRNA), and ANGPTL2 Li-pshRNA group), and cell viability in all five groups was greater than that of cells treated with amphotericin B (P < 0.01 for comparison, Figure 1).
Figure 1.
WST-1 assay of Li-pshRNA. WST-1 assay was performed to confirm cell viability after treatment with Li-pshRNA. There was no significant difference in cell viability among the treatment groups (n = 4, **P < 0.01). ANGPTL2, angiopoietin-like protein 2; Li-pshRNA, proline-modified short hairpin RNA wrapped with liposome.
Drug delivery and efficacy in normal mouse eyes
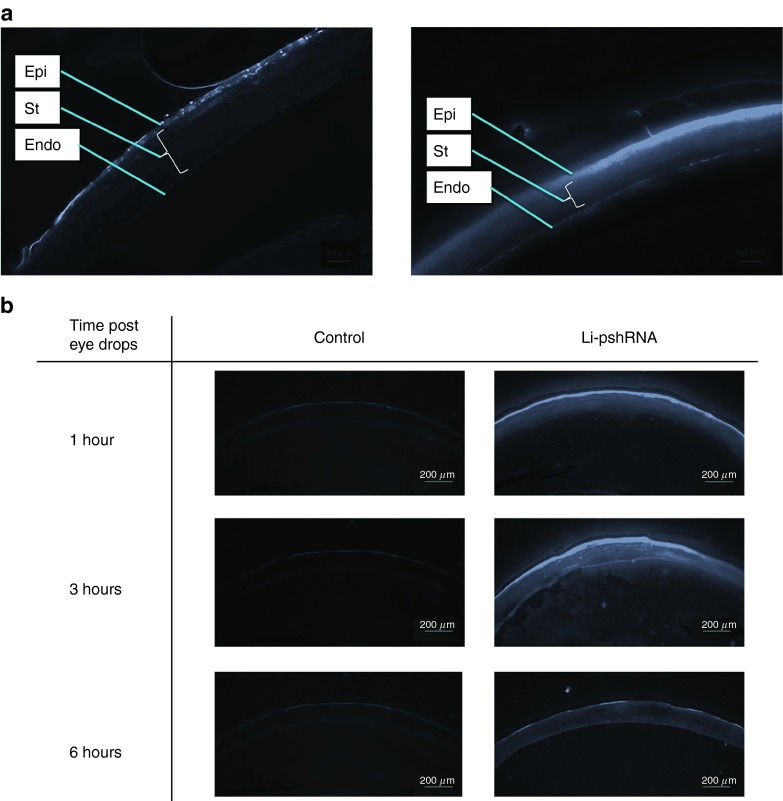
Fluorescence-labeled siRNA was distributed only in the superficial layer of the corneal epithelium, while fluorescence-labeled Li-pshRNA showed almost full-thickness distribution (Figure 2a) and was detected in the stroma and the endothelium at 3 and 6 hours after instillation of the eye drops (Figure 2b).
Figure 2.
Delivery of fluorescence-labeled pshRNA eye drops. (a) Left panel: bare siRNA with fluorescent label; right panel: fluorescence-labeled pshRNA enclosed in the lipid nanoparticle. The pshRNA was distributed only in the superficial layer of the corneal epithelium, while the Li-pshRNA was observed in almost all layers. (b) Delivery of fluorescence-labeled Li-pshRNA drops to mouse eyes. Samples were extracted at 1, 3, and 6 hours after instillation of the eye drops. Left panel: saline as control. Right panel: fluorescence-labeled Li-pshRNA. The Li-pshRNA was distributed to all layers as time advanced. Epi, epithelium; Endo, endothelium of cornea; St, stroma. Li-pshRNA, proline-modified short hairpin RNA wrapped with liposome.
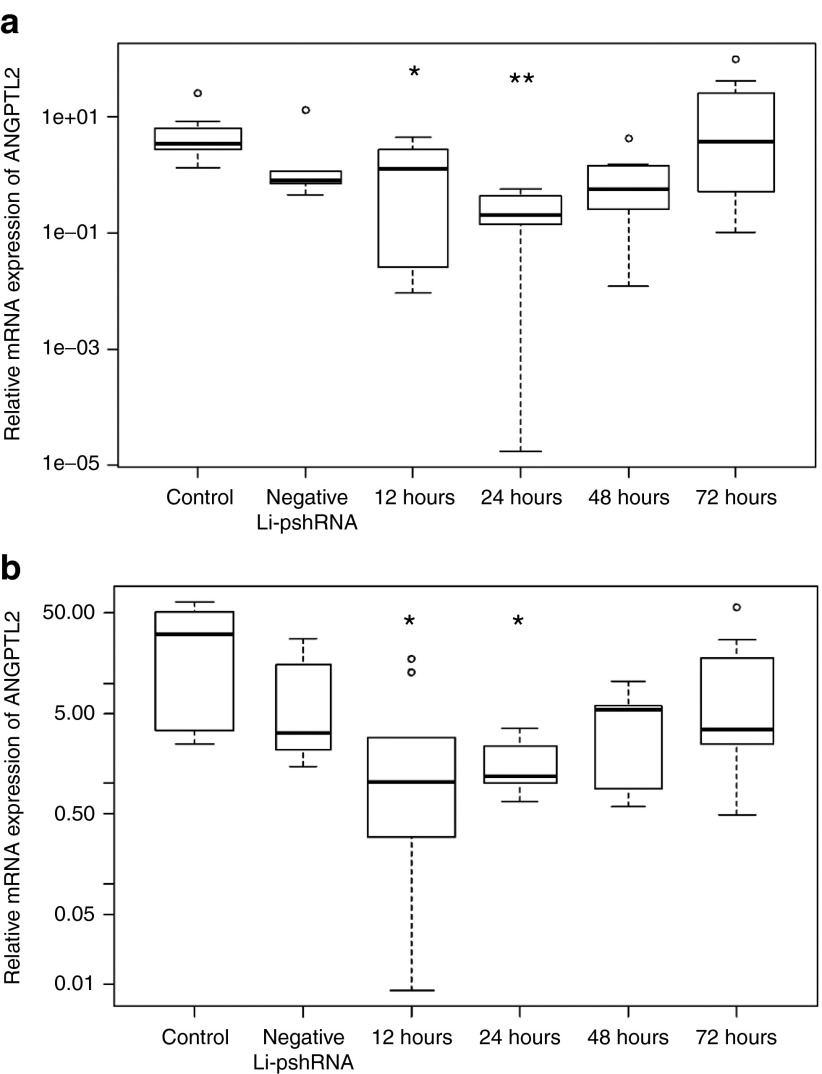
ANGPTL2 mRNA expression was strongly inhibited in both epithelial and stromal cells at 12 and 24 hours (P = 0.049 and <0.001, respectively) after instillation of eye drops in mice, but normal expression of ANGPTL2 mRNA was restored by 72 hours (Figure 3, n = 7 for all experiments). In contrast, there was no change in ANGPTL2 mRNA expression in the corneas of control mice (Figure 3).
Figure 3.
Reduction of ANGPTL2 mRNA expression in normal cornea after topical administration of ANGPTL2 Li-pshRNA. Reverse transcriptase PCR showed that ANGPTL2 mRNA expression was significantly reduced in the corneal epithelium (a) at 12 and 24 hours (P = 0.049 and <0.001, respectively; n = 7) and in stromal cells (b) at 12 and 24 hours (P = 0.0030 and 0.0021, respectively, n = 7). Expression returned to baseline levels by 72 hours. *P < 0.05, **P < 0.01. ANGPTL2, angiopoietin-like protein 2; Li-pshRNA, proline-modified short hairpin RNA wrapped with liposome.
CNV in alkali-burn models
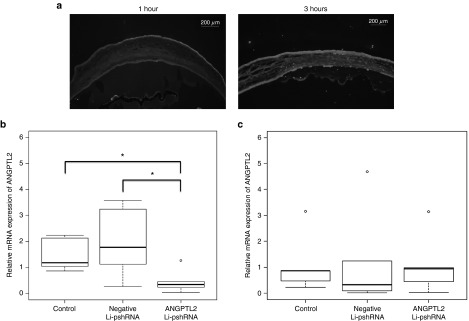
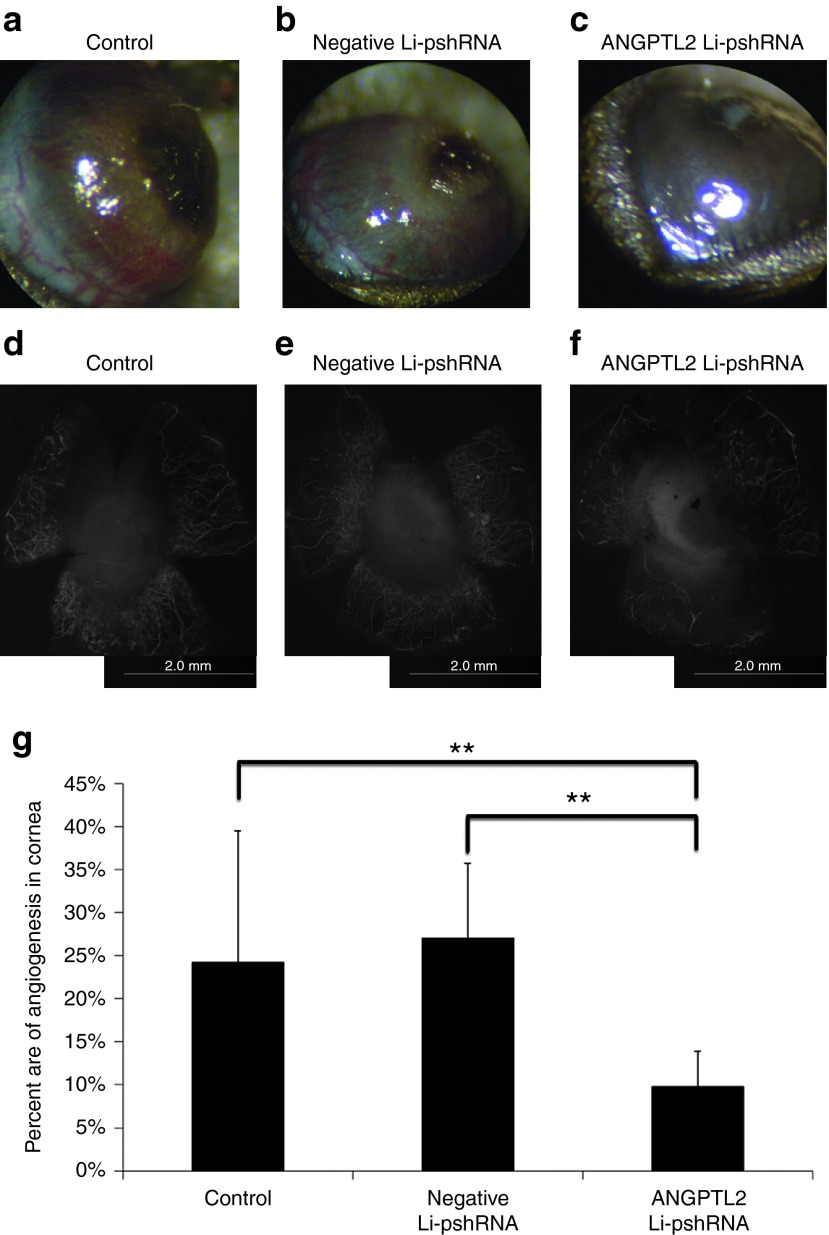
The effect of Angptl2 Li-pshRNA on CNV after alkali injury was investigated. Drug delivery in the alkali model was also evaluated by fluorescence assay, which confirmed that ANGPTL2 Li-pshRNA was distributed in all layers of the injured cornea, while ANGPTL2 pshRNA alone was not (Figure 4a). Expression of ANGPTL2 mRNA was inhibited at 36 hours after alkali injury by topical treatment with ANGPTL2 Li-pshRNA, but not by topical administration of negative Li-pshRNA or by pshRNA alone (P = 0.043, n = 6; Figure 4b). However, there was no significant difference in the levels of expression of ANGPTL2 mRNA expression between the ANGPTL2 Li-pshRNA group and the control group at 48 hours after alkali injury (Figure 4c). After 10 days of once-daily treatment, the area of CNV was markedly reduced in medicated eyes compared to the controls (P < 0.01, n = 7; Figure 5).
Figure 4.
Inhibition of ANGPTL2 mRNA by ANGPTL2 Li-pshRNA eye drops after chemical injury. (a) Fluorescence-labeled topical ANGPTL2 Li-pshRNA permeated the entire thickness of the injured cornea at 1 and 3 hours after injury. (b) ANGPTL2 mRNA expression was reduced after topical administration of ANGPTL2 Li-pshRNA eye drops 36 hours after injury (P = 0.017 and 0.032 for saline as control and negative Li-pshRNA, respectively; n = 6). ANGPTL2 mRNA expression was not inhibited by negative Li-pshRNA. *P < 0.05. (c) At 48 hours after injury, levels of ANGPTL2 expression were the same among all three groups. ANGPTL2, angiopoietin-like protein 2; Li-pshRNA, proline-modified short hairpin RNA wrapped with liposome.
Figure 5.
Inhibition of corneal neovascularization by ANGPTL2 Li-psh RNA eye drops. Corneal neovascularization after alkali injury was markedly reduced after 10 days of once-daily treatment with ANGPTL2 Li-pshRNA eye drops (c) compared to eyes treated with control (a) or negative Li-pshRNA (b). Corneas treated with ANGPTL2 Li-pshRNA showed smaller areas of neovascularization (f) compared to control (d) and negative Li-pshRNA (e) corneas. The samples were stained with CD31 to visualize the neovascularization. (g) Corneal neovascularization (CNV) was quantified by area (vascularized area/total cornea area) using image J software. The extent of CNV after ANGPTL2 treatment was significantly smaller compared to the other groups (P < 0.01, n = 9). **P < 0.01. ANGPTL2, angiopoietin-like protein 2; Li-pshRNA, proline-modified short hairpin RNA wrapped with liposome.
Discussion
Several researchers have evaluated the therapeutic potential of gene silencing by RNAi for CNV in animal models. Kim et al., using a corneal micro-pocket assay and herpetic stromal keratitis models in mice, reported that the subconjunctival injections and systemic administration of vascular endothelial growth factor pathway-specific siRNAs inhibited CNV,11 and Qazi et al. reported that intrastromal injection of poly nanoparticles loaded with plasmid-expressing anti-vascular endothelial growth factor siRNA also inhibited CNV in a more sustained and robust manner than the naked plasmid alone.12 Thus, the concept of using RNAi agents against CNV seems promising. However, the invasiveness of subconjunctival or intrastromal injections could pose a hurdle to repeated treatments. A topical formulation would be a more desirable and convenient approach. In this study, we have shown that effective topical delivery of a modified RNAi agent (ANGPTL2 Li-pshRNA) can be accomplished using a lipid nanoparticle, and that this agent reduced the expression of ANGPTL2 mRNA and inhibited CNV in a mouse alkali-burn model.
It is crucial to confirm the safety of any therapy before clinical application. RNAi is generally thought to have minimal cellular toxicity because of its endogenous nature, and in a previous study, the intravitreal injection of RNAi did not cause any obvious adverse effects in mouse retina.13 In the present study, Li-pshRNA did not affect cell viability (Figure 1), and we believe that the highly biocompatible nature of RNAi-loaded lipid nanoparticles could be ideal for clinical application.
We used single-stranded RNAi (pshRNA) because double-stranded siRNAs have been associated with adverse off-target effects due to activation of innate immunity, specifically due to Toll-like receptor 3 activation.10 Further, annealing is mandatory for generation of double-stranded siRNA, but not for pshRNA, which is a single-stranded RNAi in which the linker region has been replaced by amino acid derivatives. This substitution and the short hairpin construct exhibit high stability in vivo and preliminary data (not shown) suggest that pshRNA is less susceptible to degeneration due to RNase activity. Because annealing is not necessary to generate the pshRNA, the cost can be also reduced. Moreover, previous research showed that pshRNA did not have off-target effects and it was more stable against nuclease than double-stranded RNAi constructs.14 Thus, pshRNA overcomes many disadvantages hitherto encountered with double-stranded siRNAs.
The corneal epithelium contains tight junctions that form a barrier to paracellular drug permeation of the inner layer of the cornea in vivo. The corneal epithelium is lipoidal in nature, which poses considerable resistance to permeation by topically administered hydrophilic agents,15 as demonstrated by the failure of unmodified pshRNA, which is hydrophilic, to penetrate the epithelial surface in our experiments (Figure 2). The charge of a drug also affects permeation. Positively charged molecules exhibit poor permeation, presumably because they bind to the negatively charged corneal and scleral proteoglycan matrix.16 To overcome this, the pshRNA was loaded into lipid nanoparticles (Li-pshRNA) that were specifically designed to facilitate drug delivery to the retina.17 In this study, we showed that ANGPTL2 Li-pshRNA could reduce in vivo corneal ANGPTL2 mRNA expression (Figures 3 and 4). This result and others, for example, that the topical administration of edaravone-loaded lipid nanoparticles can protect against light-induced retinal dysfunction,18 support the potential usefulness of lipid nanoparticle-loaded topical agents in the treatment of corneal diseases.
We found that the marked reduction in the level of ANGPTL2 mRNA was not sustained beyond 48 hours after alkali injury. Even in the control group, ANGPTL2 expression dropped to the same level as that of GAPDH, suggesting that ANGPTL2 is produced in the very early stage of inflammation. As alkali damage progresses, the cells are not able to produce as much ANGPTL2, and it becomes more difficult to detect the effects of ANGPTL2 RNAi.
In conclusion, extensive corneal injuries such as chemical burns cause conjunctivalization of the corneal surface and massive neovascularization, leading to corneal opacity and severe reduction in and visual acuity. We have successfully generated a topical RNAi agent (Li-pshRNA) that inhibits corneal inflammation and CNV by inhibiting expression of ANGPTL2. Li-pshRNA eye drops show promise for preventing CNV and subsequent visual impairment without the side effects commonly experienced with steroids or immunosuppressive agents.
Materials and methods
Animals. All animal experiments were approved by the University of Tokyo Hospital Animal care committee and conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All surgical procedures were performed under ketamine hydrochloride (35 mg/kg) and xylazine chloride (5 mg/kg) anesthesia, and all efforts were made to minimize suffering. C57BL/6 mice aged 8–12 weeks were used in all experiments.
Reagents. The ANGPTL2-specific single-stranded RNAi segment was contained in a short hairpin structure (ANGPTL2 pshRNA) with a stem loop, which was synthesized in the solid phase.14 The stem loop contained proline derivatives similar to PnkRNA, as previously described.19 The base sequence of the ANGPTL2 pshRNA was 5′-CCA GAA AGC GAG UAC UAU AUU CC proline modified-3′, 5′-GGA AUA UAF UAC UCG CUU UCU GGU U-3′ (structure shown in Figure 6), and the negative control was 5′-UAC UAU UCG ACA CGC GAA GUU CC proline modified-3′, 5′-GGA ACU UCG CGU GUC GAA UAG UAU U-3′. The reagents were supplied by BONAC (Fukuoka, Japan). The linker region of the pshRNA was replaced by a fluorescent pigment for drug delivery experiments.
Figure 6.
ANGPTL2 pshRNA platform. PshRNA was synthesized in solid phase as single-stranded RNAs that self-anneal after synthesis into a helical structure containing a single stem loop. Red characters indicated guide sequence, and green characters showed nucleotide cassette sequence of pshRNA. ANGPTL2, angiopoietin-like protein 2; pshRNA, proline-modified short hairpin RNA. A, adenine; C, cytosine; G, guanine; U, uracil; P, proline derivative.
The lipid nanoparticle was prepared as described previously.17 1,2-Distearoyl-sn-glycero-3-phosphocholine, cholesterol, and stearylamine (7:3:1 molar ratio, lipid concentrations: 20 mmol/l) were dissolved in a small amount of chloroform and dried in a rotary evaporator under reduced pressure at 40 °C to form a thin lipid film. The film was dried in a vacuum oven overnight to ensure complete removal of the solvent. After hydration of the film with a suitable amount of Tris–ethylenediaminetetraacetic acid buffer containing RNAi, the resultant lipid suspension was incubated in a water bath at 70 °C. The multilamellar vesicle suspension was incubated at room temperature for 15 minutes. Submicron-sized liposomes were prepared using an extruder (LipoFast-Pneumatic, Avestin, Ottawa, Ontario, Canada) equipped with a polycarbonate membrane (0.1-µm membrane filter pore size; Whatman Japan KK, Tokyo, Japan). Extrusion was performed 41 times with nitrogen pressure (200 psi). The final phospholipid and RNAi concentrations in liposomal suspensions were 20 mmol/l and 0.5 or 1 mol/l, respectively.
Cell toxicity of Li-pshRNA in vitro. Cellular viability was evaluated using a nonradioactive colorimetric assay (WST-1; TAKARA Bio, Shiga, Japan) according to the manufacturer's protocol. Simian virus 40-immortalized human corneal epithelial cells were cultured in Dulbecco's modified Eagle medium (Wako Pure Chemical Industry, Osaka, Japan)/F12 containing 10% fetal bovine serum supplemented with cholera toxin (100 ng/ml), human recombinant epidermal growth factor (10 µg/ml), and insulin (5 µg/ml). After the culture reached confluence, ANGPTL2 Li-pshRNA, negative control Li-pshRNA, Amphotericin B (0.1% Fungizone, Bristol-Myers, Tokyo, Japan), or saline was applied for 30 minutes followed by WST-1 reagent in phenol red-free Dulbecco's modified Eagle medium/F12 medium for 1 hour at 37 °C, and the dye was measured at 450 nm by a Victor 3V Multilabel Counter model 1420 plate reader (Perkin Elmer, Waltham, MA). The results were expressed as the mean ± SD of the percentage of cell viability compared with that of the control-treated controls.
Drug delivery to the cornea. Drops of Li-pshRNA labeled with fluorescent pigment (5 µl) were instilled on the corneal surface of anesthetized mice, after which the eyes were enucleated. Specimens were embedded in optimal cutting temperature compound (Sakura Finetechnical, Tokyo, Japan) after 1, 3, and 6 hours. The samples were immediately frozen in liquid nitrogen and cut into 10-µm sections. Corneal distribution of the labeled pshRNA was examined by fluorescence microscopy (BZ-9000; Keyence, Osaka, Japan). The same procedure was performed in the alkali-burn models with drops instilled 1, 3, and 6 hours after alkali burn.
To examine changes in mRNA expression after administration of the Li-pshRNA RNAi agent, 5 µl of ANGPTL2 Li-pshRNA was instilled on the corneal surface in anesthetized mice. Eyes were enucleated at 12, 24, 48, and 72 hours after administration, and the corneal epithelium was separated from the corneal stroma using trypsin-ethylenediaminetetraacetic acid. The total RNA of each sample was extracted and ANGPTL2 expression was quantified by quantitative real-time reverse transcriptase PCR.
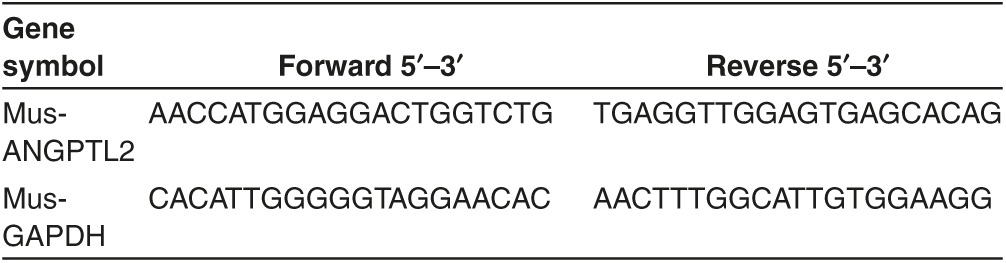
Real-time reverse transcriptase PCR. cDNA was synthesized using PrimerScript RT Master Mix (TAKARA Bio). The PCR amplifications were performed in an instrument using Takara PCR Thermal Cycler Dice (TAKARA Bio) as described previously.9 The primer sets are shown in Table 1.
Table 1. Quantitative reverse transcriptase PCR primers.
Mouse model of alkali-induced inflammatory CNV. The mouse alkali-burn models were produced as previously described20,21,22 with modifications to suit our experiment. Chemical injury was induced by placing 5 µl of 0.5 N sodium hydroxide (NaOH) on the cornea of anesthetized mice for 30 seconds. The eyes were then washed with 20 ml saline (day 0). On day 1, the injured eyes were treated with 5 µl of ANGPTL2 Li-pshRNA or negative Li-pshRNA for 5 minutes, after which reagents were absorbed (KimWipe: Nikkei Products, Osaka, Japan) and eyes were subsequently treated with ofloxacin ophthalmic ointment (Talibit, Santen, Osaka, Japan). The RNAi reagent and antibiotic treatments were repeated daily until day 10, then the mice were euthanized and the cornea with limbus was excised.
CNV was quantified using CD31 staining as previously described.9 Briefly, corneas were fixed in acetone for 10 minutes at −20 °C, washed three times in phosphate-buffered saline, stained with rat anti-mouse CD31 antibody (BD Biosciences, Franklin Lakes, NJ), and incubated at 4 °C overnight. The samples were washed again with phosphate-buffered saline and stained with the secondary antibody (Alexa Fluor 594 donkey anti-rat IgG, Invitrogen, San Diego, CA) for 5 hours at room temperature. The stained samples were evaluated by fluorescence microscopy (BZ-9000, Keyence), and the CD31-positive blood vessels were quantified by area using Image J software (http://rsb.info.nih.gov/ij/). The results are expressed as vessel area/total field.
To examine changes in mRNA expression in our model, the corneas were extracted 36 and 48 hours after injury, and quantitative real-time reverse transcriptase PCR of ANGPTL2 was performed for each group (control, negative Li-pshRNA, and ANGPTL2 Li-pshRNA) using the conditions described above.
Statistical analysis. All of the data were analyzed by Mann–Whitney U-test using statistical software R (Ver. 3.1.1), and values of P < 0.05 were considered statistically significant after Holm's correction.
Acknowledgments
This work has been supported by a grant-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We would like to specially thank Azusa Kamikawa, assistant researcher, for her assistance in conducting our experiments. This work was done in Tokyo, Japan.
References
- Bachmann, B, Taylor, RS and Cursiefen, C (2010). Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology 117: 1300–5. [DOI] [PubMed] [Google Scholar]
- Epstein, RJ, Stulting, RD, Hendricks, RL and Harris, DM (1987). Corneal neovascularization. Pathogenesis and inhibition. Cornea 6: 250–257. [DOI] [PubMed] [Google Scholar]
- Petsoglou, C, Balaggan, KS, Dart, JK, Bunce, C, Xing, W, Ali, RR et al. (2013). Subconjunctival bevacizumab induces regression of corneal neovascularisation: a pilot randomised placebo-controlled double-masked trial. Br J Ophthalmol 97: 28–32. [DOI] [PubMed] [Google Scholar]
- Chang, JH, Garg, NK, Lunde, E, Han, KY, Jain, S and Azar, DT (2012). Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol 57: 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, TI, Chung, JL, Hong, JP, Min, K, Seo, KY and Kim, EK (2009). Bevacizumab application delays epithelial healing in rabbit cornea. Invest Ophthalmol Vis Sci 50: 4653–4659. [DOI] [PubMed] [Google Scholar]
- Kadomatsu, T, Endo, M, Miyata, K and Oike, Y (2014). Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab 25: 245–254. [DOI] [PubMed] [Google Scholar]
- Thorin-Trescases, N and Thorin, E (2014). Angiopoietin-like-2: a multifaceted protein with physiological and pathophysiological properties. Expert Rev Mol Med 16: e17. [DOI] [PubMed] [Google Scholar]
- Kanda, A, Noda, K, Oike, Y and Ishida, S (2012). Angiopoietin-like protein 2 mediates endotoxin-induced acute inflammation in the eye. Lab Invest 92: 1553–1563. [DOI] [PubMed] [Google Scholar]
- Toyono, T, Usui, T, Yokoo, S, Kimakura, M, Nakagawa, S, Yamagami, S et al. (2013). Angiopoietin-like protein 2 is a potent hemangiogenic and lymphangiogenic factor in corneal inflammation. Invest Ophthalmol Vis Sci 54: 4278–4285. [DOI] [PubMed] [Google Scholar]
- Kleinman, ME, Yamada, K, Takeda, A, Chandrasekaran, V, Nozaki, M, Baffi, JZ et al. (2008). Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B, Tang, Q, Biswas, PS, Xu, J, Schiffelers, RM, Xie, FY et al. (2004). Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol 165: 2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi, Y, Stagg, B, Singh, N, Singh, S, Zhang, X, Luo, L et al. (2012). Nanoparticle-mediated delivery of shRNA.VEGF-a plasmids regresses corneal neovascularization. Invest Ophthalmol Vis Sci 53: 2837–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama, T, Yoshida, S, Ishikawa, K, Kobayashi, Y, Zhou, Y, Nakao, S et al. (2015). Inhibition of choroidal fibrovascular membrane formation by new class of RNA interference therapeutic agent targeting periostin. Gene Ther 22: 127–137. [DOI] [PubMed] [Google Scholar]
- Takanashi, M, Sudo, K, Ueda, S, Ohno, S, Yamada, Y, Osakabe, Y et al. (2015). Novel types of small RNA exhibit sequence- and target-dependent angiogenesis suppression without activation of Toll-like receptor 3 in an age-related macular degeneration (AMD) mouse model. Mol Ther Nucleic Acids 4: e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudana, R, Ananthula, HK, Parenky, A and Mitra, AK (2010). Ocular drug delivery. AAPS J 12: 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, SH, Lutz, RJ, Wang, NS and Robinson, MR (2007). Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res 39: 244–254. [DOI] [PubMed] [Google Scholar]
- Murata, M, Yonamine, T, Tanaka, S, Tahara, K, Tozuka, Y and Takeuchi, H (2013). Surface modification of liposomes using polymer-wheat germ agglutinin conjugates to improve the absorption of peptide drugs by pulmonary administration. J Pharm Sci 102: 1281–1289. [DOI] [PubMed] [Google Scholar]
- Shimazaki, H, Hironaka, K, Fujisawa, T, Tsuruma, K, Tozuka, Y, Shimazawa, M et al. (2011). Edaravone-loaded liposome eyedrops protect against light-induced retinal damage in mice. Invest Ophthalmol Vis Sci 52: 7289–7297. [DOI] [PubMed] [Google Scholar]
- Hamasaki, T, Suzuki, H, Shirohzu, H, Matsumoto, T, D'Alessandro-Gabazza, CN, Gil-Bernabe, P et al. (2012). Efficacy of a novel class of RNA interference therapeutic agents. PLoS One 7: e42655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotozono, C, He, J, Matsumoto, Y, Kita, M, Imanishi, J and Kinoshita, S (1997). Cytokine expression in the alkali-burned cornea. Curr Eye Res 16: 670–676. [DOI] [PubMed] [Google Scholar]
- Zhou, SY, Xie, ZL, Xiao, O, Yang, XR, Heng, BC and Sato, Y (2010). Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol Vis 16: 1389–1398. [PMC free article] [PubMed] [Google Scholar]
- Anderson, C, Zhou, Q and Wang, S (2014). An alkali-burn injury model of corneal neovascularization in the mouse. J Vis Exp (86),e51159, doi:10.3791/51159(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]