Abstract
Gene electrotransfer is a safe and efficient nonviral technique for the transfer of nucleic acids of all sizes. Using a small reporter plasmid (3.5 kbp), electrotransfer of more than 90% of the cells, with ~70% viability, can be routinely achieved even in primary cells like mesenchymal stem cells. However, under the same experimental conditions, electrotransfer of larger plasmids (from 6 to 16 kbp) results in very low viability and transfection efficacy. Here, we show that these strong decreases are directly linked to the physical size of the plasmid molecule. Moreover, large plasmids are toxic only when the cells are exposed to electrotransfer pulses. This specific toxicity of large plasmids during electrotransfer is not due to transgene expression and occurs within less than 45 minutes. Indeed, postpulses recovery times of up to 45 minutes are able to entirely abolish the specific toxicity of large plasmid electrotransfer, resulting in a survival and transfection efficacy identical to that of small plasmids. Finally, electrotransfer of small and large plasmids can reach 90–99% of transfection with 60–90% survival considering the findings here reported.
Keywords: electroporation, electropermeabilization, electrotransfer, large plasmids, mesenchymal stem cells
Introduction
In gene therapy, the use of large plasmids is sometimes required, as for example for the transfer of large coding sequences such as the full-length dystrophin gene1 or for the transfer of large plasmids containing multiple genes (like those used for induced pluripotent stem cell reprogramming).
For the transfection of large genes, viruses are usually not the best option as they are limited in the size of the total DNA that they can carry. Moreover, they may present many safety limitations in particular for clinical applications. On the opposite, electrogene transfer is safe and not limited by the size of the plasmid as electrogene transfer can even deliver 240 kbp DNAs.2 However, in vitro, electrotransfection efficiency drops dramatically with the increase of the plasmid size.2,3 Indeed for DNA fragments smaller than 2,000 bp, cytoplasmic diffusion depends strongly on DNA size.4,5 On the other hand, DNA fragments larger than 2 kbp are unable to diffuse in the cytoplasm due to the complex environment and viscoelastic properties of the cytosol. In this case, the DNA is actively transported along microtubules to reach the nucleus.4,5 Since this mechanism is independent of the DNA size, the decreased electrotransfection efficiency observed with increasing size of the DNA is probably due to a decreased delivery of larger DNA across the plasma membrane. A drastic decrease in transgene expression was also observed in vivo, using intramuscular naked DNA injection, when the size of the plasmid increased.6
Very few data are available about the effect of large plasmids on electrotransfer and the underlying mechanisms are not completely known. This knowledge would be crucial to optimize the electrotransfer of large plasmids. Therefore, in this work, we studied the differences between the electrotransfer of small and large plasmids and we investigated the origin of the increased toxicity and the reduced transfection efficiency that was observed during the electrotransfer of large plasmids.
Results
Effect of plasmid size on electrotransfer toxicity and efficacy
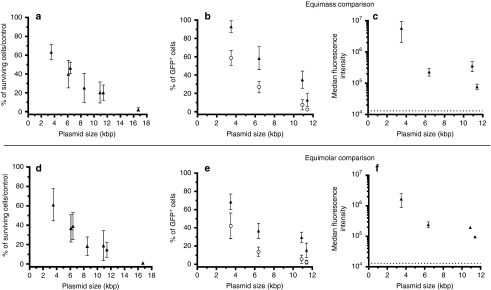
Adipose tissue-derived mesenchymal stem cells (AT-MSCs) were electrotransferred with plasmids of various sizes, ranging from 3,487 to 16,680 bp at equimolar or equimass concentrations (Figure 1). Twenty-four hours after the electrotransfer, the cells were analyzed by flow cytometry. The survival, percentage of transfection, and expression per cell were all inversely correlated to the plasmid size in both equimolar and equimass experiments (P < 0.001, Kendall's rank correlation). The percentage of surviving cells expressing green fluorescent protein (GFP) and the percentage of initial cells expressing GFP were both decreasing with the plasmid size increase.
Figure 1.
Effect of plasmid size on electrotransfer toxicity and efficacy. Adipose tissue-derived mesenchymal stem cells (AT-MSCs) were analyzed by flow cytometry 24 hours after treatment. Equimass experiment (a–c): AT-MSCs were electrotransferred (eight pulses, 1,500 V/cm, 100 µs, 1 Hz) with 50 µg of plasmids of various sizes. Equimolar experiment (d–f): AT-MSCs were electrotransferred with ~6.7 pmol of plasmids of various sizes (equivalent to 50 µg of the 11.4 kbp plasmid pCAGMKOSiE). In panels b and e, the open circles represent the percentage of treated cells expressing green fluorescent protein (GFP) and the filled triangles represent the percentage of surviving cells expressing GFP. In panels c and f, the dotted line represent the background fluorescence intensity of the controls. Data are representative of 4–10 independent experiments. The survival, percentage of transfection, and expression per cell are inversely correlated to the plasmid size in both equimolar and equimass experiments (P < 0.001, Kendall's rank correlation).
These adverse effects of large plasmids were also observed in mouse embryonic fibroblasts and human adult fibroblasts for the large plasmid pCAGMKOSiE (11.4 kbp) (Supplementary Figure S1).
Large plasmids are toxic only in combination with electric pulses
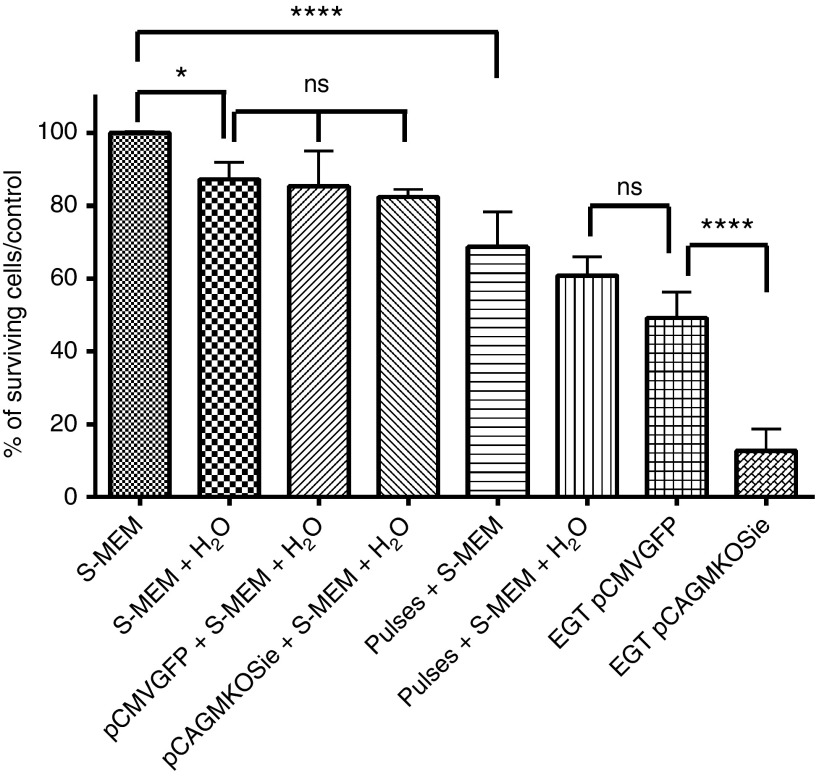
We then investigated the origin of the acute toxicity observed for the electrotransfer of large plasmids. The toxicity of each component of the electrotransfer protocol (pulses, buffer, osmotic pressure, and plasmids) was evaluated individually or in combination 24 hours after treatment (Figure 2).
Figure 2.
Specific toxicity of each component of the electrotransfer protocol for a small and a large plasmid. Adipose tissue-derived mesenchymal stem cells were exposed to each component of the electrotransfer alone or in combination: pulsing buffer (S-MEM), improved pulsing buffer (S-MEM with 50% H2O), small (pCMV-GFP, 3.5 kbp, 50 µg) or large plasmid (pCAGMKOSiE, 11.4 kbp, 50 µg), eight electric pulses (1,500 V/cm, 100 µs, 1 Hz), complete EGT (S-MEM + 50% H2O + plasmid + pulses). For each condition, cells were counted with the flow cytometer 24 hours after treatment and survival expressed as the percentage of cells counted in the control (S-MEM). Data are representative of three to five independent experiments. (*P < 0.05 and ****P < 0.0001, one-way analysis of variance with Holm-Šídák multiple comparison test). CMV, cytomegalovirus; EGT, electrogene transfer; GFP, green fluorescent protein; ns, nonsignificant; S-MEM, minimum essential medium modified for suspension cultures.
The hypoosmotic electrotransfer buffer alone induced ~10% loss of cell viability (P < 0.01). The addition of the plasmid to the electrotransfer buffer did not further increase the toxicity, whether the plasmid was small or large (P > 0.05), proving that the plasmids are not toxic by themselves regardless of their size. In the absence of DNA, the electric pulses were responsible for an additional 30% loss of cell viability (P < 0.001). A plasmid size-dependent toxicity was observed only when the plasmids were used in combination with the pulses. This demonstrates that the cells need to be exposed to the electric pulses in order to be affected by the DNA.
Toxicity of large plasmid electrotransfer increases with the plasmid quantity
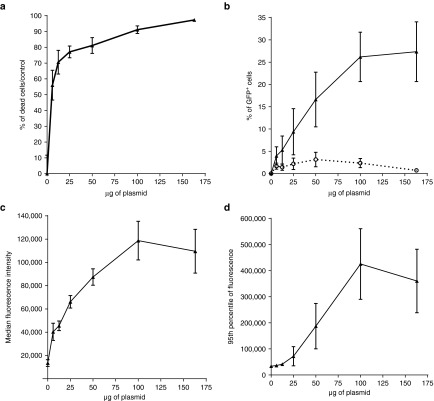
To evaluate the effect of the plasmid quantity, AT-MSCs were electrotransferred with various quantities of the large plasmid pCAGMKOSiE (11.4 kbp). The volumes were the same as before, only the concentration of the plasmid was changed. Twenty-four hours after electrotransfer, the cells were analyzed by flow cytometry (Figure 3). The toxicity (Figure 3a), percentage of transfection (Figure 3b), and levels of expression (Figure 3c,d) were all positively correlated to the plasmid quantity (P < 0.001, Kendall's rank correlation). On the one hand, increasing the plasmid concentration reduces the number of surviving cells (Figure 3a), on the other hand, these surviving cells are more efficiently transfected (Figure 3b). The two phenomena compensate each other, resulting in a low and more or less stable percentage of treated cells expressing GFP whatever the plasmid concentration used, at least up to 100 µg of plasmid (Figure 3b).
Figure 3.
Effect of the quantity of large plasmid on electrotransfer toxicity and efficacy. Adipose tissue-derived mesenchymal stem cells were electrotransferred (eight pulses, 1,500 V/cm, 100 µs, 1 Hz) with various quantities of the large plasmid pCAGMKOSiE (11.4 kbp) and analyzed by flow cytometry 24 hours after treatment. In panel (a) the percentage of dead cells was assessed by comparing the number of cells recovered in the treated groups versus the number of cells recovered in the control group. In panel (b), the open circles and dotted lines represent the percentage of treated cells expressing green fluorescent protein (GFP) and the filled triangles and solid lines represent the percentage of surviving cells expressing GFP. Panel (c) represent the median fluorescence intensity per cell. In panel (d), the 95th percentile of fluorescence is represented instead of the maximum fluorescence to limit the effects of possible outliers. Data are representative of six independent experiments. The toxicity, percentage of surviving transfected cells, and expression per cell are all positively correlated to the plasmid quantity (P < 0.001, Kendall's rank correlation).
Specific toxicity of large plasmid electrotransfer is observed within 2 hours post-treatment
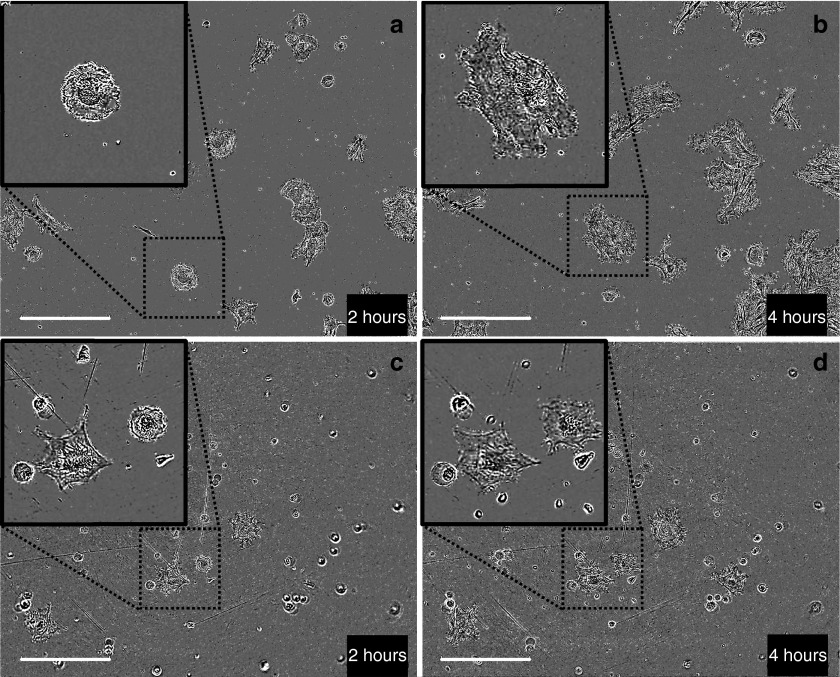
Two hours after electrotransfer, most of the cells transfected with the small pCMV-GFP plasmid were already attached and spreading while the cells transfected with the large pCAGMKOSiE plasmid were still floating. Moreover, blebs and debris were visible (Figure 4). At 2 hours after the electrotransfer, the plasmid expression is minimal; however, the toxicity is already fully visible, suggesting an absence of correlation between the two.
Figure 4.
Toxicity of small and large plasmid 2 and 4 hours after electrotransfer. Adipose tissue-derived mesenchymal stem cells were electrotransferred (eight pulses, 1,500 V/cm, 100 µs, 1 Hz) with 50 µg of either the small pCMV-GFP plasmid (3.5 kbp) (a,b) or the large pCAGMKOSiE plasmid (11.4 kbp) (c,d) and observed 2 hours (a,c) and 4 hours (b,d) after electrotransfer using a phase contrast objective. Within less than 2 hours, the cells were attaching and at 4 hours, they were well spread on the surface. For large plasmid, a significant amount of cells did not attach and some were blebbing. Bar = 400 µm. Pictures are representative of three experiments. CMV, cytomegalovirus; GFP, green fluorescent protein.
Reduced mobility of large plasmids?
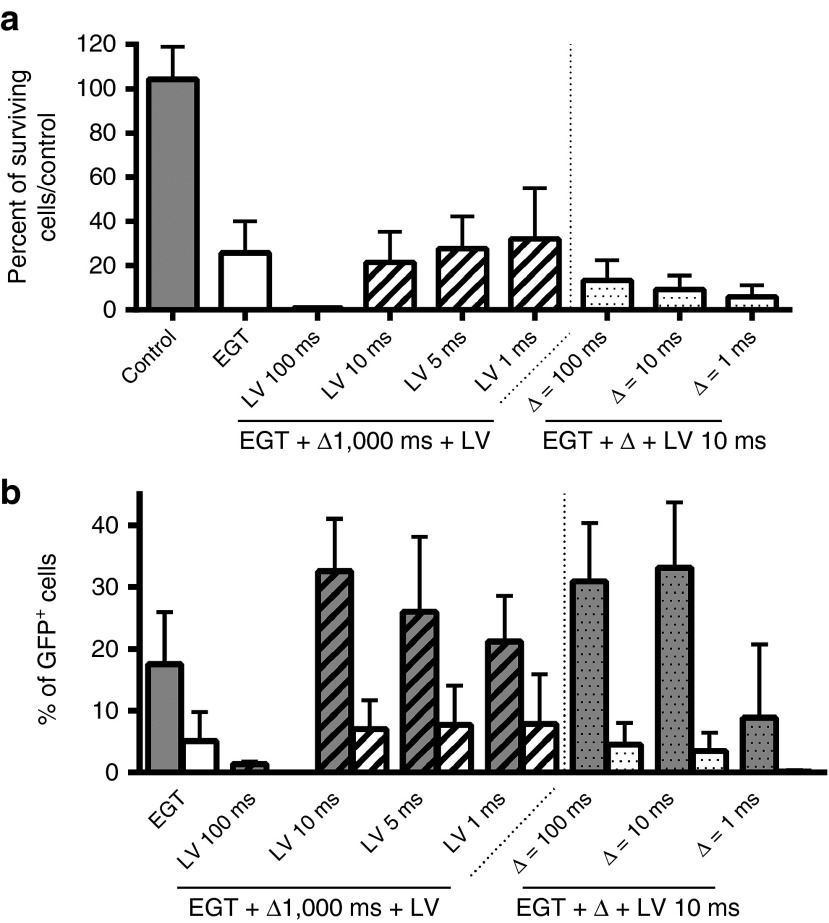
We assessed the possible benefit of adding a low-voltage electrophoretic pulse within 1 second after the electrotransfer of a large plasmid to create an electrophoretic-driven transfer across the membrane.
No improvement of cell survival was observed (Figure 5a). Concerning the transfection efficacy, only a nonsignificant small increase could be obtained with the electrophoretic pulses of 10-ms duration, while the 100-ms low-voltage pulses were very toxic (Figure 5b). This could be due to the high toxicity of the 1,500 V/cm pulses used for the electrotransfer which might hide a possible increase in transfection. Since electrotransfer with pulses of 1,000 V/cm allows a survival of ~55% for large plasmids and represents the best compromise between survival and transfection efficiency (Supplementary Figure S2), we therefore reduced the amplitude of the pulses used for the electrotransfer of the large pCAGMKOSiE plasmid. However, even with high voltage of 1,000 V/cm, no improvement of the transfection efficacy was reached by the addition of the same range of electrophoretic pulses as those used in Figure 5 (data not shown).
Figure 5.
Effect of an electrophoretic pulse on large plasmid electrotransfer toxicity and efficacy. Adipose tissue-derived mesenchymal stem cells were electrotransferred (eight pulses, 1,500 V/cm, 100 µs, 1 Hz) with 50 µg of the large plasmid pCAGMKOSiE (11.4 kbp). A single electrophoretic pulse (LV) of 200 V/cm and a duration of 1, 5, 10, or 100 ms was applied 1–1,000 ms (Δ) after the EGT. Panel (a) represent the number of cells recovered in the treated groups versus the number of cells recovered in the control group. In panel (b), the grey columns represent the percentage of surviving cells expressing GFP and the white columns represent the percentage of treated cells expressing GFP. Data are representative of three independent experiments. The electrophoretic pulses improved neither the survival nor the transfection efficiency (one-way analysis of variance with Dunnett's multiple comparison test). EGT, electrogene transfer; GFP, green fluorescent protein; LV, low voltage.
Increasing the postpulses recovery time improves large plasmid electrotransfer efficacy and survival
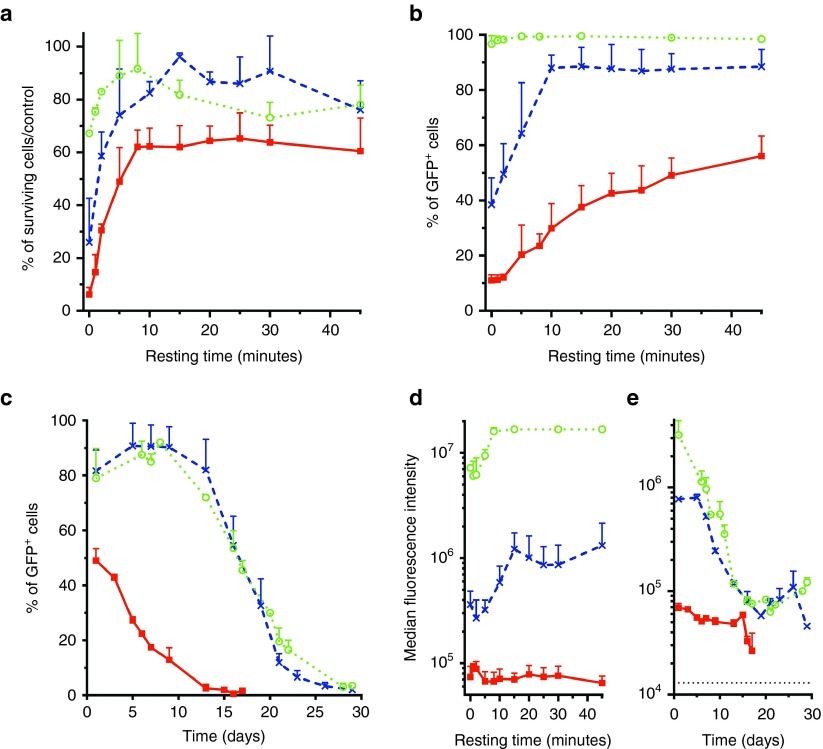
We then evaluated the benefit of a recovery time after the pulses delivery. After electrotransfer of a large plasmid (pCAGMKOSiE: 11.4 kbp or pCXLE-eGFP: 10.9 kbp) or a small plasmid (pCMV-GFP: 3.5 kbp), the cells were allowed to rest in the cuvette at room temperature for various amounts of time before being put back in culture. Twenty-four hours later, the cells were analyzed by flow cytometry for survival and electrotransfer efficacy (Figure 6a,b,d).
Figure 6.
Effect of a resting time on electrotransfer efficacy and toxicity in presence of small or large plasmids. Adipose tissue-derived mesenchymal stem cells were electrotransferred (eight pulses, 1,500 V/cm, 100 µs, 1 Hz) with 50 µg of either pCAGMKOSiE (11.4 kbp, 6.7 pmol) (filed squares and solid lines), pCXLE-eGFP (10.9 kbp, 7 pmol) (crosses and dashed line), or pCMV-GFP (3.5 kbp, 22 pmol) (open circles and dotted lines). After electrotransfer, cells were allowed to recover for 45 minutes (b,e) or for different amounts of time (a,c,d) before being put back in culture. Survival and transfection efficiency were assessed by flow cytometry 24 hours after treatment (a,c,d) or at several different days (b,e). In panel e, the dotted line represents the background fluorescence intensity of the controls. Data are representative of 3–11 independent experiments. CMV, cytomegalovirus; GFP, green fluorescent protein.
The addition of a recovery time massively increased the cell survival after the electrotransfer of large plasmids (from 5% to more than 60% for pCAGMKOSiE and from 25% to ~90% for pCXLE-eGFP for a 10-minute recovery time). An increase in survival, even though smaller, was likewise observed with the small plasmid (from 65 to 90%). Finally, even the low toxicity of the electric pulses alone was slightly reduced after a recovery time (Supplementary Figure S3). Beyond 10 minutes of recovery time, no more improvement in cell survival was observed for either the small or large plasmids.
The addition of a recovery time also resulted in a vastly improved electrotransfer efficiency for the large plasmids (from 10 to 55% for pCAGMKOSiE and from 40 to ~90% for pCXLE-eGFP). The electrotransfer of the small plasmid was already maximized and it only marginally benefitted from the recovery time (from 95 to 99%). Beyond 10 minutes of recovery time, no more improvement in transfection was observed for pCXLE-eGFP, whereas for pCAGMKOSiE, the maximum transfection efficiency required ~45 minutes of recovery (no improvement of electrotransfer efficacy was observed above 45 minutes; Supplementary Figure S4).
With the addition of a recovery time, the kinetic of transfection in the presence of the large 10.9 kbp pCXLE-eGFP was similar to the one of the small 3.5 kbp pCMV-GFP plasmid without a recovery time (Figure 6c,e). The percentage of cells electrotransfected remained stable at ~90% for ~10 days and GFP remained detectable for up to 30 days. With the addition of a recovery time, for the large pCAGMKOSiE plasmid, the percentage of transfected cells increased from 10 to 55% (Figure 6b) and remained above 10% for more than 10 days (Figure 6c). Nevertheless, the percentage of transfection and level of expression both remained all the time below the ones of the small pCMV-GFP plasmid without a recovery time (Figure 6c,e).
This positive effect of a recovery time on the electrotransfer survival, percentage, and kinetic of transfection was also observed with the 6.3 kbp pCDNA3.3_eGFP plasmid resulting in levels similar to the ones of the small 3.5 kbp pCMV-GFP plasmid (Supplementary Figure S5).
Discussion
Survival and transfection efficiency are inversely correlated to the plasmid size
We previously developed a very efficient in vitro electrotransfer protocol (90% transfection and ~70% survival) for human MSC transfection, using 50 µg of a small reporter plasmid (pCMV-GFP, 3.487 kbp) in an hypoosmotic electrotransfer buffer.7 However, when we used this optimized protocol with a larger plasmid (pCAGMKOSiE, 11.4 kbp), we observed drastic decreases in cell survival, transfection efficiency, and level of expression per cell.
We then assessed if the significantly larger size of the plasmid pCAGMKOSiE could explain some of the severe decreases observed. We thus electrotransferred AT-MSCs with plasmids of various sizes. The survival, percentage of transfection, and level of expression all proved to be very significantly correlated to the size of the plasmids (P < 0.001, Kendall's rank correlation), irrespective of their sequences (Figure 1).
The tremendously reduced number of cells recovered 24 hours after large plasmids electrotransfer was due to a massive cell death and not to a lower cell proliferation rate since: (i) the AT-MSC mean doubling time is ~3 days, meaning that in 24 hours, the difference in cell number would have been only 25% and (ii) a higher number of floating dead cells was visible with larger plasmids (Figure 4). This increased toxicity was predominantly due to the increase in plasmid size and not to the differences in nucleotides sequence (Table 1). For example, the plasmids pCX-cMyc and pEphrin-B2/IRES-eGFP had approximately comparable sizes but different promoters, genes, and backbones and yet resulted in identical toxicities (Figure 1a,d). Indeed, the toxicity is occurring in the first 2 hours following the plasmid electrotransfer (Figure 4) and therefore cannot be linked to the plasmids expression. Similarly, the toxicity observed can neither be due to specific sequence toxicity like the CpG content since the frequency of CpG sites is not correlated to the plasmid size (Table 1) and equimolar and equimass experiments showed similar trends. Moreover, if the increased toxicity was due to differences in sequences, it would be hard to explain how the postpulse recovery time was able to abolish this sequence toxicity and in the same time improve transfection and expression. Finally, the increased toxicity observed with larger plasmids cannot be explained by an increased overall mass of nucleic acids used since the same increased toxicity was observed in the equimass experiment.
Table 1. Name, size, promoters, genes, and CpG frequencies of the plasmids used in Figure 1.
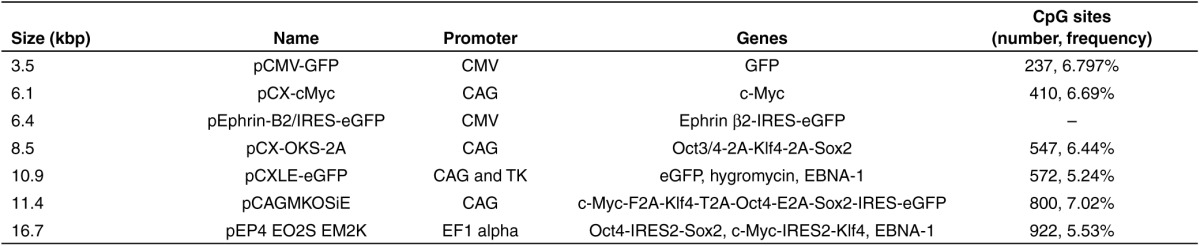
The percentage of transfection and level of expression were also predominantly affected by the plasmid size. The decreased transfection efficiency observed with larger plasmids cannot be explained by a decreased number of molecules of plasmid used since the same reduction in transfection was observed in the equimolar experiment. On the other hand, a few minor fluctuations in transfection efficiency could be linked to some differences in the plasmids' sequences. For example, the 10.9 kbp plasmid pCXLE-eGFP was slightly better expressed than it would be expected from just its size (Figure 1b,c,e,f).
Finally, none of the differences observed could be due to changes in the osmolarity or conductivity of the plasmid solutions since (i) in the equimass experiments, the conductivities should be the same, (ii) in the equimolar experiments, the osmolarities should be the same, and (iii) the 50 µg (or less) of plasmids used correspond to a few picomoles which are negligible in terms of osmolarity compared to the 100 mmol of ions in the solution.
Therefore, the decreases in survival and transfection efficiency observed are directly linked to the physical size of each individual molecule of plasmid: a single copy of a large plasmid is more toxic and harder to transfect than a single copy of a small plasmid or than the number of copies of a small plasmid equivalent in mass to the single copy of the large plasmid.
These conclusions are in agreement with previous reports describing the improved electrotransfection efficiency and safety of minicircles8,9,10,11 with respect to the corresponding complete plasmid. However, minicircles are relatively expensive and complex to produce and they do not solve the problem of the cotransfer of various genes as a minicircle carrying several genes would not be “mini” anymore. Even though the detrimental effect of the plasmid size on the electrotransfection efficacy was previously described in vivo6,9 and in vitro,9 the effect of the size of the plasmids on the toxicity of electrotransfer had never been reported nor analyzed.
Large plasmids induce cell death only in conjunction with electric pulses
We then investigated the origin of the specific cell death induced by the electrotransfer of large plasmids and found that (i) the hypoosmotic electrotransfer buffer was by itself responsible for 10% of the toxicity, (ii) the addition of the plasmids was not toxic regardless of their size, and (iii) the electric pulses by themselves were responsible for an additional 30% increase in cell death (Figure 2). Moreover, we observed an enhanced toxicity of the electric pulses in presence of the plasmids which is in agreement with previous reports.9,12 In the case of mouse lymphoid cells, the authors were able to explicitly link the apoptosis induced by electroporation in presence of DNA to the uptake of DNA molecules independently of the conformation of the introduced DNA or the transfection method used.12 In addition, they showed that electrotransfer of another polyanion such as dextran sulfate was not toxic demonstrating that toxicity resulted specifically from the uptake of DNA and not just from the uptake of relatively large polyanions.12
In our experiments, the increased toxicity of the electric pulses in the presence of plasmids was dependent on the plasmid size. However, in one of the aforementioned reports, no significant difference in cellular viability could be detected between a large and a small plasmid.9 This is most likely attributable to the small difference in size between the two plasmids used in that report (2.257 and 3.487 kbp).
Toxicity of large plasmids electrotransfer is not due to plasmids expression
Since plasmids are not toxic by themselves but only in combination with the electric pulses, the increased toxicity with large plasmids could come from: (i) the large plasmid just getting in contact with the electrically affected plasma membrane or (ii) from the large plasmid simply being electrotransferred inside the cells or (iii) from the large plasmid being electrotransferred inside the cells and being expressed.
If the toxicity was linked to the expression of the large plasmid, increasing the quantity of large plasmid electrotransfected would result in more plasmid being expressed per cell and therefore more cell death, but the maximum level of transgene expression observed in the surviving cells should remain the same and just below the toxicity threshold. In our experiments (Figure 3a), with 12 µg of plasmid, 70% of the cells were already dying. Increasing the quantity of plasmid not only resulted in a small increase in toxicity (Figure 3a) but also in a large increase of the median (Figure 3c) and maximum level of transgene expression (95th percentile) (Figure 3d) in the surviving cells. This indicates that the toxicity of large plasmids electrotransfer is not linked to the level of expression. Moreover, 2 hours after the electrotransfer, the plasmid expression was minimal (protein expression and accumulation takes several hours); however, differences in toxicity due to plasmid size were already visible: for small plasmids, most of the cells were already attached and spreading, while for large plasmids, a lot of floating cells, blebs, and debris were noticeable (Figure 4). This suggests again an absence of correlation between the toxicity of large plasmids electrotransfer and transgene expression. Lastly, if the toxicity was linked to plasmid expression, the toxicity would also have been visible with the small pCMV-GFP plasmid as this plasmid features the same GFP transgene and its expression is even higher (Figure 1). These observations are in agreement with a previous report showing that apoptosis is triggered in mouse lymphoid cells when electric pulses are delivered in presence of DNA, even without transgene expression.12
Consequently, we can conclude that the toxicity of large plasmids electrotransfer is not caused by plasmid expression. On the other hand, we cannot exclude the fact that this toxicity comes from the large plasmid getting in contact with the electrically affected plasma membrane.
Reduced survival and transfection due to reduced mobility of the large plasmids?
Plasmid translocation inside the cell cytoplasm during electrotransfer is a multistep process13: (i) the electric field applied permeabilizes the plasma membrane for several minutes allowing the free diffusion of small molecules but not of large ones such as plasmids, (ii) plasmids interact with the electropermeabilized plasma membrane and slowly cross it either by slow diffusion or by endocytosis or both. Thirty minutes after the electric pulses delivery, plasmids start to be found inside the cytoplasm.13 Large plasmids have a reduced mobility which could decrease the interaction with the electropermeabilized membrane and/or decrease the diffusion through the electropermeabilized membrane, thus explaining the loss in electrotransfer efficiency that we have observed with the large plasmids. If large plasmids are taking more time to diffuse through the plasma membrane, this could also result in increased permeabilization level and duration, as observed by Sukharev and colleagues,14 or even prevent the cells from resealing, thus explaining the increased toxicity observed. We therefore attempted to increase the large plasmid extracellular mobility by adding long electrophoretic pulses (200 V/cm, 1–100 ms) that have been shown to improve transfection in vitro14 and in vivo.15,16 Since the permeabilization induced by electrotransfer can last from seconds to hours,17,18 and the electrophoretic pulses have been shown to be efficient in vitro when added in the first 10 seconds after electroporative electric pulses delivery,14 we added the electrophoretic pulses between 1 and 1,000 ms after the electroporative pulses. However, no improvement of the survival or transfection efficacy was observed (Figure 5). Even with a lower and less toxic field amplitude for the train of eight short pulses (1,000 V/cm), no benefit of the added electrophoretic pulses was observed whatever the length and delay used (data not shown).
The absence of effect of the electrophoretic pulses is therefore either an indication that the lower mobility of large plasmids is not involved in their toxicity and reduced electrotransfer efficacy or that the electrophoretic pulses delivered here were not able to sufficiently improve the large plasmids mobility.
Long postpulses recovery times increase cell survival and transfection efficacy after large plasmids electrotransfer
Postpulses recovery times of more than 10 minutes greatly improved electrotransfer survival and transfection efficacies for all the plasmids tested. Furthermore, except for one plasmid, the long postpulses recovery times were actually able to entirely abolish the specific electrotransfer toxicity of large plasmids, resulting in survival and transfection efficacies similar to the ones obtained for small plasmids electrotransfer (Figure 6). Moreover, even though the electrotransfer of small plasmids was already very efficient (90% transfection), a recovery time after the electric pulses was nevertheless able to further increase the transfection to 99%. These benefits from a long postpulses recovery time are not specific to AT-MSCs as we have also observed them in human fibroblasts (data not shown).
Our results are in agreement with previous reports showing an increase in the number of transfected cells depending on the length of the incubation time after the pulsing.19 As in our case, the maximum benefit was reached after about 10 minutes of postincubation. Moreover, several standard electroporation protocols feature 5 or 10 minutes of recovery time before transferring the cells back in culture. However, the improvement of the cell survival had never been reported yet. This is most probably due to the fact that, as we show here, the benefit from of a postpulses delay is reduced with the small plasmids commonly used for electrotransfer. The recovery time after electrotransfer appears to be mandatory for large plasmids electrotransfer due to their specific electrotransfer toxicity. To our knowledge, this is the first time that the need for a delay postpulse has been linked to the size of the plasmid used.
The benefit of a recovery time after pulses delivery is not obvious as many commercial electrotransfer protocols recommend to transfer the cells back in culture immediately after electrotransfer. The advantage of a delay postpulse is usually assumed to come from the fact that the electroporated cells have the membranes transiently damaged and could be more sensitive to any chemical or physical (mechanical) stress like the pipetting required to transfer the cells to the culture flasks. Allowing some time for the cells to recover could perhaps reduce their sensitivity to the shearing forces exerted by the pipetting. We also tested this hypothesis: we observed the same strong improvement in survival and electrotransfer efficacy with large plasmids if the cells were pipetted and transferred into an empty tube immediately after pulses delivery and then allowed to rest in the tube for several minutes before being transferred to the culture flasks (data not shown). This result rules out the hypothesis of a higher sensitivity to the mechanical stress of the pipetting in the case of large plasmids electrotransfer in AT-MSC.
Sukharev and colleagues14 showed that the electropermeabilization level and duration are proportional to the length of the DNA molecules present during the electric pulses delivery. Even though they did not assess the consequences on the toxicity and efficiency of the electrotransfer, this could explain the increased toxicity observed with longer plasmids, as well as the improved survival with longer recovery times. Indeed, without a postpulses recovery time, the cells are still highly electropermeabilized (particularly in presence of large plasmids) when they are put back in culture, resulting in a massive intracellular uptake of possibly toxic compounds presents in the classical culture medium (antibiotics, undefined animal serum components…), but absent from the electrotransfer medium in which the cells were exposed to the electric pulses. Long postpulses recovery times allow the cells to reseal before being put back in culture media resulting in improved survival. The increases in percentage of transfection and transgene expression observed with a long recovery time after large plasmid electrotransfer are most likely the direct consequence of the increased survival (Figure 6). Indeed, it is possible that, without recovery time, the cells that are the most permeabilized, and therefore taking up the biggest amounts of large plasmids, are dying, whereas with a recovery time, they are able to reseal and survive and, consequently, strongly express the large plasmids they had massively internalized. However, for pCAGMKOSiE, the maximum transfection efficiency required ~45 minutes of recovery, whereas survival was already maximal after only 10 minutes. This suggests that a recovery time can further improve transfection efficiency by an additional mechanism other than the direct consequence of the increased survival.
Even with a long recovery time, the percentage of transfection for the large pCXLE-eGFP plasmid (10.9 kbp) remained slightly lower than for the small pCMV-GFP (3.487 kbp). This was probably entirely due to the fact that equimass amounts of plasmids were used resulting in 3.1 times less moles for the pCXLE-eGFP plasmid. Indeed, when equimass amounts of the 6.3 kbp pCDNA3.3_eGFP plasmid were used (corresponding this time to only 1.8 times less moles compare to the pCMV-GFP), the resting time was able to improve the electrotransfer efficiency up to 98%, similar to that of the small pCMV-GFP (Supplementary Figure S5).
For pCAGMKOSiE (11.4 kbp), with the recovery time, the maximum transfection efficiency achieved was only 55% and the maximum survival only 60%, which is much less than the 90% transfection and 90% survival enhancement observed with the pCXLE-eGFP (10.9 kbp) which nevertheless has a similar size. The lower increase in survival explains only partially the lower enhancement of transfection. The difference in sequences of the plasmids is probably involved. Indeed in the pCAGMKOSiE, the GFP gene is located downstream of an IRES sequence which is known to result in a significantly less efficient expression of the gene compared to a classical cap-dependent gene expression.20,21 On the contrary, the pCXLE-eGFP features the Epstein–Barr virus replication origin (oriP) and nuclear antigen (EBNA-1 gene) which have been reported to accelerate the plasmid delivery and expression.22 This was shown to be predominantly due to the promotion of cytoplasm-to-nuclear recruitment as well as enhancement of transcription, while the episomal replication of the plasmid was not essentially involved.22
With long postpulses recovery times, the percentage of transfection for pCXLE-eGFP, pCMV-GFP, and pCDNA3.3_eGFP was maximized and stable for ~10 days (Figure 6 and Supplementary Figure S5), but their median level of expression started to decrease after one or five days (Figure 6e). The gradual loss of transgene expression over time can be due to either de novo DNA methylation preventing reporter gene transcription, gradual loss of the plasmids through the nuclear pores, gradual dilution of the plasmids at each mitosis, or gradual plasmid degradation by endonucleases.
Conclusion
In this study, we found that plasmids are toxic only in combination with electric pulses (Figure 2) and proportionally to their size and concentration (Figures 1 and 3). More precisely, the decreases in survival and transfection efficiency observed are directly linked to the physical size of each individual molecule of plasmid: a single copy of a large plasmid being more toxic and harder to transfect than a single copy of a small plasmid or than the number of copies of a small plasmid equivalent in mass to the single copy of the large plasmid. Moreover, our data show that this toxicity of large plasmids electrotransfer is not caused by plasmid expression.
Finally, we found that a long recovery time after the electric pulses delivery was able to greatly improve the viability and transfection observed in the case of the electrotransfer of large plasmids. This is of remarkable practical importance to reach high levels of transfection and survival for both small and large plasmids.
Materials and methods
AT-MSC isolation. AT-MSCs were isolated from lipoaspirates as previously described.7 Briefly, lipoaspirates were extensively washed with phosphate-buffered saline (Gibco, Life Technologies, Saint Aubin, France) and digested with 0.2% collagenase (type I; Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline for 30 minutes at 37 °C. Digestion was then blocked with 10% fetal bovine serum (Gibco, Life Technologies, Saint Aubin, France). The stromal vascular fraction was separated by centrifugation (400 × g for 5 minutes) and the stromal pellet was resuspended in phosphate-buffered saline and filtered through a 100-µm mesh. The collected cells were plated and cultured at 37 °C with 95% humidity and 5% CO2 in their culture medium (Dulbecco's modified Eagle medium containing 100 U/ml penicillin and 100 µg/ml streptomycin and 10% fetal bovine serum, all coming from Gibco, Life Technologies). After 5 days, the cells were washed with phosphate-buffered saline and fresh medium was added. At 70–80% of confluency, cells were detached with TrypLE Express (Gibco, Life Technologies) and divided in half. Cells were passaged this way every 3–4 days and expanded up to passage 8 for experimentations. AT-MSCs were characterized between passages 1 and 5 by flow cytometric analysis of surface antigens and their adipogenic and osteogenic differentiation potential was also checked as previously described.7
Plasmids used. Our previous studies were performed with pCMV-GFP (3,487 bp).7 It was here used as a reference to compare with pCX-cMyc (6,131 bp; Addgene plasmid 19772),23 pcDNA3.3_eGFP (6,303 bp; Addgene plasmid 26822),24 pCX-OKS-2A (8,495 bp; Addgene plasmid 19771),23 pCXLE-eGFP (10,912 bp; Addgene plasmid 27082),25 pCAGMKOSiE (11,395 bp; Addgene plasmid 20865),26 pEP4 EO2S EM2K (16,680 bp; Addgene plasmid 20923),27 pEphrin-B2/IRES-eGFP (6,408 bp) was kindly provided by Pr. Timothy O'Brien.28
The plasmid pCMV-GFP containing the GFP reporter gene under the cytomegalovirus (CMV) promoter was produced by PlasmidFactory (Bielefeld, Germany). All the other plasmids were purified from Escherichia coli-transformed cells using NucleoBond PC2000 EF kit (Macherey-Nagel, Hoerdt, France) and then diluted in endotoxin-free water. Light absorption at 260 nm was used to determine DNA concentration and the quality of the plasmid was assessed by calculating the ratios of light absorption at 260/280 nm and 260/230 nm. Control experiments using dialyzed preparations of plasmids showed no effects on survival or on transfection efficiency of the possible remaining salts (data not shown).
Electrotransfer. Cells were detached with TrypLE Express, centrifuged at 300 × g for 10 minutes, and then resuspended at 107 cells per milliliter in minimum essential medium, modified for suspension cultures, without calcium and without glutamine (S-MEM, ref. 11380037, Gibco, Life Technologies, Saint Aubin, France). About 50 µl of AT-MSC suspension were transferred to a 1-mm electroporation cuvette (Cell Projects, Harrietsham, UK) and mixed with 50 µl of the desired quantity of plasmid in water. Electrotransfection was then performed using the Cliniporator device (IGEA, Carpi, Italy) by applying a train of eight square electric pulses (100 µs) at amplitude ranges between 500 and 1,500 V/cm at a 1 Hz repetition frequency. For experiments using electrophoretic pulses, one electrophoretic pulse of 200 V/cm and lasting from 1 to 100 ms was applied 1 to 1,000 ms after the train of eight pulses. All steps were performed at room temperature. Immediately after the electric pulses delivery (except for the experiments with a post-treatment recovery time), the cells were collected and put back in culture in a T25 flask with 5 ml of complete culture medium. The cuvette was washed twice with culture medium to collect all the cells.
Flow cytometry analysis and measurement of viability. At different time points after the electrotransfer, the culture media was removed (with all the floating dead cells). Living cells were then detached with TrypLE Express and analyzed by flow cytometry on a BD Accuri C6 flow cytometer (BD Biosciences, Erembodegem, Belgium) to evaluate the levels of GFP expression and the number of living cells using the BD Accuri CFlow Plus software. The percentage of surviving cells was determined 24 hours after treatment and expressed as a percentage of the number of cells counted in the control. Unless otherwise specified, the percentage of GFP-positive cells presented in the figures corresponds to the percentage of surviving cells expressing GFP. The counting accuracy of the BD Accuri C6 was assessed by comparing with manual counting (hemocytometer) and no significant difference was found.
Statistical analysis. All data are presented as mean plus SD. Kendall's rank correlation was used to measure the association between the variable measured and the parameters tested. One-way analysis of variance with Holm-Šídák (to compare the mean of every group with each other's) or Dunnett's (to compare the mean of every group with the mean of a reference group) multiple comparison test was used for statistical analysis for multiple comparisons among the groups. P <0.05 was considered to be statistically significant. Statistics were performed using GraphPad Prism 4 and R Statistical Software.
SUPPLEMENTARY MATERIAL Figure S1. Effect of plasmid size on electrotransfer toxicity and efficacy in human fibroblasts (a and b) and mouse embryonic fibroblasts (c). Figure S2. Percentage of survival and transfection for a large plasmid depending on the amplitude of the electrotransfer pulses. Figure S3. Effect of a resting time on electrotransfer pulses toxicity. Figure S4. Effect of a resting time on the large plasmid pCAGMKOSiE electrotransfer efficacy and toxicity. Figure S5. Kinetic of transfection for the pCDNA3.3_eGFP plasmid after electrotransfer with 25 minutes of resting time.
Acknowledgments
We thank Bassim Alsaker for providing the biological samples for AT-MSC isolation. We also thank Laurence MICHEL (INSERM U976) for kindly providing the human fibroblasts. We acknowledge the funding support from the ITMO Cancer in the frame of the Plan Cancer 2015-2019 (project PC201517), as well as the ANR through the projects IPSIOAT (ANR-11-BS09-031-02), INTCELL (ANR-10-BLAN-916) and MEMOVE (ANR-11-BS01-006-01). This research was conducted in the scope of the EBAM European Associated Laboratory (LEA). The authors declare no conflict of interest.
Supplementary Material
References
- Quenneville, SP, Chapdelaine, P, Rousseau, J, Beaulieu, J, Caron, NJ, Skuk, D et al. (2004). Nucleofection of muscle-derived stem cells and myoblasts with phiC31 integrase: stable expression of a full-length-dystrophin fusion gene by human myoblasts. Mol Ther 10: 679–687. [DOI] [PubMed] [Google Scholar]
- Sheng, Y, Mancino, V and Birren, B (1995). Transformation of Escherichia coli with large DNA molecules by electroporation. Nucleic Acids Res 23: 1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, S, Mairhofer, J, Madeira, C, Diogo, MM, Lobato da Silva, C, Monteiro, G et al. (2012). Plasmid DNA size does affect nonviral gene delivery efficiency in stem cells. Cell Reprogram 14: 130–137. [DOI] [PubMed] [Google Scholar]
- Vaughan, EE, DeGiulio, JV and Dean, DA (2006). Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr Gene Ther 6: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs, GL, Haggie, P, Seksek, O, Lechardeur, D, Freedman, N and Verkman, AS (2000). Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem 275: 1625–1629. [DOI] [PubMed] [Google Scholar]
- Bloquel, C, Fabre, E, Bureau, MF and Scherman, D (2004). Plasmid DNA electrotransfer for intracellular and secreted proteins expression: new methodological developments and applications. J Gene Med 6 (suppl. 1): S11–S23. [DOI] [PubMed] [Google Scholar]
- Liew, A, André, FM, Lesueur, LL, De Ménorval, MA, O'Brien, T and Mir, LM (2013). Robust, efficient, and practical electrogene transfer method for human mesenchymal stem cells using square electric pulses. Hum Gene Ther Methods 24: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darquet, AM, Cameron, B, Wils, P, Scherman, D and Crouzet, J (1997). A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther 4: 1341–1349. [DOI] [PubMed] [Google Scholar]
- Chabot, S, Orio, J, Schmeer, M, Schleef, M, Golzio, M and Teissié, J (2013). Minicircle DNA electrotransfer for efficient tissue-targeted gene delivery. Gene Ther 20: 62–68. [DOI] [PubMed] [Google Scholar]
- Joubert, V, André, FM, Schmeer, M, Schleef, M and Mir, LM (2013). Increased efficiency of minicircles versus plasmids under gene electrotransfer suboptimal conditions: an influence of the extracellular matrix, in minicircle and miniplasmid DNA vectors: the future of nonviral and viral gene transfer. (M. Schleef, ed.), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. doi: 10.1002/9783527670420.ch13.
- Kreiss, P, Cameron, B, Darquet, AM, Scherman, D and Crouzet, J (1998). Production of a new DNA vehicle for gene transfer using site-specific recombination. Appl Microbiol Biotechnol 49: 560–567. [DOI] [PubMed] [Google Scholar]
- Li, LH, Sen, A, Murphy, SP, Jahreis, GP, Fuji, H and Hui, SW (1999). Apoptosis induced by DNA uptake limits transfection efficiency. Exp Cell Res 253: 541–550. [DOI] [PubMed] [Google Scholar]
- Chabot, S, Rosazza, C, Golzio, M, Zumbusch, A, Teissié, J and Rols, MP (2013). Nucleic acids electro-transfer: from bench to bedside. Curr Drug Metab 14: 300–308. [DOI] [PubMed] [Google Scholar]
- Sukharev, SI, Klenchin, VA, Serov, SM, Chernomordik, LV and Chizmadzhev YuA, (1992). Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J 63: 1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André, FM, Gehl, J, Sersa, G, Préat, V, Hojman, P, Eriksen, J et al. (2008). Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum Gene Ther 19: 1261–1271. [DOI] [PubMed] [Google Scholar]
- Satkauskas, S, André, F, Bureau, MF, Scherman, D, Miklavcic, D and Mir, LM (2005). Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum Gene Ther 16: 1194–1201. [DOI] [PubMed] [Google Scholar]
- Kinosita, K Jr. and Tsong, TY (1977). Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim Biophys Acta 471: 227–242. [DOI] [PubMed] [Google Scholar]
- Lopez, A, Rols, MP and Teissie, J (1988). 31P NMR analysis of membrane phospholipid organization in viable, reversibly electropermeabilized Chinese hamster ovary cells. Biochemistry 27: 1222–1228. [DOI] [PubMed] [Google Scholar]
- Neumann, E, Schaefer-Ridder, M, Wang, Y and Hofschneider, PH (1982). Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi, H, Xu, Z, Ishii-Watabe, A, Uchida, E and Hayakawa, T (2000). IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 1: 376–382. [DOI] [PubMed] [Google Scholar]
- Ngoi, SM, Chien, AC and Lee, CG (2004). Exploiting internal ribosome entry sites in gene therapy vector design. Curr Gene Ther 4: 15–31. [DOI] [PubMed] [Google Scholar]
- Kishida, T, Asada, H, Kubo, K, Sato, YT, Shin-Ya, M, Imanishi, J et al. (2008). Pleiotrophic functions of Epstein-Barr virus nuclear antigen-1 (EBNA-1) and oriP differentially contribute to the efficiency of transfection/expression of exogenous gene in mammalian cells. J Biotechnol 133: 201–207. [DOI] [PubMed] [Google Scholar]
- Okita, K, Nakagawa, M, Hyenjong, H, Ichisaka, T and Yamanaka, S (2008). Generation of mouse induced pluripotent stem cells without viral vectors. Science 322: 949–953. [DOI] [PubMed] [Google Scholar]
- Warren, L, Manos, PD, Ahfeldt, T, Loh, YH, Li, H, Lau, F et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7: 618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita, K, Matsumura, Y, Sato, Y, Okada, A, Morizane, A, Okamoto, S et al. (2011). A more efficient method to generate integration-free human iPS cells. Nat Methods 8: 409–412. [DOI] [PubMed] [Google Scholar]
- Kaji, K, Norrby, K, Paca, A, Mileikovsky, M, Mohseni, P and Woltjen, K (2009). Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458: 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J, Hu, K, Smuga-Otto, K, Tian, S, Stewart, R, Slukvin, II et al. (2009). Human induced pluripotent stem cells free of vector and transgene sequences. Science 324: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, GP, D'Arcy, S, Ahsan, T, Nerem, RM, O'Brien, T and Barry, F (2010). Mesenchymal stem cells overexpressing ephrin-b2 rapidly adopt an early endothelial phenotype with simultaneous reduction of osteogenic potential. Tissue Eng Part A 16: 2755–2768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.