Abstract
The advantages of lipid-based transfection reagents have permitted their widespread use in molecular biology and gene therapy. This study outlines the effect of cryo-manipulation of a cationic lipid-based formulation, Lipofectamine 2000, which, after being frozen and thawed, showed orders of magnitude higher plasmid delivery efficiency throughout eight different cell lines, without compromising cell viability. Increased transfection efficiency with the freeze-thawed reagent was also seen with 2'-O-methyl phosphorothioate oligonucleotide delivery and in a splice-correction assay. Most importantly, a log-scale improvement in gene delivery using the freeze-thawed reagent was seen in vivo. Using three different methods, we detected considerable differences in the polydispersity of the different nucleic acid complexes as well as observed a clear difference in their surface spreading and sedimentation, with the freeze-thawed ones displaying substantially higher rate of dispersion and deposition on the glass surface. This hitherto overlooked elevated potency of the freeze-thawed reagent facilitates the targeting of hard-to-transfect cells, accomplishes higher transfection rates, and decreases the overall amount of reagent needed for delivery. Additionally, as we also saw a slight increase in plasmid delivery using other freeze-thawed transfection reagents, we postulate that freeze-thawing might prove to be useful for an even wider variety of transfection reagents.
Keywords: freezing, Lipofectamine 2000, lipofection, lipoplex, transfection
Introduction
The delivery of biologically active compounds into target cells in vitro and in vivo is of fundamental importance for scientific research as well as for therapeutic applications. Among numerous delivery systems, much emphasis has been placed on liposomes due to their composition of naturally occurring lipids, permitting biodegradation, high biocompatibility, and relatively low immunogenicity.1,2 Furthermore, liposomes can act as vehicles for the transportation of various types of bioactive cargos into cells, including nucleic acids, antigens, proteins, and small-molecule compounds.3,4,5
In the late 1980s, it was reported that cationic lipids (CLs) were able to facilitate intracellular DNA delivery.6 Since then, a number of commercial and noncommercial carriers have been developed by combining different CLs with a variety of neutral helper lipids (e.g., DOPE or cholesterol) that can be mixed with nucleic acid to form a positively charged complex (lipoplex) for the delivery of negatively charged molecules.7,8 Lipoplexes are often unstable in solution, resulting in the formation of aggregates, which contribute to lower transfection efficiency.9,10,11 Therefore, increasing their storage stability either on their own or when complexed with cargo molecules is of great interest.12,13 One way to potentially increase storage time and/or affect transfection efficiency could be to subject the lipids to a freeze-thaw cycle prior to use. On the other hand, it is believed that in terms of bioactive delivery, freezing has undesirable consequences for lipid-based transfection, since it might introduce complex fusion or compromise physical integrity.14 Hence, many, if not all, commercially available lipid-based formulations are, according to the manufacturers' recommendations, not to be frozen but rather be stored at +4 °C. Nevertheless, to our knowledge, there has not been a comprehensive study focusing on the effect of such physical manipulation of commercially available lipid-based transfection reagents.
One of the most widely used commercially available lipid-based transfection reagent to date is Lipofectamine 2000 (LF2000), well-known for its high transfection efficiency in a broad range of cell lines. Here, we describe the beneficial effect of freeze-thawing Lipofectamine 2000 on plasmid and oligonucleotide transfection efficiency both in vitro and in vivo. By varying the transfection conditions in different assays, we were able to optimize the LF2000 transfection protocol for increased efficiency and uncover the underlying mechanisms for such a positive result.
Results
Freeze-thawing of LF2000 results in log-scale increase in plasmid transfection efficiency
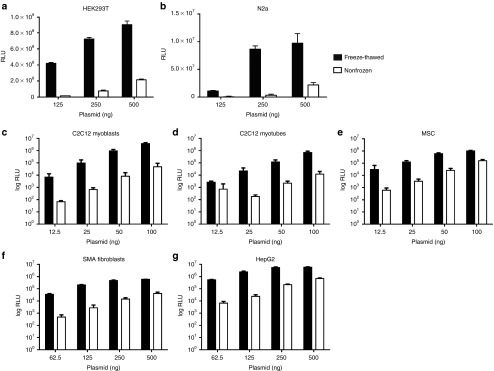
Our initial observation of the increased transfection efficiency with freeze-thawed LF2000 was both serendipitous and surprising, as the manufacturers' instructions suggest a decrease in transfection performance after freezing the reagent. To confirm whether the observed increase in efficiency was indeed due to freeze-thawing, freeze-thawed LF2000 was used to transfect HEK293T cells with luc-plasmid at different doses. This resulted in a luciferase signal of two exponential magnitudes above the non-frozen LF2000, which had been stored at +4 °C according to the manufacturers' recommendations (Figure 1a). The improvement in efficiency led us to further investigate the phenomenon in a range of other cell lines, including mouse neuroblastoma cells (N2a), myoblasts (C2C12), myotubes (differentiated C2C12), immortalized human mesenchymal stromal cells (hTERT MSCs), spinal muscular atrophy patient fibroblasts (SMA), human hepatoma (HepG2), basal breast cancer (MDA-MB-468), and epidermoid carcinoma (A431) cell lines (Figure 1b–g and data not shown for MDA-MB-468 and A431). Consistently, freeze-thawed LF2000 showed a log-scale improvement in plasmid transfection efficiency in all tested cell types. The effect was most prominent at lower doses and was observed throughout different batches of LF2000.
Figure 1.
Representative experiments comparing luc-plasmid transfection with nonfrozen versus freeze-thawed LF2000 in the following cell-lines: HEK293T (a), N2a (b), C2C12 myoblasts (c), C2C12 myotubes (d), hTERT MSCs (e), spinal muscular atrophy fibroblasts (f), and HepG2 (g). (c–g) Relative luminescence units (RLU) are depicted in log-scale. Each graph shows a representative experiment (mean + SD) in triplicates (24-well format; a,b,f, and g) or quintuplicates (96-well format; c–e).
For any transfection agent, one of the most important parameters affecting transfection efficiency is the DNA/reagent ratio that ensures optimal complexation. In order to address this, we carried out a ratio-titration study using different ratios of plasmid versus LF2000 (starting from 1:1 up to 1:4) to transfect 0.5 µg of luc-plasmid into C2C12 cells. Freeze-thawed LF2000 showed superior efficiency over the nonfrozen reagent at all ratios (Supplementary Figure S1a). As high amounts of transfection reagent compromise cell viability, the 1:2.5 ratio was found to be optimal for performing the experiments for both freeze-thawed and nonfrozen LF2000, in accordance with the instructions of the manufacturer.
Freeze-thawed LF2000 significantly increases the fraction of transfected cells
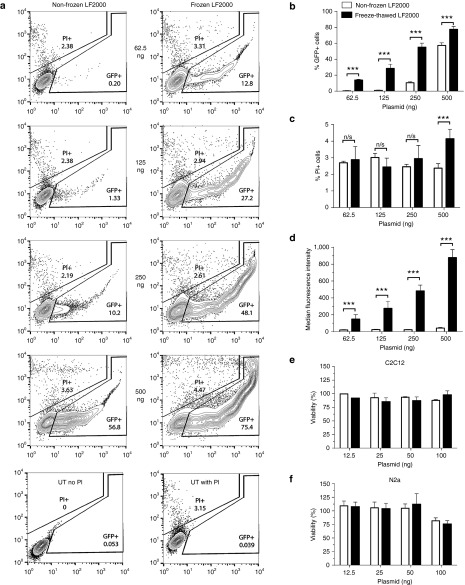
Since bioluminescent assays can only provide a quantitative measure of the transfection efficiency in positively transfected cells (i.e., the total amount of protein being expressed), flow cytometry measurement of GFP expression was further performed. Cells were transfected with a GFP plasmid to quantify the fraction of cells affected by the altered transfection efficiency, with simultaneous PI staining. These results further confirmed the significantly (P < 0.001) better performance of freeze-thawed over nonfrozen LF2000, with up to 45% increase in the number of transfected cells (Figure 2a,b). At the highest plasmid concentration, PI staining for the freeze-thawed formulation indicated a decreased cell viability (Figure 2c), nonetheless exhibited significantly (P < 0.001) higher median fluorescence intensity values at all tested concentrations (Figure 2d). In addition, cell viability was also evaluated using the WST-1 cytotoxicity assay, showing a slight decrease in viability at the highest dose (100 ng) in N2a cells, although not in C2C12 cells using both formulations (Figure 2e,f). Taken together, these results show that freeze-thawed LF2000 can be used to increase transfection efficiency in numerous cell lines, resulting in higher rates of delivery at lower doses, without marked increase in toxicity.
Figure 2.
Evaluation of nonfrozen and freeze-thawed LF2000 plasmid transfection efficiency and toxicity by flow cytometry analysis and WST-1 assay. (a) Representative contour plots showing the % of GFP+ and PI+ cells together with the fluorescence intensity after GFP plasmid transfection using nonfrozen and freeze-thawed LF2000 in N2a cells. (b–d) GFP expression, cell viability, and median fluorescence intensity values for the dose-titrated plasmid (n = 2; 24-well format; triplicates). (e,f) WST-1 toxicity assay 24 hours post-transfection with luc-plasmid in C2C12 (e) and N2a (f) cell-lines (n = 2; 96-well format; quintuplicates). Statistical significance was evaluated by a two-tailed Student's t-test (n.s., not significant; ***P < 0.001).
Enhanced transfection of cryo-manipulated LF2000 is not dependent on a specific promoter
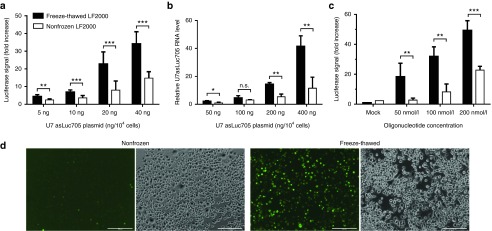
In order to ensure that the observed effect was not a result of a specific plasmid, expression cassette and/or type of promoter used, cells were similarly tested with a plasmid expressing a U7 snRNA construct from a minicircle plasmid.15 These constructs were used in a HeLa cell line (HeLa Luc705) stably expressing a reporter gene encoding a luciferase transcript interrupted by a splicing-deficient intron.16 Expression of the modified U7 snRNA can correct the splicing defect, thus giving rise to elevated luciferase expression.17 Consistent with the previous results, cells transfected with freeze-thawed LF2000 displayed significantly higher luciferase signals than those transfected with nonfrozen LF2000 (Figure 3a). In addition, according to RT-qPCR analysis, higher concentrations of the U7 snRNA showed a more prominent increase with freeze-thawed LF2000 in comparison to nonfrozen LF2000 (Figure 3b).
Figure 3.
Transfection of splice-correcting U7 snRNA minicircle plasmid and splice-correcting oligonucleotides. (a) Luciferase expression of HeLa Luc705 cells after transfection with the indicated amount of plasmid in 96-well format. Luciferase activity was measured from cell lysates and normalized to total protein content. Mean values + s.d. from seven replicates are shown. (b) U7 asLuc705 snRNA levels after transfection, as measured by RT-qPCR. Cells were transfected in 24-well format. After RNA extraction, U7 asLuc705 snRNA expression was measured by qPCR and normalized to that of RNU24. Mean + SD from three replicates are shown. (c) Mean fold increase in luciferase signal 24 hours post-transfection of HeLa Luc705 cells with a 18-mer 2'-O-Me-PS splice-correcting oligonucleotide (n = 2) (d) Representative fluorescence microscopy images of N2a cells 24 hours post-transfection with 100 nmol/l FAM-labeled 18-mer oligonucleotide using nonfrozen or freeze-thawed LF2000 (additional time points in Supplementary Figure S2. Results in (a) and (c) are presented as fold increase over untreated cells. In all cases, statistical significance was evaluated by a two-tailed Student's t-test (n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001).
To further establish the general effect of freeze-thawed LF2000 on nucleic acid delivery, the HeLa Luc705 cells were transfected with splice-correcting 2'-O-methyl phosphorothioate (2'-O-Me-PS) oligonucleotides. Again, a clear increase in luciferase signal was observed with freeze-thawed over nonfrozen LF2000 (Figure 3c). Furthermore, the uptake of a fluorescein-labeled 18-mer 2'-O-Me-PS and a Cy3-labeled 20-mer 2'-O-Me-PS complexed with LF2000 was monitored by fluorescence microscopy at different concentrations and time points after transfection. Consistent with previous results, we observed an elevated uptake of the oligonucleotide complexes with the freeze-thawed LF2000 (Figure 3d; Supplementary Figures S2 and S3). Thus, our results show that freeze-thawing results in higher transfection efficiency with different types of nucleic acid-LF2000 complexes, regardless of the plasmid organization or the chemical composition of the nucleic acid.
Freeze-thawed LF2000 increases in vivo plasmid delivery
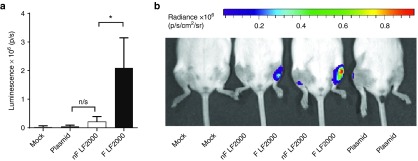
The aforementioned results describe the beneficial effects of freeze-thawing LF2000 for the delivery of nucleic acids in vitro. As the delivery of genetic material by lipoplexes is also of great value for animal studies and therapeutic strategies, we further wanted to establish whether freeze-thawing of LF2000 could have an impact on in vivo delivery. This was investigated using intramuscular injection (i.m.) of a luciferase plasmid complexed with nonfrozen or freeze-thawed LF2000. Luciferase expression analyzed by the IVIS imager 24 hours postinjection revealed a log-scale higher luminescence in the muscle treated with freeze-thawed LF2000, further corroborating our in vitro findings (Figure 4).
Figure 4.
Intramuscular (i.m.) injections of plasmid (PT2/C-fluc) complexed with nonfrozen (nF) or freeze-thawed (F) LF2000. (a) Mean luminescence readouts of different treatments + SD; n = 4; *P < 0.05. (b) IVIS image of i.m. injected mice 24 hours postinjection of an identical plasmid dose. NF and F complexes were injected into contralateral muscles to rule out interindividual differences between treated mice.
The enhancement in delivery persists over time and through several freeze-thaw cycles
From a user-friendly perspective, it would be favorable to retain the enhanced transfection efficiency over a longer period of time. Hence, transfection efficiency was examined by freezing and thawing LF2000 upon arrival, and then storing it for 2 weeks at +4 °C. Improved transfection efficiency was most accentuated for the freeze-thawed reagent used immediately after O/N storage at −20 °C. Nonetheless, the transfection efficiency remained notably better over nonfrozen LF2000 even after 2 weeks at +4 °C (Supplementary Figure S4a,b).
To understand whether the transfection efficiency of freeze-thawed LF2000 changes with repeated freeze-thaw cycles, aliquots of LF2000 were subjected to one to three freeze-thaw cycles with intermediate thawing at RT. The results demonstrated that despite exposing the reagent to repeated physical stress, these freeze-thawed formulations still retained higher transfection efficiency over the nonfrozen agent throughout the study (Supplementary Figure S4c).
Next, we aimed to evaluate the effect that the freezing rate might have on the lipid-based formulation. Therefore, LF2000 was briefly snap-frozen in liquid nitrogen followed by thawing at room temperature prior to use. Subsequent luminescence readouts showed that even snap-freezing of the reagent helped to elevate the transfection efficiency, although not to the same level as slow freezing O/N at −20 °C (Supplementary Figure S4d).
Freeze-thawing effect is most prominent for lipid-based formulations
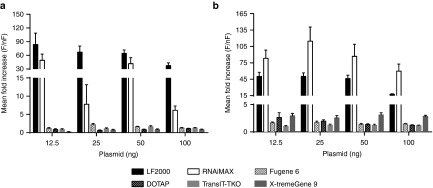
In order to start dissecting the underlying mechanism behind the increased efficiency, we first wanted to investigate whether the freezing effect is dependent on the composition of the transfection reagent. To elucidate this, a comparison in plasmid transfection efficiency between LF2000 and other lipid-based (Lipofectamine RNAiMAX, DOTAP methosulfate) and nonlipid-based (X-tremeGENE 9 DNA Transfection Reagent, TransIT-TKO and Fugene 6) reagents was conducted in HEK293T and N2a cells. Overnight freezing of nonlipid formulations and DOTAP resulted in a modest increase in plasmid expression (Figure 5a,b), while the usage of freeze-thawed Lipofectamine RNAiMAX increased the transfection efficiency to a similar level as that for freeze-thawed LF2000. Thus, the outstanding transfection efficiency seems to be related to the specific structure or composition of both Lipofectamine products.
Figure 5.
Representative experiments on luc-plasmid transfection efficiency using transfection reagents of different composition. Mean fold increase (freeze-thawed (F) over nonfrozen (nF)) in transfection efficiency using reagents of lipid-based (LF2000, Lipofectamine RNAiMAX, DOTAP) and nonlipid-based (X-tremeGene 9 DNA Transfection Reagent, TransIT-TKO Transfection Reagent) composition in N2a (a) and HEK293T (b) cells. Mean + SD from quintuplicates (96-well format) are depicted.
Complexes with freeze-thawed LF2000 have higher heterogeneity than their nonfrozen counterparts
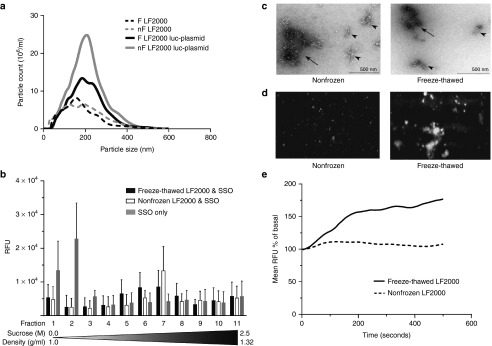
It is known that smaller molecules pass the cell membrane more easily than larger ones. This also applies to lipoplexes that help the bioactive reagents traverse the membrane (reviewed in ref. 18). Therefore, to assess whether the improvement in transfection efficiency of freeze-thawed LF2000 was due to a simple reduction of overall size, we carried out nanoparticle tracking analysis (NTA) on these complexes. Plasmid complexes formed with freeze-thawed LF2000 (mean size 212.7 ± 9.8 nm) displayed a similar polydispersed size distribution pattern as complexes with nonfrozen LF2000 (mean size 211.3 ± 11.8 nm) (Figure 6a). Hence, the size of particles could not explain the enhanced transfection rate for the freeze-thawed LF2000. Interestingly, we did observe a significant (P < 0.001) increase in overall particle concentration after complex formation with nonfrozen LF2000 (8.21 × 108 ± 5.83 × 107 particles/ml) versus for freeze-thawed LF2000 (5.40 × 108 ± 6.37 × 107 particles/ml).
Figure 6.
Analysis of nonfrozen (nF) and freeze-thawed (F) LF2000 lipoplexes by nanoparticle tracking analysis (NTA) (a), density gradient centrifugation (b), transmission electron microscopy (TEM) (c), TIRF (d), and TIRF-M (e). (a) NTA analysis of LF2000 alone or complexed with luc-plasmid at 21 °C. A significant difference (P < 0.001) in mean particle count between F and nF was detected (n = 3). (b) Sucrose gradient centrifugation of Cy3-labeled oligonucleotide complexes with F LF2000 displaying a more heterogeneous population of lipoplexes than with the nF reagent (n = 4). (c) TEM images of plasmid DNA complexes with nF and F LF2000. Irregular clusters of fine fibers (arrowheads) and their agglomerates (arrows) are pointed out. (d) TIRF snapshot of duplex RNA complexes with nF and F LF2000, showing vast differences in particle spreading rate upon contact with the glass surface. (e) TIRF-M time course of precipitation analysis of F and nF LF2000 complexes with duplex-RNA show an increased sedimentation of the F complexes on the glass surface.
In order to further examine the size distribution of the LF2000 lipoplexes, we employed dynamic light scattering (DLS) analysis, which showed a substantial heterogeneity of the plasmid complexes (Supplementary Figure S5a). Also, we noticed a higher intensity weighted harmonic mean size (z-average) of the nonfrozen LF2000 lipoplexes, hinting to their aggregation and leading to the necessity of further analysis by transmission electron microscopy (TEM).
In addition, the heterogeneity of the freeze-thawed LF2000 Cy3-labeled oligonucleotide complexes was confirmed by density gradient centrifugation, where the buoyant density of the particular complexes ranged from 1.12–1.20 g/ml (fractions 5–8) compared to the nonfrozen lipoplexes showing a much more homogenous population around 1.17 g/ml (fraction 7; Figure 6b).
Nonfrozen and freeze-thawed LF2000 plasmid complexes show a similar morphology and cargo release potential
To further examine the dispersity of the lipoplexes, TEM analysis of LF2000 complexed with a plasmid was employed using a negative staining technique. Plasmid DNA nanocomplexes with both nonfrozen and freeze-thawed LF2000 appeared as irregular clusters of fine fibers with the diameter in the range of 150–250 nm (arrowheads in Figure 6c). These clusters had often associated with each other forming bigger agglomerates with heterogeneous shape and diameter up to 1 µm (arrows in Figure 6c). Visual estimation in electron microscope revealed that the complexes with both nonfrozen and freeze-thawed LF2000 have rather similar morphology. Still, the nanocomplexes with freeze-thawed LF2000 formed somewhat smaller clusters than nonfrozen LF2000. To ensure that the nanostructures observed in TEM indeed represent the plasmid and LF2000 complexes, we performed identical experiment with plasmid labeled with colloidal gold. As indicated in Supplementary Figure S6a, similar complexes, tagged by gold particles, were formed, confirming the identity of structures. In parallel with labeled complexes, free unattached gold particles (empty arrowheads in Supplementary Figure S6a) and plasmid were also present in the specimen. LF2000 itself, either nonfrozen or freeze-thawed (Supplementary Figures S6b,c, respectively), did not form stable particles that could be detected in electron microscope, whereas naked plasmid DNA yielded small compact bundle-like structures with a diameter of 60–100 nm (arrowheads in Supplementary Figure S6d).
Freeze-thawed LF2000 complexes disperse on surfaces more readily
Differences in cargo release are another potential mechanism that could affect transfection efficiency. To study if this was an underlying cause for the observed effect, we evaluated the stability of the complexes through zeta-potential measurements by DLS, and also assayed them on agarose gel in the absence or presence of different concentrations of heparin. The results revealed neither a significant (P = 0.28) difference in the measured zeta potential (Supplementary Figure S5a) nor showed a distinction in plasmid release between the nonfrozen and freeze-thawed LF2000 complexes with regard to the availability of the competing polyanions (Supplementary Figure S5b).
As an alternative method to study the effect of decomplexation in more detail, we investigated the behavior of LF2000 in contact with surfaces using total internal reflection fluorescence (TIRF) microscopy. From the TIRF movies and “stills” it is evident that the duplex RNA complexes formed with freeze-thawed LF2000 spread out on the surface of the glass to a greater extent than those formed with nonfrozen LF2000 (Figure 6d; Supplementary Movie S1). This behavior was more evident at 37 °C than at 20 °C (data not shown). In addition, we observed an increase in complex precipitation on the glass surface over time (Figure 6e). For freeze-thawed LF2000 complexes, the signal reached a maximal of 180% of the basal signal, calculated as the background at time zero, whereas with nonfrozen LF2000 the signal peaked at 112%. Given that the camera signal is relatively linear in the measured interval, sevenfold more of the labeled siRNA settled on the bottom of the cell-culture vessel with freeze-thawed versus nonfrozen LF2000. Both the formulations reached a plateau after 400 seconds. Thus, it appears that the freeze-thawed complexes more readily disperse on a glass surface as well as sediment to a higher degree than the nonfrozen LF2000, potentially explaining the increased uptake of nucleic acids in cells and increased cellular activity.
Discussion
Lipoplexes are structures that are spontaneously formed in solution by the assembly of amphipathic molecules. Due to their dynamic properties and easy manipulation, they are widely used in biological research for the delivery of biologically active compounds. For this reason, the effect of different storage conditions is of major interest, as improper storage could potentially damage these lipid-based compounds and compromise the transfection efficiency. In this study, we demonstrate that by prefreezing the commercially available liposomal formulation LF2000, one can unexpectedly obtain tremendously improved transfection efficiency, both in vitro and in vivo.
The enhanced delivery by freeze-thawed LF2000 was observed regardless of the cell type used (HEK293T, N2a, C2C12, hTERT-MSC, SMA patient fibroblasts, HepG2, MDA-MB-468, A431), the type of plasmid (conventional or minicircle) or the type of promoter in the expression cassette (CMV, U7). Moreover, this freeze-thawed reagent could be extended for the delivery of oligonucleotides into the cells.
It is known that conditions optimized for in vitro transfections are often not applicable to efficient in vivo delivery.19 LF2000 has been used in both settings20,21,22,23 we performed i.m. injections in mice, which rendered a substantially higher luminescence from the muscles treated with freeze-thawed LF2000 than with nonfrozen LF2000, further supporting our in vitro findings.
One of the potential drawbacks of the cryo-manipulation could be increased toxicity. Based on PI staining with flow cytometry, we observed increased cytotoxicity at the highest plasmid amount. However, we propose that some cytotoxicity might arise as an indirect result of the highly efficient transfection, which saturates the gene expression machinery with plasmid-derived transcripts and proteins, thus reducing the amount of cellular processing complexes available for the expression of essential endogenous genes. Most importantly, no increase in toxicity was observed with the luc-plasmid transfections assayed with the WST-1 assay, nor with the oligonucleotide transfections using a comparable amount of transfected material (up to 120 ng in 96-well format). This further strengthens our interpretation that the cell death observed by flow cytometry with the plasmid transfections was due to the excessively high levels of GFP transgene.
One potential explanation for the increased transfection efficiency could be that freezing changes the size of the complexes. For large liposomes (5–150 µm diameter), it has been observed that, due to osmotic pressure, slow freezing alters the membrane structure24 by inducing dehydration contraction by about 20% of the liposomes' precooling diameter.25 In order to see if this was the case here, we used NTA to detect any changes in particle numbers or size while using nonfrozen or on their own. We noticed a significantly lower number of particles after plasmid complexation with freeze-thawed LF2000. However, we could not detect a difference in particle size between the two formulations. Therefore, we employed DLS analysis to evaluate the intensity weighted mean size of the particular complexes. The substantial postcomplexation increase in z-average of the nonfrozen lipoplexes revealed that these complexes may have undergone aggregation to a greater extent than the freeze-thawed ones. Although DLS and NTA size measurements showed discrepancies, this has been noted before.26 Indeed, DLS is known to perform well mainly for homogeneous samples since the presence of aggregates strongly contributes to the intensity of the scattered light and therefore to size overestimation.27
One additional parameter related to lipoplex size that we decided to measure was the polydispersity of the lipoplexes. We indeed observed a lower polydispersity index for the nonfrozen as opposed to freeze-thawed LF2000 lipoplexes, which was also confirmed by density gradient centrifugation showing a wider variation in buoyant density for latter complexes. As the polydispersity usually arises due to aggregation and fusion of complexes during lipoplex formation, we visualized the structure of the lipoplexes in more detail by using TEM. We saw a heterogeneous population of nanoparticles having a similar size to the one seen by NTA, together with a propensity to form aggregates that were smaller for freeze-thawed LF2000 clusters, supporting the z-average measurements by DLS. Yet, it still remains to be explored, whether the liposomal complex could undergo internal structural changes after freezing. Although we cannot detect this change by standard particle size analysis, we hypothesise that this could still contribute to higher transfection efficiency.28 Also, there still are conflicting views on the optimal size of a lipoplex, and how this physical aspect may determine its pathway of entry and ultimately impact on its efficiency in traversing the cell membrane. While large complexes have an advantage in being actively endocytosed in cells due to their facilitated membrane contact and fusion, there may be a preference for smaller particles in other cells, and yet in some cells there is no correlation between the lipoplex size and uptake efficiency (reviewed in ref. 18).
Another important aspect contributing to efficient transfection is cargo release through lipoplex decomplexation.29 The surface charge of lipoplexes, which also is crucial for the passage through the cell membrane,30 greatly affects both complexation and decomplexation. As we neither observed any substantial changes in surface charge by DLS nor in decomplexation in the presence of a competing polyanion, other factors were explored, including in vitro sedimentation of the complexes that might promote the uptake of the freeze-thawed lipoplexes. Indeed, by using TIRF, we observed a higher surface spreading as well as a sevenfold higher precipitation of complexes with the freeze-thawed LF2000 in the cell culture vessel. Therefore, an appealing hypothesis is that the elevated transfection efficiency is brought about through the increased sedimentation and spreading of the freeze-thawed lipoplexes. This allows them to target a larger number of cells, with a larger percentage of cell-complex interactions resulting in productive cargo release. However, since an increased plasmid delivery was also observed in vivo, where the correlation between increased sedimentation and uptake efficiency is much less elaborated, other factors may contribute to the phenomena.
Our results also indicate that the observed increase in transfection efficiency is related to a specific composition of the transfection reagent. Freeze-thawing of nonlipid-based transfection reagents or the well-described liposomal compound DOTAP had only modest impact on transfection efficiency, whereas freeze-thawing of Lipofectamine RNAiMAX had an effect similar to that of LF2000. Therefore, it is plausible that the effect is related to distinct components or shared structure of these two proprietary formulations.31 Yet, without a detailed knowledge about the functional headgroups of the present cationic formulations,32 we would like to keep from further speculations and hope that future studies will complement our findings.
This study is the first comprehensive overview of the freeze-thawing effect of a commercial transfection reagent. By using various methods we were able to show the beneficial effect of freeze-thawing of a lipid-based transfection reagent on plasmid as well as oligonucleotide delivery, and also provide some insight into the underlying causes of this phenomenon. Although we did observe batch to batch variation in the performance of the reagent after freeze-thawing, the present findings are still important for the general understanding of transfection reagent manipulation, as well as for redefining the parameters for the applicability and efficiency of a widely used commercial cationic lipofection reagent. Importantly, our results underpin the fact that the amount of reagent needed for cellular transfections can be drastically reduced while maintaining activity by simple freeze-thawing.
Materials and methods
Reagents. LF2000 (Thermo Fisher Scientific, Waltham, MA) was either stored according to the manufacturer's instructions (non-frozen LF2000), subjected to overnight (O/N) freezing at −20 °C (freeze-thawed LF2000) or snap-frozen in liquid nitrogen (snap-frozen LF2000). The reagent was thawed at room temperature (RT) immediately prior to use. Several batches of LF2000 from different lots were tested over a period of 2 years. Batch differences did occur.
Other liposomal (Lipofectamine RNAiMAX, Thermo Fisher Scientific; DOTAP methosulfate, Sigma-Aldrich, St. Louis, MO) and nonliposomal transfection reagents (X-tremeGene 9 DNA Transfection Reagent, Roche Diagnostics, Mannheim, Germany; TransIT-TKO Transfection Reagent, Mirus Bio LLC, Madison, WI; Fugene 6 Transfection Reagent, Promega Corporation, Madison, WI) underwent O/N freezing at −20 °C followed by thawing at RT directly prior use are correspondingly denoted as freeze-thawed reagents.
Cell culture and transfection conditions. All cell lines were incubated in a humidified atmosphere at 37 °C containing 5% CO2. Cells were maintained in high-glucose (4.5 g/l) Dulbecco's modified Eagle's medium with GlutaMax (DMEM GlutaMax, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific). SMA patient fibroblasts (GM03813) were maintained in low-glucose (1 g/l) Dulbecco's Modified Eagle Medium (DMEM, Thermo Fisher Scientific). C2C12 myoblasts were initially grown in DMEM GlutaMax with 20% FBS. For differentiation into C2C12 myotubes, DMEM medium supplemented with 2% FBS was applied 24 hours after seeding. The myotubes were used for transfection 7–9 days later, while changing the medium every 2–3 days. The human telomerase reverse transcriptase-immortalized human mesenchymal stromal cells (hTERT MSCs)33 were maintained in Roswell Park Memorial Institute (RPMI) GlutaMAX Supplement, HEPES-buffered medium (RPMI 1640 Medium, Thermo Fisher Scientific) in the presence of 10% FBS. For transfections in 96- or 24-well format, 104 or 5 × 104 cells were seeded 24 hours prior to transfection, respectively. 2.5 µl of LF2000 was used for every 1 µg of transfected material, if not specified otherwise. The amount of plasmid was scaled up according to the plate format used.
WST-1 assay. Cells were transfected with nonfrozen or freeze-thawed LF2000 in a 96-well format as described above. After 24 hours, cell proliferation reagent (Roche Diagnostics) was added to both treated and untreated cells, while fresh medium was added to control wells for background normalization. Fluorescence measurements were taken at 440 nm/630 nm at 15 minutes, 30 minutes, 1 hour, 2 hours, and 4 hours to verify the linear range of color development. The optimal incubation period was specifically verified for each cell line (1 hour for N2a and 30 minutes for C2C12) and used for subsequent cytotoxicity calculations.
Flow cytometry and fluorescent microscopy. 5 × 104 cells were seeded in 24-well plate wells 1 day before the transfection with a CD63-EmGFP (Emerald Green Fluorescent Protein) expressing plasmid using either nonfrozen or freeze-thawed LF2000. Cells were imaged after 24 hours with fluorescence microscopy (Olympus IX81, Olympus America, Center Valley, PA) or harvested for flow cytometry. Prior to the analysis with flow cytometry, cells were resuspended in Dulbecco's phosphate-buffered saline (Thermo Fisher Scientific) solution containing 2% FBS, 1 mmol/l ethylenediaminetetraacetic acid and 1 µl/ml propidium iodide (PI) (Thermo Fisher Scientific). Flow cytometry analysis was performed by BD FACSCalibur in the Center for Hematology and Regenerative Medicine (HERM) at Karolinska Institutet. Data analysis was done by using FlowJo Software (FlowJo, LLC, Ashland, OR).
NTA. The NanoSight NS500 nanoparticle analyzer (Malvern Instruments, Malvern, UK) was used to measure the size distribution and concentration of particles of LF2000 alone or complexed with R4B4G3-luc plasmid (hereafter referred to as luc-plasmid) in Opti-MEM I Reduced Serum Medium (Thermo Fisher Scientific). The mixtures were loaded on the NS500, where the number of particles as well as their movement was recorded for 5 × 60 seconds. The analysis was conducted using the NTA Software 2.3 (Malvern Instruments).
Splice-correction with minicircle plasmids and oligonucleotides. HeLa cells stably expressing the gene for luciferase with the mutated 705 human β-globin mini-intron sequence inserted (HeLaLuc705 (ref. 16)) were used to monitor the transfection and expression efficiency of splice-correcting agents. These were either 705Luc-18mer-2́-O-methyl-phosphorothioate-FAM (705Luc-18mer-2́-O-Me-PS-FAM) oligonucleotides described in ref. 16, Cy3-labeled 20-mer 2́-O-Me-PS oligonucleotides (hereafter Cy3-labeled oligonucleotides) or modified U7 snRNA constructs (U7 asLuc705) expressed from minicircle plasmids, as described in ref. 15. In brief, the β-globin intron contains a point mutation that generates an aberrant 5'splice site (5'ss) that subsequently activates a cryptic 3'splice site (3'ss). Consequently, a part of the intron is included in the spliced mRNA and no functional protein is produced. Treatment with oligonucleotides or the U7 constructs can block these splice sites and concomitantly restore the correct splicing and thus generate a functional luciferase.
Luciferase assays. Transfection efficiencies of plasmids (luc-plasmid) or splice-correcting agents were assessed by using the Luciferase Assay System (Promega Corporation) and luminescence was measured with GloMax-96 Microplate Luminometer (Promega Corporation). In case of the MC plasmids, the luciferase activity was further normalized to the amount of total protein in each sample, as measured by a Protein Assay (Bio-Rad Laboratories, Hercules, CA).
RT-qPCR. RNA was extracted from the cells transfected with the U7 snRNA construct after 24 hours by following a standard Trizol (Thermo Fisher Scientific) extraction protocol. The modified U7 snRNA was detected by real-time reverse transcription polymerase chain reaction (RT-qPCR) and normalized to small nucleolar C/D box 24 RNA (RNU24) as described previously.15
Animal experiments. 5 µg of PT2/C-fluc plasmid (Addgene plasmid 20203 (ref. 34)) was used for intramuscular (i.m.) injections in mice, either on its own or complexed with 12.5 µg freeze-thawed or non-frozen LF2000 in OptiMEM medium, in a total volume of 50 µl. The luminescence was imaged 24 hours postinjection using the IVIS Imaging System (PerkinElmer, Waltham, MA). All animal experiments were approved by The Swedish Local Board for Laboratory Animals. The experiments were performed in accordance with the ethical permission and designed to minimize the suffering and pain of the animals.
TIRF microscopy. TIRF microscopy experiments were performed on a Zeiss Laser TIRF 3 system using a 100× objective. Glass inserts (P35G-1.5-14-C) were from MatTek Ashland, MA. TIRF angle was set at 70 degrees (depth of penetration = 86 nm) For experiments performed at 37 °C, Opti-MEM I Reduced Serum was preheated and added to glass inserts mounted in a microscope heating stage held at 37 °C, after which nonfrozen or freeze-thawed LF2000 complexed with Alexa-568-labeled 21-mer duplex RNA was added. Images were recorded after a brief refocusing.
Sucrose gradient. 0.12 nmol of Cy3-labeled 20-mer oligonucleotides were loaded either on their own, or complexed with nonfrozen/freeze-thawed LF2000, on top of a discontinuous 0.0–2.5 M sucrose gradient and centrifuged at 200,000 g for 16 hours at 4 °C in a SW40 swinging bucket rotor (Beckman Coulter, Fullerton, CA). 1 ml fractions were collected through bottom puncture and weighed to determine the density of the specific fractions. 100 µl from each fraction was transferred to a 96-well plate and measured for fluorescence using FLUOstar OPTIMA Microplate Reader (BMG Labtech GmbH, Ortenberg, Germany) using excitation/emission wavelength of 544 nm/570 nm.
Heparin displacement assay. The relative stability of the LF formulations was evaluated by measuring the release of plasmid in the presence of heparin. Formulations with nonfrozen or freeze-thawed LF2000 containing 100 ng plasmid were incubated for 30 minutes at 37 °C in the absence or presence (0.0018 or 18 µg/µl) of heparin sodium (Sigma-Aldrich). Loading buffer was then added and the reactions were analyzed on 0.8% agarose gels in 1× TAE buffer and visualized by staining with SYBR Gold (Thermo Fisher Scientific).
Dynamic light scattering analysis. The z-average diameter, polydispersity and zeta potential of mock freeze-thawed or non-frozen LF2000, Fugene 6, DOTAP or their lipoplexes with luc-plasmid were determined by dynamic light scattering using Zetasizer Nano ZS (Malvern Instruments). The complexes were prepared as stated above and diluted 10 times in 0.2 µm filtered distilled water. 500 μl of each sample was measured in folded capillary cells (Malvern Instruments). All measurements were performed at 22 °C using 8 × 10 seconds runs for size and polydispersity measurements or 10 zeta runs, consisting of six submeasurements. The data was analyzed using Zetasizer software (Malvern Instruments).
TEM. TEM analysis of transfection mixtures was carried through on complexes of 0.5 µg of pGL3 plasmid (Promega Corporation) with 1.25 µl of LF2000 in OptiMEM medium, incubated at room temperature for 30 minutes. The particles of pDNA and LF2000 were visualized by negative staining with uranyl acetate. In some experiments, biotinylated plasmid and colloidal gold labeled neutravidin were used to visualize the formed complexes for their identification. Labeling of pDNA with colloidal gold was performed as described in ref. 35, using biotin tagged pGL3 plasmid (Label IT Nucleic Acid Labeling Kit, Biotin, Mirus Bio LLC) and neutravidin-colloidal gold conjugate (10 nm in diameter). Electron microscopy analysis was performed using FEI Tecnai G2 Spirit BioTWIN microscope run at 120 kV. The images were recorded with Orius SC1000 CCD camera and processed using Adobe Photoshop CS4.
Statistical analysis. Statistical analysis was performed by GraphPad Prism v6 (GraphPad Software, La Jolla, CA) by using two-tailed Student's t-test, with two-sample unequal variance.
SUPPLEMENTARY MATERIAL Figure S1. Dose titration of LF2000 vs luc-plasmid in C2C12 cells. Figure S2. Uptake of FAM-labelled oligonucleotides at 1 h, 6 h and 24 h post-transfection in N2a and HEK293T cells. Figure S3. Uptake of Cy3-labelled oligonucleotides at 1 h, 6 h and 24 h post-transfection in N2a and HEK293T cells. Figure S4. The effect of long-term storage, multiple freeze-thawing and snap-freezing of LF2000 on luc-plasmid transfection efficiency. Figure S5. DLS measurements and heparin displacement assay of non-frozen or freeze-thawed LF2000 either nakedly or in plasmid complexes. Figure S6. Transmission electron microscopy images of non-frozen or freeze-thawed LF2000 complexes with biotin labelled plasmid DNA. Movie S1. TIRF microscopy videos of non-frozen and freeze-thawed LF2000 complexes with duplex RNA.
Acknowledgments
This research was supported by the Swedish Research Council (SELA, CIES), The Swedish Society for Medical Research (SELA), postdoctoral grants from Sigrid Jusélius Foundation and Osk Huttunen Foundation (Helsinki, Finland; JJT), and from Hjärnfonden (The Brain Foundation, Stockholm, Sweden; EMZ), the national scholarship program Kristjan Jaak, which is funded and managed by Archimedes Foundation in collaboration with the Estonian Ministry of Education and Research (HS), KID-funding (Karolinska Institutet doctoral co-financing agreement with the Stockholm County Council; GC), European Research Council Starting Grant 260627 ‘MINDS' in the FP7 ideas program of the EU (RM), by a VENI Fellowship from the Netherlands Organisation for Scientific Research (PV) and the Estonian Ministry of Education and Research (0180019s11, MP). The authors declare no competing financial interest.
Supplementary Material
References
- Smyth Templeton, N (2002). Liposomal delivery of nucleic acids in vivo. DNA Cell Biol 21: 857–867. [DOI] [PubMed] [Google Scholar]
- Immordino, ML, Dosio, F and Cattel, L (2006). Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 1: 297–315. [PMC free article] [PubMed] [Google Scholar]
- Fraley, R, Straubinger, RM, Rule, G, Springer, EL and Papahadjopoulos, D (1981). Liposome-mediated delivery of deoxyribonucleic acid to cells: enhanced efficiency of delivery related to lipid composition and incubation conditions. Biochemistry 20: 6978–6987. [DOI] [PubMed] [Google Scholar]
- Gregoriadis, G and Florence, AT (1993). Liposomes in drug delivery. Clinical, diagnostic and ophthalmic potential. Drugs 45: 15–28. [DOI] [PubMed] [Google Scholar]
- Zuris, JA, Thompson, DB, Shu, Y, Guilinger, JP, Bessen, JL, Hu, JH et al. (2015). Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol 33: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner, PL, Gadek, TR, Holm, M, Roman, R, Chan, HW, Wenz, M et al. (1987). Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA 84: 7413–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y, Mounkes, LC, Liggitt, HD, Brown, CS, Solodin, I, Heath, TD et al. (1997). Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol 15: 167–173. [DOI] [PubMed] [Google Scholar]
- Gregoriadis, G (1988). Liposomes as a drug delivery system: optimization studies. Adv Exp Med Biol 238: 151–159. [DOI] [PubMed] [Google Scholar]
- Lai, E and van Zanten, JH (2002). Real time monitoring of lipoplex molar mass, size and density. J Control Release 82: 149–158. [DOI] [PubMed] [Google Scholar]
- Ross, PC and Hui, SW (1999). Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther 6: 651–659. [DOI] [PubMed] [Google Scholar]
- Almofti, MR, Harashima, H, Shinohara, Y, Almofti, A, Li, W and Kiwada, H (2003). Lipoplex size determines lipofection efficiency with or without serum. Mol Membr Biol 20: 35–43. [DOI] [PubMed] [Google Scholar]
- Bergström, LM and Bramer, T (2008). Synergistic effects in mixtures of oppositely charged surfactants as calculated from the Poisson-Boltzmann theory: a comparison between theoretical predictions and experiments. J Colloid Interface Sci 322: 589–595. [DOI] [PubMed] [Google Scholar]
- Hinrichs, WL, Manceñido, FA, Sanders, NN, Braeckmans, K, De Smedt, SC, Demeester, J et al. (2006). The choice of a suitable oligosaccharide to prevent aggregation of PEGylated nanoparticles during freeze thawing and freeze drying. Int J Pharm 311: 237–244. [DOI] [PubMed] [Google Scholar]
- Harrigan, PR, Madden, TD and Cullis, PR (1990). Protection of liposomes during dehydration or freezing. Chem Phys Lipids 52: 139–149. [DOI] [PubMed] [Google Scholar]
- Stenler, S, Wiklander, OP, Badal-Tejedor, M, Turunen, J, Nordin, JZ, Hallengärd, D et al. (2014). Micro-minicircle Gene Therapy: Implications of Size on Fermentation, Complexation, Shearing Resistance, and Expression. Mol Ther Nucleic Acids 2: e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, SH, Cho, MJ and Kole, R (1998). Up-regulation of luciferase gene expression with antisense oligonucleotides: implications and applications in functional assay development. Biochemistry 37: 6235–6239. [DOI] [PubMed] [Google Scholar]
- Gorman, L, Suter, D, Emerick, V, Schümperli, D and Kole, R (1998). Stable alteration of pre-mRNA splicing patterns by modified U7 small nuclear RNAs. Proc Natl Acad Sci USA 95: 4929–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, B, Zhang, S, Jiang, H, Zhao, B and Lv, H (2007). Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J Control Release 123: 184–194. [DOI] [PubMed] [Google Scholar]
- Pedroso de Lima, MC, Simoes, S, Pires, P, Faneca, H and Duzgunes, N (2001). Cationic lipid-DNA complexes in gene delivery: from biophysics to biological applications. Adv Drug Deliv Rev 47: 277–294. [DOI] [PubMed] [Google Scholar]
- Hunt, MA, Currie, MJ, Robinson, BA and Dachs, GU (2010). Optimizing transfection of primary human umbilical vein endothelial cells using commercially available chemical transfection reagents. J Biomol Tech 21: 66–72. [PMC free article] [PubMed] [Google Scholar]
- Wang, YL, Liu, W, Wada, E, Murata, M, Wada, K and Kanazawa, I (2005). Clinico-pathological rescue of a model mouse of Huntington's disease by siRNA. Neurosci Res 53: 241–249. [DOI] [PubMed] [Google Scholar]
- Qian, L, Van Laake, LW, Huang, Y, Liu, S, Wendland, MF and Srivastava, D (2011). miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 208: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B, Lin, H, Xiao, J, Lu, Y, Luo, X, Li, B et al. (2007). The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 13: 486–491. [DOI] [PubMed] [Google Scholar]
- Siminovitch, D and Chapman, D (1971). Liposome bilayer model systems of freezing living cells. FEBS Lett 16: 207–212. [DOI] [PubMed] [Google Scholar]
- Kobayashi, M, Nemoto, K, Tanaka, G and Hishida, M (2011). Study of the freezing behavior of liposomes. J Therm Sci Tech-Jpn 6: 57–68. [Google Scholar]
- Tuoriniemi, J, Johnsson, ACJH, Holmberg, JP, Gustafsson, S, Gallego-Urrea, JA, Olsson, E et al. (2014). Intermethod comparison of the particle size distributions of colloidal silica nanoparticles. Sci Technol Adv Mat 15; 035009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiskoot, W and Crommelin, DJA. Methods for Structural Analysis of Protein Pharmaceuticals. AAPS Press, Arlington, VA, 2005. [Google Scholar]
- Stark, B, Pabst, G and Prassl, R (2010). Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effects of cryoprotectants on structure. Eur J Pharm Sci 41: 546–555. [DOI] [PubMed] [Google Scholar]
- Caracciolo, G, Marchini, C, Pozzi, D, Caminiti, R, Amenitsch, H, Montani, M et al. (2007). Structural stability against disintegration by anionic lipids rationalizes the efficiency of cationic liposome/DNA complexes. Langmuir 23: 4498–4508. [DOI] [PubMed] [Google Scholar]
- Wasungu, L and Hoekstra, D (2006). Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release 116: 255–264. [DOI] [PubMed] [Google Scholar]
- Zhi, D, Zhang, S, Cui, S, Zhao, Y, Wang, Y and Zhao, D (2013). The headgroup evolution of cationic lipids for gene delivery. Bioconjug Chem 24: 487–519. [DOI] [PubMed] [Google Scholar]
- Chu, Y, Masoud, M and Gebeyehu, G (2009). US7479573 B2 Transfection reagents. Google Patents.
- Mihara, K, Imai, C, Coustan-Smith, E, Dome, JS, Dominici, M, Vanin, E et al. (2003). Development and functional characterization of human bone marrow mesenchymal cells immortalized by enforced expression of telomerase. Br J Haematol 120: 846–849. [DOI] [PubMed] [Google Scholar]
- Wiesner, SM, Decker, SA, Larson, JD, Ericson, K, Forster, C, Gallardo, JL et al. (2009). De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res 69: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arukuusk, P, Pärnaste, L, Margus, H, Eriksson, NK, Vasconcelos, L, Padari, K et al. (2013). Differential endosomal pathways for radically modified peptide vectors. Bioconjug Chem 24: 1721–1732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.