Abstract
Introduction
The incidence of urinary tract infections caused by Extended-Spectrum Beta Lactamase (ESBL) producing Escherichia coli (E. coli) strains due to long term and overuse of broad-spectrum cephalosporine is on the rise. CTX beta-lactamase type, a broad-spectrum beta-lactamase, has been expanding in many countries. The ctx gene is harbored on a plasmid that is spread between Enterobacteriaceae family, especially in E. coli. The aim of this study was to determine the pattern of antimicrobial resistance and investigate the prevalent ESBL phenotype and the ctx-M gene in E. coli isolated from patients with urinary tract infections (UTI) in Semnan.
Methods
A cross sectional study was performed on 109 strains of E. coli isolated from the urine culture of patient suffering from a UTI referred to Shafa hospital (Semnan, Iran) during March–July 2015. Antimicrobial susceptibility testing was applied and the prevalence of the ESBL phenotype was confirmed using combination disk. PCR methods were completed for amplification of the bla ctx gene. Data were analyzed using SPSS version 18 software.
Results
One hundred ninety samples (4.16%) were identified as E. coli. Twenty one (26.6%) of E. coli were ESBL positive and 73.4% were ESBL negative. There was 100% susceptibility to imipeneme. Twenty (68.97%) out of 29 isolates were positive for the ctx-M gene, as detected by PCR.
Conclusion
In urinary tract infections, antibiotic treatment was experimental and detailed information regarding the sensitivity of bacteria in the area can be useful to achieve the best treatment.
Keywords: E. coli, ctx-M gene, urinary tract infection, ESBL
1. Introduction
Escherichia coli (E. coli) is an important and common pathogen of urinary tract infections (UTIs) (1, 2). Treatment of UTIs caused by E. coli is becoming difficult due to antibiotic resistance (3). Resistance by the various mechanisms, such as altered target sites, enzymatic inactivation of antibiotics, active efflux pump, and decreased permeability by the porins, are known in Gram negative bacteria (4). One of the most common resistance mechanisms is the production of Extended–Spectrum Beta-Lactamase enzymes (ESBL) that hydrolyze all penicillins, early cephalosporins, oximino-cephalosporins, and monobactames, but they cannot hydrolyze carbapenemes or cephamycins (5–7). These enzymes are susceptible to inhibitors, such as clavolanic acid, sulbactam, and tazobactam (8, 9). The prevalence of ECBLs in pathogens continues to be associated with higher rates of healthcare costs and mortality (10). Until now, 400 types of enzymes are known to be seen in the Enterobacteriacea family (11). The CTX-M beta lactamase types have been expanding in many countries and are currently the most widespread enzymes (12).
At present, CTX-M-Beta Lactamases are encoded in a plasmid and can hydrolyze both ceftazidime and cefotaxime, but give a high level of resistance to cefotaxime and a low level of activity against ceftazidime (13, 14). These preplasmic enzymes were reported for the first time in the late 1980s (7). The CTX-M enzymes are a group of class A ESBL that have disseminated among a wide range of clinical bacteria within and across the species in the world since 1995 (12). Nowadays, based on >130 amino acid sequences, CTX-M allelic variant have been categorized in five major phylogenetic groups: CTX-M1, CTX-M2, CTX-M8, CTX-M9, and CTX-M25 (15). The incidence of urinary tract infections caused by ESBL producing E. coli strains that result from long term and overuse of broad-spectrum cephalosporine is on the rise. Prevalence of CTX-M Beta Lactamase in commonly isolated organisms, such as E. coli, is now a serious public health problem worldwide. In urinary tract infections, antibiotic treatment was experimental and few details exist about the sensitivity and molecular epidemiology of E. coli in Semnan. Current knowledge of antibiotic resistance patterns and the frequency of CTX-M B-Lactamase between E. coli isolates from patients with UTI in every region could help with rapid treatment. With this background, the aim of this study was to determine the pattern of antimicrobial resistance and investigate the prevalence of the ESBL phenotype and ctx-M gene in E. coli isolated from patients with UTIs in Semnan.
2. Material and Methods
2.1. Study design, sampling and bacterial isolates
A total of 2618 urine samples of early morning mid-stream were collected in sterile, wide mouthed bottles from patients between March and July of 2015. Samples were analyzed within one hour after collection. Samples were inoculated using an inoculating loop with a 10 μl volume calibration on nutrient, blood, and Mac Conkey agar plates and incubated in aerobic condition at 37 °C for 24 hours. Colony counts equal or more than 105 per ml were considered as positive UTIs and at less than 105 per ml was considered a suspected UTI and the assay repeated. Identification was done based on routine standard biochemical tests, including oxidase, catalase, motility and gas production, reduction of nitrates, Indol, methyl red, voges-proskauer, citrate, lactose fermenter, and lysine dexarboxylatate tests. Samples that were confirmed as E. coli were stored in BHI Broth containing 15% glycerol at −70 °C and were subjected to further molecular tests.
2.2. Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was applied using the Kirby Bauer disk diffusion method. E. coli ATCC 25922 was used to control the quality of the applied antimicrobial agents. The susceptibility of isolates to the following antibiotics was evaluated; Briefly, a 0.5 McFarland suspension was prepared from pure culture of E. coli in a nutrient broth and inoculated on Muller-Hinton agar. The antibiotic discs were applied at distances of 24 mm from center to center. The plates were incubated aerobically for 24h at 37 °C. Based on the diameter of the inhibition zone, the results were interpreted as susceptible, intermediate, or resistant.
2.3. Phenotypic detection of ESBLs
The prevalence of the Extended-Spectrum Beta Lactamase (ESBL) phenotype was confirmed with a Cefotaxime (30 μg) or Cefotaxime disk with or without clavolanate (10μg), as per the CLSI 2013 guidelines. A positive ESBL phenotype meant that the zones produced by the disks with clavolanate were ≥5 mm larger than those without inhibitor.
2.4. DNA extraction and PCR of bla-ctx-M gene
Genomic DNA was extracted by boiling a suspension of ESBL producing bacterial cells in sterile distilled water. Ten μl of DNA template was use in the PCR reaction containing 1X buffer, MgCl2, dNTP, Taq polymerase, and primers for detection of the bla-CTX-M gene. The primer sequences were as follows: bla-CTX-M-F 5′-ACCGCCGATAATTCGCAGAT-3′ and bla-CTX-M-R 5′-GATATCGTTGGTGGTGCCATA-3′. The PCR conditions for amplification of the bla-CTX-M gene were done as follows: Initial denaturation at 94 °C for 5 minutes, denaturation at 94 °C for 1 minute, 30 seconds for annealing at 59.2 °C, 1 minute at 72 °C for elongation, and the final extension was conducted at 72 °C for 5 minutes. A negative control was used in every PCR assay. The PCR product was subjected to electrophoresis in a 1% agarose gel and staining with Ethidium bromide (0.5 mg/ml).
2.5. Research ethics
This research was conducted in accordance with the ethical principles of clinical specimens.
2.6. Statistical analysis
The degree of significance was calculated using the Chi-Square test. P-values less than 0.05% were considered significant. Statistical analysis was carried out using SPSS version 18 (SPSS Inc., Chicago, IL, USA).
3. Results
In this study, among 2618 urine samples tested, 109 (4.16%) were identified as E. coli. Based on the results of the combination disc method, twenty nine (26.6%) of the E. coli samples were ESBL positive and 73.4% were ESBL negative. Twenty six (89.65%) of the positive samples were female and three (10.34%) were male. The average age was 32 years old and patient age ranged from 6 days to 87 years old. According to the results obtained from disc diffusion antimicrobial susceptibility testing, which is represented in Figure 1, the majority of ESBL positive isolates, showed a high level of resistance to Trimetoprim-Sulfametaxazol (46.8%). There was 100% susceptibility to Imipeneme. PCR detection of the blactx-M gene in ESBL producing isolates revealed that 20 (68.97%) out of 29 isolates were positive (Figure 2 and Table 1).
Figure 1.
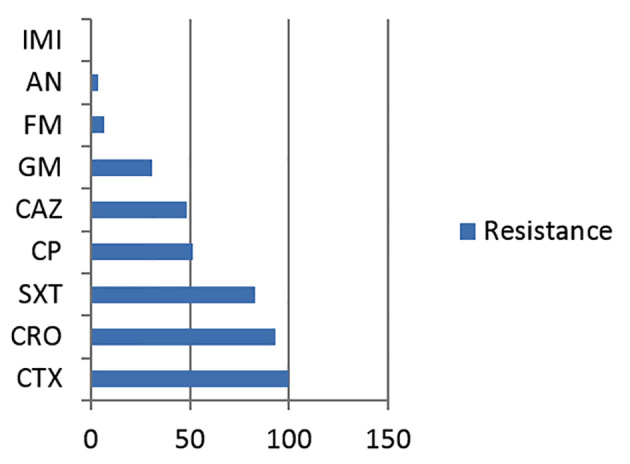
The resistance pattern of 29 ESBL- producing E. coli isolates
Figure 2.
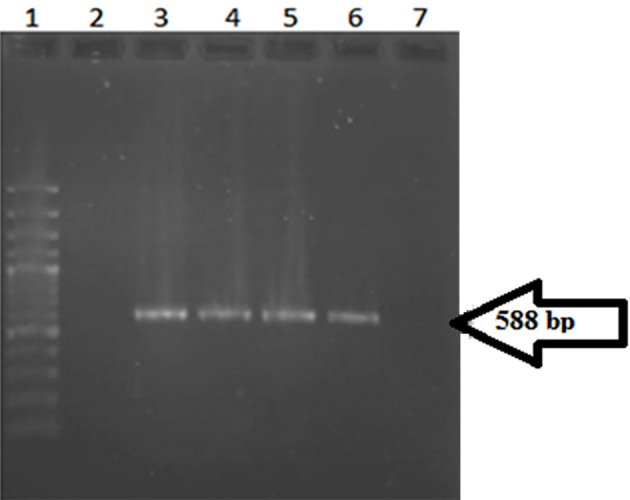
Electrophoresis of PCR products on a 1% agarose gel.Lane1. Ladder 100 bp. Lane 2. Negative control. Lane 3. Positive control. Lanes 4, 5, 6. Positive samples that indicated a 588 bp PCR product. Lane 7. Negative sample.
Table 1.
The antibiotic resistance pattern in Positive ESBL strains and prevalence of the ctx_M gene in every pattern.
| Pattern | CTX | CRO | SXT | CP | CAZ | GM | FM | AN | IMI | Number of isolates | Number of positive CTX gene |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | R | 1 | 1 | |||||||
| 2 | R | R | R | 6 | 2 | ||||||
| 3 | R | R | R | R | 3 | 2 | |||||
| 4 | R | R | R | 2 | 2 | ||||||
| 5 | R | R | R | R | 3 | 3 | |||||
| 6 | R | R | R | R | 1 | 0 | |||||
| 7 | R | R | R | R | 1 | 1 | |||||
| 8 | R | R | R | R | R | 1 | 1 | ||||
| 9 | R | R | R | R | R | 3 | 1 | ||||
| 10 | R | R | R | R | R | 3 | 2 | ||||
| 11 | R | R | R | R | R | 1 | 1 | ||||
| 12 | R | R | R | R | R | R | 3 | 3 | |||
| 13 | R | R | R | R | R | R | 1 | 1 |
4. Discussion
UTIs are second only to infections of the respiratory tract as the most common bacterial infections and need for antimicrobial therapy. In most cases, the cause of community and hospital UTI was found to be associated with E. coli. Increasingly, resistance to antimicrobials among uropathogens, practically E. coli, has limited treatment choices. The worldwide spread of ESBL producing E. coli has been strikingly rapid, especially in communities, and is an important case of invasive infection (16, 17). Age over 60, previous antibiotic treatment, long hospitalization, and diabetes were found to be the factors associated with ESBL (18). Identification of betalactamase is essential for a reliable epidemiological investigation of antimicrobial resistance. In the present study, we surveyed antimicrobial drug resistance, the ESBL phenotype, and ctx gene detection in E. coli strains isolated from urinary tract infections in Semnan. Following the work on 2618 urine samples, we found that 109 of them were infected with E. coli. One hundred isolates were from outpatient and the remaining nine were from inpatients. In the present study, the ESBL producing E. coli were 26.6% of all E. coli strains isolated from urinary tract infections. Our results indicated that females had a higher rate of isolation of ESBL producing E. coli, which is consistent with the findings of other studies (19). In our study, the pattern of antimicrobial susceptibilities was determined in all isolates and results showed a 46.8% resistant to Trimetoprime-Solfometxazol and 30.3% were resistant to Cefotaxime and Ciprofloxcin. In accordance with other studies, a 100% sensitivity was seen with Imipenem, which has been found to be the most effective antibiotic (20). This is an important result of this study because many infections are caused by Gram positive and Gram negative that can be treat with carbapenemes. This result may be related to the low use of these antibiotics in this region. The presence of an ESBL phenotype is a good marker of the MDR pattern and resistance to newer beta lactam antibiotics. It is important to note that all of isolates showed an MDR pattern. The resistance rates to Cefotaxime and Ceftazidim were 100% and 48.27%, respectively. Additionally, 68.97% of ESBL producing E. coli and 18.35% of all isolates carried the ctx-M gene. Edelstein and colleagues reported that CTX-M beta lactamases have a more destructive effect on Cefotaxime and Cefteriaxon than Ceftazidim. This is in agreement with our findings (13). In this study another worrisome characteristic of ESBL strains was the resistance rate to non-beta lactam antibiotics.
5. Conclusions
The rate of ESBL-producing E. coli isolated from urinary tract infection was 26.6% with multiple drug resistance, resulting in limited treatment options. Therefore, investigating of the molecular and epidemiological traits of bacteria will help prevent and reduce the spread of resistance.
Acknowledgments
The authors would like to acknowledge to the Semnan University of Medical Sciences and Shafa hospital for supporting this research.
Footnotes
iThenticate screening: May 14, 2016, English editing: July 01, 2016, Quality control: July 04, 2016
Conflict of Interest:
There is no conflict of interest to be declared.
Authors' contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Thomas M, Hooton MD. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028–37. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 2.Dormingy JA, Nabeth P, Juergens-Behr A, Perrier-Gros-Claude JD. Risk factors for antibiotic resistance Escherchia coli isolated from community–aquired urinary tract infections in Dakar, Senegal. J Antimicrob Chemother. 2005;56(1):236–9. doi: 10.1093/jac/dki158. [DOI] [PubMed] [Google Scholar]
- 3.Giske CG, Sundsfjord AS, Kahlmeter G, Woodford N, Nordmann P, Paterson DI, et al. Redefining extended-spectrum beta lactamase: Balancing science and clinical need. J Antimicrob Chemother. 2009;63(1):1–4. doi: 10.1093/jac/dkn444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao SP, Rama PS, Gurushanthappa V, Manipura R, Srinivasan K. Extended–Spectrum Beta lactamase producing Escherchia coli and Klebsiellapneumoniae: A multi-centric study across Karnataka. J Lab Phyicians. 2014;6(1):7–13. doi: 10.4103/0974-2727.129083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert C, Gautier V, Arlet G. DNA sequence analysis of the genetic environment of various bla CTXM genes. J Antimicrob chemother. 2006;57(1):14–23. doi: 10.1093/jac/dki398. [DOI] [PubMed] [Google Scholar]
- 6.Gupta V. An update on newer bata-lactamase. Indian J Med Res. 2007;126(5):417–27. [PubMed] [Google Scholar]
- 7.Bonnet R. Growing group of extended–spectrum B-lactamase the CTXM enzymes. Antimicrob Agents Chemother. 2004;48(1):1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canton R, Gonzalez-Alba JM, Galan JC. CTX-M Enzymes origion and diffusion. Front Microbiol. 2012;3:10. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naseer U, Sundsfjord A. The CTX-M conundrum: dissemination of plasmids and Escherchia coli clones. Microb Drug resist. 2011:83–97. doi: 10.1089/mdr.20100132. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Bonome RA. Extended–Spectrum beta lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feizabadi MM, Delfani S, Raji N, Majnooni A, Aligoli M, Shahcheraghi F, et al. Distribution of beta TEM, bla SHV, bla CTXM genes among clinical isolates of klebsiellapneumoniae at labafinejad hospital, Tehran, Iran. Microb drug resist. 2010;16(1):49–53. doi: 10.1089/mdr.2009.0096. [DOI] [PubMed] [Google Scholar]
- 12.Ruppe E, Hem S, Lath S, Gautier V, Arieg F, sarthau JL, et al. CTX B-lactamase in Escherchia coli from community–acquired urinary tract infections, Cambodia. Emerg Infect Dis. 2009;15(5):741. doi: 10.3201/eid1505.071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchoun Sh. Prevalence and molecular epidemiology of CTXM extended–Spectrum B-lactamase-producing Escherchia coli and Klebsiellapneumoniae in Russian hospitals. Antimicrob Agents Chemother. 2003;47(12):3724–32. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzouvelekis LS, Tzelepi E, Tassios PT, Legakis NJ. CTX-M type B-lactamases: an emerging group of extended–Spectrum enzymes. Int J Antimicrob Agents. 2000;14(2):137–42. doi: 10.1016/S0924-8579(99)00165-X. [DOI] [PubMed] [Google Scholar]
- 15.Rossolini GM, Anderson MM, Mugnaioli C. The spread of CTX M type extended-spectrum–beta lactamases. Clin Microbiol Infect. 2008;14(1):33–41. doi: 10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Cerero L, Pascual A. Epidemiology of ESBL in the community an increasing problem. Enferm Infect MicrobiolClin. 2007;25(52):23–8. [Google Scholar]
- 17.Garcia-Hernandez AM, Garcia Nazquez E, Hernandez-Torroes A, et al. Bacteremia due to E. coli producing Extended- Spectrum beta-lactamase (ESBL): clinical relevance and today’s insights. Rev Esp Quimioter. 2011;24(2):57–66. [PubMed] [Google Scholar]
- 18.Silvia N, Oliveira M, Bandeiral AC, Brites C. Risk factors for infection by extended spectrum beta lactamase producing Klebsiellapneumoniae in a tertiary hospital in Salvador, Brazil. Braz J infects Dis. 2006;10(3):191–3. doi: 10.1590/S1413-86702006000300007. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes R, Amador P, Oliveira C, Prudencio C. Molecular characterization of ESBL, producing Enterobacteriaceae in Northern Portugal. Sci World J. 20142014:6. doi: 10.1155/2014/782897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multi drug resistant gram-negaative bacilli. Antimicrob Agents Chemother. 2008;52(3):813–21. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


