Abstract
Triantennary N-acetyl galactosamine (GalNAc3)-conjugated antisense oligonucleotides (ASOs) have greatly improved potency via receptor-mediated uptake. In the present study, the in vivo pharmacology of a 2′-O-(2-methoxyethyl)-modified ASO conjugated with GalNAc3 (ISIS 681257) together with its unmodified congener (ISIS 494372) targeting human apolipoprotein (a) (apo(a)), were studied in human LPA transgenic mice. Further, the disposition kinetics of ISIS 681257 was studied in CD-1 mice. ISIS 681257 demonstrated over 20-fold improvement in potency over ISIS 494372 as measured by liver apo(a) mRNA and plasma apo(a) protein levels. Following subcutaneous (SC) dosing, ISIS 681257 cleared rapidly from plasma and distributed to tissues. Intact ISIS 681257 was the major full-length oligonucleotide species in plasma. In tissues, however, GalNAc sugar moiety was rapidly metabolized and unconjugated ISIS 681257 accounted > 97% of the total exposure, which was then cleared slowly from tissues with a half-life of 7–8 days, similar to the half-life in plasma. ISIS 681257 is highly bound to plasma proteins (> 94% bound), which limited its urinary excretion. This study confirmed dose-dependent exposure to the parent drug ISIS 681257 in plasma and rapid conversion to unconjugated ASO in tissues. Safety data and the extended half-life support its further development and weekly dosing in phase 1 clinical studies.
Keywords: antisense oligonucleotide, disposition, mice, pharmacokinetics
Introduction
Antisense oligonucleotides (ASOs) are short, chemically modified, synthetic, single-strand DNA/RNA like oligonucleotides (typically of 14–22 nucleotides in length), that have the ability to target any gene product of interest by specifically hybridizing with the target complementary RNA via sequence-specific Watson-Crick base paring. After binding, mRNA may be modulated by several mechanisms including degradation by RNase H, or by redirecting post-transcriptional splicing processes,1,2 and ultimately, regulating target protein biosynthesis. As a platform of therapeutics, ASOs have been in drug development over two decades, and have been developed in numerous chemical classes. One of the most leading modifications is 2′-O-methoxyethyl (2′-MOE) modified ASOs, owing to its higher affinity target mRNA binding while maintaining RNase H activity, decreasing general nonhybridization toxicities, and enhanced pharmacokinetic profile by increasing nuclease resistance.3,4,5,6,7
A significant number of 2′-MOE ASOs have been tested clinically for the treatment of a wide variety of diseases including viral disease, cancer, cardiovascular, metabolic, and inflammatory diseases. Several recent clinical studies have documented that the antisense concept works in man. These studies include reductions in circulating apoB and low-density lipoprotein (LDL)-cholesterol (LDL-C) levels by mipomersen, reductions in clusterin levels in prostate tumor tissue by custirsen sodium, and reductions in apoC-III and triglyceride levels by ISIS 304801.8,9,10 Mipomersen is now approved by the US FDA and registered worldwide as Kynamro in multiple countries as an adjunct to lipid-lowering medications and diet to reduce LDL-C, apolipoprotein B (apo B), total cholesterol, and non–high-density lipoprotein-cholesterol in patients with homozygous familial hypercholesterolemia (HoFH).
The pharmacokinetics of 2'-MOE ASOs are well understood as a class due to their similar physiochemical properties, such as hydrophilicity, good water solubility, poly-anionic and comparable molecular weight in the range of 5,000–8,000 Da. The pharmacokinetics of 2'-MOE ASOs are remarkably similar across sequence and species, which makes their pharmacokinetics in humans highly predictable.11,12,13,14,15 Following SC administration, 2'-MOE ASOs can be rapidly absorbed into the systemic circulation, extensively distribute to tissues including liver and kidneys, and metabolized in tissues via the ubiquitous nucleases prior to renal excretion as short oligonucleotides. 2'-ASOs generally have an apparent plasma elimination half-life of 2–4 weeks. Currently, 2′-MOE ASO therapeutics have used simple formulation and administration of the oligonucleotide and relied on the bulk cellular uptake into certain tissues to target selected mRNA. As a result, the field has primarily focused on cells that inherently take up the 2′-MOE ASO, such as hepatocytes, over the last 15 years. Improving the efficiency of uptake into hepatocytes, or gaining the ability to target cells that do not naturally take up the 2′-MOE ASO would greatly improve their therapeutic utility. Recent studies have shown that conjugation with triantennary N-acetyl galactosamine (GalNAc3), a high-affinity ligand for the hepatocyte-specific asialoglycoprotein receptor (ASGPR) can substantially improve drug distribution to target cells, thus, it greatly enhances the targeted distribution of these molecules to hepatocytes and resulted in significant improvements in potency.16,17,18
ISIS 681257 is a GalNAc3-conjugated 2′-MOE-modified ASO, targeting to apolipoprotein (a) (apo(a)), a protein produced in hepatocytes. The hybridization of ISIS 681257 to apo(a) mRNA results in RNase H-mediated degradation of the cognate mRNA; thus, inhibiting the translation of the apo(a) protein, a distinct protein component of lipoprotein(a) (Lp(a)), which is a genetic variant of LDL. Elevated Lp(a) levels in humans are associated with increased risk of cardiac death, myocardial infarction, stroke, and peripheral arterial disease.19,20 The goal of treatment with ISIS 681257 is to reduce the production of apo(a) in the liver and as a consequence, the level of Lp(a) lipoprotein in blood, potentially slowing down or reversing cardiovascular disease by reducing thrombotic, atherogenic or inflammatory events in patients with elevated Lp(a) levels. The mechanism of action has been confirmed in LPA transgenic (Tg) mice, which express the entire human apo(a) genomic sequence21 and nonhuman primates.
There has been an abundance of information published regarding the pharmacokinetic properties of second-generation ASOs.11,12,14,22 GalNAc-conjugated ASOs just recently advanced to preclinical and clinical development. This is the first time that the in vivo toxicokinetics, disposition, and metabolism of a GalNAc-conjugated ASO are being characterized and reported. The pharmacokinetic results obtained in this study will provide guidance for the development of future GalNAc-conjugated oligonucleotides in the chemical class.
Results
Pharmacodynamics in transgenic mice
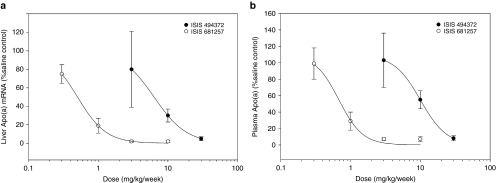
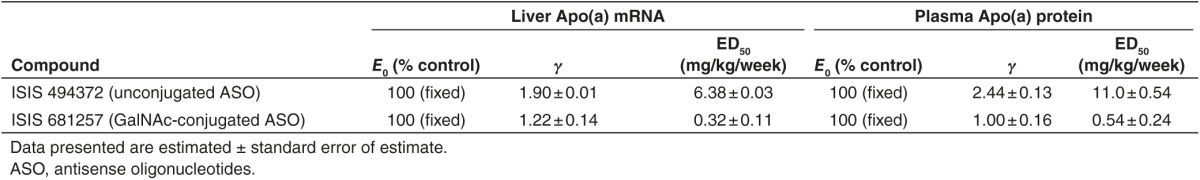
Mice dosed with ISIS 681257 (Figure 1) at dose levels of 0.3, 1, 3, and 10 mg/kg, or ISIS 494372 (unconjugated) at dose levels of 3, 10, and 30 mg/kg once weekly for 6 weeks had dose-dependent reductions in target mRNA expression (Figure 2). An inhibitory effect sigmoidal Emax model was fitted to the data and ED50 were estimated (Table 1). The model-estimated doses for unconjugated apo(a) ASO (ISIS 494372) that produced 50% of maximum drug-induced inhibitory effect (ED50) for liver apo(a) mRNA and plasma apo(a) protein levels were 6.38 and 11.0 mg/kg/week, respectively (Table 1). In contrast, the ED50 for GalNAc-conjugated apo(a) ASO (ISIS 681257) was 0.32 and 0.54 mg/kg/week for liver apo(a) mRNA and plasma apo(a) protein levels, respectively. Therefore, GalNAc-conjugation improved the potency of the apo(a) ASO over 20-fold as measured by direct target knock down in the apo(a) liver mRNA and plasma apo(a) protein levels (Figure 2).
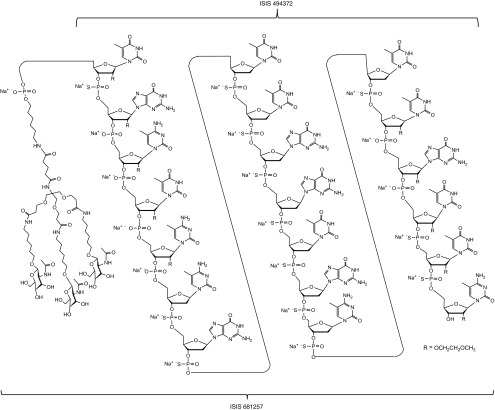
Figure 1.
Chemical structure of ISIS 681257 and ISIS 494372. Note, ISIS 494372 is uncongugated ISIS 681257, i.e., contains the 20 nucleotides only without GalNAc3-THA. ISIS 494372 has uniform phosphorothioate linkages.
Figure 2.
Comparison of dose–response curves of GalNAc-conjugated antisense oligonucleotides (ASO) (ISIS 681257) and uncongugated ASO (ISIS 494372) for liver Apo(a) mRNA (a) and plasma Apo(a) protein (b) (2 days after last treatment) following 6 weeks of subcutaneous administrations in human transgenic mice.
Table 1. Summary of pharmacodynamic parameters of unconjugated and GalNAc-conjugated ASO in human LPA transgenic mice.
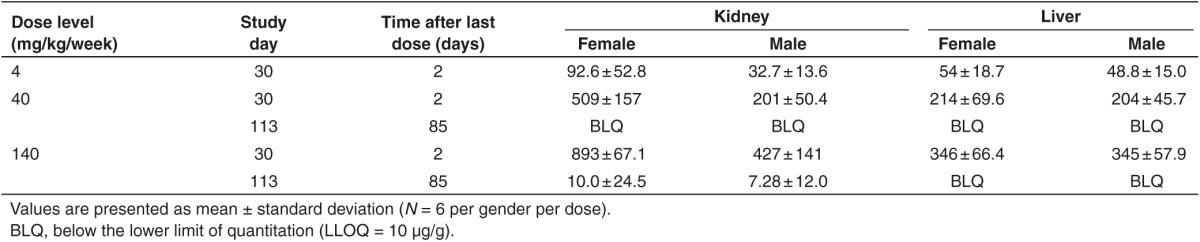
Toxicokinetics and metabolism in plasma
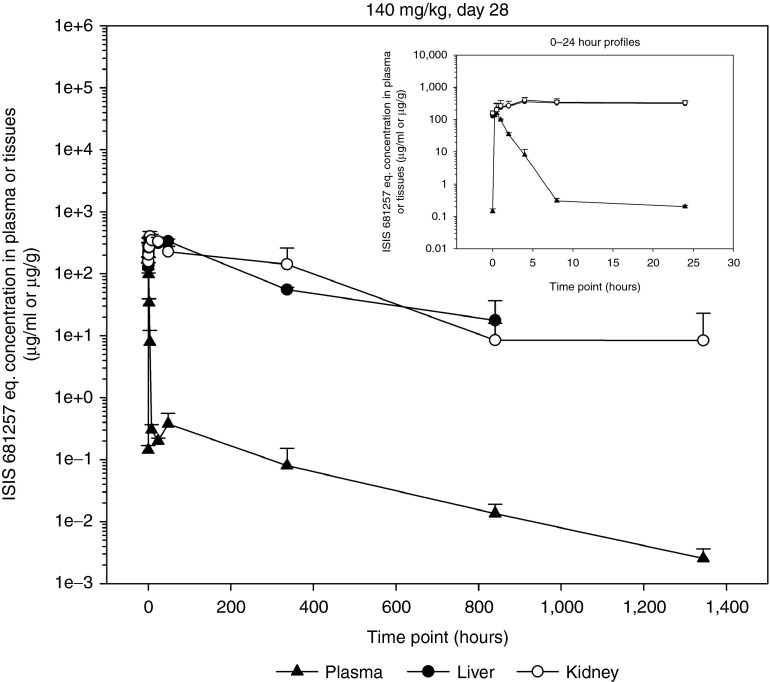
Following SC injection in mice, the time to plasma Cmax (Tmax) was observed at 0.25 to 1 hours postdose (Table 2). Following a single SC dose, mean peak (Cmax) and total area under the curve from time 0 to 48 hours (AUC0–48 hours) was dose-dependent, and increased greater than dose proportionally between 4 and 40 mg/kg while increasing near dose proportionally from 40 to 140 mg/kg. The nonlinearity in dose-exposure relationship at the lowest dose level studied (4 mg/kg) was also reflected in the threefold higher apparent plasma clearance compared to the higher toxicology dose levels evaluated. After attaining Cmax, plasma concentrations decreased in an apparent multi-exponential fashion with time (Figure 3). When compared to the single dose, mean Cmax and AUC0–48 hours appeared to be similar after repeated doses on day 28 (seven SC doses), suggesting little or no plasma accumulation and time invariant toxicokinetics (TK). Following SC administration, the mean mean residence time (MRT)0–48 hours values were in the range of 1.5 to 4 hours (Table 2), reflecting the initial rapid and almost complete distribution of total full-length ASOs from plasma to tissues within the first 24 hours postdose. The initial rapid distribution phase from plasma was followed by a much slower postdistribution elimination phase with an apparent terminal elimination half-life (t1/2λz) of approximately 7 to 8 days following 4 weeks of treatment, and appeared to be independent of dose (Table 2). The plasma concentrations in the postdistribution phase were several magnitudes less than Cmax, and were in parallel with elimination phase in tissues (Figure 3).
Table 2. Summary of plasma toxicokinetic parameters of total full-length ASO (ISIS 681257-equivalent) in mice following first and last subcutaneous administration.
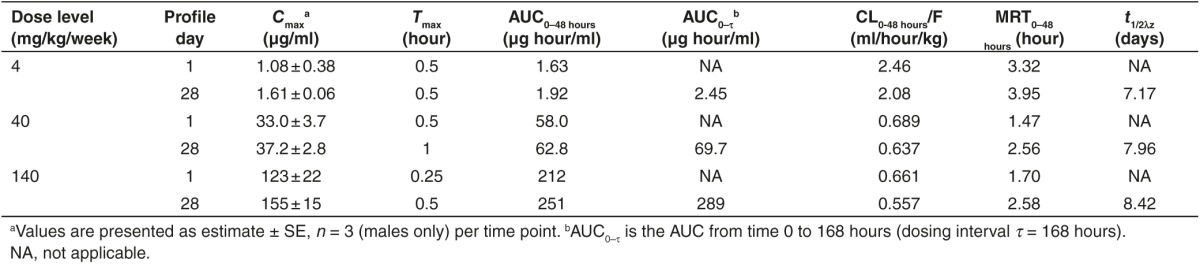
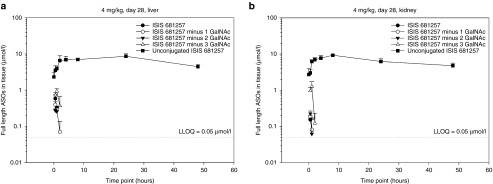
Figure 3.
Concentration–time profiles of ISIS 681257 in plasma, liver, and kidneys following the last dose of multiple subcutaneous administrations of ISIS 681257 at 140 mg/kg for 4 weeks in mice. 0-24 hour profiles. Error bars represent standard deviation (N = 3 per time point).
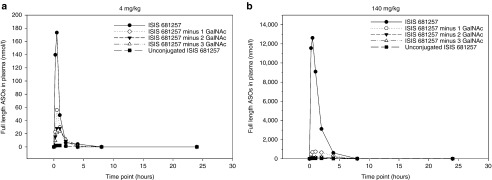
Intact ISIS 681257 (fully conjugated ISIS 681257) was the most abundant full-length oligonucleotide in plasma, which accounted for greater than 60 and 90% of total full-length oligonucleotide at Cmax following 4 and 140 mg/kg dose, respectively (Figure 4a,b). Total exposure of intact ISIS 681257 as measured by AUC accounted for 50 and 80% of total full-length ASOs detected following 4 and 140 mg/kg dose, respectively (Supplementary Table S1). Lower abundance of ISIS 681257 metabolites, with 1, 2, and/or 3 GalNAc sugar deletions or unconjugated ISIS 681257 were also detected and total exposure (AUClast) of each of its full-length oligonucleotide metabolites accounted for 17% or less of total full-length oligonucleotide (Supplementary Table S1). Although AUClast for unconjugated ISIS 681257 accounted for less than 5% of total full-length oligonucleotide, it was the major oligonucleotide species at later time points (24 hours and later), reflecting a postdistribution equilibrium between plasma and tissue. Following repeated treatment on day 28, there were no shortmer oligonucleotides associated with nuclease-mediated metabolism detected in plasma, examined in the 2 hours (near peak) and predose (trough) samples (data not shown).
Figure 4.
Plasma concentration–time profiles of fully conjugated, partially conjugated (with 1, 2, or 3 sugar deletions), and unconjugated ISIS 681257 following repeated subcutaneous injections of 4 mg/kg (a) and 140 mg/kg (b) ISIS 681257 in mice.
Toxicokinetics and metabolism in tissues
Rapid plasma clearance was attributed to a rapid and extensive uptake by liver and kidney, with peak tissue levels observed at 2 to 48 hours after a single dose, and 4 to 24 hours after multiple doses (Table 3, Figure 3). Following a single SC administration, up to 70% of administered dose distributed to liver and 10% to the kidney (assuming liver and kidney weight of 1.75 and 0.32 g, respectively, for a 20 g mouse23 at the 4 mg/kg dose, which reduced to 10 and 4% in liver and kidney, respectively, as dose increased to 140 mg/kg, reflecting organ saturation at higher dose levels. Following once weekly repeated administrations, a nearly twofold accumulation was observed at 4 mg/kg in kidney, and at all the dose levels tested in liver. However, no additional accumulation was observed in kidney at 40 and 140 mg/kg dose upon repeated administrations (Table 3). Following 4 weeks of repeated treatment, oligonucleotide concentrations in liver and kidney were dose-dependent but less than proportional to dose, reflecting saturation of tissue uptake at higher dose levels. Less than 2% of oligonucleotide remained in tissues after a 12-week recovery period, consistent with tissue half-life of 6–9 days (Tables 3 and 4). Although kidney concentrations in female mice were consistently higher (two- to threefold) compared to males, no gender difference was observed in mouse liver concentrations (Table 4).
Table 3. Summary of tissue toxicokinetic parameters of unconjugated ISIS 681257 in mice following first and last subcutaneous administration of ISIS 681257.
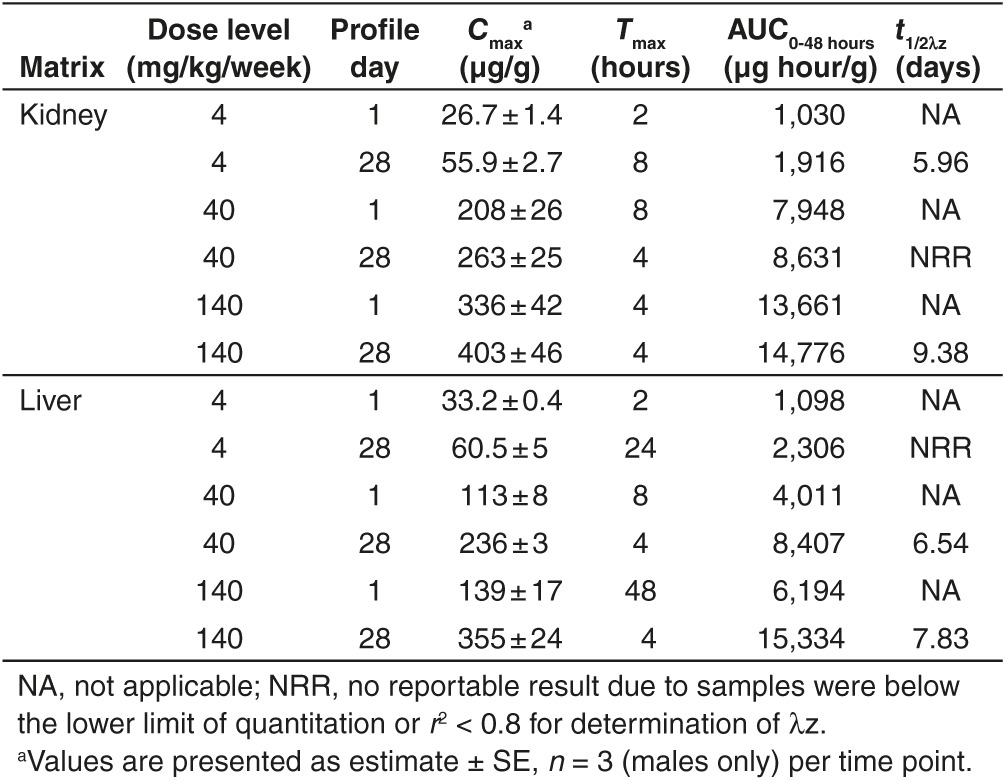
Table 4. Tissue concentrations (µg/g) of unconjugated ISIS 681257 following repeated subcutaneous administrations of ISIS 681257 in mice for 4 weeks.
In contrast to plasma, the major oligonucleotide species in tissues is unconjugated ISIS 681257, which accounted for > 97% of total full-length oligonucleotide (Figure 5 and Supplementary Table S2). Low levels of fully conjugated ISIS 681257 and its full-length oligonucleotide metabolites with 1-, 2-, and/or 3-GalNAc sugar deletions were typically observed within the first 4 hours following dose administration, and total exposure (AUClast) of either ISIS 681257 or each of its full-length oligonucleotide metabolites with 1, 2, and/or 3 GalNAc sugar deletions accounted for < 1.2% of total full-length oligonucleotides detected (Figure 5 and Supplementary Table S2). These data suggest that the 5ʹ-GalNAc3-trishexylamino-C6 conjugate on ISIS 681257 is rapidly metabolized once distributed to tissues, releasing the unconjugated ASO to produce its pharmacologic effect.
Figure 5.
Concentration–time profiles of fully conjugated, partially conjugated (with 1, 2, or 3 sugar deletions), and unconjugated ISIS 681257 in liver (a) and kidney (b) following multiple (day 28) subcutaneous injections of 4 mg/kg fully conjugated ISIS 681257 in mice. Error bars represent standard deviation (n = 3).
Lastly, full-length oligonucleotides (primarily unconjugated ISIS 681257) were detected at 48 hours after repeated administrations and accounted for > 80% total detected oligonucleotide. Low abundance of shortmer oligonucleotides (each less than 5%) associated with nuclease-mediated metabolism was detected in tissues following repeated administrations over 4 weeks (Figure 6).
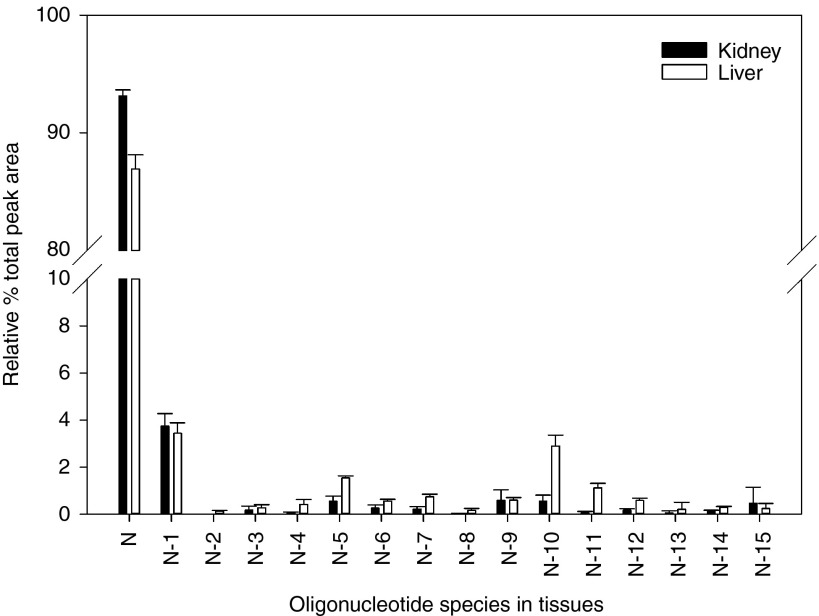
Figure 6.
Mean relative percent of unconjugated ISIS 681257 (full-length) and shortmer metabolites in liver and kidney cortex 48 hours following 4 weeks of treatment (day 30) of 140 mg/kg/week ISIS 681257 in mice. N = Unconjugated ISIS 681257. Error bars represent standard deviation (n = 9).
Plasma protein binding
The extent of in vitro protein binding of ISIS 681257 in whole mouse plasma was 96.3 ± 0.1 and 94.1 ± 0.4% when evaluated at 5 and 150 µg/ml ISIS 681257, respectively.
Discussion
ISIS 681257 is a 2′-MOE ASO conjugated via a trishexylamino-C6 (THA) cluster with three galactosamine sugars (GalNAc3), a high-affinity ligand to the hepatocyte-specific ASGPR. The GalNAc3-THA cluster has a molecular weight of approximately 1,520 Da, which is approximately 21.4% of the size compared to unconjugated ASO (7117 Da). Oligonucleotides detected in plasma were mostly intact conjugated form (GalNAc3-THA-ASO), which facilitated the asialoglycoprotein receptor-mediated uptake into hepatocytes. Once internalized, the GalNAc sugars and the THA cluster were rapidly cleaved from GalNAc-conjugated ASOs within minutes to a few hours. Thus, the unconjugated ASOs were almost exclusively full-length oligonucleotide within the liver by 48 hours after dosing. Furthermore, it is believed that the unconjugated ASOs exhibit the pharmacological effects. Therefore, it was hypothesized that ISIS 681257 would act similar to a prodrug to facilitate uptake into hepatocytes, and rapidly be converted to an active drug (unconjugated ASO) once internalized. The unconjugated ASO, i.e., the 2′-MOE modified antisense oligonucleotide, would then be free to interact with the target mRNA and subsequently metabolized slowly via nuclease-mediated metabolic pathway with elimination half-life in weeks.12,14,24
Following a single SC administration, ISIS 681257 was rapidly cleared from plasma with MRT less than 4 hours. This rapid plasma clearance was attributed to rapid and efficient uptake into liver and kidney tissues, primarily liver hepatocyte as reported previously.18 ISIS 681257 is highly bound to plasma proteins, which restricts glomerular filtration thus limiting urinary excretion. Plasma clearance was dose-dependent, and was more rapid at the low, clinically relevant doses, suggesting a more rapid and greater distribution/uptake to the liver, also supported by the higher percentage of the administered dose observed in liver at lower dose levels. As determined, approximately 70% of administered dose distributed to liver at 4 mg/kg compared to 10% at 140 mg/kg. These results were consistent with the high affinity, but saturable, ASGPR-mediated liver uptake.16,17,18 Nonetheless, two- to fourfold accumulation was observed over the entire dose range evaluated following repeated dosing, i.e., 4 to 140 mg/kg, likely attributed to uptake by nonparenchymal cells, which could serve as a lower affinity but higher capacity (referred to as nonproductive) site for unconjugated 2′-MOE distribution.24 These phenomena were not observed in kidney.
No significant metabolism of the conjugate or the ASO in plasma was observed due to the rapid distribution to tissue (primarily liver), favoring the intended ASGPR-mediated hepatic uptake. Once in tissues, GalNAc sugars were rapidly cleaved from the molecule, releasing unconjugated ISIS 681257, which binds to target mRNA to exhibit its pharmacological effect. While fully conjugated ASOs represented the primary circulating component in plasma at the first 4 hours following SC dosing, the unconjugated ISIS 681257 appeared to be the major circulating species at later times, albeit several orders of magnitude lower than the peak concentrations of parent ISIS 681257 in plasma. The parallel elimination phase and similar terminal elimination half-lives of unconjugated ASOs in plasma and tissues (liver and kidney) suggests that there was equilibrium between plasma and tissues for unconjugated ASOs. These results suggest that plasma elimination can be used as a surrogate for tissue elimination of the active unconjugated ASO species, and postdistribution plasma concentrations can be used to estimate target tissue liver concentrations.
As with other 2′-MOE ASOs, unconjugated ISIS 681257 was slowly metabolized via nuclease-mediated metabolism to shortened metabolites. Nuclease metabolism was the rate limiting step, and no shortmer metabolites accumulated within tissues or plasma. Due to being less bound to plasma and tissue proteins, the shortened endonucleolytic products were rapidly eliminated in urine once generated.6,12
In summary, ISIS 681257, a GalNAc-conjugated ASO, demonstrated unique and favorable pharmacokinetic and disposition profiles similar to an ideal prodrug following SC administration in mice. The pharmacokinetics of ISIS 681257 in mice are characterized by rapid distribution of the fully conjugated ASO into tissues, resulting in the highest concentrations in liver, the target organ for pharmacology, and the kidney. Once distributed to tissues, the GalNAc sugars and THA cluster on ISIS 681257 were rapidly cleaved within minutes to hours, leaving the fully unconjugated form as the only full-length oligonucleotide detected in tissues within 48 hours after dosing, and accounted for greater than 97% of total exposure in liver and kidney. Unconjugated ISIS 681257 was cleared slowly from tissues with a half-life of 6–9 days, consistent with the long elimination half-life in plasma. These favorable pharmacokinetic properties for ISIS 681257 support further development in humans and can serve as model compound for development of other drug candidates of this chemical class.
Materials and Methods
Materials. ISIS 681257 is a synthetic antisense oligomer of 20 nucleotides (i.e., a 20-mer) (ASO) that are connected sequentially by phosphorothioate and phosphodiester linkages (mixed backbone design), with a total of ten 2′-O-methoxyethyl modified ribofuranosyl nucleotides, five on each end of the oligonucleotide, and conjugated covalently with a triantennary cluster of N-acetyl galactosamine (GalNAc3) sugars via trishexylamino-C6 (THA) linker at the 5ʹ-end via a phosphodiester linkage (GalNAc3-THA-ASO) (Figure 1). The comprehensive structure activity relationship of the N-Acetylgalactosamine conjugated ASO has been described previously (Prakash TP et al., manuscript submitted). ISIS 494372 is unconjugated ISIS 681257, i.e., contains the 20 nucleotides only without GalNAc3-THA. In addition, ISIS 494372 has uniform phosphorothioate linkages.
The purity of ISIS 681257 determined by LC-MS (MW 8635.9 ± 1.3 amu) was 88.0% (major impurities that greater than 1%: sodium counter ion 5%, full-length mono PO 2.0%, total N-1 1.5%), with the full-length test compound (LC/UV) purity of 97.9%. The purity of ISIS 494372 based on LC-MS (MW 7212.0 ± 1.2 amu) was 92.1%, with the full-length test compound (LC/UV) purity of 98%. Dosing was performed based on the quantity of full-length 20-mer oligonucleotide.
Animal experiments
Pharmacology study in human LPA transgenic mice. Human LPA transgenic mice which express the entire human apo(a) genomic sequence21 were obtained as a gift from Dr. Knut Eliassen (The Norwegian School of Veterinary Science, Oslo, Norway). All animal procedures were conducted utilizing protocols and methods approved by the Institutional Animal Care and Use Committee and were in compliance with Animal Welfare Act and Guide for the Care and Use of Laboratory Animals (by ILAR publication). Animals were maintained on a normal chow diet were given access to food and water ad libitum during the study period. Female human LPA transgenic mice received either SC injection of saline, ISIS 681257 at dose levels of 0.3, 1, 3, and 10 mg/kg, or ISIS 494372 at dose levels of 3, 10, and 30 mg/kg once weekly for 6 weeks. At 48 hours after the last dose, mice were sacrificed for human hepatic apo(a) mRNA and human apo(a) plasma protein quantification. Apo(a) primers used in qRT-PCR assay for the analysis of hepatic apo(a) mRNA were 5′-GCCGTGGCTGCCTGAG-3′ and 5′-AGGAGCTCGCAGGATGGAT-3′; and fluorescent probe used was 5′-CCTCAATACCCCAAGTCCACCTGCC-3′.
Plasma apo(a) protein levels were quantified using a Randox Lp(a) turbiometric assay (Kearneysville, West Virginia, LP2757) derived by Olympus Clinical Analyzer.
Disposition study in CD-1 mice. Male and female CD-1 mice (CrljOri:CD1 (ICR)) were obtained from Orient Bio, Republic of Korea (Seongnam, South Korea). All animal procedures were conducted utilizing protocols and methods approved by the Institutional Animal Care and Use Committee and were in compliance with Animal Welfare Act and Guide for the Care and Use of Laboratory Animals (by ILAR publication).
Male and female CD-1 mice received either a SC injection of saline or ISIS 681257 at dose levels of 4, 40, and 140 mg/kg/dose on days 1, 3, 5, and 7 (loading doses) followed by once weekly doses of 4, 40, and 140 mg/kg/week thereafter through day 28. Drug concentrations were measured in plasma for toxicokinetic (TK) animals (three males per time point per dose). Blood samples were collected for ISIS 681257 (total full-length ASOs) quantitation in plasma by cardiac puncture at sacrifice in tubes containing EDTA at 15, 30, and 60 minutes, 2, 4, 8, 24, and 48 hours following the first dose and last dose (day 28) (three males per time point). Additionally, plasma samples were also collected at 14, 35, 56, and 85 days after the last dose (day 28) (three males per time point) to determine elimination kinetics. Drug concentrations were measured in liver and kidney samples collected at approximately 48 hours after the last dose (day 30) for all toxicology dose groups (six males and six females per dose), and TK animals (three males per time point) at time points that coincided with plasma sample collection as listed above. Lastly, concentrations were also measured in liver and kidney tissues of mice (six males and six females per dose) receiving the 40 and 140 mg/kg/dose sacrificed 12 weeks after completion of the 4-week treatment regimen (on day 113) to assess clearance during a 3-month recovery phase.
Analytical methods
Hybridization enzyme-linked immunosorbant assay (ELISA) for quantitation of full-length ASOs in plasma. All plasma samples collected were analyzed at Korea Institute of Toxicology (KIT) using a quantitative, sensitive hybridization ELISA method with a minor variation of the previously reported method.25 Plasma sample analyses were performed based on the principles and requirements described in 21 CFR Part 58. The assay was validated for precision, accuracy, selectivity, sensitivity, and stability of ISIS 681257 prior to analysis of mouse plasma samples. In addition, the assay conducted with synthesized putative shortened oligonucleotide metabolite standards showed no measurable cross-reactivity, confirming the assay's specificity for the parent full-length oligonucleotide. The quantitation range was 1.00 to 100 ng/ml, with the low and high ends of the range defining the lower (LLOQ) and upper (ULOQ) limit of quantitation, respectively. This assay quantitated full-length ASOs (including fully conjugated, partially conjugated with 1, 2, or 3-sugar deletions, and unconjugated ISIS 681257) using ISIS 681257 standard curves. Therefore, total full-length ASOs were reported as ISIS 681257-equivalent mass concentrations (µg eq./ml) in plasma.
Ion-pairing UPLC-UV for quantitation of unconjugated ISIS 681257 in tissues and profiling of shortmer oligonucleotide metabolites in plasma and tissues. Tissue samples (50 mg) were weighed, minced, homogenized, and extracted first using a liquid-liquid extraction (LLE) with phenol:chloroform:isoamyl alcohol (25:24:1), followed by a solid phase extraction (SPE) using a 96-well Strata X packed plate (Phenomenex, Torrance, CA). Samples were then analyzed by ion-pairing (IP) UPLC with ultraviolet (UV) detection (IP-UPLC-UV). A 27-mer oligonucleotide was added prior to extraction as internal standard. Chromatographic separation was performed with a gradient system at a flow rate of 1.0 to 0.8 ml/minute on a Waters Acquity UPLC system, using an ACQUITY UPLC OST C18 column heated to 60 °C, with 10 mM/l tributylammonium acetate (TBAA) in 32.5% Acetonitrile and 10 mM/l TBAA in 90.7% Acetonitrile as the mobile phase with a 10-µl injection volume. Oligonucleotide peaks were detected by UV absorbance at 260 nm. Tissue sample analyses were conducted at CMIC (Hoffman Estates, IL) and were performed based on the principles and requirements described in 21 CFR part 58. The quantitation range was 10.00 µg/g to 1,500 µg/g (1.405 to 210.8 µmol/l), with the low and high ends of the range defining the LLOQ and ULOQ, respectively. Samples were stored at −80 °C prior to sample extraction and analysis.
A subset of extracted plasma and tissue samples from the high dose group (140 mg/kg) were subjected to qualitative analysis for metabolite profiling to determine the relative proportions (percent of total peak area) of full-length oligonucleotides (primarily conjugated ISIS 681257 in plasma and unconjugated ISIS 681257 in tissues) and shortmer metabolites using IP-UPLC with UV detection using the method described above. The chromatograms were monitored for full-length oligonucleotides (N) and metabolites with one to fifteen nucleotide deletions (N-1 to N-15). Each metabolite observed was provisionally identified based on the comparison of their relative retention times with the relative retention times of the putative N-1, N-5, and N-15 metabolite reference materials.
Ion-pairing HPLC-MS/MS method for quantitation of fully conjugated, partially conjugated, and unconjugated ISIS 681257 in plasma and tissues. Plasma (pooled) and tissue samples from the 4 and 140 mg/kg dose groups were further analyzed for quantitation of each full-length oligonucleotide species (i.e., fully conjugated, partially conjugated with 1, 2, or 3-sugar deletions, and unconjugated ISIS 681257) using an ion-pairing IP-HPLC-MS/MS method. Plasma (50 µl) or tissue (50 mg) samples were extracted in the same manner as the IP-UPLC-UV samples listed above.
Separation of the extracted plasma samples was accomplished on a Waters Acquity UPLC system, using an ACQUITY UPLC OST C18 column heated to 60 °C (Waters, Milford, MA). The column was maintained at 60 °C and flow rate on the column was 0.3 to 0.5 ml/minute. The column was equilibrated with 400 mM/l hexafluoroisopropanol (HFIP)/15 mM/l triethylammonium (TEA) in water. A gradient from 20 to 40% methanol over 9 minutes was used to elute ISIS 681257 and its various metabolites with one or more sugar deletions. Mass measurements were made on-line using an API5500 mass spectrometer. Peaks on the MRM chromatogram were identified based on their eluting position. Concentrations were determined by the internal standard (IS) method based on the peak area ratios of analyte to IS. MRM transitions of m/z 1079, 1016, 936, 913, 891, and 1,050, all with a product ion of 94.8 for ISIS 681257, ISIS 693407, ISIS 681257-1GAlNAc, ISIS 681257-2GAlNAc, ISIS 681257-3GAlNAc, and IS were respectively monitored. Mass spectra were obtained in the negative mode with an ion spray voltage of −4.5 kV, a nebulizer and turbo gas flow of 50 psi, a curtain gas flow of 12 psi, and heater gas temperature of 650 °C. The LLOQ for plasma was 8.63 ng/ml (1.00 nmol/l).
Separation of the extracted tissue samples was accomplished on an Agilent triple quadrupole mass spectrometry system consisting of a 1290 binary, a column oven, an auto sampler, and a 6460 triple quadrupole mass spectrometer (Agilent, Wilmington, DE). The tissue extracts were injected onto a Kinetex analytical column (Phenomonex, 100 × 2.1 mm, 2.5 µm particle size; 200 Å). The column was maintained at 55 °C and flow rate on the column was 0.3 ml/minute. The column was equilibrated with 400 mM/l HFIP/15 mM/l TEA in water. A gradient from 10 to 80% methanol over 7 minutes was used to elute ISIS 681257 and its various metabolites with one or more sugar deletions. Mass measurements were made on-line using an Agilent 6460 mass spectrometer. Peaks on the MRM chromatogram were identified based on their eluting position. Concentrations were determined by the internal standard method based on the peak area ratios of analyte to IS. MRM transitions are the same as listed above. Mass spectra were obtained using a spray voltage of −1,500 V, a nebulizer gas flow of 25 psig, a sheath gas flow rate of 12 l/minute at 350 °C, a drying gas flow rate of 5 l/minute at 350 °C, and a capillary voltage of −3,750 V. Chromatograms for tissue samples were analyzed using Agilent Mass Hunter software in the same manner as the plasma samples. The LLOQ for tissues was 0.432 µg/g (0.05 µmol/l).
Protein binding assay. An ultrafiltration method26 was used to assess whole plasma protein binding characteristics of ISIS 681257. Briefly, Millipore (Bedford, MA) Ultrafree-MC filters with low-binding regenerated cellulose membrane (MW cutoff of 30 K daltons) were used. Whole mouse plasma protein binding was evaluated at two concentrations (5–150 µg/ml) to bracket the Cmax expected over the dose range evaluated in the study. Aliquots (50 µl per aliquot) of ultrafiltrates (containing unbound ISIS 681257) and initial plasma samples (containing total (bound and unbound) ISIS 681257) were assayed using nuclease-dependent hybridization ELISA to obtain the drug concentration in each sample. Three replicates at each concentration were run to determine % unbound means and standard deviations, using the following equation:
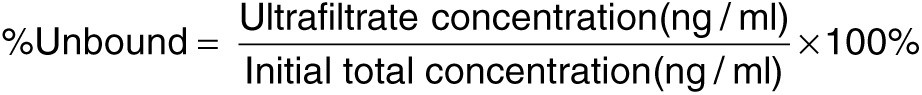 |
Pharmacodynamic analysis. The relationship between liver apo(a) mRNA levels and plasma apo(a) protein levels with dose of unconjugated (ISIS 494372) and GalNAc-conjugated (ISIS 681257) ASOs in human LPA transgenic mice following 6 weeks of treatments (2 days after the last treatment) was analyzed with an inhibitory effect sigmoidal Emax model using Phoenix Winnonlin version 6.3 (Certara, St. Louis, MO) with uniform weighting by the following equation:
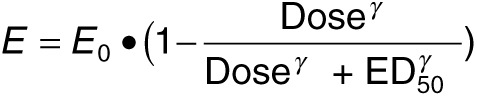 |
Where E is the measured response (% saline control), E0 is the baseline response of saline control animals (fixed to 100%), ED50 is the dose that produced 50% of maximum drug-induced effect, and γ is the sigmoidal factor.
Pharmacokinetic analysis. Noncompartmental analysis methods (spare sampling module) were used to analyze the plasma and tissue concentration data (Phoenix WinNonlin 6.3, Pharsight Corporation, Mountainview, CA). Plasma PK parameters for total full-length ASOs (ISIS 681257-equivalent) using the data obtained from the hybridization ELISA method included peak plasma concentration (Cmax), time to Cmax (Tmax), AUC0–48 hours and AUC0–τ (calculated using the linear trapezoidal method), clearance (CL/F), MRT, and terminal elimination half-life (t1/2λz). Tissue PK parameters for unconjugated ISIS 681257 obtained from the high performance liquid chromatography with ultraviolet detection (HPLC-UV) assay included Cmax, Tmax, AUC0–48 hours, and terminal elimination half-life (t1/2λz).
Relative abundance of each full-length ASO analyte in plasma and tissues from the HPLC-MS/MS assay was calculated by AUClast for each analyte divided by AUClast of total full-length ASO multiplied by 100. Relative abundance of full-length versus shortmer oligonucleotide associated with nuclease-mediated metabolism in plasma and tissues from the HPLC-UV assay was summarized using descriptive statistics.
SUPPLEMENTARY MATERIAL Table S1. Exposure and relative abundance of each full-length ASO species in plasma following first and last subcutaneous administration in mice. Table S2. Summary of relative abundance of each full-length ASO species in tissues following first and last subcutaneous administration in mice.
Acknowledgments
The authors wish to thank Dan Norris and Ken Luu for their scientific discussion and critical review of the manuscript. Finally, this manuscript would not be possible without the administrative support provided by Robert Saunders, for which we are grateful.
Supplementary Material
References
- Bennett, CF and Swayze, EE (2010). RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 50: 259–293. [DOI] [PubMed] [Google Scholar]
- Crooke, ST (2004). Antisense strategies. Curr Mol Med 4: 465–487. [DOI] [PubMed] [Google Scholar]
- Manoharan, M (1999). 2'-carbohydrate modifications in antisense oligonucleotide therapy: importance of conformation, configuration and conjugation. Biochim Biophys Acta 1489: 117–130. [DOI] [PubMed] [Google Scholar]
- McKay, RA, Miraglia, LJ, Cummins, LL, Owens, SR, Sasmor, H and Dean, NM (1999). Characterization of a potent and specific class of antisense oligonucleotide inhibitor of human protein kinase C-alpha expression. J Biol Chem 274: 1715–1722. [DOI] [PubMed] [Google Scholar]
- Henry, SP and Danis, RP (2001). Potential therapeutic application of antisense oligonucleotides in the treatment of ocular diseases. Expert Opinion on Pharmacotherapy 2: 277–291. [DOI] [PubMed] [Google Scholar]
- Yu, RZ, Geary, RS, Kim, TW and Levin, AA (2007). Mouse and monkey toxicokinetics of a second generation antisense oligonucleotide (ASO) targeting human ApoB-100, following chronic treatment for up to 1 year. Annul Meeting of American Association of Pharmaceutical Scientists, vol. Abstract #: T2492: San Diego, CA.
- Geary, RS (2009). Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol 5: 381–391. [DOI] [PubMed] [Google Scholar]
- Chi, KN, Eisenhauer, E, Fazli, L, Jones, EC, Goldenberg, SL, Powers, J et al. (2005). A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2'-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst 97: 1287–1296. [DOI] [PubMed] [Google Scholar]
- Graham, MJ, Lee, RG, Bell, TA 3rd, Fu, W, Mullick, AE, Alexander, VJ et al. (2013). Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 112: 1479–1490. [DOI] [PubMed] [Google Scholar]
- Kastelein, JJ, Wedel, MK, Baker, BF, Su, J, Bradley, JD, Yu, RZ et al. (2006). Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 114: 1729–1735. [DOI] [PubMed] [Google Scholar]
- Geary, RS, Khatsenko, O, Bunker, K, Crooke, R, Moore, M, Burckin, T et al. (2001). Absolute bioavailability of 2'-O-(2-methoxyethyl)-modified antisense oligonucleotides following intraduodenal instillation in rats. J Pharmacol Exp Ther 296: 898–904. [PubMed] [Google Scholar]
- Geary, RS, Yu, RZ, Watanabe, T, Henry, SP, Hardee, GE, Chappell, A et al. (2003). Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2'-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos 31: 1419–1428. [DOI] [PubMed] [Google Scholar]
- Geary, RS, Norris, D, Yu, R and Bennett, CF (2015). Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87: 46–51. [DOI] [PubMed] [Google Scholar]
- Yu, RZ, Kim, TW, Hong, A, Watanabe, TA, Gaus, HJ and Geary, RS (2007). Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab Dispos 35: 460–468. [DOI] [PubMed] [Google Scholar]
- Yu, RZ, Grundy, JS and Geary, RS (2013). Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert Opin Drug Metab Toxicol 9: 169–182. [DOI] [PubMed] [Google Scholar]
- Akinc, A, Querbes, W, De, S, Qin, J, Frank-Kamenetsky, M, Jayaprakash, KN et al. (2010). Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 18: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasty, R, Dorkin, JR, Vegas, A and Anderson, D (2013). Delivery materials for siRNA therapeutics. Nat Mater 12: 967–977. [DOI] [PubMed] [Google Scholar]
- Prakash, TP, Graham, MJ, Yu, J, Carty, R, Low, A, Chappell, A et al. (2014). Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res 42: 8796–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erqou, S, Kaptoge, S, Perry, PL, Di Angelantonio, E, Thompson, A, White, IR, et al. (2009). Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 302: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erqou, S, Thompson, A, Di Angelantonio, E, Saleheen, D, Kaptoge, S, Marcovina, S et al. (2010). Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol 55: 2160–2167. [DOI] [PubMed] [Google Scholar]
- Frazer, KA, Narla, G, Zhang, JL and Rubin, EM (1995). The apolipoprotein(a) gene is regulated by sex hormones and acute-phase inducers in YAC transgenic mice. Nat Genet 9: 424–431. [DOI] [PubMed] [Google Scholar]
- Sewell, KL, Geary, RS, Baker, BF, Glover, JM, Mant, TG, Yu, RZ et al. (2002). Phase I trial of ISIS 104838, a 2'-methoxyethyl modified antisense oligonucleotide targeting tumor necrosis factor-alpha. J Pharmacol Exp Ther 303: 1334–1343. [DOI] [PubMed] [Google Scholar]
- Davies, B and Morris, T (1993). Physiological parameters in laboratory animals and humans. Pharm Res 10: 1093–1095. [DOI] [PubMed] [Google Scholar]
- Geary, RS, Wancewicz, E, Matson, J, Pearce, M, Siwkowski, A, Swayze, E et al. (2009). Effect of dose and plasma concentration on liver uptake and pharmacologic activity of a 2'-methoxyethyl modified chimeric antisense oligonucleotide targeting PTEN. Biochem Pharmacol 78: 284–291. [DOI] [PubMed] [Google Scholar]
- Yu, RZ, Baker, B, Chappell, A, Geary, RS, Cheung, E and Levin, AA (2002). Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal Biochem 304: 19–25. [DOI] [PubMed] [Google Scholar]
- Watanabe, TA, Geary, RS and Levin, AA (2006). Plasma protein binding of an antisense oligonucleotide targeting human ICAM-1 (ISIS 2302). Oligonucleotides 16: 169–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.