Abstract
Airway epithelium defends the invasion from microorganisms and regulates immune responses in allergic asthma. Thymic stromal lymphopoietin (TSLP) from inflamed epithelium promotes maturation of dendritic cells (DCs) to prime Th2 responses via CCL17, which induces chemotaxis of CD4+ T cells to mediate inflammation. However, few studies have investigated the regulation of epithelial CCL17. In this study, we used shRNA against TSLP to clarify the role of TSLP in the airway inflammation and whether TSLP affects the airway inflammation via epithelial CCL17. Specific shTSLP was delivered by lentivirus and selected by the knockdown efficiency. Allergic mice were intratracheally pretreated with the lentivirus and followed by intranasal ovalbumin (OVA) challenges. The sera antibody levels, airway inflammation, airway hyper-responsiveness (AHR), cytokine levels in bronchoalveolar lavage fluids, and CCL17 expressions in lungs were determined. In vivo, TSLP attenuation reduced the AHR, decreased the airway inflammation, inhibited the maturations of DCs, and suppressed the migration of T cells. Furthermore, the expression of CCL17 was particularly decreased in bronchial epithelium. In vitro, CCL17 induction was regulated by TSLP. In conclusion, TSLP might coordinate airway inflammation partially via CCL17-mediated responses and this study provides the vital utility of TSLP to develop the therapeutic approach in allergic airway inflammation.
Keywords: airway inflammation, asthma, CCL17, shRNA, thymic stromal lymphopoietin
Introduction
Airway epithelium protects the airway from exposure to allergens and participates in the initiation and progression of allergic inflammation.1 The activation of airway epithelium induces secretion of chemokines and cytokines that attract monocytes and immature dendritic cells (DCs) to the inflamed location and prime immune responses.2 Therefore, a major therapeutic approach treating allergic asthma is to develop an effective attenuation on epithelium-mediated inflammatory responses.
Thymic stromal lymphopoietin (TSLP) is critical for the development of allergic airway inflammation,3,4 including the generation of Th2 differentiation directly or through DCs,5,6 the enhancement of basophil and eosinophil hematopoiesis,7,8 and the inhibition of antigen-specific regulatory T cells.9 The functional receptor is composed of the TSLPR subunit and the IL-7 receptor α chain.10 In the airway of asthmatic patients, the TSLP mRNA and protein in the epithelium are expressed and are highly correlated with disease severity.11,12 In the animal model, overexpression of TSLP in the lungs developed into an asthma-like disease.13 Although blockage of the TSLP pathway with specific antibodies could reduce the severity of allergic airway inflammation,14,15 the detailed effects of TSLP are still unclear.
CCL17 plays a dominant role in Th2-related diseases, such as atopic dermatitis and asthma.16,17 The level of CCL17 is significantly increased in bronchoalveolar lavage (BAL) fluids18,19 and in the airway of asthmatic patients.20 It was found that CCL17 expressed in the bronchial epithelium during ovalbumin (OVA) challenges and the treatment with anti-CCL17 antibodies in asthmatic mice reduced airway inflammation, eosinophil infiltration, and airway hyper-responsiveness (AHR).21 The bronchial cells produced CCL17 after the stimulation of proinflammatory factors and allergens.22,23 On DCs, TSLP increased the expression of TSLP receptors24 and enhanced the expression of CCL17 to augment Th2 responses in the inflamed location.25,26 However, it remains unclear whether the production of epithelial CCL17 is regulated by TSLP.
In this study, airway epithelium was administrated with lentivirus containing the specific small hairpin RNA (shRNA) that targeted TSLP to clarify the functional role of TSLP in the expression of epithelial CCL17 and allergic inflammation. The results showed that the attenuation of TSLP in the airway of asthmatic mice significantly reduced AHR and airway inflammation, which might be mediated by inhibiting the activation of DCs, reducing the production of CCL17 in bronchial epithelium and BAL fluids, and suppressing the migration of T cells. TSLP regulated the expression of CCL17 and TSLPR in primary epithelial cells in vitro. Taken as a whole, these data demonstrated that epithelium-derived TSLP modulated airway inflammation might be partially through a CCL17 axis.
Results
Lentivirus containing constructed-shRNA against TSLP significantly decreased TSLP secretion
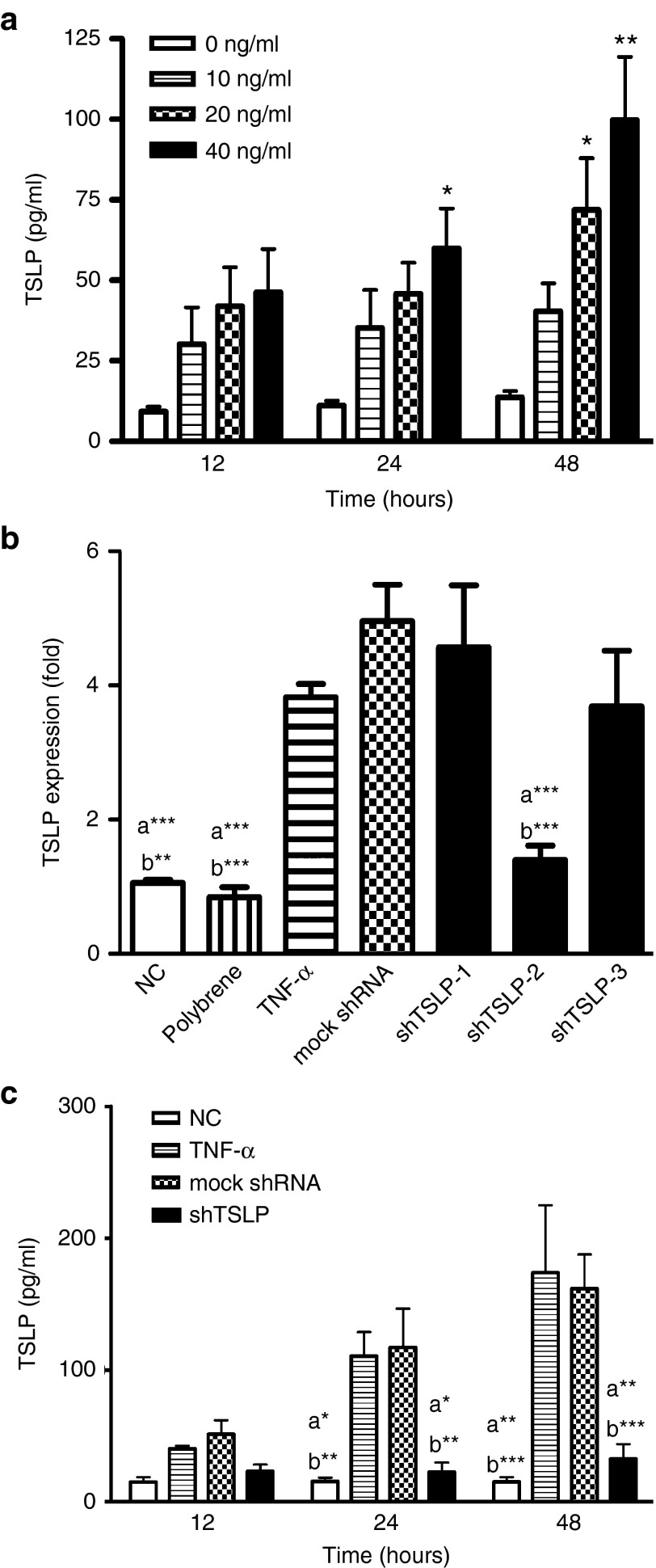
In our previous study, the primary lung cells were cultured and clarified with the phenotype as alveolar type II cells.27 Recently, RNAi is used as a tool in animal models to study the detailed functions of the targeted gene. In order to obtain a useful shRNA against TSLP, an induced-TSLP condition was set-up and used to determine the efficiency of lentivirus containing the shRNA in vitro. The expression of TSLP was induced by different dosages of TNF-α following time courses. The data showed that TSLP was obviously induced by high dosages of TNF-α (20 and 40 ng/ml) in 48 hours (Figure 1a). As a result, 40 ng/ml TNF-α was used as the stimulator in vitro system. Three shRNAs against TSLP (shTSLP-1, 2, and 3) were constructed and packaged into lentivirus for gene silencing. To determine the knockdown efficiency of shTSLP, the mRNA expressions of TSLP in epithelial cells were analyzed by real-time PCR during preinfection with lentivirus and the TNF-α stimulation. In order to increase infective efficiency, the treatment of lentiviral infection was combined with polybrene, which is a cationic polymer. Compared with the result in mock shRNA, the mRNA expression was decreased by about 50% in the shTSLP-2 group (Figure 1b). According to this finding, lentivirus containing shTSLP-2 was used as the therapeutic material. The levels of TSLP protein were measured following the time-course protocol. The results showed that the knockdown of TSLP could significantly appear in 24 hours of postinfection while the reduction was more obvious in 48 hours (Figure 1c).
Figure 1.
Lentivirus containing shRNA against thymic stromal lymphopoietin (TSLP) decreased the production of TSLP in vitro. (a) The induction of TSLP in primary lung cultures. Primary lung cells were stimulated by different doses of TNF-α (0, 10, 20, and 40 ng/ml) and the culture supernatants were collected following time course. *P < 0.05 and **P < 0.01 versus no TNF-α. The Knockdown efficiency of shTSLP in mRNA levels (b) and proteins (c). Cells were preinfected with lentivirus containing the mock shRNA or shTSLP (multiplicity of infection = 10) for 48 hours and stimulated by TNF-α (40 ng/ml). RNA was collected after TNF-α stimulation for 4 hours and the expression of mRNA was assessed by real-time polymerase chain reaction. Proteins in the culture supernatants were collected at time course (12, 24, and 48 hours) and analyzed by enzyme-linked immunosorbent assay. a*P < 0.05, a**P < 0.01, and a***P < 0.001 versus TNF-α. b*P < 0.05, b**P < 0.01, and b***P < 0.001 versus the mock shRNA. Data are shown as mean ± standard error of the mean and representative of six independent experiments.
Local administration with shTSLP in OVA-sensitized mice reduced the severity of AHR but did not affect the levels of antigen-specific antibodies in sera
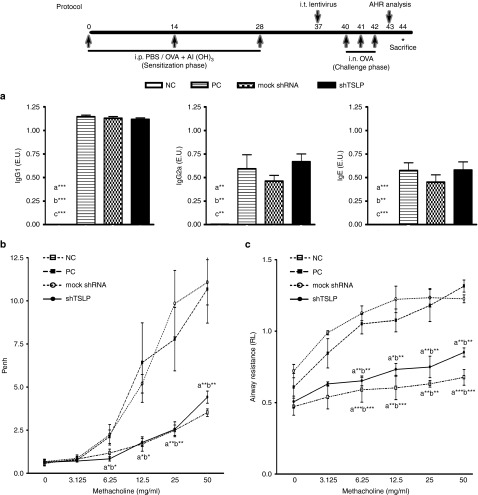
Following the sensitization and challenge protocol (Figure 2), a well-established murine model of OVA-induced asthma was used to evaluate the therapeutic effect of shTSLP. In our previous study, lentivirus during intratracheal administration could infect the bronchus, alveoli, and macrophages in the airway.27 In the OVA-induced asthmatic mice treated with lentivirus or not, there was no significant difference in OVA-specific IgG1, IgG2a, and IgE in OVA-sensitized groups among the positive control (PC) group, the shTSLP, and the mock shRNA group. The levels of OVA-specific IgG1, IgG2a, and IgE in the negative control (NC) group which was not received OVA sensitization were too low to be detectable (Figure 2a). The treated mice were analyzed for the degrees of constriction in airway after methacholine challenges in two systems, including the whole-body plethysmography and the invasive plethysmography. The airway constrictions in the OVA-sensitized and challenged mice were increased during methacholine challenges. The severity of AHR was significantly reduced in the shTSLP-treated mice but not in the mock shRNA-treated and PC mice (Figure 2b). The difference could be obviously noted in the low dosage (6.25 mg/ml) of methacholine challenges. The similar trend was also presented in the results of airway resistance (Figure 2c). These data suggested that local administration of shTSLP affected the progression of AHR but did not sway the systemic immune response.
Figure 2.
shTSLP significantly reduced the degree of airway hyperresponsiveness. The protocol of the asthmatic animal model is summarized in the figure. Mice were sacrificed after OVA sensitization and OVA challenges combining with or without lentiviral pretreatment. (a) The expression of OVA specific-Abs in the sera. Data are presented as ELISA units (E.U.). Following protocol, airway function of the treated mice was determined by (b) whole-body plethysmography or (c) invasive plethysmography after OVA challenges. Results in whole-body plethysmography were expressed as the baseline Penh value and results in invasive plethysmography were expressed as the airway resistance value. N = 6–8 per group. a*P < 0.05, a**P < 0.01, a***P < 0.001 versus the PC. b*P < 0.05, b**P < 0.01, b***P < 0.001 versus the mock shRNA. Data are shown as mean ± standard error of the mean and representative of five independent experiments. Data of invasive plethysmography were representative of three independent experiments. AHR, airway hyper-responsiveness; i.t., intratracheal; NC, negative control; OVA, ovalbumin; PC, positive control; TSLP, thymic stromal lymphopoietin.
shTSLP administration reduced the levels of Th2 cytokines and the maturation of airway DCs locally
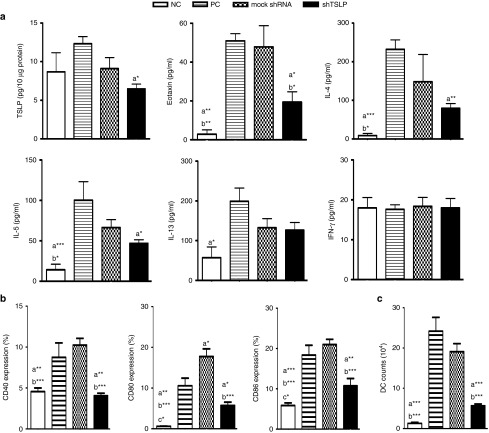
To evaluate the effect of shTSLP in the airway inflammation, the cytokine profile was analyzed. The production of TSLP was significantly decreased in the lung homogenization of the shTSLP-treated group but obviously increased in the PC group. The cytokine levels of IL-4 and IL-5 in the shTSLP-treated group were lower than that in the PC group. Meanwhile, the level of eotaxin, the key chemoattractant of eosinophil, in the shTSLP-treated group was lower than that in the PC or mock shRNA-treated groups respectively. However, the levels of IFN-γ and IL-13 among all groups were not statistically different (Figure 3a). Matured DCs play a very important role in the differentiation of T cells via the cooperation of cytokines and surface molecules. Following the procedure of the animal model, airway DCs isolated from lungs were analyzed for the expression of costimulatory molecules, including CD40, CD80, and CD86 by flow cytometry. The results showed that not only the expression of costimulatory molecules but also the number of DCs in the shTSLP-treated group was obviously lower than that in the PC and mock shRNA-treated groups (Figure 3b,c). These findings suggested that local attenuation of TSLP in the airway affected airway inflammation through modulating the recruitment and activation of DCs.
Figure 3.
shTSLP decreased the productions of Th2 cytokines and the expression of costimulatory molecules on lung dendritic cells (DCs). (a) The productions of thymic stromal lymphopoietin (TSLP) and proinflammatory cytokines in lungs. Lungs were homogenized in lysis buffer and the protein concentrations of homogenates were measured by bicinchoninic acid assay. The expressions of TSLP in lung homogenates (10 μg) and the levels of cytokines in bronchoalveolar lavage fluids were measured by enzyme-linked immunosorbent assay. (b) The expression of CD40, CD80, and CD86 on airway DCs. (c) The number of airway DCs. DCs were isolated from lungs and the expressions of costimulatory molecules were determined by flow cytometry. N = 6–8 per group. a*P < 0.05, a**P < 0.01, a***P < 0.001 versus the PC. b*P < 0.05, b**P < 0.01, b***P < 0.001 versus the mock shRNA. c*P < 0.05, c**P < 0.01, c***P < 0.001 versus shTSLP. Data are shown as mean ± standard error of the mean and representative of five independent experiments. NC, negative control; PC, positive control.
shTSLP administration decreased eosinophil and neutrophil recruitment in lungs
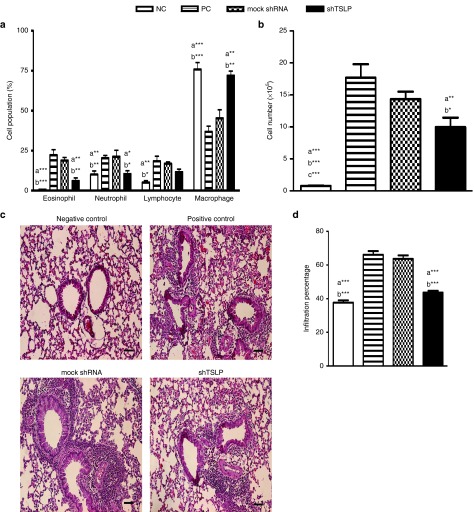
To assess the effect of shTSLP in the degrees of inflammatory infiltration, the cell numbers from BAL fluids and the cell populations in the airway were analyzed. The percentage of eosinophils in the PC or mock shRNA-treated group was higher than that in the shTSLP-treated and NC groups (Figure 4a). The decreased eosinophils probably resulted from the lower level of eotaxin (Figure 3a). Meanwhile, the percentage of neutrophils in the shTSLP-treated group was lower than the PC and mock shRNA-treated groups. The treatment of shTSLP enhanced the percentage of macrophages in BAL fluids, compared with those of the PC and mock shRNA-treated groups. The cell numbers of the shTSLP-treated and NC groups were lower than those of the PC and mock shRNA-treated groups (Figure 4b). In the pathological findings from the lung section, the inflammatory infiltration in the mock shRNA-treated and PC groups was much more severe than that in the NC and shTSLP-treated groups (Figure 4c,d). These data suggested that TSLP modulated the recruitment of inflammatory cells in the airway.
Figure 4.
shTSLP decreased the infiltration of inflammatory cells. After sacrifice, (a) the percentage of inflammatory cells and (b) total cell counts in the bronchoalveolar lavage fluids were analyzed. N = 6–8 per group. a*P < 0.05, a**P < 0.01, a***P < 0.001 versus the PC. b*P < 0.05, b**P < 0.01, b***P < 0.001 versus the mock shRNA. Data are shown as mean ± SEM and representative of five independent experiments. (c) Histological examination of lung tissue. (d) Quantitative analysis of histological sections. The paraffin sections of the treated mice were prepared and stained with hematoxylin and eosin. Bar = 100 μm (magnification: ×100). NC, negative control; PC, positive control; TSLP, thymic stromal lymphopoietin.
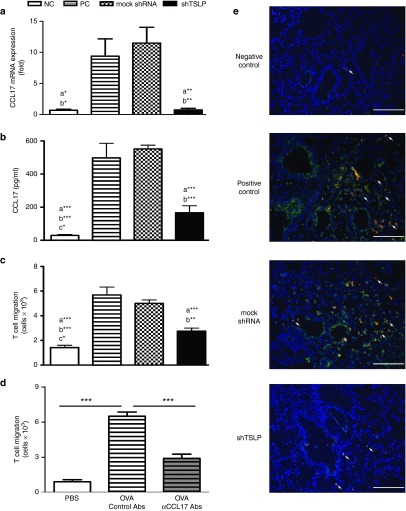
Reduced TSLP in the airway diminished the expressions of CCL17 in bronchial epithelium and the migration of T cells
Not only the levels of proinflammatory cytokines but also the expression of CCL17 was affected during the shRNA treatment. The data showed that the levels of CCL17 mRNA and protein in lungs and in BAL fluids were increased respectively by OVA challenges but decreased by the combination with the shTSLP treatment (Figure 5a,b). CCL17 is crucial for Th2 recruitment to asthmatic airways. To investigate whether the decrease of TSLP affects the T-cell migration, CD4+ T cells were cocultured with BAL fluids from the treated mice. The results showed that attenuated TSLP decreased the T-cell migration (Figure 5c). To further confirm whether the reduced T-cell migration was due to reduced CCL17, the BAL fluids were supplied anti-CCL17 antibodies in a migration assay. The result presented about 50% decrease and the trend was similar to the results of shRNA treatment (Figure 5d). These results suggested that CCL17 played a major role in an infiltration of T cell and CCL17 might be regulated by TSLP. To clarify the source of CCL17 in asthmatic airways, immunofluorescent staining was used to detect the distribution of CCL17. The results showed that CCL17 significantly expressed in the bronchial epithelium and DCs (CD11c+ cells) of asthmatic mice after airway challenges. The expression of CCL17 producing DCs in the shTSLP group was less than that in the PC and mock shRNA groups (Figure 5e). These findings indicated that TSLP might modulate the production of CCL17 to regulate the inflammation and cell infiltration.
Figure 5.
shTSLP downregulated CCL17 in lungs and T-cell migration. (a) The expression of CCL17 in lungs and (b) bronchoalveolar lavage (BAL) fluids. The lungs were used for RNA preparation and mRNA expressions of CCL17 were analyzed by quantitative real-time polymerase chain reaction. The levels of CCL17 in BAL fluids were measured by enzyme-linked immunosorbent assay. (c) The migration of T cells was induced by BAL fluids. (d) The migration of T cells was reduced by CCL17 neutralization. BAL fluids were cultured with control antibodies or anti-CCL17 antibodies (500 ng/ml) for migration assay. **P < 0.01, ***P < 0.001 versus the OVA plus control antibodies. (e) The expression of CCL17 in lung sections. The sections from the treated mice were prepared and stained by immunofluorescent staining. The arrows pointed out CCL17 and CD11c-expressing cells. Bar = 200 μm (magnification: ×100). N = 6–8 per group. a*P < 0.05, a**P < 0.01, a***P < 0.001 versus the PC. b*P < 0.05, b**P < 0.01, b***P < 0.001 versus the mock shRNA. c*P < 0.05 versus shTSLP. Data are shown as mean ± standard error of the mean and representative of five independent experiments. NC, negative control; OVA, ovalbumin; PC, positive control; TSLP, thymic stromal lymphopoietin.
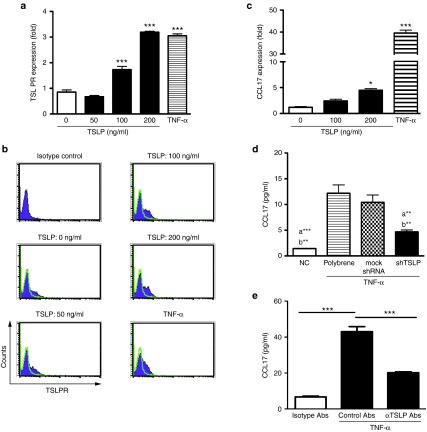
Attenuated the level of TSLP decreased the production of CCL17
Since the level of CCL17 was increased in the asthmatic airways, we further examined whether CCL17 was directly induced by TSLP in airway epithelial cells. It is well known that proinflammatory cytokines induce the expression of CCL17 in airway epithelium cells.28 Therefore, the treatment of TNF-α was used as the positive control. We found that TSLPR expressed on primary epithelial cells and TSLP and TNF-α could enhance the expression (Figure 6a,b). After a high dose of TSLP, the mRNA expression of CCL17 was induced (Figure 6c). However, TSLP alone could not induce a detectable level of CCL17 protein (data not shown). To clarify the correlation between TSLP and CCL17 under an inflammatory condition, primary epithelial cells were pretreated with shTSLP and then stimulated with TNF-α. CCL17 levels were reduced in the shTSLP group (Figure 6d). To further define whether TSLP mediated in CCL17 induction, the TNF-α stimulation was treated with neutralizing TSLP antibodies. The results showed that the induction of CCL17 was regulated by TSLP-dependent pathway (Figure 6e). In summary, these results demonstrated that epithelial CCL17 was regulated by TSLP in epithelial cells.
Figure 6.
Thymic stromal lymphopoietin (TSLP) regulated the expression of CCL17 in vitro. (a) mRNA levels and (b) expressions of TSLPR were induced by TSLP. (c) The expression of CCL17 was TSLP depend on. Primary epithelial cells (105/well) were stimulated by different doses of TSLP (0, 50, 100, and 200 ng/ml) or TNF-α (40 ng/ml). RNA was collected after TSLP stimulation for 6 hours and the expression of mRNA was assessed by real-time polymerase chain reaction. The TSLPR expressions on cells after TSLP stimulation for 48 hours were analyzed by flow cytometry. *P < 0.05, ***P < 0.01 versus medium only. (d) Attenuated TSLP reduced the expression of CCL17. a **P < 0.01, a ***P < 0.001 versus polybrene plus TNF-α. b**P < 0.01 versus the mock shRNA plus TNF-α. (e) Neutralizing TSLP decreased CCL17 induction. Data representative of three independent experiments are shown as means ± SEM.
Discussion
This study investigated the therapeutic effect of shRNA against TSLP in allergenic asthma and the crosstalk between TSLP and CCL17 in airway inflammation. The attenuation of TSLP derived from epithelium significantly reduced airway inflammation by lessening the activation of DCs and decreasing the production of epithelial CCL17.
For the complicated immune network, researchers further investigated the mechanisms to develop an effective therapy for allergic diseases. Monoclonal antibody is an effective therapy to specifically target the molecular pathways involved in the pathogenesis of various inflammatory disorders. Studies suggested that TSLP endows DCs with an ability to prime naïve T cells to Th2 differentiation and to produce chemokines CCL17 and CCL22, recruiting Th2 cells to the draining LNs. Local blockage of TSLP receptor before antigen sensitization or challenges significantly reduced eosinophil infiltration, goblet cell hyperplasia, and Th2 cytokine production via inhibition of maturation and migration of airway DCs.14,15,29 Neutralization of TSLP in infection of RSV also inhibited inflammation and AHR.30 These reports suggested that TSLP played a key role in airway inflammation via regulating the function of DCs. However, some challenges of engineered monoclonal antibodies, particularly in immunity-related adverse effects and allergy (rash, infusion reactions), suggested that a detailed understanding of molecular mechanisms is crucial.31,32 The advantages of the RNAi technique include less immunological responses and a better penetration into tissues to clear the unique characteristics of the specific genes. In this study, we found the alleviation in the degree of airway inflammation, the severity of AHR, and in the maturation of DCs was similar to those of antibody treatment. Although the levels of IL-4 and IL-5 in the airways were reduced by shTSLP treatment, the level of IL-13 was not. This might be resulted from IL-13 produced by airway epithelial cells. In addition to its expression in Th2 cells, IL-13 was consistently produced by the airway epithelium and was enhanced by TSLP.33 In this study, the airway epithelial cells were increased the expression of TSLPR by TSLP and TNF-α stimulation (Figure 6a,b). Even though epithelial TSLP was attenuated, the effect of IL-13 induction by TSLP in epithelium probably would not completely be eliminated. Furthermore, we found that TSLP affected the expression of epithelial CCL17 and this regulation might play a mediating role in airway inflammation.
Many studies have suggested that CCL17 was involved in AHR, airway inflammation, and cell infiltration.21,34 CCL17 was not only released from DCs but also promoted the maturation of DCs via a positive feedback to enhance the polarization of Th2 cells.35,36 Knock-down of CCL17 during the maturation of DCs decreased the recruitment of CD4+ T cells and Tregs.37 Reports showed that the interaction of RSV and Th2 cytokines tends to induce the expression of CCL17 via NF-κB and STAT6 pathway respectively.38 In cooperation with IL-4, TGF-β and Der p induced the expression of CCL17 in airway epithelium.23 This study found that TSLP alone could not induce a detectable level of CCL17 but TNF-α could do so in vitro. The results suggest that more severe inflammation is required for the induction of CCL17, and this might explain why in the literature no article has reported the ability of TSLP in regulating epithelial CCL17. Research showed that the eosinophil in the peripheral blood expressed CCR4 (the CCL17 receptor) and the recruitment of eosinophil induced by TSLP is dependent on CCL17 (ref. 39). This study found that the bronchial epithelium was the main source of CCL17 during allergen challenges and attenuated TSLP reduced the production of CCL17 to decrease the cell recruitment. Therefore, TSLP might regulate CCL17-mediated inflammation.
Targeting Th2 response is the major therapeutic approach for asthma but the pathophysiological role of Th2 cytokines in these complex airway diseases remains unclear. Recent studies showed that Th2 cytokines released from antigen-specific T cells could enhance the secretions of epithelial TSLP and production of CCL17 in DCs to augment inflammation.40 In addition, Th2 cytokines could abrogate TSLP-mediated induction of CCR7, which plays a key role in the migration of DCs from inflamed tissues to the draining lymph nodes and retain active DCs in the inflamed tissue to further exacerbate local inflammation.41 Furthermore, TSLP signaling in CD4+ T cells was required for the Th2 memory formation and recall response to local antigen challenges.42 The Elimination of CCR4+ cells via CCL17-PE38 treatment sufficiently reduced airway inflammation and AHR.34 This study further found that TSLP regulated not only the productions of Th2 cytokines but also the expression of epithelial CCL17 to modulate Th2 responses. These findings might provide the connections of a complicated network in immune responses and intensify the therapeutic potency of TSLP in allergic asthma.
In summary, the results of this study clarified the relationship between TSLP and CCL17 in airway inflammation. Epithelium-derived TSLP induced by allergens and proinflammatory cytokines was found to regulate epithelial CCL17 which mediates Th2 recruitment in airway inflammation. Overall, the data provided further evidence of the vital role of TSLP in airway inflammation and its therapeutic value in treating allergic asthma.
Materials and methods
Protocol of CCL17 and TSLP induction. Female Balb/c mice were obtained from the National Laboratory Animal Center in Taiwan, Taiwan. Lungs were isolated from Balb/c mice and cut into 2–3 mm3 and ground in Minimum Essential Medium-α complete medium (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS), 2 mmol/l L-glutamine, 100 unit/ml penicillin, 1 mg/ml streptomycin, and 0.25 mg/ml amphotericin (Biological industries, Kibbutz Beit Haemek, Israel) and 25 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.2). After blood cell deletion, cells were incubated for 10–14 days. The primary cells were harvested and prepared for assay. The phenotypic determination of primary epithelial cells has been described previously.27 In the culture system, cells (105/well) were starved overnight in the FBS-free medium. CCL17 induction was treated with different doses of TSLP (0 ~ 200 ng/ml, R&D, Minneapolis, MN) and TNF-α (40 ng/ml, R&D) for 4 hours in a fresh FBS-free medium and the RNA extractions were collected accordingly. TSLP induction was treated with different doses of TNF-α (0 ~ 40 ng/ml, R&D) for 4 hours in a fresh FBS-free medium and the RNA extractions were collected accordingly. Total RNA was performed with random hexamer primers and Super-Script III RNase H- reverse transcriptase (Invitrogen, Carlsbad, CA) to convert to cDNA. The expression of mouse CCL17 and TSLP was measured by quantitative real-time polymerase chain reaction with TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA) in ABI PRISM 7400 Sequence Detector (Applied Biosystems, Foster City, CA). All levels of reported mRNA were normalized to the glyceraldehyde-3-phosphate dehydrogenase mRNA level. For the protein assay, cells were starved and stimulated following time course (CCL17: 72 hours; TSLP: 12, 24, and 48 hours). For TSLP neutralization, the primary epithelial cells were stimulated by TNF-α (40 ng/ml) containing control antibodies or anti-TSLP antibodies (1 μg/ml, R&D) for 72 hours. The level of CCL17 or TSLP in the cultured supernatant was measured by enzyme-linked immunosorbent assay (ELISA; R&D) according to the manufacturer-recommended protocols.
Preparation of lentivirus containing constructed shRNA against TSLP. The targeting sequences of mouse tslp gene were shTSLP-1: AAGATTGTGGTATTCCTTCAT, shTSLP-2: AACTTCACGTCAATTACGAAA and shTSLP-3: AAACCTAACTGTAGTAGGAAG. The control sequence of mock shRNA was GTCAGACTGTGCCATGACTG. The control shRNA had no homologous gene in any mouse gene. The procedure of lentiviral preparation and the viral titer were conducted as previously described.27
Determination of suppressed efficiency of shRNA. For RNA expression, the primary epithelial cells were infected with a mixture, including polybrene (8 μg/ml, Sigma, St Louis, MO) and lentiviruses containing shRNA (multiplicity of infection: 10) for 48 hours and were stimulated by TNF-α (40 ng/ml) for 4 hours. The RNA extractions were collected and the mRNA of TSLP was analyzed by real-time PCR. For the protein assay, cells were infected with lentivirus and stimulated by TNF-α at different time intervals (12, 24, and 48 hours).
Establishment of murine model of asthma. Female BALB/c mice (6 weeks old, n = 6–8 mice/group) were obtained from the Animal Center of the College of Medicine, National Taiwan University, maintained on 12 hours light/dark cycle and provided food and water ad libitum. All animal studies were approved by the College of Medicine of Institutional Animal Care and Use Committee. The mice were intraperitoneally sensitized by injection of 200 μl phosphate-buffered saline (PBS) containing 50 µg of OVA (Sigma) and 2 mg of aluminum hydroxide (Sigma) on day 0. Following two times of boost with 25 μg OVA in the same dosage of adjuvant on days 14 and 28. After equally ranking in the levels of OVA-specific IgE, OVA-sensitized mice were challenged daily by intranasal administration with OVA (100 µg) in 50 µl PBS for 3 days. Lentivirus containing shTSLP or mock shRNA (3 × 106 infectious units) was intratracheally delivered into the anesthetized animals 3 days before the first challenge with OVA. The PBS (positive control) and lentivirus containing mock shRNA were used as the controls. The protocol was described in Figure 2. During the 3-day OVA challenges, airway resistance and inflammatory indications were analyzed.
Determination of the airway resistance and function. After performing a tracheostomy, the airway resistance of the treated mice was measured as an increase in pulmonary resistance after challenges with aerosolized β-methacholine (MCh, Sigma). The setting and parameters were as previously described.27 The resistance of the orotracheal tube (0.45 cmH2O.s.ml−1) was subtracted from all airway resistance measurements. Data were expressed as the pulmonary resistance (RL) of the five independent experiments.
Airway reactivity was expressed as Penh and measured in unrestrained animals by barometric whole-body plethysmography (Buxco, Troy, NY). In the beginning, the mice were placed in the chambers respectively and baseline readings were taken and averaged for 3 minutes. Aerosolized PBS or β-methacholine in increasing concentrations (3.125–50 mg/ml) was administrated for 3 minutes. The readings were taken and averaged for 3 minutes after each nebulization. Airway reactivity was expressed as enhanced pause (Penh) of the five independent experiments.
Determination of airway inflammation and histological examination. Cells were harvested from the BAL fluids, depleted RBC with ACK reagent. The cells (105/ml) were resuspended in PBS containing 1% bovine serum albumin and performed by cytospin. The cell slides were stained with the Liu staining and a total of 300 cells on the slides were distinguished on cytologic preparations under microscopes. The supernatants of BAL fluids were assayed by the ELISA assay. The lungs were fixed with 10% formalin-PBS buffer and paraffin sections were prepared and stained with hematoxylin and eosin to evaluate the degree of infiltrating inflammatory cells under microscopes. The photomicrographs of lung sections were quantified by Image J software (NIH, v1.45). The infiltration percentages were calculated according to the formula:
(%) = (the number of pixels within the defined area of cell particles/the total number of pixels within the field of view) × 100
The average of the four fields (100× magnification) of view was used to determine cell infiltration in each lung analyzed.
Determination of cytokine production. The levels of IL-4, IL-5, IL-13, IFN-γ, CCL17, and eotaxin in the BAL fluids were determined by the ELISA assays (R&D) according to the manufacturer-recommended protocols. The partial lungs were frozen and samples of the frozen lungs were collected by homogenization in lysis buffer (Cell Signaling Technology, Danvers, MA). Protein concentrations were measured by a bicinchoninic acid kit (Thermo scientific, Rockford, IL). The levels of TSLP and CCL17 in lung homogenates were determined by the ELISA assays (R&D).
Determination of surface markers on DCs. The lungs were isolated form treated mice and digested as described.43 Tissues were dissociated and single cells suspensions were obtained. The following antibody was purchased from BD Pharmingen (Franklin Lakes, NJ): purified CD16/CD32 (Fc block). The following antibodies were purchased from eBioscience (San Diego, CA): anti-CD11c APC, antiCD40 PE, anti-CD80 PE, anti-CD86 PE, and isotype control antibodies. Lung DCs were stained with antibodies and analyzed on a FACSCalibur flow cytometer (BD Immunocytometry Systems).
Migration assay. CD4+ T cells were isolated from the spleens of Blab/c mice using magnetic beads (BD Bioscience, San Jose, CA). Migration assay was performed using 96-well Transwell plates with a 5.0 µm polycarbonate membrane (Corning Life Sciences, Corning). The lower chamber was filled with the culture medium (200 μl) containing 100 μl supernatants of BAL fluids. CD4+ T cells (1 × 105) in the 100 μl culture medium were placed in triplicate in the upper chamber. After incubation at 37 °C for 4 hours, migrated cells in the lower chamber were counted by flow cytometry. For CCL17 neutralization, control antibodies or anti-CCL17 antibodies (500 ng/ml, R&D) were added into the lower chamber which was filled with the culture medium (200 μl) containing 100 μl supernatants of BAL fluids.
Immunofluorescent staining. Lungs were perfused with PBS, fixed in 4% paraformaldehyde and embedded in OCT. The frozen sections of 8 μm thickness were prepared for staining. The slides were washed with PBS following methanol fixation for 10 minutes at −20 °C and rinsed with PBS. Slides were incubated in blocking solution (Biogenex, Fremont, CA) for 2 hours and permeabilized by 0.3% Triton X-100 in PBS for 30 minutes at RT. During incubation with polyclonal rabbit anti-mouse CCL17 (Dilution: 1:100, Bioss, Woburn, MA) in a humid chamber overnight at 4 °C, the antigen detection was carried out with Alexa-488 goat antirabbit IgG (Invitrogen) and anti-CD11c PE (eBioscience) for 2 hours. Negative control sections were processed by omitting the specific primary antibody. The sections were added mounting media with DAPI (Vector Laboratories) and coverslip. The Immunofluorescent labelings were analyzed using fluorescence microscope (Zeiss Axio Imager) and were visualized with Axio Vision.
Determination of antigen-specific antibody. The levels of anti-OVA antibody in sera were determined by the ELISA assay according to the protocols set in the previous study.27
Statistical analysis. All values were the mean ± standard error of the mean using one-way analysis of variance followed by the Fisher protected least significant difference test. The minimal level of significance was a P value <0.05.
Author contributions
Y-L.C. performed the experiments, analyzed the data, and wrote the manuscript. B-L.C. directed the project.
Acknowledgments
We would like to acknowledge the service provided by the DNA Sequencing Core of the First Core Laboratory, National Taiwan University College of Medicine. The authors declare no competing financial interests.
References
- Lloyd, CM and Saglani, S (2015). Epithelial cytokines and pulmonary allergic inflammation. Curr Opin Immunol 34: 52–58. [DOI] [PubMed] [Google Scholar]
- Hammad, H and Lambrecht, BN (2008). Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 8: 193–204. [DOI] [PubMed] [Google Scholar]
- Zhou, B, Comeau, MR, De Smedt, T, Liggitt, HD, Dahl, ME, Lewis, DB et al. (2005). Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6: 1047–1053. [DOI] [PubMed] [Google Scholar]
- Al-Shami, A, Spolski, R, Kelly, J, Keane-Myers, A and Leonard, WJ (2005). A role for TSLP in the development of inflammation in an asthma model. J Exp Med 202: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori, M and Ziegler, S (2007). Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol 178: 1396–1404. [DOI] [PubMed] [Google Scholar]
- Soumelis, V, Reche, PA, Kanzler, H, Yuan, W, Edward, G, Homey, B et al. (2002). Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 3: 673–680. [DOI] [PubMed] [Google Scholar]
- Hui, CC, Rusta-Sallehy, S, Asher, I, Heroux, D and Denburg, JA (2014). The effects of thymic stromal lymphopoietin and IL-3 on human eosinophil-basophil lineage commitment: Relevance to atopic sensitization. Immun Inflamm Dis 2: 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa, MC, Saenz, SA, Hill, DA, Kim, BS, Headley, MB, Doering, TA et al. (2011). TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477: 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, L, Zhang, Y, Yao, W, Kaplan, MH and Zhou, B (2011). Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol 186: 2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, SF (2012). Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol 130: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, S, O'Connor, B, Ratoff, J, Meng, Q, Mallett, K, Cousins, D et al. (2005). Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 174: 8183–8190. [DOI] [PubMed] [Google Scholar]
- Nguyen, KD, Vanichsarn, C and Nadeau, KC (2010). TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley, MB, Zhou, B, Shih, WX, Aye, T, Comeau, MR and Ziegler, SF (2009). TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol 182: 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F, Huang, G, Hu, B, Song, Y and Shi, Y (2011). A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol 164: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, DT, Ma, C, Niewoehner, J, Dahl, M, Tsai, A, Zhang, J et al. (2013). Thymic stromal lymphopoietin receptor blockade reduces allergic inflammation in a cynomolgus monkey model of asthma. J Allergy Clin Immunol 132: 455–462. [DOI] [PubMed] [Google Scholar]
- Saeki, H and Tamaki, K (2006). Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci 43: 75–84. [DOI] [PubMed] [Google Scholar]
- Romagnani, S (2002). Cytokines and chemoattractants in allergic inflammation. Mol Immunol 38: 881–885. [DOI] [PubMed] [Google Scholar]
- Bochner, BS, Hudson, SA, Xiao, HQ and Liu, MC (2003). Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol 112: 930–934. [DOI] [PubMed] [Google Scholar]
- Katoh, S, Fukushima, K, Matsumoto, N, Matsumoto, K, Abe, K, Onai, N et al. (2003). Accumulation of CCR4-expressing CD4+ T cells and high concentration of its ligands (TARC and MDC) in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. Allergy 58: 518–523. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon, P, Papi, A, Mariani, M, Di Lucia, P, Casoni, G, Bellettato, C et al. (2001). The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest 107: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, S, Takizawa, H, Yoneyama, H, Nakayama, T, Fujisawa, R, Izumizaki, M et al. (2001). Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol 166: 2055–2062. [DOI] [PubMed] [Google Scholar]
- Sekiya, T, Miyamasu, M, Imanishi, M, Yamada, H, Nakajima, T, Yamaguchi, M et al. (2000). Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol 165: 2205–2213. [DOI] [PubMed] [Google Scholar]
- Heijink, IH, Marcel Kies, P, van Oosterhout, AJ, Postma, DS, Kauffman, HF and Vellenga, E (2007). Der p, IL-4, and TGF-beta cooperatively induce EGFR-dependent TARC expression in airway epithelium. Am J Respir Cell Mol Biol 36: 351–359. [DOI] [PubMed] [Google Scholar]
- Tanaka, J, Watanabe, N, Kido, M, Saga, K, Akamatsu, T, Nishio, A et al. (2009). Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy 39: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima, M and Ziegler, SF (2013). Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J Immunol 191: 4903–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleck, B, Kazeros, A, Bakal, K, Garcia-Medina, L, Adams, A, Liu, M et al. (2015). Coexpression of type 2 immune targets in sputum-derived epithelial and dendritic cells from asthmatic subjects. J Allergy Clin Immunol 136: 619–627.e5. [DOI] [PubMed] [Google Scholar]
- Chen, YL, Huang, HY, Lee, CC and Chiang, BL (2014). Small interfering RNA targeting nerve growth factor alleviates allergic airway hyperresponsiveness. Mol Ther Nucleic Acids 3: e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada, N, Nomura, T, Kim, WJ, Otsuka, Y, Takahashi, R, Kishi, H et al. (2001). Expression of C-C chemokine TARC in human nasal mucosa and its regulation by cytokines. Clin Exp Allergy 31: 1923–1931. [DOI] [PubMed] [Google Scholar]
- Shi, L, Leu, SW, Xu, F, Zhou, X, Yin, H, Cai, L et al. (2008). Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol 129: 202–210. [DOI] [PubMed] [Google Scholar]
- Han, J, Dakhama, A, Jia, Y, Wang, M, Zeng, W, Takeda, K et al. (2012). Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J Allergy Clin Immunol 130: 1175–1186.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake, H, Wirth, T, Schenkwein, D, Räty, JK and Ylä-Herttuala, S (2009). Challenges in monoclonal antibody-based therapies. Ann Med 41: 322–331. [DOI] [PubMed] [Google Scholar]
- Guan, M, Zhou, YP, Sun, JL and Chen, SC (2015). Adverse events of monoclonal antibodies used for cancer therapy. Biomed Res Int 2015: 428169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlali, A, Jacques, E, Koussih, L, Gounni, AS and Chakir, J (2010). Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol 125: 844–850. [DOI] [PubMed] [Google Scholar]
- Honjo, A, Ogawa, H, Azuma, M, Tezuka, T, Sone, S, Biragyn, A et al. (2013). Targeted reduction of CCR4⁺ cells is sufficient to suppress allergic airway inflammation. Respir Investig 51: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Yahia, S, Azzaoui, I, Everaere, L, Vorng, H, Chenivesse, C, Marquillies, P et al. (2014). CCL17 production by dendritic cells is required for NOD1-mediated exacerbation of allergic asthma. Am J Respir Crit Care Med 189: 899–908. [DOI] [PubMed] [Google Scholar]
- Qiao, J, Li, A and Jin, X (2011). TSLP from RSV-stimulated rat airway epithelial cells activates myeloid dendritic cells. Immunol Cell Biol 89: 231–238. [DOI] [PubMed] [Google Scholar]
- Kang, S, Xie, J, Ma, S, Liao, W, Zhang, J and Luo, R (2010). Targeted knock down of CCL22 and CCL17 by siRNA during DC differentiation and maturation affects the recruitment of T subsets. Immunobiology 215: 153–162. [DOI] [PubMed] [Google Scholar]
- Monick, MM, Powers, LS, Hassan, I, Groskreutz, D, Yarovinsky, TO, Barrett, CW et al. (2007). Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol 179: 1648–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, F, Liu, LB, Shang, WQ, Chang, KK, Meng, YH, Mei, J et al. (2015). The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett 364: 106–117. [DOI] [PubMed] [Google Scholar]
- Hui, CC, Murphy, DM, Neighbour, H, Al-Sayegh, M, O'Byrne, S, Thong, B et al. (2014). T cell-mediated induction of thymic stromal lymphopoietin in differentiated human primary bronchial epithelial cells. Clin Exp Allergy 44: 953–964. [DOI] [PubMed] [Google Scholar]
- Melum, GR, Farkas, L, Scheel, C, Van Dieren, B, Gran, E, Liu, YJ et al. (2014). A thymic stromal lymphopoietin-responsive dendritic cell subset mediates allergic responses in the upper airway mucosa. J Allergy Clin Immunol 134: 613–621.e7. [DOI] [PubMed] [Google Scholar]
- Wang, Q, Du, J, Zhu, J, Yang, X and Zhou, B (2015). Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol 135: 781–91.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer, KA, Scholtes, P, Karwot, R and Finotto, S (2006). Isolation of CD4+ T cells from murine lungs: a method to analyze ongoing immune responses in the lung. Nat Protoc 1: 2870–2875. [DOI] [PubMed] [Google Scholar]