Abstract
Differences in thrombosis rates have been observed clinically between different drug eluting stents. Such differences have been attributed to numerous factors, including stent design, injury created by the catheter delivery system, coating application technologies, and the degree of thrombogenicity of the polymer. The relative contributions of these factors are generally unknown. This work focuses on understanding the thrombogenicity of the polymer by examining mechanistic interactions with proteins, human platelets, and human monocytes of a number of polymers used in drug eluting stent coatings, in vitro. The importance for blood interactions of adsorbed albumin and the retention of albumin was suggested by the data. Microscopic imaging and immunostaining enhanced the interpretation of results from the lactate dehydrogenase cell counting assay and provided insight into platelet interactions, total quantification, and morphometry. In particular, highly spread platelets may be surface-passivating, possibly inhibiting ongoing thrombotic events. In many of the assays used here, poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) showed a differentiated protein deposition pattern that may contribute to the explanation of the consistently thromboresistant blood–materials interaction for fluororpolymers cited in literature. These results are supportive of one of several possible factors contributing to the good thromboresistant clinical safety performance of PVDF-HFP coated drug eluting stents.
I. INTRODUCTION
Drug-eluting stents (DES), designed to reduce restenosis by releasing drugs that inhibit implantation injury-induced vascular smooth muscle cell migration and proliferation, have been successful in reducing in-stent restenosis rates to below 10%.1 However, DES have been recently associated with late (>30 days postimplantation) or very late (>12 months postimplantation) thrombosis,1,2 justifying ongoing efforts to further our understanding of blood–biomaterial interactions. Mechanistic aspects of the blood–materials interaction in the acute phase may affect the clinical performance of DES along with other important factors such as stent design, catheter delivery system, balloon material, and coating application technologies. The relative contributions of those factors are generally unknown. For example, upon contact with blood,3 a layer of plasma proteins almost instantly adsorbs onto the implanted biomaterial, and its makeup is largely driven by the biomaterial's surface chemistry.4,5 Platelets and inflammatory cells then come in contact with this adsorbed protein layer, which can lead to cell adhesion,6 platelet activation, and thrombosis.7,8
Much effort has been invested in understanding the involvement of various adsorbed adhesive proteins, such as fibrinogen (Fg),9–11 fibronectin (Fn),9 vitronectin (Vn),9,12 and von Willebrand factor (vWF),9,13,14 on platelet adhesion and activation. Through a series of studies measuring platelet adhesion to protein preadsorbed surfaces from either pure protein solutions9 or selectively depleted plasmas,10 the primary role of Fg in mediating platelet adhesion and activation has been established. Platelets can bind to Fg through their GP IIb/IIIa receptor at three different sites. One binding site is a dodecapeptide located at the carboxyl-terminus of the γ-chain at γ400–411 (HHLGGAKQAGDV), with two additional binding sites in the α-chain at α95–98 (RGDF) and α572–575 (RGDS).15,16 While the total amount of adsorbed Fg on a surface may be related to platelet interaction, recent studies indicate that Fg conformation may be a better indicator of platelet adhesion.10,11,17,18 Monoclonal antibody binding studies to adsorbed Fg (Ref. 19) and GP IIb/IIIa receptor blocking studies11 have been used to establish the primary importance of the dodecapeptide binding site on Fg in mediating platelet adhesion, while the RGDS and RGDF sites have been found to play a secondary, supportive role in platelet adhesion.18,19
Fluorinated polymers have had a long history in blood contacting applications and have been found by many researchers to reduce platelet adhesion and activation compared to controls.20–26 For instance, tetrafluoroethylene glow discharge treatment on woven and knit Dacron vascular prostheses improved graft patency in a baboon ex vivo model by reducing the rate of thrombotic occlusion at all time points tested, up to 1 week.20 In addition, there was a significant reduction in microembolization in vitro observed for the tetrafluoroethylene treated grafts as compared to the untreated grafts.21 Modifying polyetherurethanes with fluorinated macromolecules resulted in a fivefold reduction in platelet adhesion as compared to unmodified polyetherurethanes, although there was no significant reduction in the amount of adsorbed Fg observed.22 Plasma-induced graft polymerization of vinylidene fluoride onto polyethylene substrates resulted in a dramatic reduction in platelet adhesion and activation as compared to uncoated polyethylene.23 In addition, by modifying polyacrylonitrile membranes through blending up to 27% poly(vinylidene fluoride) (PVDF), a reduction in protein adsorption, platelet adhesion, and thrombus formation was observed.24 Fluorinated polymers have been suggested for vascular grafts because of reported evidence of lower thrombogenicity, decreased inflammatory response, and more rapid endothelialization, all desirable characteristics for a stent coating. Finally, expanded polytetrafluoroethylene vascular grafts are the clinical standard of care for blood vessel replacements down to 5 mm diameter. Due to the demonstrated blood compatibility of fluoropolymers, they have been deemed a promising group of polymers for use in blood and vascular tissue contacting applications.25,26
The aim of this study is to evaluate the blood compatibility of some polymers currently used in DES applications, independent of the effects of the antiproliferative drugs they elute. Blood compatibility and its assessment has been the subject of hundreds of papers and still there is lack of clarity as to the significance of the various assays.27,28 However, it is clear that measurement of many relevant parameters will more meaningfully profile the blood interactions of a particular blood-contacting material.29 Since poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) polymer-coated XIENCE V® DES (Abbott Vascular) have demonstrated low thrombogenicity in large scale clinical studies,30 assessment parameters can be compared to clinical stent performance to develop hypotheses to advance our understanding of blood compatibility.
Thrombus associated with DES implantation may be related not only to material surface thrombogenicity but also to numerous other factors, including stent design, vascular injury created by the catheter delivery system, and use of specialized coating application technologies. Thin struts and more flexible stent designs, for example, may be related to better strut apposition and more rapid endothelialization.31,32
To assess blood interactions relevant to blood compatibility, we used glass coverslips spin coated with relevant stent coating polymers. Parameters assessed included protein adsorption, platelet adhesion, morphology, and monocyte adhesion. Physical surface characterization was performed to ensure relevant, high-quality surfaces. While Fg, Fn, and human serum albumin (Alb) adsorption were measured from pure and plasma solutions, competitive adsorption from Fg/Alb and Fn/Alb binary solutions was also measured. For blood contacting biomaterials, it has been proposed that surfaces with high levels of adsorbed Alb are desirable.8 Alb, the most abundant blood plasma protein, could passivate polymer surfaces, preventing more reactive proteins, especially Fg, from adsorbing, thus reducing subsequent thrombosis.8,33,34 The Alb/Fg ratio, in particular, might be related to a biomaterial's blood compatibility, as a higher ratio may lead to a reduction in adherent platelets.35 In addition, protein retention after sodium dodecyl sulfate (SDS) elution was evaluated to estimate protein-surface affinity and the potential for removal by blood elements. While the amount of adsorbed protein is important, the conformational and orientational state of the adsorbed protein on the surface may be a better indicator of a surface's thrombogenicity. The accessibility and reactivity of bioactive epitopes within the adsorbed Fg was measured by monoclonal antibody binding.
II. MATERIALS AND METHODS
PVDF-HFP, poly(butyl methacrylate) (PBMA) and polystyrene-b-polyisobutylene-b-polystyrene (Kaneka; SIBS1—102T 15% styrene 85% isobutylene, molecular weight (MW) 100 000; SIBS2—103T 30% styrene 70% isobutylene, MW 100 000) were provided by Abbott Vascular. 316 stainless steel (SS) sheets were purchased from McMaster-Carr (Atlanta, GA).
A. Buffers and reagents
Citrate phosphate buffered saline (CPBS) contained 10 mM Na2HPO4, 10 mM citric acid, and 120 mM NaCl, pH 7.4. CPBS containing 3 mM NaN3 and 10 mM NaI (CPBSzI) (pH 7.4) was used for protein adsorption. Phosphate buffered saline (PBS), human albumin (>99% purity), Histopaque®, Triton-X 100, and trypan blue stain were purchased from Sigma-Aldrich (St. Louis, MO). Heparinized and acid citrate dextrose (ACD) vacutainers were purchased from Becton Dickinson (Franklin Lakes, NJ). Purified human fibrinogen (>95% clottable) was purchased from Enzyme Research Laboratories (South Bend, IN). Pooled normal citrated human plasma was purchased from Innovative Research (Novi, MI). RPMI-1640 with l-glutamine and ethylenediaminetetraacetic acid (EDTA) was purchased from Invitrogen (Carlsbad, CA). Monocyte cell culture media for monocyte adhesion studies consisted of RPMI-1640 (Invitrogen) supplemented with 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Gibco BRL), 1 mM modified eagle medium (MEM) sodium pyruvate (Sigma), 0.1 mM MEM nonessential amino acids (Sigma), and 1% penicillin-streptomycin (PEN-STREP, Sigma). The antifibrinogen monoclonal antibodies, R1, R2, and M1, were generously provided by Dr. Thomas Horbett. R1 binds to the Aα chain (87–100) near the N-terminal region with an RGDF sequence at 95–98, while R2 binds to the Aα chain (566–580) near the C-terminal region with an RGDS sequence at 572–575, and both originated from Dr. Zaverio Ruggeri's lab (Scripps Clinic, La Jolla, CA). M1 binds to the C terminus of the Fg γ chain, including the platelet dodecapeptide binding region at 402–411, and originated from Gary Matsueda's lab (Bristol Meyers Squibb, NJ).
B. Sample preparation
Glass cover slips (8 mm, ProSci Corp) were ultrasonicated for 10 min in a 1/64 dilution of Isopanasol in DI water (C.R. Callen Corp, Seattle, WA). They were then rinsed five times under ultrasonication in ultrapure water for 10 min, replacing the wash liquid with fresh water each time. The cover slips were air dried in a laminar flow hood overnight and stored in a Petri dish until ready to use.
The dry coverslips were spin-coated with 2.5% (w/v) ethyl methacrylate-methacryoxysilane copolymer (EMA-silane) in ethyl acetate on both sides. Coverslips were placed vertically in a rack to hold coverslips separate from each other during processing and baked at 60 °C overnight. They were then soaked twice in ethyl acetate for 1 h and once for 5 min, replacing the liquid with fresh ethyl acetate each time. The coverslips were then allowed to air dry in a laminar flow hood overnight. The EMA-silane pretreatment has been found to prevent delamination of polymers on glass.
Polymer solutions (2.5% w/v concentration) in various solvents were prepared (see Table I). The EMA-silane coated coverslips were spin coated with the different polymer solutions at 4000 rpm for 20 s. They were allowed to dry, flipped over, and coated on the backside using the same settings. Once dry, the polymer samples were vapor annealed in acetone for 45 min. All samples were heat-annealed at 50 °C for 17 h on a metal stage in a glass rack.
Table I.
Polymer solution concentrations used for spin coating samples on glass coverslips.
| Polymer | Solvent used | Volume/drop (μl) |
|---|---|---|
| SIBS-1 | 2.5% (w/v) in chloroform | 7 |
| SIBS-2 | 2.5% (w/v) in chloroform | 7 |
| PBMA | 2.5% (w/v) in toluene | 9.5 |
| PVDF-HFP | 2.5% (w/v) in acetone | 9.5 |
316 SS sheets were cut into 7 mm2 and subjected to successive ultrasonication for 10 min in hexane, dichloromethane, acetone, and methanol (repeated twice). SS samples were then allowed to air dry in a laminar flow hood overnight.
Prior to cell adhesion studies, the polymer-coated glass coverslip samples and stainless steel samples were soaked for 2 h in 70% ethanol (a nonsolvent for the polymers used here). They were then soaked twice in sterile PBS for 30 min. After removing the last rinse buffer, samples were stored in sterile buffer overnight, until ready to use.
C. Surface analysis using electron spectroscopy for chemical analysis
Composition and integrity of the spin-coated surfaces were characterized by electron spectroscopy for chemical analysis (ESCA) at the National ESCA and Surface Analysis Center for Biomedical Problems (NESAC/BIO, University of Washington, Seattle). Spectra were collected using a Surface Science Instruments S-Probe, with a monochromatic Al Kα1,2 x-ray source. The samples were analyzed at a 55° take-off angle so that data reflected the composition of the topmost 50–80 Å of the surface. Compositional survey scans were acquired using an analyzer pass energy of 150 eV and an x-ray spot size of 1000 μm. High-resolution (Hi-Res) spectra were acquired at a pass energy of 50 eV and resolved into individual Gaussian peaks using a least-squares fitting routine in the Service Physics ESCAVB Graphics Viewer.
Film stability was evaluated by collecting survey scans (0–1000 eV) and high-resolution C1s scans of samples soaked in ultrapure water and unsoaked samples (n = 3 for soaked and unsoaked). All binding energies (BE) were referenced to the lowest resolved C1s peak BE, the hydrocarbon peak (CHx) at 285.0 eV. Compositions are reported as the average of the collected spots for one type of sample, and presented as atomic percent.
D. Protein radiolabeling
We used the ICl method, developed by Helmkamp et al.36 as modified by Horbett,37 to radiolabel human Fg and human Alb with Na125I (Perkin Elmer, Waltham, MA). A 2:1 ICl to protein molar ratio was used for labeling Fg, while a 3:1 ICl to protein molar ratio was used for labeling Alb. Unbound 125I was separated from the labeled protein by two passes through desalting chromatography columns (Econo-Pac 10DG, BioRad, Hercules, CA). Radiolabeled Fg and Alb were stored at −20 °C and used within 2 weeks.
E. Protein adsorption and elution
Polymer-coated glass coverslips and SS samples were equilibrated by submersion in degassed CPBSzI for 1 h at room temperature. Shortly before use, single protein solutions (0.6 mg/ml Alb or 0.06 mg/ml Fg), binary solutions (0.6 mg/ml Alb and 0.06 mg/ml Fg), and 2% citrated pooled plasma solutions were made and enough 125I protein was added to achieve a specific activity of no less than 50 cpm/ng. Because the specific activities of the stock 125I protein was high, only a small amount of radiolabeled protein was added to these solutions, and did not significantly change the protein concentration.
Enough protein solution was added to samples soaking in CPBSzI to yield a final protein concentration consistent with 1% plasma solution. Samples were then incubated for 2 h at 37 °C, and rinsed of bulk protein with CPBSzI with 1 ml of fresh buffer on each sample three times. Samples were placed in tubes containing 1 ml of 2% SDS, and the bound 125I was measured using the Cobra II gamma counter. Protein adsorption was calculated from the bound 125I using the specific activities of the protein and the surface area of both sides of the sample, with corrections for background radioactivity and decay.
Samples were stored for 24 h in 2% SDS. They were then removed from the gamma counter tubes, dip-rinsed of bulk proteins and SDS sequentially in fresh CPBSzI three times, and placed in fresh gamma counter tubes. The amount of bound 125I was then measured using the Cobra II gamma counter, and the amount of eluted protein was calculated from the bound 125I using the specific activities of the protein and the surface area of both sides of the sample, with corrections for background radioactivity and decay.
F. Anti-Fg monoclonal antibody binding
The accessibility and reactivity of specific epitopes of the adsorbed Fg to blood platelets was evaluated using three different mouse antifibrinogen monoclonal antibodies: R1 (used at a concentration of 0.17 μg/ml), R2 (used at a concentration of 5 μg/ml), and M1 (used at a concentration of 0.34 μg/ml).
Spin-coated polymers on glass coverslips and SS samples were soaked in PBS for 1 h at room temperature. Samples were then adsorbed with 1% citrated pooled human plasma or 0.03 mg/ml Fg for 2 hs at 37 °C. Epitope exposure was also evaluated on samples that were exposed to proteins for only a few minutes, to evaluate the effect of residence time on epitope exposure. Samples were then rinsed by pipetting 1 ml of Tris-buffered saline plus 0.001% Tween (TBS-T) three times. Blocking for nonspecific binding on the protein preadsorbed surfaces was achieved by incubating samples with a 2.5% nonfat milk solution in TBS-T overnight at 4 °C. The next morning, samples were rinsed three times with TBS-T, and one of three monoclonal mouse anti-Fg antibodies was used to measure platelet-binding site exposure on the adsorbed Fg.
Upon addition of the primary antibody, samples were incubated for 90 min at 37 °C. After rinsing samples three times as previously described, a 1:3000 dilution of horseradish-peroxidase-conjugated goat antimouse antibody (BioRad, Hercules, CA) was added, and samples were incubated for 90 min at 37 °C. At the end of the incubation, samples were once again rinsed and moved to a fresh plate. Then, 300 μl of freshly prepared 3.3′, 5.5′-tetramethylbenzidine (BioRad, Hercules, CA) substrate solution was added to each sample and incubated for 15 min at room temperature while being shaken on an orbital shaker at 200 rpm. Next, 200 μl was collected from each well and transferred to a fresh 96-well plate, and the absorbance at 650 nm was read using a microplate reader (Molecular Devices Corporation, Union City, CA).
G. Platelet rich plasma preparation and platelet adhesion
Human blood from healthy donors was drawn by venipuncture into vacutainers containing ACD anticoagulant by trained phlebotomists at the University of Washington Medical Center, under a protocol approved by the UW Human Subjects Committee. The blood was transferred into 50 ml polyethylene conical tubes and centrifuged at 250 g for 15 min at room temperature. The platelet rich plasma (PRP) layer was collected, and the number of platelets in an aliquot of PRP was determined using a coulter counter. Average platelet concentrations were 2–4 × 108 platelets/ml, depending on the donor.
Samples were sterilized by soaking in 70% ethanol as previously described. They were preadsorbed with 1% plasma or 0.03 mg/ml Fg for 1 h and then rinsed to remove bulk protein prior to adding 200 μl of PRP to each sample. After a 2-h incubation period at 37 °C, samples were rinsed with sterile PBS to remove unbound or loosely bound platelets. One set of the samples were prepared for scanning electron microscopy (SEM), a second set were prepared for immunocytochemistry, and a third set were used to quantify the number of adherent platelets using a lactate dehydrogenase assay (LDH).6
After rinsing with PBS, samples were moved to fresh wells and 200 μl 1% Triton-X was added to each well. Platelets were lysed for 1 h. Next, 100 μl of the lysate was collected and transferred to a fresh 96-well plate, where 100 μl of LDH substrate (cytotoxicity kit, Roche Applied Science, Indianapolis) was added to each well. Samples were incubated for 30 min, and 50 μl 1 N HCl was added to stop the reaction. Absorbance at 490 nm with a reference wavelength of 650 nm was read using a Vmax optical plate reader. A calibration curve related the number of platelets to absorbance using known numbers of cells and the LDH assay described above. The calibration curve was linear (R2 > 0.95) and reproducible in the range of 0–1 × 106 platelets.
H. Evaluation of platelet morphology
Platelet activation based on cell morphology was evaluated using SEM at the Washington NanoTech User Facility (Seattle, WA), a member of the NSF National Nanotechnology Infrastructure Network. After platelet adhesion experiments as previously described, samples were fixed at room temperature using a 2.5% glutaraldehyde solution for 2 h. They were then rinsed three times with sterile PBS and soaked in fresh PBS overnight at 4 °C. The day before SEM imaging, fixed samples were dehydrated using a sequence of ethanol soaks: 50% ethanol, 70% ethanol, 80% ethanol (10 min each), 95% ethanol (two changes within 10 min), and 100% ethanol (three changes within 15 min). Immediately after completing the ethanol dehydration, samples were critical point dried and stored in a desiccator until ready to image. Samples were sputter coated with gold and SEM-imaged using an accelerating voltage of 5 kV. Three images were randomly selected for each material and protein pretreatment, and the degree of platelet activation was evaluated and categorized as either round, dendritic, spread dendritic, spreading, and fully spread.38
I. Immunocytochemical staining and optical microscopy
Mouse antihuman platelet GP IIIa antibody was paired with a labeled streptavidin biotin alkaline phosphatase (LSAB + System AP) kit to specifically stain bound platelets on surfaces using a protocol modified from Haycox and Ratner.39 After conducting the platelet adhesion assay as previously described, samples were fixed in 1:1 methanol–acetone for 5 min. They were then rinsed 3× with PBS and stored at 4 °C overnight. Subsequent steps for the immunocytochemical staining of bound platelets and platelet-derived material were conducted at room temperature. Immediately before staining, PBS was removed from the samples and replaced with TBS. An equal volume of 1:50 dilution of mouse antihuman platelet GP IIIa antibody in 2% BSA was added to each sample, and incubated 5 min. The antibody was removed, and samples were rinsed 3× by adding 1 ml TBS. Next, biotinylated secondary antibody was added to cover each sample and incubated for 5 min. The samples were then rinsed 3× with TBS, as previously described. Streptavidin-AP was added to cover each sample, and incubated for 5 min. Samples were again rinsed 3× with TBS, and soaked in the last rinse. Once mixed, 100 μl of the substrate-chromogen solution was added to each sample, and samples incubated for 10 min. After the last incubation step, samples were rinsed with distilled water, and cover slipped prior to optical microscopy. Fetal bovine serum (FBS) was included as a negative control. Polymer samples preadsorbed with protein but not exposed to platelets were included to test for nonspecific staining. Platelets adherent onto polystyrene were included as a positive control. Samples were then imaged using a Nikon E800 microscope, and six randomly selected regions were imaged at 10× and 40×.
The images collected at 10× magnification were then analyzed using imagej image processing software. Because platelets and platelet material on the surfaces had been specifically stained red, the degree of surface coverage by adherent platelets was determined by setting a color threshold in imagej. The same process was applied to all images (n = 6 for each material and protein preadsorption treatment). The percent surface coverage was calculated by dividing the selected pixels as a result of setting a color threshold by the total number of pixels in the image and multiplying by 100.
J. Monocyte isolation and adhesion
Monocytes were isolated by magnetic activated cell sorting (MACS®, Miltenyi Biotec, Auburn, CA) from human blood collected from venipuncture by a trained phlebotomist at the University of Washington Medical Center, under a protocol approved by the UW Human Subjects Committee. The following protocol for the depletion of all nonmonocyte cells was adapted from the MACS negative selection protocol provided by Miltenyi Biotec. Heparinized blood (143 USP units of sodium heparin, BD Biosciences), diluted 1:1 in sterile DPBS containing 2 mM EDTA (Invitrogen, Carlsbad, CA) was carefully layered over 15 ml Histopaque 1077 (Sigma-Aldrich, St. Louis, MO) and centrifuged at 400 g for 30 min at 25 °C. The leukocyte-rich buffy coat at the interface was collected and washed twice in Dulbecco's phosphate-buffered saline. The washed cells were resuspended with 10 ml MACS buffer, containing DPBS plus 2 mM EDTA and 0.5% (v/v) FBS (Invitrogen), and counted using a hemocytometer. Nonmonocyte cells in the fraction were depleted using the biotin-conjugated antibody cocktail against CD3, CD7, CD16, CD19, CD56, CD123, and CD235a antibodies, following the manufacturer's instructions. Isolated cells were more than 95% viable, as determined by Trypan blue exclusion, and monocytes typically made up more than 90% of the cells collected, as determined by light microscopy.
Samples were sterilized by soaking in 70% ethanol as previously described. They were preadsorbed with 1% citrated human plasma for 1 h and then rinsed with sterile PBS to remove bulk proteins. Samples were transferred to a fresh 48-well tissue culture plate.
Monocytes were suspended in RPMI-1640 (Invitrogen) supplemented with 25 mM HEPES (Gibco BRL), 1 mM MEM sodium pyruvate (Sigma), 0.1 mM MEM nonessential amino acids (Sigma), and 1% PEN-STREP at a concentration of 1 × 106 cells per ml. Next, 400 μl of the cell suspension was added to each of the preadsorbed samples. Monocytes were allowed to adhere for 2 h at 37 °C and 5% CO2. After adhesion, samples were rinsed by pipetting and removing 400 μl fresh RPMI-1640 at 37 °C into each well three times in order to remove non- or loosely-adherent monocytes.
Adherent monocytes were quantified using a modified lactate dehydrogenase (LDH) method.6 Samples were moved to a fresh 48-well plate. Cells were lysed by adding 200 μl of a 1% Triton-X solution (v/v) for 30 min at room temperature, while shaking on an orbital shaker at 100 rpm. The cell lysate was then mixed with 200 μl of the LDH substrate (cytotoxicity kit, Roche Applied Science, Indianapolis, IN), and allowed to incubate for 15 min at 37 °C. Next, 100 μl of 1 N HCl was added to stop the reaction. Then, 250 μl of the solution was transferred from each well to a fresh 96-well plate, and the absorbance read at 490 nm, reference wavelength of 650 nm (A490–650 value), using a microplate reader (Molecular Devices Corp., Union City, CA). A calibration curve relating number of cells to absorbance measurements was generated by lysing known numbers of cells, and conducting the LDH assay as described above. The calibration curve was linear (R2 > 0.95) and reproducible in the range of 0–2 × 105 cells.
K. Statistical analysis
To determine statistical significance, the data were analyzed using one-way analysis of variance, and the means compared using Bonferroni's posthoc correction with α = 0.05, unless otherwise noted.
III. RESULTS
A. Surface characterization of polymer films
Survey and Hi-Res ESCA spectra (Tables II and III, respectively) for all the polymers largely reflected expectations based on their structures (Fig. 1). The polymer samples did not show Si, a common contaminant, and there were no indications of postsoak delamination. PVDF-HFP samples displayed a 5% increase in ESCA fluorine signal postsoaking (51.4 ± 0.7–54.4 ± 1.4), which was also observed in secondary ion mass spectrometry spectra collected from soaked XIENCE V stents (unpublished). We cannot explain this small increase in fluorine signal. However, the high resolution ESCA spectra of PVDF-HFP remain largely unchanged after soaking, with no peaks other than CH2 and CF2 (note—the Hi-Res scan to 293 eV did not permit observation of the CF3-peak closer 294 eV). PBMA signals displayed a slight increase in C1s signal as a result of soaking, from 79.8 ± 1.6 to 81.9 ± 0.1. However, no major change was observed in PBMA Hi-Res spectra. In particular, there was no significant increase in the C–O (286.5 eV) or O–C=O (289 eV) peaks. The spectra collected for both SIBS-1 and SIBS-2 were found to contain only carbon (C–H) and had a single peak at 285 eV.
Table II.
Compositional ESCA survey scan of unsoaked and soaked polymer samples spin coated onto glass coverslips (data are displayed as mean ± SEM, n = 3).
| Polymer | Unsoaked | Soaked | ||||
|---|---|---|---|---|---|---|
| F1s | C1s | O1s | F1s | C1s | O1s | |
| PVDF-HFP | 51.4 ± 0.7 | 48.7 ± 0.7 | 54.4 ± 1.4 | 45.4 ± 1.3 | 0.7a | |
| PBMA | 79.8 ± 1.6 | 20.2 ± 1.6 | 81.9 ± 0.1 | 18.1 ± 0.1 | ||
| SIBS-1 | 100 | 100 | ||||
| SIBS-2 | 99.3 ± 0.1 | 0.7 ± 0.1 | 99.4 ± 0.2 | 0.7 ± 0.2 | ||
O1s signal found on a single spot.
Table III.
High resolution ESCA scans of soaked and unsoaked polymer samples spin coated onto glass coverslips show no change or degradation as a result of soaking in purified water.
| Polymer | Peak ID | Binding energy | Unsoaked (at. %) | Soaked (at. %) |
|---|---|---|---|---|
| PVDF-HFP | CH2 | 286 | 49.11 | 48.95 |
| CF2 | 290.5 | 50.89 | 51.05 | |
| PBMA | C–C/C–H | 285 | 69.13 | 68.58 |
| C–O | 286.4 | 17.99 | 18.47 | |
| O–C=O | 288.7 | 12.87 | 12.95 | |
| SIBS-1 | C–C/C–H | 285 | 100 | 100 |
| SIBS-2 | C–C/C–H | 285 | 100 | 100 |
Fig. 1.
Polymer structures for (a) PVDF-HFP, (b) poly(n-butyl methacrylate) (PBMA), and (c) polystyrene-b-polyisobutylene-b-polystyrene (SIBS1 and SIBS2).
B. Protein adsorption
1. Protein adsorption from single protein solutions
Protein adsorption to the various polymer samples and SS was measured from pure Alb and Fg solutions in CPBSzI (0.3 and 0.03 mg/ml, respectively). The concentrations used for the studies were chosen to correspond with the protein concentrations in 1% plasma solutions. Alb adsorption (Fig. 2) was greatest to SIBS1, and decreased in the order of SIBS1 > SIBS2 > PBMA > PVDF-HFP > SS. PVDF-HFP adsorbed a statistically lower amount of Alb as compared to the other polymer surfaces evaluated (α = 0.05). After elution with SDS, a statistically greater amount of retained Alb was observed on PVDF-HFP samples as compared to all other materials tested (α = 0.05), and SS samples showed the weakest retention of Alb.
Fig. 2.
Two-hour albumin adsorption from a pure Alb solution (0.3 mg/ml) in CPBSzI (black) and the retained Alb on the surfaces after a 24-h elution with 2% SDS (white). Data are expressed as mean ± SEM (n = 4). Single asterisks denote statistically significant differences in the amount of adsorbed Alb on to PVDF-HFP as compared to all other materials (α = 0.05). Double asterisks denote a significantly higher amount of retained Alb on PVDF-HFP as compared to all other materials studied (α = 0.05).
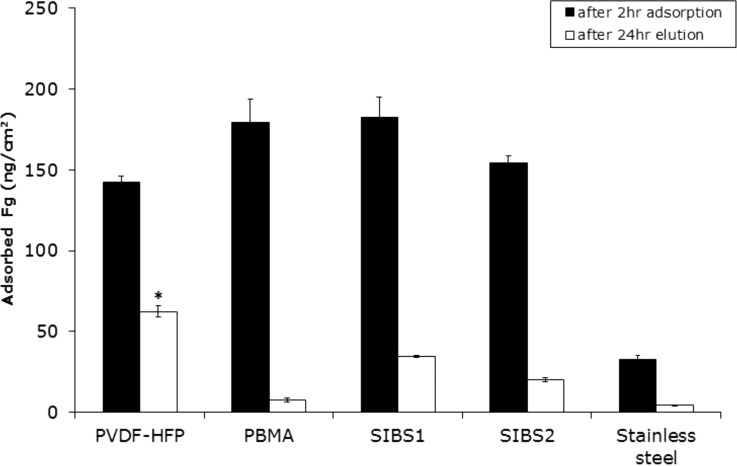
The trend for 2 h Fg adsorption from a pure protein solution (Fig. 3) resembled that observed with Alb, though the overall amounts of adsorbed Fg largely exceeded the adsorbed Alb on all the surfaces. For instance, while 85 ng/cm2 of Alb adsorbed onto PVDF-HFP, 142 ng/cm2 Fg adsorbed. Although the amount of Fg adsorbed onto PVDF-HFP after 2 h was the lowest of any of the polymer surfaces tested, the difference in Fg adsorption between the different polymers was not statistically significant. The trend observed for retained Fg was similar to that observed with retained Alb, with a greater amount of Fg retained on to PVDF-HFP than others material tested.
Fig. 3.
Two-hour fibrinogen adsorption from a pure Fg solution (0.03 mg/ml) in CPBSzI (black) and the retained Fg on the surfaces after a 24-hour elution with 2% SDS (white). Data are expressed as mean ± SEM (n = 4). The asterisk denotes the significantly greater amount of retained Fg on PVDF-HFP as compared to all other materials tested (α = 0.05).
2. Competitive protein adsorption from binary solutions
Next, we measured Alb and Fg adsorption from binary solutions generated using the proteins in the ratios observed in normal human blood plasma (Fig. 4). Two identical Alb/Fg solutions were made in PBS, adding a radiolabeled Alb tag to one solution and a radiolabeled Fg tag to the other.
Fig. 4.
Competitive protein adsorption from binary solutions (0.3 mg/ml Alb and 0.03 mg/ml Fg). Two hour protein adsorption data are represented by black bars or red bars, while 24 h elution data are represented by the white bars or pink bars. Data are presented as mean ± SEM (n = 4). There is a statistically significant higher amount of retained Alb on PVDF-HFP as compared to all other materials. There is a statistically significant higher of adsorbed Fg on SIBS-1 and SIBS-2 as compared to PVDF-HFP. There is a statistically significant higher amount of retained Fg on PVDF-HFP as compared to all other materials tested (α = 0.05 for all).
Albumin adsorption from an Alb-Fg binary solution was highest on PBMA and decreased in the order of PBMA >SIBS2 > SIBS1 > PVDF-HFP > stainless steel. The amount of retained Alb after elution with SDS followed the same trend observed with retained protein from pure protein solutions: the highest amount of retained Alb was observed on PVDF-HFP, while the lowest amount of retained protein was on SS
In the case of Fg adsorption from an Alb-Fg solution (Fig. 4), protein adsorption was highest on SIBS2 and decreased in the order of SIBS2 > SIBS1 > PBMA > PVDF-HFP > SS. The amount of retained Fg after SDS elution was once again greatest on PVDF-HFP and decreased in the order of PVDF-HFP > SIBS1 ≥ SIBS2 > stainless steel > PBMA.
In both cases, the percent of originally adsorbed protein retained after a 24-h elution in 2% SDS was numerically larger on PVDF-HFP than on other surfaces tested (Table IV). After elution, 22% of adsorbed Alb was retained on PVDF-HFP, while less than 5% was retained on all of the other surfaces. Similarly, 49% of adsorbed Fg was retained on PVDF-HFP, while less than 10% was retained on all other surfaces.
Table IV.
Percent of originally adsorbed protein retained on surfaces after 24-h elution with 2% SDS.
| Material | Albumin (%) | Fibrinogen (%) |
|---|---|---|
| PVDF-HFP | 22 | 49 |
| PBMA | 5 | 1 |
| SIBS1 | 4 | 10 |
| SIBS2 | 3 | 7 |
| Stainless steel | 2 | 8 |
3. Protein adsorption from normal human plasma
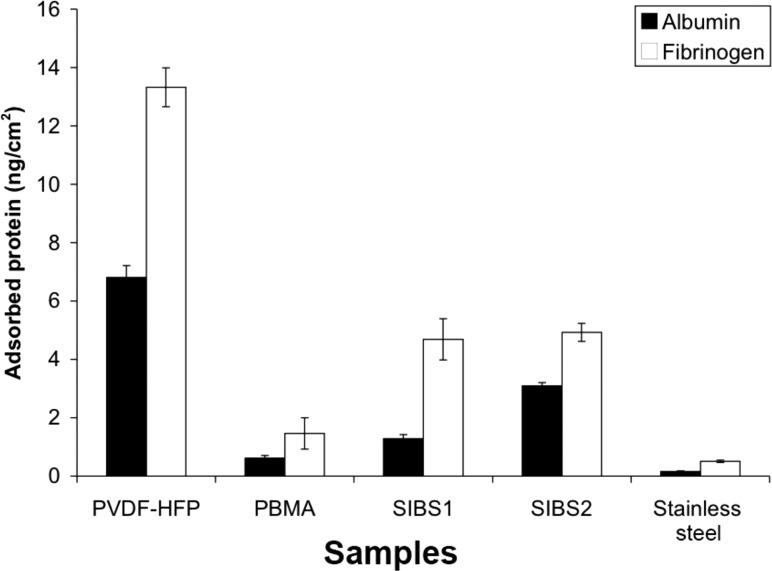
Alb and Fb adsorption from 1% citrated pooled human plasma was also measured after 2 h (Fig. 5) to simulate the complex protein solutions these surfaces might encounter in vivo. As expected, the amount of adsorbed Alb and Fg from plasma was much lower than that observed from pure protein solutions for all the surfaces and proteins tested. PVDF-HFP had higher Alb adsorption than Fg, while SIBS2 adsorbed a slightly higher amount of Fg than Alb. After elution with 2% SDS (Fig. 6), PVDF-HFP retained a larger amount of both Alb and Fg than any other material tested, consistent with that observed in single protein and binary protein adsorption experiments. As in the single protein and binary protein adsorption experiments, Fg was retained to a greater degree than Alb on all surfaces, with 13.3 ± 0.7 ng/cm2 Fg and 6.8 ± 0.4 ng/cm2 Alb retained on PVDF-HFP. SIBS1 and SIBS2 retained similar amounts of Fg, but SIBS2 retained a higher amount of Alb than SIBS1. Of the polymers tested, PBMA retained the lowest amount of all proteins in the study, though SS retained the least adsorbed protein overall.
Fig. 5.
Two hour protein adsorption from 1% citrated normal human plasma. Alb adsorption is shown in black and Fg adsorption is shown in white. Data are displayed as mean ± SEM (n = 4). The single asterisk denotes statistically significant differences in Alb adsorption as compared to PVDF-HFP. Double asterisks denote statistically significant Fg adsorption as compared to PVDF-HFP (α = 0.05 for both).
Fig. 6.
Amount of retained protein from 1% citrated human plasma on the surface of the various materials after a 24-h elution with 2% SDS. Adsorbed Alb is shown in black and Fg is shown in white. Data are displayed as mean ± SEM (n = 4). Statistically significant differences in the amount of retained Alb and Fg, respectively, on PVDF-HFP as compared to all the other materials tested were noted (α = 0.05 for both).
High Alb/Fg is thought to be an indication of a surface's low thrombogenicity.8 PVDF-HFP was initially found to have the highest Alb/Fg ratio, 1.6, of all the surfaces tested (Table V). The Alb/Fg ratio of other surfaces ranged from 0.8 for SS to 1.2 for SIBS2. Upon elution, the Alb/Fg ratio was reduced for all materials tested, and ranged from 0.3 for SS and SIBS1 to 0.6 for SIBS2. PVDF-HFP had a postelution Alb/Fg ratio of 0.5.
Table V.
Alb/Fg ratio after 2 h adsorption from 1% normal human citrated plasma and 24-h elution in 2% SDS.
| Material | 2 h adsorption | 24-h elution |
|---|---|---|
| PVDF-HFP | 1.6 | 0.5 |
| PBMA | 1.1 | 0.4 |
| SIBS1 | 1.2 | 0.3 |
| SIBS2 | 0.9 | 0.6 |
| Stainless steel | 0.8 | 0.3 |
C. Anti-Fg monoclonal antibody binding
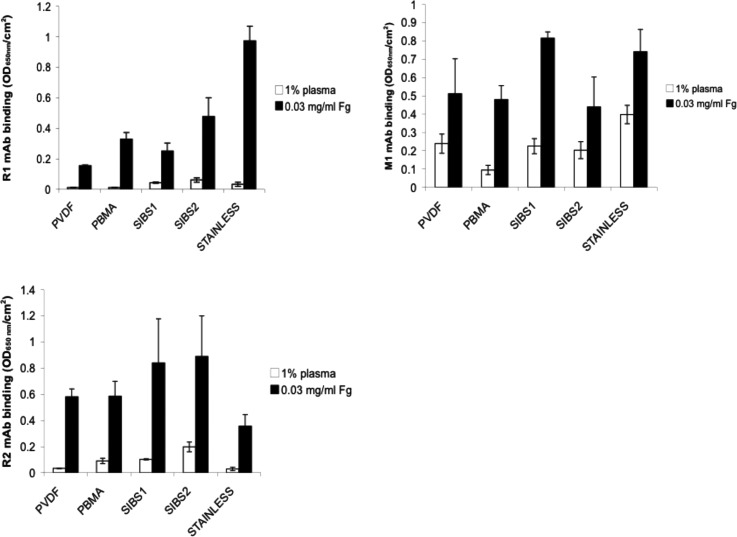
While the amount of Fg on a surface is important, the orientation and conformation of adsorbed protein on the surface may be a better indicator of thrombogenicity.18,40,41 We chose three monoclonal antibodies specific to identified platelet binding sites on the Fg molecule,42,43 R1, R2, and M1, to evaluate epitope accessibility to platelets on adsorbed Fg from pure Fg solutions and 1% plasma19 (Fig. 7). R1 and R2 bind specifically to regions in the alpha chain of the Fg molecule, which include RGD binding sites, and have been found to play a secondary, supportive role in platelet adhesion.19 M1 binds specifically to a dodecapeptide region in the gamma chain of Fg, which has been shown to be primarily responsible for platelet adhesion to surface bound Fg.19
Fig. 7.
Monoclonal antibody binding to samples preadsorbed with 1% pooled normal human citrated plasma (white bars) or 0.03 mg/ml Fg (black bars). The three antibodies used were selected for their specificity to three platelet binding sites on fibrinogen. R1 (a) and R2 (b) bind to RGD-containing regions on the alpha chain, while M1 (c) binds to the platelet binding dodecapeptide region on the gamma chain. Nonspecific binding was found to be minimal (data not shown). Data are displayed as mean ± SEM (n = 3).
For all antibodies tested, binding was lower on samples preadsorbed with 1% plasma than to the same samples preadsorbed with 0.03 mg/ml pure Fg. R1 binding to samples preadsorbed with 1% plasma was lowest on PVDF-HFP and PBMA. SIBS1, SIBS2, and SS samples had a higher, similar R1 binding. When samples were preadsorbed with pure Fg, R1 binding increased in the order of PVDF-HFP < SIBS1 < PBMA < SIBS2 < SS. R2 binding to samples preadsorbed with 1% plasma was lowest on SS and PVDF-HFP, higher on PBMA and SIBS1, and highest on SIBS2. When samples were preadsorbed with pure Fg, the lowest R2 binding was observed on SS. It was higher and about the same on PBMA and PVDF-HFP, and highest on SIBS1 and SIBS2. Finally, M1 binding to 1% plasma preadsorbed samples was lowest on PBMA, higher and approximately the same on PVDF-HFP, SIBS1, and SIBS2, and highest on SS. When samples were preadsorbed with pure Fg, M1 binding was similarly low on PVDF-HFP, PBMA, and SIBS2, and higher and about the same on SIBS1 and SS. Nonspecific binding to these surfaces was tested (results not shown) and found to be negligible. Thus, the results reflect the specific binding of the given antibodies to the binding sites on Fg, exposed as a result of protein conformational changes upon adsorption to the surfaces.
D. Platelet adhesion
Static platelet adhesion from PRP was measured on samples preadsorbed with either 1% citrated normal human plasma (Fig. 8) or 0.03 mg/ml Fg (Fig. 9) using the lactate dehydrogenase (LDH) assay.6 When samples were preadsorbed with 1% plasma, the number of adherent platelets was relatively low, and decreased in the order of SIBS1 > SS > PBMA >SIBS2 > PVDF-HFP, though the differences observed were not statistically significant at an α = 0.05 or 0.1 level. When samples were preadsorbed with pure Fg, however, the number of adherent platelets was significantly higher than that observed on samples preadsorbed with plasma.
Fig. 8.
Platelet adhesion to samples preadsorbed with 1% normal human citrated plasma measured using LDH assay. Data are displayed as mean ± SEM (n = 4).
Fig. 9.
Platelet adhesion on samples preadsorbed with pure 0.03 mg/ml pure Fg solution measured using LDH assay. Data are displayed as mean ± SEM (n = 4).
For example, the greatest number of adherent platelets as measured by the LDH assay, on samples preadsorbed with plasma, was on SIBS1, 10 000 platelets/cm2, while the same material preadsorbed with pure Fg had 28 500 adherent platelets/cm2. This is likely due to the reduced amount of Fg on surfaces preadsorbed with plasma as compared to the same surfaces preadsorbed from pure Fg solution. Platelet adhesion on surfaces preadsorbed with pure Fg decreased in the order of stainless steel > SIBS2 > SIBS1 > PBMA > PVDF-HFP. The differences observed, however, were not statistically significant at an α = 0.05 or 0.1 level.
E. Evaluation of platelet morphology
Although all samples displayed nonuniform platelet coverage, SEM analysis showed a marked difference in platelet morphology on the various substrates. Trends in total platelet adhesion and general platelet morphology were observed based on protein pretreatment of the samples. All samples preadsorbed with 0.03 mg/ml pure Fg displayed a large number of flattened, fully spread platelets. Preadsorption of the samples with 1% plasma resulted in a significant decrease in the number of flattened platelets on all samples. Samples that had not been preadsorbed with protein prior to platelet adhesion from PRP had a lower number of flattened platelets than samples preadsorbed with pure Fg, though the number of flattened platelets was still too high to count on PVDF-HFP.
Platelet morphology distributions are presented in Table VI. While these counts provide insight to the general level of platelet activation based on morphology, the high variability in platelet adhesion on a given sample is noted. To eliminate bias in the selection of images used for cell counts, the images used, all collected at 2000× magnification, were randomly selected. All samples, regardless of protein pretreatment, had few round platelets. The relative distribution (expressed as percentage of total platelets) of round, dendritic, spread dendritic, and spreading samples were observed to be similar on all samples. However, due to the extremely large number of flattened adherent platelets on all of the Fg preadsorbed polymers, counting the individual flattened platelets was not possible. Thus, the percent platelets assigned to each activation category does not take into account the flattened platelets, and is likely overestimated in these numbers.
Table VI.
Percent of cells in each activation state as determined from visual examination using SEM (n = 3 per material and protein preadsorption).
| Protein pretreatment | Material | % Round | % Dendritic | % Spread dendritic | % Spreading | % Fully spread | |
|---|---|---|---|---|---|---|---|
| 0.03 mg/ml pure Fg | PBMA | Average | 0.85 | 21.55 | 47.26 | 30.33 | a |
| Std error | 0.44 | 2.16 | 9.19 | 7.46 | |||
| PVDF-HFP | Average | 2.90 | 22.14 | 46.95 | 28.02 | a | |
| Std error | 2.90 | 5.62 | 6.99 | 5.31 | |||
| SIBS1 | Average | 0.00 | 22.71 | 56.41 | 20.87 | a | |
| Std error | 0.00 | 4.14 | 0.43 | 4.57 | |||
| SIBS2 | Average | 0.41 | 27.03 | 48.02 | 24.55 | a | |
| Std error | 0.41 | 2.64 | 0.76 | 1.47 | |||
| 1% plasma | PBMA | Average | 0.00 | 37.44 | 38.83 | 20.80 | 2.94 |
| Std error | 0.00 | 7.43 | 4.05 | 3.01 | 1.57 | ||
| PVDF-HFP | Average | 2.38 | 37.55 | 34.25 | 16.12 | 9.71 | |
| Std error | 2.38 | 13.74 | 8.61 | 1.83 | 4.58 | ||
| SIBS1 | Average | 1.33 | 21.79 | 11.52 | 11.03 | 54.32 | |
| Std error | 1.33 | 7.35 | 3.80 | 2.02 | 9.28 | ||
| SIBS2 | Average | 4.76 | 19.05 | 19.05 | 23.81 | 33.33 | |
| Std error | 1.59 | 22.11 | 12.51 | 21.95 | 41.85 | ||
| No protein preadsorption | PBMA | Average | 0.00 | 39.08 | 22.69 | 20.56 | 17.67 |
| Std error | 0.00 | 2.38 | 6.33 | 2.76 | 5.28 | ||
| PVDF-HFP | Average | 0.00 | 17.82 | 5.75 | 6.32 | a | |
| Std error | 0.00 | 17.82 | 5.75 | 6.32 | |||
| SIBS1 | Average | 0.00 | 30.02 | 22.32 | 23.29 | 24.37 | |
| Std error | 0.00 | 2.48 | 1.20 | 3.81 | 3.29 | ||
| SIBS2 | Average | b | b | b | b | b | |
| Std error | b | b | b | b | b |
Dense surface coverage, making it difficult to count individual cells.
Images that were too poor in quality to determine individual platelet activation state.
When samples were preadsorbed with 1% plasma (Fig. 10), a larger percentage of platelets adherent on PBMA and PVDF-HFP were dendritic or spread dendritic than spreading or fully spread. Conversely, a larger percentage of adherent platelets on plasma preadsorbed SIBS1 and SIBS2 were spreading, or fully spread. When samples were not preadsorbed with protein, however, a large number of fully spread platelets were observed on PVDF-HFP samples confounding accurate counts of fully spread platelets. This was not observed on the other materials, though a small percentage of platelets were spread on SIBS1 and PBMA (24% and 17%, respectively). We had difficulty collecting clear images of the SIBS2 samples, which made the flattened platelets difficult to identify. Thus, these surfaces were excluded from analysis.
Fig. 10.
Scanning electron micrographs (2000× magnification) of adherent platelets onto samples preadsorbed with 1% plasma prior to exposing to PRP for 2 h.
F. Immunocytochemical staining and optical microscopy
Micrographs representative of platelet coverage as measured by staining for cell surface GP IIIa receptors are presented in Fig. 11. Stained samples showed uneven platelet coverage (or uneven immunoreactivity) on all samples regardless of protein pretreatment, with certain regions displaying dense platelet adhesion and other regions displaying little to no platelet material present. This accounts for the high standard error observed in the percent coverage data presented in Table VII, as determined through the use of image processing software. The percent surface platelet coverage on samples preadsorbed with 1% normal human plasma was lower than on the same samples preadsorbed with 0.03 mg/ml pure Fg (except for SIBS-2). The percent coverage on samples without a protein preadsorption step was higher than that for plasma preadsorbed samples, with the values closely resembling those observed for Fg preadsorbed samples, except for SIBS2. Because protein adsorption occurs almost immediately upon exposing a biomaterial to a protein solution, the amount and type of protein adsorbed onto the various materials from the PRP was expected to largely resemble that adsorbed from 1% plasma. Thus, it was surprising to find large differences in percent surface coverage of plasma preadsorbed samples compared to samples without a protein preadsorption step.
Fig. 11.
Optical micrographs (40× magnification) of immunostained adherent platelets on PVDF-HFP, PBMA, SIBS1, and SIBS2 on 1% plasma preadsorbed surfaces.
Table VII.
Percent of surface covered by adherent platelets or platelet membrane-like material after a 2-h incubation with PRP (n = 6 for each material and protein pretreatment).
| Protein pretreatment | Material | % Coverage | Standard error |
|---|---|---|---|
| 0.03 mg/ml fibrinogen | PBMA | 19.05 | 6.09 |
| PVDF-HFP | 47.15 | 7.25 | |
| SIBS1 | 40.06 | 8.96 | |
| SIBS2 | 20.61 | 2.90 | |
| 1% plasma | PBMA | 14.93 | 3.79 |
| PVDF-HFP | 29.17 | 7.07 | |
| SIBS1 | 25.28 | 6.84 | |
| SIBS2 | 30.27 | 5.06 | |
| No protein preadsorption | PBMA | 18.57 | 5.07 |
| PVDF-HFP | 54.94 | 4.13 | |
| SIBS1 | 40.00 | 6.38 | |
| SIBS2 | 41.41 | 6.62 |
PBMA had the lowest percent surface coverage of spread platelets of all the polymers tested, regardless of protein pretreatment. Conversely, PVDF-HFP had the highest percent surface coverage for all protein treatments, except for plasma preadsorption, where the surface coverage of SIBS2 was approximately the same as that of PVDF-HFP. Percent surface coverage on SIBS1 followed the same trends observed on PVDF-HFP and PBMA samples, with a higher percentage of the surface being covered by platelets or platelet material on SIBS1 samples preadsorbed with pure Fg or not preadsorbed at all, than on samples preadsorbed with 1% plasma. The overall percent coverage values for SIBS1, however, were lower than PVDF-HFP and higher than PBMA. SIBS2 samples did not follow these same trends, however. Platelet and platelet material coverage on these surfaces varied largely based on protein preadsorption treatment, with coverage increasing in the order of pure Fg < 1% plasma < no protein preadsorption.
G. Monocyte adhesion
Monocyte adhesion was highest on SS, and decreased in the order stainless steel > SIBS2 > SIBS1 > PBMA = PVDF-HFP (Fig. 12). However, the differences in monocyte adhesion to the different surfaces were not deemed to be statistically significant at the α = 0.05, and only the differences observed between PVDF-HFP and stainless steel and PBMA and stainless steel were deemed statistically significant at the α = 0.10 level.
Fig. 12.
Monocyte adhesion on samples preadsorbed with 1% plasma measured using LDH assay. Data are displayed as mean ± SEM (n = 4). Asterisks denote statistical significance as compared to stainless steel (α = 0.10).
IV. DISCUSSION
While DES have reduced in-stent restenosis rates to lower than 10%,1 stent thrombosis issues have highlighted the importance of assessing the various contributing factors, including stent and balloon catheter design, coating application technologies, and blood compatibility of the materials used. This study focuses specifically on biomaterials blood compatibility. There has been much controversy surrounding the methods for measuring blood compatibility.28,44–46 Generally, studies of platelet interaction, activation of the intrinsic clotting system, and measurement of adsorbed proteins (the precursor event to platelet attachment or clotting) dominate efforts to rank biomaterials as nonthrombogenic or thrombogenic. Since activation of the intrinsic clotting system is associated with clotting in low blood flow or no-flow environments,7 this work focuses on platelets and protein adsorption events preceding the arrival of platelets to the surface. The high wall shear rate coronary arterial environment in which DES are implanted (approximately 400 s−1) justifies the focus on platelets rather than the intrinsic clotting system.
Relating protein adsorption events to their impact on surface-induced blood coagulation requires tests that address two issues. First, the type and amount of adsorbed protein must be evaluated. Second, the conformation of the adsorbed protein must be assessed, to determine whether cell interaction sites become exposed as a result of surface-induced changes in the protein.
In this paper, we have studied the blood compatibility of four polymers used in drug eluting stents (PVDF-HFP, PBMA, SIBS1, and SIBS2) and SS. As our focus was on the material–blood interactions, we simplified our model by spin casting the polymers (without drug) onto flat cover slips. This approach eliminated geometry effects and drug effects, simplifying our understanding of the thrombogenicity of the biomaterials. As stated above, platelet reaction with surfaces occurs in vivo in high wall shear rate regimes. The high flow, particularly in the presence of red cells, serves to transport platelets to the surface. In vitro, under no-flow conditions, this transport can be simulated by permitting sufficient time to allow platelets to gravitationally sediment to the surface where they then can react.
Fg has been shown to play a key role in platelet adhesion and clotting,9,10 and even low levels of adsorbed Fg support platelet adhesion.6 Albumin, on the other hand, has historically been used as a passivating protein, and Alb-platelet binding sites have not been identified, though Alb may support platelet adhesion to some self-assembled monolayers.47 Nevertheless, Alb is thought to be a passivating protein, and it has been hypothesized that a higher Alb/Fg ratio is desirable for less thrombogenic surfaces.8 Adsorption measurements from binary solutions and from blood plasma measure the relative affinity of a material for a specific protein competing with one or more proteins.
When protein adsorption was evaluated from single protein solutions using concentrations equivalent to that in 1% plasma, 0.3 mg/ml Alb, and 0.03 mg/ml Fg (Figs. 2 and 3), SS had the lowest adsorbed Fg and Alb. Of the polymers, PVDF-HFP had the lowest Alb and Fg adsorption, but also had the highest retention of both proteins after 2% SDS elution. These observations of strong retention of proteins are consistent with reports by others with fluorinated polymers.21,48,49 When adsorption from binary Alb/Fg solutions was evaluated (Fig. 4), Alb adsorption to all polymers was 90–110 ng/cm2, with adsorption to PVDF-HFP being the lowest. Fg adsorption from the binary solutions was also lowest on PVDF-HFP, as compared to the other polymers. SS adsorbed the lowest amount of both Alb and Fg, and retained the lowest level of protein as well. The percentage of retained Alb after 24-h elution with 2% SDS (Table IV) was highest on PVDF-HFP (22%), with no more than 5% of the originally adsorbed Alb retained on any of the other surfaces. Like Alb, Fg was also retained to a greater extent on PVDF-HFP, 49%. Retention of Fg was generally higher than Alb retention on all surfaces, though that is not the case for PBMA, which retained 5% of the originally adsorbed Alb but only 1% of the adsorbed Fg.
While adsorption studies using simple protein solutions offer insights into the affinity of individual proteins for a given surface, adsorption studies using complex protein solutions provide a more realistic picture of what might occur at biomaterial surfaces upon implantation (Figs. 5 and 6). Thus, we used 1% pooled normal citrated human plasma to evaluate both Alb and Fg adsorption in a competitive environment. As expected, the total amount of adsorbed Alb and Fg was lower when 1% plasma was used for the adsorption. Nevertheless, results largely followed those observed with the binary protein solutions, despite the fact that plasma is a complex solution containing hundreds of different proteins. The adsorption values suggest that Alb and Fg make up the largest fraction of proteins on these surfaces. No attempt was made to identify other proteins adsorbed from plasma on the various surfaces, as we were primarily interested in Alb and Fg. As was previously observed, Alb and Fg adsorption to PVDF-HFP was relatively low, with a greater amount of Alb than Fg being adsorbed. After the 2-h adsorption time, the Alb/Fg ratio (Table V) on PVDF-HFP was found to be the highest at 1.6, though upon elution this value dropped to 0.5. SIBS2 displayed the highest Alb/Fg adsorption ratio postelution, 0.6, with the remaining samples' Alb/Fg ratio increasing from 0.3 to 0.5, in the order of SS = SIBS1 < PBMA < PVDF-HFP. PVDF-HFP's high Alb/Fg ratio after initial adsorption suggests a lower thrombogenic potential for the surface. The change in the ratio postelution will be discussed shortly.
Adsorbed fibrinogen has been strongly implicated in platelet adhesion to surfaces. However, the conformation of the protein on the surface should ultimately dictate its reactivity to platelets since recognition epitopes may be sterically inaccessible or denatured. Thus, we evaluated the accessibility of known platelet binding sites on Fg as a result of protein adsorption onto surfaces studied here. Samples preadsorbed with pure Fg and from 1% plasma were included. The three antibodies selected for this study, R1, R2, and M1, have been previously used to evaluate the platelet binding activity of adsorbed Fg on surfaces,6,17,19 and a correlation between M1 binding and platelet adhesion was established.19 As expected, a lower antibody binding was observed for all samples and all antibodies when the samples were preadsorbed with Fg from 1% plasma solutions. This is likely due to two factors. First, there is a lower amount of adsorbed Fg on the surfaces as a result of competition with other proteins during the adsorption process. Second, the specific composition and density of the adsorbed protein layer from a 1% plasma solution may affect the accessibility of platelet binding sites.
R1 and R2 are specific to RGD binding sites and play a secondary, supportive role in platelet adhesion to Fg.19 M1 binding, on the other hand, is of great interest, as it is the dodecapeptide that M1 binds to that is believed to be responsible for most platelet adhesion to adsorbed Fg through interactions with the GP IIb IIIa receptor on platelets. The low R1 and R2 binding on plasma preadsorbed PVDF-HFP is notable. The PVDF-HFP's relatively higher binding of M1 is also of interest. Taken together, these monoclonal binding studies suggest that the Fg adsorbed on PVDF-HFP is in a lower platelet-binding state than Fg adsorbed on SIBS1 and SIBS2. Interestingly, SS showed high R1 and M1 accessibility, but low R2 exposure. To better relate these data to blood compatibility, platelet adhesion and activation must be evaluated.
Platelet adhesion from platelet rich plasma onto plasma or pure Fg preadsorbed samples was therefore measured using the LDH assay (Figs. 8 and 9). After allowing the platelets to adhere for 2 h, samples were rinsed to remove loosely adherent cells, and then adherent platelets were lysed to release their LDH. Thus, by measuring the amount of released LDH and then relating it back to LDH in a known number of cells, we can quantify adherent cells on our surfaces. Using this method, the results suggested that platelet adhesion to PVDF-HFP preadsorbed with both 1% plasma or pure Fg was lower than to other materials assessed here. As with protein adsorption, platelet adhesion numbers were found to be lower on samples preadsorbed with plasma than with Fg, presumably due to a lower amount of Fg present on the surfaces preadsorbed with plasma as well as a lower number of exposed platelet binding sites. However, it is important to note that adhesion trends did not follow the trends observed for total Fg adsorption or elution. In fact, stainless steel, which had the lowest amount of protein under all conditions tested had the highest number of adherent platelets. PVDF-HFP, on the other hand, retained the largest amount of Fg, but had the fewest number of visually countable adherent platelets. These observations support the idea that it is not the total amount of adsorbed protein driving a surface's potential for supporting platelet adhesion, but rather it is the surface protein conformation (accessibility to important binding epitopes) that is important.
We did not observe a correlation between M1 antibody binding and platelet adhesion as suggested in the literature (Fig. 13). When we analyzed the surfaces using SEM (Fig. 10) and immunocytochemistry (Fig. 11), however, we find that LDH might underestimate the total number of adherent cells on these samples, particularly PVDF-HFP. We found that the density of adherent cells was nonuniformly distributed between different regions of the same sample. We observed that there is extensive spreading of adherent platelets on PVDF-HFP, and significantly less spreading and cell adhesion on PBMA. Upon extensive spreading, it is likely that these cells release their LDH, being washed away during the rinsing step of the assay, thus yielding low LDH platelet counts.
Fig. 13.
No correlation was found between number of adherent platelets and M1 monoclonal antibody binding to surfaces preadsorbed with (a) plasma or (b) fibrinogen. Data are displayed as mean ± SEM (n = 3).
It has been suggested that certain polymers induce initial platelet binding and flattening—that highly spread platelets later act as a passivating layer on the material surface, thus inhibiting additional platelet activation.39 Since highly spread, adherent platelets are difficult to quantify (see images in Fig. 10), platelet counts by SEM may not be a reliable method to assess numbers of adherent platelets. In addition LDH assays, too, raise concerns (see previous paragraph). These complications continue to justify studies on the precipitating events leading to thrombotic complications. Thus, protein adsorption, occurring before platelets ever reach the surface, remains a viable area for study. In this regard, consider the data in Fig. 6 obtained with citrated plasma, a relevant medium for studying such adsorption. Based upon the hypothesis that albumin enrichment at surfaces is desirable for reducing platelet reaction, Fig. 6 shows the highest level of retained albumin on PVDF-HFP. However, retained fibrinogen is also highest on PVDF-HFP. The tight binding of proteins consistently noted with PVDF-HFP may equate to extreme surface denaturation. Albumin, without binding sites known to interact with platelets, should not be much affected by such conformational loss. On the other hand, fibrinogen would lose the conformationally displayed specific reactive sites for platelet interactions from strong surface binding and subsequent chain unfolding. Thus, fibrinogen, too, under these conditions may become nonreactive and passivate the surface. Such loss of protein recognizability by severe surface denaturation may also account for the difficulty in correlating platelet events with antibody binding (Fig. 13).
Additional studies will be required to address the unique protein adsorption behavior of PVDF-HFP and how that relates to the clinical observation of satisfactory fluoropolymer performance.30,50–52 In fact, fluoropolymers have long been a preferred group of materials for blood contact applications.53–55 Furthermore, PVDF-HFP has favorable mechanical properties to perform as a durable coating for substrates that are subject to deformation (as are stents).56 In large clinical studies, PVDF-HFP coated stents have demonstrated lower levels of acute and late state thrombosis30 compared to other stents, although release of antiproliferative agents, variability in stent design, and coating processing technologies used complicate simple conclusions about the contribution of the biomaterials themselves.
Monocyte adhesion was measured (Fig. 12) because inflammation has been often implicated in thrombosis. The low monocyte adhesion to the PVDF-HFP is consistent with this idea, though statistically with a sample size of n = 4, the level of uncertainty is too high to draw strong conclusions about this potential marker of stent reactivity.
V. CONCLUSIONS
Our results highlight important points regarding the assessment of the blood compatibility of stent coating materials. The in vivo phenomena observe in the evaluation of blood compatibility are complex and involve both biological and physical factors. Thus, it is important to develop well-controlled test systems that separate effects such as stent placement, geometry, flow, and patient-to-patient variability from biological processes such as protein adsorption, protein conformation, and platelet adhesion and activation. It is also equally important to understand and acknowledge the limitations of in vitro tests and the concerns with decoupling parameters that are, in fact, convoluted. The surface-induced thrombus activation process is sufficiently complex that observation of a single parameter (for example, platelet adhesion) is never satisfactory.
The specific blood compatibility assessment parameters to be measured that correlate with clinical performance have yet to be identified. However, our work highlights differences in the biological reactivity of stent polymers. PVDF-HFP retained albumin more than other polymers and in some cases showed higher levels of albumin adsorption. This observation of Alb and Fg protein deposition and retention on PVDF-HFP polymer (used on the XIENCE V drug eluting stent) is consistent with the albumin/fibrinogen ratio hypotheses proposed first proposed in the 1970s (Ref. 8) and reinforced by studies in the 1980s.57,58
In this study, we have seen consistently differentiated protein adsorption and platelet interaction behavior compared to other relevant stent coating polymers and stainless steel. To accentuate these differences, see Table VIII that directly compares all materials and assays from this study. These differences suggest new hypotheses as to protein and surface factors important for blood compatibility. Further, these hypotheses may help the understanding of the long unexplained blood compatibility of fluoropolymers that has led to its use for cardiovascular implants such as vascular grafts. It is possible that high albumin retention may passivate surfaces against acute thromboembolic events in two ways: (1) by presenting a surface with few specific binding sites to the blood, (2) by cooperatively influencing the conformation of relevant epitopes on the culprit proteins such as fibrinogen, VwF, etc. The same acute protein interaction phenomena may also modulate the long-term clinical safety performance by interaction of proteins with endothelial cell, inflammatory cells, and smooth muscle cells.
Table VIII.
In most cases, the PVDF-HFP polymer exhibited significantly different behavior from other polymers tested (bold type numbers highlight results substantially different compared to other materials).
| PVDF-HFP | PBMA | SIBS1 | SIBS2 | Stainless steel | |
|---|---|---|---|---|---|
| Albumin adsorption from a pure Alb solution, adsorbed/retained | 3.0 | 73 | 17 | 27 | 20 |
| Fibrinogen adsorption from a pure Fg solution, adsorbed/retained | 2.3 | 24 | 5.3 | 7.7 | 7.9 |
| Competitive protein adsorption from binary solutions (0.3 mg/ml Alb and 0.03 mg/ml Fg), Alb vs Fg, adsorbed/retained | 4.6 | 21 | 23 | 38 | 65 |
| Competitive protein adsorption from binary solutions (0.3 mg/ml Alb and 0.03 mg/ml Fg), Fg vs Alb, adsorbed/retained | 2.1 | 82 | 11 | 14 | 12 |
| Two hour protein adsorption from 1% citrated normal human plasma Alb/Fg | 1.6 | 1.1 | 1.2 | 0.9 | 0.8 |
| retained surface protein of the various materials after a 24-hour elution with 2% SDS, Alb/Fg | 0.5 | 0.5 | 0.3 | 0.7 | 0.3 |
| total amount of retained Alb (ng/cm2) | 7.0 | 0.6 | 1.3 | 3.1 | 0.2 |
| Monocyte adhesion (cells/cm2) | 2450 ± 60 | 2350 ± 50 | 5650 ± 1590 | 7950 ± 2060 | 13670 ± 1100 |
| Platelet adhesion (LDH) 1% plasma preadsorbed | 6000 ± 820 | 8000 ± 820 | 10000 ± 2450 | 7500 ± 500 | 9500 ± 4200 |
| 0.03 mg/ml Fg preadsorbed | 9300 ± 960 | 20170 ( 11420 | 28500 ( 6860 | 29330 ( 9570 | 78780 ( 61230 |
Multiple assays to examine protein adsorption, protein reactivity, and platelet interactions are necessary to get a full picture of the blood reaction to materials. The meaning and significance of each individual test must also be considered. For example, the discrepancy observed between the LDH data and immunostaining for platelet membrane fragments emphasized that the two assays for platelet interaction give strikingly different views of the platelet behavior. The observation of a surface coated with highly spread platelet membranes, where most of the LDH had been released immediately after surface contact, has been seen before in a hydrophobic C18-rich surface.35 Thus, such hydrophobic surfaces as the PVDF-HFP and the C18-rich surface may, after a short, initial period of high reactivity become passivated and exhibit low platelet reactivity. Further studies on this passivation phenomenon will be undertaken.
ACKNOWLEDGMENTS
Funding for this study was provided by Abbott Vascular, Inc., and by the UWEB21 Engineering Research Center. Surface analysis was performed in the NESAC/BIO center, and NIBIB Research Resource.
References
- 1. Luscher T. F., Steffel J., Eberli F. R., Joner M., Nakazawa G., Tanner F. C., and Virmani R., Circulation 115, 1051 (2007). 10.1161/CIRCULATIONAHA.106.675934 [DOI] [PubMed] [Google Scholar]
- 2. Inoue T. and Node K., Circ. J. 73, 615 (2009). 10.1253/circj.CJ-09-0059 [DOI] [PubMed] [Google Scholar]
- 3. Vroman L., Adams A. L., and Fischer G. C., Adv. Chem. 199, 265 (1982). 10.1021/ba-1982-0199 [DOI] [Google Scholar]
- 4. Slack S. M. and Horbett T. A., Proteins at Interfaces II: Fundamentals and Applications, edited by Brash J. L. and Horbett T. A. ( American Chemical Society, Washington, D.C., 1995). [Google Scholar]
- 5. Brash J. L., “ Role of plasma protein adsorption in the response of blood to foreign surfaces,” in Blood Compatible Materials and Devices: Perspectives Towards the 21st Century, edited by Sharma C. P. and Szycher M. ( Technomic, Lancaster, PA, 1991). [Google Scholar]
- 6. Tsai W.-B., Grunkemeir J. M., and Horbett T. A., J. Biomed. Mater. Res. 44, 130 (1999). [DOI] [PubMed] [Google Scholar]
- 7. Gorbett M. B. and Sefton M. V., Biomaterials 25, 5681 (2004). 10.1016/j.biomaterials.2004.01.023 [DOI] [PubMed] [Google Scholar]
- 8. Lyman D. J., Metcalf L. C., D. Albo, Jr. , Richards K. F., and Lamb J., Trans. - Am. Soc. Artif. Intern. Organs 20, 474 (1974). [PubMed] [Google Scholar]
- 9. Grunkemeier J. M., Tsai W.-B., McFarland C. D., and Horbett T. A., Biomaterials 21, 2243 (2000). 10.1016/S0142-9612(00)00150-2 [DOI] [PubMed] [Google Scholar]
- 10. Tsai W.-B., Shi Q., Grunkemeier J. M., McFarland C., and Horbett T. A., J. Biomater. Sci. Polymer. Ed. 15, 817 (2004). 10.1163/1568562041271093 [DOI] [PubMed] [Google Scholar]
- 11. Chiumiento A., Lamponi S., and Barbucci R., Biomacromolecules 8, 523 (2007). 10.1021/bm060664m [DOI] [PubMed] [Google Scholar]
- 12. Tsai W. B. and Horbett T. A., J. Biomater. Sci. Polym. Ed. 10, 163 (1999). 10.1163/156856299X00117 [DOI] [PubMed] [Google Scholar]
- 13. Wu Y., Zhang M., Hauch K. D., and Horbett T. A., J. Biomed. Mater. Res. 85A, 829 (2008). 10.1002/jbm.a.31505 [DOI] [PubMed] [Google Scholar]
- 14. Zhang M. and Horbett T. A., J. Biomed. Mater. Res. 89A, 791 (2009). 10.1002/jbm.a.32085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawinger J., Timmons S., Loczewiak M., Strong D. D., and Doolittle R. F., Proc. Natl. Acad. Sci. U. S. A. 79, 2068 (1982). 10.1073/pnas.79.6.2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kloczewiak M., Timmons S., Lukas T. J., and Hawiger J., Biochemistry 23, 1767 (1984). 10.1021/bi00303a028 [DOI] [PubMed] [Google Scholar]
- 17. Tsai W. B., Grunkemeier J. M., and Horbett T. A., J. Biomed. Mater. Res. 67A, 1255 (2003) 10.1002/jbm.a.20024. [DOI] [PubMed] [Google Scholar]
- 18. Sivaraman B. and Latour R. A., Biomaterials 31, 832 (2010). 10.1016/j.biomaterials.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Y., Simonovsky F. I., Ratner B. D., and Horbett T. A., J. Biomed. Mater. Res. 74A, 722 (2005). 10.1002/jbm.a.30381 [DOI] [PubMed] [Google Scholar]
- 20. Garfinkle A. M., Hoffman A. S., Ratner B. D., Reynolds L. O., and Hanson S. R., Trans. - Am. Soc. Artif. Intern. Organs 30, 432 (1984). [PubMed] [Google Scholar]
- 21. Kiaei D., Hoffman A. S., Ratner B. D., Horbett T. A., and Reynolds L. O., J. Appl. Polym. Sci. 42, 269 (1988). [Google Scholar]
- 22. Massa T. M., McClung W. G., Yang M. L., Ho J. Y. C., Brash J. L., and Santerre J. P., J. Biomed. Mater. Res. 81A, 178 (2007). 10.1002/jbm.a.30936 [DOI] [PubMed] [Google Scholar]
- 23. Lin J. C., Tiong S. L., and Chen C. Y., J. Biomater. Sci. Polym. Ed. 11, 701 (2000). 10.1163/156856200743968 [DOI] [PubMed] [Google Scholar]
- 24. Liu T. Y., Lin W. C., Huang L. Y., Chen S. Y., and Yang M. C. Polym. Adv. Technol. 16, 413 (2005). 10.1002/pat.592 [DOI] [Google Scholar]
- 25. Paton D. M., Ashton T. R., and Roshan M., “ Fluorinating polymer surfaces,” U.S. patent 5,356,668 (18 October 1994).
- 26. Lilenfeld R., Popadiuk N. M., Steinheuser P., and Menezes E., “ Surgical filaments from vinylidene fluoride copolymers,” U.S. patent 4,564,013 (14 January 1986).
- 27. Ratner B. D., J. Biomed. Mater. Res. 27, 283 (1993). 10.1002/jbm.820270302 [DOI] [PubMed] [Google Scholar]
- 28. Ratner B. D., Biomaterials 28, 5144 (2007). 10.1016/j.biomaterials.2007.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao L., Sukaveshvar S., Ratner B. D., and Horbett T. A., J. Biomed. Mater. Res. A 79A, 788 (2006). 10.1002/jbm.a.30908 [DOI] [PubMed] [Google Scholar]
- 30. Grube E. et al. , JACC: Cardiovasc. Interventions 4, 168 (2011). 10.1016/j.jcin.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 31. Kolandaivelu K. et al. , Circulation 123, 1400 (2011). 10.1161/CIRCULATIONAHA.110.003210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim B.-K., Kim J.-S., Park J., Ko Y.-G., Choi D., Jang Y., and Hong M.-K., Yonsei Med. J. 53, 524 (2012). 10.3349/ymj.2012.53.3.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kottke-Marchant K., Anderson J. M., Umemura Y., and Marchant R. E., Biomaterials 10, 147 (1989). 10.1016/0142-9612(89)90017-3 [DOI] [PubMed] [Google Scholar]
- 34. Goncalves I. C., Martins M. C. L., Barbosa M. A., and Ratner B. D., Biomaterials 30, 5541 (2009). 10.1016/j.biomaterials.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 35. Dion I., Baquey C., Candelon B., and Monties J. R., Int. J. Artif. Organs 15, 617 (1992). [PubMed] [Google Scholar]
- 36. Helmkamp R. W., Goodland R. L., Bale W. F., Spar I. L., and Mutschler L. E., Cancer Res. 20, 1495 (1960). [PubMed] [Google Scholar]
- 37. Horbett T. A., J. Biomed. Mater. Res. 15, 673 (1981). 10.1002/jbm.820150506 [DOI] [PubMed] [Google Scholar]
- 38. Goodman S. L., J. Biomed. Mater. Res. 45, 240 (1999). [DOI] [PubMed] [Google Scholar]
- 39. Haycox C. L. and Ratner B. D., J. Bioomed. Mater. Res. 27, 1181 (1993). 10.1002/jbm.820270909 [DOI] [PubMed] [Google Scholar]
- 40. Lindon J. K., McManama G., Kushner L., Merrill E. W., and Saltzman E. W., Blood 68, 355 (1986). [PubMed] [Google Scholar]
- 41. Koh L. B., Rodriguez I., and Venkatraman S. S., Phys. Chem. Chem. Phys. 12, 10301 (2010). 10.1039/c001747g [DOI] [PubMed] [Google Scholar]
- 42. Kloczewiak M., Timmons S., and Hawiger J., Biochem. Biophys. Res. Commun. 107, 181 (1982). 10.1016/0006-291X(82)91686-2 [DOI] [PubMed] [Google Scholar]
- 43. Savage B. and Ruggeri Z. M., J. Biol. Chem. 266, 11227 (1991). [PubMed] [Google Scholar]
- 44. Ratner B. D., “ Evaluation of the blood compatibility of synthetic polymers: consensus and significance,” in Contemporary Biomaterials: Material and Host Response, Clinical Applications, New Technology and Legal Aspects, edited by Boretos J. W. and Eden M. ( Noyes, Park ridge, NJ, 1984) pp. 193–204. [Google Scholar]
- 45. Hanson S. R. and Ratner B. D., “ Evaluation of blood-materials interactions,” in Biomaterials Science: An Introduction to Materials in Medicine, edited by Ratner B. D., Hoffman A. S., Schoen F. L., and Lemons J. E. (Academic, San Diego, CA, 2004) pp. 367–378. [Google Scholar]
- 46. Courtney J. M., Lamba N. M. K., Sundaram S., and Forbes C. D., Biomaterials 15, 737 (1994). 10.1016/0142-9612(94)90026-4 [DOI] [PubMed] [Google Scholar]
- 47. Sivaraman B. and Latour R. A., Biomaterials 31, 1036 (2010). 10.1016/j.biomaterials.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bohnert J. L., Fowler B. C., Horbett T. A., and Hoffman A. S., J. Biomater. Sci. Polym. Ed. 1, 279 (1990). 10.1163/156856289X00154 [DOI] [PubMed] [Google Scholar]
- 49. Hoffman A. S., Horbert T. A., Bohnert J., Fowler B. C., and Kiael D., U.S. patent 3,055,316 (8 October 1991).
- 50. Laroche G., Marois Y., Guidoin R., King M. W., Martin L., How T., and Douville Y., J. Biomed. Mater. Res. 29, 1525 (1995). 10.1002/jbm.820291209 [DOI] [PubMed] [Google Scholar]
- 51. Chu C. C., Textile-based Biomaterials for Surgical Applications in Polymeric Biomaterials, Revised and Expanded, edited by Dumitru S. ( Marcel Dekker, New York, 2002). [Google Scholar]
- 52. Erli H. J., Marx R., Paar O., Niethard F. U., Weber M., and Wirtz D. C., Biomed. Eng. Online 2, 15 (2003). 10.1186/1475-925X-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stone G. W. et al. , JAMA 299, 1903 (2008). 10.1001/jama.299.16.1903 [DOI] [PubMed] [Google Scholar]
- 54. Elberg J. P. et al. , Eur. J. Vasc. Endovasc. Surg. 32, 431 (2006). 10.1016/j.ejvs.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 55. Pedrini L., Dondi M., Magagnoli A., Magnoni F., Pisano E., Del Giudice E., and Santoro M. Ann. Vasc. Surg. 15, 679 (2001). 10.1007/s100160010129 [DOI] [PubMed] [Google Scholar]
- 56. Lo E. S., “ Fluorine-containing polymers and preparation thereof,” U.S. patent 3,178,399 (13 April 1965).
- 57. Eberhart R. C., Munro M. S., Frautschi J. R., Lubin M., F. J. Clubb, Jr. , Miller C. W., and Sevastianov V. I., Ann. N. Y. Acad. Sci. 516, 78 (1987). 10.1111/j.1749-6632.1987.tb33032.x [DOI] [PubMed] [Google Scholar]
- 58. Tsai C. C., Huo H. H., Kulkarni P., and Eberhart R. C., ASAIO Trans. 36, M307 (1990). [PubMed] [Google Scholar]