Abstract
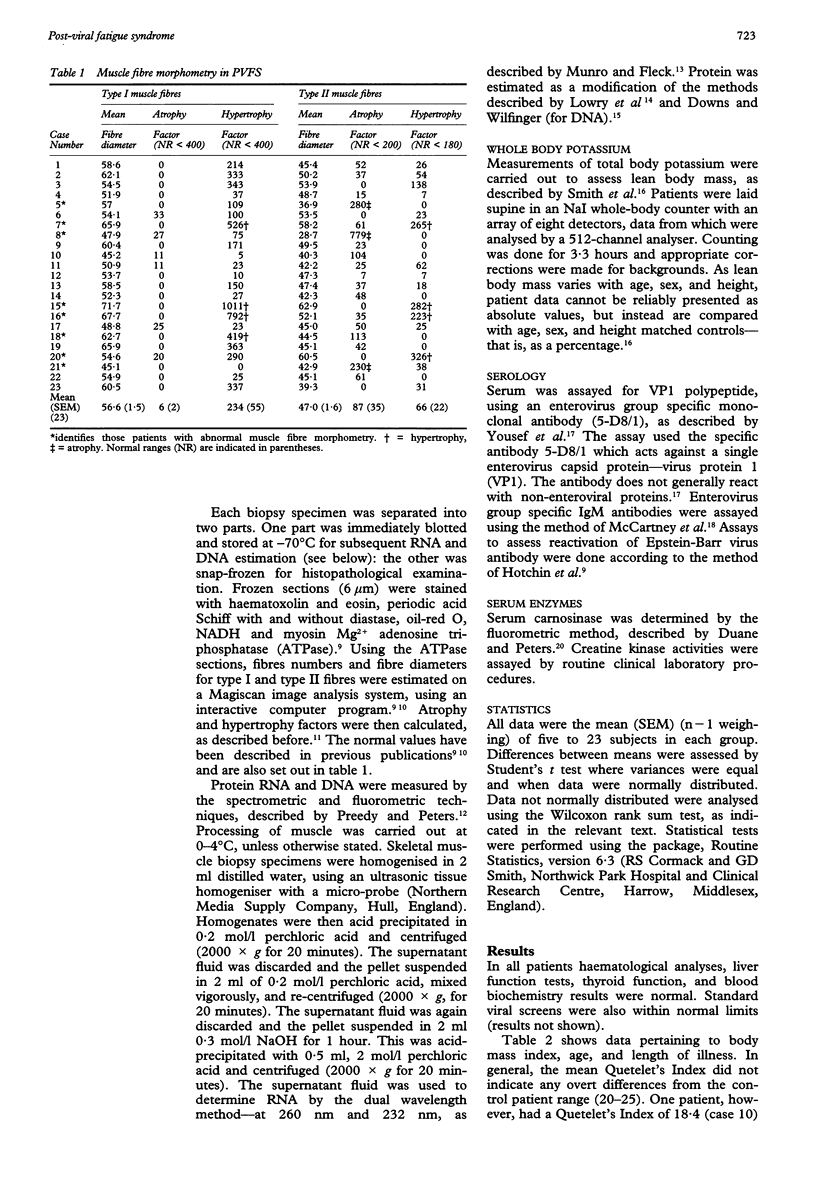
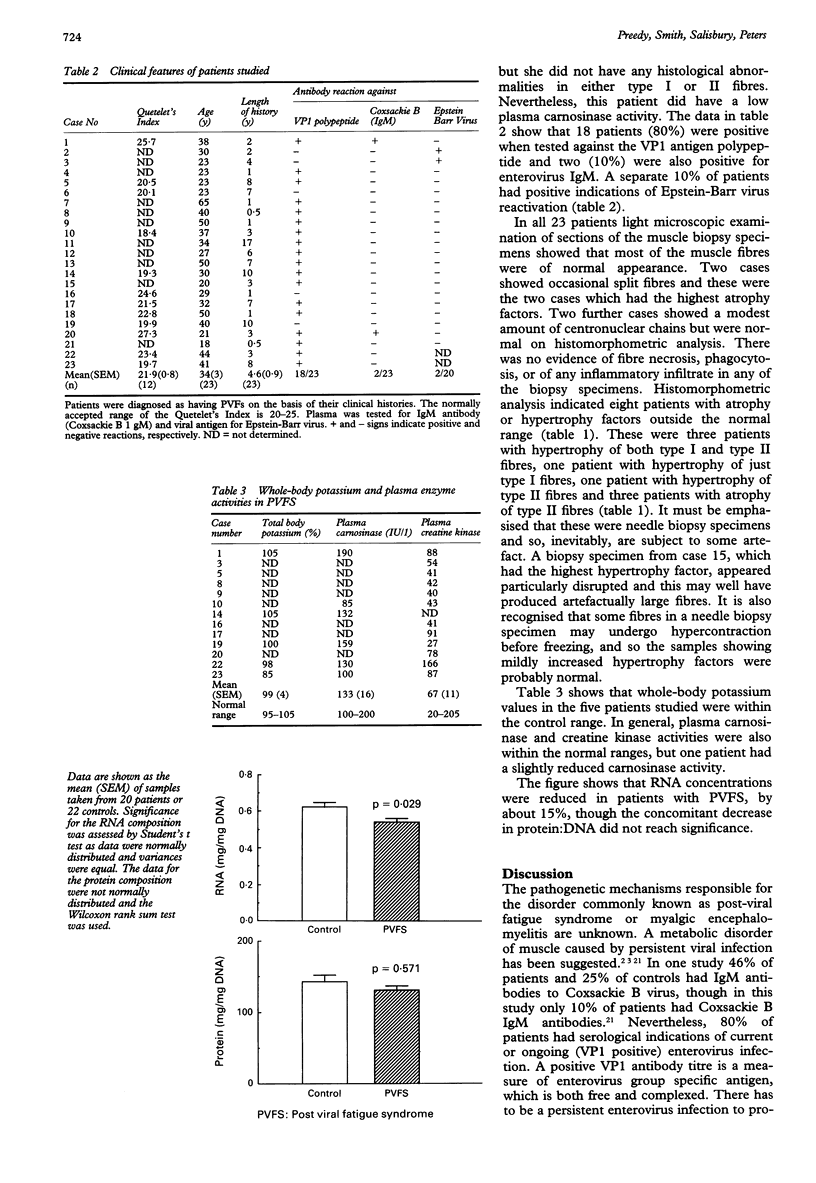
AIMS--To investigate in detail various biochemical and pathophysiological indices of muscle pathology in acute onset post-viral fatigue syndrome (PVFS). METHODS--Twenty three patients with PVFS (of mean duration 4.6 years) were subjected to needle biopsy for histomorphometry and total RNA contents. Plasma analysis included serology and creatine kinase activities. Indices of whole body mass were also measured--namely, whole body potassium content and plasma carnosinase activities. RESULTS--About 80% of the patients had serology indicative of persistent enteroviral infection as determined by VP1 antigen assay. Only about 10% of that same group of patients had serological indications of current enterovirus infection by IgM assay; a separate subset of 10% showed antibody changes suggestive of reactivation of Epstein-Barr virus. Quantitative morphometric analysis of skeletal muscle fibres indicated that the quadriceps muscle was normal or displayed only minor abnormalities in 22 patients. The Quetelet's Index (body mass index) and whole-body potassium values (index of lean body mass) were not affected in PVFS. The mean plasma carnosinase and creatinine kinase activities were also generally normal in these patients. The mean muscle RNA composition--mg RNA/mg DNA--was significantly reduced in acute onset PVFS by about 15%. The protein:DNA ratio was not significantly affected. CONCLUSIONS--Patients with acute onset PVFS, therefore, lose muscle protein synthetic potential, but not muscle bulk. Histopathology is consistent with these observations. These perturbations may contribute to the apparent feature of perceived muscle weakness associated with the persistent viral infection in the muscle themselves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Bowles N. E., Behan P. O., Bell E. J., Doyle D. Postviral fatigue syndrome: persistence of enterovirus RNA in muscle and elevated creatine kinase. J R Soc Med. 1988 Jun;81(6):326–329. doi: 10.1177/014107688808100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer M. I. The post-viral syndrome: a review. J R Coll Gen Pract. 1987 May;37(298):212–214. [PMC free article] [PubMed] [Google Scholar]
- Arnold D. L., Bore P. J., Radda G. K., Styles P., Taylor D. J. Excessive intracellular acidosis of skeletal muscle on exercise in a patient with a post-viral exhaustion/fatigue syndrome. A 31P nuclear magnetic resonance study. Lancet. 1984 Jun 23;1(8391):1367–1369. doi: 10.1016/s0140-6736(84)91871-3. [DOI] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Behan P. O., Behan W. M., Bell E. J. The postviral fatigue syndrome--an analysis of the findings in 50 cases. J Infect. 1985 May;10(3):211–222. doi: 10.1016/s0163-4453(85)92488-0. [DOI] [PubMed] [Google Scholar]
- Behan W. M., More I. A., Behan P. O. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83(1):61–65. doi: 10.1007/BF00294431. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Engel W. K. The histographic analysis of human muscle biopsies with regard to fiber types. 1. Adult male and female. Neurology. 1969 Mar;19(3):221–233. doi: 10.1212/wnl.19.3.221. [DOI] [PubMed] [Google Scholar]
- Byrne E., Trounce I., Dennett X. Chronic relapsing myalgia (? Post viral): clinical, histological, and biochemical studies. Aust N Z J Med. 1985 Jun;15(3):305–308. doi: 10.1111/j.1445-5994.1985.tb04042.x. [DOI] [PubMed] [Google Scholar]
- Calder B. D., Warnock P. J., McCartney R. A., Bell E. J. Coxsackie B viruses and the post-viral syndrome: a prospective study in general practice. J R Coll Gen Pract. 1987 Jan;37(294):11–14. [PMC free article] [PubMed] [Google Scholar]
- Cunningham L., Bowles N. E., Lane R. J., Dubowitz V., Archard L. C. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol. 1990 Jun;71(Pt 6):1399–1402. doi: 10.1099/0022-1317-71-6-1399. [DOI] [PubMed] [Google Scholar]
- Downs T. R., Wilfinger W. W. Fluorometric quantification of DNA in cells and tissue. Anal Biochem. 1983 Jun;131(2):538–547. doi: 10.1016/0003-2697(83)90212-9. [DOI] [PubMed] [Google Scholar]
- Duane P., Peters T. J. Serum carnosinase activities in patients with alcoholic chronic skeletal muscle myopathy. Clin Sci (Lond) 1988 Aug;75(2):185–190. doi: 10.1042/cs0750185. [DOI] [PubMed] [Google Scholar]
- Edwards R., Young A., Wiles M. Needle biopsy of skeletal muscle in the diagnosis of myopathy and the clinical study of muscle function and repair. N Engl J Med. 1980 Jan 31;302(5):261–271. doi: 10.1056/NEJM198001313020504. [DOI] [PubMed] [Google Scholar]
- Gow J. W., Behan W. M., Clements G. B., Woodall C., Riding M., Behan P. O. Enteroviral RNA sequences detected by polymerase chain reaction in muscle of patients with postviral fatigue syndrome. BMJ. 1991 Mar 23;302(6778):692–696. doi: 10.1136/bmj.302.6778.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin N. A., Read R., Smith D. G., Crawford D. H. Active Epstein-Barr virus infection in post-viral fatigue syndrome. J Infect. 1989 Mar;18(2):143–150. doi: 10.1016/s0163-4453(89)91150-x. [DOI] [PubMed] [Google Scholar]
- Jamal G. A., Hansen S. Electrophysiological studies in the post-viral fatigue syndrome. J Neurol Neurosurg Psychiatry. 1985 Jul;48(7):691–694. doi: 10.1136/jnnp.48.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. R., Hales J. P., Gandevia S. C. Muscle strength, endurance and recovery in the post-infection fatigue syndrome. J Neurol Neurosurg Psychiatry. 1988 Oct;51(10):1316–1322. doi: 10.1136/jnnp.51.10.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney R. A., Banatvala J. E., Bell E. J. Routine use of mu-antibody-capture ELISA for the serological diagnosis of Coxsackie B virus infections. J Med Virol. 1986 Jul;19(3):205–212. doi: 10.1002/jmv.1890190302. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Peters T. J. The effect of chronic ethanol ingestion on protein metabolism in type-I- and type-II-fibre-rich skeletal muscles of the rat. Biochem J. 1988 Sep 15;254(3):631–639. doi: 10.1042/bj2540631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M. C., Archard L. C., Banatvala J. E., Borysiewicz L. K., Clare A. W., David A., Edwards R. H., Hawton K. E., Lambert H. P., Lane R. J. A report--chronic fatigue syndrome: guidelines for research. J R Soc Med. 1991 Feb;84(2):118–121. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin G., Sowter C., Ward P., Paton K. Measurement of striated muscle fibre diameters using interactive computer-aided microscopy. J Clin Pathol. 1982 Nov;35(11):1268–1271. doi: 10.1136/jcp.35.11.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Hesp R., Mackenzie J. Total body potassium calibrations for normal and obese subjects in two types of whole body counter. Phys Med Biol. 1979 Jan;24(1):171–175. doi: 10.1088/0031-9155/24/1/016. [DOI] [PubMed] [Google Scholar]
- Stokes M. J., Cooper R. G., Edwards R. H. Normal muscle strength and fatigability in patients with effort syndromes. BMJ. 1988 Oct 22;297(6655):1014–1017. doi: 10.1136/bmj.297.6655.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers A. J., Coakley J. H., Edwards R. H. The metabolic consequences of reduced habitual activities in patients with muscle pain and disease. Ergonomics. 1988 Nov;31(11):1519–1527. doi: 10.1080/00140138808966801. [DOI] [PubMed] [Google Scholar]
- Yousef G. E., Bell E. J., Mann G. F., Murugesan V., Smith D. G., McCartney R. A., Mowbray J. F. Chronic enterovirus infection in patients with postviral fatigue syndrome. Lancet. 1988 Jan 23;1(8578):146–150. doi: 10.1016/s0140-6736(88)92722-5. [DOI] [PubMed] [Google Scholar]


