Abstract
Tissue concentrations of persistent organochlorine pesticides in laboratory-exposed largemouth bass (Micropterus salmoides) and in bass collected from Lake Apopka, FL were determined by both total mass and lipid normalized mass to better understand the bioaccumulation pathways of contaminants. In the laboratory study, male bass were orally administered a single dose of a mixture of two pesticides (p, p’-dichlorodiphenyldichloroethylene (p, p’-DDE) and dieldrin) and then fed uncontaminated food for 28 days. Gastrointestinal tract, liver, brain, gonad, kidney, spleen, and muscle were collected for chemical analysis. Different profiles were observed by total mass in tissues compared to lipid normalized mass. On a lipid normalized basis, p, p’-DDE was highest in the gastrointestinal tract followed by the liver, gonad, spleen, muscle, kidney and then brain. Dieldrin, on the other hand, was highest in the gastrointestinal tract and spleen and then followed by the gonad, muscle, liver, kidney and brain. Distribution of the chemicals to the organs differed by their log KOW and generally followed the blood flow path after the gastrointestinal tract. The low levels in kidney and brain suggest insufficient time for equilibration into these tissues, especially into the brain where the blood-brain barrier may be slow to traverse. In Lake Apopka fish, dichlorodiphenyltrichloroethanes (DDXs, sum of p, p’-DDE, p, p’-DDD, and p, p’-DDT), Drins (sum of aldrin, dieldrin, and endrin), and hexachlorocyclohexanes (HCHs) were found. For DDXs, the lipid normalized concentrations in each tissue were about the same, as predicted from theory. For Drins and HCHs, the lipid normalized concentrations were similar for kidney, spleen, brain, gonad and muscle, but much lower in the gastrointestinal tract and liver, probably because of metabolism occurring in those tissues.
Keywords: organochlorine pesticides, tissue concentration, oral dosing, largemouth bass, Lake Apopka
Graphical Abstract
1. Introduction
Organochlorine pesticides (OCPs) are chlorinated hydrocarbons that were widely used in agriculture and public health as insecticides and biocides from the 1940s through the 1960s (DHSS, 2010). OCPs that were used during that period include dichlorodiphenyltrichloroethane (DDT), methoxychlor, toxaphene, dieldrin, chlordanes, and lindane. Although their application has been banned or restricted in many countries, these pesticides, in particular DDT, continue to be used in some developing countries like Ghana and China (Hogarh et al., 2014; Li et al., 2016). OCPs are intractable in the environment and can act as endocrine disruptors, which interfere with reproduction and developmental processes in wildlife and humans (Johnson, 1999; Colborn et al., 1993; Kavlock et al., 1996).
In aquatic environments, OCPs are bioavailable to organisms and are transferred up the food chain to top predators including predatory fish, where they bioaccumulate in fatty tissues. Trophic transfer via food is often an important source of OCPs in fish since these contaminants have octanol-water partitioning coefficient (log KOW) >5 and are not freely dissolvable in water. Because most OCPs are not appreciably biotransformed in biological tissues, fish can be a suitable indicator for the contamination of their surrounding environments (Fisk et al., 1998; Lanfranchi et al., 2006). It is evident that consumption of contaminated fish is one of the most important pathways for transfer of pesticides into humans, encouraging many studies to focus on the magnitude of OCPs in edible tissues of fish species (Jiang et al., 2005; Yang et al., 2006; Li et al., 2008; Yohannes et al., 2014). In addition, understanding the distribution pattern of OCPs in other tissues (e.g., liver, kidney, gonad, brain, and spleen) provides insight into pathways of OCP bioaccumulation and helps define primary exposure in exposed animals and resultant human exposures from given levels of environmental contamination. OCP concentrations are normally found to be the lowest in muscle tissues but the highest in either hepatobiliary-related tissues such as liver, bile, and heart (Guo et al., 2008; Zhao et al., 2013, 2014) or brain (Sahagun et al., 1997; Zhou et al., 2007). These results have been primarily observed in studies where fish are chronically exposed to contaminants resulting in uptake of contaminants by different tissues in proportion to their lipid content at equilibrium. In addition, distribution of lipophilic contaminants among animal tissues can also depend on lipid content of that tissue, blood flow and time to reach equilibrium between blood and this compartment (Ondarza et al., 2011; Boon et al., 1994). Reaching to the brain, however, may take longer than other tissues since the contaminants need to transverse the blood-brain barrier.
The north shore area of Lake Apopka, FL has been extensively contaminated with OCPs from heavy agricultural activities from the 1940s to the 1970s. In 2011, a good 40 years after cessation of use, heavy levels of contaminants were still measured in the sediments (e.g., 1.0 ± 0.3, 9.5 ± 7.7, and 0.3 ± 0.1 µg/g dry wt for p, p’-DDE (the major metabolite of DDT), toxaphene, and dieldrin, respectively) (SJRWMD, 2011). Specifically, control largemouth bass (Micropterus salmoides, LMB) introduced into ¼ acre-ponds (mesocosms) from October 2007 to January 2008 in the highly OCP-contaminated north shore area of the lake contained a mean whole-body burden of 363.1, 185.1, and 11.5 µg/g lipid for p, p’-DDE, toxaphene, and dieldrin, respectively (Martyniuk et al., accepted). Although there have been extensive efforts by St. Johns River Water Management District (SJRWMD) to remediate the area since 2002 (SJRWMD, 2011), our previous study indicates that OCPs remain present in sediments of the north shore area and are bioavailable to blackworms (Lumbriculus variegatus) (Dang et al., 2016). The contaminated diets from benthic organisms and/or smaller fish could be a potential source of OCPs for LMB in mesocosms and possibly LMB in the lake. To our knowledge, there is a paucity of information about tissue accumulation of OCPs in LMB given that LMB are top predators in most aquatic food chains as well as sought-after catch for anglers and subsistence fishermen (Soupir et al., 2000). Our main objective for this study was to better understand tissue-specific accumulation of pesticides in LMB exposed to 1) an acute dose of two OCPs (p, p’-DDE and dieldrin), and 2) a historical contamination of OCPs. We excluded toxaphene in this study due to complexity of its isomers that could be present in the environment. In addition, we administered individual LMB species with a single dose of a mixture of p, p’-DDE and dieldrin to minimize toxicity and variability of chemical doses among individuals as well as individual variations in tissue-specific concentrations of OCPs.
2. Materials and methods
2.1. Standards and solvents
Neat standards of p, p’-DDE (CAS# 72-55-9, 99% pure) and dieldrin (CAS# 60-57-1, 98.7% pure) were purchased from Aldrich (Milwaukee, WI). A stock solution of 18 OCPs containing α-, β -, γ -, δ-hexachlorocyclohexane (HCH) isomers, α- and β- chlordane isomers, p, p’-DDD, p, p’-DDE, p, p’-DDT, dieldrin, aldrin, endosulfan I, endosulfan II, endosulfan sulfate, endrin, heptachlor, heptachlor epoxide, and methoxychlor at a concentration of 2000 µg/ml in hexane:toluene (1:1 v/v) were purchased from Phenomenex (Torrance, CA). Isotope internal standards (13C12-p, p’-DDE and 13C12-dieldrin) and a mixture of deuterated internal standards (acenaphtlene-d10, chrysene-d12, and phenanthrene-d10) were obtained from Cambridge Isotope Laboratories (Tewksbury, MA) and Ultra Scientific (North Kingstown, RI), respectively. Organic solvents (hexane, acetone, and acetonitrile) were purchased from Fisher Scientific (Fair Lawn, NJ).
2.2. Oral dosing
Commercial salmon feed (sinking pellets, 4 mm in size) was purchased from Skretting USA (Tooele, UT) and ground into powder using a mortar and pestle. A mixture of p, p’-DDE and dieldrin (~10 mg of each) dissolved into 2 ml ethanol was mixed with 4 ml menhaden fish oil (Jandell Corp., Boyd, TX) to facilitate the coating of chemicals onto the feed. The mixture was then slowly added dropwise into 10 g of homogenized feed while mixing to obtain a nominal concentration of 1 mg chemical/g feed. The pesticide-laden feed was mixed thoroughly in a 60 ml amber glass vial using a clean stainless steel spatula and then placed inside a fume hood overnight to allow complete evaporation of ethanol. Unspiked feed also was prepared by adding 6 ml ethanol:oil (1:2 v/v), equal to the amount of vehicle added to the spiked feeds, into 10 g of the homogenized feed.
Male largemouth bass, 1–2 years of age, were purchased from American Sports Fish Hatchery (Montgomery, AL) in May 2013. Size of fish averaged 26.3 (±1.8) cm in length and 232.1 (±45.6) g in weight. Fish were maintained in a 120 gal, flow-through round tank supplied by filtered water (municipal water passed through granular activated carbon) and aerated on-site at the Center for Environmental and Human Toxicology (CEHT), University of Florida, FL. Fish were starved 24 hr prior to oral dosing. On the day of dosing (early August 2013), fish (n=5) were anesthetized in tricaine-methanesulfonate (MS 222, 150 mg/L) and orally introduced with a spiked feed pellet paste, which was prepared by compressing about 0.2 g of spiked feed (0.2 mg OCPs) into a 3 ml syringe with cut-off end to push the plug passed the esophagus. Total ingestion was verified visually in the tank for each fish. This method was used to obtain a nominal concentration of 1 µg chemical/g fish, which was 5 times lower than the concentration of p, p’-DDE and 2 times higher than the concentration of dieldrin, respectively, measured in bass introduced into the mesocosms on the north shore area of Lake Apopka, FL. Fish (5 per group) were also orally dosed with approximately 0.2 g (~0.1% of their body weight) of unspiked (control) pellet paste one time at the beginning of the experiment. Twenty-four hours following this one time exposure, fish were fed control commercial food (sinking pellets, 4 mm in size) for up to 30 days to ascertain that spiked feed containing OCPs was cleared out of the intestine and to give fish enough time to distribute the OCPs into tissues. All experiments were carried out at a water temperature of 24.8 (±1.7) °C with a photoperiod of 16h:8h (light:dark). Fish were starved for 24 hr prior to sample collection to make sure that any food residues were flushed out of the intestine. After euthanasia with an overdose of MS 222, fish were measured for length and weight, and dissected to collect liver, gonad, muscle (with skin), gastrointestinal tract (GIT), kidney, spleen, and brain. Tissue samples were further stored frozen at −20 °C until analysis. All animals were treated as per the protocol by the University of Florida Institutional Animal Care and Use Committee.
2.3. Lake Apopka fish sampling
Lake Apopka LMB (n=10, 5 males and 5 females) were collected by electroshocking in March 2013 and body measurements of fish (total length and weight) were recorded. An additional 10 LMB (5 males and 5 females) were collected from Wildcat Lake, FL, which is in the Ocala National Forest Recreation area and served as the reference lake. Lake Apopka and Wildcat Lake LMB were about 34.1 (±4.3) cm in length and 559.5 (±193.2) g in weight, which were ~2 times bigger than bass used in the oral dosing experiment due to unavailability of adult bass in the laboratory. Fish were immediately dissected in the field to collect tissues. Samples were stored in either Whirpak bags or cryovials, placed in ice during transportation back to the laboratory, and further kept at −20 °C before analysis. To minimize the variability of concentrations in tissues between male and female fish, male fish were only used for chemical analysis resulting in the small sample size (n=5) in the current study.
2.4. Extraction of OCPs from fish tissues
Samples were thawed and GIT samples were further cleaned using a 10 ml syringe with clean water (3×10 ml) to remove any food residues. Tissue samples were transferred to a mortar containing liquid nitrogen and then pulverized into smaller particles using a pestle. The samples were freeze-dried for 24 hr prior to extractions. Approximately 0.1 – 0.3 g of the dried samples were spiked with 10 ng internal standards and extracted following the method of Hong et al. (2004) with minor modifications. In brief, samples were solvent-extracted with 7 ml of acetone:hexane (5:2, v/v) by vortexing for 1 min followed by sonication for 30 min and centrifugation in a Beckman centrifuge (5 min at 1300 rpm). The extractions were performed twice and the combined supernatants were transferred to glass tubes, concentrated to near dryness under a gentle stream of nitrogen, and reconstituted into 2 ml acetonitrile. The acetonitrile extracts were stored frozen at −20 °C for 1 hr to precipitate unwanted lipids. Cold soluble extracts were immediately transferred to clean glass tubes and the remaining material was subjected to a second freezing step using an additional 2 ml of acetonitrile. The freezing lipid filtration could remove up to 90% of the lipids extracted from the fish without any significant loss of OCPs in fish (Hong et al., 2004). The combined acetonitrile extracts (~4 ml) were concentrated to a volume of 1 ml that was subjected to a cleanup step using a Florisil SPE column (PreSep, 6 cc, 1 g). Final extracts were solvent exchanged into 1 ml hexane prior to analysis.
2.5. Lipid quantification
The lipid content of GIT, liver, gonad, and muscle was determined using the Bligh and Dyer (1959) method. There was not enough mass of other tissues (brain, kidney, and spleen) to measure the lipid content by this method. In general, for lipid quantification about 0.5 – 1 g of wet tissue was accurately weighed in a 50 ml glass vial and mechanically mixed with 3 ml of a mixture of chloroform:methanol (1:2 v/v) for 2 min. About 1 ml of chloroform was added to the mixture, homogenized for 1 min followed by 1 ml of distilled water, and homogenization was continued for an additional min. The homogenate was then transferred to a clean glass centrifuge tube, and centrifuged for 10 min at 1300 rpm. The upper alcoholic layer was pipetted out and discarded. The lower chloroform layer was collected, transferred to a pre-weighed clean tube, and evaporated to dryness under nitrogen. The tube was re-weighed and the mass difference was determined to be the lipid content.
2.6. Sample analysis
Analysis of OCPs was conducted on an Agilent 7000 gas chromatography-tandem mass spectrometry (GC-MS/MS) (Santa Clara, CA) using a HP-5MS column (Zebron, Torrance, CA; 30 m length, 0.25 mm diameter, and 0.25 µm film thickness). Helium in ultra high purity (99.999%) was used as a carrier gas with a flow rate of 1 ml/min and nitrogen was used as a collision gas. The GC temperature program started at 70 °C, increased in increments of 40 °C/min to 170 °C, followed by 4 °C/min to 200 °C, and 10 °C/min to a final temperature of 280 °C, and held for 2 min. The injector and MS source temperatures were set at 280 °C and 230 °C, respectively. Analytes were introduced into the mass spectrometer operating in an electron ionization mode, and multiple reaction monitoring (MRM) was used for quantitative analysis. Transition ions for target analytes are presented in Table 1 and the first and second most intense transitions for each analyte were used as the quantifier and qualifier ions, respectively.
Table 1.
Retention times (tr), multiple reaction monitoring (MRM) transitions, and collision energy (CE) for each transition.
| Analyte | tr (min) | Quantifier m/z | CE (V) | Qualifier m/z | CE (V) |
|---|---|---|---|---|---|
| Acenapthalen-d10 | 5.91 | 164 → 160 | 40 | 164 → 158 | 30 |
| α-HCH | 7.65 | 219 → 183 | 35 | 219 → 109 | 30 |
| β-HCH | 8.18 | 219 → 183 | 35 | 219 → 109 | 30 |
| γ-HCH | 8.29 | 219 → 183 | 35 | 219 → 109 | 30 |
| Phenanthrene-d10 | 8.47 | 188 → 160 | 25 | 188 → 158 | 30 |
| δ-HCH | 8.82 | 219 → 183 | 35 | 219 → 109 | 30 |
| Heptachlor | 9.94 | 337 → 266 | 20 | 272 → 117 | 25 |
| Aldrin | 10.83 | 263 → 193 | 25 | 263 → 191 | 30 |
| Heptachlor epoxide | 11.86 | 353 → 263 | 30 | 353 → 317 | 40 |
| Trans-chlordane | 12.44 | 375 → 303 | 30 | 375 → 226 | 25 |
| Endosulfan I | 12.73 | 241 → 206 | 25 | 241 → 170 | 25 |
| Cis-chlordane | 12.81 | 375 → 303 | 35 | 375 → 226 | 40 |
| p, p’-DDE | 13.35 | 246 → 176 | 25 | 246 → 175 | 25 |
| 13C12p, p’-DDE | 13.35 | 330 → 258 | 30 | 330 → 260 | 30 |
| Dieldrin | 13.84 | 263 → 193 | 30 | 263 → 191 | 35 |
| 13C12-Dieldrin | 13.84 | 393 → 270 | 40 | 270 → 200 | 35 |
| Endosulfan II | 14.05 | 195 → 159 | 30 | 195 → 125 | 40 |
| p, p’-DDD | 14.29 | 235 → 165 | 20 | 235 → 199 | 25 |
| Endrin | 14.47 | 263 → 193 | 30 | 263 → 191 | 35 |
| Endosulfan sulfate | 15.00 | 272 → 237 | 35 | 387 → 253 | 40 |
| p, p’-DDT | 15.11 | 235 → 165 | 20 | 235 → 199 | 25 |
| Chrysene-d12 | 16.10 | 240 → 236 | 30 | 240 → 212 | 30 |
| Methoxychlor | 16.34 | 227 → 141 | 40 | 227 → 212 | 35 |
OCP identification was accomplished by matching retention times to reference compounds in the corresponding stock standard. Calibration standards were prepared by a series of dilutions of the stock solution in hexane. A calibration standard curve was constructed using 7 levels of concentrations (0.5, 2, 20, 50, 100, 200, and 500 µg/L). Values of the coefficient of determination (r2) for the calibration curves for all OCPs were greater than 0.996. A field control (fish from Wildcat Lake, FL), matrix spike (OCP standards spiked into control tissues), and matrix spike duplicates were included with every 10 samples to check for interference and cross-contamination. The limit of quantitation (LOQ) was calculated as the mean plus three times the standard deviation of concentrations measured in control samples. The LOQ was between 0.1 and 0.5 ng/g ww for all target OCPs. The average recoveries of OCPs in matrix spikes were α -, β -, γ -, δ -HCH isomers: 80.9 ± 11.3%; heptachlor: 80.7 ± 3.0%; aldrin: 67.3 ± 4.5%; heptachlor epoxide: 63.4 ± 1.5%; α-, β-chlordanes: 63.4 ± 1.5%; endosulfans: 65.1 ± 3.7%; p, p’-DDE: 86.7 ± 3.5%; dieldrin: 64.6 ± 6.8%; endrin: 60.7 ± 6.9%; p,p’-DDD: 92.6 ± 2.8%; endosulfan sulfate: 118.2 ± 13.5%; p, p’-DDT: 94.9 ± 3.6; and methoxychlor: 122.1 ± 10.2%.
2.7. Data analysis
OCP concentrations in tissues were expressed as the mean ± standard deviation (SD). Since concentrations of lipid-soluble OCPs in the tissues of an animal might be proportional to the concentrations of lipid which is often variable among tissues, data were normalized to both wet weight (ww) and lipid weight. For samples with the concentration below LOQ, values were assigned as a half of the LOQ. Statistical analyses were performed with GraphPad software (Prism 5.0) for Windows. Although the sample size (n=5) is limited for both the laboratory exposure and Lake Apopka studies, differences in residual levels measured for each pesticide in the different tissues were analyzed for statistical significance using one-way analysis of variance (ANOVA) with the Tukey honestly-significant-difference test (p<0.05).
3. Results and discussion
Concentrations of investigated OCPs in LMB collected from the reference lake were below LOQ in all tissues. Untreated fish in the laboratory also showed unquantifiable background levels (< LOQ) of OCPs in tissues, except for p, p’-DDE, which averaged at 32.1 ± 23.8 ng/g ww. This value was not subtracted from the sample measurements for the laboratory exposed fish as this background level was ~3 orders of magnitude less than tissue concentrations measured in exposed fish.
Lipid content for muscle, gonad, GIT, and liver were not different between laboratory-exposed and wild-caught fish with averages of 0.9 ± 0.2, 2.3 ± 0.2, 4.5 ± 3.0, and 5.5 ± 1.6%, respectively. Lipid content for other tissues (e.g., kidney, spleen, and brain) was not measured due to the small mass of samples. Because lipid data for these tissues in LMB or similar freshwater fish species are not available, we used the ratio of 0.06, 0.1, and 0.6 for kidney, spleen, and brain to liver, respectively, calculated for cod fish (Gadus morhua) from a previous study (Mitchell et al., 1977).
3.1. p,p’-DDE and dieldrin in oral dosing LMB
Fish mortality or behavior changes were not observed after the single oral administration of OCPs or during the course of feeding with commercial food. Measured concentrations of p, p’-DDE and dieldrin in feed were 0.7 ± 0.01 and 1.0 ± 0.1 mg/g ww, respectively. The growth (e.g., length and body weight) did not vary significantly 30 days after the treatment (data not shown). In treated fish, concentrations of p, p’-DDE and dieldrin were above the LOQ in all tissues and their concentrations are presented in Figure 1. Concentrations of p, p’-DDE and dieldrin in each tissues are also provided in Table 2.
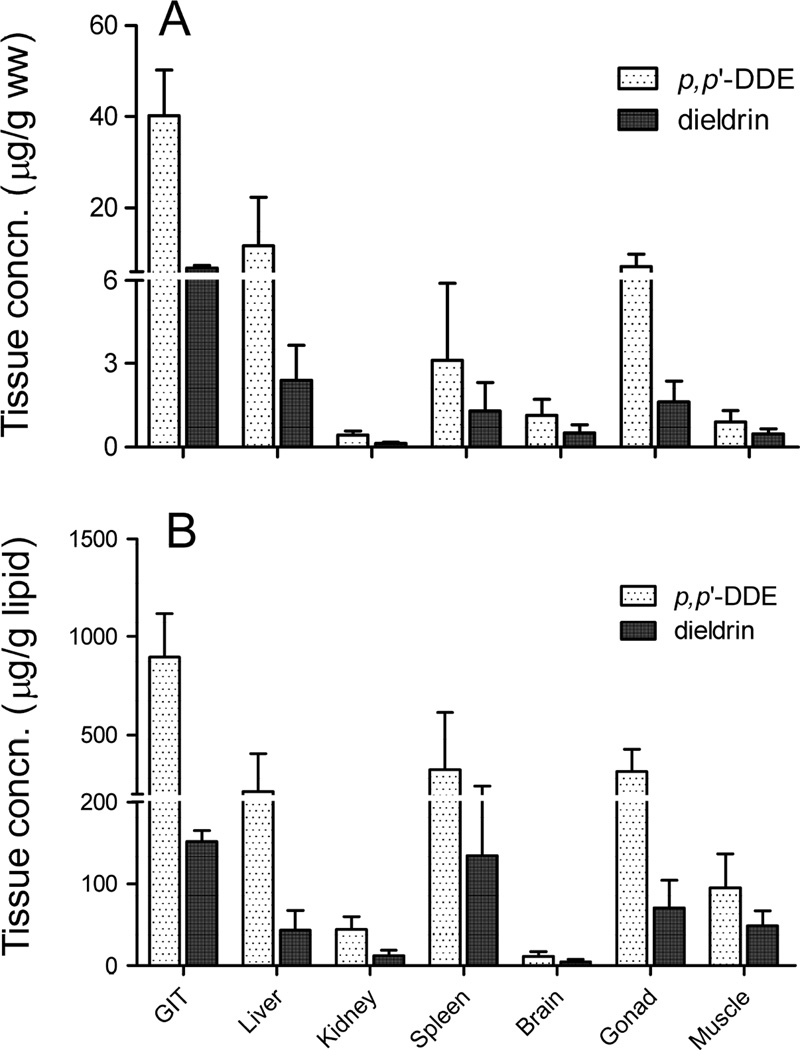
Figure 1.
Tissue concentrations of p, p’-DDE and dieldrin in largemouth bass following the oral dosing in laboratory experiments. Contaminant levels are based on A) fresh weight (µg/g ww) and B) lipid weight (µg/g lipid). Values are means of 5 replicates and error bars indicate standard deviation of mean tissue concentrations.
Table 2.
Residues of p, p’-DDE and dieldrin (n=5, mean ± SD) in the different tissues of largemouth bass received a single oral dose.
| Concn. (µg/g ww) | Concn. (µg/g lipid) | |||
|---|---|---|---|---|
| p, p’-DDE | dieldrin | p, p’-DDE | dieldrin | |
| GIT | 40.2 ± 10.1 | 6.8 ± 0.6 | 896.1 ± 224.3 | 151.9 ± 13.3 |
| Liver | 11.7 ± 10.5 | 2.4 ± 1.3 | 213.4 ± 191.2 | 43.6 ± 23.5 |
| Kidney | 0.4 ± 0.1 | 0.1 ± 0.05 | 44.5 ± 15.2 | 12.3 ± 6.7 |
| Spleen | 3.1 ± 2.8 | 1.3 ± 1.0 | 324.7 ± 290.2 | 134.4 ± 106.4 |
| Brain | 1.1 ± 0.6 | 0.5 ± 0.3 | 11.4 ± 5.8 | 5.0 ± 2.9 |
| Gonad | 7.2 ± 2.6 | 1.6 ± 0.7 | 315.7 ± 114.3 | 70.7 ± 33.2 |
| Muscle | 0.9 ± 0.4 | 0.5 ± 0.2 | 94.7 ± 41.9 | 48.7 ± 18.3 |
On a wet weight basis, concentrations were the highest in the GIT (average of 40.2 ± 10.1 and 6.8 ± 0.6 µg/g ww for p, p’-DDE and dieldrin, respectively) followed by liver (average of 11.7 ± 10.5 and 2.4 ± 1.3 µg/g ww for p, p’-DDE and dieldrin, respectively) (Fig. 1A). The lowest concentrations were measured in kidney with an average of 0.4 ± 0.1 and 0.1 ± 0.05 µg/g ww for p, p’-DDE and dieldrin, respectively (Fig. 1A). Concentrations in liver, spleen, brain, muscle, and gonad were not statistically different (p<0.05) from each other. Concentrations of p, p’-DDE were always higher than the concentrations of dieldrin in tissues. p, p’-DDE and dieldrin have log KOW of 6.9 and 5.4, respectively (Bruijn et al., 1989) indicating that p, p’-DDE is more hydrophobic and prone to accumulate in tissues to a higher extent than dieldrin. Tissue residues of p, p’-DDE in the current study are consistent with previous studies that show an increase in concentration or a potential for biomagnification of highly hydrophobic organic chemicals (e.g., polychlorinated biphenyls (PCBs) and DDT) in the GIT of fish upon ingestion of contaminated food (Gobas et al., 1988; Connolly and Pederson, 1988; Clark et al., 1990). Kelly et al. (2004) proposed a digestion model to explain the increased chemical residues in the GIT and attributed this phenomenon to passive molecular diffusion and/or facilitated diffusion that incorporates an additional advective transport mechanism. In addition, Dang et al. (2016) reported that the bioaccumulation potential of p, p’-DDE and dieldrin in blackworm (Lumbriculus variegatus) corresponded to their log KOW regardless of whether the contaminants were given to worms as single chemicals or as a mixture of contaminants.
On a lipid weight basis, concentration of p, p’-DDE was again the highest in GIT (average of 896.1 ± 224.3 µg/g lipid) followed by liver, spleen, and gonad with an average of 213.4 ± 191.2, 324.7 ± 290.4, and 315.7 ± 114.3 µg/g lipid, respectively (Fig. 1B). For dieldrin, the highest concentration was measured in both the GIT and the spleen with average of 151.9 ± 13.3 and 134.4 ± 106.4 µg/g lipid, respectively (Fig. 1B). Kidney and brain showed the least accumulation of p, p’-DDE and dieldrin. It is generally considered that bioaccumulation of contaminants in animal tissues are proportional to their lipid content. Fish brain contains high lipid content similar to the GIT and liver and therefore, concentrations of OCPs in these tissues are expected to be correlated. However, results from our study did not support this projected correlation, but instead the distribution of contaminants into tissues seemed to depend on the direction of blood flow after the GIT. Once ingested via food, contaminants will be carried by blood from the GIT to the liver and spleen, then to the kidney and muscle, and subsequently to the brain, where bioaccumulation is limited due to the blood-brain barrier. It may be slow to establish full equilibrium in the lipid compartments among the tissues. Another possible explanation, however, for the low accumulation of OCPs in kidney and brain could result from the estimation of lipid content in these tissues as we used the lipid ratio for kidney, brain, and spleen to liver calculated for cod fish from a previous study (Mitchell et al., 1977). LMB is freshwater fish while cod is a marine fish species that is lipid rich, possibly resulting in variability of tissue-to-tissue lipid ratio between the two species.
3.2. OCPs in Lake Apopka bass
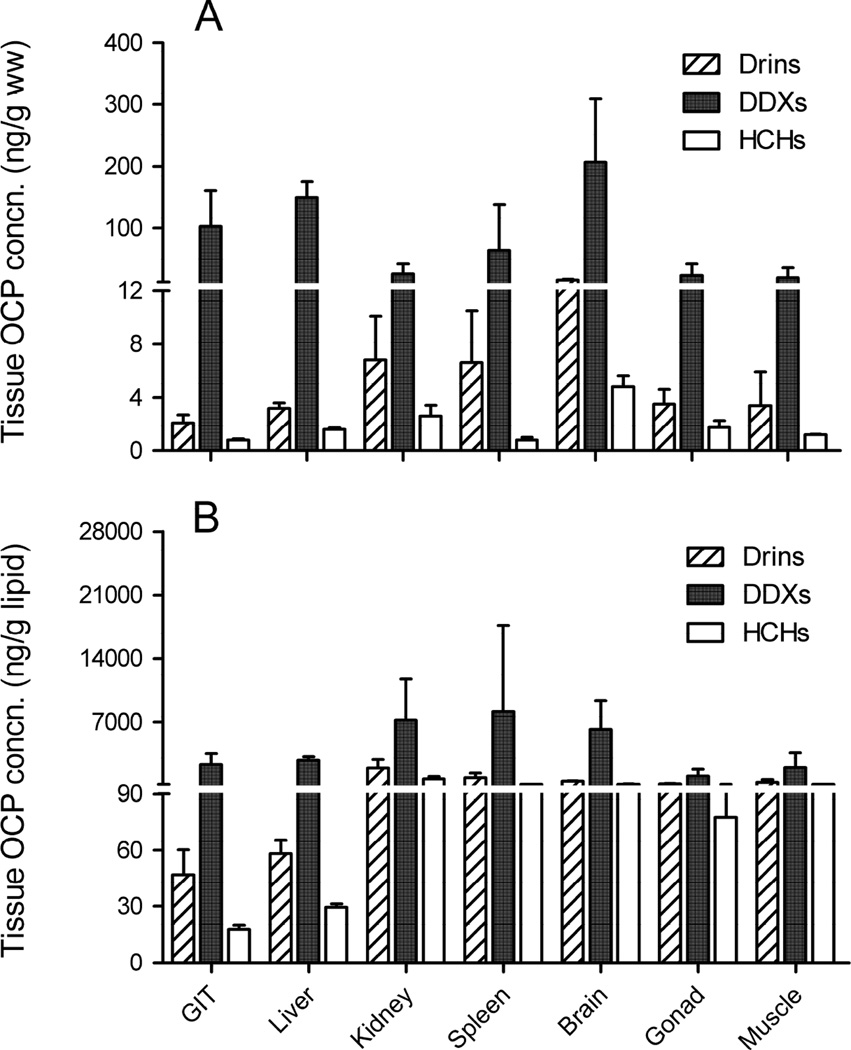
Among the 18 OCPs analyzed in Lake Apopka LMB, Drins (sum of aldrin, dieldrin, and endrin), DDXs (sum of p, p’-DDE, p, p’-DDD, and p, p’-DDT), and HCHs (sum of α -, β -, γ -, and δ -HCH) were measured in fish tissues. Other pesticides (e.g., α -, β -chlordane, heptachlor, heptachlor epoxide, endosulfan I, endosulfan II, endosulfan sulfate, and methoxychlor) were below the LOQ in LMB tissues. The levels of measured OCPs in tissues of LMB collected from Lake Apopka are presented in Figure 2 and their concentrations in each tissues are provided in Table 3. Among OCPs, DDTs were the most predominant contributing 76 – 99% of the total OCP load in tissues. On a wet weight basis, OCPs had the highest concentrations in brain (average of 15.2 ± 1.8, 206.3 ± 103.0, and 4.8 ± 0.8 ng/g ww for Drins, DDXs, and HCHs, respectively) (Fig. 2A). The tissue distribution of OCPs in Lake Apopka LMB was different from the results seen in the dosing experiment. The Apopka bass had the highest levels of OCPs in the brain, but the highest residues of OCPs were measured in GIT in the orally dosed bass suggesting that over time, OCPs can cross the blood-brain barrier and accumulate in the brain.
Figure 2.
Tissue concentrations of OCPs detected in wild-caught largemouth bass from Lake Apopka, FL. Contaminant levels are based on A) fresh weight (ng/g ww) and B) lipid weight (ng/g lipid). Values are means of 5 replicates and error bars indicate standard deviation of mean tissue concentrations. (DDXs = p, p’-DDE + p, p’-DDD + p, p’-DDT; Drins = aldrin + dieldrin + endrin, HCHs = α-HCH + β-HCH + γ-HCH + δ-HCH)
Table 3.
OCP concentrations (n=5, mean ± SD) in different tissues of Lake Apopka bass
| Concn. (ng/g ww) | Concn. (ng/g lipid) | |||||
|---|---|---|---|---|---|---|
| Drins | DDXs | HCHs | Drins | DDXs | HCHs | |
| GIT | 2.1 ± 0.6 | 102.9 ± 58.2 | 0.8 ± 0.1 | 46.8 ± 13.4 | 2291.8 ± 1296.3 | 117.8 ± 2.2 |
| Liver | 3.2 ± 0.4 | 150.0 ± 25.6 | 1.6 ± 0.1 | 58.1 ± 7.3 | 2723.6 ± 464.8 | 29.4 ± 1.8 |
| Kidney | 6.8 ± 3.3 | 25.4 ± 16.0 | 2.6 ± 0.8 | 1920.0 ± 931.7 | 7171.8 ± 4517.6 | 742.6 ± 225.9 |
| Spleen | 6.6 ± 3.9 | 64 ± 74.6 | 0.8 ± 0.2 | 841.6 ± 496.7 | 8160.7 ± 9512.3 | 102.0 ± 25.5 |
| Brain | 15.2 ± 1.8 | 206.3 ± 103.0 | 4.8 ± 0.8 | 457.4 ± 54.0 | 6196.3 ± 3093.2 | 144.1 ± 24.0 |
| Gonad | 3.5 ± 1.1 | 23.2 ± 18.1 | 1.8 ± 0.5 | 153.5 ± 48.0 | 1017.4 ± 793.8 | 77.6 ± 21.9 |
| Muscle | 3.4 ± 2.5 | 18.7 ± 16.4 | 1.3 ± 0.03 | 354.8 ± 260.4 | 1951.6 ± 1711.6 | 125.2 ± 3.1 |
High concentrations of DDTs were also measured in the GIT and liver with an average of 102.9 ± 58.2 and 150.0 ± 25.6 ng/g ww, respectively while the concentrations of Drins and HCHs were low in the GIT and liver ranging from the 0.8 – 3.2 ng/g ww (Fig. 2A). Due to their degree of lipophilicity, DDXs are highly persistent in lipid-rich tissues (e.g., GIT and liver) and bioaccumulated to a higher extent than Drins and HCHs. When normalized on a lipid weight basis, concentrations of DDXs in tissues were not significantly different from each other (p<0.05), but concentrations of Drins and HCHs were lowest in the GIT and liver (Fig. 2B) suggesting a high metabolism of Drins and HCHs as compared to persistent DDXs in these tissues.
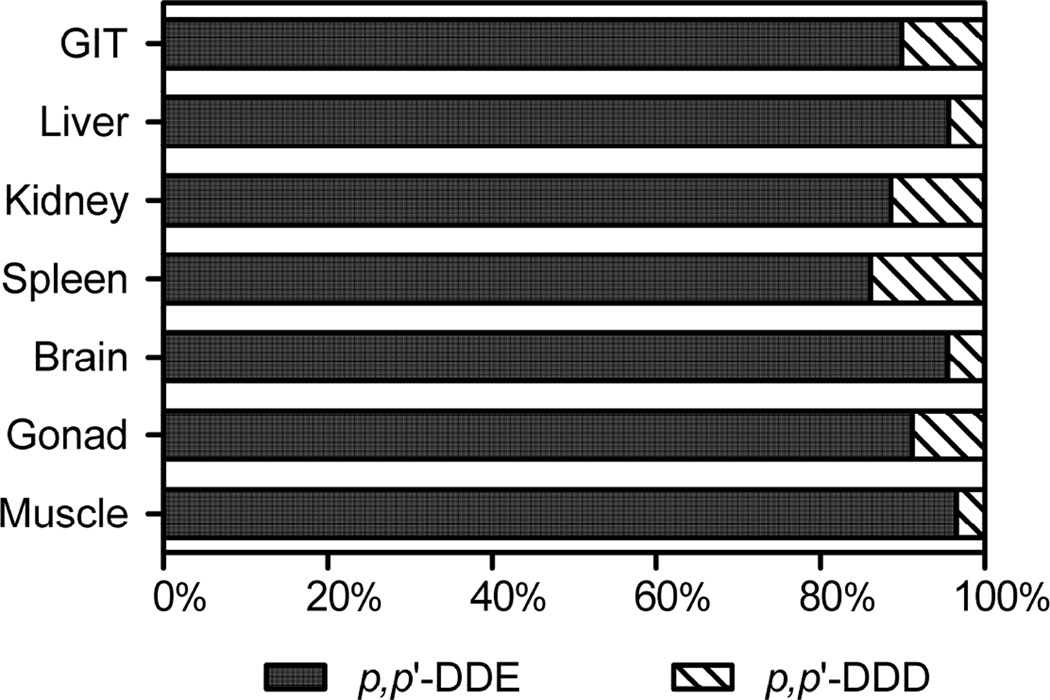
The composition of DDXs in Lake Apopka LMB is shown in Figure 3. p, p’-DDT was below the LOQ suggesting no current source of DDT in the lake. p, p’-DDD contributed <10% of total DDXs, while p, p’-DDE contributed >90% of total DDXs in tissues. Tissue distribution of p, p’-DDE among other DDT congeners were similar to results from previous studies indicating that p, p’-DDE represents 90 – 97% of DDXs in biological samples (Storelli et al., 2009; Weijs et al., 2010). Because DDT is dechlorinated to p, p’-DDE under anaerobic condition (Huang et al., 2001) and fish are also known to metabolize DDT (Vives et al., 2005; Kwong et al., 2008), the prevalence of p, p’-DDE in Lake Apopka LMB might reflect both direct uptake and the metabolic transformation of p, p’-DDT to p, p’-DDE in fish. For HCHs, only γ-HCH isomer was measured while the other three isomers were not detected in any of the fish tissues. Concentrations of γ-HCH in GIT and spleen were below the LOQ while for the other tissues the concentration of γ-HCH ranged from 0.6 to 4.3 ng/g ww. Technical HCH usually contains α-HCH (55–80%), β-HCH (5–14%), γ-HCH (8–15%), and δ-HCH (2–16%), while lindane contains more than 98% of γ-HCH (Ge et al., 2013). The composition of HCHs in LMB tissues did not appear to follow the technical grade composition. Since the degradation rate trend of HCH isomers is as follows: β> γ> α (Kouras et al., 1998), the measurement of γ-HCH in LMB may indicate a recent contamination and/or the higher historical application of γ-HCH in lindane rather than technical HCH deposition into Lake Apopka, FL.
Figure 3.
Composition of DDT related compounds in different tissues of wild-caught largemouth bass from Lake Apopka, FL. Composition was calculated as the percent of total concentration of each DDT congeners. Levels of p, p’-DDT, if detectable at all, were insignificant.
The north shore area of Lake Apopka, FL, has been the subject of many studies, but information about OCPs in Lake Apopka fish is still limited. Concentrations of p, p’-DDE in muscle tissues of Lake Apopka LMB in the current study (average of 15.3 ± 8.5 ng/g ww) were similar to concentrations of p, p’-DDE measured in muscle tissue of Lake Apopka brown bullheads (average of 23.0 ± 9.4 ng/g ww) (Gallagher et al., 2001). The St. Johns River Water Management District (SJRWMD) also conducted a Lake Apopka fish tissue monitoring survey for different types of species (e.g., LMB, brown bullhead catfish, black crappie, bluegill, killifish, and tilapia) between 2004–2006. This study showed that p, p’-DDE and p, p’-DDD were the most abundant chemicals in LMB (average of 14.9 ± 6.9 and 2.4 ± 1.8 ng/g ww in muscle for p, p’-DDE and p, p’-DDD, respectively) while the other OCPs were below the LOQ (SJRWMD, 2006). Concentrations of p, p’-DDE in muscle tissue measured from 2004 to 2006 were not different from the concentrations measured in fish collected in 2013 in our study, but levels of p, p’-DDD in 2004–2006 fish muscle samples (average of 2.4 ± 1.8 ng/g ww) were 5-fold higher than those of p, p’-DDD in our study (average of 0.5 ± 0.3 ng/g ww). The discrepancy could be attributed to the different species investigated in Lake Apopka as the previous study measured p, p’-DDD in brown bullheads and catfish which are bottom feeder as compared to bass that feed above the bottom and/or p, p’-DDD has been further metabolized, but p, p’-DDE is persistent. The tissue distribution of OCPs in Lake Apopka LMB reported here are, however, similar to results from other water bodies, which showed the highest OCP residues on a ww basis in the brain of wild-caught rainbow trout (Sahagun et al., 1997) and silver carp (Zhou et al., 2007).
4. Conclusions
Wild fish bioaccumulate contaminant loads mainly through their diet from chronic exposures. Although the liver receives toxicant via blood directly from the GIT, it is a major site of metabolism and detoxification. However, lipid stored in the liver may equilibrate with hydrophobic metabolites and it is known to be relatively high in contaminant load. Our results suggest that the brain also should be considered as a suitable organ for monitoring of long-term accumulation of contaminants in the environment. Our data also suggest that chemicals may take time to reach the brain and cross the blood-brain barrier and thus this might be a good organ to monitor for chronic exposure. To our knowledge, little information is available about the bioaccumulation of OCPs and absorption/desorption rate of OCPs into the brain of organisms like fish, and this warrants further study. The concentrations of OCPs in the brain will depend on the KOW and the ability of the chemical to cross the piscine blood-brain barrier, and this will likely be compound specific.
Tissue distribution of organochlorine pesticides in laboratory exposed and wild-caught largemouth bass was investigated.
Chemical concentrations were the highest in gastrointestinal tract for orally dosed bass.
Bioaccumulation of pesticides in tissues was associated with their KOW values.
Lipid normalized concentrations of pesticides were similar in each tissue of wild-caught fish.
Acknowledgments
We thank Derek Bolser, Ignacio Rodriguez, and Wes Porak (Florida Fish and Wildlife Research Institute, Florida Fish and Wildlife Conservation Commission, Eustis, FL 32726 USA for help during sampling. Funding for this research was supported by the National Institute of Environmental Health Sciences (NIEHS) (Award Numbers R01-ES020899).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boon JP, Oostingh I, van der Meer J, Hillebrand MTJ. A model for the bioaccumulation of chlorobiphenyl congeners in marine mammals. Eur. J. Pharmacol. 1994;270:237–251. doi: 10.1016/0926-6917(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Bruijn JD, Busser F, Seinen W, Hermens J. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environ. Toxicol. Chem. 1989;8:499–512. [Google Scholar]
- Clark KE, Gobas FA, Mackay D. Model of organic chemical uptake and clearance by fish from food and water. Environ. Sci. Technol. 1990;24:1203–1213. [Google Scholar]
- Colborn T, von Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health. Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JP, Pedersen CJ. A thermodynamic-based evaluation of organic chemical accumulation in aquatic organisms. Environ. Sci. Technol. 1988;22:99–103. doi: 10.1021/es00166a011. [DOI] [PubMed] [Google Scholar]
- Dang VD, Kroll KJ, Supowit SD, Halden RU, Denslow ND. Bioaccumulation of legacy and emerging organochlorine contaminants in Lumbriculus variegatus. 2016 doi: 10.1007/s00244-016-0264-x. In press http://dx.doi.org/10.1007/s00244-016-0264-x. [DOI] [PMC free article] [PubMed]
- Delaware Health and Social Services-Division of Public Health (DHSS) Organochlorine pesticides. 2010 http://dhss.delaware.gov/dhss/dph/files/organochlorpestfaq.pdf.
- Fisk AT, Norstrom RJ, Cymbalisty CD, Muir DC. Dietary accumulation and depuration of hydrophobic organochlorines: bioaccumulation parameters and their relationship with the octanol/water partition coefficient. Environ. Toxicol. Chem. 1998;17:951–961. [Google Scholar]
- Gallagher EP, Gross TS, Sheehy KM. Decreased glutathione S-transferase expression and activity and altered sex steroids in Lake Apopka brown bullheads (Ameiurus nebulosus) Aquat. Toxicol. 2001;55:223–237. doi: 10.1016/s0166-445x(01)00158-8. [DOI] [PubMed] [Google Scholar]
- Ge J, Woodward LA, Li QX, Wang J. Composition, distribution and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Sci. Total. Environ. 2013;452–453:421–426. doi: 10.1016/j.scitotenv.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Gobas FA, Muir DC, Mackay D. Dynamics of dietary bioaccumulation and faecal elimination of hydrophobic organic chemicals in fish. Chemosphere. 1988;17:943–962. [Google Scholar]
- Guo Y, Meng X, Tang H, Zeng EY. Tissue distribution of organochlorine pesticides in fish collected from the Pearl River Delta, China: implications for fishery input source and bioaccumulation. Environ. Pollut. 2008;155:150–156. doi: 10.1016/j.envpol.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Hong J, Kim H, Kim D, Seo J, Kim Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A. 2004;1038:27–35. doi: 10.1016/j.chroma.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hogarh JN, Seike N, Kobara Y, Ofosu-Budu GK, Carboo D, Masunaga S. Atmospheric burden of organochlorine pesticides in Ghana. Chemosphere. 2014;102:1–5. doi: 10.1016/j.chemosphere.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Liu SM, Kuo CE. Anaerobic biodegradation of DDT residues (DDT, DDD, and DDE) in estuarine sediment. J. Environ. Sci. Health B. 2001;36:273–288. doi: 10.1081/PFC-100103569. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee T, Chen K, Wong H, Zheng J, Giesy J, Lo K, Yamashita N, Lam P. Human health risk assessment of organochlorines associated with fish consumption in a coastal city in China. Environ. Pollut. 2005;136:155–165. doi: 10.1016/j.envpol.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Johnson WE. Native sport fish enhancement. III. Effects of pesticide contamination on reproductive hormone concentrations in sport fish. Eustis, FL: Report to the Florida Fish and Wildlife Conservation Commission; 1999. [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the USEPA-sponsored workshop. Environ. Health. Perspec. 1996;104(suppl. 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BC, Gobas FA, McLachlan MS. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ. Toxicol. Chem. 2004;23:2324–2336. doi: 10.1897/03-545. [DOI] [PubMed] [Google Scholar]
- Kouras A, Zouboulis A, Samara C, Kouimtzis Th. Removal of pesticides from aqueous solutions by combined physicochemical processes-the behavior of lindane. Environ. Pollut. 1998;103:193–202. [Google Scholar]
- Kwong RWM, Yu PKN, Lam PKS, Wang W. Uptake, elimination, and biotransformation of aqueous and dietary DDT in marine fish. Environ. Toxicol. Chem. 2008;27:2053–2063. doi: 10.1897/07-608.1. [DOI] [PubMed] [Google Scholar]
- Lanfranchi A, Menone M, Miglioranza K, Janiot L, Aizpun J, Moreno V. Striped weakfish (Cynoscion guatucupa): a biomonitor of organochlorine pesticides in estuarine and near-coastal zones. Mar. Pollut. Bull. 2006;52:74–80. doi: 10.1016/j.marpolbul.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Li W, Yang H, Jiang X, Liu Q, Sun Y, Zhou J. Residues and distribution of organochlorine pesticides in water and suspended particulate matter from Hangzhou Bay, East China Sea. Bull. Environ. Contam. Toxicol. 2016;96:295–302. doi: 10.1007/s00128-016-1739-1. [DOI] [PubMed] [Google Scholar]
- Li X, Gan Y, Yang X, Zhou J, Dai J, Xu M. Human health risk of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in edible fish from Huairou Reservoir and Gaobeidian Lake in Beijing, China. Food. Chem. 2008;109:348–354. doi: 10.1016/j.foodchem.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Doperalski NJ, Prucha MS, Kroll KJ, Conrow R, Barber DS, Denslow ND. High contaminant loads in Lake Apopka’s riparian wetland disrupt gene network involved in reproduction and immune function in largemouth bass. Accepted in Comp. Biochem. Physiol. D. doi: 10.1016/j.cbd.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Plack P, Thomson I. Relative concentrations of 14C-DDT and of two polychlorinated biphenyls in the lipids of cod tissues after a single oral dose. Arch. Environ. Contam. Toxicol. 1977;6:525–532. doi: 10.1007/BF02097791. [DOI] [PubMed] [Google Scholar]
- Ondarza PM, Gonzalez M, Fillmann G, Miglioranza KSB. Polybrominated diphenyl ethers and organochlorine compound levels in brown trout (Salmo trutta) from Andean Patagonia, Argentina. Chemosphere. 2011;83:1597–1602. doi: 10.1016/j.chemosphere.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Sahagun AM, Teran MT, Garcia JJ, Sierra M, Fernandez N, Diez MJ. Organochlorine pesticide residues in rainbow trout, Oncorhynchus mykiss, taken from four fish farms in Spain. Bull. Environ. Contam. Toxicol. 1997;58:779–786. doi: 10.1007/s001289900402. [DOI] [PubMed] [Google Scholar]
- Soupir CA, Brown ML, Kallemeyn LW. Trophic ecology of largemouth bass and northern pike in allopatric and sympatric assemblages in northern boreal lakes. Can. J. Zool. 2000;78:1759–1766. [Google Scholar]
- St. Johns River Water Management District (SJRWMD) Human health risk assessment update, Lake Apopka north shore restoration area (NSRA) Lake and Orange Counties, FL: 2011. AMEC Project#: 6063110187. [Google Scholar]
- SJRWMD. Lake Apopka organochlorine pesticide (OCP) fish tissue monitoring, Lake Apopka pesticide phase 2. Lake and Orange Counties, FL: 2006. [Google Scholar]
- Storelli MM, Losada S, Marcotrigiano GO, Roosens L, Barone G, Neels H, Covaci A. Polychlorinated biphenyl and organochlorine pesticide contamination signatures in deep-sea fish from the Mediterranean Sea. Environ. Res. 2009;109:851–856. doi: 10.1016/j.envres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Vives I, Grimalt JO, Ventura M, Catalan J, Rosseland BO. Age dependence of the accumulation of organochlorine pollutants in brown trout (Salmo trutta) from a remote high mountain lake (Redó, Pyrenees) Environ. Pollut. 2005;133:343–350. doi: 10.1016/j.envpol.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Weijs L, Elk C, Das K, Blust R, Covaci A. Persistent organic pollutants and methoxylated PBDEs in harbor porpoises from the North Sea from 1990 until 2008: Young wildlife at risk? Sci. Total. Environ. 2010;409:228–237. doi: 10.1016/j.scitotenv.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Yang N, Matsuda M, Kawano M, Wakimoto T. PCBs and organochlorine pesticides (OCPs) in edible fish and shellfish from China. Chemosphere. 2006;63:1342–1352. doi: 10.1016/j.chemosphere.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M. Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake-Lake Ziway, Ethiopia. Ecotoxicol. Environ. Saf. 2014;106:95–101. doi: 10.1016/j.ecoenv.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang L, Wu J, Fan C. Residual levels, tissue distribution and risk assessment of organochlorine pesticides (OCPs) in edible fishes from Taihu Lake, China. Environ. Monit. Assess. 2013;185:9265–9277. doi: 10.1007/s10661-013-3249-5. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wang Y, Zhang L, Cai Y, Chen Y. Bioaccumulation and tissue distribution of organochlorine pesticides (OCPs) in freshwater fishes: a case study performed in Poyang Lake, China’s largest lake. Environ. Sci. Pollut. Res. 2014;21:8740–8749. doi: 10.1007/s11356-014-2805-z. [DOI] [PubMed] [Google Scholar]
- Zhou RB, Zhu LZ, Kong Q. Persistent chlorinated pesticides in fish species from Qiantang River in East China. Chemosphere. 2007;68:838–847. doi: 10.1016/j.chemosphere.2007.02.021. [DOI] [PubMed] [Google Scholar]