Abstract
One carbon metabolism or methyl transfer, a crucial component of metabolism in all cells and tissues, supports the critical function of synthesis of purines, thymidylate and methylation via multiple methyl transferases driven by the ubiquitous methyl donor s-adenosylmethionine. Serine is the primary methyl donor to the one carbon pool. Intracellular folates and methionine metabolism are the critical components of one carbon transfer. Methionine metabolism requires vitamin B12, B6 as cofactors and is modulated by endocrine signals and is responsive to nutrient intake. Perturbations in one carbon transfer can have profound effects on cell proliferation, growth and function. Epidemiological studies in humans and experimental model have established a strong relationship between impaired fetal growth and the immediate and long term consequences to the health of the offspring. It is speculated that during development, maternal environmental and nutrient influences by their effects on one carbon transfer can impact the health of the mother, impair growth and reprogram metabolism of the fetus, and cause long term morbidity in the offspring. The potential for such effects is underscored by the unique responses in methionine metabolism in the human mother during pregnancy, the absence of transsulfuration activity in the fetus, ontogeny of methionine metabolism in the placenta and the unique metabolism of serine and glycine in the fetus. Dietary protein restriction in animals and marginal protein intake in humans causes characteristic changes in one carbon metabolism. The impact of perturbations in one carbon metabolism on the health of the mother during pregnancy, on fetal growth and the neonate are discussed and their possible mechanism explored.
Keywords: Protein restriction, Homocysteine, Pregnancy, Fetus, Growth, One carbon metabolism
1. Introduction
Pregnancy related clinical disorders such as eclampsia, spontaneous fetal loss, premature birth, birth defects, and fetal growth retardation are major contributors to perinatal morbidities worldwide. A large body of data exists in the literature relating a number of environmental, metabolic and endocrine signals to these morbidities. Amongst these, intrauterine growth retardation and small for gestational age is perhaps a major contributor both for its immediate effects on the neonate and for its relationship with the long term health of the offspring. Low birth weight, defined as weight less than 2500gm at term gestation, a result of intrauterine growth restriction (IUGR) remains a critical public health problem in developing countries. For example, in India it represents almost 25 to 30% of all births and is a major contributor to perinatal and neonatal morbidity and mortality and to subsequent impaired growth and stunting (Sachdev, 2001). The impact of intrauterine growth retardation and consequent low birth weight on the long term health of the offspring has now been established in large epidemiological studies in humans from different parts of the world and shown in animal models (Barker, 1994; Hales, 1997; Yajnik et al, 1995; Godfrey KM, 1998; Ozanne and Hales, 2002; Whincup et al, 2008; Warner and Ozanne, 2010; Kalhan and Wilson-Costello, 2013). The relationship between fetal growth and its regulation and nutritional, and endocrine interactions between the mother, the placenta and the fetus has been studied extensively (Murphy et al, 2006; Fowden and Forhead, 2009). In relation to fetal mass, these studies have examined the regulation of nutrient (glucose, amino acids and fatty acids) transport across the placenta, their regulation and the direct contribution of these nutrients to fetal carbon accretion (Riskin-Mashiah et al, 2009; Higgins et al, 2010; Resnik R, 2002; HAPO study, 2009; ). However, certain amino acids, such as methionine, serine, glycine, not only contribute to protein mass, but also by their role in one carbon metabolism, play a unique role in the regulation of cellular metabolism, cell proliferation and may impact fetal growth. Methionine, an essential or indispensable amino acid and a component of all proteins, is also the immediate source of the methyl (one carbon) groups required for the methylation of nucleic acids, proteins, biogenic amines, and phospholipids (Brosnan and Brosnan, 2006). Methionine and folate are the key constituents of one carbon transfer, providing the one carbon units for the numerous methyl transferase reactions. Since the methionine and folate cycles are ubiquitously present in every cell in the body and participate in key metabolic reactions, in DNA synthesis and by methylation of DNA in gene expression, perturbation in their metabolism either by nutrient deficiency, or by nutrient, hormonal and environmental interactions can have profound impact on the cell function, metabolism, growth and proliferation. This may have its greatest impact on the growing embryo and the fetus. The purpose of this review is to present the physiological adaptations in one carbon metabolism in pregnancy, its perturbations by nutritional influences and the consequences to the mother and the neonate. Since the long term consequences of intrauterine growth restriction have been reviewed several times in recent years, the present review focuses on the effects of nutrition mediated perturbations in one carbon metabolism and their impact on the health of the mother and the newborn infant.
2. One Carbon metabolism
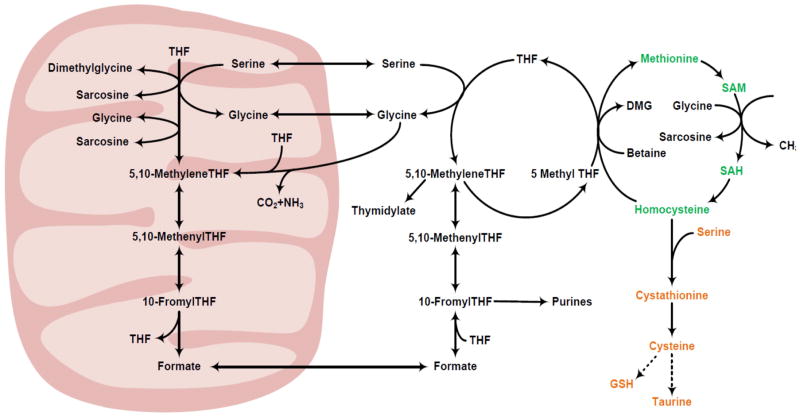
One carbon transfer, a crucial component of cellular metabolism, is comprised of folate and methionine cycles and supports the critical function of the synthesis of thymidylate, purines and methyl transferase reactions. The methionine and folate cycles are ubiquitously present in eukaryote cells, and participate in key metabolic reactions, in DNA synthesis and via methylation reactions in the expression and regulation of numerous genes and their activity and may cause epigenetic changes. Nutritional, environmental, endocrine and other disruptions that can affect one carbon metabolism may result in profound change in cell function, metabolism, growth and proliferation. This may be most conspicuous during cellular growth and proliferation such as growing embryo, fetus and malignancy. A brief description of folate mediated one carbon transfer and of methionine metabolism follows. The reader is referred to outstanding scholarly reviews for details (Tibbetts and Appling, 2010; Christensen and MacKenzie, 2006; Stover and Field, 2011; Fox and Stover, 2008, Lu and Mato, 2012). The key features of one carbon metabolism are displayed in figure 1. As shown, the metabolism of folate and methionine are closely entwined and results in the transfer of methyl groups of serine and glycine for the numerous methyltransferase reactions. Methionine, an indispensable or essential amino acid is the immediate source of the methyl (one carbon units) groups required for the methylation of proteins, phospholipids, biogenic amines, nucleic acids and synthesis of creatine. The metabolism of methionine is composed of the ubiquitously present transmethylation cycle and the transsulfuration pathway. The transmethylation cycle involves the initial conversion of methionine and ATP into s-adenosylmethionine (SAM or AdoMet) catalyzed by methionine adenosyltransferase. SAM is the universal bioactive methyl donor and donates its methyl group to a large number of methyl acceptors catalyzed by methyltransferases. S-adenosylhomocysteine (SAH) is the byproduct of the methyltransferase reactions. SAH is reversibly cleaved into homocysteine and adenosine by SAH hydrolase. Homocysteine is remethylated to form methionine either by methionine synthase which requires vitamin B12 as a cofactor or by betaine homocysteine methyltransferase which uses betaine as the methyl donor. The methyl group for the remethylation of homocysteine by methionine synthase is donated by 5-methyl tetrahydrofolate (5 methyl THF), an intermediary in the folate cycle. Methionine is catabolized via transsulfuration cascade, present in the liver, pancreas, intestine, and kidney and possibly in the brain. Transsulfuration involves the transfer of sulfur (thiol) of homocysteine to serine to form cysteine and alpha ketobutyrate. The reactions are catalyzed by B6 dependent enzymes, cystathionine beta synthase and cystathionine gamma lyase. The carbon skeleton of homocysteine enters the TCA cycle as propionyl CoA formed by the decarboxylation of alpha ketobutyrate. Cysteine is the precursor of taurine as well as a component of glutathione. It is significant to note that the transsulfuration pathway is not active in the human fetus in utero and appears rapidly immediately after birth (reviewed by Kalhan and Bier, 2008; Kalhan and Marczewski, 2012). In addition to the requirement of B12, folate and B6 as cofactors, methionine metabolism is also effected by dietary protein intake (discussed below) and by the redox state. In addition insulin and glucagon modulate the metabolism of methionine by regulating the transsulfuration cascade, or by remethylation of homocysteine and indirectly by their effect on whole body turnover of proteins.
Figure 1.
Pathways of cellular one carbon metabolism (details in text).Green: transmethylation cycle; yellow: transsulfuration cascade; black; mitochondrial and cytosolic folate metabolism pathway.
Intracellular metabolism of folate is compartmentalized between the cytoplasm and the organelles. (Tibbetts and Appling, 2010; Christensen and MacKenzie, 2006). Parallel cytosolic and mitochondrial pathways of folate mediated one carbon transfer and connected by one carbon donors serine and glycine have been described. It is postulated that serine, glycine and other methyl donors contribute to the mitochondrial one carbon pool of tetrahydrofolate (THF) to generate 5,10 methylene THF which is then oxidized by the mitochondria to formate and carbon dioxide and this formate is the source of methyl groups for the methylation of homocysteine in the cytoplasm. The expression of the mitochondrial metabolism varies in tissues during development and in other physiological and pathological states. The exact role of the interaction between mitochondrial and cytosolic one carbon transfer in human health and development remains to be identified.
2.1 Dietary protein and one carbon metabolism
Dietary protein restriction during pregnancy has been used extensively in rodents to examine the influence of maternal metabolic and nutritional environment on the immediate and long term health of the offspring. A number of outstanding studies have shown that dietary protein restriction early or late in pregnancy or during the lactation period causes fetal and neonatal growth restriction and predisposes the offspring to the development of obesity, type 2 diabetes and insulin resistance. The mechanism/s responsible for these long term consequences has not been delineated. In this context it is of interest that lower protein intake in both humans and animals has been shown to cause hyper-homocysteinemia and perturbations in one carbon metabolism. In the following section a brief discussion of these data are presented.
It is important to underscore the difference in metabolic and physiologic responses to isocaloric protein malnutrition and protein energy malnutrition. While the former is more often the consequence of lack of understanding of good nutrient habits and cultural constraints, the latter is the result primarily of poverty and economic adversity. Protein energy malnutrition results in responses that are similar to those of starvation i.e. increased gluconeogenesis, lipolysis, proteolysis, and negative nitrogen balance leading to loss of body (fat and protein) mass. A number of carefully done physiological studies have documented in detail the temporal metabolic responses to starvation in healthy and obese humans (Reviewed by Cahill GF Jr, 2006). In contrast iso-caloric protein malnutrition leads to suppression of proteolysis and maintenance of lean body mass and may lead to actual gain in fat mass. The metabolic responses in particular those related to protein and amino acid metabolism have been examined in both humans and animals. Data from studies of kwashiorkor in children had described alterations in plasma amino acid concentration in response to lower protein intake (Antener et al, 1981;Whitehead and Dean, 1964;1964). While data of human observational studies of kwashiorkor are not easy to interpret because of varied duration of protein malnutrition, and because of associated comorbidities such as infections, nonetheless these data showed an increase in plasma concentration of non-essential amino acids (NEAA) and decrease in essential amino acids (EAA) so that the ratio of NEAA/EAA was increased at the time of admission to the hospital and decreased in response to nutritional rehabilitation. The potential mechanism/s of such responses could not be examined in these early studies. Adibi (1968) examined the amino acid responses to dietary protein restriction in humans. In a study of four young subjects placed on completely protein free but isocaloric diet for five days, there was a marked increase in plasma concentration of alanine, glycine and serine (Adibi SA, 1968). More recent data have further characterized these responses and elucidated the potential mechanism of such metabolic changes. In a carefully conducted observational study, Ingenbleek and colleagues (2002) demonstrated that subclinical protein malnutrition in a goiterous population of sub-Saharan Africa was a significant risk factor for the development of hyperhomocysteinemia. Additionally the plasma levels of transthyretin, a measure of protein malnutrition, progressively decreased with the severity of goiter. The plasma transthyretin levels were significantly related to plasma homocysteine levels. The increase in the size of goiter was related to a decrease in BMI, Plasma albumin and transthyretin levels. It is significant to note that the plasma levels of B6 and folate were in the normal range and that plasma B12 levels were lower (165+/−35 pmoles/l) in the most severe (stage 3) goiter. The authors concluded that since cystathionine levels were low, and homocysteine levels were high, their data suggested an acquired defect of cystathionine beta synthase activity as a consequence of subclinical protein malnutrition. They speculated that these adaptive responses were aimed at preserving methionine homeostasis because of the critical role of methionine in one carbon transfers and other critical metabolic functions. The authors based their conclusion upon the observation that plasma methionine levels were preserved in spite of low levels of other essential amino acids in the most malnourished subjects. However their data could not definitively separate the role lower B12 status from that of protein malnutrition.
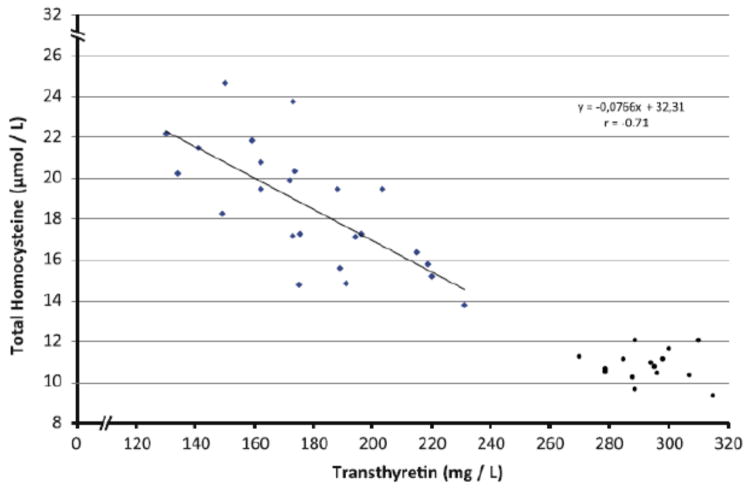
Ingenbleek and McCully (2012) addressed this question in a later study of vegetarian subjects from the Sahel region of Chad, Africa. They compared 24 rural young (18 to 30 yrs old) male subjects with 15 urban male control subjects.The rural subjects were mostly vegetarian in their dietary habits with ocassional intake of meat. The body weight, body mass index, and markers of protein status were lower in the rural subjects as compared with the urban controls. However, there was no difference in the plasma levels of vitamins B6, B12 and folate amongst the two groups. Interestingly the plasma levels of homocysteine were markedly increased and those of cysteine and glutathione significantly decreased in the study subjects suggesting inhibition of transsulfuration pathway possibly related to lower intake of protein and methionine in the diet. As shown in the Figure 2, a strong negative correlation was observed between plasma transthyretin and homocysteine levels suggesting that protein malnutrition and possibly lean body mass may be critical determinant of hyperhomocysteinemia. Other invetigators have made similar observations in vegetarian subjects, although under different clinical and experimental conditions and their data are confounded by somewhat lower levels of B12 in the study group (Huang et al, 2003; Mann et al, 1999;Hung et al, 2002).
Figure 2.
Relationship between transthyretin and total homocysteine concentration in the plasma in subjects with protein malnutrition (diamonds) and control (circles) subjects. Reproduced from Ingenbleek et al, 2012 with permission.
Studies using various animal models have confirmed several of these observations in humans and have further explored the possible mechanism/s and biological mediators. However these data should be examined with care because of the large variations in study design such as ad libitum feeding vs pair feeding, protein restriction vs protein free diet, and the duration of dietary protocol. In a similar experimental design of protein free iso-caloric diet in rats, Adibi et al (1973) showed a continuous decrease in essential amino acids starting at day 2 and a marked increase in plasma serine levels.
The mechanism of hepatic amino acid and fatty acid responses to decreased protein intake remain to be fully elucidated. Studies in-vitro and in in-vivo animal models of feeding strategies and of gene knockout are providing some interesting insights into the complex interactions. As summarized above, gene expression data in the livers of rats exposed to low protein intake suggested decreased lipid synthesis, increased fatty acid oxidation, decreased urea synthesis and high rate of synthesis of serine (Kalhan et al, 2011). The authors attributed the changes in the lipid metabolism to the higher expression of Peroxisome Proliferator Activated Receptor alpha (PPARα) observed in their study while the mechanism of changes in the hepatic amino acid metabolism could not be determined. PPARα, a member of a subfamily of nuclear receptors and highly expressed in the liver, plays a key role in the regulation of multiple metabolic pathways (Kersten, 2014). These include regulation of hepatic lipid metabolism by regulation of a number of genes involved in fatty acid uptake, intracellular fatty acid binding, fatty acid oxidation, gluconeogenesis, and bile synthesis and secretion (Kersten 2014). More recently, studies using PPARα knock-out mice and PPARα agonist show that activation of PPARα in addition to its effect on lipid metabolism also markedly impacts amino acid metabolism (Sheikh et al 2007, Kersten FASEB 2001). Oligonucleotide microarray analysis of livers of rats exposed to PPARα agonist showed that PPARα modulates a number of genes involved in transamination and deamination of amino acids and urea synthesis (Kersten et al, 2001) In a recent study Ericsson and colleagues (2014) observed that administration of PPARα agonist WY 14,643 to rats resulted in 38% increase in total amino acid concentration in the plasma, largely explained by increases in serine, glycine and threonine. In addition the concentration of arginine in the plasma was lower and that of citrulline and the concentration of ornithine higher in the experimental group.
Interestingly hepatic arginase activity was also lower in the animals receiving the PPARα agonist, although the total urinary nitrogen in the urine was unchanged. Tracer isotope data showed a higher rate of appearance of serine and glycine in the plasma of animals treated with PPARα agonist. Thus several of the changes in amino acid metabolism, especially those of glycine and serine seen with dietary protein restriction can be attributed to the increased hepatic expression of PPARα, also seen in those animals (Kalhan et al, 2011).
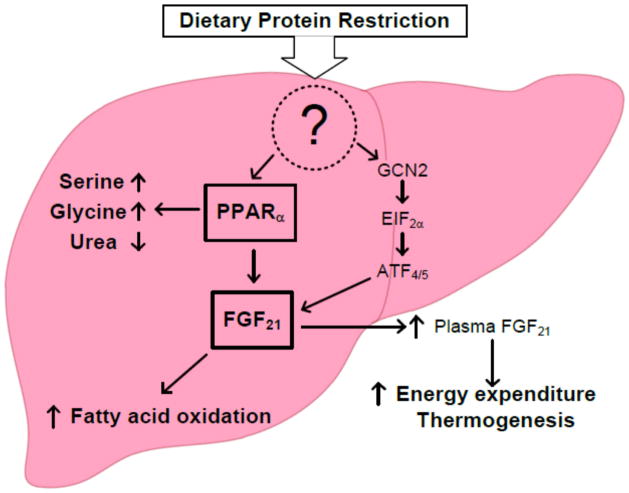
Laeger and colleagues identified FGF21 as another endocrine signal to dietary protein restrictions in rodents and in humans. FGF21, a peptide hormone secreted by many tissues including the liver has a number of effects on energy metabolism. These include thermogenesis in the brown adipose tissue, increased hepatic fatty acid oxidation, weight loss, and improved glycemia (reviewed by Fisher and Maratos-Flier, 2016). In a series of interesting studies, Laeger and colleagues (Laeger JCI 2014) show that dietary restriction of proteins, in the presence of maintained energy intake, in ad lib fed rodents, there was a marked increase in plasma levels of FGF21 in mice and in rats. This was associated with a rapid expression of FGF21 mRNA in the liver within 24 hours and sustained through day 2 and day 4. Interestingly, there was no effect of low protein diet on the expression of FGF21 in the skeletal muscle or white adipose tissue. Additionally the previously observed changes in hepatic amino acid metabolism i.e. increased expression of 3-phosphoglycerate dehydrogenase and asparagine synthase and changes in plasma amino acids (increase in glycine, serine, glutamine and alanine) were confirmed in their studies. These data were confirmed in human subjects participating in a controlled trial and placed on dietary protein restriction without energy restriction. The authors measured the plasma levels on day 28 of the study protocol and observed a 171% increase in plasma FGF21 levels in LP-fed subjects. Surprisingly they did not see any change in the expression of PPARα or any change in the expression of PPARα responsive genes in the liver. However, there was significant increase in the phosphorylation of the eukaryotic initiation factor 2a (EIF2a) in their studies showing increase in FGF21. Since serine\threonine kinase general control nonderepressible 2(GCN2) has been shown to phosphorylate EIF2a in response to amino acid depletion (starvation), and leads to inhibition of general protein translation, the authors examined whether GCN2 was involved in the observed responses. By using GCN2 knockout and PPARα knockout mice they show that both GCN2 and PPARα contribute to the regulation of FGF21 response to protein restriction. Figure 3 summarizes the possible effects of decreased dietary protein intake on hepatic metabolism.
Figure 3.
Effects of dietary protein restriction upon hepatic metabolism. A low protein intake, via an unknown mechanism possibly via change in intracellular amino acid levels, causes changes in hepatic amino acid and lipid metabolism. The changes in amino acid metabolism, mediated by PPARα, result in increased biosynthesis of serine and glycine aimed at provision of one carbon units and a decreased production of urea aimed at conservation of nitrogen. Dietary protein restriction also induces FGF21 either via PPARα or via a general stress response. Increased FGF21 causes an increase in fatty acid oxidation and (not shown) decreases fatty acid synthesis. Increase in hepatic FGF21 results in higher levels of FGF21 in the plasma which cause an increase in thermogenesis and energy expenditure in the brown adipose tissue. GCN2: general control nonderepressible 2; EIF2α: eukaryotic initiation factor 2 alpha; ATF4/5: activating transcription factor 4 and 5.
Although it is difficult to compare the many studies cited above because of differences in the study design and the experimental perturbations, a compelling description of the responses to and mechanism of protein restriction in humans and animals can be discerned from these data. These studies show that subclinical protein intake along with isocaloric energy intake, in humans and animals, result in distinct metabolic responses characterized by decreased protein turnover and oxidation (skeletal muscle), increased fatty acid oxidation and decreased fatty acid synthesis in the liver and down regulation of urea cycle enzymes in the liver. In relation to one carbon metabolism, these data show an induction of serine biosynthesis in the liver (and possibly kidney), high rate of transmethylation, and a decreased rate of transsulfuration. In humans such a paradigm results in increased plasma levels of homocysteine and decreased levels of cysteine and glutathione and an increases in plasma levels of key one carbon donors, glycine and serine (Katre et al, 2015).
Few studies have examined the effects of high protein intake on the whole body metabolism. These data show adaptive responses to dispose of the additional carbon as increased gluconeogenesis and lipogenesis and increase in the activity of cystathionine beta synthase, the catabolic pathway of methionine/homocysteine (Azzout-Marniche et al, 2007; Sarr et al, 2011; Yamamoto et al 1996)’
3. One Carbon Metabolism in Pregnancy
Detailed adaptive responses in one carbon metabolism have not been interrogated in healthy women during pregnancy. Most of these studies have been aimed at measurements of plasma levels of markers of one carbon metabolism such as homocysteine and regulatory cofactors in relation to pregnancy related complications and in relation to fetal health, specifically birth defects and birth weight. Additionally longitudinal changes during pregnancy have been evaluated only in cross-sectional studies. These data are confounded by clinically recommended supplementation with vitamins during pregnancy and by the mandatory fortification of cereal and flour with folic acid in some countries.
3.1 Vitamins
Specific responses in concentration of vitamins and other substrates in the plasma have been observed in healthy women with advancing gestation. Few studies have examined these changes longitudinally in nutritionally sufficient population who were not receiving supplemental vitamins. Since the current clinical practice recommends supplemental vitamins for pregnant women and with the increasing use of fortification of food, such studies cannot be done at the present time. Cikot and colleagues (Cikot et al, 2001) examined longitudinal changes in plasma concentration of a number of vitamins in healthy women with uncomplicated pregnancy and healthy outcomes. The study subjects were not taking vitamins or food supplements. A progressive decline in the plasma concentration of vitamin B12 was observed through gestation, reaching marginal or what would be considered deficient levels (150 picomoles/L) by 28–32 weeks gestation. The levels reached preconceptional levels by six weeks after delivery. A similar response was seen in plasma concentration of pyridoxal 5-phosphate. Serum folate showed a small decrease while red blood cell folate increased slightly during pregnancy. Stable isotopic tracer studies did not show any pregnancy related changes in the estimates of body folate pool size, catabolism, or and other measured components of folate metabolism (Gregory et al, 2001). Similar changes in plasma concentration of vitamins were observed by other investigators (Lopez-Quesada et al, 2003; Bruinse and van den Berg, 1995). These data are critical for the evaluation of studies examining the association of maternal nutritional status with perinatal health and in the design of clinical trials examining the impact of various intervention strategies. These physiological adaptive responses in changes in vitamin levels were not seen by us in healthy pregnant women receiving supplemental vitamins (Katre et al, unpublished data). The concentrations of B12, folate and B6 are markedly higher in the cord blood as compared with simultaneously obtained maternal blood suggesting an active transport of these vitamins across the placenta against a concentration gradient (Obeid et al, 2005; Ba et al, 2011). Placental folate transport has been shown to be established early in pregnancy providing folate for placental and fetal one carbon metabolism (Solanky et al, 2010). These data have been interpreted as suggesting a high demand by the fetus for these cofactors.
3.2 Amino acids
Serine Glycine
Serine, a dispensable or non-essential amino acid, plays a key role in one carbon metabolism in-vivo (Kalhan and Hanson, 2012). It is a major contributor to the one carbon pool and also is required for the formation of glycine, cysteine, taurine, phospholipids etc. Studies in rat and in humans show that during fasting, serine is synthesized in the kidney and released into circulation and is taken up by almost all organs including the liver and the skeletal muscle. Liver is quantitatively the most important consumer of serine (Brosnan and Hall, 1989; Brundin and Wahren, 1994). Stable isotopic tracer studies from Gregory’s group have shown that almost all of the methyl groups required for the whole body remethylation of homocysteine in humans are contributed by serine (Davis et al, 2004; Gregory JF III, 2000). In healthy pregnant women, a decrease in concentration of serine in the plasma occurs early in pregnancy and is sustained through gestation (Plasma serine conc. Non-pregnant 113+/− 24.5; Pregnant early, 71.9+/−6.2; late,68.5+/−9.6 μmole/l). The decrease in plasma concentration is accompanied with a decrease in whole body rate of serine turnover (Non-pregnant 152.9+/−42.8; pregnant early 123.7+/−21.5; late102.8+/−18.2 μmoles.kg-1.h-1.The physiological significance of these changes in relation to one carbon metabolism are not easy to delineate and primarily may represent the maternal responses aimed at conservation and accretion of nitrogen (Kalhan et al 2002, Kalhan SC, 2000). The decrease in turnover of serine in late pregnancy may also be the consequence of high turnover of serine and glycine in the fetal compartment resulting in high recycling of the isotopic tracer used in these studies and resulting in an underestimation of the flux (Kalhan SC, 2013). Such an inference will be consistent with the reported data in sheep fetus and discussed below.
Studies in the sheep have shown a high rate of one carbon transfer in the fetal liver and the placenta (Geddie et al, 1996; Cetin et al, 1991; 1992; Moores et al, 1994). In a series of studies in chronically catheterized fetal sheep preparation in-vivo, using various isotopic tracers and the application of Fick’s principle, Battaglia and colleagues have shown that: the uptake of serine and glycine by the uterus/placenta from the maternal circulation is low when compared with an essential amino acid like leucine; the serine taken up by the uterus/placenta is entirely metabolized (converted to glycine) in this compartment and is not transferred to the fetus; the concentration of serine and glycine in the fetal circulation is remarkably high ( serine ~600–700μM, glycine~400–600μM); there is a unique inter-organ flux of serine and glycine between fetal liver and placenta so that glycine released by the placenta into the fetal circulation is taken up by the liver and converted into serine which is then transported back to the placenta and converted to glycine. In addition the estimated serine flux in the fetal compartment using tracer dilution methods was remarkably high (42 μmol.min−1,kg−1 fetus) and approximately 8% of serine flux was decarboxylated to CO2. These high rates suggest an increased rate of entry of methyl groups into the one carbon pool in order to support methylation demands and nucleotide synthesis. The ontogenic pattern of the activity and expression of serine hydroxymethyltransferase in the fetal liver and in the placenta would suggest a high rate of one carbon metabolism in the fetus with advancing gestation (Narkewicz et al 1999). As discussed below, recent data of high formate in the fetal sheep are consistent with the high rate of turnover of the one carbon pool in the fetus. Whether a similar situation occurs in the human is not known, the high rate of transmethylation in late gestation in humans would be consistent with these observations. However, the relatively low activity of serine hydroxymethyltransferase in the human placenta (Lewis et al 2005) will also suggest that placenta may not play a big a role in serine-glycine metabolism in humans as in sheep.
Methionine
In contrast to serine, distinct changes in whole body metabolism of methionine are evident in healthy pregnant women during pregnancy. The fractional rate and the total rate of transsulfuration of methionine is shown to be significantly higher during the first trimester of pregnancy as compared with the non-pregnant women. In contrast, the rate of transmethylation was significantly higher in the third trimester of pregnancy (Dasarathy et al, 2010). The high rate of transmethylation may be related to high methylation demands in late gestation
3.3 Homocysteine
Homocysteine, the demethylation product of methionine, is the key branch point intermediate of methionine cycle and does not participate in protein synthesis (Figure 1). Data in healthy adults show that approximately 50% of homocysteine generated in the whole body is remethylated to form methionine and the rest is metabolized via the transsulfuration cascade (Young et al, 1991; MacCoss et al, 2001). The changes in plasma concentration of homocysteine are related to its intracellular metabolism. An increase in intracellular concentration as a result of decrease in metabolism (decreased transmethylation due to folate or B12 deficiencies or decreased transsulfuration due to B6 deficiency) will result in its release in circulation. A number of studies have shown a decrease in plasma homocysteine concentration during pregnancy (Dasarathy et al, 2010; Cikot et al, 2001; Ubeda et al, 2011; Murphy et al, 2002; Walker et al, 1999). The decrease is evident at 8–16 weeks of gestation with a further decrease during the second trimester (Walker et al, 1999). The reported data during the third trimester have been variable, some studies showing an increase in the third trimester relative to the levels in the second trimester while others show no significant change in the concentration (Walker et al, 1999;, Lopes-Queseda, et al, 2003; Cikot et al, 2001; Dasarathy et al, 2010). These differences may be related to the longitudinal vs. cross-sectional nature of the studies. Since 70 to 80% of plasma homocysteine is bound to albumin, Walker and colleagues suggested that the decrease in homocysteine levels may be related to the lower albumin concentration in the pregnant subjects (Walker et al, 1999). In their study, supplementation of pregnant subjects with folic acid resulted in higher plasma and red blood cell folate levels and lower plasma homocysteine concentration. Homocysteine is transported across the placenta to the fetus along a downward concentration gradient (Malinov et al, 1998; Molloy et al, 2002). The significantly lower concentration of total homocysteine in the umbilical artery vs. umbilical vein suggests utilization of homocysteine by the human fetus at term gestation (Malinov et al, 1998). Studies of isolated microvillous plasma membrane of human placenta show that homocysteine is transported across the placenta using system L, A, and y+L amino acid transporters with affinities similar to endogenous amino acids (Tsitsiou et al, 2009, 2011). Of these system L constitutes the primary transport system. Interestingly homocysteine could competitively inhibit transport of other amino acid raising the possibility of impacting syncitiotrophoblast metabolism and function as well as impacting fetal supply of essential nutrients (Tsitsiou et al, 2009).
3.4 Formate
Formate is a key intermediate in the cellular folate-mediated one carbon metabolism. Interest in the metabolism of formate was generated with the recognition of compartmentalization of folate in the cytosol and mitochondria of most living organisms (Appling; Christiansen). As discussed by Lamarre and colleagues (Morrow et al., 2015, Lamarre et al, 2013), serine, glycine, sarcosine and dimethylglycine are the major donors of the one carbon units to the tetrahydrofolate pool in the mitochondrium. The 5, 10-methylene tetrahydrofolate formed is oxidized through a series of reactions to form formate which can exit the mitochondrium and combine with THF to form 10-formyl-THF. As shown in figure 1, 10-formy-THF can be used for purine synthesis or can be further reduced and participate in the synthesis of thymidine or in methylation reactions. Folate and B12 deficiency by attenuating the methylation of homocysteine has been shown to result in high levels of homocysteine and formate in the plasma and urine of experimental animals (Lamarre et al, 2011; Deacon et al, 1990, Rabinowitz et al, 1958)
There are no published data on metabolism of formate in human pregnancy or neonate. Washburn and colleagues (Washburn et al, 2015) examined the concentration of formate in the mother, fetus and the neonate in the sheep. The animals were chronically cannulated and were studied at days 119–121 of gestation (full term ~147 days). They observed markedly high formate levels in the fetal plasma and in the amniotic fluid as compared with the maternal plasma. In addition the plasma formate levels were higher in the newborn lamb and persisted for approximately 8 weeks after birth. The mechanism/s of this increase could not be discerned in this study and was not related to deficiency of folate or B12 in the mother or the fetus. Since late gestation and the newborn period are times of maximal growth, there is a great demand for the one carbon groups for the synthesis of purines, nucleotides and for the many methylation reactions. The authors hypothesized that the high circulating formate in the fetus may be a means of providing one-carbon group to several tissues. The high levels of formate may be related to high rate of mitochondrial folate-mediated one carbon flux and as suggested by Fan and colleagues (Fan et al, 2014), it also may contribute NADPH for reductive biosynthesis. In the sheep fetus the high formate may be related to the high flux of serine and glycine in the feto-placental compartment (Kalhan and Hanson, 2012). Although a similarly high serine-glycine flux has not been observed in studies in human, it is of interest that the tracer isotope measured rates of transmethylation have been reported to be markedly high in pregnant women during the third trimester and in the human neonates studied soon after birth ( Dasarathy et al,2010; Thomas et al,2008). Future studies will define the role of formate in fetal one carbon metabolism and ascertain the perturbation in formate metabolism caused by nutrient (folate, B12, protein) insufficiencies and their impact on maternal and fetal health.
4. One carbon metabolism and the health of the mother, fetus and newborn
A number of nutrients that impact one carbon metabolism, have been examined extensively in human pregnancy, in both developed and underdeveloped countries representing well-nourished and undernourished populations, over many years and critically reviewed in the literature. These studies have examined either the habitual dietary intakes or responses to various nutrient supplements. The supplements have either been used singly or in combination as a nutrient mixture in both nutritionally sufficient and nutritionally insufficient subjects. Often the difference in dose and the design of the study makes the comparison of the data difficult. Most of these studies have used infant’s birth weight as the outcome measure. Baby’s birth weight, although an easily measurable outcome index, is a composite response to a large number of nutritional, endocrine and other maternal, placental and fetal contributors and may not necessarily represent the impact of nutrient examined. In the following, the data of nutrient that could impact one carbon metabolism in the mother or the fetus are presented.
4.1 Homocysteine
Increased levels of homocysteine in the plasma are an integrated response to a number of factors such as folate, B12, B6 and B2 deficiencies or a consequence of low dietary protein intake, certain gene polymorphisms such as methyl tetrahydrofolate and methionine synthase (Barbosa et al, 2008; Verhoef et al, 2006), renal disease or the result of endocrine disturbances. Non pregnant subjects with marginal intake of B vitamins show a correlation between the plasma concentration of homocysteine and folate, B12 and B6 (Katre et al 2015; Yajnik et al, 2014). Such a relationship is not consistently seen in nutritionally sufficient populations. Data from studies in adults show plasma homocysteine as an independent risk factor for cardiovascular, thromboembolic and other vascular disorders by impacting endothelial cell function (Hajjar, 2001; Werstuck et al 2001; Medina et al, 2001; Perla-Kajan et al, 2007). Elevated levels of homocysteine have been related to a number of pregnancy related disorders including early pregnancy loss (Nelen et al, 2000; Ronnenberg et al, 2007), preeclampsia (Lopez-Quesada et al, 2003, Vollset et al, 2000; Patrick et al, 2004), premature birth (Vollset et al, 2000), placental abruption or infarction (Goddijn-Wessel et al, 1996) and congenital heart defects (Hobbs et al, 2005). These effects of homocysteine have been attributed to its impact on vascular endothelial cell function, increased thromboembolic activity and oxidant activity (Eskes TKAB, 2000).
The impact of elevated homocysteine levels in the maternal plasma on fetal growth and birth weight in the humans has been of interest for a long time. It has been speculated that higher levels of homocysteine in the maternal plasma could impact fetal growth either directly by increased transport to the fetus or indirectly by its impact on maternal health and pregnancy related disorders. As discussed above, it has been difficult to evaluate the independent effect of homocysteine alone from that of co-existing nutrient insufficiencies and other confounders. A large study of nutritionally sufficient Dutch general population showed a negative correlation between maternal plasma homocysteine concentration measured at 30–34 weeks gestation and birth weight (Hogeveen et al, 2010). However the correlation was lost in a multivariate analysis. Regression analysis revealed maternal smoking, gestational age, and female sex were the strong determinants of birth weight in this study. A number of studies from nutritionally sufficient and nutritionally insufficient populations have shown an association between plasma homocysteine concentration of the mother and infant’s birth weight ( Vollset et al, 2000;Yajnik et al, 2005; Lindblad et al, 2005; Takimoto et al, 2007). A meta-analysis of 19 studies of over twenty one thousand subjects revealed a small increased risk for small for gestation age offspring in association with higher maternal homocysteine (Hogeveen et al, 2012). When expressed as a linear effect, a 1-SD increase in maternal homocysteine corresponded to a decrease of 31g in birth weight. The authors concluded that the small decrease in birth weight might be of little clinical relevance for the individual newborn; however it could be of importance at a population level. In contrast to all these data, one study from Montreal, Canada, showed that mothers with small babies had lower homocysteine concentrations than those giving births to larger ones (Infante-Rivard et al, 2003). Observational associations between homocysteine and birth weight, as discussed here are confounded by various nutritional and life style factors and may suffer from reverse causality. Mendelian randomization, as proposed by Katan, is an alternative method that uses genetic variations associated with lifestyle and other environmental exposures to account for these limitations (Smith and Ebrahim, 2004). Two studies have used Mendelian randomization to examine the relationship between maternal total homocysteine and offspring birth weight. Yajnik and colleagues (2014), in a study of two independent cohorts from India showed that increase in maternal homocysteine concentration resulted in a small decrease in birth weight and established a causal role for maternal homocysteine in fetal growth. A similar analysis by lee et al from Korea also showed a negative association between maternal homocysteine levels and birth weight; however the association did not reach statistically significant level (Lee et al 2013). Taken together these studies show that elevated total homocysteine concentrations in the mother appear to have a small but significant effect on fetal growth and birth weight. The mechanism of such effect and its long term impact on the health of the offspring remains to be determined.
4.2 Folate and B12 Vitamins
The effect of the alterations in the status of vitamins, folate, B12, B6 on one carbon metabolism during pregnancy and on maternal health has not been examined in humans or animal models in detail. From the published data it is difficult to extract the effect of individual vitamin, B12 alone or folate alone, on perinatal health because of the high frequency of low multi-nutrient status.
Only recently, interest in the impact of vitamin B12 on maternal and infant health has surged following the identification of low vitamin B12 status in the vegetarian populations and underscored by studies of developmental programing led by Yajnik and colleagues from Pune, India. A review of the literature relating B12 status and perinatal health has been published recently (Finkelstein et al, 2015). In relation to maternal health, Krishnaveni and colleagues (2009) suggested that low plasma vitamin B12 in pregnancy was associated with gestational ‘diabesity’ and later diabetes The authors observed that women with low vitamin B12 levels (B12 <150pmol/l) had significantly higher body mass index, sum of skin-fold thickness, insulin resistance and a higher incidence of gestational diabetes mellitus (GDM) as compared with non-deficient women. However the association with GDM became non-significant after adjusting for BMI. Interestingly, among B12 deficient women the incidence of GDM increased with increasing folate concentration. Furthermore vitamin B12 deficiency during pregnancy was positively associated with prevalence of diabetes at 5 years postpartum. The authors interpreted these data to suggest that vitamin B12 deficiency may be an important factor underlying the high risk of ‘diabesity’ in south Asian Indians. These are interesting data. However since the large proportion of their subject population was vegetarian with possibly lower protein and higher fat/carbohydrate intake, the observation could also be the result of their dietary habits leading to higher BMI, sum of skin fold thickness and lower B12. A compromised vitamin B12 status has been reported in a number of studies of lactovegetarian subjects (Antony AC, 2003, Pawlak et al, 2014). Plasma choline, betaine and dimethylglycine were lower at 36 weeks gestation in women who were folate replete but had low plasma B12 levels (Wu et al, 2013). Since dietary choline can be a source of methyl groups for one carbon metabolism, the authors suggested that the B12 and choline interaction could be a determinant of infant growth. Low vitamin B12 status has been associated with intrauterine growth retardation, but the data were confounded by a number of other contributors such as low socioeconomic status and pre-pregnancy body weight (Muthayya et al, 2006). Other studies from nutritionally sufficient populations have not observed any relationship between maternal B12 and IUGR (Takimoto et al, 2007). Low maternal B12 also has been shown to be associated with insulin resistance in the offspring (Yajnik et al, 2008; Stewart et al, 2011) and with neural tube defects (Molloy et al, 2009). Future studies examining the relationship between vitamin B12 and one carbon metabolism during pregnancy and childhood would be critical in defining the mechanism of these observations. Because of the established relationship between pre-conceptional folate status and neural tube defects, folate supplement during pregnancy has become the standard of care. Therefore few studies have examined directly the relationship between folate status and maternal and fetal health. These data show a positive correlation between maternal folate levels and baby’s birth weight (Lindblad et al, 2005; Relton et al, 2005; Villalpando S, 2008; Timmermans et al, 2009). Important interactions between maternal folate and B12 status and perinatal health have been reported, however they require further detailed mechanistic evaluation (Dwarkanath et al, 2013; Sargoor et al, 2010).
4.3 Protein
The impact of high and low protein diet during pregnancy and lactation has been studied extensively in animal models. In contrast the data in humans have been limited. Although studies of protein supplement and therefore higher protein intake can be conducted in humans, it is not ethically acceptable to restrict protein intake in the vulnerable pregnant population. Therefore the data in humans have been restricted to observational studies and are often confounded by associated changes in total energy and other nutrients. These data are not comparable with the experimental data in animals where most reported studies have examined the impact of dietary protein in the range of 6–8% of total energy intake. Such a drastic reduction in dietary protein is not seen in humans in the absence of associated decrease in energy intake, as seen in famine conditions or mass starvation. As discussed above the physiological responses to isocaloric protein restriction are markedly different from those seen with protein-energy malnutrition.
The effects of isocaloric protein restriction during pregnancy on maternal health and metabolic responses have not been examined in detail. In a previous study in rat, we had examined the effect of isocaloric protein restriction on the metabolism of the mother and translation initiation factors as indices of protein synthesis in the maternal and fetal liver (Parimi et al, 2004). These data showed that protein restriction in pregnancy resulted in a higher rate of oxygen consumption and lower levels of branched chain amino acids, lysine and histidine early in gestation suggestive of a lower rate of whole body protein turnover as was seen in the non- pregnant animals (Kalhan et al, 2011). Correspondingly and as anticipated the plasma urea nitrogen was lower in the protein restricted animals from day 10 to day 18 of gestation, followed by a marked increase till parturition. Interestingly, the concentration of non-essential amino acids, serine, glycine, and glutamine markedly increased on day 18 and 21, a period of rapid growth of fetal mass in the rat. Similar changes in glycine, serine and other amino acids were observed in other studies (Rees et al, 1999; Petrie et al, 2002). The phosphorylated 4E-BP1 (gamma form) in the maternal liver was 4-fold higher in the LP group. The phosphorylated eIF2alpha was higher in the livers of IUGR fetuses. The data on translation initiation factors suggest a higher rate of protein synthesis in the maternal liver and a lower rate in the fetal liver in response to protein restriction, the later could be part of a systemic response resulting in decrease in growth of the fetus. These data are in contrast to the non-pregnant animals where a simultaneous decrease in essential amino acids, suggesting a lower rate of whole body proteolysis, and an increase in serine and glycine suggestive of higher methylation demands or higher requirements of one carbon units and underscore the moderating influence of pregnancy on these responses. In summary, the initial maternal responses to protein restriction in rat could be aimed at conserving maternal nitrogen in the face of decreased intake and only later in the presence of high fetal demands, during the period of rapid increase in fetal mass that changes in amino acids related to one carbon metabolism were evident. Such responses may not be apparent in humans with relatively low fetal/maternal mass ratio as compared with rodents. Corresponding data of isocaloric protein restriction for human pregnancy cannot be developed because of associated risk. Additionally observational studies in humans also are confounded by accompanying lower energy intake and changes in the intake of other macro and micronutrients.
Studies in animal models, in particular rodents, have clearly demonstrated the effect of dietary protein restriction on the long term health of the offspring. Although the impact on the fetal growth and on birth weight of the pup was inconsistent in various studies, these data show that dietary protein restriction to 6–8% of metabolisable energy during pregnancy or lactation resulted in adiposity and insulin resistance (Snoeck et al, 1990),altered beta cell proliferation, islet size, islet insulin content and vascularization in the pancreas (Dahri et al, 1991; Fernandez-Twinn et al, 2004;), decreased renal mass and hypertension ( Chen et al, 2010;Jackson et al, 2002) ),impact longevity by affecting metabolic pathways implicated in the regulation of life span ( Chen et al, 2009) , alter circadian physiology, and appetite (Sutton et al, 2010). The mechanism/s of these programming effects of low protein intake have been examined and attributed to alterations in gene expression and epigenetic (methylation and acetylation of genes and histones) modifications in the fetal liver and placenta. Similar to dietary protein restriction, isocaloric high protein (40% protein) diet through pregnancy in rat, caused lower body weight of the pups on day 2 after birth (Daenzer et al, 2002). At age 3, 5 and 6 weeks, the pups born to high protein fed mothers exhibited higher body weight when compared with pair fed controls. In addition, these pups had greater relative and total fat mass and lower energy expenditure at age 9 weeks. In another study (Thone-Reineke et al, 2006) the effect of high protein feeding during pregnancy was noted to sex-dependent, increased blood pressure and glomerulosclerosis in the male offspring and higher body weight and increased fat pads in the female offspring. Other investigators have also shown programing effects of high protein exposure during pregnancy (Desclee de Maredsous et al, 2016). The maternal mediators of the observed programing effects and the impact of high protein diet on one carbon metabolism has not been studied in detail during pregnancy.
Most studies in humans are either observational of habitual nutrient intake or clinical trials of nutrient supplement. By their very nature, these studies are difficult to conduct and the data not easy to interpret because of a number of confounding variables even after statistical adjustments.
The association between maternal protein intake and fetal growth was examined by Blumfield and colleagues in healthy pregnant women (Blumfield et al 2012, Blumfield and Collins 2014). Fetal growth and composition was documented by serial ultrasound measurements and maternal diet was quantified by a validated food frequency questionnaire administered during first and third trimester. Their data showed an inverse correlation between maternal intake of protein and abdominal subcutaneous fat of the fetus at 36 week gestation. The abdominal fat was highest with low protein intake, less than 16% of energy, and the mid-thigh fat was highest when protein intake was 18–21% of energy, and high fat and low carbohydrate intake. A previous study of low income urban women had found a significant quadratic relationship between maternal protein intake and infant’s birth weight (Sloan et al 2001). These investigators observed the mean birth weight of infants born to mothers in the low (<50g/day) and high (>85g/day) protein groups were 67grams and 88 grams less than those mothers whose dietary intake of proteins was in the intermediate range. Other studies have shown a relationship between lower protein intake in late gestation and lower placental weight and neonatal birth weight (Godfrey et al 1996). The effect was more pronounced when lower protein intake in the third trimester was associated with higher carbohydrate intake in early pregnancy. A careful analysis of a number of clinical trials of high protein supplement by Kramer and recently updated by Ota and colleagues (Kramer 1993, Kramer 2000, Kramer and Kakuma 2003, Ota et al 2015) show that high protein supplement does not seem to be beneficial to the mother or the fetus and was associated with significant increased risk of small for gestational age infants. In summary, data from studies in human suggests that both high and low protein diet in pregnancy impacts fetal growth and results in lower birth weight. The mechanism of these effects remains to be delineated. Whether the observed changes are mediated via changes in maternal or fetal one carbon metabolism cannot be determined since none of the markers of one carbon metabolism were examined. In this context it is of interest that a high protein (21% of energy) supplement in healthy men caused a significant increase in postprandial total homocysteine concentration in the plasma (Verhoef et al 2005). Additionally energy and protein supplement in Indian underweight pregnant women did not impact serine and glycine kinetics (Dwarkanath et al 2016). The physiological and biological implication of these studies, in relation to maternal and neonatal health, requires further evaluation.
4.4 Multinutrient deficiency
Single nutrient deficiencies for example B12 deficiency (pernicious anemia) although occur in otherwise well-nourished populations are uncommon in the developing world particularly in relation to vitamins and protein, the nutrients that impact one carbon metabolism. In the undernourished populations of the world, more often there are multiple nutrient deficiencies along with decreased intake of energy and protein (Bhardwaj et al, 2013; Food and Nutrition Bulletin, 2008; Sukla et al, 2014). This is confounded by environment, economy, cultural and other societal factors that result in unique situations in different populations. While the incidence of low folate status has decreased markedly as a result of mandatory fortifications in some parts of the world, such is not the case where fortification is not mandated. Studies from India have shown lower folate, B12, iron status in children, adolescents and pregnant women. Particularly in children the lower micronutrient status has been associated with stunting, lower BMI and other parameters suggestive of compromised protein energy intake. Additionally there is a strong correlation between undernutrition in children and adults. In India interestingly, perhaps because of the vegetarian dietary habits, folic acid deficiency is not as common, however simultaneous deficiency of iron and B12 along with stunting and lower BMI are common (Refsum et al, 2001).
Both observational and interventional studies suggest that maternal nutritional status, rather than the pregnancy related disorders are the major determinants of IUGR. Although a number of intervention strategies (single or multiple micronutrients, protein, energy supplements) have been tested to prevent IUGR, the effect size has been small (Sachdev HPS, 2001; Christian et al, 2003), so that low birth weight remains a significant and persistent problem in the developing world. The small effect size in part may be due to the insufficiency of a number of micro and macronutrients, rather than a single nutrient, so that new approaches to evaluate integrated response to multiple nutrient insufficiencies are required in order to effectively prevent IUGR. Interventions based upon a single biomarker and a single nutrient are not likely to result in optimal outcomes. In relation to methionine and one carbon metabolism, three vitamins folate, B12 and pyridoxine are directly involved and insulin and glucagon exert their effect directly by regulating transsulfuration cascade or by effecting methionine synthase and indirectly by their effect on the whole body turnover of proteins (Jacobs et al,2001; Ratnam et al, 2002; Kalhan SC,2009a; Kalhan SC,2009b). Dietary restriction of proteins influences one carbon metabolism by increasing the biosynthesis of serine, increasing transmethylation and decreasing transsulfuration (Kalhan et al, 2011). These later responses are opposite of those due to folate and B12 deficiencies. Thus in case of multinutrient deficiencies, the expected responses would be an integration of responses to individual micronutrient deficiency or insufficiency. In a preliminary study, we have examined the effect of multinutrient insufficiency on parameters of one carbon metabolism in Indian women and compared these with nutritionally sufficient western women (Katre et al, 2015). The Indian women who were mostly vegetarian, were shorter and lighter compared with the Americans and had lower plasma levels of B12, folate and B6 also had lower plasma levels of essential amino acids and glutathione. The concentration of serine, glycine and homocysteine in the plasma was higher in the Indian subjects. There was a negative correlation between plasma B6 and homocysteine and between folate and serine and glycine. The observed changes in amino acids and markers of one carbon metabolism were likely the result of marginal protein intake and lower B12, folate and B6 status. Such integrated responses have not been interrogated during pregnancy. Only a comprehensive evaluation of these kinds of responses combined with isotopic tracer studies will allow the evaluation of the relationship between perturbations in one carbon metabolism and maternal and fetal health.
In summary, a unique adaptive response in nitrogen metabolism aimed at conserving nitrogen for protein accretion by the mother in anticipation of fetal demands is evident in early pregnancy in humans. In addition characteristic changes in methionine and one carbon metabolism are evident during gestation in the mother and during development in the fetus. The requirement of vitamin B12, folate, B6 and its regulation by environmental and endocrine signals makes methionine and one carbon metabolism particularly susceptible to these influences. Additionally the ubiquitous expression of components of methionine and one carbon metabolism in the body and its role in the numerous cellular processes including cell proliferation, methylation, and epigenetic modifications suggest that alterations in one carbon metabolism are likely to have profound effects on the health of the mother, the fetus with long term consequences for the offspring. Only rigorously conducted clinical studies in combination with appropriate animal models are likely to inform the mechanism of these sequelae and help develop intervention strategies.
Highlights.
One carbon transfers are critical component of intracellular metabolism of most eukaryotes.
Characteristic adaptations in one carbon metabolism are observed in the mother, fetus and placenta.
Perturbations in one carbon transfer can have profound impact on cell function, proliferation and growth.
Decreased dietary protein intake has profound effects on one carbon metabolism.
Environmental and nutritional influences, by affecting one carbon metabolism, impact the health of the mother, fetus and neonate with long term consequences.
Acknowledgments
The expert help of Ms. Manoa Hui in drawing the figures is gratefully appreciated. I apologize to the authors whose work was not cited in the manuscript. This was simply related to constraints of space and not to the importance of their work.
Funding: The cited work from the author’s laboratory was supported by grants from the National Institutes of Health, USA, HD11089, HD042154, DK079937, and HD073880.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi SA. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968;25:52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- Adibi SA, Modesto TA, Morse EL, Amin PM. Amino acid levels in plasma, liver, and skeletal muscle during protein deprivation. Am J Physiol. 1973;225:408–414. doi: 10.1152/ajplegacy.1973.225.2.408. [DOI] [PubMed] [Google Scholar]
- Antener I, Tonney G, Verwilghen AM, Mauron J. Biochemical study of malnutrition. Part IV. Determination of amino acids in the serum, erythrocytes, urine and stool ultrafiltrates. Int J Vitam Nutr Res. 1981;51:64–78. [PubMed] [Google Scholar]
- Antony AC. Vegetarianism and vitamin B-12 (cobalamin) deficiency. Am J Clin Nutr. 2003;78:3–6. doi: 10.1093/ajcn/78.1.3. [DOI] [PubMed] [Google Scholar]
- Azzout-Marniche D, Gaudichon C, Blouet C, Bos C, Mathe V, Huneau JF, Tome D. Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? Am J Physiol Regul Integr Comp Physiol. 2007;292:R1400–R1407. doi: 10.1152/ajpregu.00566.2006. [DOI] [PubMed] [Google Scholar]
- Ba Y, Yu H, Liu F, Geng X, Zhu C, Zhu Q, Zheng T, Ma S, Wang G, Li Z, Zhang Y. Relationship of folate, vitamin B12 and methylation of insulin-like growth factor-II in maternal and cord blood. Eur J Clin Nutr. 2011;65:480–485. doi: 10.1038/ejcn.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa PR, Stabler SP, Machado AL, Braga RC, Hirata RD, Hirata MH, Sampaio-Neto LF, Allen RH, Guerra-Shinohara EM. Association between decreased vitamin levels and MTHFR, MTR and MTRR gene polymorphisms as determinants for elevated total homocysteine concentrations in pregnant women. Eur J Clin Nutr. 2008;62:1010–1021. doi: 10.1038/sj.ejcn.1602810. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Kumar D, Raina SK, Bansal P, Bhushan S, Chander V. Rapid Assessment for Coexistence of Vitamin B12 and Iron Deficiency Anemia among Adolescent Males and Females in Northern Himalayan State of India. Anemia. 2013;2013:959605. doi: 10.1155/2013/959605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB, Raubenheimer D, Collins CE. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr. 2012;96:1032–1041. doi: 10.3945/ajcn.111.033241. [DOI] [PubMed] [Google Scholar]
- Blumfield ML, Collins CE. High-protein diets during pregnancy: healthful or harmful for offspring? Am J Clin Nutr. 2014;100:993–995. doi: 10.3945/ajcn.114.096511. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. The sulfur containing amino acids: an overview. J Nutr. 2006;136:1636s–1640s. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Hall B. Renal serine production in vivo: effects of dietary manipulation of serine status. Can J Physiol Pharmacol. 1989;67:1058–1061. doi: 10.1139/y89-167. [DOI] [PubMed] [Google Scholar]
- Bruinse HW, van den Berg H. Changes of some vitamin levels during and after normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1995;61:31–37. doi: 10.1016/0028-2243(95)02150-q. [DOI] [PubMed] [Google Scholar]
- Brundin T, Wahren J. Renal oxygen consumption, thermogenesis, and amino acid utilization during i.v. infusion of amino acids in man. Am J Physiol. 1994;267:E648–E655. doi: 10.1152/ajpendo.1994.267.5.E648. [DOI] [PubMed] [Google Scholar]
- Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- Cetin I, Fennessey PV, Quick AN, Jr, Marconi AM, Meschia G, Battaglia FC, Sparks JW. Glycine turnover and oxidation and hepatic serine synthesis from glycine in fetal lambs. Am J Physiol. 1991;260:E371–E378. doi: 10.1152/ajpendo.1991.260.3.E371. [DOI] [PubMed] [Google Scholar]
- Cetin I, Fennessey PV, Sparks JW, Meschia G, Battaglia FC. Fetal serine fluxes across fetal liver, hindlimb, and placenta in late gestation. Am J Physiol. 1992;263:E786–E793. doi: 10.1152/ajpendo.1992.263.4.E786. [DOI] [PubMed] [Google Scholar]
- Chen JH, Martin-Gronert MS, Tarry-Adkins J, Ozanne SE. Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS One. 2009;4:e4950. doi: 10.1371/journal.pone.0004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Tarry-Adkins JL, Matharu K, Yeo GS, Ozanne SE. Maternal protein restriction affects gene expression profiles in the kidney at weaning with implications for the regulation of renal function and lifespan. Clin Sci (Lond) 2010;119:373–384. doi: 10.1042/CS20100230. [DOI] [PubMed] [Google Scholar]
- Christensen KE, MacKenzie RE. Mitochondrial one-carbon metabolism is adapted to the specific needs of yeast, plants and mammals. Bioessays. 2006;28:595–605. doi: 10.1002/bies.20420. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, Adhikari RK, Sommer A, West KP., Jr Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326:571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikot RJ, Steegers-Theunissen RP, Thomas CM, de Boo TM, Merkus HM, Steegers EA. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85:49–58. doi: 10.1079/bjn2000209. [DOI] [PubMed] [Google Scholar]
- Daenzer M, Ortmann S, Klaus S, Metges CC. Prenatal high protein exposure decreases energy expenditure and increases adiposity in young rats. J Nutr. 2002;132:142–144. doi: 10.1093/jn/132.2.142. [DOI] [PubMed] [Google Scholar]
- Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(Suppl 2):115–120. doi: 10.2337/diab.40.2.s115. [DOI] [PubMed] [Google Scholar]
- Dasarathy J, Gruca LL, Bennett C, Parimi PS, Duenas C, Marczewski S, Fierro JL, Kalhan SC. Methionine metabolism in human pregnancy. Am J Clin Nutr. 2010;91:357–365. doi: 10.3945/ajcn.2009.28457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., III Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–E279. doi: 10.1152/ajpendo.00351.2003. [DOI] [PubMed] [Google Scholar]
- Deacon R, Perry J, Lumb M, Chanarin I. Formate metabolism in the cobalamin-inactivated rat. Br J Haematol. 1990;74:354–359. doi: 10.1111/j.1365-2141.1990.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Desclee de MC, Oozeer R, Barbillon P, Mary-Huard T, Delteil C, Blachier F, Tome D, van der Beek EM, Davila AM. High-Protein Exposure during Gestation or Lactation or after Weaning Has a Period-Specific Signature on Rat Pup Weight, Adiposity, Food Intake, and Glucose Homeostasis up to 6 Weeks of Age. J Nutr. 2016;146:21–29. doi: 10.3945/jn.115.216465. [DOI] [PubMed] [Google Scholar]
- Dwarkanath P, Hsu JW, Tang GJ, Anand P, Thomas T, Thomas A, Sheela CN, Kurpad AV, Jahoor F. Energy and Protein Supplementation Does Not Affect Protein and Amino Acid Kinetics or Pregnancy Outcomes in Underweight Indian Women. J Nutr. 2016;146:218–226. doi: 10.3945/jn.115.218776. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Turner N, Hansson GI, Wallenius K, Oakes ND. Pharmacological PPARalpha activation markedly alters plasma turnover of the amino acids glycine, serine and arginine in the rat. PLoS One. 2014;9:e113328. doi: 10.1371/journal.pone.0113328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes TK. Homocysteine and human reproduction. Clin Exp Obstet Gynecol. 2000;27:157–167. [PubMed] [Google Scholar]
- Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and Perinatal Health. Adv Nutr. 2015;6:552–563. doi: 10.3945/an.115.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Bulletin. Folate and vitamin B12 deficiencies: Proceedings of a WHO Technical Consultation; 18–21 October, 2005; Geneva, Switzerland. Jun, 2008. [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72:257–265. doi: 10.1159/000245927. [DOI] [PubMed] [Google Scholar]
- Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- Geddie G, Moores R, Meschia G, Fennessey P, Wilkening R, Battaglia FC. Comparison of leucine, serine and glycine transport across the ovine placenta. Placenta. 1996;17:619–627. doi: 10.1016/s0143-4004(96)80080-4. [DOI] [PubMed] [Google Scholar]
- Goddijn-Wessel TA, Wouters MG, van de Molen EF, Spuijbroek MD, Steegers-Theunissen RP, Blom HJ, Boers GH, Eskes TK. Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur J Obstet Gynecol Reprod Biol. 1996;66:23–29. doi: 10.1016/0301-2115(96)02383-4. [DOI] [PubMed] [Google Scholar]
- Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312:410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM. Maternal regulation of fetal development and health in adult life. Eur J Obstet Gynecol Reprod Biol. 1998;78:141–150. doi: 10.1016/s0301-2115(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Gregory JF, III, Cuskelly GJ, Shane B, Toth JP, Baumgartner TG, Stacpoole PW. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am J Clin Nutr. 2000;72:1535–1541. doi: 10.1093/ajcn/72.6.1535. [DOI] [PubMed] [Google Scholar]
- Gregory JF, III, Caudill MA, Opalko FJ, Bailey LB. Kinetics of folate turnover in pregnant women (second trimester) and nonpregnant controls during folic acid supplementation: stable-isotopic labeling of plasma folate, urinary folate and folate catabolites shows subtle effects of pregnancy on turnover of folate pools. J Nutr. 2001;131:1928–1937. doi: 10.1093/jn/131.7.1928. [DOI] [PubMed] [Google Scholar]
- Hajjar KA. Homocysteine: a sulph'rous fire. J Clin Invest. 2001;107:663–664. doi: 10.1172/JCI12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN. Metabolic consequences of intrauterine growth retardation. Acta Paediatr Suppl. 1997;423:184–187. doi: 10.1111/j.1651-2227.1997.tb18410.x. [DOI] [PubMed] [Google Scholar]
- HAPO study group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M, Mc AF. A review of maternal and fetal growth factors in diabetic pregnancy. Curr Diabetes Rev. 2010;6:116–125. doi: 10.2174/157339910790909431. [DOI] [PubMed] [Google Scholar]
- Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr. 2005;81:147–153. doi: 10.1093/ajcn/81.1.147. [DOI] [PubMed] [Google Scholar]
- Hogeveen M, Blom HJ, van der Heijden EH, Semmekrot BA, Sporken JM, Ueland PM, den HM. Maternal homocysteine and related B vitamins as risk factors for low birthweight. Am J Obstet Gynecol. 2010;202:572–576. doi: 10.1016/j.ajog.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Hogeveen M, Blom HJ, den HM. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr. 2012;95:130–136. doi: 10.3945/ajcn.111.016212. [DOI] [PubMed] [Google Scholar]
- Huang YC, Chang SJ, Chiu YT, Chang HH, Cheng CH. The status of plasma homocysteine and related B-vitamins in healthy young vegetarians and nonvegetarians. Eur J Nutr. 2003;42:84–90. doi: 10.1007/s00394-003-0387-5. [DOI] [PubMed] [Google Scholar]
- Hung CJ, Huang PC, Lu SC, Li YH, Huang HB, Lin BF, Chang SJ, Chou HF. Plasma homocysteine levels in Taiwanese vegetarians are higher than those of omnivores. J Nutr. 2002;132:152–158. doi: 10.1093/jn/132.2.152. [DOI] [PubMed] [Google Scholar]
- Infante-Rivard C, Rivard GE, Gauthier R, Theoret Y. Unexpected relationship between plasma homocysteine and intrauterine growth restriction. Clin Chem. 2003;49:1476–1482. doi: 10.1373/49.9.1476. [DOI] [PubMed] [Google Scholar]
- Ingenbleek Y, Hardillier E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition. 2002;18:40–46. doi: 10.1016/s0899-9007(01)00783-3. [DOI] [PubMed] [Google Scholar]
- Ingenbleek Y, McCully KS. Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition. 2012;28:148–153. doi: 10.1016/j.nut.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- Jacobs RL, Stead LM, Brosnan ME, Brosnan JT. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276:43740–43747. doi: 10.1074/jbc.M107553200. [DOI] [PubMed] [Google Scholar]
- Kalhan SC. Protein metabolism in pregnancy. Am J Clin Nutr. 2000;71:1249S–1255S. doi: 10.1093/ajcn/71.5.1249s. [DOI] [PubMed] [Google Scholar]
- Kalhan SC, Gruca LL, Parimi PS, O'Brien A, Dierker L, Burkett E. Serine metabolism in human pregnancy. Am J Physiol Endocrinol Metab. 2003;284:E733–E740. doi: 10.1152/ajpendo.00167.2002. [DOI] [PubMed] [Google Scholar]
- Kalhan SC. Fatty acids, insulin resistance, and protein metabolism. J Clin Endocrinol Metab. 2009;94:2725–2727. doi: 10.1210/jc.2009-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC. Metabolism of methionine in vivo: impact of pregnancy, protein restriction, and fatty liver disease. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:121–131. doi: 10.1159/000209977. [DOI] [PubMed] [Google Scholar]
- Kalhan SC, Uppal SO, Moorman JL, Bennett C, Gruca LL, Parimi PS, Dasarathy S, Serre D, Hanson RW. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J Biol Chem. 2011;286:5266–5277. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino Acid. J Biol Chem. 2012;287:19786–19791. doi: 10.1074/jbc.R112.357194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC, Wilson-Costello D. Prematurity and programming: contribution of neonatal Intensive Care Unit interventions. J Dev Orig Health Dis. 2013;4:121–133. doi: 10.1017/S204017441200061X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC. One-carbon metabolism, fetal growth and long-term consequences. Nestle Nutr Inst Workshop Ser. 2013;74:127–138. doi: 10.1159/000348459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC, Bier DM. Protein and amino acid metabolism in the human newborn. Annual Reviews in Nutrition. 2008;28:389–410. doi: 10.1146/annurev.nutr.28.061807.155333. [DOI] [PubMed] [Google Scholar]
- Kalhan SC, Marczewski S. Methionine, homocysteine, one carbon metabolism and fetal growth. Reviews endocrine Metabolic Disorders. 2012;13:109–119. doi: 10.1007/s11154-012-9215-7. [DOI] [PubMed] [Google Scholar]
- Katre P, Joshi S, Bhat DS, Deshmukh M, Gurav N, Pandit S, Lubree H, Marczewski S, Bennett C, Gruca L, Kalyanaraman K, Naik SS, Yajnik CS, Kalhan SC. Effect of multi-nutrient insufficiency on markers of one carbon metabolism in young women: response to a methionine load. Eur J Clin Nutr. 2015 doi: 10.1038/ejcn.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Mandard S, Escher P, Gonzalez FJ, Tafuri S, Desvergne B, Wahli W. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J. 2001;15:1971–1978. doi: 10.1096/fj.01-0147com. [DOI] [PubMed] [Google Scholar]
- Kersten S. Integrated physiology and systems biology of PPARalpha. Mol Metab. 2014;3:354–371. doi: 10.1016/j.molmet.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. Effects of energy and protein intakes on pregnancy outcome: an overview of the research evidence from controlled clinical trials. Am J Clin Nutr. 1993;58:627–635. doi: 10.1093/ajcn/58.5.627. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Balanced protein/energy supplementation in pregnancy. Cochrane Database Syst Rev. 2000:CD000032. doi: 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev. 2003:CD000032. doi: 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- Krishnaveni GV, Hill JC, Veena SR, Bhat DS, Wills AK, Karat CL, Yajnik CS, Fall CH. Low plasma vitamin B12 in pregnancy is associated with gestational 'diabesity' and later diabetes. Diabetologia. 2009;52:2350–2358. doi: 10.1007/s00125-009-1499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre SG, Molloy AM, Reinke SN, Sykes BD, Brosnan ME, Brosnan JT. Formate can differentiate between hyperhomocysteinemia due to impaired remethylation and impaired transsulfuration. Am J Physiol Endocrinol Metab. 2012;302:E61–E67. doi: 10.1152/ajpendo.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre SG, Morrow G, MacMillan L, Brosnan ME, Brosnan JT. Formate: an essential metabolite, a biomarker, or more? Clin Chem Lab Med. 2013;51:571–578. doi: 10.1515/cclm-2012-0552. [DOI] [PubMed] [Google Scholar]
- Lee HA, Park EA, Cho SJ, Kim HS, Kim YJ, Lee H, Gwak HS, Kim KN, Chang N, Ha EH, Park H. Mendelian randomization analysis of the effect of maternal homocysteine during pregnancy, as represented by maternal MTHFR C677T genotype, on birth weight. J Epidemiol. 2013;23:371–375. doi: 10.2188/jea.JE20120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RM, Godfrey KM, Jackson AA, Cameron IT, Hanson MA. Low serine hydroxymethyltransferase activity in the human placenta has important implications for fetal glycine supply. J Clin Endocrinol Metab. 2005;90:1594–1598. doi: 10.1210/jc.2004-0317. [DOI] [PubMed] [Google Scholar]
- Lindblad B, Zaman S, Malik A, Martin H, Ekstrom AM, Amu S, Holmgren A, Norman M. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet Gynecol Scand. 2005;84:1055–1061. doi: 10.1111/j.0001-6349.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Quesada E, Vilaseca MA, Lailla JM. Plasma total homocysteine in uncomplicated pregnancy and in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;108:45–49. doi: 10.1016/s0301-2115(02)00367-6. [DOI] [PubMed] [Google Scholar]
- Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiological Reviews. 2012;92:1515–1542. doi: 10.1152/physrev.00047.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947–E955. doi: 10.1152/ajpendo.2001.280.6.E947. [DOI] [PubMed] [Google Scholar]
- Malinow MR, Rajkovic A, Duell PB, Hess DL, Upson BM. The relationship between maternal and neonatal umbilical cord plasma homocyst(e)ine suggests a potential role for maternal homocyst(e)ine in fetal metabolism. Am J Obstet Gynecol. 1998;178:228–233. doi: 10.1016/s0002-9378(98)80005-7. [DOI] [PubMed] [Google Scholar]
- Mann NJ, Li D, Sinclair AJ, Dudman NP, Guo XW, Elsworth GR, Wilson AK, Kelly FD. The effect of diet on plasma homocysteine concentrations in healthy male subjects. Eur J Clin Nutr. 1999;53:895–899. doi: 10.1038/sj.ejcn.1600874. [DOI] [PubMed] [Google Scholar]
- Medina M, Urdiales JL, Amores-Sanchez MI. Roles of homocysteine in cell metabolism: old and new functions. Eur J Biochem. 2001;268:3871–3882. doi: 10.1046/j.1432-1327.2001.02278.x. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Mills JL, McPartlin J, Kirke PN, Scott JM, Daly S. Maternal and fetal plasma homocysteine concentrations at birth: the influence of folate, vitamin B12, and the 5,10-methylenetetrahydrofolate reductase 677C-->T variant. Am J Obstet Gynecol. 2002;186:499–503. doi: 10.1067/mob.2002.121105. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, Scott JM, Mills JL. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics. 2009;123:917–923. doi: 10.1542/peds.2008-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores RR, Jr, Carter BS, Meschia G, Fennessey PV, Battaglia FC. Placental and fetal serine fluxes at midgestation in the fetal lamb. Am J Physiol. 1994;267:E150–E155. doi: 10.1152/ajpendo.1994.267.1.E150. [DOI] [PubMed] [Google Scholar]
- Morrow GP, MacMillan L, Lamarre SG, Young SK, MacFarlane AJ, Brosnan ME, Brosnan JT. In vivo kinetics of formate metabolism in folate-deficient and folate-replete rats. J Biol Chem. 2015;290:2244–2250. doi: 10.1074/jbc.M114.600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Scott JM, McPartlin JM, Fernandez-Ballart JD. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76:614–619. doi: 10.1093/ajcn/76.3.614. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, Mhaskar R, Thomas A, Vaz M, Bhat S, Fawzi WW. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801. doi: 10.1038/sj.ejcn.1602383. [DOI] [PubMed] [Google Scholar]
- Narkewicz MR, Moores RR, Jr, Battaglia FC, Frerman FF. Ontogeny of serine hydroxymethyltransferase isoenzymes in fetal sheep liver, kidney, and placenta. Mol Genet Metab. 1999;68:473–480. doi: 10.1006/mgme.1999.2932. [DOI] [PubMed] [Google Scholar]
- Nelen WL, Blom HJ, Steegers EA, den HM, Eskes TK. Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril. 2000;74:1196–1199. doi: 10.1016/s0015-0282(00)01595-8. [DOI] [PubMed] [Google Scholar]
- Obeid R, Munz W, Jager M, Schmidt W, Herrmann W. Biochemical indexes of the B vitamins in cord serum are predicted by maternal B vitamin status. Am J Clin Nutr. 2005;82:133–139. doi: 10.1093/ajcn.82.1.133. [DOI] [PubMed] [Google Scholar]
- Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015;6:CD000032. doi: 10.1002/14651858.CD000032.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN. Pre- and early postnatal nongenetic determinants of type 2 diabetes. Expert Rev Mol Med. 2002;4:1–14. doi: 10.1017/S1462399402005240. [DOI] [PubMed] [Google Scholar]
- Parimi PS, Cripe-Mamie C, Kalhan SC. Metabolic responses to protein restriction during pregnancy in rat and translation initiation factors in the mother and fetus. Pediatr Res. 2004;56:423–431. doi: 10.1203/01.PDR.0000136277.10365.84. [DOI] [PubMed] [Google Scholar]
- Patrick TE, Powers RW, Daftary AR, Ness RB, Roberts JM. Homocysteine and folic acid are inversely related in black women with preeclampsia. Hypertension. 2004;43:1279–1282. doi: 10.1161/01.HYP.0000126580.81230.da. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr. 2014;68:541–548. doi: 10.1038/ejcn.2014.46. [DOI] [PubMed] [Google Scholar]
- Perla-Kajan J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32:561–572. doi: 10.1007/s00726-006-0432-9. [DOI] [PubMed] [Google Scholar]
- Petrie L, Duthie SJ, Rees WD, McConnell JM. Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br J Nutr. 2002;88:471–477. doi: 10.1079/BJN2002695. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ JC, TABOR H. The urinary excretion of formic acid and formiminoglutamic acid in folic acid deficiency. J Biol Chem. 1958;233:252–255. [PubMed] [Google Scholar]
- Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM. The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr. 1999;81:243–250. [PubMed] [Google Scholar]
- Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Guttormsen AB, Joglekar A, Sayyad MG, Ulvik A, Ueland PM. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–241. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- Relton CL, Pearce MS, Parker L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br J Nutr. 2005;93:593–599. doi: 10.1079/bjn20041395. [DOI] [PubMed] [Google Scholar]
- Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care. 2009;32:1639–1643. doi: 10.2337/dc09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnenberg AG, Venners SA, Xu X, Chen C, Wang L, Guang W, Huang A, Wang X. Preconception B-vitamin and homocysteine status, conception, and early pregnancy loss. Am J Epidemiol. 2007;166:304–312. doi: 10.1093/aje/kwm078. [DOI] [PubMed] [Google Scholar]
- Sachdev HPS. Low Birth Weight in South Asia. Int J Diab Dev Countries. 2001;21:13–31. [Google Scholar]
- Sarr O, Gondret F, Jamin A, Le Huerou-Luron I, Louveau I. A high-protein neonatal formula induces a temporary reduction of adiposity and changes later adipocyte physiology. Am J Physiol Regul Integr Comp Physiol. 2011;300:R387–R397. doi: 10.1152/ajpregu.00459.2010. [DOI] [PubMed] [Google Scholar]
- Sheikh K, Camejo G, Lanne B, Halvarsson T, Landergren MR, Oakes ND. Beyond lipids, pharmacological PPARalpha activation has important effects on amino acid metabolism as studied in the rat. Am J Physiol Endocrinol Metab. 2007;292:E1157–E1165. doi: 10.1152/ajpendo.00254.2006. [DOI] [PubMed] [Google Scholar]
- Sloan NL, Lederman SA, Leighton J, Himes JH, Rush D. The effect of perinatal dietary protein intake on birth weight. Nutrition research. 2001;21:129–139. [Google Scholar]
- Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate. 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- Solanky N, Requena JA, D'Souza SW, Sibley CP, Glazier JD. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31:134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, West KP., Jr Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011;141:1912–1917. doi: 10.3945/jn.111.144717. [DOI] [PubMed] [Google Scholar]
- Stover PJ, Field MS. Trafficking of intracellular folates. Adv Nutr. 2011;2:325–331. doi: 10.3945/an.111.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukla KK, Nagar R, Raman R. Vitamin-B12 and folate deficiency, major contributing factors for anemia: A population based study. e-SPEN Journal. 2014;9:e45–e48. [Google Scholar]
- Sutton GM, Centanni AV, Butler AA. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology. 2010;151:1570–1580. doi: 10.1210/en.2009-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto H, Mito N, Umegaki K, Ishiwaki A, Kusama K, Abe S, Yamawaki M, Fukuoka H, Ohta C, Yoshiike N. Relationship between dietary folate intakes, maternal plasma total homocysteine and B-vitamins during pregnancy and fetal growth in Japan. Eur J Nutr. 2007;46:300–306. doi: 10.1007/s00394-007-0667-6. [DOI] [PubMed] [Google Scholar]
- Thomas B, Gruca LL, Bennett C, Parimi PS, Hanson RW, Kalhan SC. Metabolism of methionine in the newborn infant: response to the parenteral and enteral administration of nutrients. Pediatr Res. 2008;64:381–386. doi: 10.1203/PDR.0b013e318180e499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thone-Reineke C, Kalk P, Dorn M, Klaus S, Simon K, Pfab T, Godes M, Persson P, Unger T, Hocher B. High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1025–R1030. doi: 10.1152/ajpregu.00898.2005. [DOI] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Timmermans S, Jaddoe VWV, Hofman A, Steegers-Theunissen RPM, Steegers EAP. Periconception folic acid suppelementation, fetal growth and the risks of low birth weight and preterm birth: the generation R study. Br J Nutr. 2009;102:777–785. doi: 10.1017/S0007114509288994. [DOI] [PubMed] [Google Scholar]
- Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta. J Physiol. 2009;587:4001–4013. doi: 10.1113/jphysiol.2009.173393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine is transported by the microvillous plasma membrane of human placenta. J Inherit Metab Dis. 2011;34:57–65. doi: 10.1007/s10545-010-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda N, Reyes L, Gonzalez-Medina A, Alonso-Aperte E, Varela-Moreiras G. Physiologic changes in homocysteine metabolism in pregnancy: a longitudinal study in Spain. Nutrition. 2011;27:925–930. doi: 10.1016/j.nut.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Muthayya S, Kurpad AV, Yajnik CS, Fall CHD. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9 to 10 year-old children in South India. J Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef P, de Groot LC. Dietary determinants of plasma homocysteine concentrations. Semin Vasc Med. 2005;5:110–123. doi: 10.1055/s-2005-872397. [DOI] [PubMed] [Google Scholar]
- Verhoef P, van VT, Olthof MR, Katan MB. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am J Clin Nutr. 2005;82:553–558. doi: 10.1093/ajcn.82.3.553. [DOI] [PubMed] [Google Scholar]
- Villalpando S. Discussion: Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant and child development. Food and nutrition Bulletin. 2008;29:S112–s115. doi: 10.1177/15648265080292S114. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71:962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- Walker MC, Smith GN, Perkins SL, Keely EJ, Garner PR. Changes in homocysteine levels during normal pregnancy. Am J Obstet Gynecol. 1999;180:660–664. doi: 10.1016/s0002-9378(99)70269-3. [DOI] [PubMed] [Google Scholar]
- Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–347. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- Washburn SE, Caudill MA, Malysheva O, MacFarlane AJ, Behan NA, Harnett B, MacMillan L, Pongnopparat T, Brosnan JT, Brosnan ME. Formate metabolism in fetal and neonatal sheep. Am J Physiol Endocrinol Metab. 2015;308:E921–E927. doi: 10.1152/ajpendo.00046.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, Austin RC. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsen T, Grill V, Gudnason V, Hulman S, Hypponen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- WHITEHEAD RG, DEAN RF. SERUM AMINO ACIDS IN KWASHIORKOR. II. AN ABBREVIATED METHOD OF ESTIMATION AND ITS APPLICATION. Am J Clin Nutr. 1964;14:320–330. doi: 10.1093/ajcn/14.6.320. [DOI] [PubMed] [Google Scholar]
- WHITEHEAD RG, DEAN RF. SERUM AMINO ACIDS IN KWASHIORKOR. I. RELATIONSHIP TO CLINICAL CONDITION. Am J Clin Nutr. 1964;14:313–319. doi: 10.1093/ajcn/14.6.313. [DOI] [PubMed] [Google Scholar]
- WHITEHEAD RG, DEAN RF. The metabolism of amino acids in kwashiorkor. East Afr Med J. 1964;41:581–583. [PubMed] [Google Scholar]
- Wu BT, Innis SM, Mulder KA, Dyer RA, King DJ. Low plasma vitamin B-12 is associated with a lower pregnancy-associated rise in plasma free choline in Canadian pregnant women and lower postnatal growth rates in their male infants. Am J Clin Nutr. 2013;98:1209–1217. doi: 10.3945/ajcn.113.060269. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Fall CHD, Vaidya U, Pandit AN, Bavdekar A, Bhat DS, Osmond C, Hales CN, Barker DJP. Fetal growth and glucose and insulin metabolism in four-year-old Indian children. Diabetic Med. 1995:330–336. doi: 10.1111/j.1464-5491.1995.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Panchanadikar AV, Naik SS, Deshpande JA, Coyaji KJ, Fall C, Refsum H. Maternal total homocysteine concentration and neonatal size in India. Asia Pac J Clin Nutr. 2005;14:179–181. [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, Joshi N, Lubree HG, Deshpande VU, Rege SS, Fall CH. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik CS, Chandak GR, Joglekar C, Katre P, Bhat DS, Singh SN, Janipalli CS, Refsum H, Krishnaveni G, Veena S, Osmond C, Fall CH. Maternal homocysteine in pregnancy and offspring birthweight: epidemiological associations and Mendelian randomization analysis. Int J Epidemiol. 2014;43:1487–1497. doi: 10.1093/ije/dyu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Tanaka T, Noguchi T. The effect of a high-protein diet on cystathionine beta-synthase activity and its transcript levels in rat liver. J Nutr Sci Vitaminol (Tokyo) 1996;42:589–593. doi: 10.3177/jnsv.42.589. [DOI] [PubMed] [Google Scholar]
- Young VR, Wagner DA, Burini R, Storch KJ. Methionine kinetics and balance at the 1985 FAO/WHO/UNU intake requirement in adult men studied with L-[2H3-methyl-1-13C]methionine as a tracer. Am J Clin Nutr. 1991;54:377–385. doi: 10.1093/ajcn/54.2.377. [DOI] [PubMed] [Google Scholar]