To the Editor
Club cell secretory protein (CC16) is a homodimeric pneumoprotein that is mainly produced by non-ciliated bronchial epithelial cells1, 2 and is found both in the airways and circulation. The biological functions of CC16 have not been conclusively determined, but in vitro, ex vivo, and animal studies indicate anti-inflammatory, immunomodulatory, and anti-toxicant properties of this molecule in the lungs1, 2.
Clinical studies have reported decreased circulating levels of CC16 in asthma3. In a population-based study4, although serum CC16 levels were not associated with asthma per se, they correlated positively with lung function in asthmatics suggesting that CC16 deficits may be related to disease severity. This scenario is in line with the relation of low circulating CC16 to prevalence and progression of chronic obstructive pulmonary disease5.
Whether deficits of circulating CC16 in patients with asthma reflect similar deficits of this protein in the airways remains controversial because of inconsistent results from previous studies6, 7. One study reported lower CC16 levels in bronchoalveolar lavage (BAL) fluid from 24 patients with asthma compared to 24 controls6. In contrast, another study found both patients with difficult-to-treat (refractory) asthma and patients with mild-to-moderate asthma to have higher CC16 in induced sputum than controls7. When BAL samples were analyzed, subjects with refractory asthma had higher CC16 than those with mild-to-moderate asthma.
Assessing airway CC16 in asthma is important because it may provide insights into whether CC16 deficits in serum from asthmatics can be explained by reduced airway CC16 production. We sought to compare CC16 levels in serum and BAL from patients with refractory asthma, non-refractory asthma, and controls.
The study population included refractory asthmatics as defined by ATS criteria8; non-refractory asthmatics who were on low to medium dose inhaled corticosteroids (ICS) with a methacholine PC20 ≤ 16 mg/ml and did not meet the refractory asthma definition; and normal healthy controls with normal lung function and a PC20 > 25 mg/ml. For statistical analyses, current ICS intake was converted into beclomethasone dipropionate equivalent. Protocol approval was granted by the National Jewish Health Institutional Review Board and informed consent was obtained from study subjects.
Bronchoscopy with BAL was performed. BAL consisted of 240 ml and was analyzed for cell count and differential as well as immunomodulating mediators including CC16. Peripheral blood was obtained within approximately 30 minutes of BAL sampling.
CC16 levels were measured in serum and BAL using a commercially available ELISA kit (BioVendor, Asheville, NC). As done previously5, in statistical analyses CC16 levels were analyzed as log-transformed, standardized values to allow comparability between effects of serum and BAL CC16. Correlations between CC16 levels in serum and BAL, ICS intake, and BAL recovery were tested using Spearman correlation coefficients. Differences in CC16 levels across the three groups were tested with ANOVA and multivariate multinomial logistic regressions. Differences among participants with asthma (non-refractory and refractory asthma combined) and controls were tested with t-test and multivariate logistic regression models.
Characteristics of participants across the three groups are shown in table E1. Participants with refractory asthma were on average older and had lower % BAL recovery, but no significant differences were found for sex and smoking.
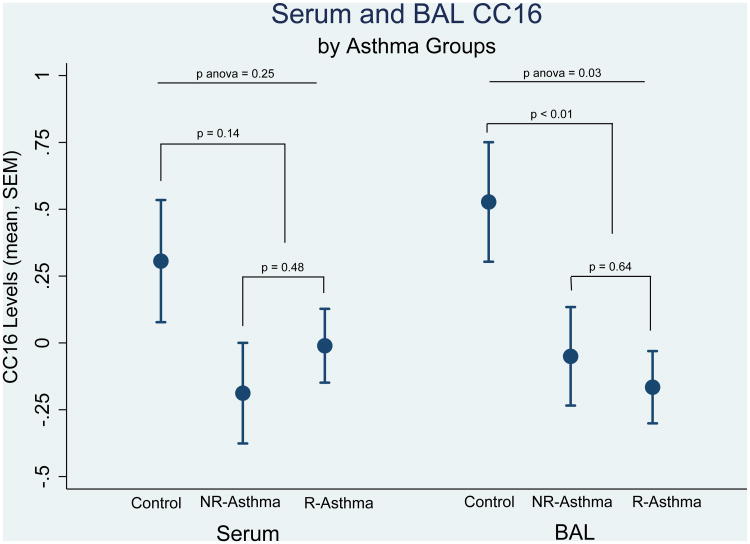
We found a significant, but weak, correlation between CC16 levels in serum and BAL (correlation coefficient: 0.22, p=0.03). When CC16 levels were compared across the three groups, lower levels of BAL CC16 were found in participants with asthma (either refractory or not) than controls (Figure 1). Similar, albeit not significant, trends were present for serum CC16. Neither serum nor BAL CC16 levels differed between the groups with non-refractory and refractory asthma. Although ICS intake was significantly higher among patients with refractory than non-refractory asthma, we did not observe an association between ICS intake and either serum or BAL CC16 levels. In addition, the lack of CC16 differences between refractory and non-refractory asthma was confirmed after adjustment for ICS intake (data not shown).
Figure 1.
Standardized CC16 levels in serum and BAL across the three groups.
NR-Asthma: non-refractory asthma
R-Asthma: refractory asthma
After adjustment for sex, age, and smoking, BAL CC16 levels were inversely associated with the risk for both non-refractory and refractory asthma (Table 1a, model 2; adjusted relative risk ratio for 1-SD increase in BAL CC16: 0.32 [p=0.02] and 0.37 [p=0.03], respectively). Similarly, they were associated with lower odds for asthma (non-refractory and refractory asthma combined) (Table 1b, model 2; adjusted odds ratio: 0.36 [p=0.02]). Associations with non-refractory, refractory, and combined asthma were generally weaker and of borderline significance for serum CC16. Consistently, in multivariate models including both serum and BAL CC16 levels (Table 1a and 1b, models 3) only the latter were significantly and inversely associated with asthma. These results were not explained by differences in BAL recovery across the three groups because there was no correlation between % BAL recovery and BAL CC16 levels, and because the association of BAL CC16 levels to asthma was confirmed after further inclusion of % BAL recovery among covariates (data not shown).
Table 1.
Univariate and multivariate associations of serum and BAL CC16 with asthma.
Table 1a includes results from multinomial logistic regression models predicting non-refractory and refractory asthma (no asthma is the reference category).
Table 1b includes results from logistic regression models predicting asthma (non-refractory plus refractory) versus non-asthma. RRRs and ORs refer to the effects of 1-unit change in standardized CC16 levels.
| Table 1a | Serum CC16 RRR (95% CI), p |
BAL CC16 RRR (95% CI), p |
|---|---|---|
|
| ||
| MODEL 1* | ||
| Non-refractory asthma | 0.57 (0.29, 1.11), 0.099 | 0.43 (0.20, 0.91), 0.028 |
| Refractory asthma | 0.68 (0.36, 1.26), 0.215 | 0.38 (0.19, 0.78), 0.008 |
|
| ||
| MODEL 2** | ||
| Non-refractory asthma | 0.45 (0.20, 1.01), 0.053 | 0.32 (0.13, 0.81), 0.016 |
| Refractory asthma | 0.47 (0.20, 1.07), 0.073 | 0.37 (0.15, 0.91), 0.030 |
|
| ||
| MODEL 3*** | ||
| Non-refractory asthma | 0.51 (0.21, 1.23), 0.133 | 0.37 (0.15, 0.96), 0.040 |
| Refractory asthma | 0.52 (0.21, 1.28), 0.154 | 0.42 (0.17, 1.05), 0.065 |
|
| ||
| Table 1b | Serum CC16 OR (95% CI), p | BAL CC16 OR (95% CI), p |
|
| ||
| MODEL 1* | ||
| Asthma | 0.64 (0.35, 1.16), 0.139 | 0.40 (0.20, 0.79), 0.009 |
|
| ||
| MODEL 2** | ||
| Asthma | 0.46 (0.21, 1.00), 0.050 | 0.36 (0.15, 0.84), 0.018 |
|
| ||
| MODEL 3*** | ||
| Asthma | 0.51 (0.22, 1.20), 0.124 | 0.41 (0.17, 0.98), 0.044 |
univariate
separate models for serum and BAL CC16, adjusted for sex, age, and smoking
single model including serum CC16, BAL CC16, sex, age, and smoking
Ns: Non-Asthmatic Controls (19), Non-refractory Asthma (28), Refractory Asthma (52)
The results of our study indicate that significant CC16 deficits are present in the airways of patients with asthma and suggest that reduced lung CC16 expression or decreased CC16-producing epithelial cells may be responsible. Although similar trends were found for serum CC16, the statistical significance and magnitude of association were stronger for CC16 measured in BAL than circulation. In addition, serum and BAL CC16 correlated only weakly with each other indicating that airway and circulating levels may differ at least partially in cellular sources and/or may be variably influenced by different factors (bronchial epithelial permeability, glomerular filtration rate, etc).
We did not observe differences between refractory and non-refractory asthma for either serum or BAL CC16, despite subjects with refractory asthma having substantial deficits in their lung function levels, a phenotype that had been linked to low circulating CC16 in previous studies4. This observation may be related to the sample size and possibly limited statistical power of our study, or to an actual lack of association between CC16 and response to treatment in asthma. Alternatively, there might have been other factors that operated as reverse confounders. For example, intake of corticosteroids is both a major criterion for the definition of refractory asthma8 and an important up-regulating factor for CC16 production9, although we did not find significant correlations between ICS intake and CC16 levels in our study.
Our finding of airway CC16 deficits in asthma is consistent with results reported by Van Vyve and colleagues6, but in apparent contrast with those from a recent study that found higher CC16 in induced sputum from patients with asthma7. The reasons for these discrepancies are unclear and may be related to differences in the clinical characteristics of participants (e.g., CC16 deficits have been shown to correlate with asthma duration3) or in the samples used to assess airway CC16 (e.g., BAL vs sputum). Nevertheless, our finding of BAL CC16 deficits in asthma is also in line with previous observations of reduced serum CC16 levels3 and decreased proportions of CC16-positive bronchial epithelial cells from the small airways in asthmatics10.
Further research is warranted to determine whether these deficits in airway CC16 have predictive value on the natural history of adult asthma and/or play a direct role in its progression.
Supplementary Material
Acknowledgments
Funding: This study was supported by awards HL107188 and HL095021 from the National Heart, Lung, and Blood Institute, US National Institutes of Health
Abbreviations
- BAL
bronchoalveolar lavage
- CC16
club cell secretory protein
- OR
odds ratio
- RRR
relative risk ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–75. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 2.Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12:445–67. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 3.Shijubo N, Itoh Y, Yamaguchi T, Sugaya F, Hirasawa M, Yamada T, et al. Serum levels of Clara cell 10-kDa protein are decreased in patients with asthma. Lung. 1999;177:45–52. doi: 10.1007/pl00007626. [DOI] [PubMed] [Google Scholar]
- 4.Rava M, Tares L, Lavi I, Barreiro E, Zock JP, Ferrer A, et al. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J Allergy Clin Immunol. 2013;132:230–2. doi: 10.1016/j.jaci.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3:613–20. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Vyve T, Chanez P, Bernard A, Bousquet J, Godard P, Lauwerijs R, et al. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J Allergy Clin Immunol. 1995;95:60–8. doi: 10.1016/s0091-6749(95)70153-2. [DOI] [PubMed] [Google Scholar]
- 7.Emmanouil P, Loukides S, Kostikas K, Papatheodorou G, Papaporfyriou A, Hillas G, et al. Sputum and BAL Clara cell secretory protein and surfactant protein D levels in asthma. Allergy. 2015;70:711–4. doi: 10.1111/all.12603. [DOI] [PubMed] [Google Scholar]
- 8.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 9.Roth FD, Quintar AA, Uribe Echevarria EM, Torres AI, Aoki A, Maldonado CA. Budesonide effects on Clara cell under normal and allergic inflammatory condition. Histochem Cell Biol. 2007;127:55–68. doi: 10.1007/s00418-006-0220-3. [DOI] [PubMed] [Google Scholar]
- 10.Shijubo N, Itoh Y, Yamaguchi T, Imada A, Hirasawa M, Yamada T, et al. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am J Respir Crit Care Med. 1999;160:930–3. doi: 10.1164/ajrccm.160.3.9803113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.