Abstract
We examined differences in regional brain activation during tests of executive function in individuals with Hoarding Disorder (HD), Obsessive Compulsive Disorder (OCD), and healthy controls (HC) using functional magnetic resonance imaging (fMRI). Participants completed computerized versions of the Stroop and Go/No-Go task. We found that during the conflict monitoring and response inhibition condition in the Go/No-Go task, individuals with HD had significantly greater activity than controls in the anterior cingulate cortex (ACC) and right dorsolateral prefrontal cortex (DLPFC). HD also exhibited significantly greater right DLPFC activity than OCD. We also observed significant differences in activity between HD and HC and between HD and OCD in regions (ACC, anterior insula, orbitofrontal cortex (OFC), and striatum) involved in evaluating stimulus-response-reward associations, or the personal and task-relevant value of stimuli and behavioral responses to stimuli. These results support the hypothesis that individuals with HD have difficulty deciding on the value or task relevance of stimuli, and may perceive an abnormally high risk of negative feedback for difficult or erroneous cognitive behavior.
Keywords: Neuroimaging, fMRI, Stroop, Go/No-go, conflict monitoring, response inhibition, hoarding disorder
1. Introduction
Hoarding Disorder (HD) is defined in the DSM-5 as an extreme and persistent inability to discard objects, regardless of their utility, often, but not always, accompanied by excessive acquisition of unneeded items (American Psychiatric Association, 2013). These behaviors lead to such excessive clutter that living or work spaces become nearly or completely unusable, resulting in severe functional impairment (Frost and Hartl, 1996; Steketee and Frost, 2003; Tolin et al., 2008). Severe hoarding can impair one’s ability to meet such basic needs as sleeping and personal hygiene, put serious mental and emotional strain on important personal relationships, and create financial and legal risks (e.g., excessive spending and eviction) (American Psychiatric Association, 2013). Hoarding behaviors can also lead to serious health and safety hazards, as they have been associated with increased risk of fires, falls, infestation, disability, and mortality (Frost et al., 2000; Frost et al., 1999; Saxena, 2007; Steketee and Frost, 2003).
HD is often characterized as an inability to make decisions regarding the categorization and discarding of possessions (Frost and Hartl, 1996; Grisham et al., 2010; Tolin et al., 2008; Tolin et al., 2012; Wincze et al., 2007), leading to the hypothesis that HD might be caused by underlying deficits in the executive functions that mediate decision making and other cognitive processes (Tolin et al., 2012). Individuals with hoarding behaviors exhibit deficits on measures of executive function such as categorization, set-shifting (Ayers et al., 2013; Mackin et al., 2011; Mackin et al., 2016; McMillan et al., 2013; Morein-Zamir et al., 2014), and sustained attention and inhibition (Blom et al., 2011; Raines et al., 2014; Tolin et al., 2011). In several studies, the severity of these executive function deficits was associated with the severity of hoarding symptoms (Ayers et al., 2013; Raines et al., 2014; Tolin et al., 2011), although the data are not always consistent across studies (Grisham et al., 2010; Tolin et al., 2011).
These executive functions are mediated by frontal brain regions, including the dorsolateral prefrontal cortex (DLPFC), ventromedial prefrontal cortex (VMPFC), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC) (Alvarez and Emory, 2006), and abnormalities or differences between groups in the activity of these brain regions can be investigated using neuroimaging techniques. While HD often co-occurs with obsessive-compulsive disorder (OCD) (Saxena, 2007; Steketee and Frost, 2003), HD and OCD appear to have separate etiologies and outcomes, as evidenced by disparate genetic contributions, ages of onset, comorbidities, and treatment responses (Mathews et al., 2014; Miguel et al., 2005; Saxena, 2008; Steketee and Frost, 2003; Tolin et al., 2014). However, hoarding was until recently classified as a subtype of OCD, and therefore most studies of the pathophysiology of hoarding, including neuroimaging studies, have focused on individuals with primary OCD and co-occurring hoarding symptoms (Alvarenga et al., 2012; Gilbert et al., 2008; Harrison et al., 2013; Mataix-Cols et al., 2004; Valente et al., 2005). Only a few neuroimaging studies have examined individuals with OCD+HD (An et al., 2009; Saxena et al., 2004; Tolin et al., 2009), and only two have directly compared HD to OCD (Tolin et al., 2012; Tolin et al., 2014).
Of the studies examining HD (with or without co-occurring OCD) rather than hoarding symptoms or dimensions in OCD, all but one (Tolin et al., 2014) examined brain activation patterns while participants completed or viewed a discarding task (An et al., 2009; Saxena et al., 2004; Tolin et al., 2009; Tolin et al., 2012). In functional magnetic resonance imaging (fMRI) studies that used discarding tasks, multiple brain regions were identified that differentiated individuals with hoarding symptoms and those without. These include the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), caudate, thalamus, posterior cingulate gyrus, ventromedial prefrontal cortex (VMPFC), insula, inferior temporal gyrus, cerebellum, inferior parietal lobe and/or precuneus, parahippocampus, fusiform gyrus, and inferior, superior and medial frontal gyri (An et al., 2009; Saxena et al., 2004; Tolin et al., 2009; Tolin et al., 2012). As can be seen by the long list of brain regions potentially implicated in hoarding and the lack of consistency regarding patterns of activation (e.g., increased vs. decreased), additional work is necessary to more definitively elucidate the neural basis of this complex disorder. Some inconsistencies may be accounted for by methodological differences, such as differences in imaging modalities (e.g., PET vs. fMRI); the study of dimensional hoarding symptoms in OCD rather than in individuals with HD; examination of previously identified regions of interest rather than whole brain analyses; and the use of discarding tasks that, while requiring decision-making, are designed to simulate complex emotional hoarding symptoms rather than tasks that tap into discrete non-emotional executive functions. Only one published study, by Tolin et al. (2014), examined patterns of brain activation in individuals with HD, OCD, and healthy controls (HC) during a non-emotional task of executive function. In this study, during response inhibition elicited by a Go/No-Go task, individuals with HD had elevated right precentral gyrus activity and reduced left middle frontal gyrus activity compared to HC, while individuals with OCD (but not HD) had elevated OFC activity. However, this study did not conduct a whole brain analysis, but only examined a limited number of regions of interest for HD, leaving open the possibility that other, unexamined brain regions also are relevant in HD pathophysiology.
Thus, the aim of this study was to expand on previous work, including that by Tolin et al. (2014), by examining differences in activation across the whole brain during emotionally neutral executive function tasks that assess error processing, conflict monitoring and response inhibition in individuals with HD, OCD and healthy controls using fMRI. A recent event-related potential study by our group found significant amplitude reduction of the error-related negativity (ERN) in response to errors committed during a flanker task in HD participants when compared to both HC and OCD participants, suggesting that deficiencies in error processing may be a key pathophysiological feature that distinguishes HD from OCD (Mathews et al., 2016). Error monitoring involves the detection of conflict between intended and executed responses (Dehaene et al., 1994; Gehring et al., 1993; Falkenstein et al., 1991), and it has been shown that the ACC and DLPFC are involved in both error monitoring and more general monitoring of response conflict, especially during tasks that require subjects to overcome prepotent response tendencies (Barber and Carter, 2005; Carter et al., 1998; Botvinik et al., 1999; Botvinick et al., 2001; Carter et al., 2000; Ford, Whitfield, and Mathalon, 2004; Carter and van Veen, 2007; MacDonald, Cohen, et al., 2000; van Veen and Carter, 2006; van Veen and Carter, 2002; ) such as the Stroop Color-Word Interference task (Carter and van Veen, 2007; van Veen and Carter, 2005; van Veen, Holroyd, et al., 2004; Milham and Banich, 2005; Kerns et al., 2004) and Go/No-Go tasks (Braver et al., 2001; Menon et al., 2001; Horn et al., 2003; Mathalon et al., 2003; Steele et al., 2013; Steele et al., 2014; Wager et al., 2005; Garavan et al., 2003). Accordingly, based on our previous findings of ERN deficits during error processing (Mathews et al., 2016), we hypothesized that HD participants would show abnormal activation in brain regions associated with error processing, conflict monitoring, and response inhibition (e.g., ACC, OFC, and DLPFC) compared to HC and non-hoarding OCD participants.
2. Methods
2.1. Participants
A total of 66 participants were recruited for this study from a larger study of neurocognition in HD and OCD. The parent study included a full clinical assessment, comprehensive neuropsychological battery, and electrophysiological (EEG) studies. All participants from this larger study who met full inclusion/exclusion criteria (as described below) and consented to participate in the fMRI study were enrolled. Participants were 18 years of age or older and were recruited through mental health clinics, the Mental Health Association of San Francisco (MHA-SF), and media advertisements. The study was approved by the Institutional Review Board at UCSF. All participants provided written informed consent and were compensated for their participation ($20 per hour, up to a total of $240 for participation in all elements of the study).
2.1.1. Clinical assessments
All participants were assessed for hoarding symptoms and HD using the Structured Interview for Hoarding Disorder (SIHD) (Mataix-Cols et al., 2013), the Saving Inventory, Revised (SI-R) (Frost et al., 2004), the UCLA Hoarding Symptom Scale (UHSS) (Saxena et al., 2007), and the Clutter Image Rating Scale (CIR) (Tolin et al., 2007). Obsessive-compulsive symptoms and OCD were assessed using the Yale Brown Obsessive Compulsive Scale (YBOCS) (Goodman et al., 1989), and the Structured Clinical Interview for Diagnosis of DSM-IV Axis I Disorders (SCID-I) (First et al., 1996). The SCID was also used to assess current and past history of other psychiatric disorders, including mood, anxiety, substance use, and psychotic disorders. Other assessments included the Obsessive Compulsive Personality Disorder (OCPD) SCID-II module (First et al., 1997), the Beck Depression Inventory (Beck et al., 1961), and the Beck Anxiety Inventory (BAI) (Beck and Steer, 1988).
2.1.2. Inclusion/exclusion criteria
With the exception of HD, all psychiatric diagnoses were assigned blinded to group status according to DSM-IV-TR criteria by two psychiatrists (CAM and AN) with experience in HD and OCD. HD diagnoses were assigned using DSM-5 criteria. For all groups, individuals with psychosis, intellectual disability, known dementia, active substance abuse, current use of antipsychotic medications, a history of head trauma with loss of consciousness, or any medical conditions known or suspected to affect cognitive function were excluded. Participants who had any incompatibility with MRI (e.g. metal implants or claustrophobia) were also excluded. All participants were asked to refrain from use of any illicit substances for at least three months prior to their participation.
HD participants were considered eligible for the study if they met DSM-5 criteria for HD. OCD participants were eligible for the study if they met DSM-IV-TR criteria for OCD without significant hoarding symptoms, defined as scores of ≤20 on the SI-R, ≤10 on the UHSS, and ≤ 8 on the CIR. Healthy control (HC) participants were included if they did not have hoarding symptoms (as defined above) or OCD symptoms (YBOCS score of <5). HC participants were excluded if they had active DSM-IV-TR Axis I diagnoses within the last year; history of Axis I diagnoses other than OCD and HD were permitted if they were in remission. HC participants were excluded from the study if they or a first-degree biological relative were determined to have clinical or subclinical OCD or HD (See Table 1 for a list of all DSM-IV-TR lifetime diagnoses within the sample). The control group (HC) was selected to match to the combined patient group (HD + OCD) on gender and education. Because of substantial differences in age between the HD and OCD groups, we specifically recruited control participants to match the age distribution of each participant group. As the total combined control sample did not differ from either the HD or the OCD groups on age, education, or gender, and in order to maximize the power of the sample, we used the entire control sample in all analyses, rather than splitting the HC group by age. All participants were right handed.
Table 1.
Lifetime DSM-IV-TR diagnoses within the participant sample.
| Hoarding Disorder (HD) (n=15) |
Obsessive-Compulsive Disorder (OCD) (n=17) |
Healthy Control (HC) † (n=25) |
|
|---|---|---|---|
| Major Depression* | 9 | 10 | 1 |
| Dysthymia | 2 | 0 | 0 |
| Generalized Anxiety Disorder | 3 | 2 | 0 |
| Social Phobia | 6 | 4 | 2 |
| Panic Disorder | 1 | 2 | 0 |
| Post-Traumatic Stress | |||
| Disorder* | 1 | 2 | 0 |
| Substance Abuse/Dependence* | 6 | 3 | 8 |
| Attention-Deficit/Hyperactivity | |||
| Disorder* | 4 | 3 | 1 |
| Psychotic Disorders | 0 | 0 | 0 |
| Obsessive-Compulsive | |||
| Personality Disorder | 1 | 3 | 0 |
All diagnoses were in remission at the time of the assessment for this participant group.
These diagnoses were in remission at the time of assessment for all participant groups. Note that no HD or OCD participants met full criteria for MDD at the time of the study, although some participants in these groups had subclinical symptoms. Similarly, no participants met full criteria for current ADHD, although several in each group had some residual symptoms (primarily inattentive), and one individual in each participant group was taking stimulants for ADHD.
Of the 66 subjects who participated in the fMRI component, 57 were included in fMRI analysis: 15 HD, 17 OCD, and 25 HC. Of the six HC subjects who were excluded, one had a first-degree relative with HD, two had fMRI data artifacts that precluded the use of their data, and three were excluded due to fMRI acquisition problems. Three OCD participants were excluded; of these, one did not meet full criteria for OCD but had subclinical symptoms, one had co-occurring subclinical hoarding symptoms, and one was excluded due to fMRI data artifacts. Three of the HD participants included in analysis had co-occurring subclinical OCD symptoms.
2.2. Measures
Stroop and Go/No-Go computerized tasks were administered during fMRI acquisition. All visual stimuli were presented on an LCD screen behind the participant’s head, which could be viewed by the participant through a mirror mounted on the head coil. Subjects who normally wear glasses were fitted with a pair of MRI-safe prescription glasses (http://cspmedical.com). Stimulus presentation was performed with Presentation® (Neurobehavioral Systems, Inc., Berkeley, CA) for the first 13 subjects and E-prime® 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA) for the remaining 44 subjects due to an institutional change in equipment. All presentation parameters remained the same. Subjects completed a practice session of each task prior to entering the scanner.
2.2.1. Go/No-Go Task
The Go/No-Go task was adapted from a paradigm used in prior studies (e.g. Mathalon et al., 2009). The visual stimuli for the Go/No-Go task consisted of a random sequence of the letters “X” or “K” (about 3 degrees of visual angle in size), each presented foveally on a computer display in white font on a black background for a duration was 100 ms. with an interstimulus interval randomly and uniformly distributed across intervals of 1000, 1500, and 2000 ms. Eighty percent of the stimuli were “Go” (=X) and 20% of the stimuli were “No-Go” (=K). Subjects were instructed to make a button press with their right index finger to the “X” and to withhold responses to the “K”. In each Go/No-Go fMRI acquisition run, 189 letter stimuli were presented. Subjects completed two runs of the Go/No-Go task (each lasting about 6 minutes), with an approximately 1 minute rest break between runs. Behavioral data from this task included “Go” reaction time and accuracy/error rates (i.e., correct response omissions and false alarms).
2.2.2. Stroop Task
The visual stimuli for the Stroop task consisted of the words “red,” “blue” and “yellow” (about 3 degrees of visual angle in size) presented foveally on a computer display in red, blue or yellow font on a black background. Subjects were instructed to make a 3-button forced-choice response to the font color on every trial. Forty percent of the trials were congruent (the word meaning and font color were the same) and 30% of the trials were incongruent (the word meaning and font color were different). Thirty percent of the trials were “null trials” in which no stimulus was presented in order to create randomized jitter in the inter-stimulus intervals between congruent and incongruent trials. Stimulus duration was 500 ms. The inter-stimulus interval was 1000 ms. (although on null trials, the effective inter-stimulus interval was 2500 ms.). Stimulus conditions were randomized. In each Stroop fMRI acquisition run lasting about 4.5 minutes, 187 trials were presented. Subjects completed four runs of the Stroop task, with approximately 1-minute breaks between runs for subjects to rest or communicate with the experimenters. Behavioral data from this task included mean reaction times to congruent and incongruent stimuli, along with incongruent and congruent trial accuracy/error rates.
2.3. Behavioral data analysis
Between-group comparisons of task performance (mean response time and error rate) during the Stroop and Go/No-Go tasks were performed using univariate analysis of covariance (ANCOVA) on SPSS v.22 (IBM Corp., Armonk, NY), including age and gender as covariates. Error rates from both tasks were log transformed for normality prior to analysis. Although accuracy data were available for all participants, Stroop response time data was not available for four OCD and eight HC participants whose Stroop fMRI data was analyzed due to acquisition problems.
2.4. fMRI acquisition and processing
A 3.0T Siemens Trio scanner was used with 41 mT/m gradients for fast echo-planar imaging. Following a localizer series, high-resolution T1-weighted structural images were obtained. Next, whole brain functional imaging was obtained using an echo-planar pulse sequence with T2*-weighted images sensitive to blood-oxygenation-level-dependent contrast (TR=2000 ms., TE=40 ms., flip angle=90°, in-plane resolution=3.75 mm2, slice thickness=3 mm). Images were prescribed on a mid-sagittal slice parallel to the anterior commissure – posterior commissure line. For the Go/No-Go task runs, 193 volumes were acquired, and for the Stroop task runs, 148 volumes were acquired. Dummy excitations were excluded from analysis.
2.5. fMRI data analysis
Whole brain statistical analysis of fMRI data was performed using MATLAB (MathWorks, Inc., Natick, MA) and Statistical Parametric Mapping-8 (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/). Before analysis, the functional images were converted to 3-D Analyze format volumes. Images were corrected for motion (i.e., spatially realigned) using a six-parameter rigid body affine transformation and corrected for differences in slice acquisition timing. The resulting images were normalized to a standard stereotaxic space (Montreal Neurological Institute EPI template using a 12-parameter affine/non-linear transformation and spatially smoothed with an 8-mm, full-width, half maximum isotropic Gaussian kernel. Image intensity was scaled to the mean global intensity of the time series. fMRI data for each subject were analyzed using a general linear model wherein the event presentation timecourse of each task condition was modeled by a regressor convolved with SPM’s canonical hemodynamic response function. For the Stroop task, events were modeled separately for the following conditions: Congruent correct, Incongruent correct, and Combined errors. Contrasts of interest were performed to compare Incongruent and Congruent correct trials and to examine Combined error trials. For the Go/No-Go task, events were modeled separately for the following conditions: Go correct, No-Go correct, Go incorrect, and No-Go incorrect. Contrasts of interest were performed to compare correct Go and No-Go trials, and to compare incorrect No-Go trials to an implicit baseline. For each task, second-level random-effects analyses were performed to examine these contrasts within and between the three groups. Age and gender were included as covariates. Additional regressors were included to model x, y, z displacements and pitch, roll, and yaw motion correction parameters as covariates. Each of these analyses was performed voxel-wise over the whole brain. Activation was considered significant at p<0.001 (uncorrected for multiple comparisons) with a minimum cluster size of 20 contiguous voxels. Cluster-extent based thresholding was used to correct for multiple comparisons (Woo et al., 2014).
Go/No-Go fMRI data were analyzed for 54 participants: 15 HD, 17 OCD, and 22 HC. Three HC participants’ scans were excluded from fMRI analysis of this task due to excessive motion or poor signal-to-noise ratio. Stroop fMRI data was analyzed for 51 participants: 13 HD, 14 OCD, and 24 HC participants. Two HD, three OCD, and one HC participants’ scans were excluded from fMRI analysis of this task due to excessive motion or poor signal-to-noise ratio.
3. Results
3.1. Sample description
There were no significant differences in gender, ethnicity, or education between the three groups, although the HD group had proportionately more female participants than the other groups (Table 2). As noted, the HC group was not significantly different in age compared to the HD (p=0.157) and OCD (p=0.188) groups. When compared to controls, both the HD and OCD groups had higher use of psychotropics (p=0.040 and <0.001, respectively) (Table 2); this difference was primarily due to antidepressant use (primarily selective serotonin reuptake inhibitors) among the HD and OCD participants (p=0.036 and <0.001, respectively), and to higher rates of benzodiazepine use in HD compared to HC (p=0.036). Additionally, the OCD group had higher antidepressant use when compared to HD (p=0.031). The HD and OCD groups did not differ in ratings of depression (BDI; p=0.568) or anxiety (BAI; p=0.709); as expected, the HC group had significantly lower BDI and BAI scores in comparison to HD (p=0.008 and 0.005, respectively) and OCD groups (p=0.014 and 0.048, respectively) (Table 2).
Table 2.
Demographic characteristics of the participant sample.
| Hoarding Disorder (HD) |
Obsessive- Compulsive Disorder (OCD) |
Healthy Control (HC) |
Three group comparison |
HD vs. OCD comparison |
||||
|---|---|---|---|---|---|---|---|---|
| n = 15 | n = 17 | n = 25 | F / X2, p value |
t / X2, p value |
||||
| N | % | N | % | N | % | |||
| Gender | 1.99, 0.369 | 1.41, 0.234 | ||||||
| Female | 11 | 73.3 | 9 | 52.4 | 13 | 52.0 | ||
| Male | 4 | 26.7 | 8 | 47.6 | 12 | 48.0 | ||
| Ethnicity | 0.02, 0.989 | 0.02, 0.893 | ||||||
| Hispanic | 2 | 13.3 | 2 | 11.76 | 3 | 12.0 | ||
| Non-Hispanic | 13 | 86.7 | 15 | 88.24 | 22 | 88.0 | ||
| Race | 2.45, 0.295 | 0.41, 0.522 | ||||||
| Caucasian | 12 | 80.0 | 15 | 88.2 | 17 | 68.0 | ||
| Non-Caucasian | 3 | 20.0 | 2 | 11.2 | 8 | 32.0 | ||
| Psychotropic use | 6 | 40 | 11 | 64.7 | 31 | 12.0 | 12.56, 0.002 | 1.95, 0.162 |
| Antidepressants2 | 4 | 26.7 | 11 | 64.7 | 1 | 4.0 | 18.49, <0.001 | 4.63, 0.031 |
| Neuroleptics | 1 | 6.7 | 2 | 11.8 | 0 | 0.0 | 2.89, 0.236 | 0.24, 0.621 |
| Mood Stabilizers | 1 | 6.7 | 1 | 5.9 | 0 | 0.0 | 1.63, 0.442 | 0.01, 0.927 |
| Benzodiazapines/ Sedative Hypnotics |
5 | 33.3 | 2 | 11.8 | 1 | 4.0 | 6.79, 0.034 | 2.17, 0.141 |
| ADHD medications/ Stimulants |
1 | 6.7 | 1 | 5.9 | 1 | 4.0 | 0.15, 0.927 | 0.01, 0.927 |
| M | SD | M | SD | M | SD | |||
| Age, years | 54.1 | 15.2 | 36.1 | 10.4 | 44.8 | 16.2 | 6.18, 0.004 | −3.95, <0.001 |
| Education, years | 15.8 | 1.8 | 16.7 | 1.9 | 15.6 | 2.4 | 1.42, 0.306 | 1.36, 0.184 |
| CIR | 12.4 | 5.5 | 4.6 | 1.4 | 4.8 | 1.4 | 38.09, <0.001 | 6.92, <0.001 |
| UHSS | 26.7 | 4.1 | 6.6 | 3.6 | 3.4 | 2.6 | 54.50, <0.001 | 8.65, <0.001 |
| SI-R | 57.9 | 11.8 | 10.9 | 6.9 | 9.0 | 9.5 | 32.84, <0.001 | 8.86, <0.001 |
| YBOCS | 8.7 | 13.9 | 27.0 | 6.7 | 0.0 | 0.0 | 63.88, <0.001 | −5.14, <0.001 |
| BAI | 11.9 | 10.7 | 10.1 | 7.9 | 3.6 | 0.9 | 4.36, 0.018 | 0.38, 0.709 |
| BDI | 13.0 | 13.7 | 9.6 | 10.0 | 3.6 | 5.6 | 4.90, 0.012 | 0.58, 0.568 |
One HC subject was using buproprion for smoking cessation, one was using zaleplon as needed for sleep, and one was using amphetamine-dextroamphetamine for treatment of ADHD.
Antidepressant use consisted primarily of selective serotonin reuptake inhibitors (SSRIs), including citalopram, fluoxetine, fluvoxamine, and sertraline. One HD subject was taking buproprion and one was taking clomipramine; one OCD subject was taking buproprion and trazodone, one was taking an SSRI (fluoxetine) and buproprion, and one was taking an SSRI (citalopram) and trazodone; the remaining subjects were taking an SSRI antidepressant only.
3.2. Behavioral data
When controlling for age and gender, there were no significant between-group differences in mean response time during incongruent and congruent trials of the Stroop task (F[2,34]=0.735, p=0.487), errors during incongruent and congruent trials of the Stroop task (F[2,46]=0.582, p=0.563), or errors of commission (i.e., false alarm button presses to No-Go stimuli) during the Go/No-Go task (F[2,49]=0.494, p=0.613). Mean response time for correct “hits” during the Go/No-Go task was significantly different between groups (F[2,49]=4.180, p=0.021). Bonferroni post-hoc analyses revealed a significant difference between the HD and OCD groups (p=0.022); there were no differences between HC and OCD (p=0.093) or HC and HD groups (p=0.833). (See Table 3 for means and standard deviations for each group). The reaction time of Stroop incongruent trials was significantly slower than congruent trials, across all groups (p<0.001).
Table 3.
Group comparisons of response time and error rate for Go/No-Go and Stroop tasks. P values are adjusted for age and sex.
| Hoarding Disorder (HD) |
Obsessive- Compulsive Disorder (OCD) |
Healthy Control (HC) |
Three group Comparison |
||||
|---|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | N | M (SD) | F statistic, p value |
|
| Go/No-Go “Go” Response Time1 (ms.) |
15 | 434.2 (64.3) | 17 | 349.8 (62.4) | 22 | 406.5 (64.6) | 4.18, 0.021 |
| Stroop Mean Response Time2 (ms.) |
13 | 809.7 (116.9) | 10 | 679.2 (60.1) | 16 | 737.5 (127.4) | 0.74, 0.487 |
| Stroop Congruent Response Time (ms.) |
13 | 724.9 (111.4) | 10 | 644.7 (51.3) | 16 | 678.6 (115.7) | 0.20, 0.823 |
| Stroop Incongruent Response Time (ms.) |
13 | 894.6 (133.2) | 10 | 713.7 (75.8) | 16 | 796.4 (146.5) | 1.32, 0.280 |
| Go/No-Go “No- Go” Error Rate3,4 (%) |
15 | 25.4 (15.4) | 17 | 26.9 (18.2) | 22 | 21.1 (14.5) | 0.49, 0.613 |
| Stroop Mean Error Rate4,5 (%) |
13 | 10.3 (7.8) | 14 | 6.9 (2.4) | 24 | 10.4 (7.2) | 0.58, 0.563 |
| Stroop Congruent Error Rate4 (%) |
13 | 5.7 (4.9) | 14 | 5.9 (2.5) | 24 | 7.7 (5.4) | 0.18, 0.833 |
| Stroop Incongruent Error Rate4 (%) |
13 | 14.9 (11.7) | 14 | 8.0 (3.5) | 24 | 13.1 (10.6) | 1.07, 0.351 |
Go/No-Go Response Time calculated based on “Go” trials only (correct hits).
Stroop Mean Response Time calculated based on mean response time of incongruent and congruent trials.
Go/No-Go Error Rate calculated based on “No-Go” trials only (errors of commission).
Error Rates for Go/No-Go and Stroop tasks were log transformed (LN) for normality prior to analysis; however, means and standard deviations are presented as raw, rather than transformed, values.
Stroop Mean Error Rates calculated based on mean error rate and response time of incongruent and congruent trials.
3.3. fMRI results
3.3.1. Conflict processing and response inhibition
During correct conflict processing and response inhibition on the Go/No-Go task (No-Go trials relative to Go trials), HD participants had greater activity in the ACC, right VLPFC, bilateral OFC, right striatum, and left temporal parietal junction (TPJ) than did HC participants (Table 4, Figure 1). OCD participants did not differ from HC participants. When we compared HD to OCD, HD participants also had greater activity in the right DLPFC, right insula, right visual cortex, and right cerebellum relative to OCD participants (Table 4, Figure 2).
Table 4.
Brain regions showing differential patterns of activation (p<0.001 with a minimum cluster size of 20 contiguous voxels) between individuals with HD, individuals with OCD, and HC participants. V=number of voxels within each cluster.
| Region | MNI coordinates | |||||
|---|---|---|---|---|---|---|
| Go/No-Go: No-Go > Go1 | t | V | x | y | z | |
| HD > HC | ||||||
| ACC | 4.75* | 219 | 0 | 36 | 28 | |
| L Sup Frontal Gyrus | 5.08 | 32 | −18 | 26 | 62 | |
| L OFC | 4.16* | 282 | −44 | 30 | 4 | |
| R OFC | 3.89 | 22 | 38 | 44 | 6 | |
| R VLPFC | 4.43 | 86 | 54 | 30 | 36 | |
| R Striatum | 3.31 | 20 | 15 | 10 | −3 | |
| L TPJ | 4.41 | 20 | −54 | −30 | 34 | |
| HD > OCD | ||||||
| R DLPFC | 4.87 | 85 | 42 | 42 | 16 | |
| R OFC | 4.65 | 23 | 40 | 30 | 6 | |
| R Insula | 4.11 | 51 | 44 | 4 | 2 | |
| L Rostral PFC | 4.65 | 62 | −24 | 54 | 26 | |
| R Visual Cx | 3.65 | 20 | 24 | −96 | −2 | |
| R Cerebellum | 4.8* | 205 | 30 | −46 | −30 | |
| Stroop: Incongruent > Congruent2 | L Premotor Cx | 3.93 | 30 | −40 | −24 | 56 |
| HD > HC | R Premotor Cx | 3.18 | 20 | 40 | 12 | 26 |
| No-Go Errors3 | ||||||
| HD > HC | ||||||
| L OFC | 3.95 | 20 | −20 | 40 | −14 | |
| R Mid CC | 4.68 | 37 | 12 | 2 | 48 | |
| R Post CC | 4.47 | 124 | 14 | −28 | 42 | |
| R Visual Cx | 4.23 | 56 | 28 | −82 | −6 | |
| R Fusiform | 3.96 | 25 | 32 | −40 | −20 | |
| L Fusiform | 4.28 | 65 | −32 | −38 | −22 | |
| OCD > HC | ||||||
| R DLPFC | 4.05 | 135 | 24 | 28 | 54 | |
| R Supramarginal Gyrus | 4.54 | 269 | 52 | −54 | 14 | |
| HD > OCD | ||||||
| R OFC | 4.5 | 73 | 28 | 38 | −8 | |
| R Striatum | 4.09 | 26 | 24 | 4 | 6 | |
| L Striatum | 3.46 | 39 | −20 | 6 | −8 | |
| OCD > HD | ||||||
| R OFC | 3.93 | 25 | 48 | 44 | −6 | |
| R Superior PFC | 4.96 | 39 | 14 | 20 | 64 | |
| L Superior PFC | 4.01 | 32 | −16 | 32 | 60 | |
| R Mid Temp | 4.21 | 96 | 52 | −30 | −4 | |
| L Mid Temp | 4.15 | 25 | −56 | −32 | −10 | |
| Stroop Errors4 | ||||||
| HD > HC | Bilateral SMA | 4.47 | 281 | −10 | 2 | 58 |
| R Sensory Cx | 4.14 | 244 | 32 | −40 | 64 | |
Clusters were significant with cluster-extent based thresholding FWE correction at p<0.05.
Go/No-Go “No-Go” trials relative to “Go” (i.e. conflict monitoring and processing).
Stroop Incongruent trials relative to Congruent trials (i.e. conflict monitoring and processing).
Go/No-Go “No-Go” errors relative to an implicit baseline (i.e. error processing).
Stroop Incongruent and Congruent errors (i.e. error processing).
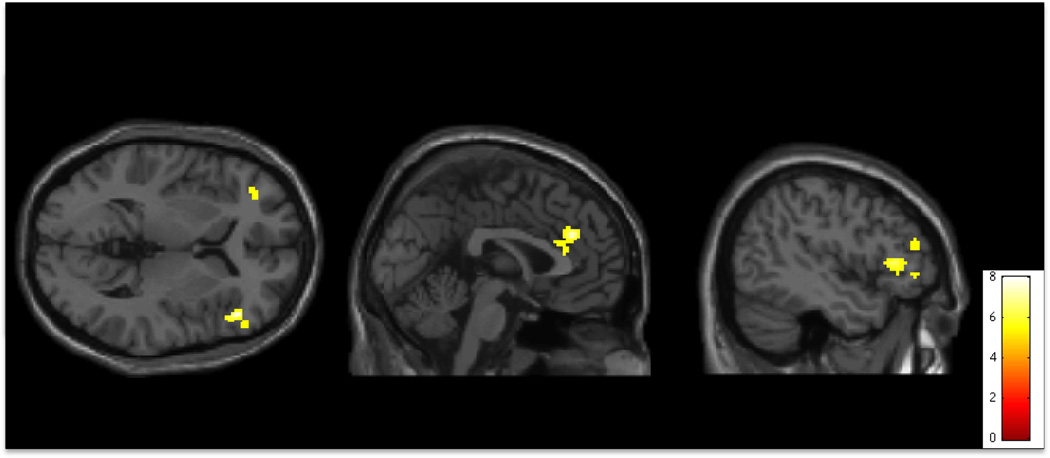
Figure 1.
Activity during conflict monitoring and processing (No-Go > Go conditions) on the Go/No-Go task. Activity is significantly greater in individuals with Hoarding Disorder than in Healthy Controls in bilateral Orbitalfrontal Cortex (OFC), Anterior Cingulate Cortex (ACC), and right Ventrolateral Prefrontal Cortex (R VLPFC) (p<0.001 uncorrected for multiple comparisons, superimposed on SPM8’s canonical single subject T1 image). See tables for coordinates of active regions in this and all subsequent figures.
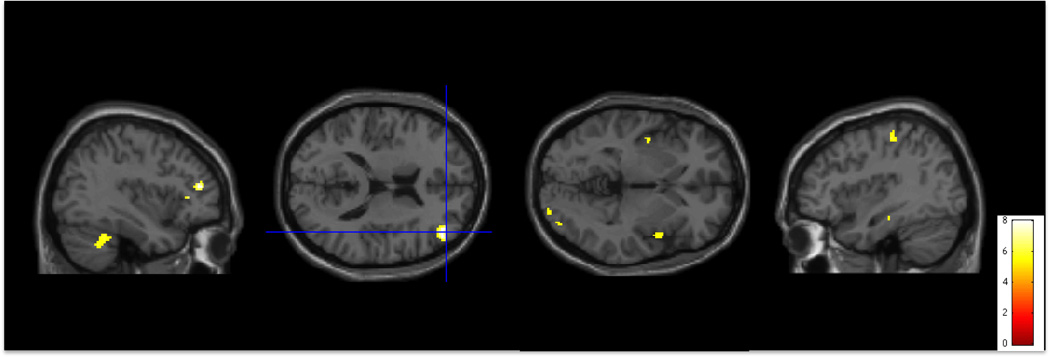
Figure 2.
Activity during conflict monitoring and processing (No-Go > Go conditions) on the Go/No-Go task. Activity is significantly greater in individuals with Hoarding Disorder than in individuals with Obsessive Compulsive Disorder in right Dorsolateral Prefrontal Cortex (R DLPFC), right Cerebellum, bilateral Insula, right Visual Cortex, and left Premotor Cortex (p<0.001 uncorrected for multiple comparisons, superimposed on SPM8’s canonical single subject T1 image).
During conflict processing on the Stroop task (incongruent trials relative to congruent trials), HD participants had greater activity than HC participants in the right premotor cortex and in right primary visual cortex (Table 4). OCD participants did not have significantly different activation patterns than HC participants. When HD and OCD participants were compared, OCD participants had greater activity in the medial PFC than did HD participants (Table 4).
3.3.2. Error processing
During No-Go error trials on the Go/No-Go task (relative to an implicit baseline), HD had greater activity than control participants in the left OFC, right insula and striatum, visual cortex, and in mid and posterior cingulate cortex (Table 4, Figure 3). OCD participants had greater activity than HC participants in the right DLPFC and right supramarginal gyrus (parietal cortex) (Table 4). Compared to OCD participants, HD had greater activity in the bilateral striatum and right VLPFC (Table 4, Figure 4).
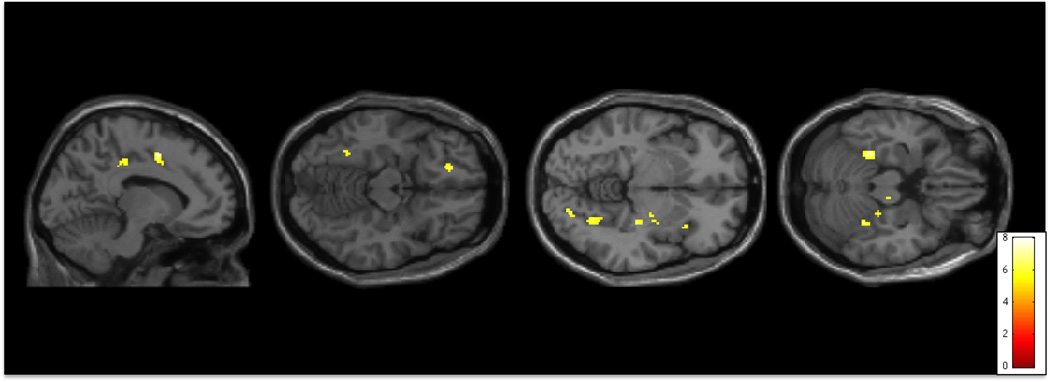
Figure 3.
Activity during error trials on the Go/No-Go task. Activity is significantly greater in individuals with Hoarding Disorder than in Healthy Controls in Mid-Cingulate Cortex (Mid-CC) and Posterior Cingulate Cortex (Posterior-CC), left Orbitofrontal Cortex (L OFC), Fusiform, Striatum, and Visual Cortex (p<0.001 uncorrected for multiple comparisons, superimposed on SPM8’s canonical single subject T1 image).
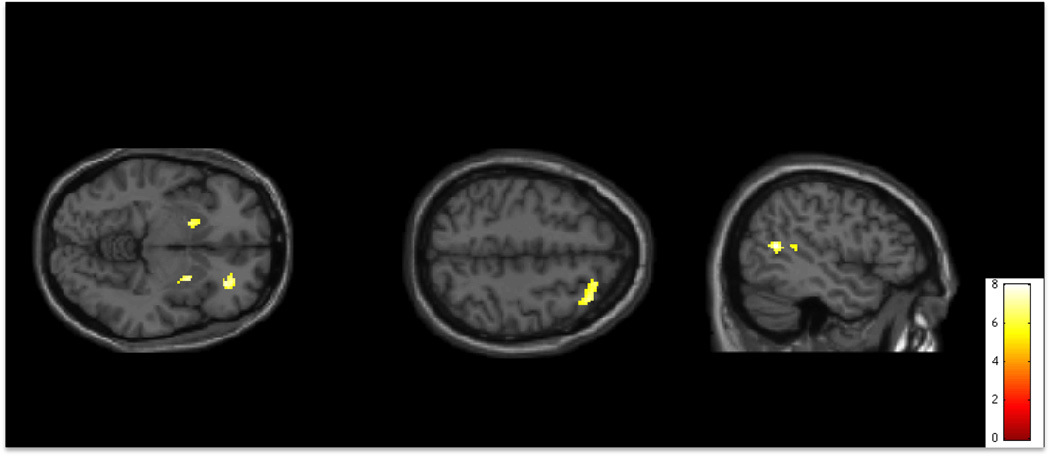
Figure 4.
Activity during error trials on the Go/No-Go task. Activity is significantly greater in individuals with Hoarding Disorder than in individuals with Obsessive Compulsive Disorder in bilateral Striatum and right Orbitofrontal Cortex (R OFC). Activity is significantly greater in individuals with Obsessive Compulsive Disorder than in individuals with Hoarding Disorder in right Dorsolateral Prefrontal Cortex (R DLPFC) and right Supramarginal Cortex (p<0.001 uncorrected for multiple comparisons, superimposed on SPM8’s canonical single subject T1 image).
HD participants had greater activity in the supplementary motor area (SMA) and right somatosensory cortex than HC participants in response to errors on the Stroop task (Table 4). There were no significant differences in brain activation patterns between OCD participants and HC participants in response to errors, or between HD and OCD participants.
4. Discussion
We examined differences in regional brain activation during conflict monitoring and processing and during error processing in individuals with HD, individuals with non-hoarding OCD, and HC participants. Our overarching theory is that HD is associated with core deficits in executive function (specifically, with abnormal self-monitoring and impaired error processing) and that these deficits are distinct from the cognitive dysfunction observed in OCD. Thus, in this study, we hypothesized that HD participants would show abnormal activation in brain regions associated with error processing and conflict monitoring (e.g., ACC, OFC, and DLPFC) compared to HC, and abnormal activation in the ACC relative to OCD participants. While we did find distinct differences in DLPFC and OFC, for HD relative to OCD participants, we did not find the differences in the ACC between HD and OCD participants that we hypothesized.
Specifically, we found that, during the conflict monitoring and response inhibition condition in the Go/No-Go task (No-Go trials relative to Go trials), individuals with HD had significantly greater activity than HC in the ACC and right DLPFC, although not in the OFC. HD also had significantly greater right DLPFC (but not ACC) activity than OCD. During error trials, there were no significant differences between groups in these regions. Although there are important limitations to this study (described further below), our results are in line with previous studies showing increased activation in these brain regions in individuals with hoarding symptoms (An et al., 2009; Saxena et al., 2004; Tolin et al., 2009; Tolin et al., 2012) with the results of our neuropsychological and electrophysiological studies of HD (Mackin et al., 2016; Mathews et al., 2016), and with our overarching theory of HD as described above.
Interestingly, although an electrophysiological measure of error monitoring, the error related negativity (ERN) was reduced in our HD participants relative to OCD and control participants (Mathews et al., 2016), and thus, we would have predicted decreased activation in the ACC, instead we saw increased activation for HD participants in the DLPFC relative to HC and OCD participants during error trials. In line with this finding, Mathalon et al. (2009) also found a reduced ERN but increased activation in the DLPFC and in the ACC in participants with schizophrenia. They suggest that the ERN may in fact be a more sensitive measure of error monitoring than brain activation patterns during the same task, in part because of the temporal precision of EEG-based event related potentials compared to fMRI (Mathalon et al., 2009).
In addition to activation abnormalities in executive control regions during the conflict and error conditions, we observed significant differences in activity for HD in comparison to HC and OCD in regions that contribute to the “salience” network/regions involved in evaluating stimulus-response-reward associations, or the personal and task-relevant value of stimuli and behavioral responses to them (ACC, anterior insula, OFC, striatum) (Craig, 2009; Etkin et al., 2011; Seeley et al., 2007). The salience network does not directly perform task-based attention and conflict monitoring and error processing per se, but appears to be the system that links cognitive processing to the reward system and determines the value or personal consequence of behavior, including cognitive behavior (e.g., responses on emotionally neutral cognitive tasks) (Bressler and Menon, 2010). In individuals with HD, regions of this salience network were hyperactive during cognitive conflict and error conditions (ACC, OFC, right anterior insula, and striatum compared to HC; bilateral anterior insula, striatum, and right VLPFC/OFC compared to OCD), even when there was no direct personal consequence of behavior performance (subjects were not rewarded for correct performance).
An et al. (2009) also reported hyperactivity in the OFC during an fMRI task in which HD subjects made discarding decisions about photos of common objects, and Tolin et al. (2012) reported that HD subjects had increased fMRI activity in the ACC and bilateral insula when making decisions about discarding possessions (An et al., 2009; Tolin et al., 2014). In conjunction with our findings, these results are consistent with the hypothesis that individuals with HD have difficulty deciding on the value or task relevance of stimuli, and may perceive an abnormally high risk of negative feedback for difficult cognitive tasks, or for commission of errors on these tasks.
Somewhat unexpectedly, we also found increased activity among individuals with HD in visual regions (right visual cortex and right fusiform cortex) during conflict tasks. Saxena et al. (2004) also reported abnormal PET glucose metabolism in the occipital cortex associated with hoarding in OCD, and An et al. (2009) reported a negative correlation between hoarding and brain activation in the visual association area (An et al., 2009; Saxena et al., 2004). We have previously reported that HD subjects have deficits in visual categorization and visual (but not verbal) learning and memory (Mackin et al., 2011; Mackin et al., 2016). These differences in visual cortex activity between HD and HC subjects suggest that visual processing deficits (specifically, inefficiencies in visual processing) may contribute to the abnormal responses to visual stimuli in the executive and salience systems in our participants. The findings that brain activation patterns in visual cortex also distinguish HD from OCD participants suggest that abnormalities in visual processing may be a unique feature of HD.
We also observed significant differences in activity between groups in motor regions. During Stroop conflict situations, HD had significantly greater activity than HC in the right premotor cortex; during Go/No-Go conflict, HD had significantly greater activity than OCD in the left premotor cortex; and during Go/No-Go errors, HD had significantly greater activity than HC in the right SMA and right sensory/motor cortex. While unexpected, these activity differences in motor regions are not unprecedented. Saxena et al. (2004) reported increased PET activity in OCD with hoarding symptoms relative to controls in the right sensory motor cortex (Saxena et al., 2004). Tolin et al. (2012) found that HD had reduced fMRI activity in the left fusiform when making decisions about discarding personal items, relative to controls, and Tolin et al. (2014) found that HD had reduced fMRI activity in the right precentral cortex during a Go/No-Go task, relative to controls (Tolin et al., 2014). While we do not yet understand the relevance of these findings, these regions should be further examined in future studies.
4.1. Limitations
There are several important limitations to this study. The biggest limitation is the sample size. After excluding participants for artifact, each disease group consisted of between 12 and 15 individuals, while the control group comprised 21 individuals. The groups also differed in terms of age, rates of psychotropic use, lifetime psychiatric history, current anxiety and depressive symptoms, and, although not statistically significant, gender distribution. Although we controlled for age and gender in all analyses, we did not control for the other potentially confounding variables, due to the small sample sizes. While we found differences among the groups with this sample size and used a cluster-based analysis to minimize type I error, it must be noted that we did not correct for multiple comparison testing in whole brain analysis, and our findings would not survive stringent correction for multiple comparisons. Therefore, our results must be considered to be preliminary only, requiring replication in in well-matched, sufficiently powered samples.
4.2. Conclusions
Although preliminary, our data provide additional evidence to support the distinction between HD and OCD as neurobiologically distinct disorders. As hypothesized, we found evidence that brain regions involved in response conflict and error processing are abnormally active (hyperactive) in HD. Our results also suggest that brain regions involved in the salience network may be hyperactive in HD; although some of these regions (e.g., the insula) have been previously identified in neuroimaging studies of hoarding, the importance of the salience network has not, to our knowledge, been discussed as being relevant in HD. Finally, we found evidence of inefficient visual processing in HD, aligning with our data from neuropsychological and electrophysiological studies, as well as evidence of increased activation in motor regions.
Supplementary Material
Highlights.
We compared fMRI during executive function tasks in hoarding (HD), OCD, and controls.
HD had differences in regions involved in executive function and visual processing.
HD had increased brain activity during conflict monitoring/response inhibition.
HD had differences in salience network, which determines value/consequence of action.
Acknowledgments
This research was supported by NIMH Grants R21 MH087748, K08 MH081065, R01 0977669, and the Althea Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
CM, DM and TL were responsible for study concept and design. CH, TL, KL, OV, SG and SF contributed to the acquisition of MRI data. CH, OV, SF, EK and CM recruited and screened subjects. TL, CH and CM performed data analysis and interpretation of findings. OV and KL assisted in performing data analysis. DM assisted in interpretation of findings. CH and TL drafted the manuscript. All authors critically reviewed content and approved the final manuscript.
Conflict of Interest
The authors report no conflict of interest.
References
- Alvarenga PG, do Rosario MC, Batistuzzo MC, Diniz JB, Shavitt RG, Duran FL, Dougherty DD, Bressan RA, Miguel EC, Hoexter MQ. Obsessive- compulsive symptom dimensions correlate to specific gray matter volumes in treatment-naive patients. J Psychiatr Res. 2012;46:1635–1642. doi: 10.1016/j.jpsychires.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: 2013. p. 235. [Google Scholar]
- An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, Brammer MJ, Phillips ML. To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Molecular psychiatry. 2009;14:318–331. doi: 10.1038/sj.mp.4002129. [DOI] [PubMed] [Google Scholar]
- Ayers CR, Wetherell JL, Schiehser D, Almklov E, Golshan S, Saxena S. Executive functioning in older adults with hoarding disorder. Int J Geriatr Psychiatry. 2013;28:1175–1181. doi: 10.1002/gps.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation; 1988. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blom RM, Samuels JF, Grados MA, Chen Y, Bienvenu OJ, Riddle MA, Liang KY, Brandt J, Nestadt G. Cognitive functioning in compulsive hoarding. J Anxiety Disord. 2011;25:1139–1144. doi: 10.1016/j.janxdis.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in cognitive sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nature reviews neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Frost RO, Hartl TL. A cognitive-behavioral model of compulsive hoarding. Behaviour research and therapy. 1996;34:341–350. doi: 10.1016/0005-7967(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behaviour research and therapy. 2004;42:1163–1182. doi: 10.1016/j.brat.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Williams L. Hoarding: a community health problem. Health & social care in the community. 2000;8:229–234. doi: 10.1046/j.1365-2524.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Youngren VR, Mallya GK. The threat of the housing inspector: a case of hoarding. Harvard review of psychiatry. 1999;6:270–278. doi: 10.3109/10673229909000339. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Mataix-Cols D, Almeida JR, Lawrence N, Nutche J, Diwadkar V, Keshavan MS, Phillips ML. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. Journal of affective disorders. 2008;109:117–126. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of general psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Norberg MM, Williams AD, Certoma SP, Kadib R. Categorization and cognitive deficits in compulsive hoarding. Behaviour research and therapy. 2010;48:866–872. doi: 10.1016/j.brat.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Cardoner N, Deus J, Alonso P, Lopez-Sola M, Contreras-Rodriguez O, Real E, Segalas C, Blanco-Hinojo L, Menchon JM, Soriano-Mas C. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biological psychiatry. 2013;73:321–328. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Arean PA, Delucchi KL, Mathews CA. Cognitive functioning in individuals with severe compulsive hoarding behaviors and late life depression. Int J Geriatr Psychiatry. 2011;26:314–321. doi: 10.1002/gps.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin RS, Vigil O, Insel P, Kivowitz A, Kupferman E, Hough CM, Fekri S, Crothers R, Bickford D, Delucchi KL, Mathews CA. PATTERNS OF CLINICALLY SIGNIFICANT COGNITIVE IMPAIRMENT IN HOARDING DISORDER. Depress Anxiety. 2016;33:211–218. doi: 10.1002/da.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Billotti D, Fernández de la Cruz L, Nordsletten AE. The London field trial for hoarding disorder. Psychol Med. 2013;43:837–847. doi: 10.1017/S0033291712001560. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Archives of general psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Jorgensen KW, Roach BJ, Ford JM. Error detection failures in schizophrenia: ERPs and FMRI. Int J Psychophysiol. 2009;73:109–117. doi: 10.1016/j.ijpsycho.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews CA, Delucchi K, Cath DC, Willemsen G, Boomsma DI. Partitioning the etiology of hoarding and obsessive-compulsive symptoms. Psychol Med. 2014;44:2867–2876. doi: 10.1017/S0033291714000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews CA, Perez VB, Roach BJ, Fekri S, Vigil O, Kupferman E, Mathalon DH. Error-related brain activity dissociates Hoarding Disorder from Obsessive Compulsive Disorder. Psychol. Med. 2016;46(2):367–379. doi: 10.1017/S0033291715001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan SG, Rees CS, Pestell C. An investigation of executive functioning, attention and working memory in compulsive hoarding. Behavioural and cognitive psychotherapy. 2013;41:610–625. doi: 10.1017/S1352465812000835. [DOI] [PubMed] [Google Scholar]
- Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, Chacon P, Pauls DL. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Molecular psychiatry. 2005;10:258–275. doi: 10.1038/sj.mp.4001617. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Papmeyer M, Pertusa A, Chamberlain SR, Fineberg NA, Sahakian BJ, Mataix-Cols D, Robbins TW. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry research. 2014;215:659–667. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines AM, Timpano KR, Schmidt NB. Effects of clutter on information processing deficits in individuals with hoarding disorder. Journal of affective disorders. 2014;166:30–35. doi: 10.1016/j.jad.2014.04.074. [DOI] [PubMed] [Google Scholar]
- Saxena S. Is compulsive hoarding a genetically and neurobiologically discrete syndrome? Implications for diagnostic classification, The American journal of psychiatry, United States; 2007. pp. 380–384. [DOI] [PubMed] [Google Scholar]
- Saxena S. Neurobiology and treatment of compulsive hoarding. CNS spectrums. 2008;13:29–36. doi: 10.1017/s1092852900026912. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Baxter LR. Paroxetine treatment of compulsive hoarding. J Psychiatr Res. 2007;41:481–487. doi: 10.1016/j.jpsychires.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, Baker SK, Baxter LR., Jr Cerebral glucose metabolism in obsessive-compulsive hoarding. The American journal of psychiatry. 2004;161:1038–1048. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Grecius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The journal of neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee G, Frost R. Compulsive hoarding: current status of the research. Clinical psychology review. 2003;23:905–927. doi: 10.1016/j.cpr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Frost RO, Steketee G. An open trial of cognitive-behavioral therapy for compulsive hoarding. Behaviour research and therapy. 2007;45:1461–1470. doi: 10.1016/j.brat.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Frost RO, Steketee G, Gray KD, Fitch KE. The economic and social burden of compulsive hoarding. Psychiatry Research. 2008;160:200–211. doi: 10.1016/j.psychres.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Kiehl KA, Worhunsky P, Book GA, Maltby N. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychol Med. 2009;39:325–336. doi: 10.1017/S0033291708003371. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Stevens MC, Villavicencio AL, Norberg MM, Calhoun VD, Frost RO, Steketee G, Rauch SL, Pearlson GD. Neural mechanisms of decision making in hoarding disorder. Archives of general psychiatry. 2012;69:832–841. doi: 10.1001/archgenpsychiatry.2011.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Villavicencio A, Umbach A, Kurtz MM. Neuropsychological functioning in hoarding disorder. Psychiatry research. 2011;189:413–418. doi: 10.1016/j.psychres.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Witt ST, Stevens MC. Hoarding disorder and obsessive-compulsive disorder show different patterns of neural activity during response inhibition. Psychiatry research. 2014;221:142–148. doi: 10.1016/j.pscychresns.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AA, Miguel EC, Castro CC, Amaro E, Duran FL, Buchpiguel CA, Chitnis X, McGuire PK, Busatto GF. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biological psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Wincze JP, Steketee G, Frost RO. Categorization in compulsive hoarding. Behaviour research and therapy. 2007;45:63–72. doi: 10.1016/j.brat.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, Wager TD. Cluster-based extent thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.