Abstract
Mast cells (MCs), which are granulated tissue-resident cells of hematopoietic lineage, contribute to vascular homeostasis, innate/adaptive immunity and wound healing. MCs are, however, best known for their roles in allergic and inflammatory diseases such as anaphylaxis, food allergy, rhinitis, itch, urticaria, atopic dermatitis and asthma. In addition to the high affinity IgE receptor (FcεRI), MCs express numerous G protein coupled receptors (GPCRs), which are the largest group of membrane receptor proteins and are the most common targets of drug therapy. Antimicrobial host defense peptides (HDPs), neuropeptides (NPs), major basic protein (MBP), eosinophil peroxidase (EPO) and many FDA approved peptidergic drugs activate human MCs via a novel GPCR known as MAS-related G protein coupled receptor-X2 (MRGPRX2; formerly known as MrgX2). Unique features of MRGPRX2 that distinguish it from other GPCRs include their presence both on plasma membrane and intracellular sites and their selective expression in MCs. In this article, we review the possible roles of MRGPRX2 on host defense, drug-induced anaphylactoid reactions, neurogenic inflammation, pain, itch and chronic inflammatory diseases such as urticaria and asthma. We propose that HDPs that kill microbes directly and activate MCs via MRGPRX2 could serve as novel GPCR targets to modulate host defense against microbial infection. Furthermore, monoclonal antibodies or small molecule inhibitors of MRGPRX2 could be developed for the treatment of MC-dependent allergic and inflammatory disorders.
Keywords: G protein coupled receptor, MRGPRX2, mast cells, host defense peptides, neuropeptides, drug-induced pseudoallergy, chronic urticaria, asthma
Introduction
Mast cells (MCs) reside primarily at sites exposed to the external environment, such as the skin, oral/gastrointestinal mucosa and respiratory tract. Activation of MCs via the cross-linking of high affinity IgE receptors (FcεRI) results in the release of preformed and newly synthesized mediators which contribute to signs and symptoms associated with hypersensitive and allergic diseases. MCs are generally classified into two types based on the protease content of their secretory granules.1 In humans, most MCs that are found in connective tissues such as the skin contain tryptase, chymase, carboxypeptidase and cathepsin and are known as MCTC. In contrast, majority of MCs that are found in lung and gut express only tryptase and are known as MCT.2–4 MCs also differ in their responses to endogenous and exogenous stimuli that promote degranulation. Thus, while both human MC types are activated via the aggregation of FcεRI, MCTC respond to complement components C3a, C5a and compound 48/80 but MCT do not.5, 6 In mice, connective tissue MCs (CTMCs) resembles MCTC while mucosal MCs (MMCs) resemble MCT.1, 7 Thus, murine CTMCs are found in the skin and MMC mature in the mucosal tissues such as the lung and gut. In addition, CTMCs are responsive to compound 48/80, C3a and C5a for degranulation but MMCs are not.8–10 Although MCs have been extensively studied for their role in allergic diseases, recent evidence suggests that they contribute significantly to vascular hemostasis, pain, itch and host defense and these responses are most likely mediated via that activation of MCTC in humans and CTMC in mice.11–15
GPCRs represent the largest family of seven transmembrane domain receptors that couple via heterotrimeric G-proteins to regulate vital cellular functions including cell proliferation, development, survival, metabolism and neuronal signal transmission. Homology cloning and bioinformatics analysis of sequence databases have led to the identification of ~800 human GPCRs.16,17 However, endogenous and/or natural exogenous ligands remain unknown for more than 100 receptors and they are collectively classified as “orphan GPCRs”. Dong and colleagues carried out a comparative analysis of the transcriptome of dorsal root ganglia (DRG) of wild type mice and neurogenin-1 deficient mice, which fails to develop a subclass of nociceptive neurons.18 This analysis led to the identification of multiple unknown transcripts, including an entire new subfamily of GPCRs related to the MAS1 oncogene and hence the encoding genes are called mas-related gene (MRG) and the receptors MAS-related GPCRs.18, 19
The MRG family comprises of approximately 50 members in mouse, rat, human, macaque and rhesus monkey that can be subdivided into several subfamilies.18, 20–22 Subfamilies A, B, C, D, E, F and H exist only in rodents, whereas the subfamily X is specific to human, macaque and rhesus monkey.23 In human, there are four MRGX genes, MRGPR X1 – X4; the mouse genome contains MrgprA (A1–A10), MrgprB (B1–B5, B8), MrgprC (C11), MrgprD, MrgprE, MrgprF, MrgprG and MrgprH genes.18, 20 It now appears that outside the DRG, human MCTC are the only cells that express MRGPRX2.24–26 In DRG, cortistatin has been identified as a potent ligand for MRGPRX2 and the receptor likely contributes to sleep regulation, locomotion activity and cortical function.27 Recent studies have shown that HDPs and NPs and activate human MCTC via MRGPRX2.28–31 In this article, we review the possible roles of MRGPRX2 on host defense, drug-induced anaphylactoid reactions, neurogenic inflammation, pain, itch and chronic inflammatory diseases such as urticaria and asthma. In addition, we discuss the signal transduction pathway via which peptide ligands activate MRGPRX2 and the mechanism of its regulation.
MRGPRX2 as a novel GPCR for NPs and other cationic peptides in human MCTC
In addition to FcεRI, MCs express a large number of GPCRs for ligands as diverse as lipids, chemokines, adenosine and the anphylatoxins C3a and C5a.8, 32–36 Although basic peptides such as MC degranulating peptide (MCDP), NPs and the synthetic histamine releaser compound 48/80 have long been known to activate MCTC via a G protein-dependent mechanism, the possible involvement of specific GPCRs has been the subject of intense debate. It was previously thought that neurokinin 1 receptor (NK1R) expressed on MCs partly mediates the response to SP.37–39 Accordingly, a NK1R inhibitor partially blocks SP-induced responses in human MCs.38, 39 Furthermore, SP translocates rapidly into rodent MCs and is able to initiate secretion when it is introduced directly into the cytosol.40, 41 Based on these findings, it was proposed that the effects of SP on MCs are mediated via two pathways, one involving NK1R and the other via the insertion of the amphiphilic SP molecule into the cell membrane, thus enabling direct activation of G proteins.42, 43
Tatemoto et al.,25 provided the first demonstration that MRGPRX2 is expressed in MCTC and showed that SP, VIP, MCDP and compound 48/80 activate MCs via this receptor. Human cord blood-derived MCs can be cultured under conditions that promote their differentiation into MCTC or MCT. Using quantitative PCR, it was found that the copy number of MRGPRX2 in in vitro-differentiated MCTC is 17,565 per 5 ng total RNA, whereas it is only 32 per 5 ng of total RNA in MCT.25 This is consistent with PCR and microarray data showing that the transcript for MRGPRX2 is expressed at high levels in human skin MCTC but at low levels in lung MCT.26,24 Interestingly, MCTC express MRGPRX1 and MRGPRX2 but not MRGPRX3 and MRGPRX4.25, 44, 45 To identify candidate ligands for MRGPRX2, peptide and chemical libraries were screened using a reporter gene assay in PC12 cells transiently transfected with cDNA encoding MRGPRX2. It was found that MCDP, compound 48/80, SP and VIP increase reporter gene expression in MRGPRX2 transfected cells.25 These ligands also induced dose-dependent Ca2+ mobilization in human embryonic kidney (HEK293) expressing MRGPRX2 but not MRGPRX1.25 The specificity of SP for MRGPRX2 over MRGPRX1 for MC degranulation was later confirmed using transfected rat basophilic (RBL-2H3) cells.30 In addition, Fijisawa et al.,26 showed that MRGPRX2 is present in both plasma membrane and at intracellular sites in human skin MCTC. Furthermore, lentiviral small hairpin RNA (shRNA)-mediated knockdown of MRGPRX2 expression results in substantial inhibition of SP-induced MC degranulation and prostaglandin D2 (PGD2) generation. In addition, a specific inhibitor of NK1R (CP-96345) does not inhibit SP-induced MC degranulation.26 These findings suggest that although both NK1R and MRGPRX2 are expressed in MCTC, SP utilizes MRGPRX2 to promote G protein-dependent MC degranulation.
MrgprB3 as the rat ortholog of human MRGPRX2
Most original studies on the effects of NPs and HDPs on MC activation were performed with rat peritoneal MCs (PMCs).46–51 Unlike the human genome, the rat genome possesses one each of the MrgprA, MrgprC, MrgprD, MrgprE, MrgprF, and MrgprH genes and six MrgprB genes.20, 19 RT-PCR analysis demonstrated that rat peritoneal MCs (PMCs) express high levels of the MrgprB3 and MrgprB8 and low levels of the MrgprB1, MrgprB2, MrgprB6, and MrgprB9 genes. MCDP and SP caused an increase reporter gene expression in MrgprB3-transfected PC12 cells. Furthermore, these peptides caused a dose-dependent increased Ca2+ mobilization in MrgprB3-transfected HEK-293 cells. In contrast, none of the peptides increased reporter gene expression or Ca2+ mobilization in cells transfected with the MrgprA, MrgprB2, MrgprB6, MrgprB8, MrgprB9, or MrgprC gene. These findings suggest that while NPs activate human MCTC via MRGPRX2 they activate rat PMCs via MrgprB3.
MrgprB2 is the mouse ortholog of human MRGPRX2
There are 22 potential MRG GPCR coding genes in mice; which makes it difficult to identify the mouse ortholog of human MRGPRX2. Using stringent reverse transcriptase-PCR (RT-PCR) in mouse PMCs, McNeil et al.,52 showed that these cells express messenger RNA for MrgprB2 and no other MRG GPCRs. Generation of a transgenic mouse with td-Tomato reporter under the control of MrgprB2 promoter demonstrated that the expression of this receptor is restricted to CTMC in the skin, gut and trachea.52 The same group also generated mice with a 4 base pair deletion in the MrgprB2 coding region, resulting in a frame shift mutation and early termination of the receptor (shortly after the first transmembrane domain). These MrgprB2 mutant mice have no defect in MC number and respond normally to IgE/FcεRI activation. However, PMCs from these mice show dramatic reduction in Ca2+ mobilization and histamine release in response to compound 48/80 and SP in vitro and reduced paw edema in vivo. These findings demonstrate that MrgprB2 is the mouse ortholog of human MRGPRX2.
Human MRGPRX2 and mouse MrgprB2 share certain unique characteristics as these receptors are expressed in MCTC/CTMC and are activated by basic ligands such as NPs and compound 48/80. Surprisingly, MRGPRX2 and MrgprB2 differ substantially with respect to the concentration of the agonists required for their activation. Thus, EC50 values (concentration required to give 50% response) of most ligands for MrgprB2 are significantly higher than those for MRGPRX2.25, 52 For example, while SP activates MrgprB2 with an EC50 value of 54 μM, it activates MRGPRX2 with EC50 of 152 nM. This difference is reflected in only ~53% overall sequence similarity between these receptors. Furthermore, sequence similarities at the N-terminal 60 amino acids and the C-terminal 80 amino acids are ~34% and ~47%, respectively. Although the GPCR superfamily comprises of about 800 human proteins, crystal structure of only four of these proteins have been resolved.53 Based on these studies, it has been proposed that modules within GPCR’s extracellular (EC) and transmembrane extracellular regions (TM-EC) domains contribute to agonist binding whereas intracellular (IC) domains are involved in G protein coupling.53 These findings suggest that differences in the amino acid sequences of MRGPRX2 and MrgprB2 contribute to differences the ability of peptide ligands to activate these receptors.
MRGPRX2 as a novel GPCR for HDPs and its role in innate immunity
HDPs such as defensins and cathelicidins are positively charged amphipathic peptides that are crucial for the clearance of microbial pathogens and thus play an important role in host defense. In humans, defensins are divided into α and β families depending on the position of the cysteine residues involved in disulfide linkages.54 α-defensins are produced by neutrophils and intestinal paneth cells while human β-defensins (hBDs) are produced primarily by epithelial cells.55, 56 Of the four members of the hBD family (hBD1–4) that have been characterized in detail; hBD1 is expressed constitutively while the others are induced by bacteria, viruses and cytokines. LL-37 is a cathelicidin produced by neutrophils as an inactive precursor (hCAP18), which is enzymatically cleaved to release the active LL-37. HDPs kill microbes by interacting with the negatively charged phospholipid moieties and by disrupting their membranes.56 Direct antimicrobial activities were originally considered to be the primary function of these peptides and, hence their name antimicrobial peptides. However, as described below, HDPs also activate MCs and other immune cells and these features likely contribute to their effectiveness as antimicrobial agents.57
It is well documented that MCs play a critical role in host defense.12, 14, 58 HDPs (LL-37 and hBDs) act as potent MC chemoattactants,46, 59, 60 and also increase the expression of toll-like receptor-4 (TLR-4) on their surface, which may enhance ability of MCs to detect invading pathogens.14, 61 MC degranulation plays an important role in host defense by causing increased vascular permeability and by initiating the recruitment of neutrophil to the sites of infection.58, 62–66 We have recently shown that hBDs, which are derived from epithelial cells, activate human MCs via MRGPRX2.29 LL-37 is produced from activated MCs and neutrophils and it’s antimicrobial activity reflects, at least in part, the activation of MCs via MRGPRX2.28, 31, 57, 67, 68 LL-37 causes the release of the Th2 cytokines IL-4 and IL-5 as well as the proinflammatory cytokine IL-β and may contribute to the development of both innate and adaptive immunity. Interestingly, lipopolysaccharide (LPS) generated from Gram-negative bacteria inhibits LL-37-induced Th2 cytokine, but not IL-β release.61 These findings raise the interesting possibility that LL-37 co-existing with bacterial infection switches MC function towards innate immunity.61
Possible roles of MRGPRX2 on the orchestration of adaptive immunity and wound healing
In addition to innate immunity, MCs orchestrate the development of adaptive immunity and play an important role in wound healing.69–72 Thus, at the sites of microbial infection, mediators released from MCs promote migration of dendritic cells, which are subsequently increased in draining lymph nodes.73–75 Furthermore, MC-derived histamine directly modulates dendritic cell activation to enhance antigen presentation to T cells.76 Interestingly, compound 48/80, which activates MCs via MRGPRX2, has been used as a safe and effective vaccine adjuvant in mice.25, 72, 77 Enterococcus faecalis has emerged as an important cause of life-threatening multidrug-resistant bacterial infections in the hospital setting. Scheb-Wetzel et al.,78 recently showed that MCs exert potent antimicrobial effect against this pathogen and that this effect is mediated via their degranulation and release of LL-37. Moreover, LL-37 protects skin from necrotic skin infections and promotes healing.79 It is therefore likely that MRGPRX2 expressed in MCs contributes not only to innate immunity but also provides an important link to adaptive immunity and promotes wound healing (Fig. 1).
Figure 1. Activation of MRGPRX2 on MCs by HDPs may orchestrate microbial clearance and promote wound healing.
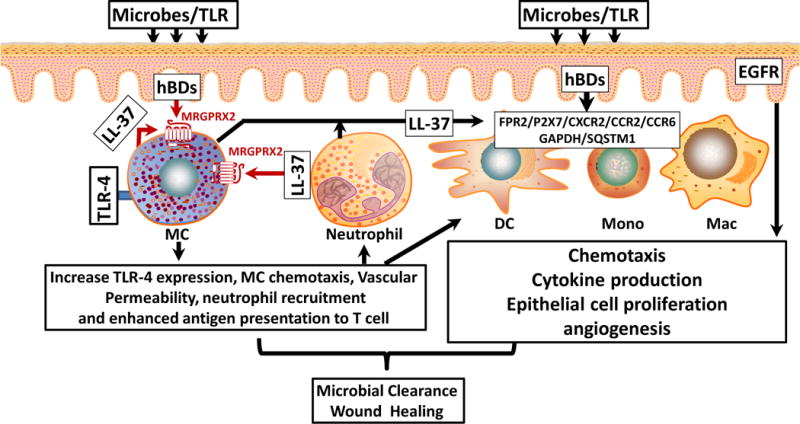
Activation of epithelial cells following microbial infection leads to the generation of hBDs which cause MC chemotaxis and degranulation via MRGPRX2. MC-derived mediators cause increased vascular permeability and promote neutrophil recruitment. LL-37 released from neutrophils also activates MRGPRX2, which enhances TLR-4 expression and causes further MC chemotaxis and degranulation. Histamine and TNF-α released from MCs activate dendritic cells (DC) leading to enhanced antigen presentation to T cells. hBDs and LL-37 produced from epithelium and neutrophil, respectively, also promote keratinocyte migration and pro-inflammatory cytokines production via the transactivation of EGFR receptor. These HDPs also activate other cell surface GPCRs and intracellular receptors (GAPDH/SQSTM1) on monocytes (Mono), and macrophages (Mac) to induce growth factor and cytokine release to promote microbial clearance and wound healing.
In addition to MCs, the effects of HDPs on host defense and wound healing may also reflect the activation of leukocytes and epithelial cells. Thus, LL-37 induces human neutrophil and monocyte chemotaxis via the activation of formyl-peptide receptor 2 (FPR2), purinergic receptor P2X7 and chemokine receptor CXCR2.80, 81,82 Furthermore, hBD3 promote monocyte chemotaxis via CCR2.83 hBD3 and LL-37 induce keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines via the transactivation of EGFR, which likely promotes the recruitment of additional leukocytes at the site of infection.84, 85 In addition to cell surface receptors, HDPs activate intracellular receptors, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and sequestosome-1 (SQSTM1).86, 87 This leads to the stimulation of multiple signaling pathways that are important in innate immunity, including p38, extracellular related kinases 1 and 2 (ERK1/2, also called MAPK3 and MAPK1, respectively), stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK), nuclear factor-κB (NF-κB), thus resulting in augmentation of chemokine production and recruitment of neutrophils and monocytes to the site of infection.57, 88 Thus, it is likely that antimicrobial activities of HDPs largely depend on their ability to activate MCs via MRGPRX2, which orchestrate the development of adaptive immunity, resulting in microbial clearance and enhanced wound healing (Fig. 1).
Roles of MRGPRX2 and MrgprB2 on anaphylactoid drug reactions
Many FDA approved peptidergic drugs are known to cause psuedoallergic drug reactions in humans. For example, icatibant, a bradykinin B2 receptor antagonist, which is used for the treatment of hereditary angioedema, causes injection site erythema and swelling in nearly every patient.89 Neuromuscular blocking drugs (NMBDs) such as artacurium, mivacurium, tubacurarine and rocurinium, which are routinely used in surgery to reduce unwanted muscle movement, induce histamine release from skin MCs and cause allergic reactions in surgical settings.90, 91 Furthermore, fluoroquinolone family of antibiotics such as ciprofloxacin and levofloxacin activate MCs to cause histamine release and are associated with allergic reactions.92–95 These three classes of drugs promote Ca2+ mobilization in HEK293 cells stably expressing MRGPRX2.52 Furthermore, they stimulate degranulation, PGD2 and TNF-α release in the human MC line, LAD2 and these responses are attenuated in cells with siRNA-mediated knockdown of MRGPRX2.52
Peptidergic drugs also cause Ca2+ mobilization and degranulation in PMCs from wild-type mice, which are dramatically reduced in MrgprB2 mutant mice.52 Additionally, MrgprB2 mutant mice display reduced paw edema in response to icatibant and atracurium and show significantly blunted systemic anaphylaxis response to the fluoroquinolone antibiotics such as ciprofloxcin, when compared to wild-type mice.52 Based on these findings, it has been proposed that human MRGPRX2 is responsible for pseudo-allergic drug reactions and that MrgprB2 may serve as a model to develop potential therapeutic target for drug-induced anaphylactic responses in vivo. However, the concentrations of ligands required to activate MrgprB2 are significantly higher than those for MRGPRX2.25, 52 For example, while ciprofloxacin and levofloxacin activate MrgprB2 with EC50 values of ~126 μg/ml and 807 μg/ml, respectively, the same drugs activate MRGPRX2 with EC50 values of ~6.8 μg/ml and 23 μg/ml. These findings suggest that there are important species-specific differences between human MRGPRX2 and mouse MrgprB2 and that MrgprB2 mutant mice may not be an appropriate model to screen drugs for human use.
Potential roles of MRGPRX2 on neurogenic inflammation, pain and itch
MCs are found in close proximity to nerve endings that release peptides such as SP and calcitonin gene-related peptide (CGRP) and appear to serve as a functional homeostatic regulatory unit.96–99 It is now generally accepted that MC activation by NPs contributes to neurogenic inflammation, pain and itch.15, 98, 100–102 Tryptase released from MCs cleaves and activates proteinase activated receptor-2 (PAR-2) on primary afferent neurons, which promotes Ca2+ influx resulting in the release of pre-stored NPs such as CGRP and SP.100 MC-derived histamine and cysteinyl leukotrienes also interact with their specific GPCRs on sensory afferent neurons to cause the release of NPs.100, 103, 104 CGRP interacts with the CGRP1 receptor to induce arteriolar dilation and hyperemia whereas SP interacts with NK1R on endothelial cells of precapillary venules to cause gap formation and plasma extravasation (Fig. 2). SP, VIP and other NPs released from sensory nerve endings and from activated MCs can provide positive feedback mechanism for further MC activation and the release of NPs.100, 104–106 The recent demonstration that NPs activate human MCTC via MRGPRX225, 26, 30, 52 suggests that this GPCR contributes to the development of neurogenic inflammation. In addition to this local MC-sensory nerve interaction, the sensation of pain is also amplified via the Ca2+-mediated axon potential that travels orthodromically along the axon to the central nervous system (CNS) (Fig. 2).
Figure 2. Potential roles for MC-MRGPRX2 on neurogenic inflammation, pain and itch.
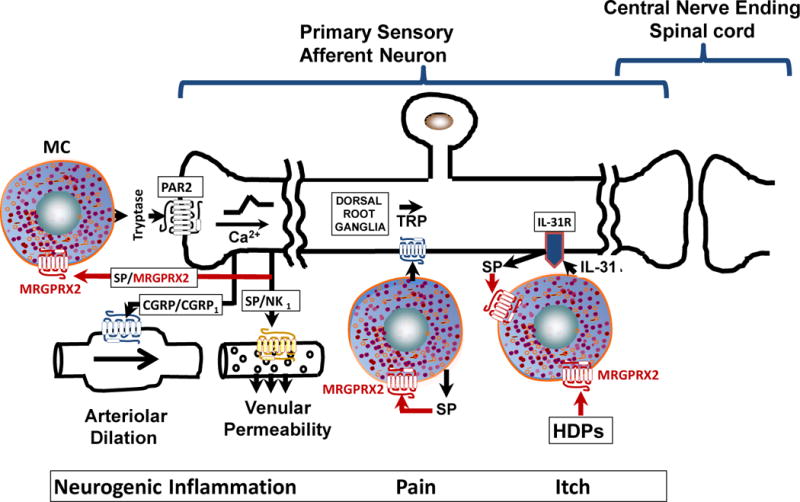
For neurogenic inflammation and pain, tryptase released from degranulated MCs activates protease activated receptor-2 (PAR-2) on sensory nerve endings resulting in the release of CGRP and SP, which interact with their receptors, CGRP1 and NK1R to promote arteriolar dilation and venular permeability, respectively. SP released from sensory nerve ending and from activated MCs also acts on MCs themselves, thus promoting a vicious cycle of MC activation via MRGPRX2. Furthermore, the Ca2+-mediated axon potential travels orthodromically along the axon to the central nervous system ultimately resulting in pain and itch responses. HDPs activate MCs via MRGPRX2, which results in the release of IL-31. Activation DRG neuron via IL-31R leads to the generation of SP, which further activates MCs via MRGPRX2 to promote the itch response.
The effects of MCs on neurogenic inflammation can occur following their activation by both IgE and non-IgE-mediated pathways. Thus, IgE-mediated activation of cutaneous MCs is markedly decreased in denervated skin when compared with normal skin.107 This suggests that sensory skin nerves augment MC-driven inflammatory responses by releasing neuropeptides that increase MC degranulation. Sickle cell anemia (SCA) is an inherited disorder, which is associated with inflammation, vascular dysfunction, severe lifelong pain and significant morbidity.108, 109 Serum level of SP is elevated SCA patients when compared with healthy controls and increase further during painful crises.110 Because NPs play an important role in cutaneous neurogenic inflammation via MC-dependent mechanisms it has been proposed that MC activation and SP release contribute to the pro-inflammatory milieu and pain in SCA.106, 111 Indeed, this hypothesis has recently been confirmed in a mouse model and it has been postulated that SP released from nerve endings and from MCs act on MCs themselves, thus promoting a vicious cycle of MC activation.112 SP activates murine and human MCs via MrgprB2 and MRGPRX2, respectively.25, 52 This raises the interesting possibility MRGPRX2 could serve as a novel target for the modulation of neurogenic inflammation and pain that are associated with both allergic and non-allergic conditions such as SCA (Fig. 2).
The MC-dependent mechanisms that promote neurogenic inflammation and pain described above also contribute to itch, a condition defined as an unconscious sensation leading to a desire to scratch. Keratinocytes release a variety of inflammatory and pruritogenic substances and also detect itch-associated signals via the expression of PAR-2, opioid, cannabinoid and histamine receptors.113–116 IL-31 is a newly identified cytokine that plays an important role in itch. Thus, mice overexpressing IL-31 develop spontaneous atopic dermatitis (AD)-like lesion with severe pruritus.117 Furthermore, in a mouse model of AD, the expression of the IL-31 receptor (IL-3-RA) is increased and a monoclonal antibody against IL-31 ameliorates the scratching behavior.118, 119 Moreover, acute allergic contact dermatitis, a skin disease featuring inflammation and pruritus, has been associated with higher IL-31 mRNA levels in DRG neurons than those seen in healthy skin.120 Furthermore, IL-3-RA is expressed on DRG neurons, and their activation leads to the release of NPs.121 Until recently, T cells were thought to be a major source of IL-31.121,101 However, Niyonsaba et al.,122 found that MCs express IL-31 and its expression is elevated in psoriatic human skin MCs. In addition, keratinocyte-derived HDPs such as hBDs induce the expression and release of IL-31 from human MCs. These HDPs also cause the release of other pruritogenic mediators such as histamine, PGE2 and SP from MCs.122 Given that HDPs activate MCs via MRGPRX2 suggest that this receptor could be targeted for the treatment of symptoms associated with itch.
Role of MRGPRX2 on chronic inflammatory diseases
Chronic urticaria (CU) is characterized by the presence of hives for at least six weeks that occurs on a daily basis.123 About 45% of these patients are known to have autoimmune CU because they produce antibodies against FcεRI and less commonly, IgE123, 124 and is associated with MC degranulation, generation of lipid-derived mediators and cytokines. The remaining ~55% of patients have idiopathic conditions.123, 124 Intradermal administration of SP and VIP causes greater wheal reactions in patients with idiopathic CU when compared to healthy control subjects.125–127 The number of MCs in the skin and their histamine content do not differ between the two groups. Based on this finding, it has been proposed that patients with CU display a defect at the level of MC function rather than their numbers. Given that SP and VIP activate human MCs via MRGPRX2, it raises the interesting possibility that MRGPRX2 expression could be upregulated in CU and that its activation by NPs contributes to the pathogenesis of CU.
Fujisawa et al.,26 recently showed that MCs in CU patients express MRGPRX2 at higher level than the healthy subjects. Furthermore, SP causes degranulation and PGD2 generation in human skin MCs and that these responses are significantly reduced in MRGPRX2-silenced MCs. These findings suggest that increased expression of MRGPRX2 in skin MCs render them more susceptible to SP and that the resulting MC degranulation and PGD2 generation contributes to the pathogenesis of idiopathic CU. However, the mechanisms responsible for MRGPRX2 upregulation in skin MCs remain unknown. Addition of histamine, SP, epithelium-derived cytokine IL-33, and thymic stromal lymphopoietin (TSLP) have no effect on the expression of MRGPRX2 on human skin MCs in vitro.26 Thus, further studies will be required to clarify the mechanisms that regulate the expression of MRGPRX2 in MCs.
Eosinophils accumulate in CU lesions and the presence of major basic protein (MBP) indicates that degranulation of eosinophils contribute to its pathogenesis.26, 128 Fujisawa et al.,26 showed that MCs and eosinophils colocalize in urticarial lesions in patients with CU. Furthermore, they demonstrated that eosinophil granule proteins, MBP and EPO induce histamine release from human skin MCs and that this response is substantially blocked in MRGPRX2-silenced cells. Thus, activation of MCs by SP via MRGPRX2 serves to initiate the wheal response and that recruitment of eosinophils and the subsequent activation of the same receptor by MBP and EPO contribute to the late or chronic phase reaction.
MCs are important effector cells that orchestrate the development of airway hyperresponsiveness and inflammation in asthma via their close interaction with airway smooth muscle (ASM) cells, T cells and leukocytes.129–133 The ability of allergen to cross-link FcεRI on MCs to induce mediator release is well documented.134–136 MCs found in the normal lung (MCT) are phenotypically different from those present in the skin (MCTC).137,138 Interestingly, while normal skin MCTC express MRGPRX2, lung MCT do not.24, 26 Mild allergic asthma is generally correlated with an increase in MCT number in both submucosa and smooth muscle but severe asthma is dominated by the presence of MCTC in the airway and increased levels of PGD2.139, 140 The level of SP is elevated in the lung of asthmatics when compared normal lung.141, 142 Furthermore, SP causes degranulation of MCs obtained from bronchoalveolar lavage.143 These findings suggest that as for CU, MRGPRX2 could participate in the pathogenesis of asthma.
Respiratory infection by rhinoviruses causes increased asthma severity in both children and adults and is associated with MC degranulation and the recruitment of eosinophils and neutrophils to the airways.144–149 Lung epithelial cells are the principal site of rhinovirus infection in both the upper and lower airways. Interestingly, rhinovirus induces the production of hBDs in bronchial epithelial cells148, 150, which activate MCs via MRGPRX2.29, 31 Thus, it is possible that MRGPRX2 expressed in human MCs contribute to rhinovirus-induced asthma exacerbation by responding to ligands generated from epithelial cells (hBDs)147, 148, eosinophils (MBP and EPO)26 and neutrophils (LL-37). It is noteworthy that LL-37 not only causes MC degranulation, and PGD2 synthesis but it also promotes their chemotaxis and induces the Th2 cytokines IL-4 and IL-5.28, 61 These findings suggest that MRGPRX2 may be involved in asthma exacerbation by rhinovirus infection.
Signaling and regulation of MRGPRX2 in human MCs
Most studies on the ability of NPs and HDPs to activate MCs were conducted with rat PMCs,46–51 human MC line, LAD2 cells28–30 or in vitro CD34+-derived human MCs.25, 29 These studies demonstrated that NPs and HDPs cause MC chemotaxis, degranulation and cytokine generation via signaling pathways that involve the activation of pertussis toxin (PTx)-sensitive G proteins. While PTx inhibits HDP-induced degranulation in human MCs endogenously expressing MRGPRX2 and transfected RBL-2H3 cells, it has no effect on Ca2+ mobilization in response to these ligands.28, 29 La3+ (an inhibitor of Ca2+ release-activated Ca2+ channels, Orai) and 2-aminoethoxydiphenyl borate (2-APB, a dual inhibitor of inositol 1,4,5-triphosphate receptor and Orai-1/Orai-2) cause substantial inhibition of hBD-induced Ca2+ mobilization and degranulation. This suggests that MRGPRX2 couples to both PTx-sensitive and insensitive signaling pathways most likely involving Gαq and Gαi to induce degranulation. In addition to degranulation, HDPs also induce the expression of the potent pruritic cytokine IL-31 via phosphatidylinositol 3-kinase (PI3K) and MAP kinases p38, JNK and ERK in human MCs. Furthermore, treatment of MCs with PTx or inhibitors of MAP kinases resulted in the substantial inhibition of hBD/LL-37-induced IL-31.122
Until the discovery of MRGPRX2 cationic amphipathic peptides (HDPs, NPs and MCDPs) were thought to directly interact with PTx-sensitive G proteins (Gαi2 and Gαi3), in a receptor-independent manner, to propagate signaling for Ca2+ mobilization and degranulation.151–153 A unique feature of MRGPRX2 that distinguishes from other GPCRs in MCs is that it is expressed both on the plasma membrane and in intracellular sites.26 Although the exact intracellular site for MRGPRX2 has not been determined it appears to co-localize with tryptase, indicating its possible expression in MC granules.26 However, the mechanism via which amphipathic peptide ligands interact with granule associated MRGPRX2 is unknown. Increased Ca2+ mobilization through Orai-1 and Orai-2 is essential for antigen-induced MC degranulation.154, 155 It is interesting to note that while Orai-1 is found in the plasma membrane, Orai-2 is mainly localized in MC granules.156 2-APB, which attenuates MRGPRX2-mediated Ca2+ mobilization and degranulation, inhibits both Orai-1 and Orai-2.29, 157 These findings suggest that MRGPRX2 utilizes plasma membrane Orai-1 and granule-associated Orai-2 to promote MC mediator release (Fig. 3).
Figure 3. MRGPRX2 signaling in MCs.
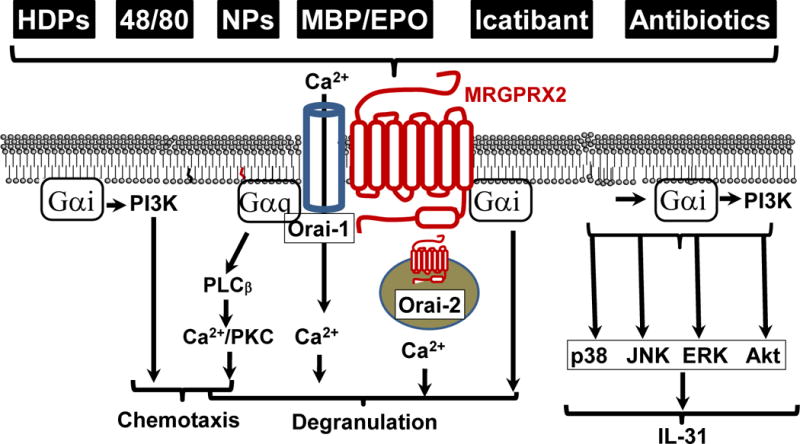
The ligands for MRGPRX2 activate pertussis toxin-dependent Gαi and pertussis toxin-independent Gαq pathways for MC responses. MCs express MRGPRX2 on the cell surface as well as intracellular sites. The extracellular receptor probably uses the Orai-1 Ca2+ channel and the pertussis toxin-independent Gαq pathway for MC degranulation whereas the intracellular receptor may use the Orai-2 Ca2+ channel to mediate MC responses. Activation of MRGPRX2 also results in Gαi-dependent production of the pruritogenic cytokine IL-31 via the MAP kinase and Akt-dependent pathways.
Most GPCRs undergo rapid agonist-induced receptor phosphorylation and this provides an important mechanism for their desensitization and internalization.158 Although human MCs express a large number of GPCRs, phosphorylation and desensitization of the anaphylatoxin C3a receptor (C3aR) has been studied in most detail. Following C3a stimulation, the receptor undergoes rapid phosphorylation, desensitization and internalization.159–161 Furthermore, silencing the expression of G protein coupled receptor kinase – 2 or 3 (GRK2 or GRK) causes a more sustained Ca2+ mobilization, attenuated C3aR desensitization, and enhanced degranulation. However, unlike C3aR and most other GPCRs, MRGPRX2 is resistant to LL-37-induced receptor phosphorylation, desensitization and internalization. Also, silencing of either GRK2 or GRK3 has no effect on LL-37 induced MC degranulation28. Thus, MRGPRX2 appears to be unique among GPCRs that are expressed in MCs with regards to its resistance to desensitization. The biological significance of this feature is unknown but could reflect the fact that unlike other GPCRs expressed in MCs, MRGPRX2 is activated by multiple peptide ligands that are likely to be present at the site of infection or infllamation. Thus, resistance to desensitization may allow the receptor to respond to multiple ligands simultaneously.
Conclusion and future directions
MRGPRX2 is a non-canonical GPCR that is expressed on human MCs and is found both at the plasma membrane and intracellular sites. It likely plays a dual role in promoting MC-mediated host defense and contributing to the pathogenesis of allergic and inflammatory diseases. In addition to the direct kill of microbes by HDPs, their ability to activate MRGPRX2 may serve to orchestrate the development of MC-mediated innate and adaptive immune responses and to promote healing (Fig. 1). Thus, harnessing this MC-activating feature of HDPs may provide novel approaches to develop antimicrobial therapy against multidrug resistant bacteria.162 Most studies evaluating clinical potentials of HDPs for antimicrobial activity involve their topical application at the site of infection.163 At these sites, it is desirable for a therapeutic agent to display antimicrobial activity and to harness MC’s host defense and wound-healing properties. Therefore, HDP-mediated activation of MCs via MRGPRX2 and the resulting inflammatory responses in the context of cutaneous microbial infection likely outweighs the risks of developing adverse reactions.
Based on the recent finding that the mouse counterpart of MRGPRX2 (MrgprB2) participates in peptidergic drug-induced pseudoallergic reactions it has been proposed that mice with functional deletion of MrgprB2 together with their wild-type counterparts could serve as useful tools in the preclinical screening of new peptidergic drugs and candidate small molecule therapeutics for symptoms of pseudoallergic reactions.153 However, given the important differences in the potency of NPs and certain peptidergic drugs for MRGPRX2 and MrgprB2, these mice may not be suitable to screen drugs for use in humans.52 Recently, a number of humanized mouse models have been developed via the engraftment of human hematopoietic stem cells in immune deficient mice.164,165, 166 These mice develop human MCs in the skin, intestine and lung. Furthermore, human MCs that are generated in these mice express human FcεRI and are responsive to human anti-IgE/IgE and serum from Japanese cedar pollinosis patients for strong passive cutaneous anaphylaxis in vivo.166 We have shown that human MCs that develop in humanized mice express MRGPRX2 (unpublished) and this model may serve as a tool to delineate the pathophysiologic effects of MRGPRX2 in vivo. In addition to pseudoallergic allergic reactions, MRGPRX2 participates in chronic urticaria and likely contributes to the pathogenesis of itch and asthma. Thus, the humanized mouse model could potentially be used to screen monoclonal antibodies and small molecule inhibitors of MRGPRX2 against MC-mediated allergic and inflammatory disorders.
Acknowledgments
This work was supported by National Institutes of Health Grant HL085774 and AI108585 to HA.
List of Abbreviation
- 2-APB
2-aminoethoxydiphenyl borate
- AD
Atopic dermatitis
- CU
Chronic urticaria
- CGRP
Calcitonin gene-related peptide
- CTMC
Connective tissue mast cell
- DRG
Dorsal root ganglia
- EGFR
Epidermal growth factor receptor
- ERK1/2
Extracellular related kinases 1 and 2
- EPO
Eosinophil peroxidase
- FcεRI
High affinity IgE receptor
- GRK
G protein coupled receptor kinase
- GPCR
G protein coupled receptor
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HDP
Host defense peptide
- hBD
human β-defensin
- IL-31RA
IL-31 receptor
- MBP
Major basic protein
- MC
Mast cell
- MCT
Tryptase-expressing MC
- MCTC
Tryptase and chymase-expressing MC
- MrgprB2
Mas-related G protein coupled receptor-B2
- MRGPRX2 (MrgX2)
Mas-related G protein coupled receptor-X2
- MMC
Mucosal mast cell
- NP
Neuropeptide
- NMBD
Neuromuscular blocking drug
- NK1R
Neurokinin-1 receptor
- PMC
Peritoneal mast cell
- PAR-2
Proteinase activated receptor-2
- PI3K
Phosphatidylinositol 3-kinase
- PGD2
Prostaglandin D2
- SP
Substance P
- SQSTM-1
Sequestosome-1
- SAPK
Stress-activated protein kinases
- TLR
Toll-like receptor
- VIP
Vasoactive intestinal peptide
Footnotes
Disclosure of potential conflict of interest: None
References
- 1.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138:4381–6. [PubMed] [Google Scholar]
- 3.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–15. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 4.Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015;8:444–63. doi: 10.1038/mi.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–8. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, Schwartz LB. Generation of anaphylatoxins by human β-tryptase from C3, C4, and C5. J Immunol. 2008;180:6307–16. doi: 10.4049/jimmunol.180.9.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264:258–60. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- 8.Schafer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol. 2013;131:541–8e1–9. doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdei A, Andrasfalvy M, Peterfy H, Toth G, Pecht I. Regulation of mast cell activation by complement-derived peptides. Immunol Lett. 2004;92:39–42. doi: 10.1016/j.imlet.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara T, Murakami M, Suzuki-Nishimura T, Uchida MK, Kudo I. Mouse bone marrow-derived mast cells undergo exocytosis, prostanoid generation, and cytokine expression in response to G protein-activating polybasic compounds after coculture with fibroblasts in the presence of c-kit ligand. J Immunol. 1997;158:393–404. [PubMed] [Google Scholar]
- 11.Ando T, Matsumoto K, Namiranian S, Yamashita H, Glatthorn H, Kimura M, et al. Mast cells are required for full expression of allergen/SEB-induced skin inflammation. J Invest Dermatol. 2013;133:2695–705. doi: 10.1038/jid.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53–9. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 13.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–6. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 14.St John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol. 2013;190:4458–63. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 16.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 17.Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003;100:4903–8. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–32. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 19.Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711–9. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 20.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–8. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, et al. Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci. 2002;5:201–9. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 22.Burstein ES, Ott TR, Feddock M, Ma JN, Fuhs S, Wong S, et al. Characterization of the Mas-related gene family: structural and functional conservation of human and rhesus MrgX receptors. Br J Pharmacol. 2006;147:73–82. doi: 10.1038/sj.bjp.0706448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solinski HJ, Gudermann T, Breit A. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol Rev. 2014;66:570–97. doi: 10.1124/pr.113.008425. [DOI] [PubMed] [Google Scholar]
- 24.Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010;125:1137–45 e6. doi: 10.1016/j.jaci.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 25.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–8. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 26.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134:622–33 e9. doi: 10.1016/j.jaci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278:44400–4. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 2011;286:44739–49. doi: 10.1074/jbc.M111.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. β-Defensins activate human mast cells via Mas-related gene X2. J Immunol. 2013;191:345–52. doi: 10.4049/jimmunol.1300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian H, Kashem SW, Collington SJ, Qu H, Lambris JD, Ali H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol Pharmacol. 2011;79:1005–13. doi: 10.1124/mol.111.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta K, Subramanian H, Ali H. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun. 2016;22:21–30. doi: 10.1177/1753425915610643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–70. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okayama Y, Saito H, Ra C. Targeting human mast cells expressing G-protein-coupled receptors in allergic diseases. Allergol Int. 2008;57:197–203. doi: 10.2332/allergolint.R-08-163. [DOI] [PubMed] [Google Scholar]
- 35.Ahamed J, Venkatesha RT, Thangam EB, Ali H. C3a enhances nerve growth factor-induced NFAT activation and chemokine production in a human mast cell line, HMC-1. J Immunol. 2004;172:6961–8. doi: 10.4049/jimmunol.172.11.6961. [DOI] [PubMed] [Google Scholar]
- 36.Ali H, Panettieri RA., Jr Anaphylatoxin C3a receptors in asthma. Respir Res. 2005;6:19. doi: 10.1186/1465-9921-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwyzer R. Membrane-assisted molecular mechanism of neurokinin receptor subtype selection. EMBO J. 1987;6:2255–9. doi: 10.1002/j.1460-2075.1987.tb02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guhl S, Lee HH, Babina M, Henz BM, Zuberbier T. Evidence for a restricted rather than generalized stimulatory response of skin-derived human mast cells to substance P. J Neuroimmunol. 2005;163:92–101. doi: 10.1016/j.jneuroim.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Chahdi A, Mousli M, Landry Y. Substance P-related inhibitors of mast cell exocytosis act on G-proteins or on the cell surface. Eur J Pharmacol. 1998;341:329–35. doi: 10.1016/s0014-2999(97)01480-5. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz D, Wiesner B, Zipper J, Winkler A, Krause E, Beyermann M, et al. Mechanism of peptide-induced mast cell degranulation. Translocation and patch-clamp studies. J Gen Physiol. 1998;112:577–91. doi: 10.1085/jgp.112.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mousli M, Bronner C, Bockaert J, Rouot B, Landry Y. Interaction of substance P, compound 48/80 and mastoparan with the alpha-subunit C-terminus of G protein. Immunol Lett. 1990;25:355–7. doi: 10.1016/0165-2478(90)90207-7. [DOI] [PubMed] [Google Scholar]
- 43.Mousli M, Bueb JL, Bronner C, Rouot B, Landry Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990;11:358–62. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- 44.Kashem SW, Subramanian H, Collington SJ, Magotti P, Lambris JD, Ali H. G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2. Eur J Pharmacol. 2011;668:299–304. doi: 10.1016/j.ejphar.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solinski HJ, Petermann F, Rothe K, Boekhoff I, Gudermann T, Breit A. Human Mas-related G protein-coupled receptors-X1 induce chemokine receptor 2 expression in rat dorsal root ganglia neurons and release of chemokine ligand 2 from the human LAD-2 mast cell line. PLoS One. 2013;8:e58756. doi: 10.1371/journal.pone.0058756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babolewska E, Brzezinska-Blaszczyk E. Human-derived cathelicidin LL-37 directly activates mast cells to proinflammatory mediator synthesis and migratory response. Cell Immunol. 2015;293:67–73. doi: 10.1016/j.cellimm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–53. [PubMed] [Google Scholar]
- 49.Foreman JC, Jordan CC, Piotrowski W. Interaction of neurotensin with the substance P receptor mediating histamine release from rat mast cells and the flare in human skin. Br J Pharmacol. 1982;77:531–9. doi: 10.1111/j.1476-5381.1982.tb09328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piotrowski W, Devoy MA, Jordan CC, Foreman JC. The substance P receptor on rat mast cells and in human skin. Agents Actions. 1984;14:420–4. doi: 10.1007/BF01973842. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, et al. Antimicrobial peptides human β-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 2007;37:434–44. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 52.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–41. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci. 2012;33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 55.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 56.Hazlett L, Wu M. Defensins in innate immunity. Cell Tissue Res. 2011;343:175–88. doi: 10.1007/s00441-010-1022-4. [DOI] [PubMed] [Google Scholar]
- 57.Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9:761–8. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 58.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–7. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 59.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human β-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421–6. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 60.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshioka M, Fukuishi N, Kubo Y, Yamanobe H, Ohsaki K, Kawasoe Y, et al. Human Cathelicidin CAP18/LL-37 Changes mast cell function toward innate immunity. Biol Pharm Bull. 2008;31:212–6. doi: 10.1248/bpb.31.212. [DOI] [PubMed] [Google Scholar]
- 62.Gekara NO, Weiss S. Mast cells initiate early anti-Listeria host defences. Cell Microbiol. 2008;10:225–36. doi: 10.1111/j.1462-5822.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- 63.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–15. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 64.Malaviya R, Navara C, Uckun FM. Role of Janus kinase 3 in mast cell-mediated innate immunity against gram-negative bacteria. Immunity. 2001;15:313–21. doi: 10.1016/s1074-7613(01)00184-4. [DOI] [PubMed] [Google Scholar]
- 65.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis [see comments] Nature. 1996;381:75–7. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 66.Malaviya R, Georges A. Regulation of mast cell-mediated innate immunity during early response to bacterial infection. Clin Rev Allergy Immunol. 2002;22:189–204. doi: 10.1385/CRIAI:22:2:189. [DOI] [PubMed] [Google Scholar]
- 67.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–8. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 68.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol. 2008;180:7565–73. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–8. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 70.Douaiher J, Succar J, Lancerotto L, Gurish MF, Orgill DP, Hamilton MJ, et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol. 2014;122:211–52. doi: 10.1016/B978-0-12-800267-4.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–41. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 73.Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–42. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 75.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006;177:1755–62. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- 76.Amaral MM, Davio C, Ceballos A, Salamone G, Canones C, Geffner J, et al. Histamine improves antigen uptake and cross-presentation by dendritic cells. J Immunol. 2007;179:3425–33. doi: 10.4049/jimmunol.179.6.3425. [DOI] [PubMed] [Google Scholar]
- 77.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27:3544–52. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheb-Wetzel M, Rohde M, Bravo A, Goldmann O. New insights into the antimicrobial effect of mast cells against Enterococcus faecalis. Infect Immun. 2014;82:4496–507. doi: 10.1128/IAI.02114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 80.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol. 2009;39:3181–94. doi: 10.1002/eji.200939496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140:103–12. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 83.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Human β-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol. 2010;184:6688–94. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 85.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 86.Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol. 2009;183:2688–96. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- 87.Yu HB, Kielczewska A, Rozek A, Takenaka S, Li Y, Thorson L, et al. Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J Biol Chem. 2009;284:36007–11. doi: 10.1074/jbc.C109.073627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nijnik A, Madera L, Ma S, Waldbrook M, Elliott MR, Easton DM, et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J Immunol. 2010;184:2539–50. doi: 10.4049/jimmunol.0901813. [DOI] [PubMed] [Google Scholar]
- 89.Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, et al. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol. 2011;107:529–37. doi: 10.1016/j.anai.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 90.Koppert W, Blunk JA, Petersen LJ, Skov P, Rentsch K, Schmelz M. Different patterns of mast cell activation by muscle relaxants in human skin. Anesthesiology. 2001;95:659–67. doi: 10.1097/00000542-200109000-00019. [DOI] [PubMed] [Google Scholar]
- 91.Mertes PM, Alla F, Trechot P, Auroy Y, Jougla E, Groupe d’Etudes des Reactions Anaphylactoides P Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011;128:366–73. doi: 10.1016/j.jaci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 92.Kelesidis T, Fleisher J, Tsiodras S. Anaphylactoid reaction considered ciprofloxacin related: a case report and literature review. Clin Ther. 2010;32:515–26. doi: 10.1016/j.clinthera.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mori K, Maru C, Takasuna K. Characterization of histamine release induced by fluoroquinolone antibacterial agents in-vivo and in-vitro. J Pharm Pharmacol. 2000;52:577–84. doi: 10.1211/0022357001774228. [DOI] [PubMed] [Google Scholar]
- 94.Mori K, Maru C, Takasuna K, Furuhama K. Mechanism of histamine release induced by levofloxacin, a fluoroquinolone antibacterial agent. Eur J Pharmacol. 2000;394:51–5. doi: 10.1016/s0014-2999(00)00147-3. [DOI] [PubMed] [Google Scholar]
- 95.Blanca-Lopez N, Ariza A, Dona I, Mayorga C, Montanez MI, Garcia-Campos J, et al. Hypersensitivity reactions to fluoroquinolones: analysis of the factors involved. Clin Exp Allergy. 2013;43:560–7. doi: 10.1111/cea.12099. [DOI] [PubMed] [Google Scholar]
- 96.Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am Rev Respir Dis. 1991;143:S55–8. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 97.Arizono N, Matsuda S, Hattori T, Kojima Y, Maeda T, Galli SJ. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990;62:626–34. [PubMed] [Google Scholar]
- 98.Alving K, Sundstrom C, Matran R, Panula P, Hokfelt T, Lundberg JM. Association between histamine-containing mast cells and sensory nerves in the skin and airways of control and capsaicin-treated pigs. Cell Tissue Res. 1991;264:529–38. doi: 10.1007/BF00319042. [DOI] [PubMed] [Google Scholar]
- 99.MacQueen G, Marshall J, Perdue M, Siegel S, Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989;243:83–5. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- 100.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 101.Cevikbas F, Kempkes C, Buhl T, Mess C, Buddenkotte J, Steinhoff M. Role of Interleukin-31 and Oncostatin M in Itch and Neuroimmune Communication. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): 2014. [PubMed] [Google Scholar]
- 102.Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in Neuroimmune Communication and Itch. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): 2014. [PubMed] [Google Scholar]
- 103.Taylor-Clark TE, Nassenstein C, Undem BJ. Leukotriene D4 increases the excitability of capsaicin-sensitive nasal sensory nerves to electrical and chemical stimuli. Br J Pharmacol. 2008;154:1359–68. doi: 10.1038/bjp.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Diest SA, Stanisor OI, Boeckxstaens GE, de Jonge WJ, van den Wijngaard RM. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim Biophys Acta. 2012;1822:74–84. doi: 10.1016/j.bbadis.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 105.Lee MG, Dong X, Liu Q, Patel KN, Choi OH, Vonakis B, et al. Agonists of the MAS-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol. 2008;180:2251–5. doi: 10.4049/jimmunol.180.4.2251. [DOI] [PubMed] [Google Scholar]
- 106.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 107.Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol. 2008;121:955–61. doi: 10.1016/j.jaci.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 108.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–56. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 109.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–51. [PubMed] [Google Scholar]
- 110.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92:3148–51. [PubMed] [Google Scholar]
- 111.Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69:S15–26. doi: 10.1016/j.jaad.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 112.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–62. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128:18–25. doi: 10.1038/sj.jid.5700965. [DOI] [PubMed] [Google Scholar]
- 114.Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–20. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamaura K, Oda M, Suwa E, Suzuki M, Sato H, Ueno K. Expression of histamine H4 receptor in human epidermal tissues and attenuation of experimental pruritus using H4 receptor antagonist. J Toxicol Sci. 2009;34:427–31. doi: 10.2131/jts.34.427. [DOI] [PubMed] [Google Scholar]
- 116.Bigliardi-Qi M, Sumanovski LT, Buchner S, Rufli T, Bigliardi PL. Mu-opiate receptor and Beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology. 2004;209:183–9. doi: 10.1159/000079887. [DOI] [PubMed] [Google Scholar]
- 117.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 118.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516:180–1. doi: 10.1016/j.ejphar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 120.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–7. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 121.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–60. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human β-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184:3526–34. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 123.Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777–87. doi: 10.1111/j.1365-2222.2009.03256.x. [DOI] [PubMed] [Google Scholar]
- 124.Vonakis BM, Saini SS. New concepts in chronic urticaria. Curr Opin Immunol. 2008;20:709–16. doi: 10.1016/j.coi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borici-Mazi R, Kouridakis S, Kontou-Fili K. Cutaneous responses to substance P and calcitonin gene-related peptide in chronic urticaria: the effect of cetirizine and dimethindene. Allergy. 1999;54:46–56. doi: 10.1034/j.1398-9995.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- 126.Smith CH, Atkinson B, Morris RW, Hayes N, Foreman JC, Lee TH. Cutaneous responses to vasoactive intestinal polypeptide in chronic idiopathic urticaria. Lancet. 1992;339:91–3. doi: 10.1016/0140-6736(92)91000-x. [DOI] [PubMed] [Google Scholar]
- 127.Bedard PM, Brunet C, Pelletier G, Hebert J. Increased compound 48/80 induced local histamine release from nonlesional skin of patients with chronic urticaria. J Allergy Clin Immunol. 1986;78:1121–5. doi: 10.1016/0091-6749(86)90260-5. [DOI] [PubMed] [Google Scholar]
- 128.Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM, et al. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: comparison of patients with and without anti-FcεRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103:484–93. doi: 10.1016/s0091-6749(99)70475-6. [DOI] [PubMed] [Google Scholar]
- 129.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, et al. Mast cells, FcεRI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004;172:6398–406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 130.Brightling CE, Bradding P. The re-emergence of the mast cell as a pivotal cell in asthma pathogenesis. Curr Allergy Asthma Rep. 2005;5:130–5. doi: 10.1007/s11882-005-0086-9. [DOI] [PubMed] [Google Scholar]
- 131.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1313–23. doi: 10.1152/ajplung.2001.281.6.L1313. [DOI] [PubMed] [Google Scholar]
- 132.Thangam EB, Venkatesha RT, Zaidi AK, Jordan-Sciutto KL, Goncharov DA, Krymskaya VP, et al. Airway smooth muscle cells enhance C3a-induced mast cell degranulation following cell-cell contact. Faseb J. 2005;19:798–800. doi: 10.1096/fj.04-2797fje. [DOI] [PubMed] [Google Scholar]
- 133.Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? J Allergy Clin Immunol. 2004;114:58–65. doi: 10.1016/j.jaci.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 134.Cai Y, Bjermer L, Halstensen TS. Bronchial mast cells are the dominating LTC4S-expressing cells in aspirin-tolerant asthma. Am J Respir Cell Mol Biol. 2003;29:683–93. doi: 10.1165/rcmb.2002-0174OC. [DOI] [PubMed] [Google Scholar]
- 135.Venkatachalam TK, Qazi S, Samuel P, Uckun FM. Inhibition of mast cell leukotriene release by thiourea derivatives. Bioorg Med Chem Lett. 2003;13:485–8. doi: 10.1016/s0960-894x(02)00992-7. [DOI] [PubMed] [Google Scholar]
- 136.Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172:7254–62. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- 137.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 138.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–63 e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–12. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nieber K, Baumgarten CR, Rathsack R, Furkert J, Oehme P, Kunkel G. Substance P and β-endorphin-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J Allergy Clin Immunol. 1992;90:646–52. doi: 10.1016/0091-6749(92)90138-r. [DOI] [PubMed] [Google Scholar]
- 142.Tomaki M, Ichinose M, Miura M, Hirayama Y, Yamauchi H, Nakajima N, et al. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med. 1995;151:613–7. doi: 10.1164/ajrccm.151.3.7533601. [DOI] [PubMed] [Google Scholar]
- 143.Heaney LG, Cross LJ, Stanford CF, Ennis M. Substance P induces histamine release from human pulmonary mast cells. Clin Exp Allergy. 1995;25:179–86. doi: 10.1111/j.1365-2222.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 144.Zhu J, Message SD, Qiu Y, Mallia P, Kebadze T, Contoli M, et al. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;145:1219–29. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Busse WW, Gern JE, Dick EC. The role of respiratory viruses in asthma. Ciba Found Symp. 1997;206:208–13. doi: 10.1002/9780470515334.ch13. discussion 13–9. [DOI] [PubMed] [Google Scholar]
- 146.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wiehler S, Proud D. Interleukin-17A modulates human airway epithelial responses to human rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2007;293:L505–15. doi: 10.1152/ajplung.00066.2007. [DOI] [PubMed] [Google Scholar]
- 148.Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, et al. Rhinovirus increases human β-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38:59–64. doi: 10.1016/S0928-8244(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 149.Proud D. The role of defensins in virus-induced asthma. Curr Allergy Asthma Rep. 2006;6:81–5. doi: 10.1007/s11882-006-0015-6. [DOI] [PubMed] [Google Scholar]
- 150.Proud D, Sanders SP, Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human β-defensin 2 both in vitro and in vivo. J Immunol. 2004;172:4637–45. doi: 10.4049/jimmunol.172.7.4637. [DOI] [PubMed] [Google Scholar]
- 151.Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 152.Ferry X, Eichwald V, Daeffler L, Landry Y. Activation of betagamma subunits of G(i2) and G(i3) proteins by basic secretagogues induces exocytosis through phospholipase Cβ and arachidonate release through phospholipase Cγ in mast cells. J Immunol. 2001;167:4805–13. doi: 10.4049/jimmunol.167.9.4805. [DOI] [PubMed] [Google Scholar]
- 153.Grimbaldeston MA. Mast cell-MrgprB2: sensing secretagogues or a means to overreact? Immunol Cell Biol. 2015;93:221–3. doi: 10.1038/icb.2015.10. [DOI] [PubMed] [Google Scholar]
- 154.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Crit Rev Immunol. 2009;29:155–86. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ikeya M, Yamanoue K, Mochizuki Y, Konishi H, Tadokoro S, Tanaka M, et al. Orai-2 is localized on secretory granules and regulates antigen-evoked Ca2+ mobilization and exocytosis in mast cells. Biochem Biophys Res Commun. 2014;451:62–7. doi: 10.1016/j.bbrc.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 157.DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW., Jr Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;283:19265–73. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–92. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 159.Gupta K, Subramanian H, Klos A, Ali H. Phosphorylation of C3a receptor at multiple sites mediates desensitization, β-arrestin-2 recruitment and inhibition of NF-κB activity in mast cells. PLoS One. 2012;7:e46369. doi: 10.1371/journal.pone.0046369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guo Q, Subramanian H, Gupta K, Ali H. Regulation of C3a receptor signaling in human mast cells by G protein coupled receptor kinases. PLoS One. 2011;6:e22559. doi: 10.1371/journal.pone.0022559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vibhuti A, Gupta K, Subramanian H, Guo Q, Ali H. Distinct and shared roles of β-arrestin-1 and β-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One. 2011;6:e19585. doi: 10.1371/journal.pone.0019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gupta K, Kotian A, Subramanian H, Daniell H, Ali H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget. 2015 doi: 10.18632/oncotarget.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang L, Falla TJ. Host defense peptides for use as potential therapeutics. Curr Opin Investig Drugs. 2009;10:164–71. [PubMed] [Google Scholar]
- 164.Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, Hasegawa T, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–77. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tanaka S, Saito Y, Kunisawa J, Kurashima Y, Wake T, Suzuki N, et al. Development of mature and functional human myeloid subsets in hematopoietic stem cell-engrafted NOD/SCID/IL2rgammaKO mice. J Immunol. 2012;188:6145–55. doi: 10.4049/jimmunol.1103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T, et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol. 2013;191:2890–9. doi: 10.4049/jimmunol.1203543. [DOI] [PubMed] [Google Scholar]


