Abstract
Objective
This study determined the efficacy of a novel point-of-care immunoflow device (POCID) for detecting matrix metalloproteinase (MMP)-8 concentrations in oral fluids in comparison with a gold-standard laboratory-based immunoassay.
Methods
Oral rinse fluid and whole expectorated saliva samples were collected from 41 participants clinically classified as periodontally healthy or diseased. Samples were analyzed for MMP-8 by Luminex immunoassay and POCID. Photographed POCID results were assessed by optical scan and visually by two examiners. Data were analyzed by Pearson correlation and receiver operator characteristics.
Results
MMP-8 was readily detected by the POCID, and concentrations correlated well with Luminex for both saliva and rinse fluids (r=0.57–0.93). Thresholds that distinguished periodontitis from health were delineated from both the optical scans and visual reads of the POCID (sensitivity 0.7–0.9, specificity 0.5–0.7; p < 0.05).
Conclusions
Performance of this POCID for detecting MMP-8 in oral rinse fluid or saliva was excellent. These findings help demonstrate the utility of salivary biomarkers for distinguishing periodontal disease from health using a rapid point-of-care approach.
Keywords: saliva, biomarker, periodontitis, point-of-care, MMP-8
Saliva contains biomarkers that have potential utility for the diagnostic assessment of periodontal disease (Lamster et al., 2003, Miller et al., 2006, Nomura et al., 2006, Ozcaka et al., 2011, Giannobile et al., 2009, Giannobile et al., 2011, Kinney et al., 2011, Ramseier et al., 2009). In theory, the clinical utility of salivary biomarkers arises from the presence of proteins and peptides that occur from the host response to inflammation, connective tissue degradation, and bone remodeling during periodontal disease. As these biomolecules can emanate from the crevicular fluid into saliva, it is not surprising that salivary levels of interleukin-1β, matrix metalloproteinase (MMP)-8, osteoprotegerin and macrophage inflammatory protein-1α have shown promise as biomarkers of periodontitis (Gursoy et al., 2009, Gursoy et al., 2010, Salminen et al., 2014, Ebersole et al., 2013, Al-Sabbagh et al., 2012, Rathnayake et al., 2013b). Additional salivary biomarkers associated with these biological phases are known and have been examined with respect to periodontal disease, smoking status, comorbid disease, and response to treatment (Zhang et al., 2009, Miller et al., 2010, Liede et al., 1999, Heikkinen et al., 2010, Mirrielees et al., 2010, Sexton et al., 2011).
Laboratory-based immunological assays are the current standard for measuring salivary biomarkers of periodontal disease (Rathnayake et al., 2013a, Gursoy et al., 2013, Thomas et al., 2009). While sensitive and specific, these gold standard assays are relatively expensive, require a several day turn-around, and generally involve collection, storage, and transport of specimens to a laboratory where costly instrumentation and technical expertise is required. As a result, efforts are ongoing to develop and translate biochemical assays to chair-side, point-of-care (POC) formats.
An ideal POC device should be rapid, sensitive, specific, accurate, reproducible, and cost effective. Lateral flow chromatography (LFC), which can demonstrate many of these features, already has commercial examples involving salivary diagnostic technology for human immunodeficiency virus POC testing (Oraquick®), and for determining nicotine exposure from smoking cigarettes and drugs of abuse (Alcolock 2015; Zachary et al., 2012, Gonzalez et al., 2011, Volkov et al., 2009, Cooke et al., 2008). Also, LFC using an oral rinse sample for assessing periodontal disease risk based on MMP-8 concentration has been tested and introduced in Europe (Nwhator et al., 2014, Heikkinen et al., 2016, Izadi Borujeni et al., 2015). However, the diagnostic utility of this device in a clinical setting is not well known, nor is this product approved by the United States Food and Drug Administration.
During the past several years our laboratory has refined a strategy for the development of a POC device for the detection of oral fluid biomarkers associated with systemic conditions as well as periodontal disease (Miller et al., 2006, Miller et al., 2014, Foley et al., 2012b, Foley et al., 2012a, Miller et al., 2010). These efforts have led to the development of a LFC assay device. This single-use disposable, POC immunoflow device (POCID) has been engineered to detect low concentrations of MMP-8 in saliva. MMP-8 was selected as a proof-of-principle, because salivary levels of this analyte: i) demonstrate a biological relationship with periodontal disease, ii) correlate with response to periodontal therapy, and iii) are within the detection range offered by LFC assay (Birkedal-Hansen, 1993, Sorsa et al., 2004, Miller et al., 2006, Buduneli & Kinane, 2011, Sexton et al., 2011, Gupta et al., 2015). The aims of this research were to determine if: i) MMP-8 is detectable in oral fluids using a POCID, and ii) concentrations of MMP-8 determined using a POCID correlate with results from a gold-standard laboratory-based immunoassay.
Materials and Methods
Patient Population and Clinical Examination
This study was conducted at the University of Kentucky, College of Dentistry (UKCD), Lexington, Kentucky between March 2013 and January 2015. Participants were recruited from the UKCD general clinic and student populations. Inclusion enrollment criteria were: 18 years and older, good systemic health, more than 17 teeth present, and were clinically categorized as periodontal health or periodontal disease. None of the periodontal disease participants had received periodontal care within the preceding 6 months.
All enrolled patients received a complete periodontal examination by one calibrated examiner (NJ). A complete medical and dental history was reviewed along with exclusion criteria. Findings from the head, neck, and oral cancer screening were recorded on a data collection sheet as normal or abnormal. Probing pocket depth (PPD) and clinical attachment loss were measured and recorded by dividing the tooth into six segments (mesial-buccal, mid-buccal, distal-buccal, mesial-lingual, mid-lingual, and distal-lingual). After PPD measurements, all sites were examined for bleeding on probing (BOP), and those measurements were recorded. Participants were categorized as clinically healthy or periodontal disease based on a modification of the criteria previously described (Albandar et al., 1999). Health was defined as < 10 % sites with BOP, < 2% sites with PPD > 3 mm, no sites with PPD ≥ 5 mm. Mild periodontal disease was determined if the participant had ≥20% of sites with BOP and ≥ 20% of sites with PPD = 4 mm; moderate periodontal disease had ≥ 20% of sites with BOP and > 20% sites with PPD = 4 mm, and ≤ 20% PPD = 5 mm; advanced periodontal disease had ≥ 20% of sites with BOP and >20% PPD ≥ 5 mm. Patients were excluded if they were pregnant, used antibiotics in the last three days, were currently using glucocorticoids or anti-inflammatory medication, had a recent fever, had an infectious, inflammatory or autoimmune condition (e.g., diabetes, rheumatoid arthritis), alcoholism, or had an oral mucosal inflammatory condition (e.g., aphthous, lichen planus, leukoplakia, and oral cancer) as detected on oral examination. The University of Kentucky Institutional Review Board approved the study on 3/27/13 (#13-0130-F6A), and all procedures were performed in accordance with the guidelines set out by the Declaration of Helsinki. Subjects received informed consent and monetary compensation as well as a clinical examination as part of the study protocol.
Sample Collection
Participants did not to eat or use oral products one hour prior to providing saliva. Five minutes prior to the start of the study, each participant swished with water and cleared the mouth of any contents. After the initial rinse, participants received 10 mL of tap water and were instructed to rinse their mouth with the water for 30 seconds, then expectorate into a sterile 15 mL tube (Sigma-Aldrich, St. Louis, MO) containing a lyophilized protease inhibitor cocktail (Evergreen Scientific Los Angeles, CA). For all expectorations, the sample tube was maintained on ice. After rinse collection, each participant provided 5 mL of unstimulated whole saliva by expectorating every 30 seconds into a provided sterile 15 mL tube with lyophilized protease inhibitor. Both the rinse and saliva samples were separated into 1 mL aliquots. One aliquot was processed immediately and used for MMP-8 detection using the POCID. The remaining aliquots were frozen at −80°C until assayed using Luminex technology.
Immunoassay Determination of MMP-8 Concentrations
An immunological beadlyte assay kit (EMD Millipore, Billerica, MA) was used to detect MMP-8 concentrations. All samples were analyzed in duplicate by Luminex® within 6 months of sample collection per our previously reported method (Sexton et al., 2011). Standards were included on all runs, and the results are reported within the linearity of the assays.
Point-of-Care Assay for MMP-8
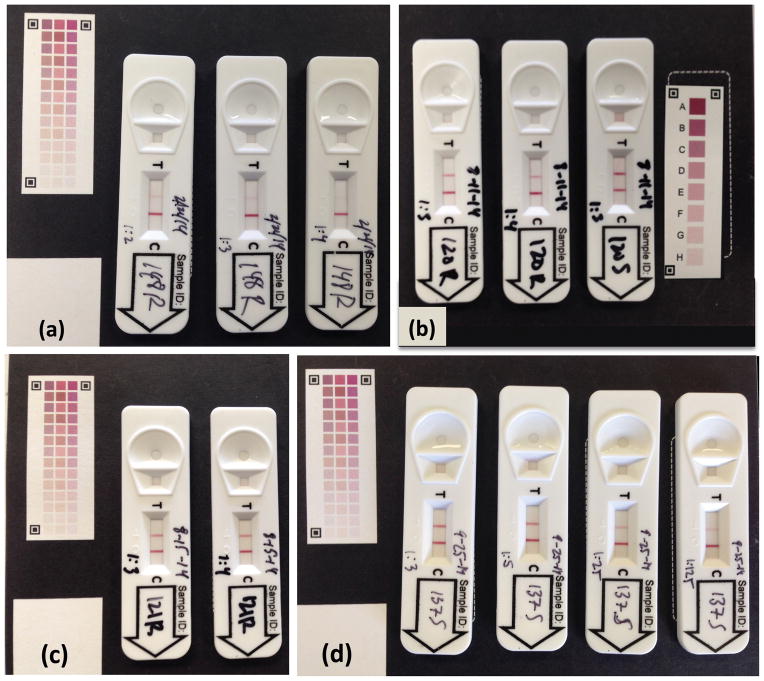
All POC assays (Rapidassays ApS, Copenhagen-S, Denmark) were performed chairside at the time of collection. Collected rinse samples were diluted 1:2, 1:3 and 1:4 and saliva samples were diluted 1:2, 1:3, 1:5, 1:25 and 1:125 in running buffer (Rapid Assays, Copenhagen-S, Denmark) to yield a specimen that flowed appropriately in the POCID. Twenty μL of the diluted sample was mixed with antibody against MMP-8, allowed to sit for 2 min, then added to the square loading well of the device, followed by the administration of 3 drops of running buffer (~100 μL) into the round buffer reservoir. Ten minutes after loading the sample, results (assay range of detection was 1 – 1000 ng/mL) were digitally recorded (Apple iPhone 5, Apple Inc., Cupertino, CA) using an adjacently placed 8-window, and/or 42-window color calibration card (WCCC). A valid test result produced a control line within the POCID window; any result not demonstrating a control line was considered invalid.
Resulting images were assessed using two methods. First, each digital photographic image (Fig. 1a) was evaluated with quantification application software designed by Rapid Assays by uploading each image to http://www.test-reader.com/m/lft/gRAD0002/aa01/ra05-095/02. This analytic website contained proprietary software that compared the test line density with the control line color and the 42-WCCC. The software generated a numerical value representative of the MMP-8 concentration for each sample, and results were emailed to the investigator. Second, each digital photographic image taken with the 8-WCCC was projected in a dark room on a ten by eight foot screen. Two examiners (NJ and CSM), seated 15 feet from the screen, independently scored the test and control line density by visually comparing the image with the 8-WCCC. According to color density (A to H), each color was assigned a numeric value from 0 to 8, with 0 being a test line not visible, and 8 being the maximum intensity score for a detected test or control line (Fig. 1b).
Figure 1.
Examples of POCIDs after ten minute assays with color calibration cards. (a) Healthy participant #148 rinse (R) at dilutions 1:2, 1:3 and 1:4. (b) Periodontal disease participant #120 saliva (S) dilution 1:3, and rinse (R) dilutions 1:3, 1:4. (c) Periodontal disease participant #121 rinse (R) dilutions 1:3, 1:4. (d) Periodontal disease participant #137 saliva (S) dilutions 1:3, 1:5, 1:25, 1:125.
Statistical Analysis
Wilcoxon rank sum tests were used to compare the MMP-8 results between the periodontal group and the healthy group for both rinse and saliva specimens. Correlations among methods (Luminex, optically scanned image, and visual determined image) were obtained using Pearson’s correlation, and multiple linear regression models were constructed to determine if the MMP-8 readings for a given method were related to age, gender, race, smoking, and number of teeth. Agreements between each of the methods and Luminex (i.e., the gold standard) were obtained using the Bland-Altman procedure. To determine how each method discriminated health from periodontitis a logistic regression model was constructed for this endpoint with each method used to make the prediction. Results were summarized as area under the receiver operator characteristic (ROC) and the sensitivity and specificity associated with the best cut-point determined from this curve. The optimal cutpoint was selected based on the point in the ROC that was closest to the ideal point 100% sensitivity and 100% specificity. All statistical analyses were performed using PC-SAS, Version 9.3. Statistical significance was determined at the α; = 0.05 level for both the multiple linear regression and the logistic regression models, but was determined at the α = 0.01 level for Pearson’s correlations due to the number of correlations determined.
Results
Demographics
Forty-one participants were evaluated (Table 1). Thirty-one had clinical measures consistent with periodontal disease (3 had mild chronic periodontitis, 19 had generalized moderate chronic periodontitis, and 9 had generalized severe chronic periodontitis). Ten participants were periodontally healthy. The periodontal disease group was older, had more tobacco smokers, had fewer teeth, and had proportionally fewer women (p = 0.004, 0.084, 0.057, and 0.23, respectively).
Table 1.
Demographics of the study population
| Category | Health | Periodontitis |
|---|---|---|
| Females | 7 | 15 |
| Males | 3 | 16 |
| Race | 2 A, 6 C, 2 H | 2 A, 3 B, 22 C, 4 H |
| Mean age (range) | 31.5 ± 5.2 (23–40) years | 44.9 ± 14.3 (23–80) years |
| Smoker | 0 | 10 |
| No. Teeth | 28.2 ± 1.2 | 26.2 ± 3.3 |
| Periodontal index (% sites; mean ± SD) | ||
| BOP | 1.29 ± 1.31 | 52.72 ± 24.50 |
| PD ≥ 4 mm | 0.003 ± 0.005 | 46.68 ± 0.15 |
| PD ≥ 5 mm | 0 | 25.61 ± 0.15 |
A, Asian; B, African American; BOP, bleeding on probing; C, Caucasian; H, Hispanic; PD, probing pocket depth
Luminex MMP-8 concentrations
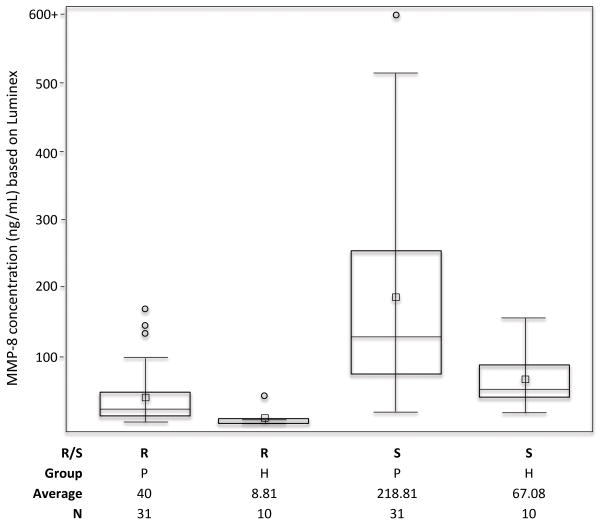
MMP-8 was detected by Luminex in all rinse and matched saliva samples. Concentrations ranged from 1.94 to 168.6 ng/mL in oral rinse samples, and from 19.8 to 1223.1 ng/mL in saliva specimens (Fig. 2). On average, saliva samples contained 7.8 times more MMP-8 than rinse samples (range 1.73 to 28.3). The median concentration of rinse samples from periodontal disease participants (median: 22.9 ng/mL; range: 4.77–168.6 ng/ml) was 4.1 times higher than the median of the rinse samples from healthy participants (median: 5.6 ng/mL; range: 1.94 – 43.28 ng/mL; p=0.001). Similarly, the median concentration of MMP-8 in saliva from the periodontal disease group (129.8 ng/mL; range 19.1 to 1223.1 ng/mL) was 2.5 times higher than from the healthy group (51.9 ng/mL; range 19.67–157.96 ng/mL; p = 0.011). ROC analysis demonstrated a cut point of 8 ng/mL MMP-8 from rinse samples detected periodontal disease participants with a sensitivity of 0.9 and specificity of 0.84 (p = 0.04, AUC = 0.86). A cut point of 89 ng/mL MMP-8 in saliva detected periodontal disease participants with a sensitivity of 0.8 and specificity of 0.74 (p = 0.04, AUC = 0.78).
Figure 2.
Box plots showing the distribution of MMP-8 levels in oral rinse (R) fluids and whole saliva (S) from participants categorized as healthy (H) or periodontitis (P).
Performance of the POCID as determined by optical scan
MMP-8 concentrations were assayed from dilutions of rinse (n=102) and saliva (n=143) samples (Fig. 1). Eighty three percent (203/245) of the samples flowed appropriately, as evident by a color-free, square loading well after the 10 minute assay, in conjunction with the detection of a red control line. Of the 17% of samples that left a red stain in the sample well, indicating ineffective flow of the sample-antibody-complex, 6% (6/102) were from diluted rinses, and 25% (36/143) were from diluted saliva specimens.
Table 2 shows the optical performance of the POCID compared with the Luminex results. Overall, the POCID performance was excellent, regardless of use of oral rinse or whole saliva. MMP-8 concentrations of the rinses and saliva from participants by POCID correlated strongly with Luminex values (r = 0.70–0.97). The best correlations were seen at a rinse dilution of 1:3 (r = 0.82) and saliva dilution of 1:5 (r=0.93).
Table 2.
Pearson correlation coefficients of Luminex with Optical POCID and Visual Read of POCID
| Luminex (ng/mL) | Optical Scan Reading of POC Device | Correlation with Luminex | P value | Visual Average POC Device | Correlation with Luminex | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution | N | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | ||||||
| PERIO | R | 1:2 | 10 | 12.29 | 8.34 | 37.8 | 11.83 | 0.7021 | 0.0236 | 1.8 | 0.98 | 0.9033 | 0.0003 |
| 1:3 | 31 | 13.33 | 14.45 | 61.68 | 47.8 | 0.8182 | <.0001 | 2.89 | 1.99 | 0.8834 | <.0001 | ||
| 1:4 | 31 | 10 | 10.84 | 61.16 | 47.37 | 0.7241 | <.0001 | 2.69 | 2.08 | 0.694 | <.0001 | ||
|
| |||||||||||||
| S | 1:2 | 10 | 86.75 | 71.12 | 97.9 | 90.59 | 0.9444 | <.0001 | 3.5 | 2.05 | 0.8314 | 0.0029 | |
| 1:3 | 30 | 74.53 | 89.66 | 82.68 | 67.42 | 0.7702 | <.0001 | 3.88 | 2.17 | 0.7041 | <.0001 | ||
| 1:5 | 31 | 43.76 | 53.16 | 90.1 | 83.93 | 0.9288 | <.0001 | 3.77 | 1.91 | 0.6955 | <.0001 | ||
| 1:25 | 21 | 9.62 | 12.35 | 55.38 | 38.65 | 0.7245 | 0.0002 | 2.83 | 1.31 | 0.6535 | 0.0013 | ||
| 1:125 | 21 | 1.92 | 2.47 | 33.29 | 19.47 | 0.6088 | 0.0034 | 1.6 | 0.64 | 0.6051 | 0.0037 | ||
|
| |||||||||||||
| HEALTH | R | 1:2 | 10 | 4.4 | 6.14 | 40.2 | 9.76 | 0.7581 | 0.0111 | 1.35 | 0.53 | 0.7963 | 0.0058 |
| 1:3 | 10 | 2.94 | 4.1 | 40.1 | 18.76 | 0.9705 | <.0001 | 1.3 | 0.95 | 0.9857 | <.0001 | ||
| 1:4 | 10 | 2.2 | 3.07 | 36.9 | 16.41 | 0.9059 | 0.0003 | 1.25 | 0.79 | 0.9857 | <.0001 | ||
|
| |||||||||||||
| S | 1:2 | 10 | 33.54 | 20.95 | 61.9 | 37.27 | 0.2868 | 0.4217 | 2.35 | 1.43 | 0.3131 | 0.3784 | |
| 1:3 | 10 | 22.36 | 13.96 | 53.8 | 18.17 | 0.3438 | 0.3308 | 2.25 | 1.16 | 0.4427 | 0.2001 | ||
| 1:5 | 10 | 13.42 | 8.38 | 42 | 11.13 | 0.1134 | 0.7552 | 1.4 | 0.81 | 0.146 | 0.6874 | ||
|
| |||||||||||||
| ALL | R | 1:2 | 20 | 8.35 | 8.2 | 39 | 10.63 | 0.5666 | 0.0092 | 1.58 | 0.8 | 0.8623 | <.0001 |
| 1:3 | 41 | 10.8 | 13.45 | 56.41 | 43.37 | 0.8283 | <.0001 | 2.5 | 1.91 | 0.8968 | <.0001 | ||
| 1:4 | 41 | 8.1 | 10.09 | 55.24 | 43.07 | 0.7479 | <.0001 | 2.34 | 1.95 | 0.7344 | <.0001 | ||
|
| |||||||||||||
| S | 1:2 | 20 | 60.15 | 57.87 | 79.9 | 69.9 | 0.8633 | <.0001 | 2.93 | 1.82 | 0.7405 | 0.0002 | |
| 1:3 | 40 | 61.49 | 80.9 | 75.08 | 59.69 | 0.7666 | <.0001 | 3.48 | 2.08 | 0.7126 | <.0001 | ||
| 1:5 | 41 | 36.36 | 48.05 | 78.08 | 75.57 | 0.9281 | <.0001 | 3.2 | 1.99 | 0.6997 | <.0001 | ||
| 1:25 | 21 | 9.62 | 12.35 | 55.38 | 38.65 | 0.7245 | 0.0002 | 2.83 | 1.31 | 0.6535 | 0.0013 | ||
| 1:125 | 21 | 1.92 | 2.47 | 33.29 | 19.47 | 0.6088 | 0.0034 | 1.6 | 0.64 | 0.6051 | 0.0037 | ||
R, rinse; S, saliva
Using this data set and ROC analyses, threshold concentrations that distinguished the periodontal disease group from the healthy group in rinse and saliva were calculated. Oral rinse fluids by POC assay had a cut-point ranging from 34–39 optical units, with a sensitivity of 0.7–0.9 and a specificity of 0.5–0.71. Saliva dilution samples analyzed using the POCID had a cut-point ranging from 47–58 optical units (sensitivity of 0.7–0.8, specificity of 0.5–0.7, AUC = 0.60 – 0.68). Best AUC results were observed with salivary dilution of 1:5 (sensitivity 0.8, specificity 0.7, p = 0.046, AUC = 0.59 – 0.74).
Performance of the POCID as determined by visualization
The relationship between the Luminex results and POCID assay results, as determined visually by two examiners was also determined (Table 2). Performance of the rinse at 1:2 and 1:3 dilutions were excellent yielding correlations of 0.79–0.99 with Luminex (p<0.0058). Saliva dilutions 1:2 and 1:3 also produced good correlations (0.7–0.83) overall and when the periodontal disease samples were analyzed.
The threshold concentration that distinguished the periodontal disease group from the healthy group was calculated using ROC analysis. Here, the visual readings by both examiners produced similar results with cut-points between 1 and 2 (i.e., A and B on the 8-WCCC) for diluted rinse, and between 2 and 3 (i.e., B and C on the 8-WCCC) for diluted saliva. Rinse dilution 1:3 performed best for both examiners (sensitivity 0.9 and specificity 0.7; p<0.066, AUC = 0.78) for identifying periodontal disease participants. Saliva diluted 1:5 performed best for both examiners (sensitivity 0.9 and specificity 0.6–0.7; p<0.02, AUC = 0.83) in discriminating periodontal disease from health.
Bland-Altman plots were performed for all dilutions. These analyses showed a linear pattern indicating that the agreement varied with the magnitude of the readings (data not shown). In multiple regression model analyses, none of the factors (age, race, gender, smoking status, or number of teeth) significantly affected the MMP-8 concentrations as determined by either Luminex or the POCID (data not shown).
Discussion
This study determined whether MMP-8 is detectable in oral fluids using a POCID, and if POCID-based concentrations of MMP-8 correlate with laboratory-based immunoassay results. We examined the utility of this device in 41 participants who provided an oral rinse and whole expectorated saliva. Our findings based on 245 individual POCID assays and confirmatory 82 oral fluid Luminex immunoassays demonstrate that: 1) the POCID performed well in the rapid measurement of MMP-8 concentrations in oral rinse and saliva samples, 2) both the optically-read and visually-read results correlated well with the laboratory-based assay system, and 3) a POCID that employs LFC for detection of a host response biomarker has the potential to discriminate persons who have clinical features of periodontal disease from healthy controls.
MMP-8 is present at elevated concentrations at sites of active periodontal inflammation compared with healthy control sites (Kinane et al., 2003, Sodek & Overall, 1992, Sorsa et al., 2006, Ingman et al., 1996, Soder et al., 2006, Leppilahti et al., 2014, Mantyla et al., 2003, Mantyla et al., 2006, Prescher et al., 2007). This, coupled with the fact that MMP-8 exists at ng/mL concentrations in saliva (Miller et al., 2006), made this analyte a good candidate for use in a LFC assay. MMP-8 was detected in all samples by Luminex, and concentrations from periodontitis participants were 4.1 times higher in oral rinse and 2.5 times higher in saliva samples compared with healthy controls. These differences permitted thresholds to be established based on the Luminex data that discriminated periodontal disease from health (p<0.044). In addition, both oral fluids assayed on the POCID produced results that distinguished periodontal disease group from the healthy group with reasonable sensitivity and specificity. The discriminatory capacity of salivary MMP-8 is consistent with our previous studies (Miller et al., 2006, Christodoulides et al., 2007), as well as the ability of an oral rinse to contain MMP-8 levels that can distinguish periodontitis from health (Nwhator et al., 2014, Leppilahti et al., 2011). Together the findings suggest that both oral fluids appear to have good sensitivity and specificity for detecting moderate to severe periodontal disease when compared against health.
A concern with the utilization of saliva in a lateral flow POCID is its viscoelastic properties. We attempted to reduce the viscosity by diluting the samples in buffer prior to performing the assay. Nevertheless, 17% of the diluted samples did not flow appropriately, based on a remnant pink-red color in the loading well after the ten minute assay. The majority of these flow-affected samples occurred with whole saliva and generally were noted at lower dilutions. However, dilutions between 1:2 and 1:5 yielded satisfactory results, with a salivary dilution of 1:5 and a rinse dilution of 1:3 providing optimal results. Overall, the oral rinse was equally efficacious as saliva in yielding detectable concentrations of MMP-8, but the rinse was more fluid, and could be provided by almost all patients; in contrast to some patients who demonstrated difficulty providing saliva because of hyposalivation.
The utility of a POCID in dental applications also needs to take into account the framework of the interaction of patients with the dental healthcare team. In the office, the POCID is envisioned to be used by a member of the professional team and could result in semi-quantitative outcomes using a simplified densitometric scanning process, as described in this report. Alternatively, the POCID could be developed to deliver a visual analytic outcome that would enable the practitioner to document normal or elevated levels of MMP-8, which could help educate the patient and be used as a tool in strategies for individual patient management. Using this visual approach, an outcome could be implemented at the patient level as part of a patient-oriented oral health wellness program that emphasizes empowerment of the patient to contribute to oral health care and the need for professional intervention. Thus, a goal of this project was to determine whether densitometric optical cut-point values and visual cut-point values were comparable to laboratory-based Luminex assay results. Here both optical and visual results were generally excellent; with the visual readings showing significant agreement between the independent examiners. The benefit of using a visual reading over optical digital uploads would translate into less expense, less technical demand, and a more rapid process. Therefore, a more consumer friendly POCID with a simple yes/no response might be expected to enhance the global availability and use of the device.
As with many studies, there are limitations to consider. Limitations included a small study population, the use of extreme conditions (periodontal health vs. disease), groups that were not specifically matched for some demographic variables, inability to address all potential confounders for detecting elevated MMP-8 levels, and a cross-sectional study design. The influences of confounders such as smoking and genetic variation are undetermined for the potential effects on POC identification strategies for MMP-8, as well as other biomarkers (Chen et al., 2001, Heikkinen et al., 2010, Heikkinen et al., 2010a). Also, because of the small sample size, smokers and non-smokers were not stratified in the data analysis. Smoking has been suggested to affect MMP-8 levels because of the effects of nicotine on circulation and inflammation (Soder et al., 2002, Soder, 1999), although high levels of MMP-8 in gingival crevicular fluid levels have been detected in clusters of smoking subjects (Mantyla et al., 2006). Therefore, it cannot be definitively stated that all periodontitis patients who smoke would be expected to have lower levels of MMP-8 in gingival crevicular fluid, oral rinses, and saliva. Moreover, it is currently unclear whether smoking would adversely impact the effectiveness of this type of POC assessment. Of note, our study reports similar findings to Mantyla et al. (2006), with smokers and non-smokers grouped together demonstrating elevated levels of MMP-8 in both rinse and whole saliva in periodontitis compared to healthy controls subjects. Finally, we recognize that focusing on a single salivary host response biomarker is a limitation, and a positive POC result does not necessarily document the extent or location of disease, or the ability of the device to discriminate gingivitis from periodontitis.
Conclusions
A disposable POCID using rinse or saliva can provide a rapid, sensitive and specific approach for detecting elevated levels of MMP-8 potentially reflective of periodontitis. Further studies are needed to confirm the potential use of this device to aid practitioners in identifying, treating, and monitoring the biologic progression of periodontal disease. Moreover, the use of additional salivary biomarkers would be expected to improve the ability of a POCID as an adjunct to clinical and radiographic examinations for detection, risk prediction, and clinical decision-making in the management of periodontal disease.
Acknowledgments
Source of Funding: Supported by Office of the Associate Dean for Research, College of Dentistry, University of Kentucky. This work was supported by a USPHS grant from the NIH/NCRR RR020145 and NIGMS GM103538, and the services of the Center for Clinical and Translational Sciences at the University of Kentucky (NCATS UL1 TR000117).
We thank Jennifer Buryzynski, DMD, Nigel Caterer (Rapidassays ApS) and Jason Stevens for their help in this project.
Footnotes
Conflict of Interest: None to declare
References
- [Accessed on November 22, 2015];Alcolock drug screeners. http://drugwipeusa.com/?gclid=CjwKEAiA7MWyBRDpi5TFqqmm6hMSJAD6GLeAun3mop9X-1k_BqoJNMNBivlqeWwiLCq-Azh8lSUJfBoCZlrw_wcB.
- Al-Sabbagh M, Alladah A, Lin Y, Kryscio RJ, Thomas MV, Ebersole JL, Miller CS. Bone remodeling-associated salivary biomarker MIP-1alpha distinguishes periodontal disease from health. J Periodontal Res. 2012;47:389–95. doi: 10.1111/j.1600-0765.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;38(Suppl 11):85–105. doi: 10.1111/j.1600-051X.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Wolff L, Aeppli D, Guo Z, Luan W, Baelum V, Fejeskov O. Cigarette smoking, salivary/gingival crevicular fluid cotinine and periodontal status. A 10-year longitudinal study. J Clin Periodontol. 2001;28:331–9. doi: 10.1034/j.1600-051x.2001.028004331.x. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Floriano PN, Miller CS, Ebersole JL, Mohanty S, Dharshan P, Griffin M, Lennart A, Ballard KL, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV, McDevitt JT. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–28. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:607–12. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Schuster JL, Stevens J, Dawson D, 3rd, Kryscio RJ, Lin Y, Thomas MV, Miller CS. Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. Journal of clinical immunology. 2013;33:271–9. doi: 10.1007/s10875-012-9771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JD, 3rd, Sneed JD, Steinhubl SR, Kolasa J, Ebersole JL, Lin Y, Kryscio RJ, McDevitt JT, Campbell CL, Miller CS. Oral fluids that detect cardiovascular disease biomarkers. Oral surgery, oral medicine, oral pathology and oral radiology. 2012a;114:207–14. doi: 10.1016/j.oooo.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JD, 3rd, Sneed JD, Steinhubl SR, Kolasa JR, Ebersole JL, Lin Y, Kryscio RJ, McDevitt JT, Campbell CL, Miller CS. Salivary biomarkers associated with myocardial necrosis: results from an alcohol septal ablation model. Oral surgery, oral medicine, oral pathology and oral radiology. 2012b;114:616–23. doi: 10.1016/j.oooo.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile WV, McDevitt JT, Niedbala RS, Malamud D. Translational and clinical applications of salivary diagnostics. Adv Dent Res. 2011;23:375–80. doi: 10.1177/0022034511420434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Foley MW, Bieber NM, Bourdelle PA, Niedbala RS. Development of an ultrasensitive immunochromatography test to detect nicotine metabolites in oral fluids. Analytical and bioanalytical chemistry. 2011;400:3655–64. doi: 10.1007/s00216-011-5051-y. [DOI] [PubMed] [Google Scholar]
- Gupta N, Gupta ND, Gupta A, Khan S, Bansal N. Role of salivary matrix metalloproteinase-8 (MMP-8) in chronic periodontitis diagnosis. Front Med. 2015;9:72–76. doi: 10.1007/s11684-014-0347-x. [DOI] [PubMed] [Google Scholar]
- Gursoy UK, Kononen E, Huumonen S, Tervahartiala T, Pussinen PJ, Suominen AL, Sorsa T. Salivary type I collagen degradation end-products and related matrix metalloproteinases in periodontitis. J Clin Periodontol. 2013;40:18–25. doi: 10.1111/jcpe.12020. [DOI] [PubMed] [Google Scholar]
- Gursoy UK, Kononen E, Pradhan-Palikhe P, Tervahartiala T, Pussinen PJ, Suominen-Taipale L, Sorsa T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010;37:487–93. doi: 10.1111/j.1600-051X.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- Gursoy UK, Kononen E, Uitto VJ, Pussinen PJ, Hyvarinen K, Suominen-Taipale L, Knuuttila M. Salivary interleukin-1beta concentration and the presence of multiple pathogens in periodontitis. J Clin Periodontol. 2009;36:922–7. doi: 10.1111/j.1600-051X.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Nwhator SO, Rathnayake N, Mantyla P, Vatanen P, Sorsa T. Pilot Study on Oral Health Status as Assessed by an Active Matrix Metalloproteinase-8 Chairside Mouthrinse Test in Adolescents. J Periodontol. 2016;87:36–40. doi: 10.1902/jop.2015.150377. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Sorsa T, Pitkaniemi J, Tervahartiala T, Kari K, Broms U, Koskenvuo M, Meurman JH. Smoking affects diagnostic salivary periodontal disease biomarker levels in adolescents. J Periodontol. 2010;81:1299–307. doi: 10.1902/jop.2010.090608. [DOI] [PubMed] [Google Scholar]
- Ingman T, Tervahartiala T, Ding Y, Tschesche H, Haerian A, Kinane DF, Konttinen YT, Sorsa T. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23:1127–32. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Izadi Borujeni S, Mayer M, Eickholz P. Activated matrix metalloproteinase-8 in saliva as diagnostic test for periodontal disease? A case-control study. Medical microbiology and immunology. 2015;204:665–72. doi: 10.1007/s00430-015-0413-2. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Darby IB, Said S, Luoto H, Sorsa T, Tikanoja S, Mantyla P. Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J Periodontal Res. 2003;38:400–4. doi: 10.1034/j.1600-0765.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, Shelburne CE, Rayburn LA, Singh AK, Giannobile WV. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90:752–8. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. Beta-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J Periodontol. 2003;74:353–9. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- Leppilahti JM, Ahonen MM, Hernandez M, Munjal S, Netuschil L, Uitto VJ, Sorsa T, Mantyla P. Oral rinse MMP-8 point-of-care immuno test identifies patients with strong periodontal inflammatory burden. Oral Dis. 2011;17:115–22. doi: 10.1111/j.1601-0825.2010.01716.x. [DOI] [PubMed] [Google Scholar]
- Leppilahti JM, Hernandez-Rios PA, Gamonal JA, Tervahartiala T, Brignardello-Petersen R, Mantyla P, Sorsa T, Hernandez M. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J Clin Periodontol. 2014;41:348–56. doi: 10.1111/jcpe.12223. [DOI] [PubMed] [Google Scholar]
- Liede KE, Haukka JK, Hietanen JH, Mattila MH, Ronka H, Sorsa T. The association between smoking cessation and periodontal status and salivary proteinase levels. J Periodontol. 1999;70:1361–8. doi: 10.1902/jop.1999.70.11.1361. [DOI] [PubMed] [Google Scholar]
- Mantyla P, Stenman M, Kinane D, Salo T, Suomalainen K, Tikanoja S, Sorsa T. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res. 2006;41:503–12. doi: 10.1111/j.1600-0765.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- Mantyla P, Stenman M, Kinane DF, Tikanoja S, Luoto H, Salo T, Sorsa T. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J Periodontal Res. 2003;38:436–9. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- Miller CS, Foley JD, 3rd, Floriano PN, Christodoulides N, Ebersole JL, Campbell CL, Bailey AL, Rose BG, Kinane DF, Novak MJ, McDevitt JT, Ding X, Kryscio RJ. Utility of salivary biomarkers for demonstrating acute myocardial infarction. J Dent Res. 2014;93:72S–79S. doi: 10.1177/0022034514537522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campbell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, Ebersole JL, McDevitt JT. Current developments in salivary diagnostics. Biomarkers in Medicine. 2010;4:1–18. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–9. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- Mirrielees J, Crofford LJ, Lin Y, Kryscio RJ, Dawson DR, 3rd, Ebersole JL, Miller CS. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol. 2010;37:1068–74. doi: 10.1111/j.1600-051X.2010.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Tamaki Y, Tanaka T, Arakawa H, Tsurumoto A, Kirimura K, Sato T, Hanada N, Kamoi K. Screening of periodontitis with salivary enzyme tests. J Oral Sci. 2006;48:177–83. doi: 10.2334/josnusd.48.177. [DOI] [PubMed] [Google Scholar]
- Nwhator SO, Ayanbadejo PO, Umeizudike KA, Opeodu OI, Agbelusi GA, Olamijulo JA, Arowojolu MO, Sorsa T, Babajide BS, Opedun DO. Clinical correlates of a lateral-flow immunoassay oral risk indicator. J Periodontol. 2014;85:188–94. doi: 10.1902/jop.2013.130116. [DOI] [PubMed] [Google Scholar]
- Ozcaka O, Nalbantsoy A, Buduneli N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46:592–8. doi: 10.1111/j.1600-0765.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- Prescher N, Maier K, Munjal SK, Sorsa T, Bauermeister CD, Struck F, Netuschil L. Rapid quantitative chairside test for active MMP-8 in gingival crevicular fluid: first clinical data. Ann N Y Acad Sci. 2007;1098:493–5. doi: 10.1196/annals.1384.019. [DOI] [PubMed] [Google Scholar]
- Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–46. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnayake N, Akerman S, Klinge B, Lundegren N, Jansson H, Tryselius Y, Sorsa T, Gustafsson A. Salivary biomarkers for detection of systemic diseases. PLoS One. 2013a;8:e61356. doi: 10.1371/journal.pone.0061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnayake N, Akerman S, Klinge B, Lundegren N, Jansson H, Tryselius Y, Sorsa T, Gustafsson A. Salivary biomarkers of oral health: a cross-sectional study. J Clin Periodontol. 2013b;40:140–7. doi: 10.1111/jcpe.12038. [DOI] [PubMed] [Google Scholar]
- Salminen A, Gursoy UK, Paju S, Hyvarinen K, Mantyla P, Buhlin K, Kononen E, Nieminen MS, Sorsa T, Sinisalo J, Pussinen PJ. Salivary biomarkers of bacterial burden, inflammatory response, and tissue destruction in periodontitis. J Clin Periodontol. 2014;41:442–50. doi: 10.1111/jcpe.12234. [DOI] [PubMed] [Google Scholar]
- Sexton WM, Lin Y, Kryscio RJ, Dawson DR, 3rd, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. 2011;38:434–41. doi: 10.1111/j.1600-051X.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J, Overall CM. Matrix metalloproteinases in periodontal tissue remodelling. Matrix Suppl. 1992;1:352–62. [PubMed] [Google Scholar]
- Soder B. Neutrophil elastase activity, levels of prostaglandin E2, and matrix metalloproteinase-8 in refractory periodontitis sites in smokers and non-smokers. Acta Odontol Scand. 1999;57:77–82. doi: 10.1080/000163599428940. [DOI] [PubMed] [Google Scholar]
- Soder B, Airila Mansson S, Soder PO, Kari K, Meurman J. Levels of matrix metalloproteinases-8 and -9 with simultaneous presence of periodontal pathogens in gingival crevicular fluid as well as matrix metalloproteinase-9 and cholesterol in blood. J Periodontal Res. 2006;41:411–7. doi: 10.1111/j.1600-0765.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- Soder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29:384–91. doi: 10.1034/j.1600-051x.2002.290502.x. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mantyla P. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- Thomas MV, Branscum A, Miller CS, Ebersole J, Al-Sabbagh M, Schuster JL. Within-subject variability in repeated measures of salivary analytes in healthy adults. J Periodontol. 2009;80:1146–53. doi: 10.1902/jop.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov A, Mauk M, Corstjens P, Niedbala RS. Rapid prototyping of lateral flow assays. Methods Mol Biol. 2009;504:217–35. doi: 10.1007/978-1-60327-569-9_14. [DOI] [PubMed] [Google Scholar]
- Zachary D, Mwenge L, Muyoyeta M, Shanaube K, Schaap A, Bond V, Kosloff B, de Haas P, Ayles H. Field comparison of OraQuick ADVANCE Rapid HIV-1/2 antibody test and two blood-based rapid HIV antibody tests in Zambia. BMC Infect Dis. 2012;12:183. doi: 10.1186/1471-2334-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Henson BS, Camargo PM, Wong DT. The clinical value of salivary biomarkers for periodontal disease. Periodontol 2000. 2009;51:25–37. doi: 10.1111/j.1600-0757.2009.00315.x. [DOI] [PubMed] [Google Scholar]