Abstract
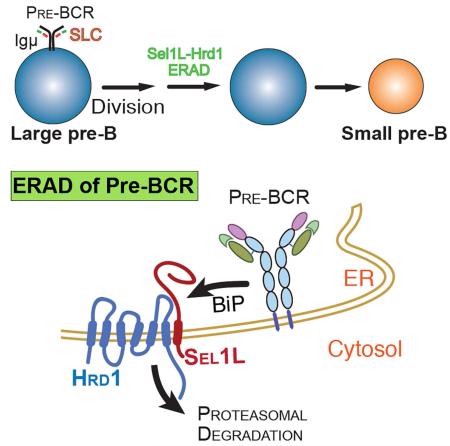
Endoplasmic reticulum (ER)-associated degradation (ERAD) is a principal mechanism that targets ER-associated proteins for cytosolic proteasomal degradation. Here our data demonstrate a critical role for the Sel1L-Hrd1 complex, the most conserved branch of ERAD, in early B cell development. Loss of Sel1L-Hrd1 ERAD in B cell precursors leads to a severe developmental block at the transition from the large to small pre-B cells. Mechanistically, we show that Sel1L-Hrd1 ERAD selectively recognizes and targets the pre-B cell receptor (pre-BCR) for proteasomal degradation in a BiP-dependent manner. The pre-BCR complex accumulates both intracellularly and at the cell surface in Sel1L-deficient pre-B cells, leading to persistent pre-BCR signaling and pre-B cell proliferation. This study thus implicates ERAD mediated by Sel1L-Hrd1 as a key regulator of B cell development and reveals the molecular mechanism underpinning the transient nature of pre-BCR signaling.
Keywords: endoplasmic reticulum-associated degradation, Sel1L, Hrd1, B cell development, pre-B cell receptor, large pre-B cells
INTRODUCTION
Proteins destined for secretion or membrane localization are folded in the endoplasmic reticulum (ER). Protein folding is a complex, error-prone process that often results in the generation of irreparable misfolded, often toxic, protein by-products. A stringent quality control system is coupled to the protein folding process, ensuring that only correctly folded proteins exit the ER to proceed to their destination. ER-associated degradation (ERAD) is a fundamental quality control mechanism responsible for the recognition and translocation of terminally misfolded or unfolded proteins in the ER, targeting them for cytosolic proteasomal degradation (Olzmann et al., 2013). The use of artificial and disease-related mutant proteins as ERAD substrates has shed important insights into ERAD biology (Guerriero and Brodsky, 2012); however, very little is known about the physiological importance of ERAD and the nature of its endogenous substrates.
The best-characterized ERAD machinery in mammals is the highly conserved Sel1L-Hrd1 complex consisting of the E3 ubiquitin ligase Hrd1 and its adaptor protein Sel1L (Olzmann et al., 2013). While many biochemical insights into ERAD biology have been gained from the degradation of model substrates such as immunoglobulin (Ig) light and heavy chains, the current challenges are to identify the nature of its endogenous protein substrates and to delineate the physiological relevance and significance of ERAD in individual cell types in vivo. Using global inducible Sel1L-deficient mouse models, we recently demonstrated that Sel1L is a key component of mammalian Hrd1 ERAD machinery by stabilizing the Hrd1 protein and that the Sel1L-Hrd1 complex plays a critical role in the maintenance of ER homeostasis and animal survival (Sun et al., 2014). While Sel1L-deficient mice are embryonic lethal (Francisco et al., 2010), acute Sel1L deletion in adult mice results in pancreatic atrophy and premature lethality (Sun et al., 2014). In adipocytes, Sel1L deficiency causes intracellular retention of lipoprotein lipase, leading to hypertriglyceridemia (Sha et al., 2014). In the small intestine, epithelial Sel1L-Hrd1 ERAD is indispensable for the secretory function of Paneth cells and protects mice from pathogen-induced ileitis (Sun et al., 2016), whereas in the colon, it modulates the progression of experimental colitis in part via the degradation of IRE1α, the sensor of unfolded protein response (UPR) (Sun et al., 2015). However, the physiological importance of Sel1L-Hrd1 ERAD in cell differentiation and development has not been explored to date.
To this end, we chose to investigate B cell development as a model system because of its complicated yet well-defined and developmentally restricted expression of many growth factors and cell surface-associated receptors required for different stages of development. We reasoned that ERAD may help reshape the membrane receptor proteome in the B cell development. To reach the antibody-producing mature stage, B cell precursors undergo a stepwise differentiation process in the bone marrow (BM) from pro-B cells, cycling large pre-B cells, resting small pre-B cells, and finally to immature B cells. This process requires a productive and sequential rearrangement of the genes encoding Ig heavy (IgH or Igμ) and light (IgL) chains that form B cell receptor (BCR). Prior to IgL chain rearrangement, developing B cells express a set of B lineage-specific genes called λ5 (CD179b) and VpreB (CD179a), which form an IgL-chain-like structure known as the surrogate light chain (SLC) to pair with Igμ heavy chain to form the “pre-BCR” complex (Clark et al., 2014; Herzog et al., 2009; Lee et al., 1999; Pillai and Baltimore, 1987).
Here we found that Sel1L-Hrd1 ERAD manages a key checkpoint at the transition from the large to the small pre-B cells. It does so by targeting the pre-BCR complex for proteasomal degradation in a BiP-dependent manner, an event that is important for the termination of pre-BCR signaling and hence differentiation. Unexpectedly, Sel1L-Hrd1 ERAD has no effect on the turnover of the BCR complex.
RESULTS
Reduced peripheral B cells in B cell-specific Sel1L-deficient mice
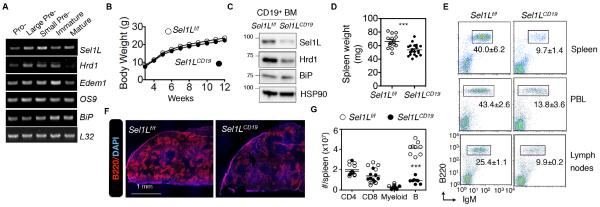
Expression of Sel1L and Hrd1 genes was induced around the pre-B cell stage in developing lymphocytes, preceding that of ER chaperones BiP and Edem1 (Fig. 1A), suggesting a possible role of Sel1L-Hrd1 ERAD in early lymphopoiesis. To investigate whether Sel1L-Hrd1 ERAD plays a role in B cell development, we crossed Sel1Lflox/flox (Sel1Lf/f) mice on the C57BL/6 background (Fig. S1A) with CD19-Cre mice to generate B cell-specific Sel1L-deficient mice (Sel1LCD19). The CD19 promoter is active specifically in B cells and throughout B cell development from the pro-B cell stage (Zhou et al., 1991). The Sel1LCD19 mice and their control Sel1Lf/f littermates were born in a normal Mendelian ratio (not shown) and appeared healthy with no obvious growth defects (Fig. 1B). Immunoblot analysis confirmed the deletion of the Sel1L protein and reduction of Hrd1 protein in the BM-derived B cells (Fig. 1C). Spleen weights were significantly reduced in Sel1LCD19 mice vs. controls (Fig. 1D and Fig. S1B). The percentage of peripheral B cells in spleen, blood, and lymph nodes was reduced by 60-75% (Fig. 1E) and the absolute numbers of splenic B cells were >75% lower in Sel1LCD19 mice compared to controls, while other cell types, including CD4+, CD8+ T and myeloid cells were not affected (Fig. 1F-G and Fig. S1C). The reduction in B cells was not due to elevated cell death (Fig. S1D). Similar to the conventional BM B cells (also known as B-2 cells), the percentage of peritoneal CD5+ B-1 cells was decreased in Sel1LCD19 mice vs. Sel1Lf/f controls (Fig. S1E). Of note, there were ~20% residual peripheral B cells in the Sel1LCD19 mice, which is in line with the fact that CD19-Cre-mediated deletion efficacy is approximately 75-80% in the early B cell compartment (Rickert et al., 1997). Hence, we concluded that Sel1L is required for B cell development.
Figure 1. Reduced peripheral B lymphocyte cellularity in Sel1LCD19 mice.
(A) RT-PCR analysis of ERAD genes in B cell subpopulations from bone marrows (BM) of C57BL/6 mice. (B) Growth curves for male littermates. (C) Immunoblots of Sel1L, Hrd1, and BiP in CD19+ BM cells from Sel1Lf/f and Sel1LCD19 mice. (D) Spleen mass. (E) Flow cytometric analysis of mature B cells (B220+/IgM+) in spleen, peripheral blood (PBL), and lymph nodes. (F) Representative confocal microscopic images of B cells (red) in the spleen. (G) Absolute numbers of splenic CD4+, CD8+ T, myeloid, and mature B cells. Data are representative of two (A,C,F) or three (E) independent experiments. n=8 C57BL/6 mice (A), 18 mice each (B), 3 mice each (C), 15 Sel1Lf/f mice and 19 Sel1LCD19 mice (D), 3 mice each (F), and 8-9 mice each (E,G). Values shown as mean ± s.e.m.; N.S., not significant; *P<0.05, ***P<0.001 by two-tailed Student’s t-test. See also Figure S1.
Developmental defect at the transition from the large to small pre-B cell stage in Sel1LCD19 mice
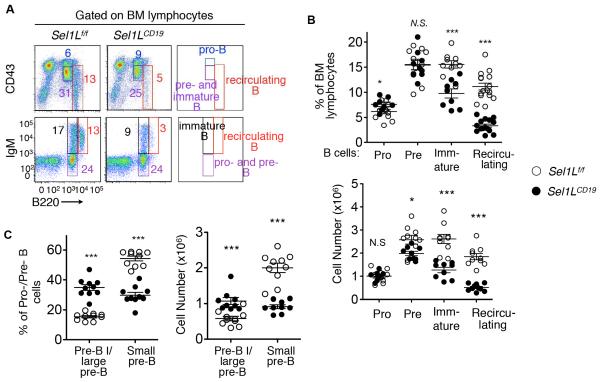
In adult BM, B lymphocytes develop from pluripotent hematopoietic stem cells through an ordered differentiation process, where Igμ and IgL genes are sequentially rearranged and the productive rearrangement of each establishes two key checkpoints that test the functionality of each Ig component (Fig. S2A). At the first checkpoint, Igμ must form a signaling competent pre-BCR with the SLC; at the second checkpoint, a functional BCR must be formed between Igμ and a nascent IgL after SLC expression is extinguished (Fig. S2A). Consistent with the reduced peripheral B cellularity in Sel1LCD19 mice, B220high IgM+ recirculating mature and B220low IgM+ immature B cells were significantly decreased in the BM of Sel1LCD19 mice vs. Sel1Lf/f controls (Fig. 2A-B). On the other hand, the percentage of B220low CD43hi pro-B cells was slightly elevated in Sel1LCD19 mice while that of pre-B cells was comparable to controls (Fig. 2A-B). These data point to a developmental defect at the pro-/pre-B cell stage in Sel1LCD19 mice. Pro-/pre-B cells can be further classified based on the expression of SLC and the differentiation marker CD2 (Fig. S2A). Within the pro-/pre-B cell compartment of the BM, SLC+ CD2− pre-B I and large pre-B (II) cells were twice as many, whereas SLC− CD2+ small pre-B cells were reduced by 50% in the Sel1LCD19 mice compared to Sel1Lf/f littermates (Fig. 2C and Fig. S2B). Cell death did not account for the changes in B cell populations of Sel1LCD19 mice as apoptotic rates for various precursor populations were comparable between the cohorts (Fig. S2C). Both Sel1L heterozygosity and CD19-Cre expression as in Sel1Lf/+;CD19-Cre (Sel1LCD19/+) mice had no effect on B cell development (Fig. S2D), splenic B cell composition (Fig. S2E) and spleen weight (Fig. S2F). Thus, B cell-specific Sel1L deficiency results in a severe developmental defect at the transition from the large to small pre-B cells.
Figure 2. Developmental blockade at the transition from the large to small pre-B cells in Sel1LCD19 mice.
(A) Flow cytometric analysis of B220-CD43 (upper) and B220-IgM (lower) in BM cells from Sel1Lf/f and Sel1LCD19 mice, with quantitation in percentage and absolute cell number shown in (B). (C) Quantitation of flow cytometric analysis of various pro-/pre-B cell populations in BM of Sel1Lf/f and Sel1LCD19 mice. Original flow data shown in Fig. S2B. Data are representative of three independent experiments with n= 9-10 mice each. Values shown as mean ± s.e.m.; *P<0.05, ***P<0.001 by two-tailed Student’s t-test. See also Figure S2-3.
The developmental defect of Sel1LCD19 mice is independent of Chop
We hypothesized that impairment of an ER quality control system like ERAD could activate a stress response, and that might account for the developmental block observed. Indeed, a subset of ER stress-responsive genes such as BiP and Chop were moderately elevated in the large pre-B cells of Sel1LCD19 mice (Fig. S3A). To determine more directly the contribution of ER stress in B cell development of Sel1LCD19 mice, we examined the role of Chop in Sel1LCD19 mice by generating Sel1LCD19;Chop−/− mice. However, Chop deficiency had no impact on the B cell developmental defects associated with the loss of Sel1L, in terms of low spleen weight (Fig. S3B), paucity of the B cell compartment within the peripheral lymphocyte pool (Fig. S3C), and the developmental block at the large pre-B cell stage in the BM (Fig. S3D-G). Thus, B cell-specific Sel1L deficiency results in a developmental block in a Chop-independent manner.
Selective accumulation of the pre-BCR in large pre-B cells
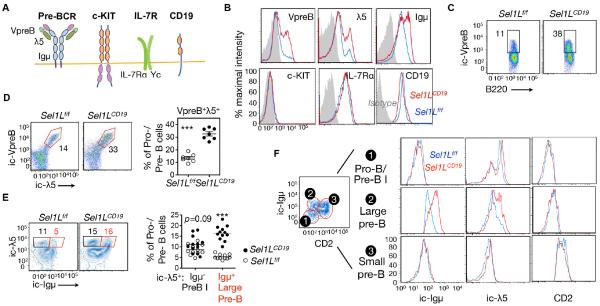
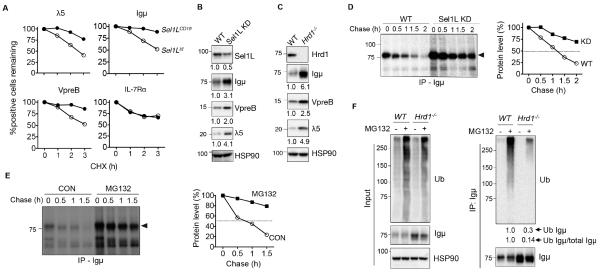
To explore the possible mechanism, we measured the protein levels of various key factors involved in B cell development at the pre-B cell stage, including c-Kit, IL-7Rα, CD19, and the pre-BCR complex (Clark et al., 2014; Herzog et al., 2009). All of these factors are transmembrane proteins synthesized in the ER (Fig. 3A). While total levels (intracellular and surface) of c-Kit and IL-7Rα protein were comparable, protein levels of three main components of the pre-BCR complex were dramatically increased in the pro-/pre-B cells of Sel1LCD19 mice (Fig. 3B). The percentage of SLC+ cells was significantly increased in Sel1LCD19 BM (Fig. 3C and Fig. S4A). Accumulation of the two SLC components VpreB and λ5 occurred within the same cells (Fig. 3D). Moreover, as expression of SLC precedes that of the Igμ heavy chain (Kudo et al., 1992; Lassoued et al., 1996), there is a λ5-single positive pre-B I cell stage in addition to a λ5+ Igμ+ large pre-B cells (Fig. S2A). Strikingly, we observed a 3-fold increase in the percent of λ5+ Igμ+ large pre-B cells in Sel1LCD19 mice compared to that of Sel1Lf/f mice, while the percent of λ5+ Igμ− pre-B I cells was not affected by ERAD deficiency (Fig. 3E). In line with this finding, measurement of λ5 and Igμ at different developmental stages revealed their accumulation only in large pre-B cells when both were co-expressed (Fig. 3F). These data demonstrate that Sel1L-Hrd1 ERAD recognizes and degrades the pre-BCR complex, rather than its individual components. Indeed, using a pre-BCR complex-specific antibody, we found that the proportion of pre-BCR complex-positive cells was doubled in the Sel1LCD19 BM (Fig. S4B). Both quantitative-PCR and RT-PCR analyses revealed comparable transcript levels of VpreB, λ5, and Igμ genes (Fig. S4C-D), suggesting that pre-BCR protein accumulation is a result of post-transcriptional regulation. Hence, our data identify the pre-BCR complex, rather than its individual components, as the possible Sel1L-Hrd1 ERAD substrate in developing B cells.
Figure 3. Accumulation of the pre-BCR complex in Sel1L-deficient large pre-B cells.
(A) Schematic diagram of various membrane receptors involved in early B cell development. (B) Flow cytometric histogram analysis of BM cells from Sel1Lf/f and Sel1LCD19 mice, stained for total (surface and intracellular) levels of indicated proteins, gated on B220lowIgM− pro-/pre-B cells. Gray-shaded area indicates isotype control. (C) Flow cytometric analysis of BM cells from Sel1Lf/f and Sel1LCD19 mice stained for B220 and intracellular (ic) VpreB, gated on pro-/pre-B cells. (D) Flow cytometric analysis of BM for ic-λ5 and ic-VpreB in pro-/pre-B cells with quantitation shown on the right. (E) Flow cytometric analysis of BM for ic-λ5 and ic-Igμ in pro-/pre-B cells with quantitation shown on the right. (F) Flow cytometric histogram analysis of CD2, ic-Igμ, and ic-λ5 in various early B cell populations in BM. Gating strategy for each population (1-3) is shown on the left. Data are representative of four (B) or two (C-F) independent experiments. n=18 mice each (B), 7 mice each (C), 7 mice each (D), 11-12 mice each (E), and 9 mice each (F). Values shown as mean ± s.e.m.; ***P<0.001 by two-tailed Student’s t-test. See also Figure S4.
The pre-BCR complex is an endogenous Sel1L-Hrd1 ERAD substrate
We next directly tested whether the pre-BCR is an ERAD substrate in pre-B cells. Protein levels of Igμ, λ5, and VpreB were significantly stabilized in the BM of Sel1LCD19 mice compared to Sel1Lf/f mice (Fig. 4A and Fig. S5A). By contrast, IL-7Rα protein stability was not affected (Fig. 4A and Fig. S5B). To further corroborate these findings in vitro, we generated Sel1L- or Hrd1-deficient pre-BCR-expressing pre-B cells, 70z/3 (Paige et al., 1978), using the RNAi and CRISPR/cas9 systems, respectively (Fig. 4B-C). Loss of either Sel1L or Hrd1 in the 70z/3 cells increased steady-state protein levels of all three components of the pre-BCR complex (Fig. 4B-C), and led to Igμ protein stabilization, with its half-life increasing from approximately 60 min to over 120 min (Fig. 4D). Inhibition of proteasomal activity using MG132 or bortezomib significantly increased Igμ protein stability in 70z/3 cells (Fig. 4E and data not shown), implicating the proteasome in pre-BCR degradation. Furthermore, ubiquitination of Igμ protein was reduced dramatically in Sel1L−/− or Hrd1−/− 70z/3 cells (Fig. 4F and not shown), pointing to the indispensable role of Sel1L-Hrd1 ERAD in pre-BCR ubiquitination and degradation. Thus, we concluded that Sel1L-Hrd1 ERAD recognizes and targets the pre-BCR complex for ubiquitination and proteasomal degradation and that Sel1L-Hrd1 ERAD deficiency leads to intracellular accumulation of the pre-BCR.
Figure 4. The pre-BCR complex is an endogenous Sel1L-Hrd1 ERAD substrate.
(A) Protein turnover for intracellular λ5, VpreB and Igμ expression in fresh BM of Sel1Lf/f and Sel1LCD19 mice treated with 50 μg/ml cycloheximide (CHX) for the indicated times. IL-7R, a control membrane protein. (B-C) Immunoblot analysis of WT, Sel1L KD (KD) (B) and Hrd1−/− (C) 70Z/3 pre-B cells with quantitation (normalized to HSP90) shown below each gel. (D) Pulse-chase analysis showing Igμ decay in WT and KD pre-B cells with quantitation shown on the right. (E) Pulse-chase analysis showing Igμ decay in WT pre-B cells treated with or without 25 μM MG132 for 0.5 h with quantitation shown on the right. (F) Immunoblot analysis of Igμ immunoprecipitates in WT and Hrd1−/− pre-B cells treated with or without 25 μM MG132 for 3 h. Quantitation of Igμ ubiquitination (with or without normalization to total Igμ) shown below the blot. Data are representative of two (A,D,E), three (B-C), and four (F) independent experiments. n=3 mice each (A). See also Figure S5.
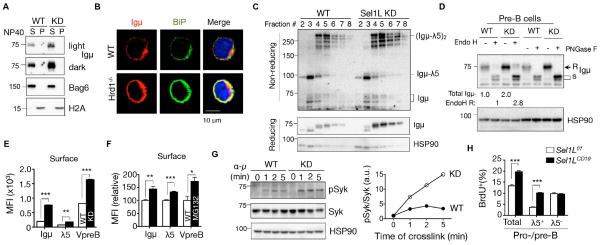
Enhanced ER exit of the pre-BCR in Sel1L-deficient pre-B cells
We next determined the effect of Sel1L-Hrd1 ERAD deficiency on pre-BCR complex formation and intracellular trafficking. The accumulated pre-BCRs remained largely soluble (Fig. 5A), colocalized with ER chaperone BiP (Fig. 5B) and exhibited similar sensitivity to trypsin digestion in ERAD-deficient 70z/3 cells (Fig. S6A). These data suggest that, in the case of pre-BCR, Sel1L-Hrd1 ERAD deficiency does not trigger a dramatic conformational change and hence protein aggregation. Moreover, accumulation of Igμ and SLCs in ERAD-deficient 70z/3 pre-B cells increased the abundance of the high molecular weight pre-BCR complexes, as revealed by non-reducing SDS-PAGE (left two lanes, Fig. S6B) and sucrose density gradient fractionation analyses (Fig. 5C). Moreover, while the majority of Ig protein in WT pre-B cells was retained in the ER and exhibited high-mannose endoglycosidase (Endo) H-sensitivity as previously reported (Brouns et al., 1996), Sel1L deficiency resulted in a nearly 3-fold increase in endoH-resistant and peptide-N-glycosidase F (PNGase F)-sensitive Igμ protein, indicative of increased ER exit of the pre-BCR complex in the absence of Sel1L-Hrd1 ERAD (Fig. 5D). Consistently, more surface pre-BCR complexes were detected in ERAD-deficient pre-B cells (Fig. 5E) or pre-B cells treated with the proteasome inhibitor MG132 (Fig. 5F). Thus, impaired pre-BCR degradation by Sel1L-Hrd1 ERAD leads to elevated surface pre-BCR in pre-B cells.
Figure 5. ERAD is indispensable for the termination of pre-BCR signaling in developing B cells.
(A) Immunoblot analysis of Igμ in the NP-40 soluble (S) and insoluble (P) fractions of the WT and Sel1L KD pre-B cells. The distribution of Bag6 and H2A marks the S and P fractions, respectively. (B) Confocal microscopic images of Igμ (red) and BiP (green) staining in WT and Hrd1−/− 70z/3 cells counterstained with DAPI. (C) Sucrose gradient followed by immunoblot analysis of Igμ in WT and KD pre-B cells under non-reducing and reducing conditions. (D) Immunoblot analysis of Igμ in WT and KD pre-B cell lysates treated with EndoH or PNGase F. (r, s= EndoH resistant, sensitive). Quantitation of total Igμ and EndoH (r) Igμ shown below the blot. (E-F) Quantitation of mean fluorescent intensity (MFI) of surface pre-BCR components in (E) WT and KD pre-B cells and (F) WT pre-B cells treated with or without 25 μM MG132 for 2 h. (G) Western blot analysis of phospho-(pSyk) and total Syk (Syk) of WT and KD cells cross-linked with α-Igμ (10 μg/ml) for the indicated times with quantitation shown on the right. (H) Quantitation of flow cytometric analysis of BrdU incorporation in pro-/pre-B cells and λ5+ or λ5− pro-/pre-B cells of Sel1Lf/f and Sel1LCD19 mice with original flow data shown in Fig. S6D. Data are representative of 2-3 independent experiments. n=6 mice each for (H). Values shown as mean ± s.e.m.; *P< 0.05, **P<0.01, ***P<0.001 by two-tailed Student’s t-test. See also Figure S6.
Persistent pre-BCR signaling and pre-B cell cycling in Sel1LCD19 mice
We next tested whether elevated surface pre-BCR results in augmented pre-BCR signaling, consequently inducing a burst of proliferation of large pre-B cells. Phosphorylation of the pre-BCR key downstream effector, spleen tyrosine kinase (Syk) (Herzog et al., 2009), was significantly increased in ERAD-deficient pre-B cells (Fig. 5G), as was overall tyrosine phosphorylation (Fig. S6C). Furthermore, the total number of BrdU-positive cycling pro-/pre-B cells in BM was increased by more than 50% in Sel1LCD19 mice compared to Sel1Lf/f mice (Fig. 5H and Fig. S6D). Among them, only λ5+ large pre-B cells were highly proliferative in Sel1LCD19 mice, increasing more than 2.5 fold; whereas λ5− pro-B cell proliferation did not increase in Sel1LCD19 mice. Intriguingly, proliferation of Sel1L-deficient large pre-B cells was over 2 fold higher than WT pre-B cells when stimulated with IL-7 (Fig. S6E-F), suggesting that Sel1L effects on the pre-B compartment may not be restricted to pre-BCR signaling and may include IL-7R signaling. Thus, Sel1L deficiency in B cells leads to elevated and sustained pre-BCR signaling and persistent proliferation of large pre-B cells. In aggregate, these findings demonstrate that the Sel1L-Hrd1 ERAD is required to attenuate pre-BCR signaling by promoting its protein turnover.
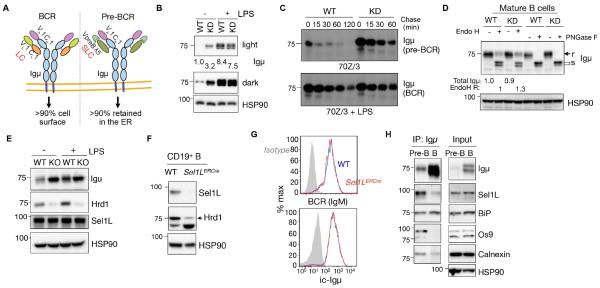
Sel1L-Hrd1 ERAD degrades the pre-BCR, not the BCR, complex
In the pre-BCR, SLC and Igμ assemble into a quaternary structure reminiscent of the BCR with VpreB and λ5 equivalent to the variable and constant regions (VL and CL), respectively, of IgL (Fig. 6A). We compared how Sel1L-Hrd1 ERAD targets pre-BCR for degradation by comparing the effect of ERAD on the pre-BCR vs. the BCR both in vitro and in vivo. Upon overnight LPS stimulation, 70z/3 pre-B cells differentiated into mature B cells expressing BCR with conventional κ chain replacing the SLC (Fig. S6G-H), as previously reported (Paige et al., 1978). This unique feature allowed us to determine how ERAD affects the trafficking and stability of the pre-BCR vs. BCR complexes containing the same Igμ heavy chain in the same cell. Unlike Igμ in the pre-BCR, neither the steady-state protein level or stability of Igμ in the BCR complex, nor its endoH sensitivity (i.e. ER exit), were affected by Sel1L-Hrd1 ERAD deficiency in mature B cells (Fig. 6B-D). This was not due to the lack of expression of ERAD components in mature B cells, as Sel1L and Hrd1 expression levels were comparable between pre- and mature B cells (Fig. 6E).
Figure 6. Sel1L-Hrd1 ERAD degrades the pre-BCR, but not the BCR complex.
(A) Diagram depicting the difference between BCR and pre-BCR complexes. (B) Immunoblot analysis of Igμ in WT and Sel1L KD cells (KD) treated with or without 20 μg/mL of lipopolysaccharide (LPS) for 18 h, with quantitation shown below. (C) Pulse-chase analysis showing Igμ decay in WT and KD 70Z/3 cells treated with or without 20 μg/mL of LPS for 18 h. (D) Immunoblot analysis of Igμ in WT and KD B cell lysates treated with EndoH or PNGase F. (r/s= EndoH resistant/sensitive). Quantitation of total Igμ and EndoH resistant (R) Igμ shown below the blot. (E) Immunoblot analysis of ERAD proteins in WT and Hrd1−/− 70z/3 pre-B cells treated with or without 20 μg/mL LPS for 18 h. (F) Western blot analysis of CD19+ splenic B cells isolated from WT and Sel1l-inducible knockout (Sel1LERCre) mice. (G) Histogram analysis of surface IgM and intracellular (ic) Igμ in splenic B cells of WT and Sel1LERCre mice (N=3 mice each). (H) Immunoblot analysis of immunoprecipitates of Igμ-agarose in pre-B 70z/3 and mature B cells. Representative of two independent experiments shown. See also Figure S7.
To further test this in vivo, we analyzed mature splenic B cells from Sel1l-inducible knockout (Sel1LERCre) mice expressing estrogen receptor-Cre recombinase fusion protein (ERCre) under the control of the actin promoter (Sun et al., 2014) (Fig. 6F). Consistent with our in vitro observations, both intracellular Igμ and surface BCR (IgM) levels were comparable between Sel1Lf/f and Sel1LERCre mature B cells (Fig. 6G). Moreover, plasma cell differentiation, cell size and total IgG levels in LPS/IL-4-treated mature primary B cells (Sel1Lf/f and Sel1LERCre) were not affected by Sel1L deficiency (Fig. S7A-C). Indeed, Igμ interacted with several components of the ERAD machinery, including Sel1L and OS9, to a greater extent in pre-B cells compared to mature B cells (Fig. 6H). Therefore, we concluded that the pre-BCR, but not the BCR, complex is an endogenous Sel1L-Hrd1 ERAD substrate.
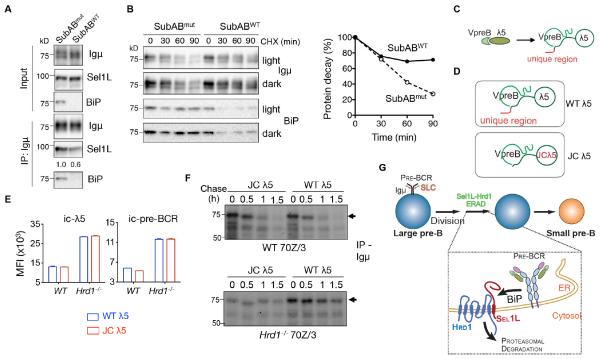
Pre-BCR ERAD requires BiP, but is independent of the unique region of λ5
As BiP is involved in the folding and intracellular trafficking of the pre-BCR complex (Minegishi et al., 1999b), we first tested whether BiP is required for pre-BCR ERAD. Depletion of BiP with the subtilase cytotoxin SubAB (Paton et al., 2006) significantly reduced the interaction between Igμ and Sel1L (Fig. 7A) and stabilized Igμ protein (Fig. 7B). Moreover, given that the pre-BCR and BCR complexes share the common Igμ heavy chain, we speculated that a key determinant in pre-BCR recognition by ERAD may lie in the binding of SLC to Igμ. We noted previous reports demonstrating that the unique region of λ5 (Fig. 7C), which is a non-Ig portion and does not pair with either VpreB or Igμ (Clark et al., 2014; Herzog et al., 2009), is known to retain pre-BCR complex in the ER (Fang et al., 2001; Minegishi et al., 1999b; Ohnishi and Melchers, 2003). To determine the role of the unique region of λ5 in pre-BCR degradation, we expressed wildtype (WT λ5) and mutant λ5 lacking the unique region (JC λ5, with only J and C parts of λ5) (Ohnishi and Melchers, 2003) in WT and Hrd1−/− pre-B cells (Fig. 7D). Surprisingly, JC λ5 expression had no effect on total steady state intracellular levels of the pre-BCR complex in both WT and Hrd1−/− pre-B cells compared to those expressing WT λ5 (Fig. 7E and Fig. S7D). Moreover, total pre-BCR complex levels were higher in Hrd1−/− pre-B cells than those of WT cells expressing JC λ5 (Fig. 7E), suggesting that the JC λ5-containing pre-BCR complex remains to be an ERAD substrate. Consistently, JC λ5 expression failed to affect Igμ protein half-life (Fig. 7F). On the other hand, JC λ5 increased surface pre-BCR in WT pre-B cells as previously reported (Fang et al., 2001; Minegishi et al., 1999b; Ohnishi and Melchers, 2003) (Fig. S7E). Hence, we concluded that BiP, but not the unique region of λ5, is required for the recognition of the pre-BCR complex as an ERAD substrate in pre-B cells.
Figure 7. Sel1L-Hrd1 ERAD-mediated pre-BCR degradation is BiP-dependent but λ5 unique region-independent.
(A) Immunoblot analysis of Igμ immunoprecipitates in 70Z/3 cells treated with 0.5 μg/ml SubABmut (SubAA272B) or SubABWT for 45 min. (B) Translation shut-off assay of Igμ in 70Z/3 cells treated by 50 μg/ml cycloheximide (CHX) together with 0.5 μg/ml SubABmut or SubABWT for the indicated times with quantitation shown on the right. (C) Schematic diagram depicting surrogate light chains (SLC). (D) Schematic diagram depicting SLC containing WT and mutant λ5 (JC λ5) lacking the unique region. (E) Quantitation of mean fluorescent intensity (MFI) of intracellular levels of λ5 (ic-λ5) and pre-BCR in WT and Hrd1−/− 70z/3 pre-B cells expressing WT or JC λ5. Values shown as mean ± s.e.m. (F) Pulse-chase analysis showing Igμ decay in WT and Hrd1−/− pre-B cells expressing WT or JC λ5. Representative of two independent experiments shown. (G) The model: The Sel1L-Hrd1 ERAD manages the checkpoint in B cell development by targeting the pre-BCR for cytosolic proteasomal degradation (via BiP), which attenuates pre-BCR signaling and allows further B cell differentiation. See also Figure S7.
DISCUSSION
This study shows that Sel1L-Hrd1 ERAD manages a key checkpoint in B cell development by selectively targeting the pre-BCR complex for proteasomal degradation. Indeed, Sel1L-Hrd1 ERAD complex is indispensable for the termination of pre-BCR signaling. B-cell-specific deficiency of the Sel1L-Hrd1 ERAD system blocks developmental progression at the large to small pre-B cell transition. Surprisingly, we show that the pre-BCR, not BCR, complex is an endogenous Sel1L-Hrd1 ERAD substrate in B cells and that Sel1L-Hrd1 ERAD has no obvious effect on plasma cell differentiation. Lastly, we show that ERAD-mediated pre-BCR degradation requires BiP, but independently of the unique region of λ5 known to be important for the ER retention of the pre-BCR complex.
Pre-BCR signaling marks a critical checkpoint in B cell development. The pre-BCR signal induces a burst of proliferation of large pre-B cells to ensure expansion of B cell precursors with a functional Igμ chain, a process known as “pre-BCR-dependent positive selection” (Hess et al., 2001; van Loo et al., 2007); and importantly, it downregulates its own expression via silencing the SLC genes, while upregulating recombination activating gene (Rag) expression and IgL rearrangement. Deficiency in VpreB (Mundt et al., 2001), λ5 (Kitamura et al., 1992), the transmembrane region of Igμ (Kitamura and Rajewsky, 1992), Igα (Pelanda et al., 2002) and Igβ (Gong and Nussenzweig, 1996) all prevents entry into the proliferating large pre-B cell stage and leads to a marked enrichment of pro-B cells in the BM, resulting in a profound decrease in mature peripheral B cells (Herzog et al., 2009). As Sel1L-deficiency leads to a B cell developmental block at a later stage, the molecular mechanism underlying the defect in the Sel1LCD19 mice is unlikely due to a loss-of-function of the pre-BCR. Instead, our data demonstrate that the Sel1LCD19 mice phenocopy those of mouse models with gain-of-function pre-BCR signaling. Persistent pre-BCR signaling, when the expression of λ5 is extended (Martin et al., 2007) or downstream negative effectors such as SLP-65 are inactivated (Jumaa et al., 1999), leads to elevated surface pre-BCR and continued cycling of large pre-B cells, preventing further differentiation. Similarly, Sel1L-deficiency in B cells results in intracellular accumulation and elevated surface pre-BCR and signaling. Consequently, Sel1LCD19 mice exhibit continued proliferation of large pre-B cells and decreased cellularity of small pre-B cells and mature B cells.
Expression and signaling from the pre-BCR complex are transient at the transition from the pro-B to the pre-B cell stage. Downregulation of pre-BCR signaling is a prerequisite for cell cycle exit and further differentiation to the resting small pre-B cell stage, such that IgL gene segment rearrangement can commence (Flemming et al., 2003; Kitamura et al., 1992; Melchers, 2005; Minegishi et al., 1998; Mundt et al., 2001; Parker et al., 2005; Shimizu et al., 2002; Wang et al., 2002). Self-limiting mechanisms include transcriptional silencing of SLC and dilution out of pre-BCRs due to large pre-B cell cycling. Our data show that the Sel1L-Hrd1 ERAD complex plays a key role in the termination of pre-BCR signaling in B cell development. Given the structural and composition similarity between the pre-BCR and the BCR complexes, our findings raise an intriguing question regarding subtrate maturation in the ER and selection by ERAD. The binding of SLC to Igμ, unlike that of LC to Igμ, is likely to be much less efficient, associated more intensively with BiP and/or may expose folding intermediates of pre-BCR to the ERAD machinery. This model is consistent with earlier reports demonstrating that the pre-BCR complex is prone to misfolding and requires the activity of ER folding machinery (Hendershot, 1990; Minegishi et al., 1999a), and that the majority of pre-BCR is retained in the ER, with less than 2% reaching to the cell surface compared to 90% of BCR reaching the cell surface in mature B cells (Brouns et al., 1996; Fagioli and Sitia, 2001; Fang et al., 2001; Hendershot et al., 1987; Pillai and Baltimore, 1987; Thorens et al., 1985). Moreover, the intrinsic ability of assembled pre-BCR complexes to exit the ER is limited by the unique region of λ5 (Fang et al., 2001; Minegishi et al., 1999b; Ohnishi and Melchers, 2003). We show in this study that, while the unique region of λ5 controls the ER exit of the pre-BCR complex, it is dispensable for pre-BCR ERAD.
Our study demonstrates that the function of ERAD could not be accurately predicted solely on the basis of the abundance of secreted protein production. We speculate that this selectivity may derive from a particularly complex requirement for the folding of specific substrates in a cell type-specific manner, (in this case, the pre-BCR), rather than from the extent and the consequence of the UPR. Indeed, there was only very mild, if any, ER stress and cell death associated with Sel1L/ERAD deficiency in differentiating B cells. Moreover, Sel1LCD19 mice exhibited phenotypes largely distinct from those with B cell-specific UPR deficiency (Hetz, 2012; Sha et al., 2011). For example, Xbp1 deficiency blocks plasma cell differentiation from B cells, whereas it has no effect on B cell development (Reimold et al., 2001; Todd et al., 2009). IRE1α deficiency causes a severe impairment of VDJ recombination of Ig genes and hence there is no expression of IgH and IgL chains of the BCR (Zhang et al., 2005). This defect is associated with reduced expression of RAG1/2 and terminal deoxynucleotidyl transferase. On the other hand, the PERK-eIF2α branch of the UPR is not required for B cell differentiation (Zhang et al., 2005). Therefore, Sel1L-Hrd1 ERAD manages B cell development in an UPR-independent manner.
Polypeptides that do not meet the quality-control standards are retained within the ER and are recognized and retrotranslocated by the ERAD machinery for proteasomal degradation in the cytosol. Mammals possess a number of ERAD complexes, with the Sel1L-Hrd1 being the most conserved and characterized biochemically. A limited number of endogenous substrates of Sel1L-Hrd1 ERAD complex have been identified in vitro, including ER luminal hedgehog, ATF6 and CD147 (Chen et al., 2011; Horimoto et al., 2013; Tyler et al., 2012). More recently, nuclear proteins such as NRF2, Blimp1 and PGC1β have been identified as cell type-specific Hrd1 substrates in vivo (Fujita et al., 2015; Wu et al., 2014; Yang et al., 2014). We recently reported IRE1α as an endogenous substrate for the Sel1L-Hrd1 ERAD in various cell types (Sun et al., 2015). Here we show that the pre-BCR complex is an endogenous ERAD substrate in developing B cells. Sel1L is required in this process likely as a key adaptor protein to stabilize Hrd1 protein as shown previously (Sun et al., 2014). Additionally, the physical interaction between the pre-BCR and Sel1L suggests that Sel1L may be intimately involved in pre-BCR turnover by recruiting the pre-BCR complex to the ligase Hrd1. As the pre-BCR is the first protein complex identified as an endogenous ERAD substrate to date, further investigation into its degradation mechanism may shed important mechanistic insights into substrate selection and protein degradation in vivo.
EXPERIMENTAL PROCEDURES
Mice
Sel1Lf/f mice on the C57B6/L background have been recently described (Sun et al., 2014) and crossed with CD19 promoter-driven Cre (CD19-Cre) mice from Jackson laboratory (B6.129P2(C)-Cd19tm1(cre)Cgn/J, #006785) to generate Sel1Lf/f;CD19-Cre+ (Sel1LCD19) and control Sel1Lf/f;CD19-Cre- (Sel1Lf/f) littermates at 1:1 ratio. Sel1Lf/+;CD19-Cre+ (Sel1LCD19/+) were generated to determine the effect of Sel1L heterozygosity and CD19-Cre effect on B cell development. Adult 6-8-week-old mice were used in most studies. In addition, Sel1LCD19 mice were crossed with Chop−/− mice (B6.129S(Cg)-Ddit3tm2.1Dron/J, JAX 005530) to generate Sel1Lflox/flox;CD19Cre+;Chop−/− double knockout (Sel1LCD19;Chop−/−) mice. Inducible Sel1L-deficient (Sel1LERCre) mice expressing estrogen-receptor-Cre (ERCre) fusion protein were previously reported (Sun et al., 2014). Sel1Lf/f;ERCre− and Sel1Lf/f;ERCre+ (Sel1LERCre) littermates were used in the study. In all the studies, the CD19-Cre allele was maintained as heterozygous. Mice were housed under specific pathogen-free conditions. Cohoused age- and gender-matched littermates were used in all in vivo experiments. All animals were sacrificed by cervical dislocation and tissues were immediately harvested. Frozen tissues were stored at −80 °C. All animal procedures have been approved by the Cornell IACUC (#2007-0051).
Flow Cytometry, Intracullar Staining, BrdU Labeling and Antibodies
Flow cytometric analysis of peripheral immune cells was performed as we previously described (Ji et al., 2014; Ji et al., 2012). To isolate bone marrow (BM), tibia and femurs were isolated from mice and BM was flushed using cold PBS supplemented with 5% FBS and 1% penicillin/streptomycin. The RBCs in the BM suspension was lysed and resuspended in cold PBS with 5% FBS and 1% penicillin/streptomycin. Cell suspension were stained with either 1:100 or 1:200 fluorochrome- or biotin conjugated antibodies against CD4 (GK1.5), CD8 (YTS169), Gr-1 (RB6-8C5), CD11b (M1/70), CD45 (30-F11), CD19 (6D5), CD138 (281-2), CD2 (RM2-5), IgM (RMM-1), IgD (11-26c.2a), CD5 (53-7.3), λ5 (C-16), CD43 (1B11), VpreB (R3), Pre-BCR (SL156), avidin-PerCP and isotype control antibodies (BioLegend or BD Biosciences). Annexin V, propidium iodide and 7-AAD were purchased from BioLegend and used per manufacturer’s protocol. Secondary antibodies such as anti-IgG FITIC and anti-Igμ PE were from Jackson Immunoresearch. For intracellular staining, cells were fixed and permeabilized by BD Cytofix/Cytoperm Fixation/Permeabilization Kit per manufacturer’s protocol. For BrdU labeling, mice were injected intraperitoneally with BrdU (Sigma, 0.6 mg/10 g of body weight) at 2 h prior to sacrifice. BM cells were collected, labeled with cell surface antibodies, and then fixed in cold 70% ethanol at −20 °C overnight. The samples were washed and diges ted with DNase I (Roche, 50 U/ml in 4.2 mM MgCl2 and 150 mM NaCl) at room temperature for 30 min. The rest of the procedures were performed as the regular flow cytometric analysis using BrdU-FITC (PRB-1, BD Biosciences). Samples were analyzed using BD LSR cell analyzer at the Flow Cytometry Core Facility at Cornell University. Data were analyzed using the CellQuest software (BD Biosciences) and Flowjo (Flowjo.com).
Western Blot and Antibodies
Preparation of whole cell lysates, endoH treatment and Western blots were performed as previously described (Sha et al., 2014; Sun et al., 2015). Antibodies used in this study were: Igμ-peroxidase (goat, 1:5,000) from Sigma; HSP90 (rabbit, 1:6,000), BiP (goat, 1:1,000) and α-Tubulin (mouse, 1:2000) from Santa Cruz; Sel1L (rabbit, 1:2,000) and OS9 (rabbit, 1:10,000) from Abcam; phospho-Syk and Syk (rabbit, 1:1000) from Cell Signaling; and Calnexin (rabbit, 1:8,000) from Assay Design. Hrd1-specific antibody (rabbit, 1:200) from Dr. Richard Wojcikiewicz at SUNY Upstate Medical University (Pearce et al., 2007); Antibodies for Bag6 (rabbit, 1:10,000) and H2A (rabbit, 1:10,000) were a kind gift from Dr. Yihong Ye (NIDDK). Band density was quantitated using the Image Lab software on the ChemiDOC XRS+ system (Bio-Rad). Protein levels were normalized to HSP90 and are presented as mean ± SEM unless otherwise specified.
Pulse Labeling
4 × 106 70z/3 cells were resuspended in cysteine and methionine-free medium (Invitrogen, 21013024) for 15 min and metabolically labeled with 50 μCi [35S]-cysteine and methionine (EasyTag, PerkinElmer) for 10 min. The labeling was stopped by the addition of 5 volumes of cold chase medium (pre-B cell culture medium supplemented with 5 mM L-methionine (Sigma, M5308) and 5 mM L-cysteine (Sigma, C7352)). Cells were collected at the indicated times of chase and lysed in 0.5 % NP40 lysis buffer with NEM (20 mM). Lysates were subjected to immunoprecipitation with Igμ agarose (Sigma). Immunoprecipitates were eluded and separated on a 7% SDS-PAGE gel. Gels were incubated with the neutralizing buffer (30% (v/v) methanol in PBS) for 10 min and then subjected to autoradiography with X-film (Kodak).
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons between groups were made by unpaired two-tailed Student’s t test, except by one-way ANOVA with Tukey post-test in Figure 6. All experiments were repeated at least twice or performed with independent samples.
Supplementary Material
HIGHLIGHTS.
ERAD deficiency blocks the transition from large pre-B cells to small pre-B cells.
ERAD manages a B cell developmental checkpoint by attenuating pre-BCR signaling.
The pre-BCR complex is an endogenous Sel1L-Hrd1 ERAD substrate in B cells.
Pre-BCR degradation by the Sel1L-Hrd1 complex requires the ER chaperone BiP.
ACKNOWLEDGEMENTS
We thank Drs. Yihong Ye, Linda Hendershot, James C. Paton and Richard Wojcikiewicz for reagents; Drs. Yihong Ye, Cynthia A. Leifer and Margaret S. Bynoe for critical comments on our manuscript, and other members of the Qi lab for comments and technical assistance. This work was supported by NIH R03AI074801-01 and support from the Dean of the College of Medicine, State University of New York-DMCNIH (C.A.R.); R01GM113188, R01DK105393, Juvenile Diabetes Research Foundation 1-SRA-2014-251-Q-R, and American Diabetes Association (ADA) 1-12-CD-04 (to L.Q.). L.Q. is the recipient of the Junior Faculty and Career Development Awards from ADA.
Footnotes
AUTHOR CONTRIBUTION
Y.J. performed most of in vivo experiments with the help of H.K.; Y.J and H.K. designed and performed most in vitro experiments; L.Y. generated the Sel1LCD19 mice and performed initial characterization; H.S. helped with some in vitro experiments; C.A.R. and Q. L. provided key reagents; L.Q. and Y.J. conceived the project and designed the experiments; L.Q. wrote the manuscript, H.K. and Y.J. wrote the figure legends and methods, everybody edited and approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brouns GS, de Vries E, Neefjes JJ, Borst J. Assembled pre-B cell receptor complexes are retained in the endoplasmic reticulum by a mechanism that is not selective for the pseudo-light chain. J Biol Chem. 1996;271:19272–19278. doi: 10.1074/jbc.271.32.19272. [DOI] [PubMed] [Google Scholar]
- Chen X, Tukachinsky H, Huang CH, Jao C, Chu YR, Tang HY, Mueller B, Schulman S, Rapoport TA, Salic A. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J Cell Biol. 2011;192:825–838. doi: 10.1083/jcb.201008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol. 2014;14:69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagioli C, Sitia R. Glycoprotein quality control in the endoplasmic reticulum. Mannose trimming by endoplasmic reticulum mannosidase I times the proteasomal degradation of unassembled immunoglobulin subunits. J Biol Chem. 2001;276:12885–12892. doi: 10.1074/jbc.M009603200. [DOI] [PubMed] [Google Scholar]
- Fang T, Smith BP, Roman CA. Conventional and surrogate light chains differentially regulate Ig mu and Dmu heavy chain maturation and surface expression. J Immunol. 2001;167:3846–3857. doi: 10.4049/jimmunol.167.7.3846. [DOI] [PubMed] [Google Scholar]
- Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol. 2003;4:38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- Francisco AB, Singh R, Li S, Vani AK, Yang L, Munroe RJ, Diaferia G, Cardano M, Biunno I, Qi L, et al. Deficiency of suppressor enhancer lin12 1 like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J Biol Chem. 2010;285:13694–13703. doi: 10.1074/jbc.M109.085340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Yagishita N, Aratani S, Saito-Fujita T, Morota S, Yamano Y, Hansson MJ, Inazu M, Kokuba H, Sudo K, et al. The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC-1beta. EMBO J. 2015;34:1042–1055. doi: 10.15252/embj.201489897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92:537–576. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L, Bole D, Kearney JF. The role of immunoglobulin heavy chain binding protein in immunoglobulin transport. Immunology today. 1987;8:111–114. doi: 10.1016/0167-5699(87)90861-9. [DOI] [PubMed] [Google Scholar]
- Hendershot LM. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J Cell Biol. 1990;111:829–837. doi: 10.1083/jcb.111.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- Hess J, Werner A, Wirth T, Melchers F, Jack HM, Winkler TH. Induction of pre-B cell proliferation after de novo synthesis of the pre-B cell receptor. Proc Natl Acad Sci U S A. 2001;98:1745–1750. doi: 10.1073/pnas.041492098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Horimoto S, Ninagawa S, Okada T, Koba H, Sugimoto T, Kamiya Y, Kato K, Takeda S, Mori K. The Unfolded Protein Response Transducer ATF6 Represents a Novel Transmembrane-type Endoplasmic Reticulum-associated Degradation Substrate Requiring Both Mannose Trimming and SEL1L Protein. J Biol Chem. 2013;288:31517–31527. doi: 10.1074/jbc.M113.476010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Sun S, Goodrich JK, Kim H, Poole AC, Duhamel GE, Ley RE, Qi L. Diet-Induced Alterations in Gut Microflora Contribute to Lethal Pulmonary Damage in TLR2/TLR4-Deficient Mice. Cell reports. 2014;8:137–149. doi: 10.1016/j.celrep.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. Short Term High Fat Diet Challenge Promotes Alternative Macrophage Polarization in Adipose Tissue via Natural Killer T Cells and Interleukin-4. J Biol Chem. 2012;287:24378–24386. doi: 10.1074/jbc.M112.371807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumaa H, Wollscheid B, Mitterer M, Wienands J, Reth M, Nielsen PJ. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity. 1999;11:547–554. doi: 10.1016/s1074-7613(00)80130-2. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Kudo A, Thalmann P, Sakaguchi N, Davidson WF, Pierce JH, Kearney JF, Reth M, Rolink A, Melchers F. The expression of the mouse VpreB/lambda 5 locus in transformed cell lines and tumors of the B lineage differentiation pathway. Int Immunol. 1992;4:831–840. doi: 10.1093/intimm/4.8.831. [DOI] [PubMed] [Google Scholar]
- Lassoued K, Illges H, Benlagha K, Cooper MD. Fate of surrogate light chains in B lineage cells. J Exp Med. 1996;183:421–429. doi: 10.1084/jem.183.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Brewer JW, Hellman R, Hendershot LM. BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol Biol Cell. 1999;10:2209–2219. doi: 10.1091/mbc.10.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Lu L, Cascalho M, Wu GE. Maintenance of surrogate light chain expression induces developmental delay in early B cell compartment. J Immunol. 2007;179:4996–5005. doi: 10.4049/jimmunol.179.8.4996. [DOI] [PubMed] [Google Scholar]
- Melchers F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat Rev Immunol. 2005;5:578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Coustan-Smith E, Rapalus L, Ersoy F, Campana D, Conley ME. Mutations in Igalpha (CD79a) result in a complete block in B-cell development. J Clin Invest. 1999a;104:1115–1121. doi: 10.1172/JCI7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Coustan-Smith E, Wang YH, Cooper MD, Campana D, Conley ME. Mutations in the human lambda5/14.1 gene result in B cell deficiency and agammaglobulinemia. J Exp Med. 1998;187:71–77. doi: 10.1084/jem.187.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Hendershot LM, Conley ME. Novel mechanisms control the folding and assembly of lambda5/14.1 and VpreB to produce an intact surrogate light chain. Proc Natl Acad Sci U S A. 1999b;96:3041–3046. doi: 10.1073/pnas.96.6.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt C, Licence S, Shimizu T, Melchers F, Martensson IL. Loss of precursor B cell expansion but not allelic exclusion in VpreB1/VpreB2 double-deficient mice. J Exp Med. 2001;193:435–445. doi: 10.1084/jem.193.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K, Melchers F. The nonimmunoglobulin portion of lambda5 mediates cell-autonomous pre-B cell receptor signaling. Nat Immunol. 2003;4:849–856. doi: 10.1038/ni959. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Kopito RR, Christianson JC. The Mammalian Endoplasmic Reticulum-Associated Degradation System. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige CJ, Kincade PW, Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978;121:641–647. [PubMed] [Google Scholar]
- Parker MJ, Licence S, Erlandsson L, Galler GR, Chakalova L, Osborne CS, Morgan G, Fraser P, Jumaa H, Winkler TH, et al. The pre-B-cell receptor induces silencing of VpreB and lambda5 transcription. EMBO J. 2005;24:3895–3905. doi: 10.1038/sj.emboj.7600850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- Pearce MM, Wang Y, Kelley GG, Wojcikiewicz RJ. SPFH2 mediates the endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors and other substrates in mammalian cells. J Biol Chem. 2007;282:20104–20115. doi: 10.1074/jbc.M701862200. [DOI] [PubMed] [Google Scholar]
- Pelanda R, Braun U, Hobeika E, Nussenzweig MC, Reth M. B cell progenitors are arrested in maturation but have intact VDJ recombination in the absence of Ig-alpha and Ig-beta. J Immunol. 2002;169:865–872. doi: 10.4049/jimmunol.169.2.865. [DOI] [PubMed] [Google Scholar]
- Pillai S, Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987;329:172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha H, He Y, Yang L, Qi L. Stressed out about obesity: IRE1alpha-XBP1 in metabolic disorders. Trends Endocrinol Metab. 2011;22:374–381. doi: 10.1016/j.tem.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha H, Sun S, Francisco AB, Ehrhardt N, Xue Z, Liu L, Lawrence P, Mattijssen F, Guber RD, Panhwar MS, et al. The ER-associated degradation adaptor protein Sel1L regulates LPL secretion and lipid metabolism. Cell Metab. 2014;20:458–470. doi: 10.1016/j.cmet.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Mundt C, Licence S, Melchers F, Martensson IL. VpreB1/VpreB2/lambda 5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. J Immunol. 2002;168:6286–6293. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- Sun S, Louri R, Cohen SB, Ji Y, Goodrich JK, Poole AC, Ley RE, Denkers EY, Mcguckin MA, Long Q, et al. Epithelial Sel1L is required for the maintenance of intestinal homeostasis. Mol Biol Cell. 2016;27:483–490. doi: 10.1091/mbc.E15-10-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Shi G, Han X, Francisco AB, Ji Y, Mendonca N, Liu X, Locasale JW, Simpson KW, Duhamel GE, et al. Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proc Natl Acad Sci U S A. 2014;111:E582–591. doi: 10.1073/pnas.1318114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Shi G, Sha H, Ji Y, Han X, Shu X, Ma H, Inoue T, Gao B, Kim H, et al. IRE1a is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nat Cell Biol. 2015;17:1546–1555. doi: 10.1038/ncb3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Schulz MF, Vassalli P. Bone marrow pre-B lymphocytes synthesize immunoglobulin mu chains of membrane type with different properties and intracellular pathways. EMBO J. 1985;4:361–368. doi: 10.1002/j.1460-2075.1985.tb03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, McHeyzer-Williams MG, Glimcher LH. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med. 2009;206:2151–2159. doi: 10.1084/jem.20090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RE, Pearce MM, Shaler TA, Olzmann JA, Greenblatt EJ, Kopito RR. Unassembled CD147 is an endogenous endoplasmic reticulum-associated degradation substrate. Mol Biol Cell. 2012;23:4668–4678. doi: 10.1091/mbc.E12-06-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo PF, Dingjan GM, Maas A, Hendriks RW. Surrogate-light-chain silencing is not critical for the limitation of pre-B cell expansion but is for the termination of constitutive signaling. Immunity. 2007;27:468–480. doi: 10.1016/j.immuni.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Wang YH, Stephan RP, Scheffold A, Kunkel D, Karasuyama H, Radbruch A, Cooper MD. Differential surrogate light chain expression governs B-cell differentiation. Blood. 2002;99:2459–2467. doi: 10.1182/blood.v99.7.2459. [DOI] [PubMed] [Google Scholar]
- Wu T, Zhao F, Gao B, Tan C, Yagishita N, Nakajima T, Wong PK, Chapman E, Fang D, Zhang DD. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qiu Q, Gao B, Kong S, Lin Z, Fang D. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J Exp Med. 2014;211:2467–2479. doi: 10.1084/jem.20140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LJ, Ord DC, Hughes AL, Tedder TF. Structure and domain organization of the CD19 antigen of human, mouse, and guinea pig B lymphocytes. Conservation of the extensive cytoplasmic domain. J Immunol. 1991;147:1424–1432. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.