SUMMARY
Binding of Ca2+-loaded calmodulin (CaM) activates eukaryotic elongation factor 2 kinase (eEF-2K) that phosphorylates eEF-2, its only known cellular target, leading to a decrease in global protein synthesis. Here, using an eEF-2K-derived peptide (eEF-2KCBD) that encodes the region necessary for its CaM-mediated activation, we provide a structural basis for their interaction. The striking feature of this association is the absence of Ca2+ from the CaM C-lobe sites, even under high Ca2+ conditions. eEF-2KCBD engages CaM largely through the C-lobe of the latter in an anti-parallel 1-5-8 hydrophobic mode reinforced by a pair of unique electrostatic contacts. Sparse interactions of eEF-2KCBD with the CaM N-lobe results in persisting inter-lobe mobility. A conserved eEF-2K residue (W85) anchors it to CaM by inserting into a deep hydrophobic cavity within the CaM C-lobe. Mutation of this residue (W85S) substantially weakens interactions between full-length eEF-2K and CaM in vitro and reduces eEF-2 phosphorylation in cells.
IN BRIEF
Using solution NMR methodology, Lee et. al. establish the structural basis for the recognition of calmodulin by eukaryotic elongation factor 2 kinase. The interaction largely occurs through the calmodulin C-lobe that lacks bound calcium ions even under high calcium conditions.
INTRODUCTION
Eukaryotic elongation factor-2 kinase (eEF-2K), a member of the atypical α-kinase family (Ryazanov, 1987; Ryazanov, 2002; Ryazanov et al., 1997), inhibits eukaryotic elongation factor 2 (eEF-2), its only known physiological substrate, by phosphorylating it on a specific threonine (T56) (Nairn and Palfrey, 1987; Ryazanov and Davydova, 1989; Ryazanov et al., 1988a). Phosphorylated eEF-2 has impaired ability to bind the ribosome resulting in an overall reduction in the rate of protein synthesis (Carlberg et al., 1990; Dumont-Miscopein et al., 1994; Ryazanov et al., 1988b). The regulation of protein synthesis by eEF-2K is critical in a variety of cellular processes, ranging from the control of cell cycle progression (Smith and Proud, 2008) to driving the synthesis of proteins necessary for synaptic plasticity (Gildish et al., 2012; Park et al., 2008; Sutton et al., 2007; Verpelli et al., 2010). Aberrant eEF-2K activity is thought to contribute to several disease states, including a variety of cancers (Liu et al., 2013; Meric-Bernstam et al., 2012; Tekedereli et al., 2012), Alzheimer’s disease (Li et al., 2005; Ma et al., 2014), depression (Kavalali and Monteggia, 2012; Monteggia et al., 2013), and heart disease (Chan et al., 2004; Horman et al., 2002; Usui et al., 2013).
Utilizing a variety of steady and pre-steady state kinetics measurements combined with in vitro and cell-based assays, Tavares et. al demonstrated that eEF-2K is activated through a unique two-step process initiated by the binding of Ca2+-loaded calmodulin (CaM) (Tavares et al., 2014). This mode of activation does not appear to occur through a “release of inhibition” mechanism (Soderling and Stull, 2001; Swulius and Waxham, 2008) and sets eEF-2K apart from other CaM-dependent kinases. In the first step, the inactive kinase undergoes a conformational transition within its catalytic domain induced by Ca2+-CaM binding. This leads to a more than a 103-fold enhancement in its ability to auto-phosphorylate on a key threonine (T348, eEF-2K residues are italicized throughout the text to distinguish them from CaM residues) in the regulatory (R-loop) immediately C-terminal to the kinase domain (Figure 1). The engagement of a basic allosteric binding pocket within the kinase domain by phosphorylated T348 enables a second conformational transition of the R-loop to generate a state with the highest activity towards substrate. Thus, the fully active conformation of eEF-2K is attained through two sequential conformational transitions initiated by Ca2+-CaM binding, which represents a critical step in the eEF-2K activation pathway.
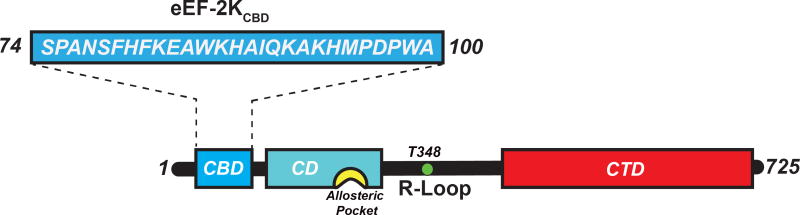
Figure 1. Schematic representation of the domain organization of eEF-2K.
The catalytic domain (CD, cyan), the basic allosteric pocket (yellow) and the critical auto-phosphorylation site T348 (green) on the regulatory loop (R-loop) are shown; the C-terminal domain (CTD, red) contains multiple Sel1 like repeats (Mittl and Schneider-Brachert, 2007). The N-terminal CaM-binding domain (CBD, 74-100, blue) is encoded by the eEF-2KCBD peptide used here.
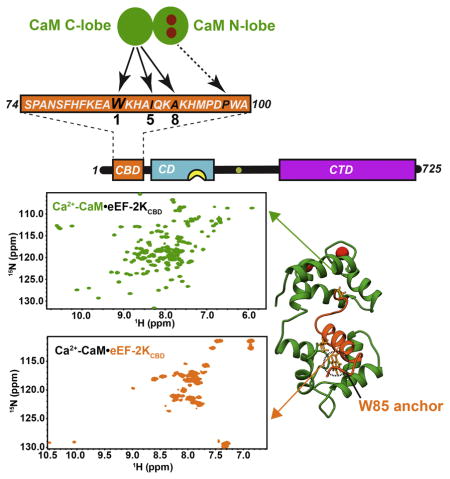
The absence of an N-terminal segment (75–100) on eEF-2K prevents its Ca2+-CaM-dependent activation, suggesting that a CaM-binding site is localized within this segment (Diggle et al., 1999). On the basis of sequence analysis together with biochemical studies, eEF-2K has been proposed to engage CaM in a 1-5-8-14 mode (numbers indicate spacing of critical hydrophobic anchors) (Hoeflich and Ikura, 2002) within the fragment encompassing residues F79 through M95 (Diggle et al., 1999). However, this sequence in eEF-2K contains a histidine rather than a canonical hydrophobic residue at the 14th position, suggesting that the overall binding mode deviates from this predicted topology. Thus, as a necessary step towards determining the structural and mechanistic basis of the regulation of eEF-2K by Ca2+-CaM, we determined the structure of CaM in complex with a 27-residue peptide (eEF-2KCBD) encompassing residues S74-A100 of eEF-2K.
Our results indicate that eEF-2KCBD interacts with CaM largely through the C-lobe of the latter, in a fashion reminiscent of a 1-5-8 mode, with several unique features, most importantly, the absence of Ca2+ ions in the C-lobe metal binding sites even at high Ca2+ concentration. NMR data suggest little difference in the conformation of the C-lobe between the apo and Ca2+-loaded states of CaM when peptide-bound. Interactions between eEF-2KCBD and the N-lobe of CaM are enhanced with the occupancy of the N-lobe Ca2+-sites, but are absent when Ca2+ is not present. These interactions, though weak in isolation, combined with those involving the C-lobe lead to a ~240-fold increase in affinity. Based on the structure of the Ca2+-CaM•eEF-2KCBD complex, disruption of the key hydrophobic anchoring interaction involving W85 on eEF-2KCBD by substitution with a polar residue (W85S) leads to a ~80-fold reduction in the affinity of full-length eEF-2K towards CaM and in its ability to phosphorylate eEF-2 in cells.
RESULTS
The Ca2+-loaded State of CaM Forms a Tight Complex with eEF-2KCBD
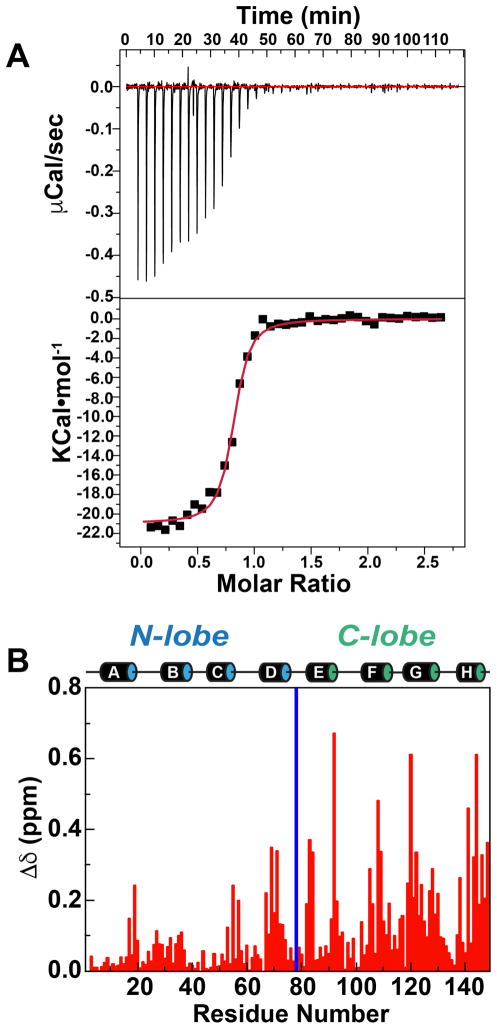
Isothermal titration calorimetry (ITC) measurements suggest that eEF-2KCBD binds Ca2+-loaded CaM with high affinity (KD=153±15 nM; average over measurements in triplicate) under conditions used in our NMR experiments (Figure 2A). Large chemical shift perturbations (CSPs) are seen upon comparing the 1H, 15N HSQC spectrum of Ca2+-CaM with that of the 1:1 Ca2+-CaM•eEF-2KCBD complex (Figure S1). The C-lobe displays larger CSPs (average over C-lobe residues, Δδ=0.17±0.15 ppm) in absolute terms compared to the N-lobe (average over N-lobe residues, Δδ=0.07±0.07 ppm) (Figure 2B). This suggests that in the Ca2+-loaded state of CaM, eEF-2KCBD engages both lobes with the C-lobe playing a more significant role, perhaps in anchoring eEF-2KCBD to CaM, while the interactions with the N-lobe are less robust. Surprisingly, addition of an excess of the eEF-2KCBD peptide leads to further perturbations largely in the N-lobe of CaM, while the C-lobe resonances display minimal further changes (Figure S2). This may indicate that in the 1:1 complex the N-lobe of CaM is still available to participate in further weak, perhaps non-specific interactions with excess peptide.
Figure 2. Interaction between eEF-2KCBD with CaM in the presence of Ca2+.
(A) ITC trace representative of three repeats is shown. For the trace shown, KD=137.2±18.8 nM, ΔH=−16.6 KCal•mol−1, ΔS=−22.5 Cal•mol−1•K−1. (B) Backbone amide chemical shift perturbations induced on Ca2+-CaM upon formation of the 1:1 Ca2+-CaM•eEF-2KCBD complex. The locations of the eight helices (A, B, C, D on the N-lobe and E, F, G, H on the C-lobe) are shown. The blue bar represents residues that are unassigned in the Ca2+-CaM•eEF-2KCBD complex.
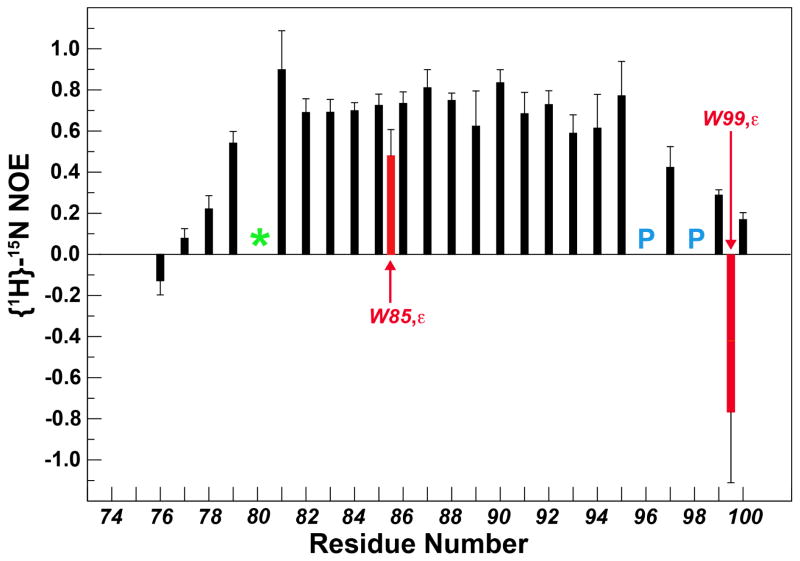
The 1H, 15N HSQC spectrum of eEF-2KCBD (Figure S3) in the context of the 1:1 Ca2+-CaM•eEF-2KCBD complex is characterized by a higher degree of spectral dispersion compared with that of free eEF-2KCBD indicating the formation of a well-defined structure upon complex formation. The F81-M95 segment of eEF-2KCBD displays {1H}-15N steady state NOEs that are modest to large (0.58–0.89, Figure 3) when bound to CaM suggesting that this segment is folded in the complex. A particularly illustrative observation is the large chemical shift change seen for the indole NH resonance of W85 upon binding. This residue has been suggested to be a key hydrophobic anchor for the interaction between CaM and eEF-2K (Diggle et al., 1999). Notably, the W85 indole NH also displays a significant (for a sidechain position) steady-state NOE value (0.48±0.13) suggesting that it is ordered in the complex, possibly due to a close interaction with CaM. This contrasts the indole NH resonance of W99 that is disordered (NOE=−0.77±0.17) in the bound state.
Figure 3. Steady-state {1H}-15N NOE for eEF-2KCBD in the Ca2+-CaM•eEF-2KCBD complex.
The NOEs for the sidechain indole NH positions for W85 and W99 are shown in red. The backbone amide resonance of H80 was not detected in the spectra and is depicted by the green ‘*’. The locations of the two prolines are shown in blue.
The Full Complement of Ca2+ ions is not Required for the CaM/eEF-2KCBD Interaction
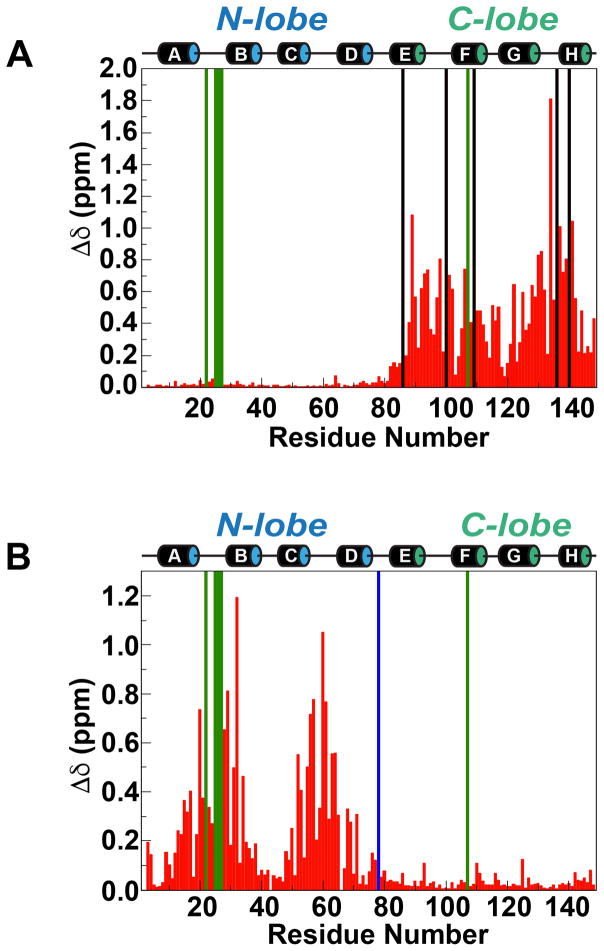
CSPs measured in Ca2+-free CaM in the presence of eEF-2KCBD indicate an interaction. In contrast to the Ca2+-loaded CaM discussed above, these CSPs are restricted to the C-lobe of CaM (Figure 4A, Δδ=0.45±0.32 ppm) with minimal changes being seen in the N-lobe (Δδ=0.02±0.01 ppm). As in the case of the interaction in the presence of Ca2+, the binding is in the slow exchange regime on the NMR chemical shift timescale. Fitting the intensities of the bound-state peaks in the presence of increasing amounts of eEF-2KCBD to Equation 2 yields a KD value (averaged over 20 residues) of 36±19 μM suggesting a ~240-fold weaker interaction in the absence of Ca2+. Given the affinity, the slow-exchange behavior is somewhat surprising though not unprecedented (Zuiderweg, 2002).
Figure 4. Structural differences in CaM in the presence of eEF-2KCBD under low and high Ca2+ conditions.
(A) Chemical shift changes induced on apo-CaM in the presence of a 3-fold molar ratio of eEF-2KCBD. The black and green bars represent residues that are unassigned in the apo-CaM•eEF-2KCBD complex and apo-CaM alone, respectively. (B) Differences in chemical shifts between the apo-CaM•eEF-2KCBD and Ca2+-CaM•eEF-2KCBD complexes. The blue and green bars represent residues that are unassigned in the Ca2+-CaM•eEF-2KCBD complex and the apo-CaM•eEF-2KCBD complex, respectively.
Titration of Ca2+ into the apo-CaM•eEF-2KCBD complex (~79% saturated) has little effect on the C-lobe resonances. In contrast, several N-lobe resonances disappear and then reappear at higher concentrations of Ca2+ at positions corresponding to those in the 1:1 Ca2+-CaM•eEF-2KCBD complex. Comparison of the spectra of the Ca2+-CaM•eEF-2KCBD and apo-CaM•eEF-2KCBD (1:3 mixture of apo-CaM and eEF-2KCBD) complexes suggests that the largest differences between the two species are in the N-lobe (Figure 4B, Δδ=0.27±0.26 ppm) with minimal differences in the C-lobe (Δδ=0.03±0.03ppm). The intensities of peaks corresponding to the Ca2+-loaded state of the complex for resonances of the Ca2+-binding loops exhibit contrasting behavior for the N- and C-lobes during the Ca2+ titration. While the intensities of C-lobe peaks are stable over the Ca2+-titration course, those of the N-lobe increase in intensity (as the Ca2+-loaded state becomes populated) and saturate at around 2-equivalents of Ca2+ (Figure S4). Analyses of chemical shift signatures known to be diagnostic for the presence/absence of Ca2+ in EF-hand containing proteins are also very revealing. The amide 1H of the conserved glycine at the 6th position (G6) of the Ca2+-binding loop is known to exhibit chemical shift values larger than 10.0 ppm in the Ca2+-bound state (Akerfeldt et al., 1996). In the present case, the 1H chemical shifts for C-lobe residues, G98 and G134, are 10.60 (10.61) and 10.27 (10.26) ppm in the apo-CaM•eEF-2KCBD complex (Ca2+-CaM•eEF-2KCBD complex), respectively. This suggests that the C-lobe EF-hands locally attain a Ca2+-bound like conformation in the presence of eEF-2KCBD even in the absence of Ca2+. In the N-lobe, G25 is broadened out (10.58 ppm in the Ca2+-CaM•eEF-2KCBD complex), but G61 has observable amide 1H chemical shifts of 9.87 ppm and 10.54 ppm in the apo-CaM•eEF-2KCBD and Ca2+-CaM•eEF-2KCBD complexes, respectively, showing the characteristic Ca2+-induced downfield shift. Similarly, the amide 15N chemical shifts of the hydrophobic residues at the 8th position (I27, I63 for the N-lobe; I100, V136 for the C-lobe) of the Ca2+-binding loops are known to undergo significant downfield shifts in the presence of Ca2+ (Biekofsky et al., 1998). I63 (I27 is broadened out) shows a 4.83 ppm downfield shift in the Ca2+-CaM•eEF-2KCBD complex compared to the apo-CaM•eEF-2KCBD complex, while the resonance positions for I100 and V136 are virtually identical presence and absence of Ca2+. Finally, it has been noted that the orientation of a large aromatic group at the -4 position of the Ca2+-binding loop leads to large upfield shifts of the 13Cβ resonance of the first Ca2+-loop aspartate (D20 for the N-lobe and D93 for the C-lobe) in the presence of Ca2+ (Atreya and Chary, 2002). We note that while D20 13Cβ resonance is shifted upfield by 1.55 ppm in the Ca2+-CaM•eEF-2KCBD state compared with the apo-CaM•eEF-2KCBD state, the D93 resonance is virtually identical in the two states. We also note that D58 (at the 3rd position of the second N-lobe EF-hand) is also upfield shifted by 1.45 ppm in the Ca2+-CaM•eEF-2KCBD complex.
Based on this multitude of data it is apparent that the N-lobe Ca2+ sites are occupied in the Ca2+-CaM•eEF-2KCBD complex. However, the minimal CSPs for the backbone and for diagnostic sidechain positions for C-lobe resonances in the presence of Ca2+ suggest that the C-lobe sites are not metal bound in the Ca2+-CaM•eEF-2KCBD complex. A scenario where eEF-2KCBD drives the conformation of the C-lobe around the Ca2+-sites to bound-like conformations even in the absence of metal (this is certainly true around G6, as discussed above) leading to minimal further perturbations upon metal binding, could be imagined. However, the presence of Ca2+ would be expected to produce local chemical shift perturbations, at the very least. These are not seen in the present case. Therefore, in the structure calculations described below, we assume that only the N-lobe Ca2+-sites are occupied in the Ca2+-CaM•eEF-2KCBD complex.
Solution Structure of the Ca2+-CaM•eEF-2KCBD Complex
In order to obtain further insight into the mode of interaction between CaM and eEF-2KCBD in the Ca2+-loaded state of the former, we solved the structure of the Ca2+-CaM•eEF-2KCBD complex using solution NMR techniques (see Table 1 for statistics). It is evident from the structural ensemble (Figure 5) that there is considerable dynamics between the N- and C-lobes of CaM when bound to eEF-2KCBD. This presence of this mobility is confirmed by a lack of inter-lobe NOE cross-peaks, by the fact that the linker residues T79 (0.33±0.03) and D80 (0.42±0.03) show low steady-state {1H}-15N NOE values (not shown), and by an analysis of residual dipolar coupling (RDC) data (see below).
Table 1.
Refinement and structure statistics for the Ca2+-CaM•eEF-2KCBD complex
| Pairwise Cartesian RMS deviation (Å) | |||
|---|---|---|---|
| N-lobe | C-lobe | C-lobe/eEF-2KCBD | |
| Global backbone heavy atoms | 0.89 ± 0.14 | 0.90 ± 0.14 | |
| Global heavy atomsa | 1.66 ± 0.14 | 1.93 ± 0.14 | |
| Ordered backbone heavy atoms | 0.80 ± 0.14 | 0.78 ± 0.14 | 0.90 ± 0.16 |
| Ordered heavy atomsb | 1.72 ± 0.16 | 1.90 ± 0.16 | 1.99 ± 0.18 |
| Restraint information | |||
| NOE derived distance restraints | |||
| Intra-residue | 1079 | ||
| Inter-residue | 1015 | ||
| Sequential | 447 | ||
| Medium | 382 | ||
| Long | 286 | ||
| Inter-molecular | 140 | ||
| Distance restraints for Ca2+c | 12 | ||
| Dihedral angle restraints | 293 | ||
| Energies (KCal•mol−1) | |||
| Total | −8209.4 ± 175.1 | ||
| NOE | 52.4 ± 7.4 | ||
| Cdih | 2.1 ± 0.6 | ||
| Ramachandran statistics (%)d | |||
| Most favored regions | 90.0 ± 1.4 | ||
| Additionally allowed regions | 8.7 ± 1.7 | ||
| Generously allowed regions | 0.5 ± 0.6 | ||
| Disallowed regions | 0.7 ± 0.9 | ||
| Restraint violations | |||
| Distance restraints (> 0.5 Å) | 0 | ||
| Dihedral restraints (> 5°) | 0 | ||
| Average RMS deviation from experimental restraints | |||
| Distance restraints (Å) | 0.0188 ± 0.0014 | ||
| Dihedral angle restraints (°) | 0.3396 ± 0.0521 | ||
| Average RMS deviation from idealized geometries | |||
| Distance restraints (Å) | 0.0141 ± 5.10×10−4 | ||
| Dihedral angle restraints (°) | 1.297 ± 0.036 | ||
| Average RMS Z-scores for deviation from current reliable structurese | |||
| Bond lengths | 0.67 ± 0.02 | ||
| Bond angles | 0.73 ± 0.02 | ||
| Omega angle | 0.64 ± 0.03 | ||
| Side-chain planarity | 0.86 ± 0.07 | ||
| Improper dihedral distribution | 0.90 ± 0.03 | ||
| Inside/Outside distribution | 1.04 ± 0.02 | ||
| Average Z-scores for deviation from current reliable structurese | |||
| 1st generation packing quality | −0.32 ± 0.19 | ||
| 2nd generation packing quality | −0.61 ± 0.30 | ||
| χ1/χ2 rotamer normality | −1.61 ± 0.41 | ||
| Backbone conformation | 0.75 ± 0.22 | ||
| Q-Factors for Residual Dipolar Couplings | |||
| Individual Lobes | 0.27 ± 0.02 (N-lobe) | 0.24 ± 0.05 (C-lobe) | |
| Pearson correlation co-efficient | .93 ± 0.02 (N-lobe) | 00.86 ± 0.04 (C-lobe) | |
| Average Q-Factors | 0.25 ± 0.03 | ||
Global RMSD: 4–76 for the N-lobe (1–77) and 83–146 for the C-lobe (82–148)
Secondary structure: 6–19, 26–39, 45–55, 65–76 (N-lobe); 82–92, 103–112, 118–128, 138–146 (C-lobe). C-lobe/peptide RMSD: 83–146 (CaM C-lobe); 82–92 (eEF-2KCBD).
Ca2+-O distances for the sidechains of D20, D22, D24, E31, D56, D58 and N60, E67, and the backbone carbonyls of T26 and T62 were set to the range 2.2–2.6 Å.
Values based on PROCHECK (Laskowski et al., 1996) analysis (1–148 for CaM and 74-100 for eEF-2KCBD).
Values based on WHATIF (Vriend, 1990).
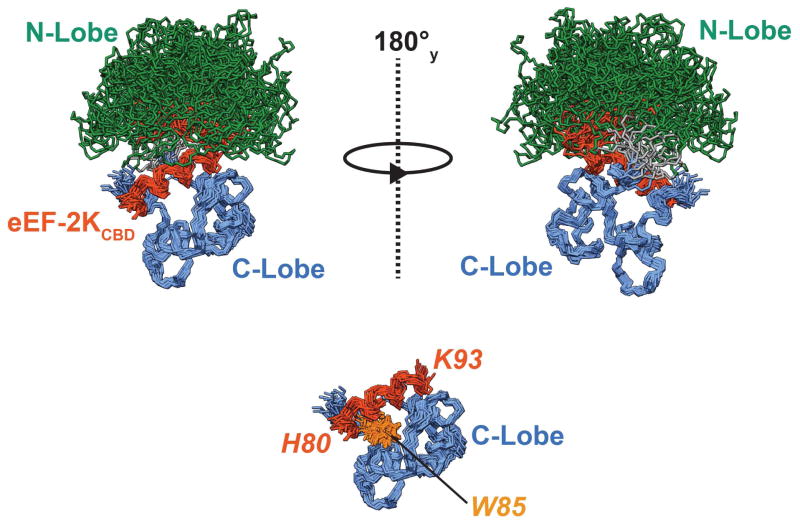
Figure 5. Solution structure of the Ca2+-CaM•eEF-2KCBD complex.
20 structures representing the final NMR ensemble have been overlaid using the C-lobe and eEF-2KCBD (see Table 1). The N-lobe (1–77) and C-lobe of CaM (82–148) have been colored green and blue respectively and the linker (78–81) is colored grey. eEF-2KCBD is colored dark orange. The N-lobe of CaM, the linker, 6 residues on the N-terminus and 7 residues on the C-terminus of eEF-2KCBD are hidden to allow better visualization of the C-lobe eEF-2KCBD interactions in the lower panel. The sidechains of the critical W85 are shown and colored light orange. The eEF-2KCBD residues are in italics and shown in orange. The Ca2+ ions occupying the N-lobe sites are not shown.
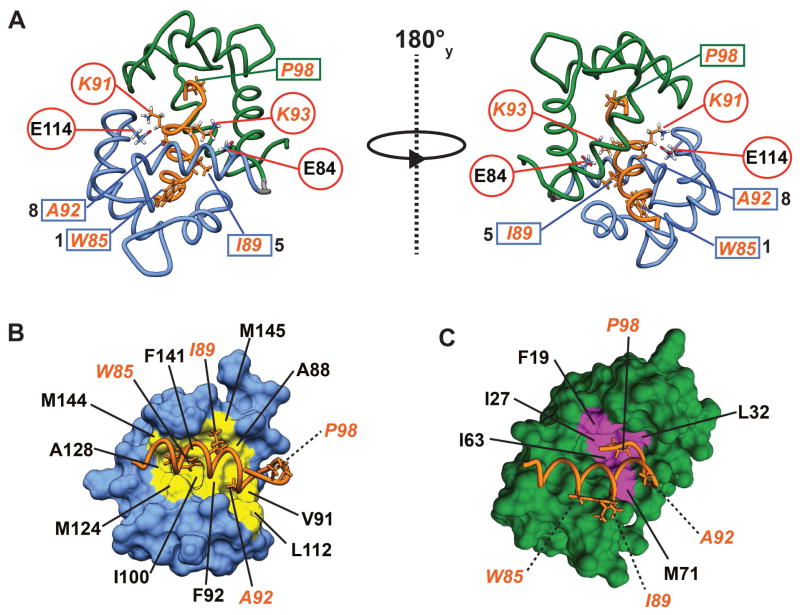
eEF-2KCBD forms a long single helix that is well-defined from F81 to A92 in line with the {1H}-15N NOE discussed above. As suggested by the CSP data, a majority of the stabilizing contacts between eEF-2KCBD and CaM involves the C-lobe of the latter with few contacts involving the N-lobe (Figure 6A). W85 on eEF-2KCBD inserts deep into a hydrophobic pocket formed by the sidechains of F92, I100, M124, I125, A128, V136, F141 and M144 (Figure 6B). Significant contacts are also seen between the sidechains of I89 and those of A88, F92, F141 and M145 on CaM. A92 on eEF-2KCBD makes multiple contacts with the sidechains of V91, F92 and L112. Thus eEF-2KCBD interacts with CaM in an anti-parallel fashion in a mode that largely conforms to a 1-5-8 binding mode with W85 being the key anchor residue at the 1-position (Figure 6A). Some contacts are also seen between the C-terminus of eEF-2KCBD and the CaM N-lobe (Figures 6A, C). These involve contacts between P98 with F19, I27, L32, I63 and M71. W99 (position 14 for a canonical 1-5-8-14 interaction)(Rhoads and Friedberg, 1997) contacts F19 and M71 through its backbone and has few consistent ring contacts < 5 Å within the NMR ensemble suggesting a high degree of disorder in this region as expected from the low {1H}-15N NOE values for the sidechain indole. While most of the interactions between CaM and eEF-2KCBD involve hydrophobic residues, K91 and K93 on eEF-2KCBD make electrostatic contacts with CaM residues E114 and E84, respectively. These interactions stabilize opposite faces of eEF-2KCBD and serve to anchor it to helices F and E on the C-lobe of CaM (Figure 6A).
Figure 6. Contacts between CaM and eEF-2KCBD.
(A) The CaM/eEF-2KCBD interface is stabilized by three sets of interactions: 1-5-8 hydrophobic docking (residues labeled and outlined using blue rectangles) of eEF-2KCBD onto the the CaM C-lobe; interactions between P98 (outlined using a green rectangle) on eEF-2KCBD and the CaM N-lobe; two sets of electrostatic interactions (outlined by red circles) involving basic residues on eEF-2KCBD and acidic residues on CaM. Detailed views of the 1-5-8 binding (B) and the interaction of P98 (C) with the C-lobe and the N-lobe of CaM, respectively, are shown. Locations of key residues on the CaM surface are colored (yellow for the C-lobe and magenta for the N-lobe) and labeled (in black). eEF-2KCBD residues are labeled in orange. The dashed lines indicate residues that point out the plane of the paper.
The angles between the helices that form the EF hands in the N-lobe and C-lobe of CaM (Table S3), calculated using an in-house protocol based on the algorithm defined by Kuboniwa et. al (Kuboniwa et al., 1995), provides insight into the differences in overall conformations of the two lobes. Based on our Ca2+ titration data, the N-lobe Ca2+ sites of CaM appear to be occupied; this is borne out by the fact that the inter-helical angles for the N-lobe are similar to the Ca2+-bound state of CaM rather than those in apo CaM. In contrast, the inter-helical angles in the C-lobe, especially those involving helix F, are unique. While the E/G, E/H and G/H angles resemble those in Ca2+-loaded CaM, the E/F angle is significantly smaller than both Ca2+-CaM and apo-CaM, and the F/H angle is larger than both states. The F/G angle is intermediate between the more closed conformation seen for apo-CaM and the more open conformation seen for Ca2+-CaM. These changes in inter-helical angles appear to be the result of the reorientation of the F-helix away from the Ca2+-bound-like state (Figure S5). Additionally, the angle between helices D and E, which defines the inter-lobe angle, is larger than that seen both for Ca2+-loaded CaM and apo-CaM suggesting a greater degree of lobe closure than both cases. However, as in the case of apo-CaM, there is a significant degree of variability, suggesting that several inter-lobe orientations are sampled within the NMR ensemble. A conformation of CaM that is more closed than the ligand-free state is also borne out by an analysis of the hydrodynamic properties of the Ca2+-CaM•eEF-2KCBD complex using 15N relaxation data. An axially-symmetric diffusion tensor is obtained upon analysis for the data for the N- and C-lobes separately with an anisotropy value, Dpara/Dperp=1.14±0.05 which is smaller than that of Ca2+-CaM (1.61±0.02) in solution (Chang et al., 2003).
Inter-lobe Dynamics in the Ca2+-CaM•eEF-2KCBD Complex
The Ca2+-CaM•eEF-2KCBD structural ensemble is characterized by an inability to simultaneously align the N- and C-lobes of CaM, and the low {1H}-15N NOE values for linker residues point to significant inter-lobe dynamics. We used a set of 1H-15N RDCs to validate the structural dynamics of the ensemble. Using intact structures i.e. data for the N-lobe and C-lobe simultaneously from the NMR ensemble to analyze the RDCs resulted in very poor Q factors (1.10±0.39, correlation co-efficient=0.47±0.14). In contrast, use of data for the N-lobe and C-lobe separately produced far better Q factors (N-lobe: 0.27±0.04, C-lobe: 0.24±0.05), and correlation co-efficients (N-lobe: 0.93±0.02, C-lobe: 0.86±0.04) suggesting that the RDC data could not be explained by a single relative orientation of the N- and C-lobes of CaM. We also noted that the absolute values of the RDCs measured for the N-lobe residues were roughly 12% smaller than those of the C-lobe. We used the ratio of the generalized degree of order (θ) values (Tolman et al., 2001) for the N- and C-lobes to define an order parameter (S2) for inter-lobe dynamics using Equation 3. A value of S2 =0.68±0.17 is indicative of significant inter-lobe dynamics.
W85 Plays a Critical Role in the Ca2+-CaM-Mediated Activation of eEF-2K
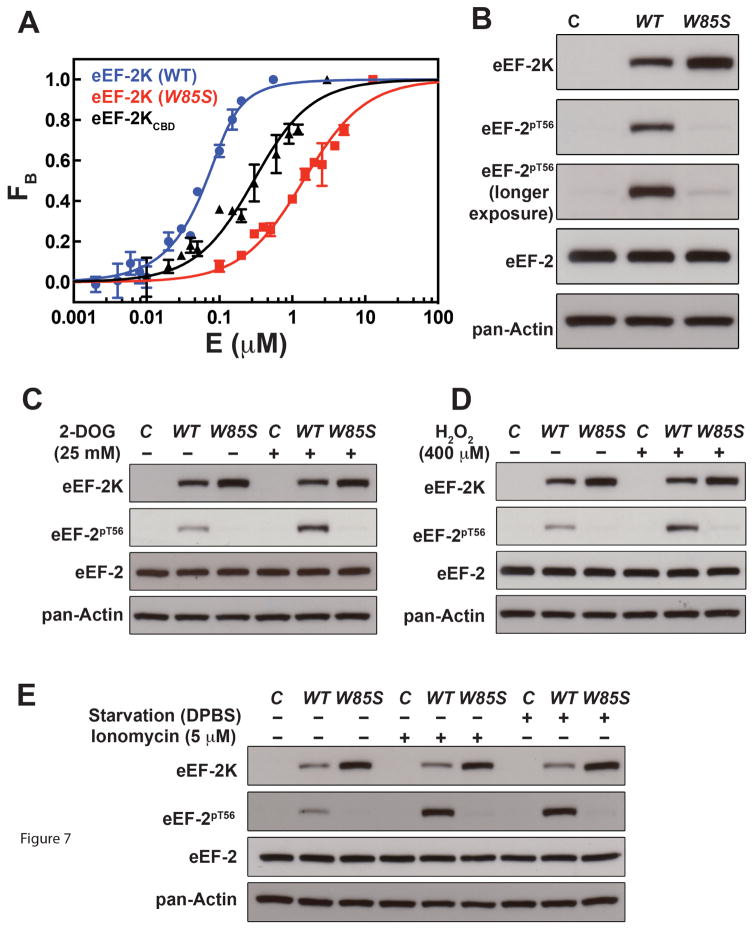
Based on the structural results it is evident that W85 plays a critical role in docking eEF-2KCBD onto CaM. In order to ascertain whether the importance of hydrophobic interactions involving W85 are maintained in full-length eEF-2K, we compared the ability of wild-type and W85S (in which W85 is replaced by a polar residue) eEF-2K to interact with, and be activated by Ca2+/CaM. Full-length eEF-2K (KD=16.7±2.8 nM, Figure 7A) binds Ca2+-CaM roughly 15-fold more tightly than eEF-2KCBD (KD=245.0±25.8 nM) under similar conditions; the latter is consistent with the value of 153 nM determined by ITC under NMR conditions. This confirms that the largest contribution to the interaction between Ca2+-CaM and eEF-2K results from the sequence encoded by eEF-2KCBD though additional weak interactions outside this segment cannot be ruled out. In contrast, the W85S mutation leads to an ~80-fold reduction in binding affinity (KD=1.4±0.1 μM) (Figure 7A) compared to full-length wild-type eEF-2K. The decreased association of a GST-tagged eEF-2K W85G and W85A mutants with CaM has been demonstrated before (Diggle et al., 1999; Pigott et al., 2012).
Figure 7. Influence of the W85S mutation on full-length eEF-2K.
(A) Fluorescence binding assays measuring interaction between wild-type full-length eEF-2K (blue), eEF-2KCBD (black) or the W85S mutant (red) and dansyl-CaM in the presence of Ca2+. The points (errors from duplicate measurements) represent the fraction of CaM in the bound state (calculated using Equation 5) at various concentrations of titrant and the solid curves represent the fits to Equation 6. (B) Results of the Western blot analysis for eEF-2 phosphorylated on T56 after transfection of knockout MCF-10A (eEF-2K−/−) cells with vectors encoding wild-type eEF-2K or the W85S mutant along with appropriate controls. Also shown is the level of eEF-2 phosphorylated on T56 upon stimulation with 25 mM 2-DOG (30 min) (C), 400 μM H2O2 (1 h) (D), 5 μM ionomycin (5 min) or starvation with DPBS (6 h) (E), along with the appropriate controls. Note that the blots shown in (B) have been cropped from those shown in (D) for untreated samples.
We tested the effect of the W85S mutation on the ability of eEF-2K to phosphorylate eEF-2 on T56 in cells. Wild-type eEF-2K and the W85S mutant were transiently expressed in an eEF-2K−/− MCF-10A cell-line (in which both eEF-2K alleles are knocked-out) by transfection with vectors encoding either wild-type eEF-2K or the W85S mutant. When cell lysates are probed for phosphorylation of eEF-2 on T56 by Western blot, no eEF-2 significant phosphorylation is seen for the W85S mutant in contrast to wild-type control (Figure 7B). Additionally, cells were subjected to a variety of stimuli known to result in enhanced eEF-2 phosphorylation through eEF-2K activation. These include treatment with 2-DOG to inhibit glycolysis, treatment with H2O2 to increase oxidative stress, starvation (Dulbecco’s PBS), and enhanced Ca2+ efflux (ionomycin) (Figures 7C–E). In all cases cells expressing wild-type eEF-2K show enhanced eEF-2 phosphorylation, while those expressing the W85S mutant show no significant increase in the levels of phosphorylated eEF-2.
DISCUSSION
Our results suggest that the most critical interactions between eEF-2KCBD and CaM occurs through the C-lobe of the latter, both in the Ca2+-loaded and Ca2+-free states. In the absence of Ca2+, the significantly weaker interaction (KD ~35 μM) involves only the C-lobe, leaving the N-lobe largely free. The N-lobe of CaM becomes engaged in the presence of Ca2+, taking part in interactions that in isolation are extremely weak (estimated to be ~4 mM if the free energies of the interactions involving the CaM N- and C-lobes are considered to be additive). However, the N-lobe interactions in combination with the already present interactions involving the C-lobe leads to a high affinity (KD ~ 0.15 μM) engagement of eEF-2KCBD in the Ca2+-loaded state. This is a possible mechanism by which eEF-2K may sense Ca2+ levels and modulate its affinity towards CaM perhaps in combination with other factors such as pH that are known to alter the Ca2+-requirement of CaM to attain Ca2+-bound like conformations (Pandey et al., 2014). Our recent results (Tavares et. al 2016, in preparation) suggest that the primary role of Ca2+ appears to be to promote the binding of CaM to eEF-2K rather than to induce an active conformation of the enzyme.
A unique feature of the Ca2+-CaM•eEF-2KCBD complex is the absence of Ca2+ in the C-lobe sites. In spite of the fact that the overall affinity of the C-lobe sites for Ca2+ has been determined to be higher than those of the N-lobe sites (Linse et al., 1991), engagement of eEF-2KCBD appears to reduce the Ca2+ affinity of the C-lobe sites. The ability of interaction partners of CaM to modulate its Ca2+-binding affinity has been discussed before(Hoffman et al., 2014; Theoharis et al., 2008).
An inspection of the structure of the Ca2+-CaM•eEF-2KCBD complex provides some clues into factors that could modulate the Ca2+ binding ability of the CaM C-lobe. A notable feature is a shift in the backbone for each of the two C-lobe Ca2+-coordinating EF hands (EF-3 and EF-4, Figure S6) compared to Ca2+-CaM alone. For EF-4, the significant inward shift of Q135 (which coordinates Ca2+ through its backbone carbonyl) would result in a clash with the metal ion if this site were occupied. This shift is likely the result of the interaction of the neighboring V135 with W85 on eEF-2KCBD. While less drastic than in the case of EF-4, the backbone of Y99 (which also coordinates Ca2+ through its carbonyl oxygen) in EF-3 undergoes an outward shift placing it a position too distant to optimally coordinate Ca2+. The adjoining I100 also makes key contacts with W85 in eEF-2KCBD. Key sidechains involved in metal co-ordination are also in sub-optimal conformations, though we hesitate to interpret this given the lower precision in determining the sidechain orientations. Additionally, as previously described, the inter-hand angle (F/G, Table S3) also has a value that is intermediate between the Ca2+-loaded and the Ca2+-free forms. It has been noted that Ca2+ binding in the C-lobe is highly co-operative (Valeyev et al., 2008). Thus, subtle changes in the Ca2+ affinities of individual C-lobe EF hands could be amplified to a large extent.
Though the C-lobe of CaM does not appear to contain Ca2+ when bound to eEF-2KCBD, its binding mode is distinct from IQ-motif-based interactions that define the binding of peptides to apo-CaM (Bahler and Rhoads, 2002). Some similarities exist between the structure of the Ca2+-CaM•eEF-2KCBD complex and that of the small-conductance Ca2+-activated K+ channel (SK) complexed to CaM (Schumacher et al., 2001). In the latter structure, that is rather unique, the dimeric CaM-binding domain (SKCBD) has a CaM molecule bound to each end forming a 2:2 complex; binding also occurs in a 1-5-8 mode but in reversed fashion. As in the Ca2+-CaM•eEF-2KCBD complex, Ca2+ is also absent from the C-lobe sites while the N-lobe sites are occupied. Comparison of the recognition motifs in the two cases suggest some similarities (Figure S7) but also several points of divergence. In the Ca2+-CaM•SKCBD complex, a W432 occupies a similar spatial position as W85 in eEF-2KCBD, though unlike the latter, the former simply sits on a hydrophobic surface formed by M124, E127, F141 and M144 rather than being inserted deep into the hydrophobic pocket. Of the two other hydrophobic prongs that define the Ca2+-free interactions in the Ca2+-CaM•SKCBD structure, the completely buried I428 occupies a different physical orientation than I89 in eEF-2KCBD. The final prong of the trident, A425, is in a roughly similar spatial orientation as A92 in eEF-2KCBD. However electrostatic interactions (between the sidechains of K91 and E114, K93 and E84) similar to those that further stabilize the interaction of eEF-2KCBD with the C-lobe of CaM are missing in the Ca2+-CaM•SKCBD complex.
The structure of the Ca2+-CaM•eEF-2KCBD complex provides insight into the critical nature of the hydrophobic interactions involving W85 on eEF-2K. This residue is fully conserved in metazoans (Phe in mollusca, Figure S8). Mutation of this residue to glycine or alanine has been previously shown to disrupt Ca2+-CaM binding to GST-tagged eEF-2K (Pigott et al., 2012). Here we demonstrate that a W85S mutation causes an ~80-fold reduction in binding to a full-length tag-free eEF-2K. Additionally, the W85S mutant is severely compromised in its ability to phosphorylate eEF-2 in cells. Recent studies have shown that hydroxylation on P98 (also fully conserved, Figure S8) by hydroxylases, that are inactivated during hypoxic conditions, lead to activation of eEF-2K and impaired protein translation (Moore et al., 2015). We have shown that P98 contributes to the engagement of the N-lobe and based on our structure it is conceivable that the introduction of a polar group on P98 would reduce its hydrophobic interactions with the N-lobe of CaM. Given that N-lobe based interactions are weaker with respect to those involving the C-lobe, and themselves do not appear to be critical, hydroxylation of P98 should have a relatively small effect on CaM binding, in agreement with the data of Moore et. al (Moore et al., 2015). In the same study it was also shown that mutation of W99 (W99A, W99L) does not lead to a significant reduction in CaM binding, as expected from our structure. However, the W99A mutant is unable to be activated by CaM through a yet-to-be understood mechanism.
Our present studies provide a clear structural basis for the recognition of Ca2+-CaM by eEF-2K. They do not however, provide insight into how this interaction serves to facilitate the two-step activation of the enzymatic activity of eEF-2K. Such insight can be only obtained upon the availability of the structure of a construct of eEF-2K that encompasses the CBD in addition to the catalytic domain and part of the R-loop that includes T348. Also intriguing is the observation that a phospho-mimetic R-loop mutant S500D makes the activity of eEF-2K Ca2+ but not CaM-independent (Tavares et al., 2012). Perhaps the interactions involving the C-lobe under Ca2+-free conditions favor additional N-lobe mediated interactions outside the region encompassed by eEF-2KCBD. We expect our present studies to lay the groundwork for deciphering the inner workings of this unique enzyme.
EXPERIMENTAL PROCEDURES
Expression, Purification and Sample Preparation
Full-length human calmodulin (CaM), the predicted calmodulin-binding segment of human eEF-2K (residues 74-100; eEF-2KCBD) and full-length eEF-2K were cloned, expressed and purified as described in the Supplemental Experimental Procedures.
NMR Sample Preparation
NMR samples of the Ca2+-CaM•eEF-2KCBD and apo-CaM•eEF-2KCBD complexes were prepared in the Ca2+-NMR buffer (20 mM Bis-Tris at pH 6.8, 150 mM KCl, and 10 mM CaCl2) or apo-NMR buffer (Ca2+-free) (20 mM Bis-Tris at pH 6.8, 150 mM KCl, and 10 mM EGTA), respectively, as described in the Supplemental Experimental Procedures.
Isothermal Titration Calorimetry
The affinity of eEF-2KCBD for Ca2+-CaM was determined using isothermal titration calorimentry (ITC) measurements. Three sets of experiments were performed under identical conditions to confirm reproducibility. Details of the procedures used can be found in the Supplemental Experimental Procedures.
Resonance Assignment
All NMR experiments were performed at 35 °C on Varian Inova (600 MHz) or Bruker Avance (500, 600, 800 and 900 MHz) spectrometers equipped with cryogenic probes capable of applying pulsed field gradients along the z-axis. Spectra were processed using NMRPipe (Delaglio et al., 1995) and analyzed using NMRViewJ (Johnson, 2004). Resonance assignments for the various states were obtained as described in the Supplemental Experimental Procedures.
Chemical shift perturbations for backbone amides between two states 1, 2 were calculated using the following equation:
| (1) |
Where δH1,2 and δN1,2 are the 1H and 15N chemical shifts, respectively, for each state.
Analysis of the Interaction between apo-CaM and eEF-2KCBD
Given the weaker affinity of eEF-2KCBD towards apo-CaM, an estimate of the binding affinity of the peptide was obtained using NMR-based titrations as described in detail in the Supplemental Materials and Methods. In brief, representative residues from the C-lobe for the eEF-2KCBD-saturated state (CaM:eEF-2KCBD ratio of 1:5.6) were chosen and their intensities (I) as a function of the final eEF-2KCBD concentration (P) at a constant CaM concentration (C0=80 μM) were fitted to Equation (2),
| (2) |
where I∞ is the peak intensity (in arbitrary units) at saturation.
Determination of the Ca2+ Requirement of the Ca2+-CaM-eEF-2KCBD Complex
The Ca2+ requirement of the CaM•eEF-2KCBD was determined by titration of Ca2+ into the Ca2+-free complex as described in the Supplemental Experimental Procedures.
Relaxation Measurements and Calculation of Hydrodynamic Properties
15N R1 and R2 experiments for U-[15N,13C]-Ca2+-CaM in the Ca2+-CaM•eEF-2KCBD complex were carried out at 600 MHz using standard pulse sequences (Cavanagh et al., 2007). Steady-state {1H}-15N NOE experiments were performed for CaM (600 MHz) and eEF-2KCBD (800 MHz) in the Ca2+-CaM•eEF-2KCBD complex using the pulse sequence of Ferrage et. al (Ferrage et al., 2009). The overall rotational diffusion tensor of CaM in the Ca2+-CaM•eEF-2KCBD complex was determined using the 15N R1, R2 and steady-state {1H}-15N NOE rates as described before (Ghose et al., 2001). Details are provided in the Supplemental Experimental Procedures.
Structure Calculations
The structure of the Ca2+-CaM•eEF-2KCBD complex was performed using ARIA2.3 (CNS2.1.2) (Habeck et al., 2004) using the PARALLHDG force field with PROLSQ non-bonded energy terms (Engh and Huber, 1991) and experimental distance and angular restraints. Details of the structure calculation and analysis are described in the Supplemental Experimental Procedures. Experimental restraints and structural statistics are summarized in Table 1.
Measurement and Analysis of Residual Dipolar Couplings
Residual dipolar couplings on the Ca2+-CaM•eEF-2KCBD complex were measured utilizing C12E5/n-hexanol (Ruckert and Otting, 2000) based partially aligned phases. The details of sample preparation and RDC analysis are provided in the Supplemental Experimental Procedures. Quality factors (Cornilescu et al., 1998) were calculated using the following equation (note that the Q-factor calculated in this fashion may exceed 1);
| (3) |
where Dobs and Dcalc are the measured residual dipolar couplings and those calculated from the structure, respectively. Da and R represent the normalized axial and rhombic components of the alignment tensor, respectively. The generalized degree of order (θ) (Tolman et al., 2001) for the data from the N- and C-lobes were used to define an order-parameter (S2) for the inter-lobe motion in the following way:
| (4) |
where i indexes the N-lobe and C-lobe data. The elements of the Saupe order matrix are defined in their respective principal axis frames.
Fluorescence Binding Assays
Binding of CaM to eEF-2KCBD, wild-type full-length eEF-2K and the W85S mutant was measured as described in the Supplemental Materials and Methods. The fraction of CaM bound (Fb) at a particular eEF-2K concentration was determined using Equation 5,
| (5) |
where is the fluorescence intensity at 490 nm in the absence of eEF-2K and is the intensity at saturation. The Fb values obtained at each eEF-2K concentration (E) at a constant CaM concentration (C0) were fitted to Equation 6 to obtain the apparent dissociation constant (KD).
| (6) |
Cell-based Assays
Cell-based assays utilizing knockout MCF-10A (eEF-2K−/−) cells (Sigma-Aldrich) were transfected with vectors encoding wild-type eEF-2K or the W85S mutant and lysed cells were analyzed by Western blotting using eEF-2K, eEF-2 or pT56-eEF-2 specific antibodies (Tavares et al., 2014). Details are provided in the Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
eEF-2K binds Ca2+-loaded CaM with high-affinity largely through the CaM C-lobe
C-lobe mediated interactions occur in a Ca2+-independent fashion
Mutation of the hydrophobic CaM anchor on eEF-2K disrupts its cellular activity
Acknowledgments
This research has been supported by the following grants from the NIH: GM084278 (to RG), GM059802 (to KND) and G12 MD007603 (partial support of the CCNY NMR facilities); Welch Foundation award F-1390 (to KND). RG is a member of the New York Structural Biology Center, a NYSTAR facility. KL acknowledges American Heart Association pre-doctoral fellowship 15PRE25760018. The authors thank Prof. Dr. Christian Griesinger and Dr. Fernando Rodriguez-Castañeda for providing assignments of apo-CaM and Ca2+-CaM obtained under conditions similar to those used here. This manuscript was written while RG was a Program Director at the NSF (MCB); his IRD activities are supported by award MCB 1557303.
Footnotes
ACCESSION NUMBERS
The atomic coordinates and experimentally derived restraints have been deposited in the Protein Data Bank (PDB:5J8H) and resonance assignments have been deposited in the Biological Magnetic Resonance Bank (CODE:30063).
Supplemental Information includes Supplemental Supplemental Experimental Procedures, 8 figures and 3 tables and can be found with this article online at http://dx.doi.org/
AUTHOR CONTRIBUTIONS
KL prepared samples and performed all NMR experiments and analyses supervised by AP. SA performed all structure calculations and analyses. CT, RW and DG performed the biochemical experiments involving full-length eEF-2K and mutants, in vitro and cell-based assays. RG and KND conceptualized the project and supervised various aspects of the research. RG wrote and edited the manuscript with input from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerfeldt KS, Coyne AN, Wilk RR, Thulin E, Linse S. Ca2+-binding stoichiometry of calbindin D28k as assessed by spectroscopic analyses of synthetic peptide fragments. Biochemistry. 1996;35:3662–3669. doi: 10.1021/bi9527956. [DOI] [PubMed] [Google Scholar]
- Atreya HS, Chary KV. New chemical shift signatures of bound calcium in EF-hand proteins. Curr Sci. 2002;83:1240–1245. [Google Scholar]
- Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- Biekofsky RR, Martin SR, Browne JP, Bayley PM, Feeney J. Ca2+ coordination to backbone carbonyl oxygen atoms in calmodulin and other EF-hand proteins: 15N chemical shifts as probes for monitoring individual-site Ca2+ coordination. Biochemistry. 1998;37:7617–7629. doi: 10.1021/bi9800449. [DOI] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygard O. Functional properties of phosphorylated elongation factor 2. Eur J Biochem. 1990;191:639–645. doi: 10.1111/j.1432-1033.1990.tb19169.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Fairbrother WJ, III, AJP, Mark R, Skelton NJ. Protein NMR Spectroscopy. 2. San Diego: Academic Press; 2007. [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Chang SL, Szabo A, Tjandra N. Temperature dependence of domain motions of calmodulin probed by NMR relaxation at multiple fields. J Am Chem Soc. 2003;125:11379–11384. doi: 10.1021/ja034064w. [DOI] [PubMed] [Google Scholar]
- Cornilescu G, Marquardt JL, Ottiger M, Bax A. Validation of protein structure from anisotropic carbonyl chemical shifts in a dilute liquid crystalline phase. J Am Chem Soc. 1998;120:6836–6837. [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Diggle TA, Seehra CK, Hase S, Redpath NT. Analysis of the domain structure of elongation factor-2 kinase by mutagenesis. FEBS Lett. 1999;457:189–192. doi: 10.1016/s0014-5793(99)01034-0. [DOI] [PubMed] [Google Scholar]
- Dumont-Miscopein A, Lavergne JP, Guillot D, Sontag B, Reboud JP. Interaction of phosphorylated elongation factor EF-2 with nucleotides and ribosomes. FEBS Lett. 1994;356:283–286. doi: 10.1016/0014-5793(94)01272-5. [DOI] [PubMed] [Google Scholar]
- Engh R, Huber R. Accurate bond and angle parameters. Acta Crystallogr A. 1991;47:392–400. [Google Scholar]
- Ferrage F, Cowburn D, Ghose R. Accurate sampling of high-frequency motions in proteins by steady-state 15N-{1H} nuclear Overhauser effect measurements in the presence of cross-correlated relaxation. J Am Chem Soc. 2009;131:6048–6049. doi: 10.1021/ja809526q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose R, Fushman D, Cowburn D. Determination of the rotational diffusion tensor of macromolecules in solution from NMR relaxation data with a combination of exact and approximate methods - Application to the determination of interdomain orientation in multidomain proteins. J Magn Reson. 2001;149:204–217. doi: 10.1006/jmre.2001.2295. [DOI] [PubMed] [Google Scholar]
- Gildish I, Manor D, David O, Sharma V, Williams D, Agarwala U, Wang X, Kenney JW, Proud CG, Rosenblum K. Impaired associative taste learning and abnormal brain activation in kinase-defective eEF2K mice. Learn Mem. 2012;19:116–125. doi: 10.1101/lm.023937.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck M, Rieping W, Linge JP, Nilges M. NOE assignment with ARIA 2.0: the nuts and bolts. Meth Mol Biol. 2004;278:379–402. doi: 10.1385/1-59259-809-9:379. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Chandrasekar A, Wang X, Putkey JA, Waxham MN. Neurogranin alters the structure and calcium-binding properties of calmodulin. J J Biol Chem. 2014;289:14644–14655. doi: 10.1074/jbc.M114.560656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Meth Mol Biol. 2004;278:313–352. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psych. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A. Solution structure of calcium-free calmodulin. Nature Struct Biol. 1995;2:768–776. doi: 10.1038/nsb0995-768. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- Linse S, Helmersson A, Forsen S. Calcium binding to calmodulin and its globular domains. J Biol Chem. 1991;266:8050–8054. [PubMed] [Google Scholar]
- Liu XY, Zhang L, Wu J, Zhou L, Ren YJ, Yang WQ, Ming ZJ, Chen B, Wang J, Zhang Y, et al. Inhibition of elongation factor-2 kinase augments the antitumor activity of temozolomide against glioma. PLoS One. 2013;8:e81345. doi: 10.1371/journal.pone.0081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid beta. J Neurosci. 2014;34:12230–12238. doi: 10.1523/JNEUROSCI.1694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric-Bernstam F, Chen H, Akcakanat A, Do KA, Lluch A, Hennessy BT, Hortobagyi GN, Mills GB, Gonzalez-Angulo AM. Aberrations in translational regulation are associated with poor prognosis in hormone receptor-positive breast cancer. Breast Cancer Res. 2012;14:R138. doi: 10.1186/bcr3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittl PR, Schneider-Brachert W. Sel1-like repeat proteins in signal transduction. Cell Signal. 2007;19:20–31. doi: 10.1016/j.cellsig.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Gideons E, Kavalali ET. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psych. 2013;73:1199–1203. doi: 10.1016/j.biopsych.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CE, Mikolajek H, Regufe da Mota S, Wang X, Kenney JW, Werner JM, Proud CG. Elongation factor 2 kinase is regulated by proline hydroxylation and protects cells during hypoxia. Mol Cell Biol. 2015;35:1788–1804. doi: 10.1128/MCB.01457-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn AC, Palfrey HC. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987;262:17299–17303. [PubMed] [Google Scholar]
- Pandey K, Dhoke RR, Rathore YS, Nath SK, Verma N, Bawa S, Ashish Low pH Overrides the need of calcium ions for the shape-function relationship of calmodulin: resolving prevailing debates. J Phys Chem B. 2014;118:5059–5074. doi: 10.1021/jp501641r. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott CR, Mikolajek H, Moore CE, Finn SJ, Phippen CW, Werner JM, Proud CG. Insights into the regulation of eukaryotic elongation factor 2 kinase and the interplay between its domains. Biochem J. 2012;442:105. doi: 10.1042/BJ20111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- Ruckert M, Otting G. Alignment of biological macromolecules in novel nonionic liquid crystalline media for NMR experiments. J Am Chem Soc. 2000;122:7793–7797. [Google Scholar]
- Ryazanov AG. Ca2+/calmodulin-dependent phosphorylation of elongation factor 2. FEBS Lett. 1987;214:331–334. doi: 10.1016/0014-5793(87)80081-9. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 2002;514:26–29. doi: 10.1016/s0014-5793(02)02299-8. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Davydova EK. Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation. Phosphorylated EF-2 is unable to catalyze translocation. FEBS Lett. 1989;251:187–190. doi: 10.1016/0014-5793(89)81452-8. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Natapov PG, Shestakova EA, Severin FF, Spirin AS. Phosphorylation of the elongation factor 2: the fifth Ca2+/calmodulin-dependent system of protein phosphorylation. Biochimie. 1988a;70:619–626. doi: 10.1016/0300-9084(88)90245-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988b;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Ward MD, Mendola CE, Pavur KS, Dorovkov MV, Wiedmann M, Erdjument-Bromage H, Tempst P, Parmer TG, Prostko CR, et al. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc Natl Acad Sci USA. 1997;94:4884–4889. doi: 10.1073/pnas.94.10.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Smith EM, Proud CG. CDC2-cyclin B regulates eEF2 kinase activity in a cell cycle- and amino acid-dependent manner. EMBO J. 2008;27:1005–1016. doi: 10.1038/emboj.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling TR, Stull JT. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem Rev. 2001;101:2341–2352. doi: 10.1021/cr0002386. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Swulius MT, Waxham MN. Ca2+/calmodulin-dependent protein kinases. Cell Mol Life Sci. 2008;65:2637–2657. doi: 10.1007/s00018-008-8086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares CDJ, Ferguson SB, Giles DH, Wang Q, Wellmann RM, O’Brien JP, Warthaka M, Brodbelt JS, Ren P, Dalby KN. The molecular mechanism of eukaryotic elongation factor 2 kinase activation. J Biol Chem. 2014;289:23901–23916. doi: 10.1074/jbc.M114.577148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares CDJCD, O’Brien JPJP, Abramczyk OO, Devkota AKAK, Shores KSKS, Ferguson SBSB, Kaoud TSTS, Warthaka MM, Marshall KDKD, Keller KMKM, et al. Calcium/calmodulin stimulates the autophosphorylation of elongation factor 2 kinase on Thr-348 and Ser-500 to regulate its activity and calcium dependence. Biochemistry. 2012;51:2232–2245. doi: 10.1021/bi201788e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekedereli I, Alpay SN, Tavares CD, Cobanoglu ZE, Kaoud TS, Sahin I, Sood AK, Lopez-Berestein G, Dalby KN, Ozpolat B. Targeted silencing of elongation factor 2 kinase suppresses growth and sensitizes tumors to doxorubicin in an orthotopic model of breast cancer. PLoS One. 2012;7:e41171. doi: 10.1371/journal.pone.0041171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharis NT, Sorensen BR, Theisen-Toupal J, Shea MA. The neuronal voltage-dependent sodium channel type II IQ motif lowers the calcium affinity of the C-domain of calmodulin. Biochemistry. 2008;47:112–123. doi: 10.1021/bi7013129. [DOI] [PubMed] [Google Scholar]
- Tolman JR, Al-Hashimi HM, Kay LE, Prestegard JH. Structural and dynamic analysis of residual dipolar coupling data for proteins. J Am Chem Soc. 2001;123:1416–1424. doi: 10.1021/ja002500y. [DOI] [PubMed] [Google Scholar]
- Usui T, Okada M, Hara Y, Yamawaki H. Eukaryotic elongation factor 2 kinase regulates the development of hypertension through oxidative stress-dependent vascular inflammation. Am J Physiol Heart Circ Physiol. 2013;305:H756–768. doi: 10.1152/ajpheart.00373.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeyev NV, Bates DG, Heslop-Harrison P, Postlethwaite I, Kotov NV. Elucidating the mechanisms of cooperative calcium-calmodulin interactions: a structural systems biology approach. BMC Syst Biol. 2008;2:48. doi: 10.1186/1752-0509-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpelli C, Piccoli G, Zibetti C, Zanchi A, Gardoni F, Huang K, Brambilla D, Di Luca M, Battaglioli E, Sala C. Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J Neurosci. 2010;30:5830–5842. doi: 10.1523/JNEUROSCI.0119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend G. WHAT IF: a molecular modeling and drug design program. J Mol Graph. 1990;8:52–56. 29. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- Zuiderweg ERP. Mapping protein-protein interactions in solution by NMR spectroscopy. Biochemistry. 2002;41:1–7. doi: 10.1021/bi011870b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.