Abstract
The miniaturization, sophistication, proliferation, and accessibility of technologies are enabling the capturing of more and previously inaccessible phenomena in Parkinson disease (PD). However, more information has not translated into greater understanding of disease complexity to satisfy diagnostic and therapeutic needs. Challenges include non-compatible technology platforms, the need for wide-scale and long-term deployment of sensor technology (in particular among vulnerable elderly patients), and the gap between the “big data” acquired with sensitive measurement technologies and their limited clinical application. Major opportunities could be realized if new technologies are developed as part of open-source and/or open-hardware platforms enabling multi-channel data capture, sensitive to the broad range of motor and non-motor problems that characterize PD, and adaptable into self-adjusting, individualized treatment delivery systems. The International Parkinson and Movement Disorders Society Task Force on Technology is entrusted to convene engineers, clinicians, researchers, and patients to promote the development of integrated measurement and closed-loop therapeutic systems with high patient adherence that also serve to: 1) encourage the adoption of clinico-pathophysiologic phenotyping and early detection of critical disease milestones; 2) enhance tailoring of symptomatic therapy; 3) improve subgroup targeting of patients for future testing of disease modifying treatments; and 4) identify objective biomarkers to improve longitudinal tracking of impairments in clinical care and research. This article summarizes the work carried out by the Task Force toward identifying challenges and opportunities in the development of technologies with potential for improving the clinical management and quality of life of individuals with PD.
Keywords: Technology, Parkinson disease, precision medicine, remote monitoring, wearable technology
INTRODUCTION
During the last decade, a multitude of technology-based objective measures of parkinsonian impairments have been developed, bringing with them the promise of substantially changing the diagnostic, monitoring and therapeutic landscape in Parkinson disease (PD). Sensors, mobile communications, cloud computing, advanced analytics, and the Internet of Things (wireless connectivity of all electronic devices),1, 2 are among the innovations that have the potential to transform healthcare and our approach to patients with chronic, complex and fluctuating disorders. With the abundance of new technologies, their growing power and versatility, and the smart algorithms upon which they increasingly rely, our field needs to ponder the basic questions of why we should even consider adding alternative measures, what clinical needs should be addressed, what instruments should be used, how to deploy new technologies with minimal burden, disruption and cost, and maximal compliance, and whether they replace or complement existing resources. Unfortunately, technology developers appear to be operating in competing “islands of expertise” whose focus may be redundant, thus increasing the risk of duplicating rather than extending progress while potentially making their technologies incompatible with those of other developers. Also, not all technologies are primarily driven by burning questions from within the clinical field, sometimes creating technical solutions that – clever as they may be – remain in search of a clinical indication.
In the absence of well-established and validated biomarkers of diagnosis or disease progression, PD remains a clinically defined disease. Today, clinical scales and traditional patient-reported outcomes continue to be the primary assessment tools or endpoints in both clinical care and research in PD. However, there is growing awareness that technology-based objective measures (TOMs) may improve the sensitivity, accuracy, reproducibility and feasibility of objectively capturing the full complexity and diversity of changes in motor and non-motor behaviors.3–7 Examples include the difficulty to reliably evaluate fluctuating events (e.g., the variable response to medication), to capture rare incidents (e.g., falls, or freezing of gait) or to assess behaviors that, by definition, take place over long periods of time outside the clinical examination room (e.g. physical activities in everyday life). The ability to remotely capture behavioral data and use it to optimize treatment strategies has the potential to finally “close the loop” and address critical knowledge gaps.8 Despite multiple ongoing projects by stakeholders in academia and industry, it remains challenging to determine what initiatives have the capacity to be scaled up, and what type of deployment would ensure the highest yield in the future.
This review summarizes the deliberations of the International Parkinson and Movement Disorders Society Task Force on Technology. As a first step, we concentrated on assessing the landscape of wearable devices and other technologies for individualized assessments, as well as the therapeutic and scientific uncertainties they stand to fill, rather than on the clinimetric properties of any of the growing list of measurement technologies that have become available in the last 10 years. For the latter purpose, a number of reviews have been recently published.9–11 The Task Force is entrusted with bringing together experts from the device and biopharmaceutical industry, clinicians, researchers, engineers, and patients to brainstorm on needed developments to advance PD research and care. We aim at appraising the extent to which technology and data analysis in general, and TOMs in particular, can bring robust granularity to the clinical complexity of PD in order to facilitate clinico-pathophysiologic phenotyping, the detection of prodromal symptoms, the improvement of diagnostic accuracy, progression monitoring, and to begin the process of integrating technology-based diagnostics and actionable pharmacological and non-pharmacological therapeutics for clinical applications.
DEFINITIONS AND OBJECTIVES
TOMs are the outcome of device-based instrumented clinical tests conducted by clinicians in standardized environments to objectively measure specific behaviors, or self-administered by patients to detect and monitor impairments in specific or overall function in everyday life. Initially, TOMs targeted motor phenomena, such as gait or balance, and were gathered in specialized movement laboratories.12 More recently, they have been extended to devices worn by the patient (i.e., wearable sensors and systems) in the clinic and –for remote monitoring– in the home or community settings (Figure 1).13–15 The goal of wearable technologies is to maximize the “ecological” validity as well as the temporal and spatial resolution of capturing motor and non-motor phenomena that are naturally expected to change over time. As such, wearable technology may provide a more “realistic” portrayal of behaviors of interest in clinical and research settings.16 In addition, in the research arena, increasing the temporal and spatial resolution of a targeted behavior is expected to reduce the sample size required to evaluate the effect of therapeutic interventions.17
Figure 1. Schematic representation of a subject undergoing monitoring in the home setting using wearable and ambient sensors.
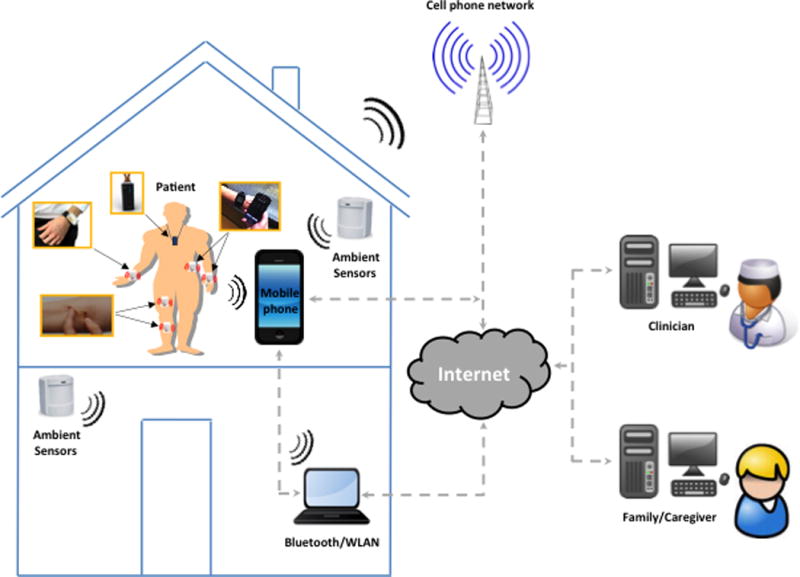
The technology shown includes a wireless unit strapped around the wrist, band-aid like sensors attached to the lower limbs, a wearable camera worn as a pendant, a smart watch, and a mobile phone clipped on the belt used as gateway to relay the data to the cloud to assess specific functions (using its embedded sensors) as well as to communicate with the subject (using customized apps). Ambient sensors and computer technologies are utilized in the home settings to gather additional information or replace wearable sensors when wearable sensors cannot be used. The integration of wearable technology with smart devices enables remote monitoring of patients with PD and real-time feedback to clinicians, family/caregivers, and the patients themselves.
Important goals of TOMs are to provide objective parameters in the detection and monitoring of motor and non-motor functions, thereby enhancing the quality of treatment delivery and allowing for personalized care (Table 1). Currently available wearable technologies (such as inertial sensors and surface electromyography [EMG]) are –with variable success– capable of capturing the number and intensity of multiple activities, such as the frequency and amplitude of movements during the day and while asleep, the frequency with which tremor and dyskinesia appear and disappear during the day, and the fluctuations in the severity of gait and balance impairments.18–22 The use of consumer wearable technologies in medicine is becoming increasingly more common. For instance, in the field of sleep medicine, the use of actigraphy for sleep monitoring may be used to supplant more traditional methods like polysomnography due to its validity, lower cost, and ability to evaluate individuals in their homes over a longer period of time.23 Advanced wearable technologies can also precisely monitor skin conductance, respiratory rate, blood pressure, oximetry and provide surface EMG, electrocardiography (ECG) and electroencephalography (EEG) tracings.24–29 Furthermore, the ability to collect TOMs using smart devices (mobile phones, tablets, and smart watches) provides additional opportunities to collect and analyze numerous clinically-relevant parameters (e.g., posture, balance, dexterity, voice and speech patterns, facial expression, eye tracking, medication and exercise compliance and adherence) and develop communication portals to improve patient engagement and self-management.
Table 1.
Examples of available and needed technologies relevant to the diagnosis and clinical management of patients with Parkinson disease
| Clinical problem | Available/needed technologies | Clinical objective |
|---|---|---|
| Improving diagnosis | Needed – Sensors for prodromal features (e.g., constipation, REM sleep behavior, anosmia); blood sensors for biomarkers (α-synuclein, proteinomics, etc) | Enable population screening for PD, including the earliest possible (prodromal) stages |
| Monitoring response to therapy and motor complications (motor fluctuations, dyskinesia) | Available – Accelerometers, gyroscopes, magnetometers, electrogoniometers, surface EMG sensors Needed: small patches onto the skin or other sensors that improve patient adherence |
Collect ecologically valid data of motor fluctuations, falls, freezing of gait episodes Implement sensor-based closed-loop technologies capable of delivering treatments (e.g., infusion pump) |
| Monitoring non-motor symptoms and progression | Available (but requiring improvements) – Sweat sensors, skin conductance sensors, heart rate sensors, blood pressure sensors | Collect ecologically valid data of non-motor symptoms and progression |
| Improving medical treatment | Available (but requiring improvements) – Oral capsules, subcutaneous and gastrointestinal infusion pumps | Implement adjustable extended-release drug formulations, smart (self-adjusting) levodopa delivery infusion systems |
| Enhancing surgical treatment | Available (but requiring improvements) – STN DBS, GPi DBS, Vim thalamus DBS | Implement closed-loop STN and GPi DBS (variable stimulation based on local field potentials) |
| Improving rehabilitation interventions | Available – Accelerometers, gyroscopes, magnetometers, electrogoniometers, surface EMG sensors, pulse oximetry sensors, respiratory rate sensors, blood pressure sensors | Implement closed-loop cueing and feedback systems validated for home use |
DBS: deep brain stimulation; EMG: electromyography; GPi: globus pallidus pars interna; PD: Parkinson disease; REM: rapid eye movement; STN: subthalamic nucleus; Vim: Ventrointermedial nucleus
Caveat emptor: Why measure at all?
The often-implied assumption that the sole existence of a PD symptom justifies its measurement and that all PD-related phenomena should be measured must be dispelled. A measure is justified if it enhances our understanding of a complex disease or aids in testing or delivering a therapy. The use of measurements to improve therapy is filled with rich examples from other branches of medicine (e.g., glucose monitoring for insulin pumps, cardiac defibrillators). It should be remembered that every qualitative clinical assessment is a form of measurement and that the use of quantitative measures carries potential for improving the decision-making process as to the need and dose of therapy. Implicit however is that what is being measured represents a therapeutic target and hence the measurement must be relevant to the treatment question (Table 1).
The case for multi-domain, multi-sensor, integrated technology
PD is characterized by considerable inter- and intra-subject clinical variability in clinical symptoms. What matters most for one patient in the motor sphere may not be as important for another (e.g., tremor vs. freezing of gait vs. sleep disturbance) given the different levels of functional disability.30 Fluctuations in daily functioning in some patients may only include non-motor phenomena (e.g., fatigue or anxiety).31 Even if we were to accurately measure the most overt deficit, most patients display a repertoire of motor and non-motor endpoints that vary within and between days, with varying impact on their quality of life.32 Thus, a multi-domain, multi-sensor, smart technology is needed to determine the source of all relevant changes, identify individualized disease fingerprints, and develop truly personalized therapeutic approaches.
CHALLENGES
The need for monitoring non-motor symptoms
The development of wearable systems to monitor individuals with PD has been focused heavily on motor aspects of the disease (e.g., tremor, bradykinesia, gait impairment, and dyskinesia)33–35 that are also, albeit with lower sensitivity and specificity, evaluated by clinical scales. Despite recent advances in the quantification of motor symptoms such as tremor, these endpoints often bear only modest quantitative agreement with measures of quality of life.36, 37 Indeed, patient priorities and sources of disability often arise from non-motor deficits (e.g., depression, anxiety, fatigue, orthostatic hypotension, sleep disturbance). Unfortunately, relatively few studies have thus far focused on capturing the fluctuations of these complex disease manifestations, marked by high variability within and between days.38, 39 The development of TOMs for non-motor endpoints has relied upon labor intensive or computerized, laboratory-based measurements (e.g., cognitive function, heart-rate variability, blood pressure changes, or sleep).40, 41 There is an urgent need for developing unobtrusive systems to monitor non-motor endpoints in the home and community settings.
Limitations of sensors used to monitor motor symptoms
Biomechanical sensors such as accelerometers, gyroscopes, and magnetometers are well suited for the detection of tremor, bradykinesia, gait impairment, and motor complications, such as dyskinesia. However, data collected in the home and community settings using these sensors do not always provide sufficient information to achieve a reliable clinical assessment of motor symptoms. For instance, it is difficult to infer from the sensor data alone if slowness of movement (as detected using biomechanical sensors) can be used as a proxy of bradykinesia, or is the result of fatigue or other factors related to the context in which a motor task is performed (e.g., slow walking due to fear of falling). Also, the resolution of biomechanical sensors is quite restricted to the anatomical area on which they are applied, which may yield low quantitative agreement with the wider range of motor disability, quality of life, and other measurable patient-relevant endpoints.36, 37
Discrepancy between clinical needs and research
Endpoints that may be ideally suited for a clinical study may not necessarily be relevant or applicable in clinical care. The relevance of specific TOMs to assessing the impact of parkinsonian symptoms on patients’ quality of life may be difficult to evaluate. For instance, fluctuations in motor symptoms and complications such as dyskinesia may have a complex quantitative relationship with measures of disability.42 In addition, the measurement target and timeline of data capture differ depending on the goals of a study. For example, an instrumented test that captures finger tapping over several hours may suffice to track the immediate response to a dopaminergic therapy. However, monitoring disease progression over time involves more complex targets and longer data collection, such as physical activity levels, gait speed, frequency of falls and near fall events, as well as a variety of periodic or continuously gathered measures of motor, cognitive, or other non-motor functions. In many cases, accuracy and reliability of these TOMs may not yet be sufficient to justify their deployment in Phase III clinical trials.
Lack of compatibility among wearable systems
Most wearable systems developed to monitor individuals with PD are not compatible with one another. As a result, it may be cumbersome or impossible to combine data gathered by TOMs developed by different manufacturers. This makes it difficult to guide behavioral changes or therapeutic interventions. Furthermore, devices developed by different manufacturers for the same purpose may not always yield the same result, raising questions about the validity of the mathematical algorithms that govern the data processing. Few currently available systems gather synchronized data from multiple body segments before transfer to a computer for whole-body analysis,43 in a way that is fully compatible with the simultaneous use of platforms developed by different manufacturers.
Limitations of available analytical methods
Despite our ability to collect and store extremely large datasets of TOMs, our ability to algorithmically analyze and synthetically display clinically and disease-relevant information to physicians and patients, remains limited. Here, clinical expertise is needed, for instance, to eliminate the “clinical noise” in the data analytical efforts. In addition, technical expertise is needed so that the field can take advantage of the tremendous advances that have been achieved over the past two decades in research areas such as signal processing and machine learning. Data analysis techniques that leverage advances in these research areas are important to achieving clinically meaningful TOMs.
Practical limitations in user engagement
Software and hardware components of wearable systems are often not as user friendly or compelling to adopt as they should be.44 Currently, patient and caregiver engagement with wearable and mobile technology is modest, as shown by a recent study demonstrating that 32% of users stop using wearables after 6 months, and 50% after just over a year.45 Similarly, there is a high dropout rate amongst smartphone apps users: 26% of apps are used only once and 74% of apps are not used more than 10 times.45, 46 Lack of motivation to use wearables/self-monitoring systems should not be underestimated, particularly in the absence of meaningful feedback provided to their users. Preliminary evidence suggests that patient empowerment and their inclusion as active players in the development of research activities may favorably impact compliance.47 Research is needed to determine the characteristics of wearable systems for long-term monitoring of motor and non-motor symptoms that would be acceptable to patients. In particular, we need to ascertain the number of sensors needed to accurately monitor PD symptoms without negatively affecting compliance in a clinical context.
OPPORTUNITIES
Wearable systems provide the opportunity to measure and monitor the individual variability of motor and non-motor phenomena, minimize rater bias, and increase sensitivity to subclinical but possibly relevant physiologic changes (Table 2).4, 10, 48, 49
Table 2.
Limitations of existing TOMs and opportunities for the development of new TOMs using wearable technology
| Limitations | Main roadblocks | Opportunities |
|---|---|---|
| Limited measurement | Low spatial resolution Single sensor data capture |
Multiple sensors Recording multiple motor and non-motor behaviors Measure their natural variability |
| Open-loop data capture | Failure to connect to other sensors or inform treatment | Continuous feedback into a closed-loop system that dictate treatment changes |
| High burden-to-value ratio | The larger the number of sensors used, the more significant is the negative impact on activities and adherence | Minimally-obtrusive, continuously-sensing systems Improve compliance Less missing data Lower cost of care |
| Non-interactivity | Off-line and unidirectional (i.e. clinician to patient) feedback modalities are not effective | Increasing real-time interaction between patient and data and between patient and clinician Decrease need for in-person clinical visits Personalize and improve patient care |
| Multiplicity of technology platforms | Too many incompatible devices Duplicative efforts Poor comparability of measures Limited interoperability |
Standardization of platform Increasing TOMs sensitivity* Increasing TOMs reliability Decreasing measurement errors More contextual information |
However, small changes of particularly sensitive measures may not necessarily be clinically relevant (e.g., levodopa-induced, peak-dose dyskinesia could vary in amplitude but not bear a significant impact on disability because of the non-linear relationship between amplitude of dyskinetic movements and disability).
Standard measurement platform
Several companies have tested or are in the process of assessing a variety of methods to probe individual motor and non-motor constructs. To avoid duplication of investments and efforts, an opportunity exists to identify the technologies and approaches with most versatility, greatest ease of deployment, least patient and physician encumbrance, and lowest cost. It should no longer be a question of whether a given motor phenomenon can be measured in yet a different manner (which it can), but how to choose a standard platform of TOMs behind which developers and end-users can coalesce. Efforts towards standardization – guided by the MDS Task Force on Technology but endorsed or sanctioned by regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) – will greatly facilitate technology adoption and the integration of different systems.
Multi-domain measurements
With different types of disabilities comes the need for tailored measurement approaches to support the design of individualized interventions. Tremor measurement may be completely irrelevant to individuals with an akinetic or postural-instability gait-disorder (PIGD) phenotype of PD. Continuous step monitoring to capture freezing of gait episodes would be futile in patients without gait impairment. Systems that are designed for multi-domain data capture could provide researchers and clinicians with the flexibility of choosing the sensors to monitor individuals with different phenotypes of PD. In exploratory studies, an approach based on multi-domain data capture would increase the likelihood of finding relevant changes in one of the many “channels” of the system. This approach would also provide the opportunity for assessing correlated effects of symptoms of interest across other domains.
Better phenotyping and subtyping
Tremor and tremorless (akinetic/PIGD) variants of PD are grossly defined clinical phenotypes, based on mainly observational evidence, with substantial heterogeneity.50 Besides these clinical phenotypes, there likely exist several disease subtypes defined by autonomic, cognitive or other domains of disability that could be captured by multichannel systems.51 In addition, it is conceivable that the greater resolution of TOMS may detect novel phenomena that could serve to more sensitively stratify certain PD subtypes and serve as (or assist in the development of) biomarkers of disease progression.
Precision medicine
By identifying areas of dysfunction and their relationship with therapy, TOMs can be used to provide customized feedback to individual patients and possibly stratify criteria that “predict” the responsiveness to distinct treatment paradigms, in a way that is similar to how consumer-based wearable devices already measure level of activity, sleep disturbances, etc. Smart algorithms could be developed to generate specific recommendations that would be made available directly to patients and clinicians to motivate changes in treatment and lifestyle-related behaviors, tailored to each person’s specific individual needs and disabilities. This approach would provide value for end-users (both patients and their care team) and thereby improve adherence.
Closed-loop (feedback) systems
Data collected using wearable sensors could be used to trigger device-based interventions. Much as ECG sensing is used in cardiac defibrillators to trigger the delivery of stimulation pulses, data collected from sensors positioned on the limbs and trunk could be used to predict, for instance, the onset of a freezing episode. The system may detect an increase in cadence with a corresponding decrease in step length or a change in frequency of the lower leg oscillations.34 The detection of such motor behaviors could trigger a device designed to deliver proprioceptive cues that could lead to a change in postural control and stepping pattern, which in turn could prevent a fall.43, 52, 53
Real-time symptom tracking
TOMs could offer real-time, continuously captured, rater-independent data, in contrast with clinical assessments that rely upon subjective information gathered during sporadic, in-clinic evaluations.54 Continuous monitoring of parkinsonian symptoms could replace diary-based recordings of fluctuations and be used to track periods of OFF, OFF with dystonia or dyskinesia (not currently captured using the Hauser diary), ON, and ON with dyskinesia over the time span of several days.
The promise of remote monitoring
TOMs based on the use of wearable systems could improve healthcare delivery by providing assessment data when patients are not in the clinic. This possibility is particularly relevant for individuals with PD who live in areas with limited access to care. TOMs could provide ecologically valid data to help clinicians monitor responses to therapy and individualize management in order to optimize outcomes.49 Remote monitoring also offers the opportunity for healthcare cost reduction.8
Better monitoring, better patient engagement, better outcomes
Innovation in sensor and communication technologies alongside mobile connectivity has enabled a process of “medical democratization”.1 The creation or support of TOMs for remote, continuous monitoring provides an opportunity for healthcare providers to scale and extend services offered to patients in order to better manage their health.55 TOMs can capture meaningful aspects of function that improve personalized patient care through an intuitive, interconnected, and energy-efficient interface.56, 57
POTENTIAL PITFALLS IN DEVELOPING TOMS
Clinimetric validation pitfall
A number of studies have been focused on developing methods to derive TOMs that parallel the clinical assessment scales commonly utilized in clinic. These are important but potentially misguided efforts toward validating new TOMs in the clinic or home settings by attempting to force a simple quantitative agreement with widely used, previously validated subjective rating scales or questionnaires. However, it could be argued that, in theory, a “perfect” objective measurement should have a complicated quantitative match with an “imperfect” subjective one. This is because a clinical rater integrates many sources of information in order to produce a subjective score, including prior experience and expectations. So there is no a priori reason to believe that assessments performed by clinical raters would lead to a simple quantitative agreement with data features derived from sensor data. Indeed, the relationship between TOMs and subjective clinical scores may be highly complex and extremely difficult to ascertain in practice.
To the extent that we are seeking more sensitive and “ecologically valid” technologies, TOMs may agree only loosely with clinical scales. An important aim of TOMs is to improve upon, rather than act as surrogates of, previously developed clinical scales. As these are developed, clinicians and regulatory agencies will need to consider that a new TOM which appears to provide clinically relevant measures of movement characteristics, but which does not correlate with the motor section of the Unified Parkinson Disease Rating Scale [UPDRS], for example, could be accepted as valid on the basis of its own merits if it can accurately represent patient-relevant endpoints. Engineers and clinicians alike should be reminded to “think outside the box” and use the power of the technology to develop new scoring paradigms rather than solely generate sensor-based versions of existing clinical scores.
Ecological validity pitfall
Efforts in ensuring validity, or the degree to which we are truly measuring what we intend to measure, increase in complexity with proliferating technologies, evolving in different platforms, and on different targets with unclear ecological and clinical relevance. To this end, before developing new TOMs, researchers would need to determine which constructs generated by routine clinical observations, and standardized by clinical scales and medical devices, are truly relevant to patients within such domains and are meaningful contributors to the performance of activities of daily living (ADLs). For example, if “dyskinesia” is not relevant as a construct to patients and is not a significant contributor to the performance of ADLs in their ecological environment, do we invest in maintaining its primacy in future technologies? Efforts to ensure system interoperability and to build open data repositories will help distinguishing relevant from futile TOMs.
“Big data” pitfall
It has been demonstrated that an abundance of behavioral data can be captured from individuals and populations using largely unstructured, “crowd-sourced” efforts. These data may differentiate populations of loosely defined “Parkinson’s disease” (on the basis of generic measures of movement abnormalities) from “normal” subjects. However, although these measures can provide valuable background information at the population and community level, they cannot substitute for a careful neurological examination, deep clinical phenotyping, and assessment via laboratory studies. At best, they complement but do not replace the phenomenological and pathophysiological granularity required for PD subtyping, much less predict the response of an individual to treatment. Ultimately, the reproducibility and responsiveness of individually selected TOMs confirmed beyond small pilot studies and accounting for contextual information and confounders, should prevail over simply obtaining a large body of population-level data.
PREPARING FOR TOMS IN CLINICAL CARE AND THE RESEARCH SETTING
Wearable systems that are used to gather TOMs in the home and community settings could generate real-time, accurate, sensitive, and rich datasets including contextual information and data such as the time of medication, food intake, and location information. While TOMs are typically derived from wearable sensors, contextual information is captured using companion applications (e.g., mobile apps and web-based applications). Wearable systems that are used to gather TOMs also provide an opportunity for multidirectional interactions among investigators/clinicians and patients/caregivers at a reasonable cost. In the clinical care setting, the use of wearable systems to generate TOMs could decrease the need for outpatient visits while maintaining high-quality care and high patient engagement. Likewise, the integration of TOMs with virtual-visit interfaces has the potential to greatly improve the accuracy and value of telemedicine visits. In the clinical research setting, the use of wearable systems could enhance protocol adherence and patient compliance, leading to fewer missing data points. Also, an appropriate choice of TOMs could lead to prospectively collecting data with high signal-to-noise ratio (with regard to the effect size of interest) hence reducing the required sample size and the resulting study costs. The composite of TOMs, companion applications designed to gather contextual information and pharmacogenomics could enable precision medicine interventions.58
Integrating technologies
The development of new TOMs is currently advancing in isolated silos rather than as part of concerted actions aimed to implement open platforms. The development of open platforms would be highly desirable in the context of obtaining comprehensive information on patients and populations of interest. An open platform may not yet be fostered by the brave new world of health-driven technologies. However, there are reasons for optimism. While in the traditional medical device market, short-term financial forces drive the creation of proprietary measuring instruments at the expense of multi-channel, interconnected systems, consumer-driven market forces are pushing heavily in the opposite direction, i.e. towards the development of open technology platforms. As consumer technologies evolve to achieve the clinimetric sophistication required for application in the clinical management of individuals with PD, the move towards shared, interoperable software and hardware for applications in research and clinical practice is also emerging.
Smart delivery of treatment
Justification for the development and adoption of TOMs is strongest when presented in the context of improving the clinical management of individuals with PD. TOMs can be used as part of closed-loop systems designed to assist in the controlled delivery of medications. The development of such systems requires manufacturing high-performance, energy-efficient and energy-harvesting sensors and storage modules.59 In this context, the development of nanomembranes and stretchable electronics on a polymeric substrate for intimate mechanical contact with soft tissue has been proposed.60 A critical unmet need is the ability to connect multi-sensor diagnostics to self-guided therapies in a closed-loop system. In the field of neuromodulation, deep brain stimulation (DBS) electrodes may also act as sensors, capable of recording local field potentials (for the presence or absence of beta-band oscillations) to automatically program the amplitude and frequency of stimulation, thus effectively closing the loop between measuring and treating.61
Objective measures of specific movement impairments can also be used to tailor therapy. Proof of concept systems have been developed to predict the onset of pathological tremor using surface electromyographic (sEMG) and acceleration data, which could inform the design of the next generation of non-invasive closed-loop predictive ON-OFF controllers for DBS.62, 63 In the realm of physical rehabilitation, subtle asymmetries in gait, limitations in joint range of motion, or excessive postural sway indicating poor balance may be difficult to observe clinically but can be addressed by rehabilitation specialists when identified using TOMs. Through TOMs, therapists could personalize the therapy prescribed to each individual.43 A simple clinical measure such as the time needed to walk a specified distance does not provide the therapist with an understanding of the spatial and temporal gait performance or the musculoskeletal and dynamic balance characteristics that cause poor mobility. These factors could be captured using TOMs and hence guide the choice of appropriate therapeutic approaches. Longitudinal monitoring of TOMs also has the potential for identifying small improvements or declines related to the intervention or the progression of the disease that could lead to changes in the prescribed rehabilitation intervention.
Regulatory needs and commercialization
We anticipate that TOMs will eventually be routinely used in both clinical practice and research settings. Despite the promise of greater sensitivity and the presumed accuracy of collected data, regulatory validation of TOMs as efficacy and safety measures will require dedicated studies. The path to marketing for TOMs appears long and risky considering the short lifecycle of technological innovation and the costs associated with their development. Unlike drug development, where there is substantial precedent and a regimented path for marketing authorization, commercialization, and license protection, the path for TOMs and digital health solutions remains to be defined. It is critical that key stakeholders share the costs and financial rewards of technology development, implementation, and maintenance in order to accelerate and preserve innovation and growth. Despite opportunities to meet all stakeholder needs, the business model for development and deployment of TOMs in healthcare remains to be determined. Currently, healthcare payers show little incentive to financially reimburse TOMs, despite the promise for healthcare cost reduction, population management and delivery of high quality, efficient care. The lack of incentive may be driven by initial expenditures and the complexity of a rapidly evolving, but not yet fully integrated, technology market. Providers are also reluctant to fully adopt TOMs despite early evidence that they can improve patient outcomes and lead to overall improved care and patient satisfaction. Clinicians may not yet view TOMs as an opportunity to support clinical decision-making and increase productivity. Patients are also reluctant to pay out of pocket despite opportunities for improved access to better care and better outcomes. Building a solid ‘business case’ –including properly designed cost-effectiveness studies– is much needed. A value-based care approach could be an attractive solution, where deployment of TOMs is funded as part of an integrated care solution where providers are rewarded for good outcomes per invested dollar, and where the decision to engage TOMs is left to the providers and patients. Finally, in addition to funding agencies, regulatory bodies such as the FDA and the EMA should increase efforts toward establishing programs that encourage the adoption of standards aimed to assure interoperability of wearable systems and the development of open data repositories.
Integration into medical care and reimbursement
Payers do not yet provide reimbursement for medical services provided by TOMs and companion apps. This limits the rate of innovation and the opportunities for integration of TOMs into medical care. Establishing reimbursement mechanisms will require demonstration that, along with the enhancements in diagnostics and therapeutics, TOMs can be integrated in quality control concepts, help reduce costs and time, as well as improve patients’ quality of life while guarding against privacy concerns. Quantifying clinical benefits of interventions using TOMs is anticipated to become increasingly important in healthcare as the allocation of resources is expected to be tied to objective outcome measures.
CONCLUSIONS AND NEXT STEPS
Despite challenges, the continuous improvements in technological sophistication, versatility, and wearability of sensors have created opportunities to collect disease-relevant data using targets consequential to patients and sensitive to PD-specific symptoms and milestones. In order to translate these opportunities into enhanced care, better self-management options for PD patients, and overall improved health care outcomes, technologies will need to be 1) developed as open platforms and integrated with electronic medical record systems, 2) suitable for the acquisition of data that captures motor and non-motor phenomena, and 3) integrated in treatment delivery systems. The International Parkinson and Movement Disorders Society Task Force on Technology aims at reversing the current model of simply adapting available technologies to meet patient management and research endpoints. As such, the Task Force will assist in improving the academic and regulatory environments for technology developers by encouraging the sanctioning of open standard platforms for technology-based measurements and treatments by, e.g., the FDA and EMA. This collaborative endeavor will encourage the development of integrated, multi-channel, and in many instances closed-loop feedback systems that can achieve more sophisticated clinico-pathophysiologic characterization, better informed tailoring of symptomatic therapy, greater patient engagement and self-assessment, and better subgroup targeting of patients for testing of future disease-modifying treatments.
Acknowledgments
Financial disclosure related to research covered in this article:
(Full financial discloses for the previous 12 months at the end of this article)
The MDS Technology Task Force assembled a group of experts from the device and biopharmaceutical industry, clinical researchers, and engineers. We have taken every effort to minimize the influence of their home institutions and industries on the summarized outcome of the deliberations.
Dr. Espay is supported by the NIH (K23MH092735) and has received grant support from CleveMed/Great Lakes Neurotechnologies, Davis Phinney Foundation, and Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay, Abbott, Chelsea Therapeutics, TEVA, Impax, Merz, Lundbeck, and Eli Lilly; honoraria from TEVA, UCB, the American Academy of Neurology, and the Movement Disorders Society; and publishing royalties from Lippincott Williams & Wilkins and Cambridge University Press.
Dr. Bonato is supported by the Michael J Fox Foundation, the National Institutes of Health, the National Science Foundation, and the Office of Naval Research.
Dr. Nahab receives funding support from the National Institute of Neurological Disorders and Stroke (1R01NS073683-01A1), the University of Miami Clinical and Translational Science Institute, the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities (1UL1TR000460), research funding support from the Dystonia Coalition (NS067501), research funding from Biotie pharmaceuticals, research funding from Avid Radiopharmaceuticals Inc., an educational grant from Medtronic Inc., and royalties from Springer Publishing.
Dr. Maetzler reports grants from the European Union (FAIR-PARK-II, Moving beyond and SENSE-PARK), from Neuroalliance, Lundbeck, Janssen and the Michael J Fox Foundation, personal fees from Licher, Roelke Pharma, UCB, GSK and Abbvie.
Mr. Dean is supported by the Davis Phinney Foundation to direct their Healthcare Strategy and Technology division.
Dr. Klucken has received grant support from the Bavarian State Ministry of Education, Science and the Arts, Bavarian Research Foundation, Intramural Funding of the FAU, from TEVA, Astrum IT, and LicherMT. Honoraria for consultant/scientific advisory board participation of UCB, TEVA, LicherMT, Desitin, Abbvie, Solvay, Ever Neuro Pharma. Dr. Klucken is Chair of the task force “sensor-based movement analysis” of the German Parkinson Society.
Dr. Eskofier has received grant support from the Bavarian State Ministry of Education, Science and the Arts, Bavarian Research Foundation, Intramural Funding of the FAU, and industry funding from TEVA, Bosch Sensortec and Astrum IT. Dr. Eskofier is an endowed professor of the adidas AG at the FAU. Dr. Eskofier is Co-chair of the task force “sensor-based movement analysis” of the German Parkinson Society.
Dr Merola has received grant support from UCB Pharma and speaker honoraria from CSL Behring, UCB Pharma, and Teva Pharmaceuticals. He has received personal compensation from Edge Consulting S.r.l., MediK S.r.l., and Sthetos S.r.l.
Dr. Horak has research grants from the VA, NIH, NMSS, DoD and Medtronic. Dr. Horak and OHSU have an equity/interest in APDM, a technology company. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU.
Dr Lang has served as an advisor for AbbVie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Ceregene, Cipla, InteKrin, Lilly, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva, and UCB; received honoraria from Medtronic, Teva, UCB, and AbbVie; received grants from Brain Canada, the Canadian Institutes of Health Research, the Edmond J. Safra Philanthropic Foundation, The Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, Physicians Services Incorporated (PSI), Tourette Syndrome Association, W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry.
Dr. Reilmann is founding director and owner of the George-Huntington-Institute, a private research institute focused on clinical and preclinical research in Huntington’s Disease, and QuantiMedis, a clinical research organization providing Q-Motor (quantitative motor) services in clinical trials and research. Dr. Reilmann provided consulting services, advisory board functions, clinical trial services, quantitative motor analyses, and/or lectures for Teva, Pfizer, Ipsen, Vaccinex, Novartis, Raptor, Omeros, Siena Biotech, Neurosearch Inc., Lundbeck, Medivation, Wyeth, ISIS Pharma, Link Medicine, Prana Biotechnology, MEDA Pharma, Temmler Pharma, Desitin, AOP Orphan, and the Cure Huntington’s Disease Initiative Foundation. Dr. Reilmann received grant support from the High-Q-Foundation, the Cure Huntington’s Disease Initiative Foundation (CHDI), the Deutsche Forschungsgemeinschaft (DFG), the European Union (EU-FP7 program), the Bundesministerium für Bildung und Forschung (BMBF), the Deutsches Zentrum für Neurodegeneration und Entzündung (DZNE), and the European Huntington’s Disease Network (EHDN)
Dr. Giuffrida is a full-time employee of Great Lakes Neurotechnologies.
Dr Nieuwboer is supported by grants from the Special Research Fund of KU Leuven, Belgium (contract OT/11/091), Malou-Malou funds of the King Baudouin foundation, Research Foundation Flanders (projects G.0906.11 and G.0867.15) and funding through the Gabe Foundation.
Dr. Horne is supported by the Australian National Health and Medical research Council and has a financial interest in Global Kinetics Corporation, a company that manufactures and supplies the Parkinson’s KinetiGraph (PKG), a wearable technology.
Dr. Little has nothing to disclose.
Dr. Litvan is supported by grants 5P50 AG005131-31, 5T35HL007491, 1U01NS086659, 1U54NS092089-01, Parkinson Study Group, Michael J Fox Foundation, CBD Solutions-CurePSP, AVID Pharmaceuticals, C2N Diagnostics, and BMS; serves in the Advisory Board of Pfizer/MJFF, Northera, Bristol-Myers Squibb; and in the Advisory Board for a PSG Biotie/Michael J Fox Foundation study.
Dr. Simuni has received consulting fees from Acadia, Abbvie, Allergan, GE Medical, Eli Lilly, Harbor, Ibsen, IMPAX, Lundbeck, Merz, Inc., the National Parkinson Foundation, Navidea, Pfizer, TEVA, UCB Pharma, US World Meds; served as a speaker and received honoraria from Allergan, GE Medical, Ibsen, Lundbeck, IMPAX, TEVA, and UCB Pharma; received research support from the National Parkinson Foundation, TEVA, Auspex, Biotie, Civitas, NIH, and the Michael J. Fox Foundation; and received funding support for educational programming from GE Medical, TEVA, Lundbeck, Medtronic, and Merz, Inc.
Dr. Dorsey is supported by the National Institute of Neurological Disorders and Stroke (P20 NS092529), has stock options in Grand Rounds, has filed for a patent related to telemedicine and neurology, has served as a consultant to the National Institute of Neurological Disorders and Stroke, Clintrex, MC10, Shire, and MedAvante, provided medico-legal advice, and has received research funding from AMC Health, Davis Phinney Foundation, Great Lakes Neurotechnologies, Huntington Study Group, Michael J. Fox Foundation, National Institute of Neurological Disorders and Stroke, National Science Foundation, Patient-Centered Outcomes Research Institute, Prana Biotechnology, Raptor Pharmaceuticals, Sage Bionetworks, and Teva Pharmaceuticals.
Dr. Burack owns stock in Novartis. She has no other commercial relationships.
Mr. Kubota directs data science and the partnership with Intel on wearable technologies and analytics enacted through the Michael J Fox Foundation for Parkinson’s Research full time.
Dr. Kamondi has nothing to disclose
Dr. Godinho has nothing to disclose
Dr. Daneault is supported by the Canadian Institute of Health Research and the Michael J Fox Foundation.
Dr. Mitsi is the Founder & CEO of Apptomics LLC.
Dr. Krinke is a full-time employee of Medtronic and serves as Board Observer at Functional Neuromodulation, Ltd.
Prof. Hausdorff has received research support from the Tel Aviv Sourasky Medical Center, NIH, Michael J Fox Foundation for Parkinson’s Research, National Parkinson Foundation, Israel Science Foundation, and FP7 European Commission. He submitted a patent application on the use of body-fixed sensors in Parkinson disease. The intellectual property rights for this patent application are held by the Tel Aviv Sourasky Medical Center.
Prof. Bloem currently serves as Associate Editor for the Journal of Parkinson’s disease, serves as editorial board member of Physiotherapy Canada, has received honoraria from serving on the scientific advisory board for Danone and Zambon, has received fees for speaking at conferences from AbbVie and Teva, and received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, the Prinses Beatrix Foundation, the Stichting Parkinson Fonds, the National Parkinson Foundation, the Hersenstichting Nederland, and the Parkinson Vereniging.
Dr. Papapetropoulos is a full-time employee of TEVA Pharmaceuticals.
Footnotes
All other authors have no financial disclosure to report related to research covered in this article.
Authors’ roles
1. Research project: A. Conception, B. Organization, C. Execution
2. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique
AJE: 1A, 1B, 1C, 2A, 2B
PB: 1B, 1C, 2C
FN, WM, JMD, JK, BME, AM, FH, AEL, RR, JG, AN, MH, MAL, IL, TS, RD, MAB, KK, AK, CG, J-FD, GM, LK, JMH: 1C, 2B
BRB: 1B, 1C, 2B
SP: 1A, 1B, 2B
References
- 1.Pasluosta CF, Gassner H, Winkler J, Klucken J, Eskofier BM. An Emerging Era in the Management of Parkinson’s Disease: Wearable Technologies and the Internet of Things. IEEE J Biomed Health Inform. 2015;19(6):1873–1881. doi: 10.1109/JBHI.2015.2461555. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Jafari R, Zhou G, et al. Guest Editorial: Special Issue on Internet of Things for Smart and Connected Health. Internet of Things Journal, IEEE. 2015;2(1):1–4. [Google Scholar]
- 3.Ozinga SJ, Machado AG, Miller Koop M, Rosenfeldt AB, Alberts JL. Objective assessment of postural stability in Parkinson’s disease using mobile technology. Mov Disord. 2015;30(9):1214–1221. doi: 10.1002/mds.26214. [DOI] [PubMed] [Google Scholar]
- 4.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov Disord. 2013;28(11):1544–1551. doi: 10.1002/mds.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani B, Jimenez MC, Vingerhoets FJ, Aminian K. On-shoe wearable sensors for gait and turning assessment of patients with Parkinson’s disease. IEEE Trans Biomed Eng. 2013;60(1):155–158. doi: 10.1109/TBME.2012.2227317. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther. 2012;36(2):51–57. doi: 10.1097/NPT.0b013e318254ba7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi F, Bee C, Duval C, et al. Using ecological whole body kinematics to evaluate effects of medication adjustment in Parkinson disease. J Parkinsons Dis. 2014;4(4):617–627. doi: 10.3233/JPD-140370. [DOI] [PubMed] [Google Scholar]
- 8.Papapetropoulos S, Mitsi G, Espay AJ. Digital Health Revolution: Is it Time for Affordable Remote Monitoring for Parkinson’s Disease? Front Neurol. 2015;6:34. doi: 10.3389/fneur.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shull PB, Jirattigalachote W, Hunt MA, Cutkosky MR, Delp SL. Quantified self and human movement: a review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture. 2014;40(1):11–19. doi: 10.1016/j.gaitpost.2014.03.189. [DOI] [PubMed] [Google Scholar]
- 10.Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov Disord. 2013;28(12):1628–1637. doi: 10.1002/mds.25628. [DOI] [PubMed] [Google Scholar]
- 11.Bonato P. Wearable sensors and systems. From enabling technology to clinical applications IEEE Eng Med Biol Mag. 2010;29(3):25–36. doi: 10.1109/MEMB.2010.936554. [DOI] [PubMed] [Google Scholar]
- 12.Espay AJ, Giuffrida JP, Chen R, et al. Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson’s disease. Mov Disord. 2011;26(14):2504–2508. doi: 10.1002/mds.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz CG, Stebbins GT, Wolff D, et al. Testing objective measures of motor impairment in early Parkinson’s disease: Feasibility study of an at-home testing device. Mov Disord. 2009;24(4):551–556. doi: 10.1002/mds.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klucken J, Barth J, Kugler P, et al. Unbiased and mobile gait analysis detects motor impairment in Parkinson’s disease. PLoS One. 2013;8(2):e56956. doi: 10.1371/journal.pone.0056956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BR, Patel S, Buckley T, et al. A web-based system for home monitoring of patients with Parkinson’s disease using wearable sensors. IEEE Trans Biomed Eng. 2011;58(3):831–836. doi: 10.1109/TBME.2010.2090044. [DOI] [PubMed] [Google Scholar]
- 16.Stamford JA, Schmidt PN, Friedl KE. What Engineering Technology Could Do for Quality of Life in Parkinson’s Disease: A Review of Current Needs and Opportunities. IEEE J Biomed Health Inform. 2015;19(6):1862–1872. doi: 10.1109/JBHI.2015.2464354. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Graber A, McBurney RN, Balasubramanian R. Sample size and statistical power considerations in high-dimensionality data settings: a comparative study of classification algorithms. BMC Bioinformatics. 2010;11:447. doi: 10.1186/1471-2105-11-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy SH, Cole BT, Gilmore LD, et al. High-resolution tracking of motor disorders in Parkinson’s disease during unconstrained activity. Mov Disord. 2013;28(8):1080–1087. doi: 10.1002/mds.25391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mera TO, Burack MA, Giuffrida JP. Quantitative assessment of levodopa-induced dyskinesia using automated motion sensing technology. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:154–157. doi: 10.1109/EMBC.2012.6345894. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Trutoiu L, Murai A, et al. Quantitative measurement of motor symptoms in Parkinson’s disease: a study with full-body motion capture data. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6789–6792. doi: 10.1109/IEMBS.2011.6091674. [DOI] [PubMed] [Google Scholar]
- 21.Shen X, Mak MK. Technology-assisted balance and gait training reduces falls in patients with Parkinson’s disease: a randomized controlled trial with 12-month follow-up. Neurorehabil Neural Repair. 2015;29(2):103–111. doi: 10.1177/1545968314537559. [DOI] [PubMed] [Google Scholar]
- 22.Weiss A, Herman T, Giladi N, Hausdorff JM. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS One. 2014;9(5):e96675. doi: 10.1371/journal.pone.0096675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21(1):122–127. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Ho C, Slappey N, et al. A Wearable Conductivity Sensor for Wireless Real-time Sweat Monitoring. Sensors and Actuators B: Chemical. 2015 [Google Scholar]
- 25.Caldara M, Colleoni C, Guido E, Re V, Rosace G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sensors and Actuators B: Chemical. 2016;222:213–220. [Google Scholar]
- 26.Cazalé A, Sant W, Ginot F, et al. Physiological stress monitoring using sodium ion potentiometric microsensors for sweat analysis. Sensors and Actuators B: Chemical. 2016;225:1–9. [Google Scholar]
- 27.Solla P, Cadeddu C, Cannas A, et al. Heart rate variability shows different cardiovascular modulation in Parkinson’s disease patients with tremor dominant subtype compared to those with akinetic rigid dominant subtype. Journal of Neural Transmission. 2015:1–6. doi: 10.1007/s00702-015-1393-5. [DOI] [PubMed] [Google Scholar]
- 28.Sommerauer M, Imbach LL, Jarallah M, Baumann CR, Valko PO. Diminished event-related cortical arousals and altered heart rate response in Parkinson’s disease. Mov Disord. 2015;30(6):866–870. doi: 10.1002/mds.26165. [DOI] [PubMed] [Google Scholar]
- 29.Hellman AM, Shah SP, Pawlowski SM, Duda JE, Morley JF. Continuous non-invasive monitoring to detect covert autonomic dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(7):723–728. doi: 10.1016/j.parkreldis.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Nieuwboer A, De Weerdt W, Dom R, Lesaffre E. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil. 1998;20(4):142–150. doi: 10.3109/09638289809166074. [DOI] [PubMed] [Google Scholar]
- 31.Brun L, Lefaucheur R, Fetter D, et al. Non-motor fluctuations in Parkinson’s disease: prevalence, characteristics and management in a large cohort of parkinsonian outpatients. Clin Neurol Neurosurg. 2014;127:93–96. doi: 10.1016/j.clineuro.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Jankovic J. Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord. 2005;20(Suppl 11):S11–16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- 33.Patel S, Lorincz K, Hughes R, et al. Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors. IEEE Trans Inf Technol Biomed. 2009;13(6):864–873. doi: 10.1109/TITB.2009.2033471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore ST, Yungher DA, Morris TR, et al. Autonomous identification of freezing of gait in Parkinson’s disease from lower-body segmental accelerometry. J Neuroeng Rehabil. 2013;10:19. doi: 10.1186/1743-0003-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SI, Daneault J-F, Golabchi FN, et al. Engineering in Medicine and Biology Society (EMBC), 2015 37th Annual International Conference of the IEEE. IEEE; 2015. A novel method for assessing the severity of levodopa-induced dyskinesia using wearable sensors; pp. 8087–8090. [DOI] [PubMed] [Google Scholar]
- 36.Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Dibble LE. Which measures of physical function and motor impairment best predict quality of life in Parkinson’s disease? Parkinsonism Relat Disord. 2011;17(9):693–697. doi: 10.1016/j.parkreldis.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berganzo K, Tijero B, Gonzalez-Eizaguirre A, et al. Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurologia. 2014 doi: 10.1016/j.nrl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Dubey H, Goldberg JC, Abtahi M, Mahler L, Mankodiya K. Proceedings of the conference on Wireless Health. ACM; 2015. EchoWear: smartwatch technology for voice and speech treatments of patients with Parkinson’s disease; p. 15. [Google Scholar]
- 39.Bayestehtashk A, Asgari M, Shafran I, McNames J. Fully automated assessment of the severity of Parkinson’s disease from speech. Computer speech & language. 2015;29(1):172–185. doi: 10.1016/j.csl.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maetzler W, Liepelt I, Berg D. Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol. 2009;8(12):1158–1171. doi: 10.1016/S1474-4422(09)70291-1. [DOI] [PubMed] [Google Scholar]
- 41.Valappil RA, Black JE, Broderick MJ, et al. Exploring the electrocardiogram as a potential tool to screen for premotor Parkinson’s disease. Mov Disord. 2010;25(14):2296–2303. doi: 10.1002/mds.23348. [DOI] [PubMed] [Google Scholar]
- 42.Hung SW, Adeli GM, Arenovich T, Fox SH, Lang AE. Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81(10):1112–1115. doi: 10.1136/jnnp.2009.173286. [DOI] [PubMed] [Google Scholar]
- 43.Horak F, King L, Mancini M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys Ther. 2015;95(3):461–470. doi: 10.2522/ptj.20140253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher JM, Hammerla NY, Rochester L, Andras P, Walker RW. Body-Worn Sensors in Parkinson’s Disease: Evaluating Their Acceptability to Patients. Telemed J E Health. 2015 doi: 10.1089/tmj.2015.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledger D, McCaffrey D. In: Inside Wearables: How the Science of Human Behavior Change Offers the Secret to Long-Term Engagement. LLC EP, editor. Endeavour Partners LLC; 2014. [Google Scholar]
- 46.Consumer Intelligence Series: The wearable future. Pricewaterhouse Coopers [Google Scholar]
- 47.Ferreira JJ, Godinho C, Santos AT, et al. Quantitative home-based assessment of Parkinson’s symptoms: the SENSE-PARK feasibility and usability study. BMC Neurol. 2015;15:89. doi: 10.1186/s12883-015-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieber B, Taylor BE, Appelboom G, McKhann G, Connolly ES., Jr Motion Sensors to Assess and Monitor Medical and Surgical Management of Parkinson Disease. World Neurosurg. 2015;84(2):561–566. doi: 10.1016/j.wneu.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Arora S, Venkataraman V, Zhan A, et al. Detecting and monitoring the symptoms of Parkinson’s disease using smartphones: A pilot study. Parkinsonism Relat Disord. 2015;21(6):650–653. doi: 10.1016/j.parkreldis.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71(4):499–504. doi: 10.1001/jamaneurol.2013.6233. [DOI] [PubMed] [Google Scholar]
- 51.Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol. 2015;72(8):863–873. doi: 10.1001/jamaneurol.2015.0703. [DOI] [PubMed] [Google Scholar]
- 52.Mazilu S, Calatroni A, Gazit E, Mirelman A, Hausdorff JM, Troster G. Prediction of Freezing of Gait in Parkinson’s From Physiological Wearables: An Exploratory Study. IEEE J Biomed Health Inform. 2015;19(6):1843–1854. doi: 10.1109/JBHI.2015.2465134. [DOI] [PubMed] [Google Scholar]
- 53.Bachlin M, Plotnik M, Roggen D, et al. Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans Inf Technol Biomed. 2010;14(2):436–446. doi: 10.1109/TITB.2009.2036165. [DOI] [PubMed] [Google Scholar]
- 54.Rampp A, Barth J, Schulein S, Gassmann KG, Klucken J, Eskofier BM. Inertial sensor-based stride parameter calculation from gait sequences in geriatric patients. IEEE Trans Biomed Eng. 2015;62(4):1089–1097. doi: 10.1109/TBME.2014.2368211. [DOI] [PubMed] [Google Scholar]
- 55.Mendiola MF, Kalnicki M, Lindenauer S. Valuable features in mobile health apps for patients and consumers: content analysis of apps and user ratings. JMIR Mhealth Uhealth. 2015;3(2):e40. doi: 10.2196/mhealth.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hubble RP, Naughton GA, Silburn PA, Cole MH. Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: a systematic review. PLoS One. 2015;10(4):e0123705. doi: 10.1371/journal.pone.0123705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del Din S, Godfrey A, Rochester L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: toward clinical and at home use. IEEE J Biomed Health Inform. 2015 doi: 10.1109/JBHI.2015.2419317. [DOI] [PubMed] [Google Scholar]
- 58.FirstWord. Patient-Centric Mobile Apps: Key Opportunities and Challenges for Pharma. Doctor’s Guide Publishing Limited. 2015 [Google Scholar]
- 59.Zheng YL, Ding XR, Poon CC, et al. Unobtrusive sensing and wearable devices for health informatics. IEEE Trans Biomed Eng. 2014;61(5):1538–1554. doi: 10.1109/TBME.2014.2309951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Son D, Lee J, Qiao S, et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat Nanotechnol. 2014;9(5):397–404. doi: 10.1038/nnano.2014.38. [DOI] [PubMed] [Google Scholar]
- 61.Quinn EJ, Blumenfeld Z, Velisar A, et al. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov Disord. 2015 doi: 10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- 62.Basu I, Graupe D, Tuninetti D, et al. Pathological tremor prediction using surface electromyogram and acceleration: potential use in ‘ON-OFF’ demand driven deep brain stimulator design. J Neural Eng. 2013;10(3):036019. doi: 10.1088/1741-2560/10/3/036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shukla P, Basu I, Tuninetti D. Towards closed-loop deep brain stimulation: decision tree-based essential tremor patient’s state classifier and tremor reappearance predictor. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:2605–2608. doi: 10.1109/EMBC.2014.6944156. [DOI] [PubMed] [Google Scholar]


