Abstract
Background
Carbon dioxide (CO2) inhalation, a biological challenge and pathological marker in Panic Disorder, evokes intense fear and panic attacks in susceptible individuals. The molecular identity and anatomical location of CO2-sensing systems that translate CO2-evoked fear remains unclear. We investigated contributions of microglial acid sensor T cell death associated gene-8 (TDAG8) and microglial pro-inflammatory responses in CO2-evoked behavioral and physiological responses.
Methods
CO2-evoked freezing, autonomic and respiratory responses were assessed in TDAG8-deficient (−/−) and wildtype (+/+) mice. Involvement of TDAG8-dependent microglial activation and pro-inflammatory cytokine IL-1β with CO2-evoked responses was investigated using microglial blocker, minocycline and IL-1β antagonist, IL- 1RA. CO2-chemosensitive firing responses using single-cell patch clamping were measured in TDAG8−/− and +/+ mice to gain functional insights.
Results;
TDAG8 expression was localized in microglia enriched within the sensory circumventricular organs (CVOs). TDAG8−/− mice displayed attenuated CO2-evoked freezing and sympathetic responses. TDAG8 deficiency was associated with reduced microglial activation and pro-inflammatory cytokine, IL-1β within the subfornical organ (SFO). Central infusion of microglial activation blocker, minocycline and IL-1β antagonist, IL-1RA attenuated CO2-evoked freezing. Finally, CO2-evoked neuronal firing in patch clamped SFO neurons was dependent on acid sensor TDAG8 and IL-1β.
Conclusions
Our data identify TDAG8-dependent microglial acid-sensing as a unique chemosensor for detecting and translating hypercapnia to fear-associated behavioral and physiological responses, providing a novel mechanism for homeostatic threat detection of relevance to psychiatric conditions such as panic disorder.
Keywords: Microglia, acid-sensing, TDAG8, CO2, fear, panic
INTRODUCTION
Fear encompasses threat-associated behavioral and physiological responses crucial to survival. Most of our current biological understanding of fear genesis comes from studies where animals are exposed to exteroceptive aversive stimuli such as pain or predator exposure (1; 2). Fear responses can also be evoked by stimuli producing an internal threat to homeostasis and imminent danger to survival. A widely-studied interoceptive stimulus, carbon dioxide (CO2) inhalation, produces intense fear, autonomic and respiratory responses that can evoke panic attacks. In humans, CO2-sensitivity lies on a continuum (3) with panic disorder (PD) patients being highly sensitive to low CO2 doses, while healthy volunteers only experience panic-like symptoms at higher concentrations (4; 5). In the extracellular space, CO2 combines with water to produce protons, leading to systemic acidosis (6; 7), which is responsible for panicogenic effects of CO2. Prior studies indicate that acid-sensing ion channels (ASICs) in the amygdala contribute to CO2-evoked fear responses (8). However, recent studies in patients with amygdala damage from Urbach-Wiethe disease indicate that the amygdala is not required for the expression of fear and panic to CO2-inhalation (9), suggesting that distinct chemosensory systems may exist for homeostatic threats such as CO2, that may not engage traditional fear mechanisms.
Microglia, innate immune cells of the central nervous system (CNS) (10) are recruited in physiological responses to homeostatic fluctuations (11). Microglia transform rapidly from a resting to a pro-inflammatory activated state upon sensing subtle imbalance in ionic homeostasis (12; 13), in accordance with their role in maintenance of CNS microenvironment. Extracellular acidification induces rapid alteration in microglial morphology and actin integrity (14; 15), suggesting their potential engagement in the effects of acidotic stimuli such as CO2. This study addresses possible mechanisms responsible for generation of panic-relevant fear responses, focusing on the T-cell death associated gene-8 (TDAG8), an acid-sensing G-protein coupled receptor (16; 17). TDAG8 is expressed in microglial cells resident in sensory circumventricular organs (CVOs). Sensory CVOs such as the subfornical organ (SFO) are integrative sites lacking a blood-brain-barrier and have access to systemic and CNS compartments for maintenance of homeostasis (18). Importantly, the SFO has been identified as a site where interoceptive stimuli can be sensed and relayed to ‘panic’-generating CNS areas (19). Previous work associates the SFO with panic-like responses to intravenous lactate (20; 21). The SFO may therefore be a primary locus for detecting interoceptive challenges relevant to panic. Given the expression of microglial acid-sensor TDAG8 in a panic-regulatory area we investigated potential recruitment of the receptor in CO2-evoked behavior and physiology. We hypothesized that acid-sensor TDAG8-mediated microglial activation within the SFO contributes to the behavioral and physiological sequelae of CO2-inhalation. Our data suggest that acid-sensor TDAG8 acts in the SFO to promote CO2-evoked behavioral (freezing) and physiological (cardiovascular) responses via a mechanism involving microglial activation and pro-inflammatory cytokine IL-1β.
METHODS AND MATERIALS
Animals
TDAG8−/− mice, a generous gift from Dr. Owen Witte, UCLA, were generated on a BALB/c background (22). All experiments reported here were performed on 8–16 week homozygous male mice carrying wild-type (TDAG8+/+) or knockout (TDAG8−/−) allele. All behavioral experiments were performed between 8am–1pm during the 12h light cycle. Study protocols were approved by the Institutional Animal Care and Use Committees of University of Cincinnati and Wright State University, in vivariums accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
CO2- behavior studies
Experimental layout is shown in Fig 2a (details in supplemental section). Mice were habituated to the CO2 chamber for 10min, one day prior to the CO2 challenge (Day 0). On day 1, mice were exposed to air, 5 and 10% CO2 concentrations for 10 min during which behavior was videotaped. This CO2 concentration range is translationally relevant to challenge studies in humans (5). The following day (Day 2) animals were returned to the chamber for 5min in the absence of CO2. Freezing, defined as the complete lack of movement except for respiration, was scored using the FreezeScan software (CleverSys Inc. ) by a trained observer blinded to genotype and treatment. Following an initial dose-response study, all subsequent experiments were conducted using 5% CO2
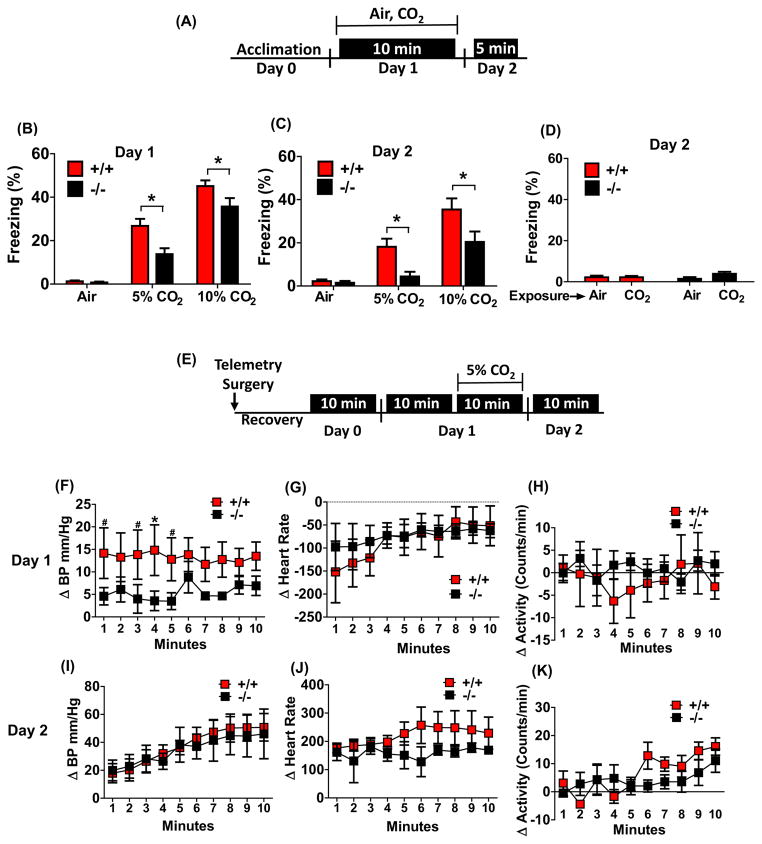
Figure 2.
TDAG8 disruption attenuates fear-associated responses to CO2 inhalation: CO2-evoked freezing behavior (panels a–d) and cardiovascular responses (panels e–k) are shown. (a) schematic of experimental design for measuring CO2-evoked freezing and conditioned fear to CO2 context. (b) A significant attenuation of freezing was observed in TDAG8−/− mice following inhalation of 5% and 10% CO2 on day 1 as compared to TDAG8+/+ mice (n/group= 9,11,12(+/+), 8,10,11(−/−). (c) On day 2, significant attenuation of conditioned freezing to CO2-context was observed in TDAG8−/− mice versus +/+ mice (n=9,11,12(+/+), 8,10,11(−/−). (d) Absence of generalized fear in TDAG8+/+ and −/− mice exposed to CO2 inhalation and placed in neutral context 24 hour post-exposure. Negligible freezing was observed in CO2 exposed mice (CO2) that was comparable to the air exposed group (air). n= 9,10,8,10. (e) Schematic of experimental design for measurement of CO2-evoked cardiovascular activation. Day1 data represent 5% CO2-evoked change in cardiovascular parameters (delta Δ) relative to mean response in context 10 minutes prior to inhalation. (f) CO2-induced elevation in blood pressure (BP) was significantly attenuated in TDAG8−/− versus TDAG8+/+ mice (n= 5). (g) No significant difference in heart rate was observed between genotypes. (h) TDAG8+/+ and −/− mice showed no differences in activity during CO2 inhalation. Day 2 data represent cardiovascular response on exposure to context alone, relative to baseline (defined as mean 2 hr response just before experimenter entry to the animal room). (i) For blood pressure a significant effect of time but no significant effect of genotype was observed. (j) No significant effect on heart rate was observed (k) Motor activity showed a significant effect of time (p<0. 05) but no effect of genotype was observed. All data are mean ± s. e. m. * p <0. 05 versus TDAG8+/+
Radio-Telemetry Surgery and Recording
Mice were implanted with telemetric devices (PA-C20; Data Sciences International) to measure blood pressure (BP) and heart-rate (HR). To assess responses to CO2-inhalation, the data were analyzed as response during CO2-inhalation minus average response 10 min prior to CO2-inhalation. This is consistent with clinical studies where cardiovascular effects during CO2 are analyzed over baseline pre-exposure measurements (23). For measurements during day 2 context exposure, delta of mean response was calculated over baseline (average of 2 hours before entry into the room) as described previously (24).
Whole Body Plethysmography
Ventilatory parameters in unrestrained, non-anesthetized mice were measured using whole-body plethysmography, as described previously (25) with modifications. For setup and data collection details see supplement.
Drug Administration
Minocycline (10μg/500nl, Sigma Aldrich) was administered intracerebroventricularly (icv) once daily for 4d prior to CO2-inhalation exposure. For the minocycline-IL-1β experiment, IL-1β (5ng/500nl, R&D Systems) was administered to minocycline-treated mice 20min prior to CO2-inhalation. For IL-1β necessity and sufficiency experiments, mouse recombinant IL-1RA (an endogenous receptor antagonist that binds selectively to the interleukin-1 receptor (IL-1R) and prevents signaling via this receptor (1. 8μg/2μl, R&D Systems) or IL-1β itself (5ng/500nl) was administered 20min prior to CO2 or air inhalation, respectively. Doses and duration of minocycline, IL-1RA and IL-1β were adapted from previous studies (26–28). For surgery details see supplement.
Immunofluorescence
Coronal brain sections were immunolabeled with primary antibodies against GFP (1:3000, Invitrogen, cat. # A-11122, USA), anti-ionized calcium binding adapter molecule (IBA-1) (1:1000, Synaptic Systems Inc. , cat. # 234-003, Germany), anti-Huc/d (1:200, Invitrogen, cat. # A-21271, USA), anti-glial fibrillary acidic protein (GFAP) (1:1000 Abcam, cat # ab4674, USA) using standard immunofluorescence procedures (see supplement for details).
Morphological Analysis
Methodology to measure morphological changes in microglia in following CO2-inhalation was adapted from previous studies (29; 30). Flattened images from Z-stacks were examined using Image J software (NIH open access) to quantify increased soma perimeter and attenuated microglial branching complexity and process length (de-ramification) that are parameters for assessing microglial activation. (For details see supplementary materials).
Measurement of Cytokines
Cytokine concentrations were measured using the Bio-Plex® Mouse Cytokine Assays (Bio-Rad, USA). For details on tissue collection see supplement.
Slice electrophysiology
Coronal SFO slices (300 μm) were used for whole-cell patch clamp recordings of CO2-evoked neuronal firing as described (31). The chemosensitive response of a neuron was determined by measuring the change in firing rate in response to a hypercapnic acidotic solution of aCSF equilibrated with either 7. 5% CO2 (pH =7. 3) or 10% CO2 (pH = 7. 15). If the firing rate of a neuron increased by greater than 20% it was deemed to be chemosensitive, otherwise it was classified as non-chemosensitive as described (31), (for methodological details, see supplement).
Data analysis and Statistics
Data are presented as mean±SEM. Normality was formally tested for all data and met assumptions of the statistical tests being used. Animals were excluded from the analysis if they were identified as outliers using the Grubb’s analysis, had unstable baseline recordings (telemetry) or were surgical “misses” as identified by cresyl staining. Planned comparisons were done using two-tailed unpaired t-test to determine statistical significance between genotypes. Analysis of variance (ANOVA) was used for analysis of genotype and treatment effects. Bonferroni’s posthoc analysis was applied where main effects were significant. The multiple t-test analysis with Sidak-Bonferroni correction for multiple comparison was used for analysis of cardiovascular data. See supplemental materials for details on electrophysiology and plethysmography data analysis. P values of <0. 05 were considered significant. Prism software was used for statistical analysis (GraphPad Software, Inc. , LA Jolla, CA).
Results
Acid-sensor TDAG8 is expressed on microglia: expression in sensory CVOs and regulation by CO2-inhalation
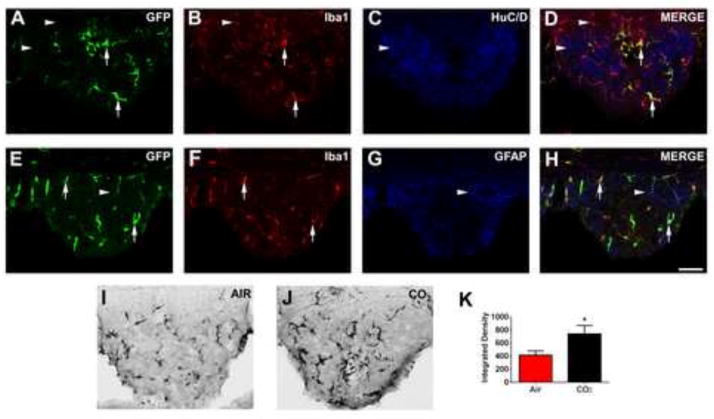
Cells expressing green fluorescent protein (GFP) downstream of the TDAG8 promoter were identified as microglia, verified by co-localization with ionized calcium binding adapter protein, (IBA-1), but not with HUC/D (neurons) or glial fibrillary acidic protein (GFAP) (astrocytes) producing cells (Fig. 1). GFP+ve-IBA-1+ve microglia were predominantly expressed in sensory CVOs, including the SFO (Fig. 1), organ vasculosum laminae terminalis (OVLT) (supplementary Fig. S1), and, area postrema (AP) (supplementary Fig. S1). GFP+ve cells were not evident in areas relevant to fear regulation such as the amygdala, prefrontal cortex and periaqueductal grey (supplementary Fig. S2). TDAG8 promoter-controlled GFP expression was significantly increased in the SFO 24h post CO2-inhalation (Fig. 1i–k, unpaired t test, t(15)=2. 177; p< 0. 05 vs air), but not in the AP or OVLT (supplementary Fig. S1q–r, p>0. 05 vs air), suggesting selective CO2-evoked regulation of TDAG8 promoter activity within the SFO and potential recruitment of the receptor in CO2 responses.
Figure 1.
Microglia within the sensory circumventricular organ (CVO), subfornical organ (SFO), express acid-sensing T cell death associated gene-8 (TDAG8) receptor (panels a–h). Regulation following CO2 inhalation (panels i–k). Using GFP knocked-in downstream of the TDAG8 promoter, abundant expression was localized in the SFO (panel a). Cellular phenotyping using triple immunohistochemistry, revealed co-localization of GFP with ionized calcium-binding adapter molecule (IBA1) positive cells within the SFO (panels b, d, f, h), but not with neuron-specific marker, HuC/D (panels c,d) or astrocytic marker, GFAP (panels g, h). Inhalation of 5% CO2 evoked a significant up-regulation of TDAG8 promoter-regulated GFP expression within the SFO area (panels i–k) Representative images showing GFP-positive cells following air inhalation (panel i) and CO2 inhalation (panel j). Quantification using Image J software revealed a significant increase in GFP expression in the CO2 group as compared to the air inhalation group (panel k) *p<0. 05 vs air controls (n=3 slices per animal from 5 mice/group). Double-labeled GFP-IBA-1 cells (arrows) and single-labeled cells (arrowheads) are indicated. Scale= 20μm.
Attenuation of CO2-evoked freezing and contextual CO2-conditioned freezing in TDAG8-deficient mice
Freezing (an established measure of fear and panic-like behavior in rodents (8; 32)) was used to evaluate behavioral responses to CO2 in TDAG8+/+/TDAG8−/− mice. Following a CO2 dose-response challenge, freezing was reduced in TDAG8−/− mice relative to TDAG8+/+ littermates on day 1 (Fig 2b). Two-way ANOVA revealed a significant main effect of genotype [F(1,54) =10. 45; p<0. 05] and treatment [F(2,54) =91. 36; p<0. 05] with no genotype x treatment interaction (p>0. 05) as both genotypes elicited higher freezing to 10% relative to 5% CO2. Bonferroni posthoc tests revealed significant differences between genotypes (p<0. 05) at both CO2 concentrations. Neither group froze during air inhalation in the test chamber (Fig 2b). TDAG8 disruption does not impact homecage or novelty-induced motor activity (33), ruling out genotype-associated motor deficits. Fear evoked by psychogenic threats (predator exposure, footshocks), neuroendocrine stress, anxiety-like or nociceptive responses were not affected by TDAG8 deficiency (supplementary Fig S3). Administration of bicarbonate (i. p. ) prior to CO2-inhalation to minimize acidosis significantly attenuated CO2-evoked freezing (supplementary Fig S4; t(10)=4. 584; p<0. 05 versus saline), suggesting that acidosis (H+) was required for CO2-evoked freezing.
Re-exposure to the testing context on day 2 evoked significant freezing in TDAG8+/+ mice with prior CO2 exposure (Fig 2c). This conditioned freezing response was significantly blunted in TDAG8−/− mice. A significant genotype [two-way ANOVA, F(1,54)=9. 613; p<0. 05] and treatment [F(2,54)=23. 84; p<0. 05] effect, but no genotype x treatment interaction (p>0. 05) was observed. Post-hoc analysis revealed significant differences between groups (p<0. 05). Negligible freezing was observed if CO2-exposed mice were placed in a neutral context on day 2 (p>0. 05) (Fig 2d) suggesting that CO2-evoked conditioned and not generalized fear.
Attenuated CO2-evoked sympathetic responses but normal ventilatory responses in TDAG8-deficient mice
Cardiovascular recordings via radio-telemetry revealed no significant genotype differences in baseline BP, HR or motor activity (p>0. 05, vs TDAG8+/+ ; supplementary Fig S5). Upon exposure to 5% CO2, TDAG8+/+ mice showed an increase in BP over mean pre-CO2 response, that was significantly attenuated in TDAG8−/− mice (Fig. 2f). Repeated measures two way ANOVA revealed a significant effect of genotype [F(1,70)=20. 13; p<0. 05], but no time or genotype x time interaction (p>0. 05 vs TDAG8+/+). CO2-evoked bradycardia was not affected by genotype (Fig 2g) (p>0. 05 vs TDAG8+/+). No significant differences in activity during CO2-inhalation were noted (Fig 2h). Re-exposure to the context (Day 2) elicited comparable increases in BP, HR and similar motor activity in TDAG8+/+ and TDAG8−/− mice (Fig 2i–k, p>0. 05 vs TDAG8+/+).
Whole-body plethysmography revealed increased ventilatory rate (VE) when inspired air was switched from room air to 5% CO2. Both genotypes exhibited similar hypercapnia-induced increase in VE of 100–150% (p>0. 05 vs TDAG8+/+; supplementary Fig. S6). Restoring room air (from 5% to 0% CO2), returned VE to the initial values in both genotypes. Thus, hypercapnic ventilatory responses appear intact in TDAG8−/− mice.
TDAG8 gates CO2-evoked activation of microglia in the subfornical organ
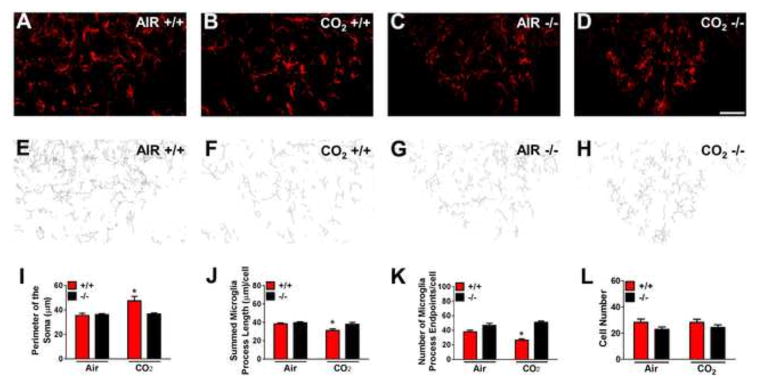
We next investigated TDAG8-mediated microglial activation within the SFO immediately following CO2-inhalation. SFO IBA1+ cells primarily represent microglia since a) they exhibit distinctive arborized features and morphological changes to CO2-inhalation consistent with microglial activation, and b) predominance of CD45low microglia (>95%) versus CD45high macrophages (<5%) was observed by flow cytometry (supplementary Fig. S7). Thus, myeloid cells within the SFO under basal conditions and following mild hypercapnia predominantly represent microglia, not systemic immune cells. As shown in Fig 3, only CO2-exposed TDAG8+/+ mice elicited an increase in soma size and reduced process length and endpoints: accepted hallmarks of de-ramified, activated microglia as compared with TDAG8−/−/CO2 and air groups (Fig 3a–k). For soma size (Fig 3i), two-way ANOVA revealed significant effects of genotype [F(1,18)=4. 82; p<0. 05], treatment [F(1,18)=7. 464; p<0. 05] and genotype x treatment interaction [F(1,18)=6. 40; p<0. 05]. For reduced process length (Fig 3j), two-way ANOVA revealed significant effect of genotype [F(1,18)=5. 98; p<0. 05] and treatment [F(1,18)=7. 68; p<0. 05]. For reduced process endpoints/cell (Fig 3k) two-way ANOVA revealed a significant effect of genotype [F(1,18)=51. 61; p<0. 05], and a genotype x treatment interaction [F(1,18)=11. 81; p<0. 05] but no significant treatment effect [p>0. 05]. Post-hoc analysis revealed significant differences between genotypes (p<0. 05). Microglial cell numbers did not differ between groups (Fig 3l; p>0. 05). TDAG8 deficiency did not impact microglial activation to other immunomodulatory agents such as lipopolysaccharide (LPS) (supplementary Fig S8) suggesting selectivity of TDAG8 to CO2-evoked microglial activation. Consistent with selective TDAG8-associated microglial activation within the SFO, we observed no significant differences (p>0. 05) in CO2-induced microglial changes in other CVOs such as the OVLT or AP (supplementary Fig S9). Thus, TDAG8 gates CO2-evoked activation of microglia within the SFO.
Figure 3.
Microglial activation within the subfornical organ following inhalation of 5% CO2 is dependent on TDAG8 receptor. TDAG8+/+ or −/− mice were sacrificed following 10 minutes of air or CO2 inhalation. (a–d) Representative fluorescent images of ionized calcium-binding adapter molecule 1 (IBA1)-labelled cells are shown. Panels (e–h) represent corresponding skeletonized illustrations constructed from maximum intensity projections of images. (i) A significant increase in microglial soma perimeter was observed only in CO2-exposed TDAG8+/+ mice versus TDAG8−/− and air exposed groups. No significant differences were present in the air inhalation cohorts. (j) Microglia process length showed significant differences between genotypes and treatment effect. (k) Process endpoints/cell revealed a significant difference between genotypes but treatment did not reach significance. (l) No significant differences were observed in the number of microglia between air and CO2 inhalation groups for both genotypes. All data are mean ± s. e. m. from 3 slices/animal, n=6. * p <0. 05 vs other groups Scale bar = 20 μm.
Microglial activation and inflammatory cytokine IL-1β are obligatory to CO2-evoked freezing
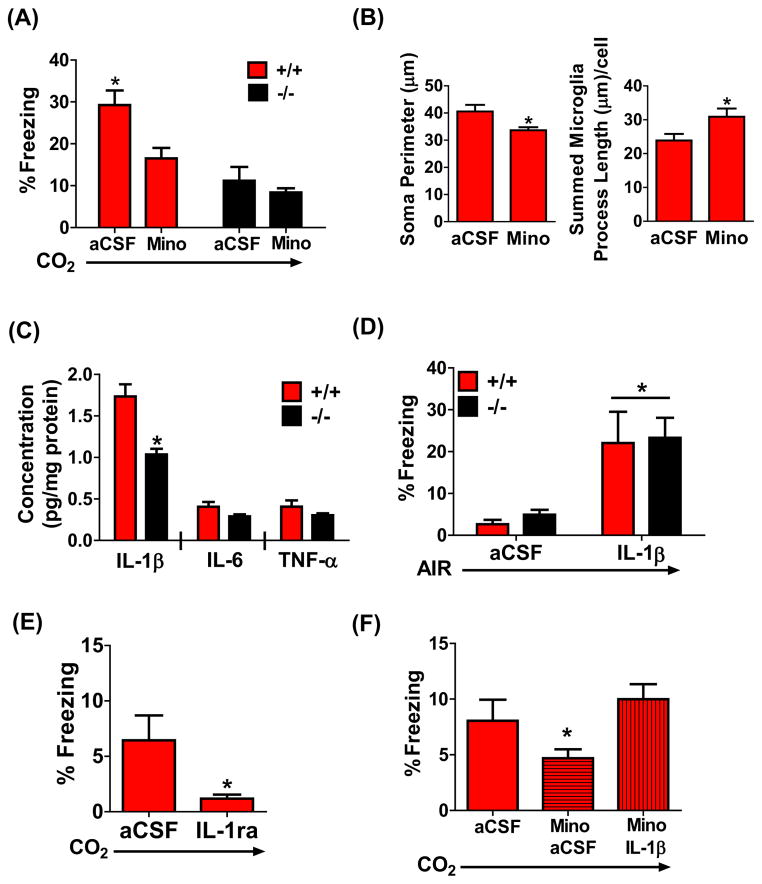
Given the association of TDAG8 with CO2-evoked behavior and microglial activation we next tested the requirement of microglial activation in CO2-responses. Central infusion of microglial blocker, minocycline, led to a significant attenuation of CO2-evoked freezing only in TDAG8+/+ mice (Fig. 4a). Two-way ANOVA revealed a significant effect of treatment [F(1,23)=6. 46; p<0. 05] and genotype [F(1,23)=18. 34; p<0. 05]. Post-hoc analysis revealed a significant treatment effect in TDAG8+/+ mice (p<0. 05). Minocycline had no significant effects in TDAG8−/− mice (p>0. 05) likely due to the absence of CO2-evoked microglial activation in these mice. Minocycline treatment also reduced CO2-evoked microglial activation in the SFO (Fig 4b). A significant reduction of microglial soma size [unpaired t test, t(10)=2. 608; p<0. 05 vs aCSF] and significant increase in process length [t(11)=2. 270, p<0. 05 vs aCSF] were observed. Collectively, these data support recruitment of TDAG8-dependent microglial activation in the expression of CO2-evoked freezing.
Figure 4.
Microglial activation and pro-inflammatory cytokine IL-1β are associated with CO2 inhalation-evoked fear. (a) Administration of minocycline (mino) attenuates 5% CO2-evoked freezing in TDAG8+/+ mice (n=6–8). No significant effect of mino was observed in TDAG8−/− mice (n=6–8). A significant genotype and treatment effect was observed. (b) CO2-evoked microglial activation in the SFO of TDAG8+/+ mice was significantly reduced in mino treated mice. A significant reduction of microglial soma size (left panel) and a significant increase in microglia process length (right panel) was observed (n=6–7/group; 3 slices/animal). (c) A significant reduction in SFO IL-1β concentration was observed in TDAG8−/− mice (n=4). Other pro-inflammatory cytokines, IL-6 and TNF-α in the SFO were not significantly different between genotypes (p>0. 05). (d) Sufficiency of IL-1β: Central (i. c. v. ) infusion of IL-1β evokes significant freezing in air exposed TDAG8+/+ and TDAG8−/− mice. Significant effect of treatment, but no genotype differences were observed (n= 8–14). (e) Necessity of IL-1β: Central (i. c. v. ) infusion of IL-1RA (antagonist of IL-1β) prior to CO2 inhalation significantly attenuates freezing (n= 5). (f) Central IL-1β infusion restores CO2-evoked fear in minocycline-treated mice. (n= 7–16). Note: Lower magnitude of freezing in all groups shown in panels (e) and (f) may be contributed by infusion of agents minutes prior to behavior. All data are mean ± s. e. m. * p<0. 05
In response to physiological insults microglia release pro-inflammatory cytokines. Significantly reduced IL-1β concentrations were observed in the SFO of TDAG8−/− mice (Fig. 4c; unpaired t test, (t(6)=4. 280; p<0. 05 vs +/+ mice). No significant genotype differences in pro-inflammatory cytokines were observed in other brain regions such as OVLT and amygdala (supplementary Fig S10a, p>0. 05 vs TDAG8+/+). Central infusion of IL-1β evoked significant freezing in air-exposed TDAG8+/+ and TDAG8−/− mice (Fig 4d). Two-way ANOVA revealed a significant effect of treatment [F(1,41)=20. 1; p< 0. 05], but no genotype, or genotype x treatment interaction (p>0. 05). ICV infusion of IL-1β antagonist, IL-1RA, resulted in a significant attenuation of CO2-evoked freezing, (Fig 4e, unpaired t test, t(8)= 2. 3 p< 0. 05 vs aCSF). Furthermore, IL-1β restored freezing behavior that was attenuated in minocycline-treated mice compared to CO2-exposed-aCSF-treated mice (Fig 4f; one way ANOVA, F2,29=8. 424; p<0. 05). Post-hoc analysis revealed that minocycline-IL-1β treated mice were significantly different from minocycline only treated mice (p<0. 05) but not with vehicle group. The increased freezing response was not due to IL-1β effects on sickness behavior as motor activity following IL-1β alone remained unchanged for the duration of our behavioral measurement (supplementary Fig S10b, p>0. 05 vs aCSF).
Microglial acid-sensor TDAG8 gates CO2-chemosensitive firing response of SFO neurons
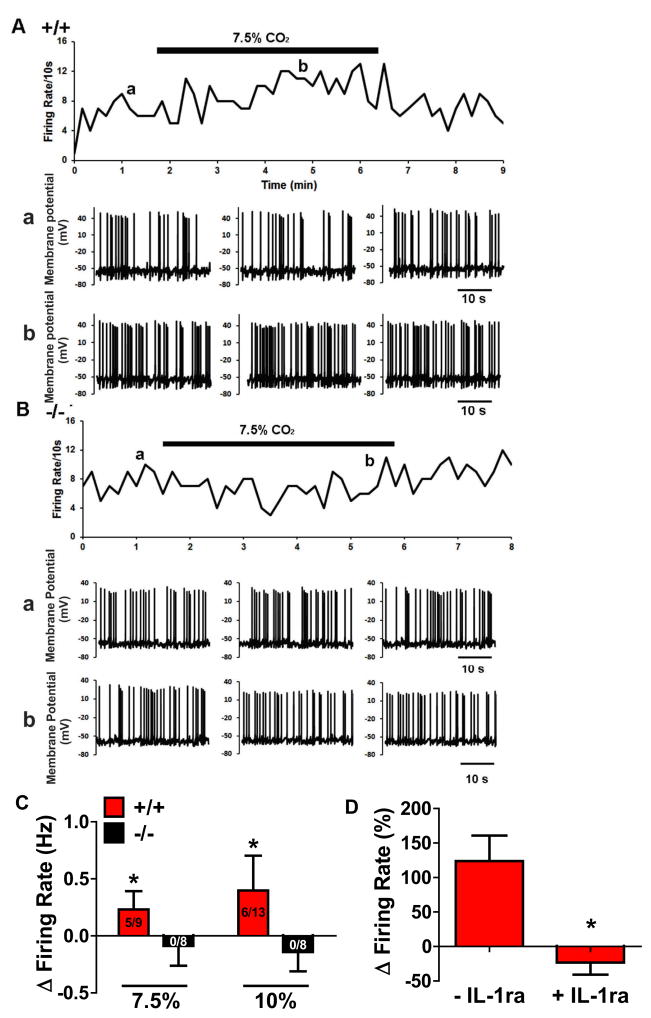
Translation of microglial acid-sensing via TDAG8 to behavior and sympathetic activation would require neuronal firing. We hypothesized that SFO neurons would exhibit CO2-chemosensitive responses, dependent on TDAG8 and IL-1β. Firing rate responses of SFO neurons to hypercapnia were studied in TDAG8+/+/TDAG8−/− mice using whole-cell patch-clamping (Fig 5A, B). Exposure to 7. 5% and 10% CO2 increased the firing rate of SFO neurons in TDAG8+/+ slices which reversed upon return to normocapnia. Similar measurements in TDAG8−/− mice showed no change in firing rate of SFO neurons to CO2 (Fig. 5B). Spontaneous firing rate was significantly increased in TDAG8+/+ mice (Fig. 5C, red bars). 5/9 cells (55%) for 7. 5% and 6/13 cells (46%) for 10% were CO2-responsive. In contrast, no CO2-responsive SFO neurons were observed in TDAG8−/− mice at either concentration (7. 5% CO2: (0/8), 10% CO2: (0/8); Fig. 5C black bars). Unpaired t-test revealed significant genotype differences for 7. 5% [t(11)=3. 314; p<0. 05 vs +/+], and 10% CO2 [t(12)=4. 25; p<0. 05 vs +/+]. The lack of firing response to hypercapnia in TDAG8−/− SFO neurons was not due to compromised neuronal integrity as similar membrane properties were observed between genotypes (supplementary Fig S11a–c; p>0. 05). CO2-chemosensitive responses were not TDAG8-dependent in other chemosensory areas such as the locus coeruleus and area postrema (supplementary Fig 11d–e; p>0. 05). Importantly, IL-1RA significantly attenuated CO2-evoked firing of SFO neurons (Fig 5D) [unpaired t test; t(8)=3. 602; p<0. 05 vs +/+], underscoring the necessity of IL-1β for CO2-evoked neuronal firing in the SFO. Collectively, CO2-chemosensitve neuronal activation in the SFO is dependent on microglial acid-sensor TDAG8 and IL-1β
Figure 5.
CO2-evoked firing of SFO neurons is dependent on TDAG8 and IL-1β. (A) Representative trace of a SFO neuron from TDAG8+/+ mouse showing an increase in spontaneous firing rate on exposure to 7. 5% CO2. The upper panel shows the time course of the effect of 7. 5% CO2 on the integrated firing rate. The lower panels show representative traces of spontaneous firing before (a) and during (b) application of 7. 5% CO2. (B) Representative trace of a SFO neuron from TDAG8−/− mouse. Spontaneous firing rate did not change in the presence of 7. 5% CO2. The lower panels show representative traces of spontaneous firing before (a) and during (b) application of 7. 5% CO2. (C) Histogram summarizing the effects of CO2 on firing rate of SFO neurons. Each bar represents the mean ± S. E. M. with n values (CO2-responsive neurons/total neurons) shown. Spontaneous firing rate was significantly increased in TDAG8+/+ mice on exposure to 7. 5% and 10% CO2 (red bars). Negligible change in firing rate of any TDAG8−/− SFO neurons (black bars) was observed after exposure to 7. 5% or 10% CO2. (D) Mean change in firing rate during exposure to 7. 5% CO2 in SFO neurons before (left) and after application of IL-1β receptor antagonist, IL-1RA (right) in TDAG8+/+ mice that show CO2-chemosensitive neuronal firing responses in the SFO (n=5). Traces shown in panels A and B are from representative experiments that were repeated at least 5 times. All data are mean ± s. e. m. * p<0. 05
Discussion
Our results delineate a novel mechanism whereby an interoceptive threat (CO2) elicits fear-relevant behavioral and physiological responses via microglia. CO2-evoked responses are mediated by a microglial acid sensing GPCR (TDAG8) in the subfornical organ, a brain region critical for monitoring the internal milieu. Microglial pro-inflammatory responses may constitute a unique chemosensory system for the detection of homeostatic threats such as CO2-inhalation of relevance to panic pathophysiology.
The acid-sensing TDAG8 receptor is localized to microglia in the sensory CVOs, areas strategically positioned near the brain ventricular system to sense homeostatic fluctuations in the body and brain (34). The SFO can detect circulating cardiovascular and metabolic signals that influence the excitability of single SFO neurons (35). Consistent with this study, previous work links the SFO with panic-like responses to intravenous lactate (20; 21). The SFO may be a primary locus for detecting interoceptive challenges relevant to panic.
TDAG8 receptor deficiency led to attenuated CO2-evoked freezing and sympathetic responses. Rising CO2 concentrations lead to acidosis, creating a state of homeostatic imbalance resulting in defensive behavioral responses (6; 8; 32). CO2-evoked freezing is not completely attenuated in TDAG8−/− mice, suggesting additional acid sensors (such as the acid-sensing ion channel 1 (ASIC1) in the amygdala (8) or acid sensing 5-HT neurons in the periaqueductal grey (36)) may also contribute. ASIC1 regulates CO2-evoked freezing at higher (10%) concentrations (8). TDAG8 may be a low-threshold CO2-sensor relevant to sensitized CO2 responding observed in PD patients. Higher prevalence of panic attacks at low (5–7. 5%) CO2 is observed in panic patients while ~10% or higher concentrations may affect healthy individuals (3). Having multiple CO2 sensors enables a broad spectrum sensing system which may “sense” a specific range of pH alteration (37).
In addition to evoking spontaneous responses during inhalation, CO2 also acts as an unconditioned stimulus to produce conditioned freezing specific to exposure context; a response attenuated in TDAG8−/− mice. CO2-evoked conditioning response is also relevant to PD, as associative learning following panic attacks results in fear and avoidance of panic associated contexts (38).
Heart rate or ventilatory responses to CO2 are not affected by TDAG8 deficiency. Modulation of pressor but not heart rate responses by TDAG8 may result from a dissociation of sympathetic and parasympathetic drives following CO2. Increased blood pressure to CO2 results from an activation of sympathetic nervous system via central chemoreceptors, while changes in heart rate are due to a secondary reflex activation of the parasympathetic nervous system via arterial baroreceptors (39). TDAG8 does not appear to regulate ventilation (or respiratory control may involve redundant mechanisms (40)). Studies with ASIC1−/− mice and delta opioid receptor (DOR)−/− mice (another model of panic-like responses) also reported intact ventilatory responses to CO2-inhalation (8; 41). Compensatory mechanisms during development may have contributed to the unaltered respiratory responsiveness to CO2.
Our study outlines a microglial mechanism responsible for CO2-sensing and its translation to fear relevant behavior. Recruitment in CO2-sensing is consistent with microglial tissue surveillance and homeostatic regulation. Our observations of rapid microglial activation in the SFO by CO2 are consistent with reports of rapid cell swelling and redistribution of actin cytoskeleton of microglia exposed to acidosis in vitro (14; 15). Microglial responsivity to an imminent homeostatic survival threat may occur rapidly. SFO microglia in TDAG8−/− mice were not activated by CO2-inhalation, suggesting that TDAG8 gates microglial activation. Furthermore, minocycline attenuated CO2-evoked freezing in a TDAG8-dependent manner highlighting the necessity for TDAG8-associated microglial activation in this effect. Moreover, minocycline treatment attenuated microglial activation within the SFO, further supporting association of microglial activation with CO2-evoked responses.
Our studies implicate IL-1β as an effector in TDAG8-microglial-CO2 evoked freezing. Physiological responses to pro-inflammatory cytokine IL-1β are primarily mediated via the SFO (42). In agreement with selective effects of CO2 on TDAG8 regulation and microglial activation in the SFO, we observed an SFO-selective reduction of IL-1β in TDAG8−/− mice. Restoration of CO2-evoked freezing in minocycline-treated mice supplemented with IL-1β further suggests that it acts downstream of microglial activation. Importantly, antagonism of IL-1β attenuated CO2-evoked freezing, suggesting necessity and IL-1β infusion was sufficient in evoking freezing in the absence of CO2. Finally, CO2-evoked activation of SFO neurons (an effect abolished in TDAG8−/− mice) was dependent on IL-1β. Collectively, our data strongly support SFO IL-1β as the primary effector in CO2-evoked freezing.
Our study provides the first evidence of CO2-chemosensing within the SFO, representing a rostral extension of the primitive brain stem CO2-chemosensory system (3). Neuroanatomical studies have mapped direct efferent connections of the SFO to principal effector sites such as the lateral and dorsomedial hypothalamus (43), and the periaqueductal grey (PAG) (44), established sites for cardiovascular control and freezing behavior, respectively. Consequently, CO2-mediated neuronal activation in the SFO may then lead to behavioral and cardiovascular responses to CO2-inhalation. Selectivity of the SFO in CO2-evoked effects is intriguing; especially given TDAG8 expression is observed in other sensory CVOs. The basis of this selectivity is not evident; however, differential expression of carbonic anhydrase isoforms (45) between CVOs may contribute to differences in the efficacy of proton production from CO2 impacting the dynamics of pH shifts within CVOs. Although our data supports the SFO as a primary site for CO2 responses mediated by microglial TDAG8 receptor, future studies on SFO-targeted interventions are warranted to further establish this area as a key acid-chemosensory site relevant to panic.
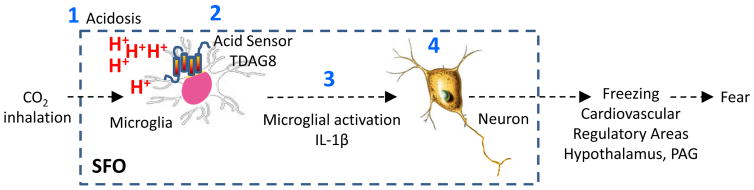
Figure 6 illustrates a potential mechanism underlying CO2-evoked responses via microglial acid sensor TDAG8. Collectively, our findings are relevant to fear and panic pathophysiology. The microglial TDAG8 acid chemosensory system provides a homeostatic threat detection mechanism housed in an area known for monitoring the internal milieu for ionic imbalance. Existence of homeostatic chemosensory systems distinct from psychogenic fear and stress modulatory pathways is supported by expression of intense fear and panic in Urbach-Wiethe subjects with damaged amygdala (9). Acid-base disturbances reported in PD subjects (46; 47) may recruit TDAG8 for translation to fear-associated responses. Although direct analysis for TDAG8 gene polymorphism localized on chromosome 14q31-q32 in PD has not been undertaken, linkage studies in probands with PD and phobias revealed a significant association of chromosome 14 with the disorder (48). Given the relevance of gene x environment interactions in the development of CO2 hypersensitivity (49), it would be important to assess TDAG8 gene-by-environment interaction effects on CO2 sensitivity. Regulation of TDAG8-mediated responses by drugs such as selective serotonin reuptake inhibitors (SSRIs) that impact panic symptoms and CO2-sensitivity will strengthen translational validity of this mechanism. Our data also highlight a potential role for microglial activation and inflammation in panic pathophysiology. Although the contribution of innate immune processes in PD subjects or its comorbidity with inflammatory conditions has not been systematically investigated, a recent National Comorbidity Survey–Replication (NCS-R) study reported significantly high comorbidity of PD with conditions associated with inflammation such as rheumatoid arthritis and chronic pain (50). Elevated pro-inflammatory cytokines were reported in PD (51), although more recently an association of pro-inflammatory markers with anxiety disorders in general but not specific to PD was reported (52). Alternatively, it is also possible that inflammation in PD is a state-dependent, transient phenomenon which may be difficult to capture in a clinical sample.
Figure 6.
Potential mechanism of CO2-evoked fear via microglial acid sensor TDAG8. (1) Acidosis (H+) generated from CO2 is the likely chemosensory signal, since bicarbonate administration attenuates CO2-evoked responses. (2) Microglial acid sensing TDAG8 receptor gates CO2-evoked activation of microglia, neuronal firing responses within the SFO and is recruited in CO2-evoked behavioral and cardiovascular manifestations of fear. In support, TDAG8-deficient mice have attenuated CO2-induced freezing and cardiovascular response, reduced microglial activation in the SFO, and negligible neuronal firing responses to CO2. (3) Microglial activation participates in CO2-evoked fear since pre-treatment with minocycline attenuates freezing behavior, as well as microglial activation in the SFO. Pro-inflammatory cytokine IL-1β is the likely effector since IL-1RA blocks CO2-evoked fear as well as CO2-induced firing of SFO neurons. Additionally, central IL-1β infusion is sufficient for evoking freezing response. Consistent with these data, IL-1β rescues CO2-evoked fear in minocycline treated mice and TDAG8−/− mice have a selective reduction of IL-1β in the SFO. 4) CO2-evoked chemosensory neuronal firing rate in the SFO is ablated in TDAG8−/− mice, suggesting that TDAG8 acid-sensing (and associated microglial inflammatory response) may gate neuronal activation in the SFO. Neurons within the SFO have efferent projections to effector areas such as hypothalamus or periaqueductal grey 43,44, that can regulate freezing and autonomic responses associated with fear.
In conclusion, our data reveal a unique acid-chemosensory mechanism gated by the acid-sensing TDAG8 receptor on microglia within the subfornical organ, a site accessible to systemic and central milieu. Active engagement of innate immune cells in the detection of homeostatic pH threat may provide novel mechanistic insights into the genesis of fear and panic attacks.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Grants R01-MH093362 and R21MH083213 to Renu Sah. We would like to acknowledge the excellent technical assistance of Laura Bailey, Kyle Brotkowski, and James B. Chambers. We thank Dr. Yvonne Ulrich-Lai (University of Cincinnati) for help with the analysis of Telemetry data, Dr. Lynn K. Hartzler (Wright State University) for advice and help on Plethysmography experiments, Alyssa Sproles (Cincinnati Children’s Hospital Medical Center) for assistance with the BioPlex cytokine assays. We gratefully acknowledge receiving TDAG8 deficient mice (TDAG8−/−) from Dr. Owen Witte and Dr. Chris Radu (University of California, Los Angeles).
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colasanti A, Esquivel G, Schruers KJ, Griez EJ. On the psychotropic effects of carbon dioxide. Curr Pharm Des. 2012;18:5627–37. doi: 10.2174/138161212803530745. [DOI] [PubMed] [Google Scholar]
- 4.Papp L, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–57. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- 5.Rassovsky Y, Kushner MG. Carbon dioxide in the study of panic disorder: Issues of definition, methodology, and outcome. J Anxiety Disord. 2003 Jan;17 doi: 10.1016/s0887-6185(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 6.Magnotta VA, Heo H-YY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, et al. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci U S A. 2012;109:8270–3. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–21. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, et al. Fear and panic in humans with bilateral amygdala damage. Nat Neurosci. 2013;16:270–272. doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanisch U-KK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 11.Walker FR, Beynon SB, Jones KA, Zhao Z, Kongsui R, Cairns M, Nilsson M. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav Immun. 2014;37:1–14. doi: 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 13.Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 14.Morihata H, Nakamura F, Tsutada T, Kuno M. Potentiation of a voltage-gated proton current in acidosis-induced swelling of rat microglia. J Neurosci. 2000;20:7220–7. doi: 10.1523/JNEUROSCI.20-19-07220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faff L, Nolte C. Extracellular acidification decreases the basal motility of cultured mouse microglia via the rearrangement of the actin cytoskeleton. Brain Res. 2000;853:22–31. doi: 10.1016/s0006-8993(99)02221-0. [DOI] [PubMed] [Google Scholar]
- 16.McGuire J, Herman JP, Ghosal S, Eaton K, Sallee FR, Sah R, et al. Acid-sensing by the T cell death-associated gene 8 (TDAG8) receptor cloned from rat brain. Biochem Biophys Res Commun. 2009;386:420–425. doi: 10.1016/j.bbrc.2009.05.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radu CG, Nijagal A, McLaughlin J, Wang L, Witte ON. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci U S A. 2005;102:1632–7. doi: 10.1073/pnas.0409415102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson AK, Gross PM, AKJ, PMG Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–86. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PL, Sajdyk TJ, Fitz SD, Hale MW, Lowry CA, Hay-Schmidt A, Shekhar A. Angiotensin II’s role in sodium lactate-induced panic-like responses in rats with repeated urocortin 1 injections into the basolateral amygdala: amygdalar angiotensin receptors and panic. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:248–56. doi: 10.1016/j.pnpbp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shekhar A, Keim SR, SRK The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekhar A, Sajdyk TS, Keim SR, Yoder KK, Sanders SK. Role of the basolateral amygdala in panic disorder. Ann N Y Acad Sci. 1999;877:747–50. doi: 10.1111/j.1749-6632.1999.tb09315.x. [DOI] [PubMed] [Google Scholar]
- 22.Radu CG, Cheng D, Nijagal A, Riedinger M, McLaughlin J, Yang LV, et al. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol Cell Biol. 2006;26:668–77. doi: 10.1128/MCB.26.2.668-677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibold NK, Viechtbauer W, Goossens L, De Cort K, Griez EJ, Myin-Germeys I, et al. Carbon dioxide inhalation as a human experimental model of panic: the relationship between emotions and cardiovascular physiology. Biol Psychol. 2013;94:331–40. doi: 10.1016/j.biopsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones Aa, Jones KR, Choi DC, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrone LGA, Bícego KC, Hartzler LK, Putnam RW, Gargaglioni LH. Cardiorespiratory effects of gap junction blockade in the locus coeruleus in unanesthetized adult rats. Respir Physiol Neurobiol. 2014;190:86–95. doi: 10.1016/j.resp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Neigh GN, Karelina K, Glasper ER, Bowers SLK, Zhang N, Popovich PG, DeVries AC. Anxiety after cardiac arrest/cardiopulmonary resuscitation: exacerbated by stress and prevented by minocycline. Stroke. 2009;40:3601–7. doi: 10.1161/STROKEAHA.109.564146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–15. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries aC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci U S A. 2010;107:16342–7. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013;10:4. doi: 10.1186/1742-2094-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutando L, Busquets-Garcia A, Puighermanal E, Gomis-González M, Delgado-García JM, Gruart A, et al. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 2013;123:2816–31. doi: 10.1172/JCI67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K-Y, Putnam RW. Transient outwardly rectifying A currents are involved in the firing rate response to altered CO2 in chemosensitive locus coeruleus neurons from neonatal rats. AJP Regul Integr Comp Physiol. 2013;305:R780–R792. doi: 10.1152/ajpregu.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mongeluzi DL, Rosellini RA, Ley R, Caldarone BJ, Stock HS. The conditioning of dyspneic suffocation fear. Effects of carbon dioxide concentration on behavioral freezing and analgesia. Behav Modif. 2003;27:620–36. doi: 10.1177/0145445503256316. [DOI] [PubMed] [Google Scholar]
- 33.Vollmer LL, Schmeltzer SN, Ahlbrand R, Sah R. A potential role for the acid-sensing T cell death associated gene-8 (TDAG8) receptor in depression-like behavior. Physiol Behav. 2015;150:78–82. doi: 10.1016/j.physbeh.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, et al. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:III–XII. 1–122. doi: 10.1007/978-3-642-55532-9. back cover. [DOI] [PubMed] [Google Scholar]
- 35.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav. 2013;121:96–102. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–40. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 37.Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–26. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y. Autonomic cardiovascular responses to hypercapnia in conscious rats: The roles of the chemo- and baroreceptors. Auton Neurosci Basic Clin. 2005;117:105–114. doi: 10.1016/j.autneu.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Nattie E. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–35. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- 41.Borkowski AH, Barnes DC, Blanchette DR, Castellanos FX, Klein DF, Wilson DA. Interaction between d opioid receptors and benzodiazepines in CO•-induced respiratory responses in mice. Brain Res. 2011;1396:54–9. doi: 10.1016/j.brainres.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei S-G, Zhang Z-H, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25. doi: 10.1161/HYPERTENSIONAHA.113.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson LW, Lind RW. Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res. 1986;379:399–403. doi: 10.1016/0006-8993(86)90799-7. [DOI] [PubMed] [Google Scholar]
- 44.Uschakov A, McGinty D, Szymusiak R, McKinley MJ. Functional correlates of activity in neurons projecting from the lamina terminalis to the ventrolateral periaqueductal gray. Eur J Neurosci. 2009;30:2347–55. doi: 10.1111/j.1460-9568.2009.07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruusuvuori E, Kaila K. Carbonic anhydrases and brain pH in the control of neuronal excitability. (S. C. Frost & R. McKenna, editors) Subcell Biochem, Subcellular Biochemistry. 2014;75:271–90. doi: 10.1007/978-94-007-7359-2_14. [DOI] [PubMed] [Google Scholar]
- 46.Friedman SD, Mathis CM, Hayes C, Renshaw P, Dager SR. Brain pH response to hyperventilation in panic disorder: preliminary evidence for altered acid-base regulation. Am J Psychiatry. 2006;163:710–5. doi: 10.1176/ajp.2006.163.4.710. [DOI] [PubMed] [Google Scholar]
- 47.Vollmer LL, Strawn JR, Sah R. Acid-base dysregulation and chemosensory mechanisms in panic disorder: a translational update. Transl Psychiatry. 2015;5:e572. doi: 10.1038/tp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gelernter J, Page GP, Bonvicini K, Woods SW, Pauls DL, Kruger S. A chromosome 14 risk locus for simple phobia: results from a genomewide linkage scan. Mol Psychiatry. 2003;8:71–82. doi: 10.1038/sj.mp.4001224. [DOI] [PubMed] [Google Scholar]
- 49.Battaglia M, Ogliari A, D’Amato F, Kinkead R. Early-life risk factors for panic and separation anxiety disorder: Insights and outstanding questions arising from human and animal studies of CO2 sensitivity. Neurosci Biobehav Rev. 2014;46P3:455–464. doi: 10.1016/j.neubiorev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R) Depress Anxiety. 2012;29:797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–55. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 52.Vogelzangs N, Beekman ATF, de Jonge P, Penninx BWJH. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.