Abstract
We report the first total syntheses of (+)-haplocidine, and its N1-amide congener (+)-haplocine. Our concise synthesis of these alkaloids required the development of a late-stage and highly selective C–H oxidation of complex aspidosperma alkaloid derivatives. A versatile, amide directed ortho-acetoxylation of indoline amides enabled our implementation of a unified strategy for late-stage diversification of hexacyclic C19-hemiaminal ether structures via oxidation of the corresponding pentacyclic C19-iminium ions. An electrophilic amide activation of a readily available C21-oxygenated lactam, followed by transannular cyclization and in situ trapping of a transiently formed C19-iminium ion, expediently provided access to hexacyclic C19-hemiaminal ether alkaloids (+)-fendleridine, (+)-acetylaspidoalbidine, and (+)-propionylaspidoalbidine. A highly effective enzymatic resolution of a non-β-branched primary alcohol (E=22) allowed rapid preparation of both enantiomeric forms of a C21-oxygenated precursor for synthesis of these aspidosperma alkaloids. Our synthetic strategy provides succinct access to hexacyclic aspidosperma derivatives, including the antiproliferative alkaloid (+)-haplocidine.
Introduction
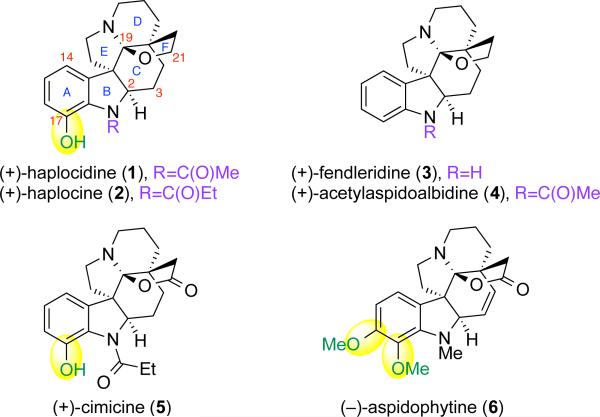
The structural complexity and diverse biological activity of the aspidosperma alkaloids have long served as an inspiration for development and application of new chemical synthesis strategies.1 A subset of these natural products are hexacyclic C19-hemiaminal ether aspidosperma alkaloids that include the potent caspase-8 inhibitor (+)-haplocidine (1, Figure 1)2,3 and the structurally related (+)-haplocine (2).2 Related natural alkaloids include (+)-fendleridine (3)4,5 and (+)-acetylaspidoalbidine (4) that possess the same hexacyclic structure without A-ring oxygenation, along with (+)-cimicine (5)6,7 and (−)-aspidophytine (6)7,8,9 as C21-lactone variants of the subfamily with A-ring oxygenation. As an outgrowth of our cyclization studies concerning synthesis of pentacyclic aspidosperma alkaloids,10,11 we sought the development of a versatile and unified strategy to secure these hexacyclic alkaloids. Herein, we describe the first total syntheses of (+)-haplocidine (1) and (+)-haplocine (2). Our synthesis features the critical use of transiently formed pentacyclic C19-iminium ions for securing the desired hexacyclic C19-hemiaminal ether architecture and a final-stage palladium catalyzed C17–H oxidation.
Figure 1.
Structure of (+)-haplocidine (1) and (+)-haplocine (2) and structurally related aspidosperma alkaloids.
The unique structure of C19-hemiaminal ether subfamily of aspidosperma alkaloids has stimulated the development of elegant synthetic strategies.1,2,4–9 Current synthetic routes to these alkaloids can be categorized into two groups based on the strategy for critical C19-hemiaminal ether introduction. One solution to this structural element is the C19-oxidation of pentacyclic intermediates.5,7,9 This approach has been applied to the syntheses of (±)-fendleridine (3) and (±)-acetylaspidoalbidine (4),4,5 in addition to (−)-aspidophytine (6)9 and related alkaloid (+)-haplophytine12 starting with A-ring oxygenated starting material.7 An alternative approach secures the desired C19-oxidation state via a cycloaddition reaction.5e,13 While this inventive solution bypasses the need for C19-methine oxidation, it does require additional reductive steps after cycloaddition, in addition to the use of chiral chromatographic separation to access enantiomerically enriched (+)-fendleridine (3) and (+)-acetylaspidoalbidine (4). We aspired to develop a unified strategy for the syntheses of (+)-haplocidine (1) and (+)-haplocine (2) that would involve regioselective late-stage A-ring functionalization and highly stereoselective cyclization events to rapidly generate their hexacyclic architecture.
Results and Discussion
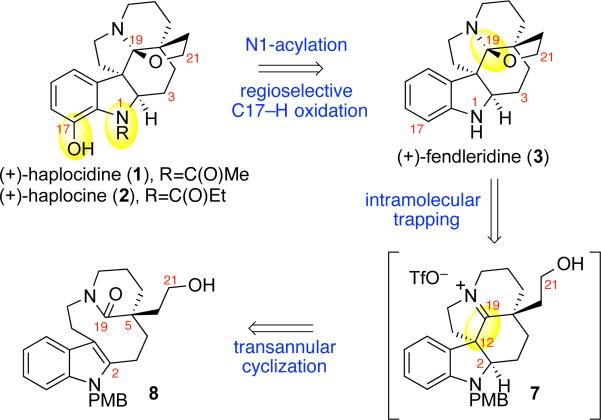
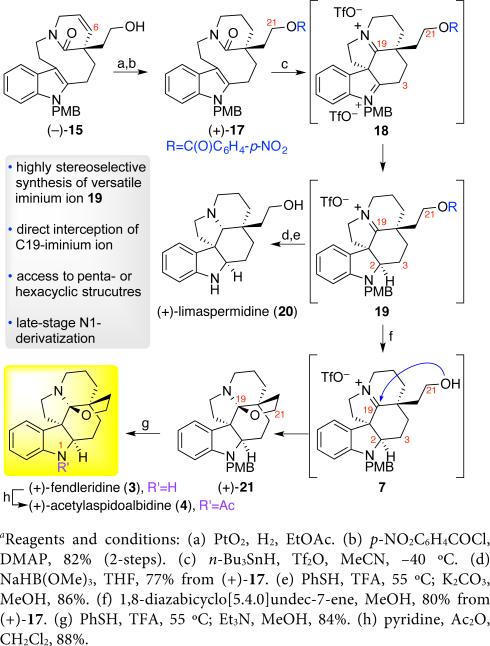
Our retrosynthetic analysis of (+)-haplocidine (1) and (+)-haplocine (2) is illustrated in Scheme 1. Structural comparison of these hexacyclic C19-hemiaminal ether alkaloids with the biogenetically related aspidosperma alkaloid (+)-fendleridine (3) highlighted the need for the development of a final-stage C17-hydroxylation. We envisioned N1-acetylation and N1-n-propionylation of (+)-fendleridine (3) followed by amide directed C–H oxidation would provide the C17-hydroxylated alkaloids (+)-1 and (+)-2, respectively. We identified an opportunity to develop a unified strategy to secure the C19-hemiaminal ether hexacycle shared by (+)-haplocidine (1), (+)-haplocine (2), (+)-fendleridine (3), (+)-acetylaspidoalbidine (4), and related aspidosperma alkaloids.6,8 Informed by our mechanistic study of the interrupted Bischler-Napieralski reaction,14,15 we planned the spontaneous interception of the transient C19-iminium ion of putative pentacycle 7 with the C21-alcohol to directly obtain the desired C19-hemiaminal ether found in this hexacyclic–genre of aspidosperma alkaloids (Scheme 1). We envisioned transannular cyclization of lactam 8, promoted by electrophilic amide activation, followed by regioselective C2-reduction to provide the necessary pentacyclic iminium ion 7. Given the natural occurrence of the characteristic aspidosperma skeleton with C21-oxygenation in both enantiomeric forms,1,16 we devised a synthetic strategy that would allow for an advanced-stage stereochemical divergence. Thus, in place of an early introduction of the C5-quaternary stereocenter via asymmetric alkylation chemistry,11 we sought the resolution of a versatile substrate to access the desired C21-hydroxylated lactam 8.
Scheme 1.
Retrosynthetic analysis of (+)-haplocidine (1) and (+)-haplocine (2)
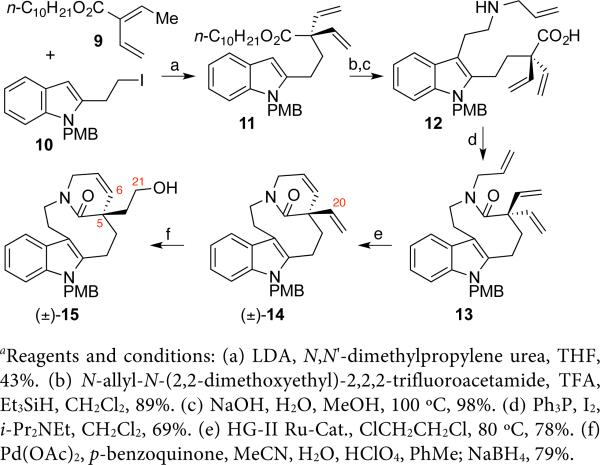
We recognized alcohol (±)-15 (Scheme 2) as an attractive precursor for enantiomerically enriched lactam 8 (Scheme 1), en route to C19-hemiaminal ether alkaloids described in this report, while offering future utility toward other aspidosperma alkaloids16a–d via C6-alkene functionalization. Deprotonation of ester 9 with lithium diisopropylamide (LDA) in the presence of N,N’-dimethylpropylene urea, followed by α-alkylation of the resulting dienolate with indole iodide 1011 afforded divinyl ester 11 in 43% yield.17 Trifluoroacetic acid (TFA) catalyzed Friedel–Crafts-type reductive alkylation18 of indole 11 followed by tandem hydrolysis of the trifluoroacetamide and n-decyl ester provided the amino acid 12 in 87% yield over two steps (Scheme 2). Slow addition of a solution of amino acid 12 and Hünig's base in dichloromethane to a solution of triphenylphosphine and iodine11,19 in dichloromethane gave lactam 13 in 69% yield. Treatment of triene 13 with Hoveyda–Grubbs II catalyst afforded lactam (±)-14 in 78% yield.20,21 Exclusive hydration of the C20-alkene of lactam (±)-14 via a Wacker-Tsuji oxidation,22 provided the corresponding C21-aldehyde that was reduced in situ to give alcohol (±)-15 in 79% overall yield (Scheme 2). The C5-quaternary center of alkene (±)-14 is likely responsible for the anti-Markovnikov mode of hydration.23
Scheme 2.
Synthesis of the C21-hydroxylated lactam (±)-15a
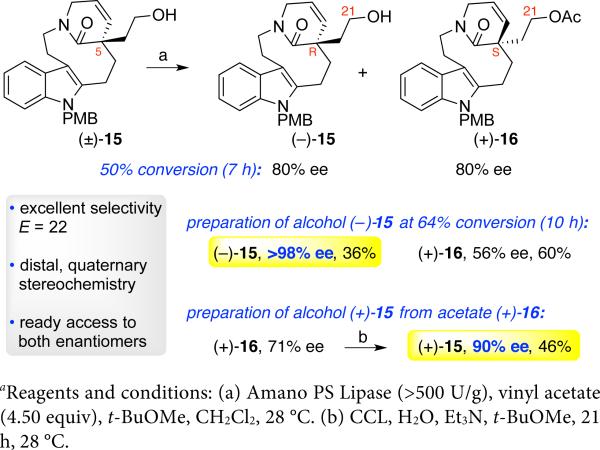
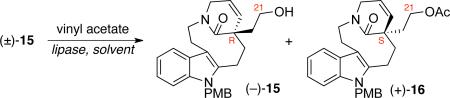
We next focused on the resolution of alcohol (±)-15 with primary interest in accessing enantiomerically enriched (−)-15 to secure the (R)-C5-stereochemistry required for the C19-hemiaminal ether aspidosperma alkaloids (+)-1–4 (Figure 1). After exploring a variety of strategies using chiral auxiliaries for preparation of alcohol (−)-15, including modifications of the synthetic route outlined in Scheme 2 and the C21-derivatization of alcohol (±)-15 to allow separation of diastereomers,24 we recognized the superiority of an enzymatic kinetic resolution of alcohol (±)-15. Whereas the kinetic resolution of primary alcohols with β-branching and a variety of secondary alcohols is well established,25 the resolution of non-β-branched primary alcohols is challenging.26 The paucity of methods for the resolution of alcohols with remote stereochemistry notwithstanding, the accommodation of the polycyclic alcohol (±)-15 as a potential substrate was of concern given the existing level of success with simpler substrates.26 We began our investigation with Candida antarctica lipase B (CAL-B) and a lipase from Candida rugosa (CCL) based on their prior success with non-β-branched primary alcohols.27 Unfortunately, we saw no acetylation of alcohol (±)-15 with CAL-B (Table 1, entry 1), likely due to the relative complexity of alcohol (±)-15 compared to prior substrates.27 We observed an encouraging level of acylation using alcohol (±)-15 and CCL (Table 1, entries 2–3)26f that prompted further examination of solvents and additive choices (Table 1, entries 4–5), resulting in alcohol (−)-15 with modest level of enantiomeric enrichment (74% ee) at 65% conversion. With further experimentation we discovered that the use of a lipase from Burkholderia cepacia with t-butyl methyl ether as solvent at 10 μM concentration of the substrate afforded the desired alcohol (−)-15 with synthetically useful level of enantiomeric enrichment (92% ee) at 55% conversion (Table 1, entry 6).
Table 1.
Enzymatic resolution of alcohol (±)-15a
| |||||
|---|---|---|---|---|---|
| entry | lipase | solvent | conversion | ee of (−)-15 | ee of (+)-16 |
| 1 | CAL-B | PhMe | – | – | – |
| 2 | CCL | PhMe | 44% | 38% | 30% |
| 3 | CCL | THF | <15% | 8% | 49% |
| 4 | CCL | PhMeb | 65% | 74% | 26% |
| 5 | CCL | t-BuOMe | 55% | 60% | 42% |
| 6 | Amano PS | t-BuOMe | 55% | 92% | 50% |
Reagents and conditions: vinyl acetate (2.0 equiv), 23 °C. Each resolution was monitored for 48 h or until approximately 50% conversion to (+)-16 (HPLC analysis), whichever occurred first.
Triethylamine (1.0 equiv) was utilized as an additive. CAL-B = Candida antarctica lipase B, CCL = Candida rugosa lipase, Amano PS = Burkholderia cepacia lipase.
With these promising conditions available for resolution of alcohol (±)-15, we turned our attention to further optimization and implementation of this strategy for synthesis of the desired lactam 8 (Scheme 1). The addition of dichloromethane as co-solvent (10:1, v/v) and use of vinyl acetate (4.50 equiv) in resolution of alcohol (±)-15 [5 μM] with Amano PS lipase led to excellent selectivity (E=22), providing one of the most effective kinetic resolutions of a complex non-β-branched primary alcohol.26 These conditions afforded the critical alcohol (−)-15 in 36% yield (10 h, 64% conversion) with an excellent level of enantiomeric excess (>98% ee)17 along with isolation of acetate (+)-16 (60%, 56% ee, Scheme 3).28 Furthermore, the enatiopurity of recovered acetate (+)-16 could be readily enhanced using a CCL hydrolytic resolution to give alcohol (+)-15 (90% ee, 46%) with excellent potential for synthesis of the characteristic (S)-C5-aspidosperma skeleton containing C21-oxygenation.6,8
Scheme 3.
Preparation of alcohols (−)-15 and (+)-15a
The versatility of lactam (−)-15 for the synthesis of C21-oxygenated aspidosperma alkaloids is illustrated in Scheme 4. Hydrogenation of lactam (−)-15 followed by p-nitrobenzoylation of the C21-alcohol gave benzoate (+)-17 in 82% yield. The electrophilic amide activation of lactam (+)-17 under our optimal conditions11 resulted in the diastereoselective formation of diiminium ion 18. Regioselective C2-reduction with tri-n-butyltin hydride in situ afforded the critical C19-iminium ion 19 as the prelude to accessing the crucial C21-hydroxy-iminium ion 7 (Schemes 1 and 4). Importantly, the presence of the electron-deficient C21-ester was found to be ideal for the desired transannular cyclization step in preparation of iminium ion 19. The absolute and relative stereochemistry of the pentacyclic C21-oxygenated iminium ion 19 was unequivocally secured via its conversion to aspidosperma alkaloid (+)-limaspermidine (20, Scheme 4).5,17,29,30 Gratifyingly, mild in situ methanolysis of iminium ion 19 resulted in the release of the C21-alcohol and spontaneous cyclization providing direct access to hexacycle (+)-21 in 80% yield from benzoate (+)-17 (Scheme 4). While unraveling of the N1-amine afforded (+)-fendleridine (3), a subsequent N1-acetylation provided (+)-acetylaspidoalbidine (4), in 84% and 88% yield, respectively (Scheme 4). All spectroscopic data as well as the optical rotation data for our synthetic (+)-fendleridine (3) (observed: [α]d24: +79 (c = 0.40, CHCl3), lit.: [α]d24: +43 (c = 1.1, CHCl3)5e,31) and (+)-acetylaspidoalbidine (4) (observed: [α]d24: +39 (c = 0.18, CHCl3), lit.: [α]d24 = +38 (c = 0.2, CHCl3)5e and [α]d24 = +46 (CHCl3)4a), were consistent with the literature.4a,17 Importantly, the application of our planned transannular cyclization followed by intramolecular trapping of a transient C19-iminium ion 7 (Scheme 1) offered a concise and completely stereoselective strategy for conversion of lactam (−)-15 to the C19-hemiaminal ether containing alkaloids (+)-fendleridine (3) and (+)-acetylaspidoalbidine (4) in only four and five steps, respectively (Scheme 4).
Scheme 4.
Concise syntheses of (+)-fendleridine (3), (+)-acetylaspidoalbidine (4), and (+)-limaspermidine (20)a
We next focused on the development of our biogenetically inspired strategy for final-stage C17-hydroxylation of fendleridine derivatives (Scheme 1) to provide (+)-haplocidine (1) and (+)-haplocine (2). We envisioned the N1-amide could provide the necessary guidance32 to afford a regioselective C–H bond oxidation, offering an exciting strategy for late-stage diversification and amplification of the molecular complexity of the C19-hemiaminal ether aspidosperma alkaloids.
Inspired by recent advances in the ortho-C–H oxidation of N-phenylacetamides,33 we sought to develop a similar transformation for the desired C17-hydroxylation of fendleridine derivatives. While conditions exist for ortho-hydroxylation of many N-acyl-azaheterocycles,34 N-acyl-indolines are a challenging set of substrates for ortho-functionalization due to the geometry and distance between the directing amide and the target C–H bond. Existing methods for introduction of the desired ortho-C–O bond in simple indolines require the use of toxic lead or thallium reagents and often give product mixtures.35 Prompted by successful indoline ortho-C–H functionalizations to give C–C,36 C–N,37 C–Se, and C–S bonds,38 we focused on development of the first transition metal catalyzed indoline ortho-C–H acetoxylation. Our most promising results involving palladium–catalyzed ortho-acetoxylation39 of N-acetylindoline (±)-22a as a model substrate are summarized in Table 2.17 The use of hexafluoroisopropanol (HFIP) and acetic anhydride provided the desired acetoxyindoline (±)-23a even at 55 °C, but required long reaction times and high catalyst loadings (Table 2, entries 1–2). Alternatively, the use of acetic acid and acetic anhydride solvent mixture (10:1, v/v) as the reaction medium at 100 °C led to faster ortho-acetoxylation (Table 2, entries 4–6). Notably, lower yields were observed when the reaction was not purged with dioxygen prior to heating (Table 2, entry 3). Under optimal conditions, treatment of N-acetylindoline (±)-22a with palladium acetate (15 mol%) and bisacetoxyiodosobenzene (2.5 equiv) in acetic acid and acetic anhydride at 100 °C under an atmosphere of dioxygen for 9 h provided acetoxy-indoline (±)-23a in 84% yield (Table 2, entry 5). While the desired oxidation product was not formed in the absence of palladium acetate (Table 2, entry 7), the exclusion of acetic anhydride led to slightly lower yield of acetoxy-indoline (±)-23a (74%, Table 2, entry 8).
Table 2.
Directed C-H oxidation of indoline (±)-22aa
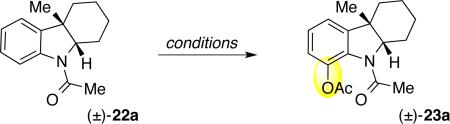
| ||||||
|---|---|---|---|---|---|---|
| entry | Pd(OAc)2 | PhI(OAc)2 | time | temperature | solvent | yield |
| 1 | 20 mol% | 4 equiv | 24 h | 70 °C | HFIP | 38% |
| 2 | 100 mol% | 2 equiv | 24 h | 55 °C | HFIP | 61% |
| 3 | 20 mol% | 2 equiv | 24 h | 100 °C | AcOH | 23% |
| 4 | 100 mol% | 2.5 equiv | 12 h | 100 °C | AcOHb | 87% |
| 5 | 15 mol% | 2.5 equiv | 9 h | 100 °C | AcOHb | 84% |
| 6 | 5 mol% | 2.5 equiv | 12 h | 100 °C | AcOHb | 67% |
| 7 | — | 2.5 equiv | 12 h | 100 °C | AcOHb | 0% |
| 8 | 20 mol% | 2.5 equiv | 13 h | 100 °C | AcOHb,c | 74% |
Reactions conducted in solvent and Ac2O mixture (10:1, v/v).
Reaction conducted under O2 atmosphere.
Reaction conducted without Ac2O.
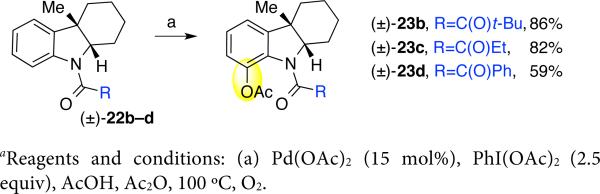
Given our interest in the application of this amide–directed ortho-acetoxylation to fendleridine derivatives with varying N1-amides (Scheme 1), we evaluated the relative effectiveness of indolines (±)-22b–d17 as substrates under the optimal conditions (Scheme 5). Notably, we were able to obtain the ortho-acetoxylation products (±)-23b–d from the corresponding N-pivaloyl, N-propionyl, and N-benzoyl indoline amides in 86%, 82%, and 59% yield, respectively.17 The oxidation products (±)-23b–d were obtained under the standard set of conditions with excellent level of regioselection. In the case of amide (±)-23b, the s-cis conformer, the optimal conformer for directed ortho-acetoxylation, was calculated to be 1.52 kcal/mol lower in energy than the corresponding s-trans conformer.17,40 In contrast, the preference calculated for the s-cis conformer for amide (±)-23d was only 0.13 kcal/mol. Thus, the greater efficacy of substrate (±)-23b compared to amide (±)-23d is consistent with its enhanced Lewis basicity and s-cis conformational preference. This N1-amide directed acetoxylation could be of broader utility given the prevalence of the N-acyl indoline substructure in various bio-active molecules and natural products.41
Scheme 5.
Directed C–H oxidation of N-acylindolinesa
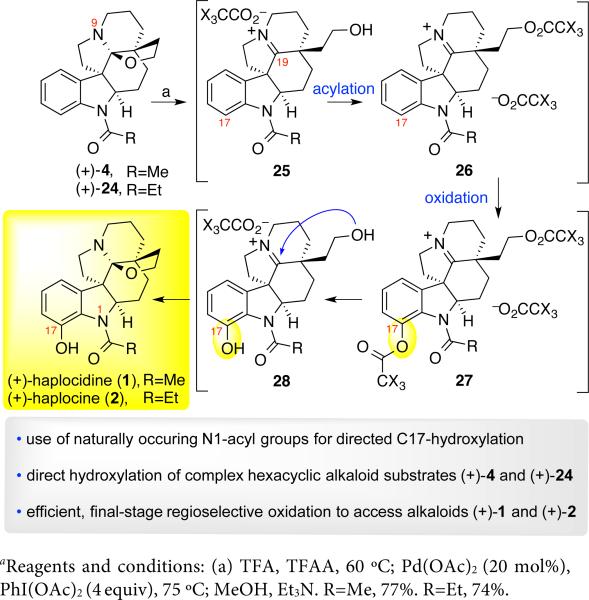
With efficient conditions for the ortho-oxidation of N-acyl indolines (±)-22a and (±)-22c at hand, we proceeded with development of a unified strategy for the first total syntheses of (+)-haplocidine (1) and (+)-haplocine (2) via a final-stage C17-hydroxylation of fendleridine derivatives (Scheme 6). Initial efforts towards the oxidation of (+)-acetylaspidoalbidine (4) to (+)-haplocidine (1) by direct application of the mildest conditions developed for N-acetylindoline (±)-22a (Table 2, entry 2) only returned the starting material. We posited that an unproductive N9-amine coordination to palladium may outcompete the N1- amide, consistent with the absence of basic nitrogens in substrates that efficiently undergo metal-catalyzed C–H functionalization.33–38,42 Thus, we envisioned a strategy to deactivate the N9-amine by exploiting the reversible opening of the C19-hemiaminal ether of (+)-acetylaspidoalbidine (4). Heating hexacyclic alkaloid (+)-4 in a mixture of acetic acid and acetic anhydride (10:1, v/v) at 100 °C for 6 h led to quantitative conversion to the corresponding C19-iminium acetate ester 26 (X=H, Scheme 6).17,43 However, application of the mildest oxidation conditions to C19-iminium acetate ester 26 (X=H) led to no observed product formation. The use of more forcing conditions with C19-iminium acetate ester 26 (X=H), involving acetic acid as solvent at 100 °C, also did not provide the product. Employing a stoichiometric amount of palladium acetate provided (+)-haplocidine (1) after hydrolysis, albeit with less than 25% conversion along with recovery of (+)-acetylaspidoalbidine (4).17 Attempts at promoting the reaction further through increased temperature, increased amounts of palladium acetate and oxidant, use of an atmosphere of dioxygen, extended reaction times and the use of alternate solvents did not result in greater conversion to (+)-haplocidine (1).17
Scheme 6.
First total syntheses of (+)-haplocidine (1) and (+)-haplocine (2)a
We surmised that the C19-iminum ion increases the energetic barrier for the C17-electrophilic palladation step compared to that involved in oxidation of N-acetylindoline (±)-22a. To our delight, a dramatic increase in the efficiency of the C17-oxidation was achieved using the C19-iminium trifluoroacetate ester 26 (X=F, Scheme 6) in place of the corresponding C19-iminium acetate ester 26 (X=H, Scheme 6).17,44 We determined that the optimal conditions involved the use of trifluoroacetic acid as the solvent, leading to clean palladium-catalyzed C17-acetoxylation of C19-iminium trifluoroacetate ester 26 (X=F, Scheme 6), likely due to more effective guidance by the N1-amide. In the event, exposure of (+)-acetylaspidoalbidine (4) to trifluoroacetic acid and trifluoroacetic anhydride (TFAA, 10:1, v/v) at 60 °C for 4 h, followed by addition of palladium acetate (20 mol%) and bisacetoxyiodosobenzene (4.0 equiv) and heating at 75 °C for 16 h gave complete conversion to the desired C17-oxidation product 27 (X=F, Scheme 6). Mild methanolysis of C17- and C21-trifluoroacetates of iminium ion 27 and spontaneous C19-hemiaminal ether formation of pentacycle 28 directly afforded (+)-haplocidine (1) in 77% overall yield. All spectroscopic data as well as the optical rotation data (observed: [α]d24: +207 (c = 0.32, CHCl3), lit.: [α]d24 = +214 ±7 (c = 0.354)) for our synthetic (+)-haplocidine (1) were in agreement with the literature.2a Furthermore, prompted by the flexibility of this oxidation reaction with respect to the N1-amide (Table 2, Scheme 5) we prepared N-propionylaspidoalbidine (+)-24 (Scheme 6)17 via N1-n-propionylation of (+)-fendleridine (3). Exposure of substrate (+)-24 to the optimal C17-oxidation conditions described above directly provided (+)-haplocine (2) in 74% yield. All spectroscopic data as well as the optical rotation data (observed: [α]d24: +180 (c = 0.23, CHCl3), lit.: [α]d24: +193 (CHCl3)) for (+)-haplocine (2) were consistent with the literature.2a Importantly, the application of this amide directed ortho-acetoxylation of indolines, combined with our strategic use of the pentacyclic C19-iminium ion 26 (X=F) provided (+)-haplocidine (1) and (+)-haplocine (2) in a single-step from (+)-acetylaspidoalbidine (4) and (+)-propionylaspidoalbidine (24), respectively. This C–H oxidation chemistry bodes well for future access to derivatives of these alkaloids.33–39,45
Conclusion
In summary, we have developed the first total syntheses of the antiproliferative alkaloid (+)-haplocidine (1), and its N1-amide congener (+)-haplocine (2). The successful implementation of our synthetic strategy for alkaloids (+)-1 and (+)-2 required a final-stage and selective C17-hydroxylation of complex aspidosperma alkaloids. The flexibility of the amide directed ortho-oxidation of indolines is promising as a new method for functionalization of related natural or designed substructures. The concise and highly stereoselective formation of pentacyclic C19-iminium ion 7 offered a unified approach to C19-hemiaminal ether alkaloids (+)-fendleridine (3), (+)-acetylaspidoalbidine (4), and (+)-propionylaspidoalbidine (24). The insights gained in the context of the development of the necessary chemistry including the versatile ortho-acetoxylation of indoline amides, the highly effective enzymatic resolution of a challenging non-β-branched primary alcohol, and strategic use of a pentacyclic C19-iminium ion are likely to be of broader utility. The critical C21-oxygenated lactam, prepared in both enantiomerically enriched forms, can serve as a branching point to access various aspidosperma alkaloids.1,16 Our strategy for the rapid formation of the C19-hemiaminal ether aspidosperma alkaloids and their biogenetically inspired late-stage C17-hydroxylation to give alkaloids (+)-1 and (+)-2 offers a highly concise route to these alkaloids.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge financial support by NIH-NIGMS (GM089732) and Amgen. We thank the NSF under CCI Center for Selective C–H Functionalization (CHE-1205646) for supporting our oxidation studies. K.L.W. acknowledges a National Science Foundation graduate fellowship and an Amgen Graduate Research Fellowship. We are grateful to Prof. Jin-Quan Yu for helpful discussions. We thank Dr. Wen-Tau T. Chang (MIT) for assistance with the DFT calculations and Dr. German F. Cabrera (MIT) for discussions related to the Wacker-Tsuji oxidation chemistry.
Footnotes
The authors declare no competing financial interests.
ASSOCIATED CONTENT
Supporting Information
Experimental procedures, spectroscopic data, and copies of NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.a Alkaloids of the Aspidospermine Group. Saxton JE. In: The Alkaloids, Chem. Biol. Cordell GA, editor. Vol. 51. Academic Press; San Diego: 1998. pp. 1–197. [Google Scholar]; b Alkaloids. O'Connor SE. In: Comprehensive Natural Products II. Mander L, Liu H-W, editors. Vol. 1. Elsevier; Amsterdam: 2010. pp. 977–1007. [Google Scholar]
- 2.a Cava MP, Talapatra SK, Nomura K, Weisbach JA, Douglas B, Shoop EC. Chem. Ind. 1963:1242. [Google Scholar]; b Cava MP, Nomura K, Talapatra SK. Tetrahedron. 1964;20:581. [Google Scholar]; c Walser A, Djerassi C. Helv. Chim. Acta. 1965;48:391. [Google Scholar]; d Dastoor NJ, Gorman AA, Schmid H. Helv. Chim. Acta. 1967;50:213. doi: 10.1002/hlca.19670500129. [DOI] [PubMed] [Google Scholar]
- 3.Hart MJ, Glicksman M, Liu M, Sharma MK, Cuny G, Galvan V. Anal. Biochem. 2012;421:467. doi: 10.1016/j.ab.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 4.a Brown KS, Budzikiewicz H, Djerassi C. Tetrahedron Lett. 1963;4:1731. [Google Scholar]; b Walser A, Djerassi C. Helv. Chim. Acta. 1965;48:391. [Google Scholar]; c Burnell RH, Medina JD, Ayer WA. Can. J. Chem. 1966;44:28. [Google Scholar]; d Mitaine AC, Mesbah K, Richard B, Petermann C, Arrazola S, Moretti C, Zeches-Hanrot M, Le Men Olivier L. Planta Med. 1996;62:458. doi: 10.1055/s-2006-957939. [DOI] [PubMed] [Google Scholar]
- 5.a Ban Y, Ohnuma T, Seki K, Oishi T. Tetrahedron Lett. 1975;16:727. [Google Scholar]; b Honma Y, Ohnuma T, Ban Y. Heterocycles. 1976;5:47. [Google Scholar]; c Yoshido K, Sakuma Y, Ban Y. Heterocycles. 1987;25:47. [Google Scholar]; d Overman LE, Robertson GM, Robichaud AJ. J. Am. Chem. Soc. 1991;113:2598. [Google Scholar]; e Campbell EL, Zuhl AM, Liu CM, Boger DL. J. Am. Chem. Soc. 2010;132:3009. doi: 10.1021/ja908819q. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Guerard KC, Guerinot CB-A, Menard M-A, Lepage M, Beaulieu MA, Canesi SJ. Org. Chem. 2012;77:2121. doi: 10.1021/jo300169k. [DOI] [PubMed] [Google Scholar]; g Tan SH, Banwell MG, Willis AC, Reekie TA. Org. Lett. 2012;14:5621. doi: 10.1021/ol3026846. [DOI] [PubMed] [Google Scholar]; h Zhang S-H, Shen X-L, Li Z-Q, Zou L-W, Wang F-Q, Zhang H-B, Shao Z-H. J. Org. Chem. 2013;78:11444. doi: 10.1021/jo402004f. [DOI] [PubMed] [Google Scholar]
- 6.a Cava MP, Talapatra SK, Yates P, Rosenberger M, Szano AG, Douglas B, Raffauf RF, Shoop EC, Weisbach JA. Chem. Ind. 1963:1875. [Google Scholar]; b Cava MP, Lakshmikantham MV, Talapatra SK, Yates P, Rae D, Rosenberger M, Szabo AG, Douglas B, Weisbach JA. Can. J. Chem. 1973;51:3102. [Google Scholar]
- 7.Satoh H, Ueda H, Tokuyama H. Tetrahedron. 2013;69:89. [Google Scholar]
- 8.a Rogers EF, Snyder HR, Fischer RF. J. Am. Chem. Soc. 1952;74:1987. [Google Scholar]; b Snyder HR, Fischer RF, Walker JF, Els HE, Nussberger GA. J. Am. Chem. Soc. 1954;76:2819. [Google Scholar]; c Snyder HR, Fischer RF, Walker JF, Els HE, Nussberger GA. J. Am. Chem. Soc. 1954;76:4601. [Google Scholar]; d Snyder HR, Strohmayer HF, Mooney RA. J. Am. Chem. Soc. 1958;80:3708. [Google Scholar]
- 9.a He F, Bo Y, Altom JD, Corey EJ. J. Am. Chem. Soc. 1999;121:6771. [Google Scholar]; b Sumi S, Matsumoto K, Tokuyama H, Fukuyama T. Org. Lett. 2003;5:1891. doi: 10.1021/ol034445e. [DOI] [PubMed] [Google Scholar]; c Sumi S, Matsumoto K, Tokuyama H, Fukuyama T. Tetrahedron. 2003;59:8571. doi: 10.1021/ol034445e. [DOI] [PubMed] [Google Scholar]; d Mejía-Oneto JM, Padwa A. Org. Lett. 2006;8:3275. doi: 10.1021/ol061137i. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Marino JP, Cao G. Tetrahedron Lett. 2006;47:7711. [Google Scholar]; f Nicolaou KC, Dalby SM, Majumder U. J. Am. Chem. Soc. 2008;130:14942. doi: 10.1021/ja806176w. [DOI] [PubMed] [Google Scholar]; g Yang R, Qiu FG. Angew. Chem. Int. Ed. 2013;52:6015. doi: 10.1002/anie.201302442. [DOI] [PubMed] [Google Scholar]
- 10.Medley JW, Movassaghi M. Angew. Chem. Int. Ed. 2012;51:4572. doi: 10.1002/anie.201200387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mewald M, Medley JW, Movassaghi M. Angew. Chem. Int. Ed. 2014;53:11634. doi: 10.1002/anie.201405609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a Ueda H, Satoh H, Matsumoto K, Sugimoto K, Fukuyama T, Tokuyama H. Angew. Chem. Int. Ed. 2009;48:7600. doi: 10.1002/anie.200902192. [DOI] [PubMed] [Google Scholar]; b Nicolaou KC, Dalby SM, Li S, Suzuki T, Chen DY-K. Angew. Chem. Int. Ed. 2009;48:7616. doi: 10.1002/anie.200904588. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Boger DL. J. Am. Chem. Soc. 2014;136:3312. doi: 10.1021/ja500548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medley JW, Movassaghi M. Org. Lett. 2013;15:3614. doi: 10.1021/ol401465y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White KL, Mewald M, Movassaghi MJ. Org. Chem. 2015;80:7403. doi: 10.1021/acs.joc.5b01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For examples of heterochiral aspidosperma alkaloids, see: Azoug M, Loukaci A, Richard B, Nuzillard J-M, Moreti C, Zeches-Hanrot M, Le Men-Olivier L. Phytochemistry. 1995;39:1223. Bui A-M, Das BC, Potier P. Phytochemistry. 1980;19:1473. Indole Alkaloids. Grundon MF. The Alkaloids. Vol. 7. The Chemical Society, Burlington House; London: 1977. p. 223. Iglesias R, Diatta L. Rev. Cenic. Cienc. Fis. 1975;6:141. Kahrl J, Gebreyes T, Djerassi C. Tetrahedron Lett. 1971:2527. Harper JK, Dunkel R, Wood SG, Owen NL, Li D, Cates RG, Grant DM. J. Chem. Soc.-Perkin Transactions 2. 1996:91.
- 17.Please see Supporting Information for more details.
- 18.Righi M, Topi F, Bartolucci S, Bedini A, Piersanti G, Spadoni G. J. Org. Chem. 2012;77:6351. doi: 10.1021/jo3010028. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Akula HK, Lakshman MK. Eur. J. Org. Chem. 2010:2709. doi: 10.1002/ejoc.200901420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168. [Google Scholar]
- 21.Sattely ES, Meek SJ, Malcolmson SJ, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:943. doi: 10.1021/ja8084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a Smidt J, Hafner W, Jira R, Sieber R, Sedlmeier S, Sabel A. Angew. Chem. Int. Ed. 1962;1:80. [Google Scholar]; b Cachoux F, Ibrahim-Ouali M, Santelli M. Tetrahedron Lett. 2000;41:1767. [Google Scholar]; c Tsuji J. Palladium Reagents and Catalysts-New Perspectives for the 21st Century. J. Wiley & Sons Ltd.; Chichester: 2004. [Google Scholar]
- 23.a Muzart J. Tetrahedron. 2007;63:7505. [Google Scholar]; b Wright JA, Gaunt MJ, Spencer JB. Chem.–Eur. J. 2006;12:949. doi: 10.1002/chem.200400644. [DOI] [PubMed] [Google Scholar]; c Weiner B, Baeza A, Jerphagnon T, Feringa BL. J. Am. Chem. Soc. 2009;131:9473. doi: 10.1021/ja902591g. [DOI] [PubMed] [Google Scholar]; d Michel BW, McCombs JR, Winkler A, Sigman MS. Angew. Chem. Int. Ed. 2010;49:7312. doi: 10.1002/anie.201004156. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Teo P, Wickens ZK, Dong G, Grubbs RH. Org. Lett. 2012;14:3237. doi: 10.1021/ol301240g. [DOI] [PubMed] [Google Scholar]; f Dong JJ, Harvey EC, Fanãnaś-Mastral M, Browne WR, Feringa BL. J. Am. Chem. Soc. 2014;136:17302. doi: 10.1021/ja510163w. [DOI] [PubMed] [Google Scholar]; g Dong JJ, Browne WR, Feringa BL. Angew. Chem. Int. Ed. 2014;54:734. doi: 10.1002/anie.201404856. [DOI] [PubMed] [Google Scholar]
- 24.The chiral auxiliaries included 1,2-diphenylethane-1,2-diamine and tryptophan, menthol, and camphor based carboxylate derivatives.
- 25.For a selection of reviews on the use of enzymatic resolution in synthesis see: Sih CJ, Wu SH. Topics Stereochem. 1989;19:63. Chen C-S, Sih CJ. Angew. Chem. Int. Ed. Engl. 1989;28:695. Bo-land W, Frössl C, Lorentz M. Synthesis. 1991:1049. Faber K, Riva S. Synthesis. 1992:895. Theil F. Chem. Rev. 1995;95:2203.
- 26.a Cambou B, Klibanov AM. J. Am. Chem. Soc. 1984;106:2687. [Google Scholar]; b Hughes DL, Bergan JJ, Amato JS, Bhupaty M, Leazer JL, McNamara JM, Sidler DR, Reider PJ, Grabowski EJ. J. Org. Chem. 1990;55:6252. [Google Scholar]; c Mizuguchi E, Takemoto M, Achiwa K. Tetrahedron: Asymmetry. 1993;4:1961. [Google Scholar]; d Soriente A, Laudisio G, Giordano M, Sodano G. Tetrahedron: Asymmetry. 1995;6:859. [Google Scholar]; e Ors M, Morcuende A, Jimenez-Vacas MI, Valverde S, Herradón B. Synlett. 1996:449. [Google Scholar]; f Goj O, Burchardt A, Haufe G. Tetrahedron: Asymmetry. 1997;8:399. [Google Scholar]; g Isleyen A, Tanyeli C, Dogan O. Tetrahedron: Asymmetry. 2006;17:1561. [Google Scholar]; h Chen H, Nagabandi S, Smith S, Goodman JM, Plettner E. Tetrahedron: Asymmetry. 2009;20:449. [Google Scholar]; i Forró E, Schönstein L, Fülöp F. Tetrahedron: Asymmetry. 2011;22:1255. [Google Scholar]
- 27.Schönstein L, Forró E, Fülöp F. Tetrahedron: Asymmetry. 2013;24:202. [Google Scholar]
- 28.Concentration of the crude reaction mixture after addition of toluene is essential to avoid an acid-mediated hydrolysis of acetate (+)-16.
- 29.Medina JD, Digenova L. Planta Medica. 1979;37:165. [Google Scholar]
- 30.a Zhang S-X, Shen X-L, Li Z-Q, Zou L-W, Wang F-Q, Zhang H-B, Shao Z-H. J. Org. Chem. 2013;78:11444. doi: 10.1021/jo402004f. [DOI] [PubMed] [Google Scholar]; b Chen Z, Zhou S, Jia Y. J. Org. Chem. 2015;80:12545. doi: 10.1021/acs.joc.5b02402. [DOI] [PubMed] [Google Scholar]; c Du J-Y, Zeng C, Han X-J, Qu H, Zhao X-H, An X-T, Fan C-A. J. Am. Chem. Soc. 2015;137:4267. doi: 10.1021/jacs.5b01926. [DOI] [PubMed] [Google Scholar]
- 31.The precise level of enantiomeric enrichment is unclear and the observed variation may be due to method of isolation and purification.
- 32.Krylov IB, Vil VA, Terent'ev AO. Beilstein J. Org. Chem. 2015;11:92. doi: 10.3762/bjoc.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.a Tellitu I. Euro. J. Org. Chem. 2007;3:437. [Google Scholar]; b Wang G-W, Yuan T-T, Wu X-L. J. Org. Chem. 2008;73:4717. doi: 10.1021/jo8003088. [DOI] [PubMed] [Google Scholar]; c Wang G-W, Yuan T-T. J. Org. Chem. 2010;75:476. doi: 10.1021/jo902139b. [DOI] [PubMed] [Google Scholar]; d Shan G, Yang X, Ma L, Rao Y. Angew. Chem. Int. Ed. 2012;51:13070. doi: 10.1002/anie.201207458. [DOI] [PubMed] [Google Scholar]; e Jiang T-S, Wang G-W. J. Org. Chem. 2012;77:9504. doi: 10.1021/jo301964m. [DOI] [PubMed] [Google Scholar]; f Yang X, Shan G, Rao Y. Org. Lett. 2013;15:2334. doi: 10.1021/ol400437a. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z-J, Quan X-J, Ren Z-H, Wang Y-Y, Guan Z-H. Org. Lett. 2014;16:3292. doi: 10.1021/ol501293c. [DOI] [PubMed] [Google Scholar]
- 35.a Taniguchi M, Yamamoto I, Nakagawa M, Hino T. Chem. Pharm. Bull. 1985;33:4783. [Google Scholar]; b Somei M, Kawasaki T, Ohta T. Heterocycles. 1988;27:2363. [Google Scholar]; c Yamada K, Somei M. Heterocycles. 1998;48:2481. [Google Scholar]; d Yamada K, Somei M. Heterocycles. 2012;84:785. [Google Scholar]
- 36.a Jiao L-Y, Oestreich M. Org. Lett. 2013;15:5374. doi: 10.1021/ol402687t. [DOI] [PubMed] [Google Scholar]; b Jiao L-Y, Oestreich M. Chem.–Eur. J. 2013;19:10845. doi: 10.1002/chem.201302140. [DOI] [PubMed] [Google Scholar]; c Kim M, Mishra NK, Park J, Han S, Shin Y, Sharma S, Lee Y, Lee E-K, Kwak JH, Kim IS. Chem. Commun. 2014;50:14249. doi: 10.1039/c4cc06929c. [DOI] [PubMed] [Google Scholar]; d Pan S, Ryu N, Shibata T. Adv. Syn. Catal. 2014;356:929. [Google Scholar]; e Pan S, Wakaki T, Ryu N, Shibata T. Chem. Asian J. 2014;9:1257. doi: 10.1002/asia.201301733. [DOI] [PubMed] [Google Scholar]; f Park J, Mishra NK, Sharma S, Han S, Shin Y, Jeong T, Oh JS, Kwak JH, Jung YH, Kim IS. J. Org. Chem. 2015;80:1818. doi: 10.1021/jo502733q. [DOI] [PubMed] [Google Scholar]; g Wang X, Tang HY, Feng HJ, Li YC, Yang YX, Zhou B. J. Org. Chem. 2015;80:6238. doi: 10.1021/acs.joc.5b00684. [DOI] [PubMed] [Google Scholar]; h Wu YX, Yang YX, Zhou B, Li YC. J. Org. Chem. 2015;80:1946. doi: 10.1021/jo502596k. [DOI] [PubMed] [Google Scholar]; i Yang D, Mao S, Gao YR, Guo DD, Guo SH, Li B, Wang YQ. Rsc Advances. 2015;5:23727. [Google Scholar]
- 37.a Ai W, Yang X, Wu Y, Wang X, Li Y, Yang Y, Zhou B. Chem.–Eur. J. 2014;20:17653. doi: 10.1002/chem.201405077. [DOI] [PubMed] [Google Scholar]; b Pan C, Abdukader A, Han J, Cheng Y, Zhu C. Chem–Eur. J. 2014;20:3606. doi: 10.1002/chem.201304236. [DOI] [PubMed] [Google Scholar]; c Shin K, Chang S. J. Org. Chem. 2014;79:12197. doi: 10.1021/jo5018475. [DOI] [PubMed] [Google Scholar]
- 38.Xie WC, Li B, Wang BQ. J. Org. Chem. 2016;81:396. doi: 10.1021/acs.joc.5b01943. [DOI] [PubMed] [Google Scholar]
- 39.a Yoneyama T, Crabtree RH. J. Mol. Catal. A-Chem. 1996;108:35. [Google Scholar]; b Dick AR, Sanford MS. Tetrahedron. 2006;62:2439. [Google Scholar]; c Liu G, Stahl SS. J. Am. Chem. Soc. 2006;128:7179. doi: 10.1021/ja061706h. [DOI] [PubMed] [Google Scholar]; d Deprez NR, Sanford MS. Inorg. Chem. 2007;46:1924. doi: 10.1021/ic0620337. [DOI] [PubMed] [Google Scholar]
- 40.Density functional theory at B3LYP level with 6-31G(d) as basis set (Spartan '14, Version 1.1.1, by Wavefunction, Inc.)
- 41.a Reina M, Ruiz-Mesia W, Lopez-Rodriguez M, Ruiz-Mesia L, Gonzalez-Coloma A, Martinez-Diaz R. J. Nat. Prod. 2012;75:928. doi: 10.1021/np300067m. [DOI] [PubMed] [Google Scholar]; b Rasoanaivo P, Palazzino G, Nicoletti M, Galeffi C. Phytochemistry. 2001;56:863. doi: 10.1016/s0031-9422(00)00475-1. [DOI] [PubMed] [Google Scholar]
- 42.For recent examples for oxidation conditions that circumvent non-productive Lewis basic nitrogen coordination, see: Howell JM, Feng KB, Clark JR, Trzepkowski LJ, White MC. J. Am. Chem. Soc. 2015;137:14590. doi: 10.1021/jacs.5b10299. Adams AM, Du Bois J, Malik HA. Org. Lett. 2015;17:6066. doi: 10.1021/acs.orglett.5b03047. Moretti RA, Du Bois J, Stack TDP. Org. Lett. 2016;18:2528. doi: 10.1021/acs.orglett.6b00518.
- 43.Conversion of (+)-acetylaspidoalbidine (4) to the corresponding iminium acetate ester 26 (X=H) is consistent with the observed 1H NMR deshielding of the C8-, C10-, and C21-resonances by approximately 1, 1, and 0.5 ppm, respectively.
- 44.Under similar conditions using excess Pd-catalyst and oxidant, the acetate 26 (X=H) and the trifluoroacetate 26 (X=F) were converted to the desired product (+)-1 to 18% and 75%, respectively.
- 45.a Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012;45:788. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang G, Lindovska P, Zhu D, Kim J, Wang P, Tang R-Y, Movassaghi M, Yu J-Q. J. Am. Chem. Soc. 2014;136:10807. doi: 10.1021/ja505737x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.