Abstract
Introduction
Outcome for glioma (GBM) remains dismal despite advances in therapeutic interventions including chemotherapy, radiotherapy and surgical resection. The overall survival benefit observed with immunotherapies in cancers such as melanoma and prostate cancer has fuelled research into evaluating immunotherapies for GBM.
Areas covered
Preclinical studies have brought a wealth of information for improving the prognosis of GBM and multiple clinical studies are evaluating a wide array of immunotherapies for GBM patients. This review highlights advances in the development of immunotherapeutic approaches. We discuss the strategies and outcomes of active and passive immunotherapies for GBM including vaccination strategies, gene therapy, check point blockade and adoptive T cell therapies. We also focus on immunoediting and tumor neoantigens that can impact the efficacy of immunotherapies.
Expert opinion
Encouraging results have been observed with immunotherapeutic strategies; some clinical trials are reaching phase III. Significant progress has been made in unraveling the molecular and genetic heterogeneity of GBM and its implications to disease prognosis. There is now consensus related to the critical need to incorporate tumor heterogeneity into the design of therapeutic approaches. Recent data also indicates that an efficacious treatment strategy will need to be combinatorial and personalized to the tumor genetic signature.
Keywords: Glioma, immunotherapy, gene therapy, cancer vaccines, passive immunotherapy, checkpoint blockade
1. Introduction
Gliomas are the most frequently diagnosed human primary brain tumors1. Glioma is derived from the greek word for “glue”, as glial tumors are derived from CNS cells that form the scaffolding and structural support for neurons. Gliomas are diagnosed according to the predominant cell population (astrocyte or oligodendrocyte) and are graded according to pathologic features that correlate with tumor aggressiveness and clinical outcome, including nuclear atypia, microvascular proliferation, hemorrhages and necrosis1. Lower-grade gliomas (WHO grade II/III) are molecularly similar and frequently progress to glioblastoma (GBM, WHO grade IV).
Recently, efforts have been made to sub-group grade II–III (“lower grade”) gliomas based on shared molecular features. In particular, sub-groups are based on the molecular status of: (1) deletion of chromosomes arms 1p and 19q (1p/19q co-deletion), (2) mutation in codon 132 of the isocitrate dehydrogenase 1 gene (IDH1), and (3) activating mutations in the promoter of the TERT gene, which encodes telomerase2, 3. Sub-grouping according to these features is predictive of histologic sub-type and prognosis, and molecular characteristics are increasingly used in the diagnostic work-up of gliomas (Table 1) 4. For example, IDH1 mutational status has become a key feature in determining the prognostic and biologic features of lower-grade glioma2, 3, 5, 6. IDH1 mutation is found in a majority of lower-grade glioma and secondary GBM, and is a positive prognostic variable2, 3. Gliomas which carry 1p/19q co-deletion are oligodendrocytic in lineage, and carry the best prognosis and response to alkylator-based chemotherapy and radiation7. Lower-grade IDH1-mutated gliomas without co-deletion are predominantly astrocytic tumors, and frequently carry concurrent mutations in the tumor suppressor TP53 and in the histone chaperone protein ATRX4, 5. Mutation (and loss) of ATRX was recently described to promote both glioma tumor growth and genetic instability8. The group of IDH mutated, not co-deleted lower-grade gliomas carry a moderate prognosis, while non-IDH1-mutated gliomas carry the worst prognosis2, 3. These molecular features are less prognostic in primary GBM, which carries a uniformly dismal prognosis2, 3.
Table 1.
Overview of molecular and genetic alterations in glioma
| Adult Primary | ||||
|---|---|---|---|---|
| Tumor Subtype | Classical | Mesenchymal | Neural | Proneural |
| overexpression | EGFR, NES168, 175 | MET, CHI3L1, CD44, MERTK175 | EGFR(1,2) | PDGFRA168, 175 |
| low expression/ablation | PTEN, CDKN2A168, 175 | NF1, PTEN168, 175 | PTEN175 | |
| mutation | EGFR168, 175 | NF1168, 175, PTEN168 | IDH1, PIK3A/R1168, 175 | |
| pathway activity | Notch168, 175, Sonic hedgehog168 | TNF, NFkB168, 175 | HIF, PI3K, PDGF175 | |
| marker expression | CHI3L1, MET168 | NEFL, GABRA1, SYT, SLC1A5168, 175 | SOX, DCX, DLL3, ASCL1, TCF4168, 175 | |
Despite progress in understanding the molecular attributes of glioma, current treatments are sub-optimal and human glioma results in significant morbidity and mortality9. Prognosis in gliomas is correlated with the degree of maximal safe resection10, 11. However, complete resection of gliomas is rarely achieved due to tumor infiltration into normal tissue and/or proximity to critical motor/sensory tracts12. Radiation is effective in prolonging survival in GBM and may be beneficial in some grade II/III gliomas13. Gliomas generally invade and grow locally; therefore radiation is given focally to involved areas.
Chemotherapy is only minimally effective in the treatment of gliomas. The addition of the alkylating agent temozolomide (TMZ), during and after radiation, prolongs survival in GBM, especially those with promoter methylation of the DNA-damage repair protein O6-methylguanine-DNA methyltransferase (MGMT)9. Other cytotoxic agents and regimens have displayed efficacy in certain lower-grade gliomas14, but the efficacy of most chemotherapeutic agents is hindered by the difficulty of delivering agents to tumor cells in the brain parenchyma15. Thus there is an urgent need for the development of efficacious multi-pronged therapies that are tailored to the unique facets of GBM biology.
Recent clinical data has caused a strong interest in the development and evaluation of immunotherapeutic approaches for GBM16–18. Approval of sipuleucel-T (Provenge) for metastatic hormone resistant prostate cancer and ipilimumab (Yervoy) for metastatic melanoma by the FDA has validated the efficacy of immunotherapies in other cancers19. In addition, a growing body of evidence has demonstrated the prognostic impact of immune cell infiltrates in the tumor20, 21.
The CNS has been traditionally considered an immune privileged system. However, a growing body of evidence has challenged this concept lately18, 22–25. It has been shown that immune cells can cross the blood brain barrier to gain access to the brain parenchyma and can leave the CNS to reach the cervical lymph nodes. Also, the ventricles, meninges, and perivascular spaces lack blood brain barrier (BBB) and their immune-reactivity is not different from that in the periphery22–25. Considering that the immune system has access to the brain and that GBM expresses multiple tumor antigens that can be targeted by immunotherapeutic approaches, the development of these therapies has gained considerable interest over the last decade. Immunotherapeutic approaches aim to induce an adaptive immune response that specifically targets and kills GBM cells without affecting normal cells within the brain parenchyma. Although these strategies trigger antitumor immunity26, the clinical benefit has been lower than hitherto anticipated. The ability of the immunotherapeutic approach to inhibit the immunosuppressive tumor microenvironment may be crucial to yield tumor regression27, 28.
1.1 Mechanisms of immune suppression in GBM
GBM cells actively contribute to the generation and maintenance of the immunosuppressive microenvironment29, 30 (Fig. 1). These cells produce anti-inflammatory cytokines, such as IL-10 and TGF-β, which inhibit effector T cell responses and APC function, while stimulating regulatory T cell (Treg) expansion and function31. Tregs are also recruited to the tumor microenvironment by the local production of chemokines, such as CCL2, by GBM cells32. Besides Tregs, myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs) also infiltrate the GBM tumor microenvironment, inhibit anti-tumor immune responses, and support tumor progression33, 34. Modulation of the expression of membrane-bound molecules that interact with tumor infiltrating immune cells is another mechanism that inhibits the adaptive antitumor immune response against GBM. Differential expression of Human Leucocyte Antigen (HLA) molecules has been involved in the immunological escape of GBM. The expression of HLA class I is downregulated in GBM specimens when compared with lower grade astrocytomas, which has been involved in the evasion of cytotoxic T lymphocyte (CTL) responses35. Although downregulation of HLA class I reduces the immunogenicity of GBM cells, it could activate NK cell response against the tumor. However, GBM cells overexpress nonclassical HLA class I molecules, such as HLA-G and HLA-E36, 37, which are involved in the inhibition of NK and CTL responses to the allogeneic fetus during pregnancy. Changes in HLA class II expression have also been reported in GBM cells and have been associated with epigenetic changes in the class II transactivator (CIITA) promoter38.
Figure 1. Role of GBM cells in the generation and maintainance of the immunosuppressive tumor microenvironment.
GBM cells have a central role in the immunosuppressive nature of tumor microenvironment. GBM cells express membrane-bound and soluble immunosuppressive proteins. FasL induces apoptosis of tumor infiltrating limphocytes. B7 molecules activate CTLA-4 and PD-L1 are ligands of immunosuppressive recetors CTLA-4 and PD-1, which inhibit effector T cells that infiltrate the tumor microenvironment. Downregulation of HLA-I reduces the immunogenicity of GBM cells, which in turn could activate NK cell response against the tumor. However, GBM cells overexpress an alternative MHCI molecule, HLA-G, which inhibits NK and CTL response. The synthesis of antiinflammatory cytokines and chemokines also contribute to the immunosuppressive microenvironment. GBM cells produce CCL2 and TGF-beta and IL-10 which recruit Tregs, myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs) that in turn inhibit the T cell response. IL-10 also inhibits the function of antigen presenting cells (APCs) within the tumor.
GBM cells can also directly inhibit T cell function through the expression of negative regulators of T cell function. FasL-expressing GBM cells induce apoptosis of tumor-infiltrating T cells that express Fas39. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Programmed death-1 (PD-1), Lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin mucin-3 (TIM-3) are negative regulators of the immune system and seem to have a crucial role in GBM immunological escape22. While CTLA-4 expression reduces the activation of naive CD4+ and CD8+ T cells40–42, PD-1 and TIM-3 are markers of T cell exhaustion in activated lymphocytes43. LAG3 is a negative regulator of activated T and NK cell expansion during chronic inflammation and tumorigenesis44. These molecules not only inhibit effector T cell and NK cell activation and function, but they also promote Treg immunossupressive function22. Considering that ligands of these proteins are overexpressed in GBM cells, i.e. PD-L145, galectin-946, they constitute valuable targets to overcome the immunosuppressive nature of GBM and improve the efficacy of immunotherapeutic strategies for GBM.
2. Immunotherapy approaches
Immunotherapeutic strategies can be broadly divided into four major classes; (1) immunomodulatory strategies like the ones that target checkpoints using ipilimumab, (2) active immunotherapy such as with cancer vaccines and immune stimulatory gene therapy, (3) passive immunotherapies utilizing antibodies and (4) adoptive strategies such as the ones using chimeric antigen receptor (CAR) T cells.
2.1 Targeting immunosuppressive checkpoints
T cells interact with Antigen-Presenting Cells (APCs) and tumor cells, providing signals to regulate immune response through stimulation or inhibition of T-cell activation47. These several ligand-receptor interactions are called checkpoint pathways. Checkpoint pathways are non-redundant and can be either co-stimulatory or co-inhibitory. While CD28, TNFRSF4 (OX40), CD40L, CD2 and CD137 enhance immune response, CTLA-4, PD-1, LAG-3, TIM-3 and TIGIT inactivate T lymphocytes48–53. Ligands for these immunosuppressive checkpoints are often overexpressed in the GBM microenvironment to inhibit T-cell response against tumor cells, and can be targets for therapies.
CTLA-4 is expressed exclusively by T cells and inhibits their activation by dephosphorylating the CD3ζ chain. CD28 and CTLA-4 share the ligands CD80 and CD86, but CTLA-4 binds them with higher affinity54, 55. Ipilimumab is a fully humanized monoclonal antibody against CTLA-4, approved in 2011 by the FDA and the European Medicines Agency for the treatment of metastatic melanoma which has shown benefits in patients with brain metastasis56–59. Extensive preclinical research has shown exciting results with CTLA-4 blockade19. Administration of CTLA-4 blocking antibodies improves the survival of animals bearing intracranial SMA-560 tumors60, anti-CTLA-4 antibodies in combination with IL-12 also led to the eradication of GL261 gliomas61, and the combination of CTLA-4 antibodies with vaccination with GMCSF expressing GBM cells was more effective than either treatment alone62. Ipilimumab has demonstrated encouraging results in patients with melanoma and brain metastases as a single agent therapy or in combination with radiotherapy18, and a clinical trial evaluating ipilimumab for recurrent and newly diagnosed GBM is currently recruiting patients (NCT02017717).
PD-1 binds to PD-L1 and PD-L2, expressed on surface of tumor cells50, 63, tumor-associated macrophages33, vascular endothelial cells64 and some neurons of the tumor microenvironment65. Particularly, PD-L1 has been found to have increased expression in GBM patients45. In 2014, the FDA approved Pembrolizumab and Nivolumab for metastatic melanoma. Later in 2015, the FDA also approved Nivolumab for non-small-cell lung cancer (NSCLC)66–69. Both agents are monoclonal antibodies that inhibit the PD-1 receptor and its interaction with ligands. These antibodies have also shown benefits in patients with other advanced cancers70, 71. The first large phase III trial of Nivolumab and Ipilimumab in GBM patients at different stages of treatment was initiated in 2014 (NCT02017717). Moreover, other PD-1/PD-L1 checkpoint inhibitors are under investigation for solid tumors and hematological cancers (AMP-224, MEDI4736, MPDL3280A, MSB0010418C)72.
TIM-3 and LAG-3 are also being investigated as possible targets for GBM therapy. TIM-3 is a glycoprotein with extracellular immunoglobulin and mucin domains that binds Galectin-9 and regulates T-cell exhaustion73. It has been shown to be highly expressed in patients with melanoma, NSCLC, lymphoma and other malignancies74. In recent investigations it was found that not only was TIM-3 expression significantly increased in peripheral blood CD4+ and CD8+ T cells of glioma patients compared with healthy controls, but that the expression of galectin-9 in tumor tissues was associated with TIM-3 expression on Tumor Infiltrating Lymphocytes (TILs) and the WHO grade of glioma46, 75. Some studies show that combination therapy with anti-PD-1, anti-TIM-3 and focal radiation results in regression of murine gliomas, concluding that the addition of a second checkpoint-blocking antibody could achieve additive or synergistic antitumor effects76. Checkpoint blockade could also prove a useful adjuvant when combined with other therapeutic strategies. Indoleamine 2, 3 dioxygenase 1 (IDO) is a tryptophan catabolic enzyme induced in GBM patients77. IDO expression by brain tumor cells is critically important in the immunosuppressive tumor microenvironment because it modulates Treg activation, expansion and recruitment78. Recent research shows that blockade of CTLA-4 and PD-L1 in combination with IDO inhibition using 1-methyltryptophan (1-MT) in murine glioblastoma models results in a highly effective survival advantage and a significantly decreased level of Tregs, in comparison with the mono or dual therapies79.
LAG-3 is a CD4 homolog that binds MHC Class II80 and seems to act synergistically with PD-L1 to control the expansion of already activated T-cells. It was found to be highly expressed on TILs from patients with melanoma, colorectal cancer or fibrosarcoma81. Currently, there is an ongoing phase I trial that studies the treatment with anti-LAG-3 mAb alone or in combination with Nivolumab in patients with recurrent glioblastoma (NCT02658981).
Of note, inhibition of immunological checkpoints not only improves the efficacy of immune-stimulant strategies, but can also be used in combination with conventional therapies, such as chemotherapy and radiotherapy16. Checkpoint inhibitors have also been shown to enhance the efficacy of anti-glioma stem cell treatments82.
2.2 Gene therapy
Gene therapy initially developed to restore the function of defective or absent genes, and has gained popularity for the treatment of glioblastoma, due in part to the failure of other treatment options and to the successful design of many vectors able to safely deliver therapeutic genes into the tumor, such as viruses (adeno- and adeno-associated, retroviruses, herpes simplex virus, measles, reovirus, poliovirus), stem cells (neural, mesenchymal or embryonic), liposomes and nanoparticles17, 83. Current gene therapeutic approaches for glioma can be classified in four categories: suicide gene therapy, oncolytic virotherapy, tumor suppressor gene therapy and immuno-stimulatory therapy84. Viral mediated gene therapies can induce immunogenic cell death of malignant cells85 and release of damage associated molecular patterns like HMGB186, 87, leading to improved antigen presentation and enhanced anti-tumor immune response.
2.2.1 Suicide gene therapy
Suicide gene therapy relies on the targeted administration of an enzyme, most commonly the viral gene thymidine kinase (TK), into dividing tumor cells. Upon administration of an inactive prodrug, for example ganciclovir (GCV), TK will convert it into a toxic metabolite, resulting in tumor cell apoptosis88–90. This strategy has shown success in numerous preclinical trials of glioma and has also been tested in several phase I and II clinical trials, which demonstrated the safety for this gene therapeutic approach91, 92. However, a large randomized phase III trial of HSV-TK/GCV in combination with surgery and radiation failed to show improved survival in newly diagnosed GBM93.
To improve upon this treatment, our group has developed a therapeutic strategy using adenovirus-mediated delivery of Flt3L, a cytokine which induces the generation and stimulates the migration of dendritic cells (DCs)94. In several preclinical studies we have shown that Ad-Flt3L in combination with AdTK/GCV induced prolonged survival of tumor-bearing animals, and that this was associated with increased recruitment of DCs into the tumor, generation of tumor specific T-cell responses and of long-term immunological memory, an effect dependent on the TLR2 activation by HMGB1 released by the dying tumor cells87, 95–99. A phase I clinical trial using this gene therapeutic approach is currently underway (NCT01811992, https://clinicaltrials.gov/show/NCT01811992).
2.2.2 Oncolytic viral therapy
Oncolytic viral (OV) therapy exploits the ability of conditionally replicative viral vectors to selectively divide within tumor cells, induce their lysis, and amplify engineered therapeutic genes within the tumor environment. In addition to the direct oncolytic activity, OVs are also very effective at inducing immune responses to themselves and to the infected tumor cells85. Oncolytic viruses tested in clinical trials for glioma include: oncolytic Herpes Simplex Virus (oHSV)100, 101, conditionally replicating adenoviruses (CRAds), oncolytic measles102 and reovirus vectors103. These vectors have shown safety when administered in phase I clinical trials and show improvement over non-replicating viruses in terms of efficiency of transduction of the therapeutic gene. A drawback however is represented by the vector-induced immune response. Further modifying these vectors can reduce their antigenic properties, and efforts have been made to increase selectivity of the vectors, using glioma specific and radiotherapy inducible promoters, like survivin104, 105. Two CRAd’s have been evaluated in clinical trials for glioma: ONYX-015, engineered to replicate in p53 deficient tumor cells, has been demonstrated to be safe when administered intracranially, but shows no clinical benefit106, 107, and Ad5Delta24, further modified into Ad5Delta24-RGD and DNX2401108 to increase its targeting to tumor cells, is currently tested in several Phase I/II trials: NCT01582516- phase I/II trial testing the convection enhanced delivery of Delta-24rgd in patients with recurrent GBM, NCT02197169 – phase I unicentric trial (Target-I) testing DNX2401 expressing IFN-γ in recurrent glioblastoma or gliosarcoma, and NCT01956734, a phase I trial testing DNX2401 in combination with temozolomide.
2.2.3 Immunomodulatory gene therapy
Immunomodulatory gene therapy is designed to create a tumor environment optimized for the induction of an effective anti-tumor immune response. This has been tried with targeted delivery of cytokines into the tumor microenvironment to achieve higher local concentrations of immuno-stimulatory molecules, such as IL-2, IL-4, IL-12, IFN-γ and IFN-β, without generating systemic side effects.
IL-2, known to induce proliferation of all immune cells and to enhance production of other immunostimulatory cytokines has first been tested in glioma patients in 1986 when recombinant IL-2 and autologous lymphokine activated killer cells (LAK cells) were directly injected into the tumor cavity, demonstrating safety and in vitro tumor specific killing by LAKs109. Systemic administration of IL-2 has shown to be poorly tolerated110. In a phase I trial, combination of suicide gene therapy and IL-2, obtained through the administration of retroviral producing cells expressing IL-2 and HSV-TK resulted in minimal side effects and partial response, evidenced by MRI, in 2 of 12 patients111.
IL-4 stimulates proliferation of lymphocytes acting synergistically with IL-2 and also induces DC maturation and production of IL-12. In a rat glioma model, subcutaneous vaccines with 9L cells transduced with HSV expressing TK, IL-4, or TK + IL-4 inhibited subcutaneous tumor growth and increased survival upon challenge with intracranial 9L tumors112. This was correlated with increased production of IFN-γ in the splenocytes of treated animals challenged with irradiated tumor cells. This strategy was tested in a phase I trial113, results of which have not been published.
The type I interferon gene IFN-β has been widely tested in treatments of several cancers, showing direct antiproliferative effect, and in glioma enhances sensitivity for TMZ treatment114, 115. In Japan, a phase I clinical trial with liposomal delivery of IFN-β showed minimal toxicity and clinical response with more than 50% reduction of the tumor in 2 out of 5 patients116. This study was followed by another phase I trial using administration of Ad-hIFNβ into the tumor cavity and surrounding tumor area post resection, demonstrating safety and induction of tumor cell apoptosis117.
2.3 Active immunotherapy
Cancer vaccines aim at stimulating adaptive immune responses that target tumor-specific antigens. Strategies utilized include delivery of tumor-associated antigens, administration of tumor antigen loaded DCs and tumor cell vaccines.
2.3.1 Peptide vaccination: tumor associated antigens and shared neoantigens
Successful immunotherapies rely on the targeting of glioma-specific antigens to selectively kill tumor cells, without inducing autoimmune reactions. To date, numerous glioma associated antigens (GAA) have been identified (Table 2), and targeted therapies have been developed and tested in recently completed and ongoing clinical trials. Several known tumor-associated antigens such as HER-2, TRP-2, gp100, MAGE-1, IL-13α2 and AIM-2 are being targeted in GBM16–18. Additionally, new immuno-therapeutic strategies use neoantigens to specifically target tumor cells. Neoantigens represent antigenic molecules, absent from normal tissues, which result from tumor specific DNA somatic mutations present within protein-coding regions; commonly occurring mutations, shared by many tumors, are attractive targets for therapy118, 119. The most extensively studied target is EGFR variant III16–18. EGFRvIII is a mutant form of the EGFR gene, found in 20–30% of GBM patients, expressing a truncated, constitutively active form of the receptor, causing increased proliferation and survival advantage of GBM tumor cells120. Evaluation of Rindopepimut/CDX-110 (14 amino acid peptide conjugated to KLH) in 18 patients with EGFRvIII mutation in combination with standard radiotherapy and chemotherapy showed a median survival of 26 months. At recurrence 82% of the tumors lost EGFRvIII expression suggesting treatment induced immunoediting. Currently a phase III (NCT01480479) multi-center trial is looking at the efficacy of CDX-110, GMCSF, TMZ and keyhole limpet hemocyanin (KLH) for the treatment of adult patients with EGFRvIII positive gliomas and a phase II study is evaluating the combination of rindopepimut, GMCSF and bevacizumab in relapsed EGFRvIII positive gliomas (NCT01498328).
Table 2.
Glioma associated antigens as therapeutic targets
| Antigen | Description | Expression | Tested in clinical trials? |
|---|---|---|---|
| ABCG 181 | ATP – Binding Cassette Group | Glioma stem cells | N/A |
| Aim-2 29, 132, 182, 183 | Antigen Isolated Melanoma-2 | High grade astrocytoma | DC vaccine pulsed with HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2 peptides 33% immune response132 ICT-107 DC Vaccine (NCT02546102) ongoing |
| Art1 29 | Antigen Recognized by T cells-1 | Adult and pediatric brain tumors | N/A |
| Art4 29 | Antigen Recognized by T cells-4 | Adult GBM | N/A |
| B-catenin 184, 185 | Beta-catenin | Astrocytomas | N/A |
| BMI1 186–188 | polycomb-group (PcG) BMI1 | Medulloblastoma, Glioblastoma, Glioma stem cells | N/A |
| B-cyclin29, 183, 189 | B-cyclin | Adult GBM | N/A |
| Cathepsin B 190–192 | Cathepsin B | Glioma stem cells, glioblastoma | N/A |
| Cav-1193, 194 | Caveolin -1 | Anaplastic astrocytoma, Glioblastoma, Oligodendroglioma, Ependymoma | N/A |
| CD133 195–198 | Cluster of differentiation 133, Prominin | Glioma stem cells | ICT-121 DC vaccine pulsed with CD133 (NCT02049489) ongoing |
| CD74 199, 200 | Cluster of Differentiation 74, HLA class II histocompatibility antigen gamma chain Binds Macrophage Migration Inhibitory Factor |
Glioblastoma | N/A |
| COX-2 201–203 | Cyclo-oxygenase 2 | Glioblastoma, CD133+ GSC cells | COX-2 inhibitor celecoxib and TMZ with thalidomide or isoretinoin (NCT00112502) No difference in PFS or OS202 |
| E-Cadherin 204, 205 | Epithelial-calcium-dependent adhesion | Glioblastoma, Pontine glioma, Medulloblastoma | N/A |
| EGFRvIII 50, 127–129, 196, 206 | Epidermal growth factor receptor variant III (truncated, constitutively active form of the receptors) | Glioblastoma |
EGFRvIII peptide vaccine ACTIVATE, ACTII (NCT00643097) 43% EGFRvIII-specific antibody response127 ACTIII (NCT00458601) 85% EGFRvIII-specific antibody response129 ACTIV (NCT01480479) ongoing ReACT (NCT01498328) ongoing EGFRvIII CARs NCT02209376 ongoing NCT01454596 ongoing |
| Ephrin A2/Eck 29, 143, 183, 207–210 | Ephrin Receptor A2 /epithelial cell kinase | Glioma Stem Cells, Glioblastoma, Adult and pediatric brain tumors | DC vaccine pulsed with EphA2, IL-13Rα2, YKL-40, and gp100 (NCT00766753) 58% immune response143 EphA2, IL-13Rα2, and survivin peptide vaccine (NCT01130077) 62% peptide specific immune response131 SL-701 peptide vaccine (NCT02078648) ongoing |
| EZH2 29, 209, 211 | Enhancer of zeste homolog 2 | Adult GBM, Glioma stem cells | N/A |
| Fra-1/Fosl 129, 207, 209 | Fos related antigen 1 | Adult GBM, highly expressed in pediatric brain tumors | N/A |
| GAGE-1 29, 212 | G antigen 1 | Low expression in adult high grade and pediatric brain tumors | N/A |
| Ganglioside/ GD2 213, 214 | Gangliosinde GD2 | Astrocytic tumors | N/A |
| GLAST 215, 216 | GLutamate ASpartate Transporter, Solute carrier family 1 (glial high-affinity glutamate transporter), member 3, SLC1A3, Excitatory Amino Acid Transporter 1 (EAAT1). | Glioma stem cells | N/A |
| Gli-1 217–219 | Glioma-associated oncogene homolog 1 | Glioblastoma, recurrent Glioblastoma after chemotherapy | IPI-926: Hedgehog pathway inhibitor 28% response217 |
| GnT-V,β1, 6-N 29, 183, 209, 220 | b 1.6 N-acetyl glucosaminotransferase | Astrocytoma, Adult GBM | N/A |
| GP-100 29, 50, 132, 143, 183, 221, 222 | Melanoma Glycoprotein-100 | Low expression in adult and pediatric brain tumors | DC vaccine pulsed with HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2 peptides 33% immune response132 DC vaccine pulsed with EphA2, IL-13Rα2, YKL-40, and gp100 (NCT00766753) 58% immune response143 DC vaccine pulsed with WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100 66% cellular immune response221 |
| Her-2/Neu 29, 132, 183, 209, 221–223 | HumanEpidermal Growth Factor Receptor 2, proto-oncogene Neu, receptor tyrosine-protein kinase erbB-2, CD340 | Adult GBM | DC vaccine pulsed with HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2 peptides 33% immune response132 DC vaccine pulsed with WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100 66% cellular immune response221 HER-2 CAR (NCT02442297) ongoing |
| IDH1R132H 224 | Isocitrate Dehydrogenase 1 gene |
Mutations present in majority of several types of malignant glioma, most prevalent in secondary GBM | PEPIDH1M: IDH1 peptide vaccine (NCT02193347) ongoing IDH1 peptide vaccine (NOA-16, NCT02454634) ongoing |
| IL-13Rα250, 132, 143, 183, 207, 210, 225, 226 | Interleukin 13 receptor alpha 2 protein chain |
High grade astrocytoma | DC vaccine pulsed with HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2 peptides 33% immune response132 DC vaccine pulsed with EphA2, IL-13Rα2, YKL-40, and gp100 (NCT00766753) 58% immune response143 EphA2, IL-13Rα2, and survivin peptide vaccine (NCT01130077) 62% peptide specific immune response131 SL-701 peptide vaccine (NCT02078648) ongoing IL13Rα2 CAR (NCT02208362) ongoing |
| Ki-67189, 227 | The nuclear cell proliferation-associated antigen of antibody Ki-67 | Prognostic marker for low grade glioma | N/A |
| Ku70/Ku80228 | Human Ku heterodimer proteins subunits | Glioblastoma | N/A |
| LICAM229, 230 | L1 Cell Adhesion molecule | Glioma stem cells | N/A |
| Mage-129, 50, 132, 183, 221, 222, 231 | Melanoma Antigen-1 | Adult GBM, Neuroblastoma | DC vaccine pulsed with HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2 peptides 33% immune response132 DC vaccine pulsed with WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100 66% cellular immune response221 |
| Mage-3221, 231 | Melanoma Antigen-3 | Adult GBM, Neuroblastoma | DC vaccine pulsed with WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100 66% cellular immune response221 |
| MART-129, 183 | Melanoma antigen recognized by T-cells | Low expression in adult and pediatric brain tumors | N/A |
| MRP329, 232 | Multidrug Resistance Protein-3 | Adult GBM | Personalized peptide vaccine mix containing MRP3 peptide 83% peptide-specific CTL response to at least one peptide233 |
| Nestin197, 198, 215, 231, 234 | Nestin | Glioma stem cells, Glioblastoma, Astrocytoma | N/A |
| NY-ESO-1231, 235 | New York esophageal squamous cell carcinoma 1 |
Neuroblastoma | CDX 1401 vaccine: DEC-205 antibody linked to NY-ESO-1 (NCT00948961) 79% NY-ESO-1-specific antibody response, 56% NY-ESO-1-specific T cell response235 CDX-1401 vaccine (NCT00948961) ongoing |
| Olig2215, 236–238 | Oligodendrocyte transcription factor 1 | Glioma stem cells, Oligodendroglioma, Oligoastrocytoma | N/A |
| Prox1239 | Prospero homeobox protein 1 | High grade glioma (also in immature neurons) | N/A |
| PTH-rP29 | Para-Thyroid Hormone-related Protein | Adult GBM | N/A |
| PSCA240 | Prostate stem cell antigen, GPI-anchored cell surface protein | WHO grade III–IV gliomas | N/A |
| Sart-129, 183 | Squamous cell carcinoma Antigen Recognized by T cells-1 | Astrocytoma, Adult GBM | N/A |
| Sart-229 | Squamous cell carcinoma Antigen Recognized by T cells-2 | Adult GBM | N/A |
| Sart-329 | Squamous cell carcinoma Antigen Recognized by T cells-3 | Adult GBM | N/A |
| Sox 2241, 242 | SRY (sex determining region Y)-box 2 | Glioma stem cells | N/A |
| Sox 6243 | SRY (sex determining region Y)-box 6 | Glioma stem cells | N/A |
| Sox 10236 | SRY (sex determining region Y)-box 10 | Oligodendroglioma, Oligoastrocytoma | N/A |
| Sox 1129, 209, 244 | SRY (sex determining region Y)-box 11 | Adult and pediatric brain tumors | N/A |
| SSEA245 | Stage-Specific Embryonic Antigen 1(SSEA-1/LeX) |
Glioma stem cells | N/A |
| Survivin29, 183, 209, 227 | Survivin | Glioblastoma | EphA2, IL-13Rα2, and survivin peptide vaccine (NCT01130077) 62% peptide specific immune response131 SL-701 peptide vaccine (NCT02078648) ongoing SurVaxM survivin peptide vaccine (NCT02455557) ongoing |
| h-Tert29, 246 | Telomerase Reverse Transcriptase | Adult GBM | N/A |
| TRP-129 | Tyrosinase Related Protein-1 | Adult GBM | N/A |
| TRP-229, 50, 132 | Tyrosinase Related Protein-2 | Adult and Pediatric brain tumors | DC vaccine pulsed with HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2 peptides 33% immune response132 |
| Tyrosinase29 | Tyrosinase | Adult GBM | N/A |
| UPAR247, 248 | Urokinase-type plasminogen activator receptor, CD87 | High grade glioma cells | N/A |
| WT-1221, 249–251 | Wilm‘s Tumor Antigen – 1, WT2725 | Glioblastoma and Medulloblastoma | DC vaccine pulsed with WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100 66% cellular immune response221 WT-1 peptide vaccine No increase in WT-1 specific CTLs post-vaccination249 WT2725: WT-1 peptide vaccine (NCT01621542) ongoing |
| YKL-4029, 143, 236, 252 | Chitinase-3-like protein 1 (CHI3L1), also known as YKL-40 | Astrocytoma, Oligoastrocytoma, High grade glioma, Glioma Stem Cells, secreted biomarker present in the serum of glioblastoma patients, Adult and pediatric brain tumors | DC vaccine pulsed with EphA2, IL-13Rα2, YKL-40, and gp100 (NCT00766753) 58% immune response143 Peptide vaccine with EGFRvIII, IL-13Rα2, EphA2, Her2/neu, YKL-40 peptides (NCT02754362) ongoing |
Mutations of IDH1 and IDH2 have been found in more than 80% of WHO grade II/III astrocytomas, oligodendrogliomas and oligoastrocytomas5. For IDH1, the most common genetic alteration is represented by a single nucleotide substitution: R132H, encompassing more than 70% of all IDH mutations. To test if this neoantigen is amenable for immunotherapeutic targeting, Schumacher et al.121 generated a peptide library encompassing this mutation and tested its ability to bind MHC-I and MHC-II. This study demonstrates that several 15mer peptides bound to the human MHC-II allele HLA-DRB1*0101 and that MHC-II humanized mice immunized with one of these peptides developed robust IFNγ T-cell responses resulting in Th1 CD4 specific responses with generation of mutation-specific anti-IDH1 antibodies detectable in the serum. Furthermore, specific IFNγ producing T cells were isolated from the serum of patients harboring IDH1 (R132H)-mutated gliomas but not from patients with wild type IDH gliomas. Also tumor growth was reduced in MHC-II humanized mice immunized with the IDH1 (R132H) peptide vaccine, effect dependent on CD4+ cells. Notably, immunization with the mutant peptide induced immuno-editing of the IDH1 (R132H) tumors, which resisted vaccination and showed marked decrease in IDH1 (R132H) expression. This study highlights the importance of CD4+ T-cell-mediated anti-tumor immune response in the absence of an MHC-class-I-restricted CD8+ T cell response and the potential therapeutic success when targeting this neoantigen with immunotherapy. Currently, an open clinical trial (RESIST, led by Dr. Vlahovicat at Duke University, NCT02193347) is testing the use of the IDH1 Peptide Vaccine (PEPIDH1M) administered with GM-CSF and tetanus toxoid preconditioning of the vaccine site in adult patients with recurrent grade II glioma positive for IDH1R132H. Another Phase 1 clinical trial (NOA-16) is testing an IDH1 Peptide Vaccine in patients with IDH1R132H mutant grade II and IV tumors with ATRX loss and without 1p/19q codeletion.
Diffuse intrinsic pontine glioma (DIPG) is often associated (70%) with missense mutation of the lysine in the histone H3.3 K27M, with the mutation also present in 30% of adult GBM patients. A recent report tested whether this mutation can serve as neoantigen for immunotherapy and if it can initiate a specific CTL response. Preliminary results show that out of 4 peptides generated by a peptide–MHC binding algorithm and a proteosomal cleavage site prediction system, one was able to induce specific CTL responses which specifically lysed HLA-A2+ DIPG cell lines harboring the mutation122. If proven safe and effective this therapeutic strategy will bring great hope for children with DIPG.
One potential drawback of single peptide vaccination is immuno-editing; the concept of immuno-editing, describing the crosstalk between the immune system and cancer, has developed over the last century from the idea of immune surveillance, which posits that the immune system is continuously monitoring and capable of eliminating cells displaying neoplastic mutations123–125. As cancer still develops in immuno-competent organisms, the immune surveillance is eventually overcome due to the aggressive growth and invasion of cancers and to immuno-suppressive mechanisms induced by malignancy126. Immuno-editing represents a major obstacle for the success of immunotherapies for glioma. Deciphering mechanisms of immuno-editing will lead to an increased understanding of glioma pathogenesis and progression and to the design of more successful immunotherapies for this disease.
As discussed above, an illustrative example of glioma immuno-editing following targeted immune therapy is represented by the recently completed Phase II multicenter study (ACTIVATE, ACTII), which used the CDX-110 vaccine (Rindopepimut) concurrent with temozolomide in patients with newly diagnosed EGFRvIII-positive GBM127. The ACTII trial demonstrated that OS was positively correlated with the development of specific antibody responses against EGFRvIII (identified in 6 of 14 patients), and that remarkably, at recurrence 82% of patients had lost EGFRvIII expression127, 128. Immuno-editing was also demonstrated in a subsequent Phase II multicenter single-arm trial (ACTIII) using the same therapeutic approach, showing decrease of EGFRvIII immunoreactivity in 67% (4 of 6 patients), and an OS of 21.8 months129. A Phase III Study (ACT IV) of Rindopepimut, GM-CSF, TMZ and KLH for patients with newly diagnosed EGFRvIII positive gliomablastoma will likely be discontinued as the interim analysis showed that the treatment is unlikely to meet statistically significant primary OS endpoint in patients with minimal residual disease130.
To reduce the risk of immuno-editing, immune tolerance and disease recurrence following single peptide vaccinations, many studies are investigating the production of effective combinations of several GAA. A recently completed study has tested the intradermal administration of a combination of three peptides: EphA2, IL-13Rα2 and survivin together with tetanus toxoid and poly [I:C] as adjuvants in pediatric brain stem and high grade gliomas (NCT01130077). Results showed that the vaccine was well tolerated, induced the development of specific GAA immune responses, and led to favorable clinical outcome, particularly in patients manifesting temporary pseudo-progression, indicative of immune cell infiltration within the tumors causing edema131. Other ongoing clinical trials are testing a combination of the immunogenic peptides IL13Rα2, EphA2 and Survivin (SL-701 vaccine in combination with bevacizumab, NCT02078648) in patients with newly diagnosed GBM. The ICT-107 vaccine is an autologous DC vaccine pulsed with six peptides (HER2, TRP-2, gp100, MAGE-1, IL13Rα2 and AIM-2). In the initial phase I trial this treatment induced a response in 33% of the 21 patients enrolled with a median OS of 38.4 months132. This vaccine is currently being tested in a randomized, two-arm, phase III study with ICT-107 or placebo control in combination with the SOC (NCT02546102). Ongoing clinical trials are testing a proprietary combination of 11 HLA-A2 restricted tumor-associated peptides IMA950 alone (NCT020278648) or in combination with GMCSF (NCT01222221) or TLR3 agonist, Poly ICLC (NCT01920191).
2.3.2 Peptide vaccination: patient-specific neoantigens
Identification of patient-specific neoantigens has been made possible by the advent of next-generation DNA and RNA deep sequencing technology, the development of prediction algorithms, MHC multimer based screens133, and high throughput sequencing of antigen-specific receptors of whole lymphocyte populations (TCRseq, BCRseq) capable of identifying patient-specific combinations of immunogenic neo-antigens. That specific T cell reactivity can be induced by neoantigens identified through cancer exome sequencing has been demonstrated in melanoma patients134, 135, results which prompted the development of clinical trials to target patient-specific neoantigens with immune therapy in several cancers. This strategy has been adopted by the European Glioma Actively Personalized Vaccine (GAPVAC) Consortium in a phase I clinical trial (NCT02149225) testing personalized peptide vaccines in combination with Poly-ICLC and GM-CSF in newly diagnosed glioblastoma. A phase-I clinical trial led by Dr. Reardon at the Dana Farber Cancer Institute is testing the safety and efficacy of neoantigen based vaccines in combination with radiation in MGMT-unmethylated newly-diagnosed GBM (NCT02287428).
An avenue of increased interest in the management of cancers is represented by the targeting of neoantigens induced by radiation therapy136, 137. Ionizing radiation induces DNA strand breaks, which will kill rapidly dividing cells and induce immunogenic cell death, generating damage associated molecular patterns, like HMGB1138 and induce phenotypic changes in normal and malignant cells. It has been shown in preclinical models of glioma that radiation therapy induces the expression of novel targets such as integrins αvβ3 and matrix metalloproteinases MMP2 and MMP9, which can promote glioma progression139, however when combined with immune checkpoint blockade using anti PD-1 antibodies, radiation therapy induces long-term survival in glioma bearing animals140. In other cancer models it has also been shown that radiation induces various cell surface receptors on tumor cells (MHC-I, ICAM-1, Fas), and that radiotherapy augmented tumor cell killing by antigen specific T cells136. Combining next generation sequencing with radiotherapy to identify immunogenic mutations141 and designing therapeutic antigenic peptides to be used as agents in combinatorial approaches with radiation and immune checkpoint blockade are attractive strategies to pursue further for the treatment of glioblastoma.
A recent study from our lab has shown that loss of ATRX increases genetic instability. Furthermore, analysis of genome-wide data for human gliomas showed that ATRX mutation is associated with increased mutation rate at the single-nucleotide variant (SNV) level8. Tumors with ATRX loss could therefore potentially be more immunogeneic due to the generation of neoepitopes or neoantigens. As pointed out by Schumacher et al, such neoantigens are crucial to tumor rejection because the T cell repertoire against these antigens is not affected by central tolerance118.
2.3.3 DC Vaccination
DCs are the most efficient APCs that can activate CD4, CD8, NK and NKT cells17. Factors that can modulate the efficacy of DC vaccination strategies include methods of DC differentiation, loading with tumor antigens, route of administration, and adjuvants used (Fig 2 and Table 3)17. The most advanced current clinical trial using an autologous DC vaccine- DCVax-L is in Phase III (NCT00045968). While the vaccine was shown to be safe, limited clinical benefit was observed. Combination of autologous DC vaccine with Imiquimod or Poly ICLC showed increased OS in patients with mesenchymal gene signature142. Increased OS was also seen in 33% of the patients treated with DC vaccine pulsed with six GAA peptides132. Treatment of 22 patients with recurrent GBM with α-type 1 polarized DCs pulsed with EphA2, IL13Rα2, YKL-40 and gp100 combined with poly ICLC showed the induction of a positive immune response in 58% of the patients143. An interesting approach using DC vaccination was utilized to target glioma stem cells (GSCs). A recently completed phase I/II trial (NCT00846456) using DCs transfected with autologous GSC mRNA demonstrated significantly longer PFS and OS than the controls. Like EGFRvIII peptide vaccines, DC vaccines pulsed with CDX-110 showed safety and efficacy in eliciting an anti-tumor immune response and improved survival in GBM patients that expressed the variant128. Results from a randomized and blinded clinical trial by the Sampson group have demonstrated that pre-conditioning the vaccine site with antigens such as tetanus/diphtheria toxoid can significantly improve the efficacy of tumor-antigen-specific DCs144. Patients given tetanus toxoid had enhanced DC migration bilaterally and significantly improved survival144.
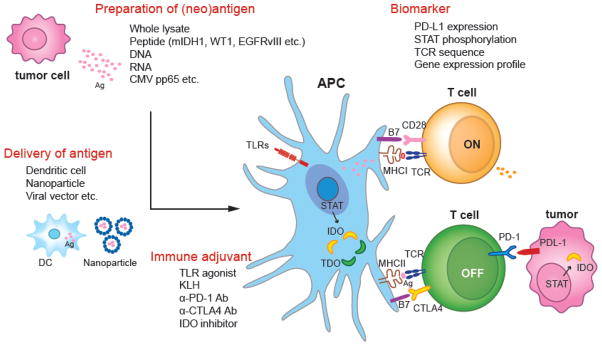
Figure 2. Schematic showing factors that impact vaccination strategy for glioblastoma.
(1) preparation and (2) delivery of tumor associated antigens, (3) immune adjuvant to boost immune reactions against tumor antigens and (4) biomarkers to precisely estimate the efficacy of vaccination and to select proper patient populations. Immune adjuvants are divided in two groups: (1) agonists for pro-inflammatory molecules such as TLR agonists or ligation of B7 and CD28 receptors, and (2) antagonists for anti-inflammatory molecules such as anti-PD-1 blocking antibodies or IDO inhibitors. DC represents dendritic cell; CMV, cytomegalovirus; APC, antigen presenting cell, TLR, toll like receptor.
Table 3.
Key factors that impact the efficacy of cancer vaccines for GBM patients
| Strategy | Items |
|---|---|
| Generation of tumor antigens143, 144, 221, 253–259 | CMV pp65 RNA, IDH1(R132H), WT1, EGFRvIII, IL13R-a2, H3F3A(K27M), autologous tumor lysate, HSPPC-96, cocktail of peptides |
| Delivery of tumor antigens87, 260–263 | systemic injection of antigens, dendritic cells, (viral vector) |
| Adjuvant142, 174, 264, 265 | tetanus, KLH, ISA51, imiquimod, polyICLC, (anti-PD-1 Ab, anti-CTLA4 Ab, anti-Gr1 MDSC inhibitor, IDO inhibitor) |
| Stratification of patients142, 169, 170 | pSTAT signaling, mesenchymal gene expression signature, TCR |
GBM, glioblastoma; CMV, cytomegalovirus; pp65, phosphoprotein 65; IDH1, isocitrate dehydrogenase 1; WT1, Wilms tumor 1; EGFR, epidermal growth factor receptor; HSPPC, heat shock protein peptide complex; PD-1, programmed death 1; CTLA4, cytotoxic lymphocyte antigen 4; MDSC, myeloid derived suppressor cell; IDO, indoleamine 2,3-dioxygenase; STAT, signal tranducer and activator of transcription; TCR, T cell receptor. Adjuvants in the parenthesis have not been tested in human trials.
2.3.4 Tumor cell vaccination
Encouraging results were seen in a prospective Phase I/II clinical trial using temozolomide, radiotherapy and autologous formalin fixed tumor cell vaccine in 24 patients with newly diagnosed GBM145. A recent phase II clinical trial of adult recurrent GBM in which vaccines were made by isolating tumor gp96 complexes from autologous-resected tumors (HSPPC-96) demonstrated the safety and OS of 42.6 weeks146. A randomized phase II trial will compare the efficacy of HSPPC-96 vaccine with or without bevacizumab in recurrent resectable GBMs (NCT01814813).
2.3.5 Immune stimulatory adjuvants
Immune adjuvants can enhance vaccine associated anti-tumor immunity through mechanisms such as antigen clustering, maintaining an antigen depot, stimulating innate immunity, promoting DC maturation, and blocking Treg induced suppression16–18. Compounds most commonly evaluated as adjuvants are poly I:C, CpG oligonucleotides, and Imiquimod that activate TLR3, TLR9 and TLR7 respectively16–18. TLRs are pathogen associated molecular pattern receptors that when activated increase the production of immune-stimulatory cytokines and costimulatory molecule expression on APCs147. In a prospective phase II clinical trial of pediatric glioma, treatment with poly ICLC was well tolerated by children. 5 out of 10 children showed long-term stable disease148. Another phase II trial testing the combination of poly ICLC with radiotherapy and TMZ concluded that poly ICLC administration may improve the efficacy of radiotherapy and TMZ treatment with no added adverse effects149. While CpG-ODN showed promise in many preclinical and phase I clinical studies, intratumoral administration of CpG ODN as a single treatment modality showed little benefit in a phase II clinical study with patients with recurrent GBM150. Besides TLR agonists, other immune stimulatory adjuvants have also been tested. Administration of tetanus toxoid was shown to improve the efficacy of DC vaccination in GBM patients and to prolong their survival144. Mitchell et al. reported that tetanus toxoid preconditioning in vaccination site in a mouse glioma model enhanced DC migration and suppressed tumor growth in a CCL3 dependent fashion144. KLH is being tested in active vaccination studies for GBM patients. GMCSF has also been used to promote vaccine immunogenicity by enhancing DC maturation151.
2.4 Passive immunotherapy: antibodies
Monoclonal antibody therapy results in tumor cell death through a variety of immune and non-immune mediated mechanisms152. Ideally the expression of the antigen recognized by the antibody would be restricted to the tumor cells, so as to minimize injury to normal tissues. GBMs are highly vascular tumors that produce large amounts of VEGF. Currently, the most prominent VEGF targeting drug is bevacizumab (BEV), a recombinant humanized monoclonal antibody that binds to human VEGF-A. Several phase II trials have tested the efficacy of anti-VEGF therapies either alone or in combination with irinotecan, etoposide, nitrosourea, or other agents153. While BEV proved to be a safe and feasible treatment option, the initially reported promising response rates might partly be attributed to imaging limitations resulting in an apparent but debatable reduction in the contrast-enhancing tumor volume [31, 32]. Recent prospective phase III trials (AVAglio & RTOG 0825) were designed to prove the efficacy of TMZ based radio chemotherapy in combination with BEV as first-line therapy for GBM. While the RTOG 0825 trial failed to show significant benefits in terms of progression free (PFS) and overall survival (OS), the AVAglio study demonstrated a significant prolongation of PFS by 4.4 months in the BEV arm. However, this PFS benefit did not translate into an improvement in OS153. Epidermal growth factor receptor gene mutation is a frequent finding in GBM with EGFR variant VIII being the most common one. Combination therapy of anti-EGFRvIII antibody, cetuximab in combination with bevacizumab/irinotecan was not found to be superior than bevacizumab/irinotecan alone. Intra-arterial infusion of cetuximab alone or in combination with BEV is currently being tested154 (NCT01884740 and NCT01238237). Another antibody against EGFR, nimotuzumab, in combination with radiation and chemotherapy in patients with diffuse intrinsic pontine gliomas has shown an increased median OS of 15 months when compared to 9.4 months for nimotuzumab and radiation alone155. A randomised, open label phase III trial was conducted to evaluate efficacy of nimotuzumab added to standard therapy for newly diagnosed glioblastoma. No statistically significant increase in overall survival was observed by the addition of nimotuzumab156. ABT-414 is an antibody-drug conjugate that delivers Monomethyl Auristatin F or MMAF to cells with active EGFR or mutant EGFRvIII. Interim results from a phase I clinical trial testing ABT 414 as a monotherapy, in combination with chemotherapy and in combination with radiation and chemotherapy showed objective responses in 4 out of 12 patients, including 2 that showed complete response (NCT02573324).
2.5 Adoptive immunotherapy: autologous T cell transfer and CAR T cells
Adoptive T cell transfer involves directly transferring T cells with high avidity against the tumor antigens to the host to generate anti-tumor immunity152. Rosenberg et al pioneered the technique in a subset of melanoma patients by expanding TILs obtained from resected melanoma tumors157. The technique however was limited by the availability of tumor-specific lymphocytes. To overcome this limitation, the same group developed patient T cells genetically modified to express a T cell receptor that recognized melanoma antigen MART-1, resulting in tumor regression158. Early approaches utilizing adoptive transfer for glioma involved administration of T cells isolated from dLNs following s.c. injection of irradiated tumor cells with GMCSF or ex vivo expansion of T cells induced by culture with tumor cells152. In a phase I clinical trial in patients with recurrent GBM and cytomegalovirus positive serology, adoptive transfer of autologous CMV-peptide expanded T cells resulted in a PFS of 243 days in 4 out of 10 patients159. Adoptive T cell transfers are restricted by the need to match the HLA type, and next generation T cell transfer strategies use CAR T cells. Chimeric antigen receptor T cells, or CAR T cells, are engineered to target a specific antigen by combining the recognition specificity of an antibody with T cell signaling through the CD3 ζ chain or FCεRIγ160. A serious concern with the use of this approach is the damage that can occur to normal tissues if the antigen expression is not tumor specific. Thus it is essential to select targets that show tumor restricted expression. Using CARs as a therapeutic strategy in brain tumors was first tested by the Jensen group, who showed that intratumoral delivery of IL-13 zetakine CAR eliminated orthotopic human glioma tumors in immune compromised mice161. The clinical trial testing the safety and feasibility of this therapy in patients with recurrent GBM has shown minimal side effects, and 2 out of 3 patients who received repeated intracranial infusions of IL-13 zetakine+ CTLs showed transient anti-glioma immune responses162. HER2-specific CAR T cells have been shown to generate a HER2-dependent antitumor response with increased production of IFN-γ and IL-2, resulting in tumor regression in a xenograft mouse GBM model163. A phase I trial will test the safety and efficacy of using HER2-specific CARs in patients with recurrent GBM (NCT02442297). The Rosenberg group at NCI (NCT01454596) and the University of Pennsylvania/Novartis (NCT02209376) are also currently recruiting participants to test the safety and feasibility of administering T cells expressing anti-EGFRvIII CAR to patients with gliomas expressing EGFRvIII. The role of engineered T cell therapy may be expanded in the future to include gene transfer mediating extended survival, enhanced tumor penetration, or resistance to immunosuppression164.
3. Predictive biomarkers for immunotherapy
Significant advances have been made in genetic and molecular characterization of tumors, allowing for the identification of predictive biomarkers for glioma immunotherapy. As mentioned above, clinical trials of EGFRvIII CAR T cell therapy (NCT02209376) and EGFR peptide vaccination (NCT00458601, NCT01480479) used glioma EGFRvIII expression as a precondition for enrollment, limiting the potential for adverse events in those who were not likely to experience therapeutic benefit165. Similarly, IL-13Rα2 expression was used as a precondition for enrollment in a clinical trial of IL-13Rα2 CAR T cells (NCT02208362). Other biomarkers such as IDH1/2 mutation, 1p/10q deletion, MGMT methylation, ATRX loss, and H3.3 K27M mutation166 are prognostically useful, and their ability to predict therapeutic efficacy of a particular immunotherapy remains open for exploration167. Large scale clustering of several biomarkers into neural, proneural, classical, and mesenchymal subtypes corresponds with differences in treatment efficacy of chemotherapy and radiotherapy, and holds significant potential for informing efficacy of immunotherapy168. In a Phase I trial testing autologous tumor lysate-pulsed DC vaccination (NCT01204684), the study population was stratified according to the genetic expression signature of tumor cells (mesenchymal, proneural and proliferative), and patients with mesenchymal signatures, the worst prognostic subtype, had significantly extended survival compared to a historical control cohort with the same signature, whereas no survival difference was observed in those with a proneural gene expression signature142. Moreover, the mesenchymal subtype showed higher numbers of CD3+ and CD8+ TILs compared with other subtypes, suggesting that gene expression profile can be used as a biomarker for choosing an efficacious immunotherapy.
Alternatively, biomarkers which are not tumor-associated may also hold significant predictive value. Hsu et al. analyzed sequence similarity of the T cell receptor (TCR) β chain gene between T cells in the tumor and those in the peripheral blood. They showed that two patients with the highest TCR sequence similarity between tumor and blood before treatment showed the longest survival among 5 patients who were treated with DC vaccine169. HLA type may be an important predictive biomarker for peptide vaccines. The EphA2, IL-13Rα2, and survivin peptide vaccine was optimized to bind HLA-A2 most efficiently; thus the clinical trial prescreened for HLA-A2 expression prior to enrollment (NCT01130077). Humoral biomarkers may also aid in monitoring biological effects of immunotherapy. Recently, Everson et al. tested the association of phospho-STAT signaling and patient survival after DC vaccine treatment170. An increased per cell phosphorylation of STAT-5 in cytotoxic T-cells following IL-2 stimulation was associated with extended survival (log rank p = 0.0015). Patients who survived longer than two years had a significantly greater phosphorylated STAT-5 ratio (p = 0.015).
4. Expert opinion
GBMs remain one of the most difficult to treat human cancers. In spite of optimal standard of care, delivered in the most complex and advanced medical institutions, its prognosis has remained dismal, with a median survival of ~12–18 months post diagnosis9, 16, 18. This is thought to be due to the fact that GBMs are usually diagnosed at late stages of the disease; the blood brain barrier precludes the effective action of chemotherapeutic agents, there is a paucity of antigen presenting cells (APCs) within the central nervous system, and GBM is highly immunosuppressive30, 31, 33, 39, 45, 49, 171. The tumor microenvironment in GBMs is composed mainly of immunosuppressive cells, i.e., regulatory T cells (Tregs), tumor associated macrophages (TAMs) and myeloid derived suppressor cells (MDCSs). These cells exhibit immunosuppressive effects, which inhibit anti-tumor specific cytotoxic T cells functions, ultimately leading to decreased efficacy of immunotherapeutic approaches. In addition, GBMs are highly heterogeneous, with tumor cells acquiring new mutations after treatment, leading to therapy resistant disease172, 173.
In GBM, tumor infiltrating T cells and Tregs exhibit high levels of the immunosuppressive receptor, CTLA4. CTLA4 binds with high affinity to CD80 and CD86, which are present on APCs, therefore inhibiting their binding to the T cells’ immune stimulatory receptor CD2822, 43, 72. This powerful immunosuppressive mechanism can be overcome by the use of antibodies which block CTLA422, 43, 72. Another powerful immunosuppressive mechanism is mediated by PD1-PDL1/PDL2 interactions, which occur during the effector phase of T cells functions leading to apoptosis of cytotoxic T cells22, 43, 72. Thus, immune checkpoint blockade has become a very attractive therapeutic target to be used in combination with other chemotherapeutic strategies, radiation, and/or immunotherapies22, 72, 174. Although these approaches are yielding very promising results in GBM preclinical models62, 76 and clinically for the treatment of immunogenic cancers, such as melanoma56, 66, no data is available yet in relation to their clinical efficacy in GBM. The clinical trial approach that we developed in our laboratory, which entails using a combination of a conditional cytotoxic gene therapy approach88, 89 together with an immune stimulatory approach to attract APCs to the tumor microenvironment87, 95–99, has proven to be highly efficacious in several rat and mouse syngeneic, intracranial GBM models and is currently being tested in the clinic (https://clinicaltrials.gov/show/NCT01811992). In summary, taking into account the formidable challenges posed by GBM, we hypothesize that in order to elicit therapeutic benefit, it will be necessary to use combination therapies. These should include, surgery (aiming to perform aggressive resections when clinically possible) chemotherapy, radiation, and immune stimulatory approaches. These could include vaccination strategies, intra-tumor gene therapy strategies, oncolytic virotherapy, and immune checkpoint blockade. Although we have a long way ahead, we believe that the basic science discoveries related to how the immune system works in GBM, coupled with well-designed clinical trials, will enable the scientific/medical community to make inroads into developing novel combination therapies that will elicit improved median survival and better prognosis for this devastating cancer.
Acknowledgments
Financial support: This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants R01-NS094804, R01-NS074387, R21-NS091555, and R37-NS094804 to M.G.C.; NIH/NINDS Grants R01-NS076991, R01-NS082311, R21-NS084275, and R01-096756 to P.R.L.; Leah’s Happy Hearts, University of Michigan Comprehensive Cancer Center, Chad Tough Foundation, and The Phase One Foundation to both M.G.C. and P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH 2UL1-TR000433; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863.
Footnotes
Declaration of interest: The authors declare no conflict of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007 Aug;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine. 2015 Jun 25;372(26):2499–508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nature genetics. 2015 May;47(5):458–68. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 4.Paulus W. Pathology, molecular mechanisms and markers of gliomas: new insight and new challenges. Acta neuropathologica. 2015 Jun;129(6):773. doi: 10.1007/s00401-015-1443-y. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009 Feb 19;360(8):765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012 Jul;3(7):709–22. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain MC, Born D. Prognostic significance of relative 1p/19q codeletion in oligodendroglial tumors. Journal of neuro-oncology. 2015 Nov;125(2):249–51. doi: 10.1007/s11060-015-1906-y. [DOI] [PubMed] [Google Scholar]
- 8.Koschmann C, Calinescu AA, Nunez FJ, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Science translational medicine. 2016 Mar 2;8(328):328ra28. doi: 10.1126/scitranslmed.aac8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005 Mar 10;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Journal of neurosurgery. 2001 Aug;95(2):190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 11.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Mar 10;26(8):1338–45. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 12.Abhinav K, Yeh FC, Mansouri A, et al. High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery. Neuro-oncology. 2015 Sep;17(9):1199–209. doi: 10.1093/neuonc/nov113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan MD. Recent Technical Advances and Indications for Radiation Therapy in Low-Grade Glioma. Seminars in radiation oncology. 2015 Jul;25(3):189–96. doi: 10.1016/j.semradonc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 14.van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Dec 10;27(35):5881–6. doi: 10.1200/JCO.2009.24.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal S, Manchanda P, Vogelbaum MA, et al. Function of the blood-brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug metabolism and disposition: the biological fate of chemicals. 2013 Jan;41(1):33–9. doi: 10.1124/dmd.112.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Jul 15;20(14):3651–9. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calinescu AA, Kamran N, Baker G, et al. Overview of current immunotherapeutic strategies for glioma. Immunotherapy. 2015;7(10):1073–104. doi: 10.2217/imt.15.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro-oncology. 2014 Nov;16(11):1441–58. doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim ES, Kim JE, Patel MA, et al. Immune Checkpoint Modulators: An Emerging Antiglioma Armamentarium. J Immunol Res. 2016;2016:4683607. doi: 10.1155/2016/4683607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galon J, Angell HK, Bedognetti D, et al. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013 Jul 25;39(1):11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Gajewski TF, Woo SR, Zha Y, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current opinion in immunology. 2013 Apr;25(2):268–76. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim JE, Lim M. The role of checkpoints in the treatment of GBM. Journal of neuro-oncology. 2015 Jul;123(3):413–23. doi: 10.1007/s11060-015-1747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldmann J, Kwidzinski E, Brandt C, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. Journal of leukocyte biology. 2006 Oct;80(4):797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 24.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain pathology. 1992 Oct;2(4):269–76. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 25.Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. Journal of anatomy. 2002 Jun;200(6):639–46. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005 Aug 1;11(15):5515–25. doi: 10.1158/1078-0432.CCR-05-0464. Describes Phase 1 clinical trial results of DC vaccination with autologous tumor peptides; vaccination induced systemic and intracranial T cell response, but this response was limited by active tumor progression and TGF-B2 expression. [DOI] [PubMed] [Google Scholar]
- 27.Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-oncology. 2011 Mar;13(3):324–33. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimberger AB, Kong LY, Abou-Ghazal M, et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- 29.Zhang JG, Kruse CA, Driggers L, et al. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. Journal of neuro-oncology. 2008 May;88(1):65–76. doi: 10.1007/s11060-008-9534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Aug 15;17(16):5473–80. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razavi SM, Lee KE, Jin BE, et al. Immune Evasion Strategies of Glioblastoma. Front Surg. 2016;3:11. doi: 10.3389/fsurg.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan JT, Sun W, Hussain SF, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008 Jan;57(1):123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 Jun 15;19(12):3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009 Mar;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005 Dec 1;11(23):8304–11. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 36.Kren L, Slaby O, Muckova K, et al. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: an unexpected prognostic significance? Neuropathology. 2011 Apr;31(2):129–34. doi: 10.1111/j.1440-1789.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 37.Wastowski IJ, Simoes RT, Yaghi L, et al. Human leukocyte antigen-G is frequently expressed in glioblastoma and may be induced in vitro by combined 5-aza-2′-deoxycytidine and interferon-gamma treatments: results from a multicentric study. Am J Pathol. 2013 Feb;182(2):540–52. doi: 10.1016/j.ajpath.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu LW, Chow KK, Lim M, et al. Current vaccine trials in glioblastoma: a review. J Immunol Res. 2014;2014:796856. doi: 10.1155/2014/796856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badie B, Schartner J, Prabakaran S, et al. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001 Nov 1;120(1–2):19–24. doi: 10.1016/s0165-5728(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 40**.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002 Jul;3(7):611–8. doi: 10.1038/ni0702-611. This review summarizes the role of CTLA-4 in negatively regulating T cell response and demonstrates the potential of CTLA-4 as an immunotherapeutic target. [DOI] [PubMed] [Google Scholar]
- 41**.Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009 Aug 3;206(8):1717–25. doi: 10.1084/jem.20082492. Demonstrates the ability of CTLA-4 blockade to produce synergistic antitumor activity through enhancement of effector T cell function and inhibition of regulatory T cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarhini AA, Kirkwood JM. CTLA-4-blocking immunotherapy with ipilimumab for advanced melanoma. Oncology (Williston Park) 2010 Dec;24(14):1302, 04. [PubMed] [Google Scholar]
- 43.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012 Apr;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007 Nov;117(11):3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-oncology. 2015 Aug;17(8):1064–75. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z, Han H, He X, et al. Expression of the galectin-9-Tim-3 pathway in glioma tissues is associated with the clinical manifestations of glioma. Oncol Lett. 2016 Mar;11(3):1829–34. doi: 10.3892/ol.2016.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013 Jul 25;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009 May;229(1):126–44. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson C, Ruzevick J, Phallen J, et al. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saikali S, Avril T, Collet B, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. Journal of neuro-oncology. 2007 Jan;81(2):139–48. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 51.Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4:53. doi: 10.3389/fimmu.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joller N, Hafler JP, Brynedal B, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011 Feb 1;186(3):1338–42. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009 Sep;39(9):2492–501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992 Dec 24;71(7):1065–8. doi: 10.1016/s0092-8674(05)80055-8. Summarizes the interactions of CD28 and CTLA-4 with B7/BB1 and describes the role of these signaling pathways in IL-2 production and T cell activation. [DOI] [PubMed] [Google Scholar]
- 55**.Linsley PS, Greene JL, Brady W, et al. Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994 Dec;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. Demonstrates that B7-1 (CD80) and B7-2 (CD86) show similar overall receptor binding and T cell costimulatory properties, but B7-1 binds CTLA4 more readily and dissociates from CTLA-4 more slowly than B7-2. [DOI] [PubMed] [Google Scholar]
- 56.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010 Aug 19;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012 May;13(5):459–65. doi: 10.1016/S1470-2045(12)70090-6. Demonstrates the activity of ipilimumab in patients with advanced melanoma and brain metastases, and shows that ipilimumab does not produce unexpected adverse effects in this population. [DOI] [PubMed] [Google Scholar]
- 58.Queirolo P, Spagnolo F, Ascierto PA, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. Journal of neuro-oncology. 2014 May;118(1):109–16. doi: 10.1007/s11060-014-1400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Company B-MS. Yervoy (ipilimumab) [prescribing information] 2013 cited; Available from: http://www.yervoy.com.
- 60.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007 Apr 1;13(7):2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 61.Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013 Dec 16;210(13):2803–11. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwalla P, Barnard Z, Fecci P, et al. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. Journal of immunotherapy. 2012 Jun;35(5):385–9. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012 Jun 28;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eppihimer MJ, Gunn J, Freeman GJ, et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002 Apr;9(2):133–45. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Carlsson R, Ambjorn M, et al. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci. 2013 Aug 28;33(35):14231–45. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013 Jul 11;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Dec 1;31(34):4311–8. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Company B-MS. Opdivo (nivolumab) [prescribing information] 2014 cited; Available from: http://www.opdivo.bmscustomerconnect.com/gateway.
- 69.Merck & Co. I. Keytruda (pembrolizumab) [prescribing information] 2015 cited; Available from: http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
- 70.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine. 2015 Jan 22;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott JA. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol. 2014;32(15 Suppl) [Google Scholar]
- 72*.Preusser M, Lim M, Hafler DA, et al. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015 Sep;11(9):504–14. doi: 10.1038/nrneurol.2015.139. Summarizes preclinical and clinical studies of immune checkpoint modulators in glioblastoma and outlines specific challenges in clinical development of checkpoint modulators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hafler DA, Kuchroo V. TIMs: central regulators of immune responses. J Exp Med. 2008 Nov 24;205(12):2699–701. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer immunology research. 2014 May;2(5):393–8. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 75.Han S, Feng S, Xu L, et al. Tim-3 on peripheral CD4(+) and CD8(+) T cells is involved in the development of glioma. DNA Cell Biol. 2014 Apr;33(4):245–50. doi: 10.1089/dna.2013.2306. [DOI] [PubMed] [Google Scholar]
- 76.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013 Jun 1;86(2):343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003 Oct;9(10):1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 78.Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Nov 15;18(22):6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Oct 15;20(20):5290–301. doi: 10.1158/1078-0432.CCR-14-0514. Reports preclinical evidence that combinatorial blockade of IDO, CTLA-4, and PDL-1 in mouse models of GBM decreases tumor-infiltrating regulatory T cells and mediates long term survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992 Aug 1;176(2):327–37. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer research. 2012 Feb 15;72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grossauer S, Koeck K, Petritsch C. Immunecheckpoint blockage - a promising strategy to overcome glioma stem cell therapy-resistance. Insights in Neurosurgery. 2016;1(1):1. [Google Scholar]
- 83.Castro MG, Candolfi M, Wilson TJ, et al. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert opinion on biological therapy. 2014 Sep;14(9):1241–57. doi: 10.1517/14712598.2014.915307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kane JR, Miska J, Young JS, et al. Sui generis: gene therapy and delivery systems for the treatment of glioblastoma. Neuro-oncology. 2015 Mar;17(Suppl 2):ii24–ii36. doi: 10.1093/neuonc/nou355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Molecular therapy : the journal of the American Society of Gene Therapy. 2014 Feb;22(2):251–6. doi: 10.1038/mt.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012 Dec;12(12):860–75. doi: 10.1038/nrc3380. Summarizes mechanisms which regulate immunogenicity of dying cancer cells. [DOI] [PubMed] [Google Scholar]
- 87**.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS medicine. 2009 Jan 13;6(1):e10. doi: 10.1371/journal.pmed.1000010. Describes a novel mechanism of anti-GBM immune stimulation through TLR2 signaling mediated by release of HMGB1 by dying tumor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duarte S, Carle G, Faneca H, et al. Suicide gene therapy in cancer: where do we stand now? Cancer letters. 2012 Nov 28;324(2):160–70. doi: 10.1016/j.canlet.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 89.Okura H, Smith C, Rutka J. Gene therapy for malignant glioma. Mol and Cell Ther. 2014 Jul 08;2(1):1–19. doi: 10.1186/2052-8426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tobias A, Ahmed A, Moon KS, et al. The art of gene therapy for glioma: a review of the challenging road to the bedside. Journal of neurology, neurosurgery, and psychiatry. 2013 Feb;84(2):213–22. doi: 10.1136/jnnp-2012-302946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prados MD, McDermott M, Chang SM, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. Journal of neuro-oncology. 2003 Dec;65(3):269–78. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 92.Germano IM, Emdad L, Qadeer ZA, et al. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis and radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer gene therapy. 2010 Sep;17(9):664–74. doi: 10.1038/cgt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000 Nov 20;11(17):2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 94.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996 Nov 1;184(5):1953–62. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Molecular therapy : the journal of the American Society of Gene Therapy. 2004 Dec;10(6):1071–84. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96**.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer research. 2005 Aug 15;65(16):7194–204. doi: 10.1158/0008-5472.CAN-04-3434. Demonstrates the requirement of tumor cell killing through HSV1-TK therapy combined with immunostimulation through Flt3L to produce an effective anti-glioma response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Candolfi M, Curtin JF, Yagiz K, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011 Oct;13(10):947–60. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.King GD, Muhammad AK, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro-oncology. 2008 Feb;10(1):19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.King GD, Muhammad AK, Larocque D, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Molecular therapy : the journal of the American Society of Gene Therapy. 2011 Oct;19(10):1793–801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005 Nov 21;24(52):7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 101.Cassady KA, Parker JN. Herpesvirus vectors for therapy of brain tumors. The open virology journal. 2010;4:103–8. doi: 10.2174/1874357901004010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008 Jul;19(7):690–8. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Molecular therapy : the journal of the American Society of Gene Therapy. 2008 Mar;16(3):627–32. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 104.Kim JW, Kane JR, Young JS, et al. A Genetically Modified Adenoviral Vector with a Phage Display-Derived Peptide Incorporated into Fiber Fibritin Chimera Prolongs Survival in Experimental Glioma. Hum Gene Ther. 2015 Sep;26(9):635–46. doi: 10.1089/hum.2015.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nandi S, Ulasov IV, Tyler MA, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer research. 2008 Jul 15;68(14):5778–84. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998 Dec;4(12):1341–2. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 107.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Molecular therapy : the journal of the American Society of Gene Therapy. 2004 Nov;10(5):958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 108.Lang FF, Conrad C, Gomez-Manzano C, et al. FIRST-IN-HUMAN PHASE I CLINICAL TRIAL OF ONCOLYTIC DELTA-24-RGD (DNX-2401) WITH BIOLOGICAL ENDPOINTS: IMPLICATIONS FOR VIRO- IMMUNOTHERAPY. Neuro-oncology. 2014 Jul 1;16(suppl 3):iii39. [Google Scholar]
- 109.Jacobs SK, Wilson DJ, Kornblith PL, et al. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: phase I trial. Cancer research. 1986 Apr;46(4 Pt 2):2101–4. [PubMed] [Google Scholar]
- 110.Holladay FP, Heitz-Turner T, Bayer WL, et al. Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. Journal of neuro-oncology. 1996 Feb;27(2):179–89. doi: 10.1007/BF00177482. [DOI] [PubMed] [Google Scholar]
- 111.Colombo F, Barzon L, Franchin E, et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer gene therapy. 2005 Oct;12(10):835–48. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 112.Okada H, Giezeman-Smits KM, Tahara H, et al. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSVtk tumor vaccine. Gene therapy. 1999 Feb;6(2):219–26. doi: 10.1038/sj.gt.3300798. [DOI] [PubMed] [Google Scholar]
- 113.Okada H, Pollack IF, Lotze MT, et al. Gene therapy of malignant gliomas: a phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum Gene Ther. 2000 Mar 1;11(4):637–53. doi: 10.1089/10430340050015824. [DOI] [PubMed] [Google Scholar]
- 114.Natsume A, Ishii D, Wakabayashi T, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer research. 2005 Sep 1;65(17):7573–9. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
- 115.Natsume A, Wakabayashi T, Ishii D, et al. A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer chemotherapy and pharmacology. 2008 Apr;61(4):653–9. doi: 10.1007/s00280-007-0520-x. [DOI] [PubMed] [Google Scholar]
- 116.Wakabayashi T, Natsume A, Hashizume Y, et al. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. The journal of gene medicine. 2008 Apr;10(4):329–39. doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 117.Chiocca EA, Smith KM, McKinney B, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Molecular therapy : the journal of the American Society of Gene Therapy. 2008 Mar;16(3):618–26. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 118.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015 Apr 3;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 119.Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. The EMBO journal. 2013 Jan 23;32(2):194–203. doi: 10.1038/emboj.2012.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heimberger AB, Suki D, Yang D, et al. The natural history of EGFR and EGFRvIII in glioblastoma patients. Journal of translational medicine. 2005 Oct 19;3:38. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121*.Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014 Aug 21;512(7514):324–7. doi: 10.1038/nature13387. Presents a novel IDH1 peptide vaccine which induces antigen-specific T cell response and tumor control in mouse models. [DOI] [PubMed] [Google Scholar]
- 122.Hou Y, Kohanbash G, Okada K, et al. Novel and shared neoantigen for glioma T cell therapy derived from histone 3 variant H3. 3 K27M mutation. Journal for immunotherapy of cancer. 2015;3(Suppl 2):P445. [Google Scholar]
- 123.Burnet FM. Immunological aspects of malignant disease. Lancet. 1967 Jun 3;1(7501):1171–4. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- 124.Baldwin RW, Robins RA. Factors interfering with immunological rejection of tumours. British medical bulletin. 1976 May;32(2):118–23. doi: 10.1093/oxfordjournals.bmb.a071342. [DOI] [PubMed] [Google Scholar]
- 125.Doll R, Kinlen L. Immunosurveillance and cancer: epidemiological evidence. British medical journal. 1970 Nov 14;4(5732):420–2. doi: 10.1136/bmj.4.5732.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Current opinion in immunology. 2016 Apr;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Nov 1;28(31):4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Babu R, Adamson DC. Rindopepimut: an evidence-based review of its therapeutic potential in the treatment of EGFRvIII-positive glioblastoma. Core evidence. 2012;7:93–103. doi: 10.2147/CE.S29001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schuster J, Lai RK, Recht LD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-oncology. 2015 Jan 13; doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.<CLDX_News_2016_3_7_General_Releases.pdf>.
- 131*.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Jul 1;32(19):2050–8. doi: 10.1200/JCO.2013.54.0526. Reports antigen-specific immune response and clinical response in children with malignant gliomas vaccinated with the combinatorial peptide vaccine containing EphA2, IL-13Rα2, and survivin peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Phuphanich S, Wheeler CJ, Rudnick JD, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013 Jan;62(1):125–35. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hadrup SR, Bakker AH, Shu CJ, et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nature methods. 2009 Jul;6(7):520–6. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- 134.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Nov 10;31(32):e439–42. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015 May 15;348(6236):803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gandhi SJ, Minn AJ, Vonderheide RH, et al. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer letters. 2015 Nov 28;368(2):185–90. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 137.Corso CD, Ali AN, Diaz R. Radiation-induced tumor neoantigens: imaging and therapeutic implications. American journal of cancer research. 2011;1(3):390–412. [PMC free article] [PubMed] [Google Scholar]
- 138.Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wild-Bode C, Weller M, Rimner A, et al. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer research. 2001 Mar 15;61(6):2744–50. [PubMed] [Google Scholar]
- 140.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. International journal of radiation oncology, biology, physics. 2013 Jun 1;86(2):343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tinhofer I, Niehr F, Konschak R, et al. Next-generation sequencing: hype and hope for development of personalized radiation therapy? Radiation oncology. 2015;10:183. doi: 10.1186/s13014-015-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Mar 15;17(6):1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jan 20;29(3):330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144**.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015 Mar 19;519(7543):366–9. doi: 10.1038/nature14320. Demonstrates the ability of vaccine site preconditioning with tetanus toxoid to enhance DC migration and improve survival in GBM patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ishikawa E, Muragaki Y, Yamamoto T, et al. Phase I/IIa trial of fractionated radiotherapy, temozolomide, and autologous formalin-fixed tumor vaccine for newly diagnosed glioblastoma. Journal of neurosurgery. 2014 Sep;121(3):543–53. doi: 10.3171/2014.5.JNS132392. [DOI] [PubMed] [Google Scholar]
- 146.Bloch O, Crane CA, Fuks Y, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro-oncology. 2014 Jan;16(2):274–9. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010 May;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 148.Hartman LL, Crawford JR, Makale MT, et al. Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. Journal of pediatric hematology/oncology. 2014 Aug;36(6):451–7. doi: 10.1097/MPH.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosenfeld MR, Chamberlain MC, Grossman SA, et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro-oncology. 2010 Oct;12(10):1071–7. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carpentier A, Metellus P, Ursu R, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro-oncology. 2010 Apr;12(4):401–8. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reardon DA, Wucherpfennig KW, Freeman G, et al. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert review of vaccines. 2013 Jun;12(6):597–615. doi: 10.1586/erv.13.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chandramohan V, Mitchell DA, Johnson LA, et al. Antibody, T-cell and dendritic cell immunotherapy for malignant brain tumors. Future oncology. 2013 Jul;9(7):977–90. doi: 10.2217/fon.13.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Niyazi M, Harter PN, Hattingen E, et al. Bevacizumab and radiotherapy for the treatment of glioblastoma: brothers in arms or unholy alliance? Oncotarget. 2016 Jan 19;7(3):2313–28. doi: 10.18632/oncotarget.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hegde M, Bielamowicz KJ, Ahmed N. Novel approaches and mechanisms of immunotherapy for glioblastoma. Discovery medicine. 2014 Mar;17(93):145–54. [PubMed] [Google Scholar]
- 155.Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. Journal of neuro-oncology. 2014 Jun;118(2):305–12. doi: 10.1007/s11060-014-1428-z. [DOI] [PubMed] [Google Scholar]
- 156.Westphal M, Heese O, Steinbach JP, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. European journal of cancer. 2015 Mar;51(4):522–32. doi: 10.1016/j.ejca.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 157.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002 Oct 25;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006 Oct 6;314(5796):126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schuessler A, Smith C, Beagley L, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer research. 2014 Jul 1;74(13):3466–76. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 160.Ikeda H, Shiku H. Antigen-receptor gene-modified T cells for treatment of glioma. Advances in experimental medicine and biology. 2012;746:202–15. doi: 10.1007/978-1-4614-3146-6_16. [DOI] [PubMed] [Google Scholar]
- 161.Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer research. 2004 Dec 15;64(24):9160–6. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 162.Brown CE, Badie B, Barish ME, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 Sep 15;21(18):4062–72. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010 Jan 15;16(2):474–85. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013 Aug;13(8):525–41. doi: 10.1038/nrc3565. print. [DOI] [PubMed] [Google Scholar]
- 165.Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti–EGFR variant III chimeric antigen receptor T cells for glioblastoma. Science translational medicine. 2015 Feb 18;7(275):275ra22–75ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta neuropathologica. 2015;130(6):815–27. doi: 10.1007/s00401-015-1478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siegal T. Clinical Relevance of Prognostic and Predictive Molecular Markers in Gliomas. In: Schramm J, editor. Advances and Technical Standards in Neurosurgery. Vol. 43. Cham: Springer International Publishing; 2016. pp. 91–108. [DOI] [PubMed] [Google Scholar]
- 168.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010 Jan 19;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hsu M, Sedighim S, Wang T, et al. TCR sequencing can identify and track glioma-infiltrating T cells after DC vaccination. Cancer immunology research. 2016 Mar 11; doi: 10.1158/2326-6066.CIR-15-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Everson RG, Jin RM, Wang X, et al. Cytokine responsiveness of CD8(+) T cells is a reproducible biomarker for the clinical efficacy of dendritic cell vaccination in glioblastoma patients. Journal for immunotherapy of cancer. 2014;2:10. doi: 10.1186/2051-1426-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Avril T, Saikali S, Vauleon E, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. Journal of neuroimmunology. 2010 Aug 25;225(1–2):22–33. doi: 10.1016/j.jneuroim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 172.Ramirez YP, Weatherbee JL, Wheelhouse RT, et al. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals. 2013;6(12):1475–506. doi: 10.3390/ph6121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang RR, Pointer KB, Kuo JS, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Neurosurgery. 2014 Dec;75(6):N9–10. doi: 10.1227/NEU.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 174.Castro MG, Baker GJ, Lowenstein PR. Blocking immunosuppressive checkpoints for glioma therapy: the more the Merrier! Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Oct 15;20(20):5147–9. doi: 10.1158/1078-0432.CCR-14-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA: a cancer journal for clinicians. 2010 May-Jun;60(3):166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jha P, Suri V, Singh G, et al. Characterization of molecular genetic alterations in GBMs highlights a distinctive molecular profile in young adults. Diagnostic molecular pathology : the American journal of surgical pathology, part B. 2011 Dec;20(4):225–32. doi: 10.1097/PDM.0b013e31821c30bc. [DOI] [PubMed] [Google Scholar]
- 177.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 Feb 15;19(4):764–72. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 178.Fontebasso AM, Liu XY, Sturm D, et al. Chromatin remodeling defects in pediatric and young adult glioblastoma: a tale of a variant histone 3 tail. Brain pathology. 2013 Mar;23(2):210–6. doi: 10.1111/bpa.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179**.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer cell. 2012 Oct 16;22(4):425–37. doi: 10.1016/j.ccr.2012.08.024. Defines three subgroups of GBM based on H3F3A and IDH1 mutation which display distinct epigenetic and anatomic characteristics. [DOI] [PubMed] [Google Scholar]
- 180.Lindroth AM, Plass C. Recurrent H3.3 alterations in childhood tumors. Nature genetics. 2013 Dec;45(12):1413–4. doi: 10.1038/ng.2832. [DOI] [PubMed] [Google Scholar]
- 181.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Molecular cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu G, Yu JS, Zeng G, et al. AIM-2: a novel tumor antigen is expressed and presented by human glioma cells. Journal of immunotherapy. 2004 May-Jun;27(3):220–6. doi: 10.1097/00002371-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 183.Zhang JG, Eguchi J, Kruse CA, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007 Jan 15;13(2 Pt 1):566–75. doi: 10.1158/1078-0432.CCR-06-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sareddy GR, Panigrahi M, Challa S, et al. Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochemistry international. 2009 Sep;55(5):307–17. doi: 10.1016/j.neuint.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 185.Liu C, Tu Y, Sun X, et al. Wnt/beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clinical and experimental medicine. 2011 Jun;11(2):105–12. doi: 10.1007/s10238-010-0110-9. [DOI] [PubMed] [Google Scholar]
- 186.Cenci T, Martini M, Montano N, et al. Prognostic relevance of c-Myc and BMI1 expression in patients with glioblastoma. American journal of clinical pathology. 2012 Sep;138(3):390–6. doi: 10.1309/AJCPRXHNJQLO09QA. [DOI] [PubMed] [Google Scholar]
- 187.Leung C, Lingbeek M, Shakhova O, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004 Mar 18;428(6980):337–41. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 188.Zakrzewska M, Zakrzewski K, Gresner SM, et al. Polycomb genes expression as a predictor of poor clinical outcome in children with medulloblastoma. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011 Jan;27(1):79–86. doi: 10.1007/s00381-010-1260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Allan K, Jordan RC, Ang LC, et al. Overexpression of cyclin A and cyclin B1 proteins in astrocytomas. Archives of pathology & laboratory medicine. 2000 Feb;124(2):216–20. doi: 10.5858/2000-124-0216-OOCAAC. [DOI] [PubMed] [Google Scholar]
- 190.Rempel SA, Rosenblum ML, Mikkelsen T, et al. Cathepsin B expression and localization in glioma progression and invasion. Cancer research. 1994 Dec 1;54(23):6027–31. [PubMed] [Google Scholar]
- 191.Gopinath S, Malla R, Alapati K, et al. Cathepsin B and uPAR regulate self-renewal of glioma-initiating cells through GLI-regulated Sox2 and Bmi1 expression. Carcinogenesis. 2013 Mar;34(3):550–9. doi: 10.1093/carcin/bgs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sivaparvathi M, Sawaya R, Wang SW, et al. Overexpression and localization of cathepsin B during the progression of human gliomas. Clinical & experimental metastasis. 1995 Jan;13(1):49–56. doi: 10.1007/BF00144018. [DOI] [PubMed] [Google Scholar]
- 193.Barresi V, Buttarelli FR, Vitarelli EE, et al. Caveolin-1 expression in diffuse gliomas: correlation with the proliferation index, epidermal growth factor receptor, p53, and 1p/19q status. Human pathology. 2009 Dec;40(12):1738–46. doi: 10.1016/j.humpath.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 194.Senetta R, Miracco C, Lanzafame S, et al. Epidermal growth factor receptor and caveolin-1 coexpression identifies adult supratentorial ependymomas with rapid unfavorable outcomes. Neuro-oncology. 2011 Feb;13(2):176–83. doi: 10.1093/neuonc/noq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003 Sep 15;63(18):5821–8. [PubMed] [Google Scholar]
- 196.Emlet DR, Gupta P, Holgado-Madruga M, et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer research. 2014 Feb 15;74(4):1238–49. doi: 10.1158/0008-5472.CAN-13-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dahlrot RH, Hansen S, Jensen SS, et al. Clinical value of CD133 and nestin in patients with glioma: a population-based study. International journal of clinical and experimental pathology. 2014;7(7):3739–51. [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang M, Song T, Yang L, et al. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. Journal of experimental & clinical cancer research : CR. 2008;27:85. doi: 10.1186/1756-9966-27-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kitange GJ, Carlson BL, Schroeder MA, et al. Expression of CD74 in high grade gliomas: a potential role in temozolomide resistance. Journal of neuro-oncology. 2010 Nov;100(2):177–86. doi: 10.1007/s11060-010-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Baron N, Deuster O, Noelker C, et al. Role of macrophage migration inhibitory factor in primary glioblastoma multiforme cells. Journal of neuroscience research. 2011 May;89(5):711–7. doi: 10.1002/jnr.22595. [DOI] [PubMed] [Google Scholar]
- 201.Shono T, Tofilon PJ, Bruner JM, et al. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer research. 2001 Jun 1;61(11):4375–81. [PubMed] [Google Scholar]
- 202.Penas-Prado M, Hess KR, Fisch MJ, et al. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro-oncology. 2015 Feb;17(2):266–73. doi: 10.1093/neuonc/nou155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Eberstal S, Badn W, Fritzell S, et al. Inhibition of cyclooxygenase-2 enhances immunotherapy against experimental brain tumors. Cancer Immunol Immunother. 2012 Aug;61(8):1191–9. doi: 10.1007/s00262-011-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lewis-Tuffin LJ, Rodriguez F, Giannini C, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PloS one. 2010;5(10):e13665. doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu W, Tian Y, Wan H, et al. Expression of beta-catenin and E- and N-cadherin in human brainstem gliomas and clinicopathological correlations. The International journal of neuroscience. 2013 May;123(5):318–23. doi: 10.3109/00207454.2012.758123. [DOI] [PubMed] [Google Scholar]
- 206.Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert opinion on biological therapy. 2009 Aug;9(8):1087–98. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wykosky J, Gibo DM, Stanton C, et al. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008 Jan 1;14(1):199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 208.Okada H, Kohanbash G, Zhu X, et al. Immunotherapeutic approaches for glioma. Critical reviews in immunology. 2009;29(1):1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Driggers L, Zhang JG, Newcomb EW, et al. Immunotherapy of pediatric brain tumor patients should include an immunoprevention strategy: a medical hypothesis paper. Journal of neuro-oncology. 2010 Apr;97(2):159–69. doi: 10.1007/s11060-009-0016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ueda R, Low KL, Zhu X, et al. Spontaneous immune responses against glioma-associated antigens in a long term survivor with malignant glioma. Journal of translational medicine. 2007;5:68. doi: 10.1186/1479-5876-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Orzan F, Pellegatta S, Poliani PL, et al. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathology and applied neurobiology. 2011 Jun;37(4):381–94. doi: 10.1111/j.1365-2990.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 212.Scarcella DL, Chow CW, Gonzales MF, et al. Expression of MAGE and GAGE in high-grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999 Feb;5(2):335–41. [PubMed] [Google Scholar]
- 213.Mennel HD, Lell B. Ganglioside (GD2) expression and intermediary filaments in astrocytic tumors. Clinical neuropathology. 2005 Jan-Feb;24(1):13–8. [PubMed] [Google Scholar]
- 214.Schulz G, Cheresh DA, Varki NM, et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer research. 1984 Dec;44(12 Pt 1):5914–20. [PubMed] [Google Scholar]
- 215.Finocchiaro G, Pellegatta S. Immunotherapy with dendritic cells loaded with glioblastoma stem cells: from preclinical to clinical studies. Cancer Immunol Immunother. 2016 Jan;65(1):101–9. doi: 10.1007/s00262-015-1754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cantini G, Pisati F, Pessina S, et al. Immunotherapy against the radial glia marker GLAST effectively triggers specific antitumor effectors without autoimmunity. Oncoimmunology. 2012 Sep 1;1(6):884–93. doi: 10.4161/onci.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jimeno A, Weiss GJ, Miller WH, Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 May 15;19(10):2766–74. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cui D, Xu Q, Wang K, et al. Gli1 is a potential target for alleviating multidrug resistance of gliomas. Journal of the neurological sciences. 2010 Jan 15;288(1–2):156–66. doi: 10.1016/j.jns.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 219.Rossi M, Magnoni L, Miracco C, et al. beta-catenin and Gli1 are prognostic markers in glioblastoma. Cancer biology & therapy. 2011 Apr 15;11(8):753–61. doi: 10.4161/cbt.11.8.14894. [DOI] [PubMed] [Google Scholar]
- 220.Yamamoto H, Swoger J, Greene S, et al. Beta1,6-N-acetylglucosamine-bearing N-glycans in human gliomas: implications for a role in regulating invasivity. Cancer research. 2000 Jan 1;60(1):134–42. [PubMed] [Google Scholar]
- 221.Akiyama Y, Oshita C, Kume A, et al. alpha-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. BMC cancer. 2012;12:623. doi: 10.1186/1471-2407-12-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu G, Ying H, Zeng G, et al. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer research. 2004 Jul 15;64(14):4980–6. doi: 10.1158/0008-5472.CAN-03-3504. [DOI] [PubMed] [Google Scholar]
- 223.Xu Q, Liu G, Yuan X, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem cells. 2009 Aug;27(8):1734–40. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro-oncology. 2016 Jan;18(1):16–26. doi: 10.1093/neuonc/nov136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mitchell DA, Cui X, Schmittling RJ, et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011 Sep 15;118(11):3003–12. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mintz A, Gibo DM, Slagle-Webb B, et al. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002 Sep-Oct;4(5):388–99. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kogiku M, Ohsawa I, Matsumoto K, et al. Prognosis of glioma patients by combined immunostaining for survivin, Ki-67 and epidermal growth factor receptor. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2008 Nov;15(11):1198–203. doi: 10.1016/j.jocn.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 228.Persson O, Salford LG, Fransson J, et al. Distribution, cellular localization, and therapeutic potential of the tumor-associated antigen Ku70/80 in glioblastoma multiforme. Journal of neuro-oncology. 2010 Apr;97(2):207–15. doi: 10.1007/s11060-009-0013-3. [DOI] [PubMed] [Google Scholar]
- 229.Bao S, Wu Q, Li Z, et al. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer research. 2008 Aug 1;68(15):6043–8. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230*.Cheng L, Wu Q, Huang Z, et al. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. The EMBO journal. 2011 Mar 2;30(5):800–13. doi: 10.1038/emboj.2011.10. Describes a mechanism of glioma stem cell radioresistance through increased DNA damage checkpoint activation by L1CAM signalling and NBS1 upregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bao L, Dunham K, Lucas K. MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother. 2011 Sep;60(9):1299–307. doi: 10.1007/s00262-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kuan CT, Srivastava N, McLendon RE, et al. Recombinant single-chain variable fragment antibodies against extracellular epitopes of human multidrug resistance protein MRP3 for targeting malignant gliomas. International journal of cancer. 2010 Aug 1;127(3):598–611. doi: 10.1002/ijc.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Terasaki M, Shibui S, Narita Y, et al. Phase I Trial of a Personalized Peptide Vaccine for Patients Positive for Human Leukocyte Antigen–A24 With Recurrent or Progressive Glioblastoma Multiforme. Journal of Clinical Oncology. 2011 Jan 20;29(3):337–44. doi: 10.1200/JCO.2010.29.7499. [DOI] [PubMed] [Google Scholar]
- 234.Ishiwata T, Teduka K, Yamamoto T, et al. Neuroepithelial stem cell marker nestin regulates the migration, invasion and growth of human gliomas. Oncology reports. 2011 Jul;26(1):91–9. doi: 10.3892/or.2011.1267. [DOI] [PubMed] [Google Scholar]
- 235.Dhodapkar MV, Sznol M, Zhao B, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Science translational medicine. 2014 Apr 16;6(232):232ra51. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rousseau A, Nutt CL, Betensky RA, et al. Expression of oligodendroglial and astrocytic lineage markers in diffuse gliomas: use of YKL-40, ApoE, ASCL1, and NKX2-2. Journal of neuropathology and experimental neurology. 2006 Dec;65(12):1149–56. doi: 10.1097/01.jnen.0000248543.90304.2b. [DOI] [PubMed] [Google Scholar]
- 237.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007 Feb 15;53(4):503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ligon KL, Alberta JA, Kho AT, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. Journal of neuropathology and experimental neurology. 2004 May;63(5):499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 239.Elsir T, Eriksson A, Orrego A, et al. Expression of PROX1 Is a common feature of high-grade malignant astrocytic gliomas. Journal of neuropathology and experimental neurology. 2010 Feb;69(2):129–38. doi: 10.1097/NEN.0b013e3181ca4767. [DOI] [PubMed] [Google Scholar]
- 240.Geiger KD, Hendruschk S, Rieber EP, et al. The prostate stem cell antigen represents a novel glioma-associated antigen. Oncology reports. 2011 Jul;26(1):13–21. doi: 10.3892/or.2011.1265. [DOI] [PubMed] [Google Scholar]
- 241.Schmitz M, Temme A, Senner V, et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. British journal of cancer. 2007 Apr 23;96(8):1293–301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Favaro R, Appolloni I, Pellegatta S, et al. Sox2 is required to maintain cancer stem cells in a mouse model of high-grade oligodendroglioma. Cancer research. 2014 Mar 15;74(6):1833–44. doi: 10.1158/0008-5472.CAN-13-1942. [DOI] [PubMed] [Google Scholar]
- 243.Ueda R, Ohkusu-Tsukada K, Fusaki N, et al. Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. International journal of cancer. 2010 Feb 15;126(4):919–29. doi: 10.1002/ijc.24851. [DOI] [PubMed] [Google Scholar]
- 244.Schmitz M, Wehner R, Stevanovic S, et al. Identification of a naturally processed T cell epitope derived from the glioma-associated protein SOX11. Cancer letters. 2007 Jan 8;245(1–2):331–6. doi: 10.1016/j.canlet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 245.Son MJ, Woolard K, Nam DH, et al. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell stem cell. 2009 May 8;4(5):440–52. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tchirkov A, Rolhion C, Kemeny JL, et al. Clinical implications of quantitative real-time RT-PCR analysis of hTERT gene expression in human gliomas. British journal of cancer. 2003 Feb 24;88(4):516–20. doi: 10.1038/sj.bjc.6600754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer research. 1994 Sep 15;54(18):5016–20. [PubMed] [Google Scholar]
- 248.Gondi CS, Lakka SS, Dinh DH, et al. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004 Nov 4;23(52):8486–96. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 249.Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. Journal of neurosurgery. 2008 May;108(5):963–71. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 250.Morita S, Oka Y, Tsuboi A, et al. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Japanese journal of clinical oncology. 2006 Apr;36(4):231–6. doi: 10.1093/jjco/hyl005. [DOI] [PubMed] [Google Scholar]
- 251.Nakahara Y, Okamoto H, Mineta T, et al. Expression of the Wilms’ tumor gene product WT1 in glioblastomas and medulloblastomas. Brain tumor pathology. 2004;21(3):113–6. doi: 10.1007/BF02482185. [DOI] [PubMed] [Google Scholar]
- 252.Shao R, Francescone R, Ngernyuang N, et al. Anti-YKL-40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis. 2014 Feb;35(2):373–82. doi: 10.1093/carcin/bgt380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer research. 2002 Jun 15;62(12):3347–50. [PubMed] [Google Scholar]
- 254.dos Santos CJ, Stangherlin LM, Figueiredo EG, et al. High prevalence of HCMV and viral load in tumor tissues and peripheral blood of glioblastoma multiforme patients. Journal of medical virology. 2014 Nov;86(11):1953–61. doi: 10.1002/jmv.23820. [DOI] [PubMed] [Google Scholar]
- 255.Libard S, Popova SN, Amini RM, et al. Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PloS one. 2014;9(9):e108861. doi: 10.1371/journal.pone.0108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lucas KG, Bao L, Bruggeman R, et al. The detection of CMV pp65 and IE1 in glioblastoma multiforme. Journal of neuro-oncology. 2011 Jun;103(2):231–8. doi: 10.1007/s11060-010-0383-6. [DOI] [PubMed] [Google Scholar]
- 257.Mitchell DA, Xie W, Schmittling R, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-oncology. 2008 Feb;10(1):10–8. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shamran HA, Kadhim HS, Hussain AR, et al. Detection of human cytomegalovirus in different histopathological types of glioma in Iraqi patients. BioMed research international. 2015;2015:642652. doi: 10.1155/2015/642652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hdeib A, Sloan AE. Dendritic cell immunotherapy for solid tumors: evaluation of the DCVax(R) platform in the treatment of glioblastoma multiforme. CNS oncology. 2015;4(2):63–9. doi: 10.2217/cns.14.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Choi Y, Chang J. Viral vectors for vaccine applications. Clinical and experimental vaccine research. 2013 Jul;2(2):97–105. doi: 10.7774/cevr.2013.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines. 2015;3(3):662–85. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Boudreau JE, Bonehill A, Thielemans K, et al. Engineering dendritic cells to enhance cancer immunotherapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2011 May;19(5):841–53. doi: 10.1038/mt.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Caruso DA, Orme LM, Neale AM, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro-oncology. 2004 Jul;6(3):236–46. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hunn MK, Bauer E, Wood CE, et al. Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme. Journal of neuro-oncology. 2015 Jan;121(2):319–29. doi: 10.1007/s11060-014-1635-7. [DOI] [PubMed] [Google Scholar]
- 265.Zussman BM, Engh JA. Outcomes of the ACT III Study: Rindopepimut (CDX-110) Therapy for Glioblastoma. Neurosurgery. 2015 Jun;76(6):N17. doi: 10.1227/01.neu.0000465855.63458.0c. [DOI] [PubMed] [Google Scholar]