Abstract
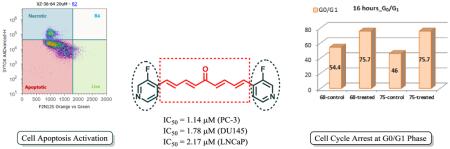
In search of more effective chemotherapeutics for the treatment of castration-resistant prostate cancer and inspired by curcumin analogues, twenty five (1E,3E,6E,8E)-1,9-diarylnona-1,3,6,8-tetraen-5-ones bearing two identical terminal heteroaromatic rings have been successfully synthesized through Wittig reaction followed by Horner-Wadsworth-Emmons reaction. Twenty-three of them are new compounds. The WST-1 cell proliferation assay was employed to assess their anti-proliferative effects toward both androgen-sensitive and androgen-insensitive human prostate cancer cell lines. Eighteen out of twenty-five synthesized compounds possess significantly improved potency as compared with curcumin. The optimal compound, 78, is 14- to 23-fold more potent than curcumin in inhibiting prostate cancer cell proliferation. It can be concluded from our data that 1,9-diarylnona-1,3,6,8-tetraen-5-one can serve as a new potential scaffold for the development of anti-prostate cancer agents and that pyridine-4-yls and quinolin-4-yl act as optimal heteroaromatic rings for the enhanced potency of this scaffold. Two of the most potent compounds, 68 and 75, effectively suppress PC-3 cell proliferation by activating cell apoptosis and by arresting cell cycle in the G0/G1 phase.
Keywords: Curcumin, Prostate cancer, Heteroaromatic ring, Cell proliferation, Cell Apoptosis, Cell Cycle Regulation
Graphical abstract
1. Introduction
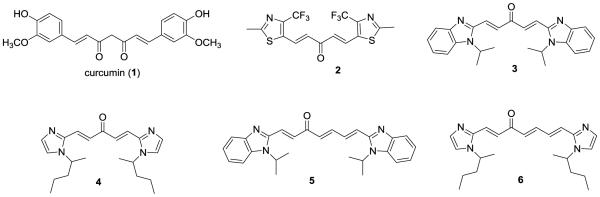
The American Cancer Society recently estimates that about 180,890 American men will be diagnosed as new prostate cancer cases and that over 26,000 men will die of prostate cancer in 2016.1 These data indicate that prostate cancer is still the most common cancer and the second leading cause of cancer-affiliated death among men in the United States. It is now well known that most prostate cancer patients die of castration-resistant prostate cancer due to the inevitable progression of resistance to first-line treatment with docetaxel.2,3 There is thus an urgent need to search for new chemotherapeutics for this deadly disease. The dietary curcumin (1) (Figure 1) was chosen by us and others as lead compound for the development of new anti-prostate cancer agents based on: i) its well-documented safety profile in humans;4 and ii) its well-evidenced therapeutic potential in treating prostate cancer, especially for castration-resistant prostate cancer, in several cell culture systems and human xenograft mouse models.4-16 To overcome its disadvantage (moderate potency and poor bioavailability) as a drug candidate,6,17,18 chemical manipulations of curcumin have been performed by us and many other research groups.5,19-21 Consequently, curcumin-based compounds with various lengths of central linkers, including 11-atom linker, 7-atom linker, 5-carbon linker, and 3-carbon linker, have so far been reported to possess similar or increased cytotoxic and anti-proliferative potency against prostate cancer cell lines, as compared with curcumin.5 However, there is no report available on the curcumin mimics containing a 9-atom linker as anti-prostate cancer agents. Our laboratory has recently discovered that basic N-containing heteroaromatic rings contribute significantly to the enhanced cytotoxic and anti-proliferative potency of curcumin-based compounds (e.g. 2-6) with 5-carbon or 7-carbon linker toward prostate cancer cells.5
Figure 1.
Structures of curcumin (1) and curcumin-based anticancer agents (2-6)
As part of our ongoing project in quest for more effective chemotherapeutics inspired by dietary natural products for the treatment of castration-resistant prostate cancer, this study aims to systematically investigate the effect of central linker length in curcumin mimics possessing terminal heteroaromatic rings on in vitro cell proliferation in prostate cancer cell models. Consequently, twenty five (1E,3E,6E,8E)-1,9-diarylnona-1,3,6,8-tetraen-5-ones (59-83) bearing two identical terminal heteroaromatic rings were designed for the evaluation of their anti-prostate cancer potential in cell-based models. Among them, 1,9-di-3-pyridinyl-1,3,6,8-nonatetraen-5-one (72) and 1,9-di-4-pyridinyl-1,3,6,8-nonatetraen-5-one (74) are known compounds.22,23 No synthesis has been reported so far. All other twenty-three (1E,3E,6E,8E)-1,9-diarylnona-1,3,6,8-tetraen-5-ones are new compounds.
2. Results and Discussion
2.1. Chemistry
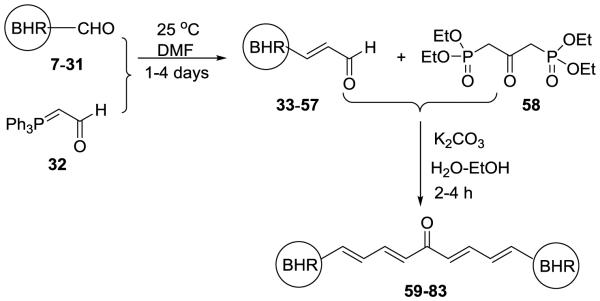
Twenty three new and two known (1E,3E,6E,8E)-1,9-diarylnona-1,3,6,8-tetraen-5-ones (59-83) bearing two identical terminal heteroaromatic rings have been successfully synthesized as illustrated in Scheme 1. Specifically, these target compounds have been achieved by Horner-Wadsworth-Emmons reaction of one equivalent of 1,3-bis(diethylphosphonato)acetone (58) with 2.2 equivalents of the appropriate (E)-3-aryl-2-propenal (33-57), using potassium carbonate as base and employing water and ethanol (3:2, v/v) as a co-solvent. The (E)-3-aryl-2-propenals (33-57) have been prepared via Wittig reaction of the appropriate carbaldedyde (7-31) with (triphenylphosphoranylidene)aldehyde (32) at room temperature in DMF for one to four days. TLC was used to detect the completion of the reaction. 1-Alkyl-1H-imidazole-2-carbaldehydes (13-17) were synthesized from 1H-imidazole-2-carbaldehydes mediated by potassium carbonate based on the procedure illustrated in the literature.24,25 1-Alkyl-1H-benzo[d]imidazole-2-carbaldehydes (27-31) were obtained from benzene-1,2-diamine and glycolic acid through a three-step transformation as previously reported by us.19 Other starting carbaldehydes were purchased from Fisher Scientific. 1,3-Bis(diethylphosphonato)acetone (58) was readily synthesized from carbazic acid methyl ester, 1,3-dichloroacetone, and triethyl phosphite according to the reported procedure.26 Curcumin, as the positive reference control, was prepared by Claisen-Schmidt condensation of vanillin with acetylacetone according to the procedure described in the literature.27
Scheme 1.
Synthesis of 1,9-diarylnona-1,3,6,8-tetraen-5-ones (59-83)
2.2. Anti-proliferative effects toward three prostate cancer cell lines
To evaluate the in vitro anticancer potential of the synthesized 1,9-diarylnona-1,3,6,8-tetraen-5-ones (59-83), we first determined their antiproliferative activity toward both androgen-sensitive (LNCaP) and androgen-insensitive (PC-3 and DU145) human prostate cancer cell lines using the WST-1 cell proliferation assay. The IC50 values, acquired based on the inhibitory rates for at least 5 concentrations, are summarized in Table 2. Curcumin was employed as a positive control for comparison in the parallel experiment. Among the series of compounds tested, eighteen out of twenty-five 1,9-diarylnona-1,3,6,8-tetraen-5-ones (59-62, 65, 66, 68, 69, 71, 72, 74, 75, 78-83) display 2-23 times higher antiproliferative potency than curcumin in three prostate cancer cell models (Table 2), suggesting that 1,9-diarylnona-1,3,6,8-tetraen-5-one can serve as a new potential scaffold of anti-prostate cancer agents. The data in Table 2 suggest that para pyridyls in 74 and 75, and quinolin-4-yl in 78 are favorable terminal heteroaromatic rings for the promising potency of these three compounds in three prostate cancer cell lines. This is different from another two promising scaffolds, (1E,4E)-1,5-diheteroarylpenta-1,4-dien-3-ones and (1E,4E,6E)-1,7-diaryl-1,4,6-heptatrien-3-ones that we previously reported, in which ortho pyridyl confers greater cytotoxic and antiproliferative potency than para pyridyl in various prostate cancer models.5 Additionally, in our previous studies, 1-alkyl-1H-imidazol-2-yl and 1-alkyl-1H-benzo[d]imidazole-2-yl serve as optimal heteroaromatic rings for the antiproliferative potency of 1,5-diheteroarylpenta-1,4-dien-3-ones (e.g. 3 and 4)19 and 1,7-diheteroarylhepta-1,4,6-trien-3-ones (e.g. 5 and 6).20 However, the current study indicates that these two groups of heteroaromatic rings can only bestow moderate antiproliferative potency to 1,9-diarylnona-1,3,6,8-tetraen-5-ones (e.g. 82 and 69). As illustrated in Table 3, the optimal 1,5-diheteroarylpenta-1,4-dien-3-one (3)19 has much greater anti-proliferative potency than 5 and 78, the optimal mimics from 1,7-diheteroarylhepta-1,4,6-trien-3-ones20 and 1,9-diarylnona-1,3,6,8-tetraen-5-ones. This indicates that the length of the linker exhibits important impact on anti-proliferative potency of these curcumin mimics and that the scaffold with 5-carbon linker is the most promising one. On the other hand, the optimal mimic with 7-carbon linker (5) is slightly more potent than the optimal mimic with 9-carbon linker (78), suggesting no potency contribution from the linker length in mimics with 9-carbon linker. The above-mentioned data collectively indicate that i) the enhanced anti-proliferative potency for 75 and 78, as compared with curcumin, may be fully due to the terminal heteroaromatic groups; and ii) 1,9-diarylnona-1,3,6,8-tetraen-5-ones with two identical terminal heteroaromatic groups can serve as a new scaffold for the development of curcumin-based anti-prostate cancer agents.
Table 2.
Anti-Proliferative Activity of Diheteroarylnona-1,3,6,8-tetraen-5-ones
| Compd. | IC50 (μM)a | IC50 (curcumin)/IC50(dienone) | ||||
|---|---|---|---|---|---|---|
| PC-3b | DU145c | LNCaPd | PC-3b | DU145c | LNCaPd | |
| Curcumin | 25.43 ± 2.15 | 26.23 ± 0.65 | 13.61 ± 2.69 | 1 | 1 | 1 |
| 59 | 6.21 ± 0.15 | 6.38 ± 0.65 | 6.85 ± 0.35 | 4 | 4 | 2 |
| 60 | 3.00 ± 1.18 | 7.37 ± 1.44 | 8.01 ± 2.87 | 9 | 4 | 2 |
| 61 | 10.47 ± 2.43 | 5.30 ± 2.36 | 8.09 ± 1.20 | 2 | 5 | 2 |
| 62 | 4.19 ± 0.24 | 4.19 ± 1.35 | 2.58 ± 1.04 | 6 | 6 | 5 |
| 63 | 24.77 ± 8.06 | 11.35 ± 2.96 | >20 | 1 | 2 | <1 |
| 64 | >20 | 18.53 ± 10.59 | >20 | <1 | 1 | <1 |
| 65 | 3.86 ± 0.23 | 2.45 ± 1.29 | 6.14 ± 2.68 | 7 | 11 | 5 |
| 66 | 7.43 ± 4.05 | 2.69 ± 0.99 | 4.94 ± 0.47 | 3 | 10 | 5 |
| 67 | >15 | >15 | > 20 | <1.7 | <1.7 | <1 |
| 68 | 2.36 ± 0.56 | 1.21 ± 0.43 | 2.43 ± 1.31 | 11 | 22 | 6 |
| 69 | 2.50 ± 1.06 | 1.75 ± 0.52 | 5.62 ± 3.57 | 10 | 15 | 2 |
| 70 | 45.69 ± 19.56 | 3.59 ± 2.63 | 17.53 ± 4.89 | <1 | 7 | <1 |
| 71 | 10.39 ± 4.71 | 8.29 ± 2.97 | 6.05 ± 4.93 | 3 | 3 | 2 |
| 72 | 5.22 ± 3.97 | 6.55 ± 3.26 | 4.94 ± 0.47 | 5 | 4 | 3 |
| 73 | >15 | >15 | 5.89 ± 2.64 | <1.7 | <1.7 | 2.3 |
| 74 | 1.79 ± 0.24 | 1.60 ± 0.08 | 1.63 ± 0.34 | 14 | 16 | 8 |
| 75 | 1.14 ± 0.12 | 1.78 ± 0.13 | 2.17 ± 0.2 | 22 | 15 | 6 |
| 76 | 11.74 ± 0.61 | 14.36 ± 0.34 | 13.62 ± 3.64 | 2 | 2 | 1 |
| 77 | >15 | >15 | 17.71 ± 4.88 | <1.7 | <1.7 | <1 |
| 78 | 1.09 ± 0.17 | 1.20 ± 0.01 | 0.98 ± 0.19 | 23 | 22 | 14 |
| 79 | 2.63 ± 0.51 | 3.50 ± 0.17 | 0.97 ± 0.34 | 10 | 8 | 14 |
| 80 | 2.48 ± 0.41 | 2.70 ± 0.28 | 1.49 ± 2.24 | 10 | 10 | 9 |
| 81 | 7.24 ± 0.58 | 9.76 ± 0.66 | 3.65 ± 0.85 | 4 | 3 | 3.7 |
| 82 | 2.67 ± 0.26 | 3.36 ± 0.36 | 3.00 ± 0.77 | 10 | 8 | 4.5 |
| 83 | 2.82± 0.38 | 4.08 ± 0.36 | 4.31 ± 0.99 | 9 | 6 | 3.2 |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay (WST-1) or trypan blue exclusion assay (TB) after 3 days exposure.
Human androgen-insensitive prostate cancer cell line
Human androgen-insensitive prostate cancer cell line
Human androgen-sensitive prostate cancer cell line
Table 3.
The Effect of Linker Length of Curcumin Mimics on Anti-Proliferative Activity
| Compd. | Linker | IC50(curcumin)/IC50(curcumin mimic) | Note | ||
|---|---|---|---|---|---|
| PC-3 | DU145 | LNCaP | |||
| 3 a | 5-carbon | 111 | 50 | 30 | The optimal candidate for the series |
| 4 a | 5-carbon | 82 | 90 | 57 | |
| 5 b | 7-carbon | 36 | 29 | 11 | The optimal candidate for the series |
| 6 b | 7-carbon | 14 | 25 | 11 | |
| 82 | 9-carbon | 10 | 8 | 4.5 | |
| 69 | 9-carbon | 10 | 15 | 2 | |
| 78 | 9-carbon | 23 | 22 | 14 | The optimal candidate for the series |
2.3. Effects of 68 and 75 on PC-3 cell apoptosis and cell cycle progression
The anti-proliferative effect of curcumin against androgen-insensitive human prostate cancer cells is linked to its capability of promoting cancer cell apoptosis and cell cycle regulation.28,29 To determine whether the suppression of PC-3 cell proliferation by the 1,9-diarylnona-1,3,6,8-tetraen-5-ones is associated with activation of cell apoptosis and cell cycle arrest, two of the most potent compounds, 75 and 68, were selected for further investigation. 74, 75, and 78 are the three most potent compounds that contain para pyridyls or quinolin-4-yl. Among them, 75 was chosen as the representative due to the presence of a fluorine atom in the aromatic ring which might bring in beneficial pharmacokinetic properties.30,31
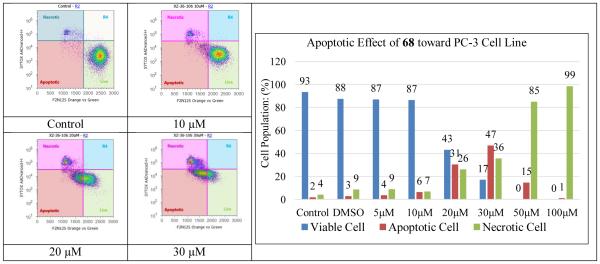
PC-3 cells were treated with 75 and 68, respectively, at 0-100 μM final concentration range for 16 h. The specific apoptotic inducer staurosporine was used as a positive control for early apoptosis in the experiments (data not shown). The early apoptotic PC-3 cells can be readily discriminated from the late apoptotic and necrotic cells by the double staining assay, consisting of the violet excitable dye F2N12S and dead cell stain SYTOX AADVanced, in a flow cytometer. Data summarized in Figures 2 and 3 demonstrate that a 16-hour treatment of both 68 and 75 can significantly promote androgen-insensitive PC-3 cell apoptosis in a dose-responsive manner. It is worth noting that 75 induced PC-3 cell apoptosis much more effectively than 68 even though they possess similar inhibitory effect on PC-3 cell proliferation, indicating that terminal para pyridyl is beneficial to cell apoptosis activation. For example, 10 μM of compound 68 induce only modest early phase of apoptosis (6%) in PC-3 cells as compared with control cells; whereas 10 μM of compound 75 activated substantial early apoptotic PC-3 cells (51%). Also, the early apoptotic cell population reached a maximum (47%) when PC-3 cancer cells were exposed to compound 68 at 30 μM concentration; in contrast, treatment of the cells with compound 75 led to a much higher maximum of early apoptosis (75%) that was reached at a lower dose (20 μM).
Figure 2.
Evolution of viable, apoptotic, and necrotic PC-3 cells populations in response to increasing dosages of compound 68.
Figure 3.
Evolution of viable, apoptotic, and necrotic PC-3 cells populations in response to increasing dosages of compound 75.
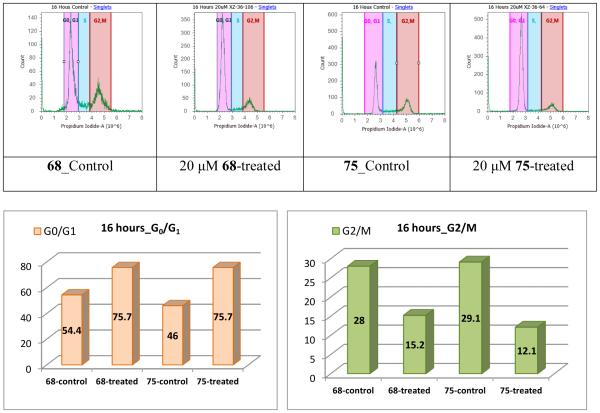
PC-3 cell cycle regulation mediated by compounds 68 and 75 was assessed by flow cytometric analysis with propidium iodide DNA staining. As illustrated in Figure 4, the PC-3 cell population in the G0/G1 phase increased by 21.3% and 29.7%, respectively, after treating with 68 and 75 for 16 h, as compared with the respective control cells. In contrast, 68 and 75 decreased the PC-3 cell population in the G2/M phase by 12.8% and 17.0%, respectively. Therefore, it can be concluded that both compounds can significantly arrest PC-3 cell cycle at the G0/G1 phase, with 75 as the slightly stronger promoter.
Figure 4.
Cell cycle analysis of PC-3 prostate cancer cells. PC-3 cancer cells were untreated or treated with 68 and 75, respectively, at 20 μM. Cells were harvested after 16 hours, fixed, stained, and analyzed for DNA content.
3. Conclusion
In summary, twenty-five symmetric 1,9-diarylnona-1,3,6,8-tetraen-5-ones with two identical nitrogen-containing terminal heteroaromatic rings have been synthesized. Eighteen 1,9-diarylnona-1,3,6,8-tetraen-5-ones exhibit significantly improved antiproliferative potency as compared with curcumin. This indicates that (1E,3E,6E,8E)-1,9-diarylnona-1,3,6,8-tetraen-5-one can serve as a potential scaffold for further exploration of the therapeutic potential for the treatment of prostate cancer. Our findings also suggest that para pyridyls and quinolin-4-yl serve as favorable heteroaromatic rings for the enhanced potency of this new scaffold. Two of the most potent compounds, 75 and 68, effectively inhibit PC-3 cell proliferation by inducing cell apoptosis and by arresting cell cycle regulation in the G0/G1 phase.
4. Experiments
4.1. General synthetic procedures
NMR spectra were obtained on a Bruker Fourier 300 spectrometer in CDCl3, or DMSO-d6. The chemical shifts are given in ppm referenced to the respective solvent peak, and coupling constants are reported in Hz. Anhydrous THF and dichloromethane were purified by PureSolv MD 7 Solvent Purification System from Innovative Technologies (MB-SPS-800). All other reagents and solvents were purchased from commercial sources and were used without further purification. Silica gel column chromatography was performed using silica gel (32-63 μm). Preparative thin-layer chromatography (PTLC) separations were carried out on thin layer chromatography plates preloaded with silica gel GF254.
4.2. General procedure for the synthesis of (E)-3-aryl-2-propenals (33-57).20,32
The reaction mixture of aldehyde (0.5 mmol) and 2-(triphenylphosphoranylidene)acetaldehyde (0.51 mmol) in DMF (0.5 mL) was stirred for 1-4 days and detected with TLC. The reaction mixture was extracted with dichloromethane (10 mL × 3). The combined organic extracts were rinsed with brine (5 mL × 3) and dried over anhydrous magnesium sulfate, and the volatile components were evaporated under vacuum to give the crude product, which was subjected to the PTLC purification using hexanes/ethyl acetate (1/1, v/v) as eluent to give the respective product. All intermediates were directly used for the next step reaction after confirming with their 1H NMR data. The 1H NMR data for the sixteen known (E)-3-aryl-2-propenals (33, 34, 36, 37, 39-46, 48, 49, 56, 57) are in consistent with those reported in the literature.20 The yields and 1H NMR spectral data for the other nine intermediates are listed below.
4.2.1. (E)-3-(Thiazol-5-yl)acrylaldehyde (35)
43% yield. 1H NMR (300 MHz, CDCl3) δ 9.64 (1H, d, J = 7.5 Hz), 8.89 (1H, s), 8.10 (1H, s), 7.65 (1H, d, J = 15.6 Hz), 6.49 (1H, dd, J = 15.6, 7.5 Hz).
4.2.2. (E)-3-(5-Chloro-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)acrylaldehyde (38)
53% yield, 1H NMR (300 MHz, CDCl3) δ 9.64 (1H, d, J = 7.5 Hz), 7.33 (1H, d, J = 16.2 Hz), 6.84 (1H, dd, J = 16.2, 7.5 Hz), 3.96 (3H, s).
4.2.3. (E)-3-(6-(Trifluoromethyl)pyridin-3-yl)acrylaldehyde (47)
85% yield, 1H NMR (300 MHz, CDCl3) δ 9.79 (1H, d, J = 7.5 Hz), 8.89 (1H, s), 8.08 (1H, d, J = 8.4 Hz), 7.78 (1H, d, J = 8.1 Hz), 7.55 (1H, d, J = 16.2 Hz), 6.85 (1H, dd, J = 16.2, 7.5 Hz).
4.2.4. (E)-3-(Quinolin-2-yl)acrylaldehyde (50)
45% yield, 1H NMR (300 MHz, CDCl3) δ 9.86 (1H, d, J = 7.8 Hz), 8.22 (1H, d, J = 8.4 Hz), 8.13 (1H, d, J = 8.4 Hz), 7.84 (1H, d, J = 8.1 Hz), 7.77 (1H, dt, J = 6.9, 1.2 Hz), 7.73 (1H, d, J = 15.9 Hz), 7.69 (1H, d, J = 8.4 Hz), 7.60 (1H, dt, J = 8.1, 0.9 Hz), 7.13 (1H, dd, J = 16.2, 7.8 Hz).
4.2.5. (E)-3-(2-Chloroquinolin-3-yl)acrylaldehyde (51)
70% yield, 1H NMR (300 MHz, CDCl3) δ 9.83 (1H, d, J = 7.5 Hz), 8.43 (1H, s), 8.02 (1H, d, J = 9.6 Hz), 7.98 (1H, d, J = 16.5 Hz), 7.89 (1H, d, J = 8.1 Hz), 7.81 (1H, d, J = 7.8 Hz), 7.62 (1H, d, J = 7.8 Hz), 6.84 (1H, dd, J = 15.9, 7.5 Hz).
4.2.6. (E)-3-(Quinolin-4-yl)acrylaldehyde (52)
72% yield, 1H NMR (300 MHz, CDCl3) δ 9.89 (d, J = 7.5 Hz, 1H), 8.96 (d, J = 4.5 Hz, 1H), 8.23 (d, J = 16.2 Hz, 1H), 8.20-8.11 (overlapped, 2H), 7.78 (t, J = 7.2 Hz, 1H), 7.65 (t, J = 7.2 Hz, 1H), 7.55 (d, J = 4.5 Hz, 1H), 6.89 (dd, J = 15.9, 7.5 Hz, 1H).
4.2.7. (E)-3-(1-Methyl-1H-benzo[d]imidazol-2-yl)acrylaldehyde (53)
46% yield, 1H NMR (300 MHz, CDCl3) δ 9.82 (1H, d, J = 7.5 Hz), 7.84 (1H, dd, J = 6.3, 3.6 Hz), 7.54 (1H, d, J = 15.6 Hz), 7.42-7.34 (4H, overlapped), 3.95 (3H, s).
4.2.8. (E)-3-(1-Ethyl-1H-benzo[d]imidazol-2-yl)acrylaldehyde (54)
54% yield, 1H NMR (300 MHz, CDCl3) δ 9.80 (1H, d, J = 7.2 Hz), 7.82 (1H, dd, J = 6.3, 3.3 Hz), 7.49 (1H, d, J = 15.6 Hz), 7.43-7.30 (4H, overlapped), 4.37 (2H, q, J = 7.5 Hz), 1.49 (3H, t, J = 7.5 Hz).
4.2.9. (E)-3-(1-Propyl-1H-benzo[d]imidazol-2-yl)acrylaldehyde (55)
72% yield, 1H NMR (300 MHz, CDCl3) δ 9.79 (1H, d, J = 6.9 Hz), 7.80 (1H, dd, J = 6.6, 5.7 Hz), 7.47 (1H, d, J = 15.6 Hz), 7.41-7.29 (4H, overlapped), 4.26 (2H, t, J = 7.2 Hz), 1.89 (2H, Hex, J = 7.5 HZ), 0.97 (3H, t, J = 7.2 Hz).
4.3. General procedure for the synthesis of 1,9-diheteroarylnona-1,3,6,8-tetra-en-5-ones (59-83)
To the solution of tetraethyl (2-oxopropane-1,3-diyl)bis(phosphonate) (1 mmol) in 4 mL ethanol, potassium carbonate (5 mmol) in 6 mL water was added dropwise. After 30 min at room temperature, the appropriate (E)-3-aryl-2-propenal (2.2 mmol) in 0.5 mL ethanol was added to the solution dropwise. The inorganic solids were removed by filtration after 2-4 h when no starting material observed by TLC, and the filtrate was diluted with with 1 M HCl (10 mL) and extracted with dichloromethane (10 mL × 3). The collected aqueous layer was neutralized by saturated aqueous NaHCO3 and then extracted with dichloromethane (10 mL × 3). The combined organic extracts were dried over anhydrous magnesium sulfate, and the volatile components were evaporated under vacuum to give the crude product, which was subjected to purification using dichloromethane/methanol (100/3, v/v) as eluent to give the respective product. Some physical and spectral data for the title compounds are listed below.
4.3.1. (1E,3E,6E,8E)-1,9-Di(thiazol-2-yl)nona-1,3,6,8-tetraen-5-one (59)
brown solid, mp 131-132 °C, 60% yield. 1H NMR (300 MHz, CDCl3) δ 7.87 (2H, d, J = 3.2 Hz), 7.44 (4H, dd, J = 15.1, 10.0 Hz), 7.36 (2H, d, J = 3.2 Hz), 7.27–7.12 (2H, overlapped), 6.66 (2H, d, J = 15.1 Hz). 13C NMR (75 MHz, CDCl3) δ 188.4, 165.4, 144.5, 141.6, 132.6, 131.9, 131.5, 120.3. IR (film) νmax: 1656, 1606, 1401, 1328, 1063 cm−1; HRMS (ESI): m/z calculated for C15H12N2OS2 [M+H]+: 301.0469; found: 301.0466.
4.3.2. (1E,3E,6E,8E)-1,9-Bis(4-methylthiazol-2-yl)nona-1,3,6,8-tetraen-5-one (60)
yellow solid, mp 138-139 °C, 54 % yield. 1H NMR (300 MHz, CDCl3) δ 7.44 (2H, dd, J = 15.0, 10.5 Hz), 7.22 (2H, dd, J = 15.3, 10.8 Hz), 7.10 (2H, d, J = 15.3 Hz), 6.94 (2H, s), 6.66 (2H, d, J = 13.8 15.3 Hz), 2.50 (6H, s). 13C NMR (75 MHz, CDCl3) δ 189.0, 165.1, 155.3, 142.2, 133.2, 131.9, 131.8, 115.7, 17.8. IR (film) νmax: 3104, 2921, 1644, 1598, 1578, 1509, 1439 cm−1. HR-MS (ESI) m/z: calcd for C17H17N2OS2 [M+H]: 329.0782; found 329.0778.
4.3.3. (1E,3E,6E,8E)-1,9-Di(thiazol-5-yl)nona-1,3,6,8-tetraen-5-one (61)
yellow solid, mp 165-166 °C, 50 % yield. 1H NMR (300 MHz, CDCl3) δ 8.75 (2H, s), 7.91 (2H, s), 7.40 (2H, dd, J = 15.0, 11.1 Hz), 7.13 (2H, d, J = 15.3 Hz), 6.73 (2H, dd, J = 15.3, 11.1 Hz), 6.54 (2H, d, J = 15.0 Hz). 13C NMR (75 MHz, CDCl3) δ 189.0, 153.9, 144.7, 142.6, 137.4, 130.69, 130.66, 130.36. IR (film) νmax: 3071, 1640, 1603, 1575, 1493, 1387 cm−1. HR-MS (ESI) m/z: calcd for C15H13N2OS2 [M+H]: 301.0469; found 301.0465.
4.3.4. (1E,3E,6E,8E)-1,9-Bis(5-methylisoxazol-3-yl)nona-1,3,6,8-tetraen-5-one (62)
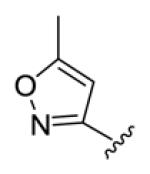
yellow solid, mp 183-184 °C, 62% yield. 1H NMR (300 MHz, CDCl3) δ 7.41 (dd, J = 15.2, 9.0 Hz, 2H), 7.00–6.84 (m, 4H), 6.60 (d, J = 15.3 Hz, 2H), 6.17 (s, 2H), 2.44 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 188.6, 170.0, 161.0, 141.8, 133.2, 131.1, 128.4, 99.2, 12.4. IR (film) νmax: 1658, 1610, 1445, 904, 725 cm−1; HRMS (ESI): m/z calculated for C17H16N2O3 [M+H]+: 297.1239; found: 297.1237.
4.3.5. (1E,3E,6E,8E)-1,9-Bis(4-bromo-1-methyl-1H-pyrazol-3-yl)nona-1,3,6,8-tetraen-5-one (63)
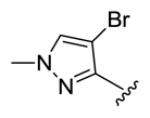
yellow oil, 46 % yield. 1H NMR (300 MHz, CDCl3) δ 7.45 (2H, dd, J = 15.0, 11.4 Hz), 7.41 (2H, s), 7.27 (2H, dd, J = 15.6, 10.8 Hz), 6.89 (2H, d, J = 15.6 Hz), 6.59 (2H, d, J = 15.0 Hz). 13C NMR (75 MHz, CDCl3) δ 189.7, 147.0, 143.5, 132.2, 130.33, 130.23, 129.3, 95.2, 40.5. IR (film) νmax: 3127, 2926, 1698, 1620, 1588, 1575, 1304, 1163, 1087, 1004 cm−1. HR-MS (ESI) m/z: calcd for C17H17N4OBr2 [M+H]: 450.9769; found 450.9762; 452.9748, found 452.9741; 454.9728, found 454.9720.
4.3.6. (1E,3E,6E,8E)-1,9-Bis(5-chloro-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)nona-1,3,6,8-tetraen-5-one (64)
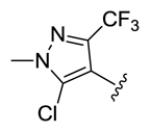
yellow solid, mp 188-189 °C, 54 % yield. 1H NMR (300 MHz, CDCl3) δ 7.41 (2H, dd, J = 15.0, 10.8 Hz), 7.03 (2H, dd, J = 15.9, 10.8 Hz), 6.82 (2H, d, J = 15.6 Hz), 6.58 (2H, d, J = 15.3 Hz), 3.94 (6H, s). 13C NMR (75 MHz, CDCl3) δ 189.3, 143.6, 139.7 (q, JCF = 37.5 Hz), 132.6, 130.1, 128.7, 127.1, 125.2 (q, JCF = 267.8 Hz), 114.5, 37.8. IR (film) νmax: 3052, 1657, 1601, 1523, 1485, 1410, 1358, 1243, 1169 cm−1. HR-MS (ESI) m/z: calcd for C19H15N4OCl2F6 [M+H]: 499.0528; found 499.0540; 501.0498; found 501.0509; 503.0468; found 503.0475.
4.3.7. (1E,3E,6E,8E)-1,9-Bis(1-isopropyl-1H-imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (65)
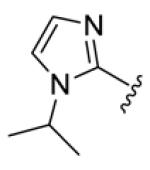
deep brown solid, mp 145-146 °C, 59 % yield. 1H NMR (300 MHz, CDCl3) δ 7.51-7.38 (4H, overlapped), 7.17 (2H, s), 7.06 (2H, s), 6.83 (2H, dt, J = 13.8, 6.3 Hz), 6.62 (2H, dt, J = 14.1, 7.2 Hz), 4.54 (2H, hept, J = 6.6 Hz), 1.49 (12H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ 189.6, 154.8, 150.5, 142.8, 140.4, 137.5, 131.7, 131.6, 124.0, 123.9. IR (film) νmax: 2977, 2928, 1642, 1599, 1463, 1417 cm−1. HR-MS (ESI) m/z: calcd for C21H27N4O [M+H]: 351.2185; found 351.2181.
4.3.8. (1E,3E,6E,8E)-1,9-Bis(1-(sec-butyl)-1H-imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (66)
deep brown solid, mp 148-150 °C, 52 % yield. 1H NMR (300 MHz, CDCl3) δ 7.48-7.43 (4H, overlapped), 7.20 (2H, s), 7.02 (2H, s), 6.82 (2H, d, J = 13.5 Hz), 6.64 (2H, d, J = 13.8 Hz), 4.27 (2H, Hex, J = 6.9 Hz), 1.68-1.73 (4H, m), 1.48 (6H, d, J = 6.9 Hz), 0.86 (6H, t, J = 7.2 Hz). 13C NMR (75 MHz, CDCl3) δ 189.4, 144.7, 142.9, 130.93, 130.87, 130.86, 125.4, 118.1, 54.0, 31.5, 22.3, 11.3. IR (film) νmax: 3028, 2964, 1642, 1459, 1418, 1400 cm−1. HR-MS (ESI) m/z: calcd for C23H31N4O [M+H]: 379.2498; found 379.2493.
4.3.9. (1E,3E,6E,8E)-1,9-Bis(1-isobutyl-1H-imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (67)
deep brown oil, 42 % yield. 1H NMR (300 MHz, CDCl3) δ 7.46 (4H, dd, J = 14.4, 7.8 Hz), 7.18 (2H, s), 6.95 (2H, s), 6.77 (2H, d, J = 14.1 Hz), 6.65 (2H, d, J = 13.8 Hz), 3.81 (4H, d, J = 7.2 Hz), 2.12-2.00 (2H, m), 0.96 (12H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ 189.4, 144.8, 142.8, 131.2, 130.0, 125.0, 122.8, 54.3, 31.2, 20.6. IR (film) νmax: 3109, 2960, 2926, 1642, 1599, 1468, 1444, 1278, 1245 cm−1. HR-MS (ESI) m/z: calcd for C23H31N4O [M+H]: 379.2498; found 379.2494.
4.3.10. (1E,3E,6E,8E)-1,9-Bis(1-(pentan-3-yl)-1H-imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (68)
brown oil, 72 % yield. 1H NMR (300 MHz, CDCl3) δ 7.46-7.41 (4H, overlapped), 7.20 (2H, s), 6.95 (2H, s), 6.80 (2H, d, J = 13.5 Hz), 6.61 (2H, d, J = 13.8 Hz), 3.98-3.93 (2H, m), 1.89-1.66 (8H, m), 0.79 (12H, t, J = 7.5 Hz). 13C NMR (75 MHz, CDCl3) δ 189.4, 145.6, 143.0, 131.2, 130.8, 130.7, 125.6, 118.1, 60.3, 29.7, 11.2. IR (film) νmax: 3071, 2967, 2934, 1640, 1597, 1507, 1458, 1420, 1275 cm−1. HR-MS (ESI) m/z: calcd for C25H35N4O [M+H]: 407.2811; found 407.2806.
4.3.11. (1E,3E,6E,8E)-1,9-Bis(1-(pentan-2-yl)-1H-imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (69)
brown oil, 61 % yield. 1H NMR (300 MHz, CDCl3) δ 7.50-7.37 (4H, overlapped), 7.18 (2H, s), 7.01 (2H, s), 6.81 (2H, d, J = 13.8 Hz), 6.62 (2H, d, J = 13.8 Hz), 4.32 (2H, hex, J = 6.9 Hz), 1.74 (4H, q, J = 7.5 Hz), 1.46 (6H, d, J = 6.6 Hz), 1.30-1.10 (4H, m), 0.9 (6H, t, J = 7.2 Hz). 13C NMR (75 MHz, CDCl3) δ 189.4, 144.6, 142.9, 131.0, 130.9, 130.7, 125.5, 118.1, 52.2, 40.5, 22.7, 20.0, 14.3. IR (film) νmax: 3104, 2959, 2932, 1641, 1597, 1506, 1457, 1418, 1402 cm−1. HR-MS (ESI) m/z: calcd for C25H35N4O [M+H]: 407.2811; found 407.2807.
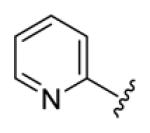
4.3.12. (1E,3E,6E,8E)-1,9-Di(pyridin-2-yl)nona-1,3,6,8-tetraen-5-one (70)
deep brown solid, mp 128-129 °C, 39% yield. 1H NMR (300 MHz, CDCl3) δ 8.63 (2H, d, J = 4.2 Hz), 7.70 (2H, t, J = 7.5 Hz), 7.55-7.43 (4H, overlapped), 7.22 (2H, dd, J = 6.6, 4.8 Hz), 7.04 (2H, dd, J = 14.1, 6.6 Hz), 6.70 (2H, d, J = 13.8 Hz). 13C NMR (75 MHz, CDCl3) δ 189.6, 154.8, 150.5, 142.8, 140.4, 137.5, 131.7, 131.6, 124.0, 123.9. IR (film) νmax: 1646, 1600, 1469, 1431, 1250, 1002 cm−1. HR-MS (ESI) m/z: calcd for C19H17N2O [M+H]: 289.1341; found 289.1338.
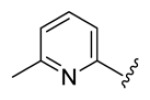
4.3.13. (1E,3E,6E,8E)-1,9-Bis(6-methylpyridin-2-yl)nona-1,3,6,8-tetraen-5-one (71)
deep brown solid, mp 85-86 °C, 46 % yield. 1H NMR (300 MHz, CDCl3) δ 7.57 (2H, t, J = 7.5 Hz), 7.49 (2H, dd, J = 15.9, 11.1 Hz), 7.44 (2H, dd, J = 15.9, 11.4 Hz), 7.19 (2H, d, J = 7.8 Hz), 7.05 (2H, d, J = 6.3 Hz), 7.00 (2H, dt, J = 13.8, 4.5 Hz), 6.67 (2H, dt, J = 13.8, 4.5 Hz), 2.59 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 189.0, 158.7, 153.6, 142.4, 140.4, 136.8, 130.7, 130.5, 123.0, 120.4, 24.6. IR (film) νmax: 3106, 2923, 1646, 1617, 1600, 1574, 1507, 1451 cm−1. HR-MS (ESI) m/z: calcd for C21H21N2O [M+H]: 317.1654; found 317.1651.
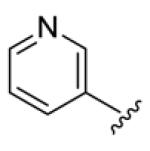
4.3.14. (1E,3E,6E,8E)-1,9-Di(pyridin-3-yl)nona-1,3,6,8-tetraen-5-one (72)
yellow solid, mp 205 °C (decomposed), 10 % yield. 1H NMR (300 MHz, CDCl3) δ 8.73 (2H, d, J = 1.5 Hz), 8.56 (2H, d, J = 8.1 Hz), 7.82 (2H, d, J = 8.1 Hz), 7.49 (2H, dd, J = 15.6, 9.9 Hz), 7.33 (2H, dd, J = 8.1, 4.8 Hz), 7.05 (1H, d, J = 15.6 Hz), 7.00 (2H, s), 6.97 (1H, d, J = 15.6 Hz), 6.63 (2H, d, J = 8.1 Hz). 13C NMR (75 MHz, CDCl3 + CD3OD) δ 189.6, 150.1, 149.2, 143.1, 138.0, 134.4, 132.7, 130.6, 129.6, 124.5. IR (film) νmax: 3028, 2923, 1642, 1620, 1580, 1479, 1415 cm−1. HR-MS (ESI) m/z: calcd for C19H17N2O [M+H]: 289.1341; found 289.1337.
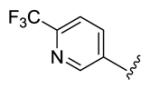
4.3.15. (1E,3E,6E,8E)-1,9-Bis(6-(trifluoromethyl)pyridin-3-yl)nona-1,3,6,8-tetraen-5-one (73)
yellow solid, mp 168-169 °C, 22 % yield. 1H NMR (300 MHz, CDCl3) δ 8.81 (2H, s), 7.96 (2H, d, J = 8.1 Hz), 7.69 (2H, d, J = 8.1 Hz), 7.48 (2H, dd, J = 15.0, 10.2 Hz), 7.10-6.98 (4H, overlapped), 6.68 (2H, d, J = 15.3 Hz). 13C NMR (75 MHz, CDCl3) δ188.9, 149.4, 148.3 (q, JCF = 34.5 Hz), 142.4, 136.3, 135.3, 135.2, 131.7, 122.0 (q, JCF = 273.3 Hz), 121.22, 121.19. IR (film) νmax: 3037, 1667, 1605, 1395 cm−1. HR-MS (ESI) m/z: calcd for C21H15N2OF6 [M+H]: 425.1088; found 425.1083.
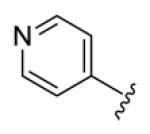
4.3.16. (1E,3E,6E,8E)-1,9-Di(pyridin-4-yl)nona-1,3,6,8-tetraen-5-one (74)
brown solid, mp 133-134 °C, 16% yield. 1H NMR (300 MHz, CDCl3) δ 8.63 (4H, d, J = 6.0 Hz), 7.48 (2H, dd, J = 15.0, 10.8 Hz), 7.35 (4H, d, J = 6.0 Hz), 7.13 (2H, dd, J = 15.6, 11.1 Hz), 6.92 (2H, d, J = 15.6 Hz), 6.66 (2H, d, J = 15.3 Hz). 13C NMR (75 MHz, CDCl3) δ 189.0, 150.9, 144.0, 142.5, 139.0, 131.8, 131.7, 121.8. IR (film) νmax: 1646, 1601, 1415, 1357, 1249, 1073 cm−1. HR-MS (ESI) m/z: calcd for C19H17N2O [M+H]: 289.1341; found 289.1336.
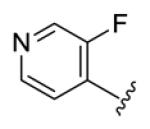
4.3.17. (1E,3E,6E,8E)-1,9-Bis(3-fluoropyridin-4-yl)nona-1,3,6,8-tetraen-5-one (75)
brown solid, mp 142-143 °C, 44 % yield. 1H NMR (300 MHz, CDCl3) δ 8.50 (2H, d, J = 1.2 Hz), 8.42 (2H, d, J = 5.1 Hz), 7.47 (2H, dd, J = 15.3, 10.8 Hz), 7.40 (2H, t, J = 5.7 Hz), 7.20 (2H, dd, J = 15.9, 10.8 Hz), 7.04 (2H, d, J = 15.6 Hz), 6.68 (2H, d, J = 15.3 Hz). 13C NMR (75 MHz, CDCl3) δ 189.0, 157.5 (d, JCF = 262.5 Hz), 146.6 (d, JCF = 5.3 Hz), 142.7, 139.7 (d, JCF = 24.8 Hz), 134.2 (d, JCF = 6.8 Hz), 131.8 (d, JCF = 46.5 Hz), 131.5, 131.4, 121.7. IR (film) νmax: 3041, 1648, 1605, 1491, 1417, 1250 cm−1. HR-MS (ESI) m/z: calcd for C19H15N2OF2 [M+H]: 325.1152; found 325.1148.
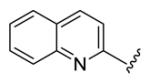
4.3.18. (1E,3E,6E,8E)-1,9-Di(quinolin-2-yl)nona-1,3,6,8-tetraen-5-one (76)
brown solid, mp 172-173 °C, 38 % yield. 1H NMR (300 MHz, CDCl3) δ 8.14 (4H, dd, J = 12.3, 8.7 Hz), 7.80 (2H, d, J = 8.1 Hz), 7.74 (2H, dt, J = 7.2, 1.2 Hz), 7.61-7.52 (8H, overlapped), 7.31-7.21 (2H, overlapped), 6.76 (2H, dt, J = 11.4, 3.0 Hz). 13C NMR (75 MHz, CDCl3) δ 189.4, 155.1, 148.7, 142.9, 141.3, 137.4, 133.0, 132.1, 130.8, 130.0, 128.4, 128.2, 127.6, 120.7. IR (film) νmax: 3053, 1654, 1619, 1609, 1569, 1504, 1429 cm−1. HR-MS (ESI) m/z: calcd for C27H21N2O [M+H]: 389.1654; found 389.1650.
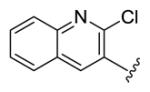
4.3.19. (1E,3E,6E,8E)-1,9-Bis(2-chloroquinolin-3-yl)nona-1,3,6,8-tetraen-5-one (77)
yellow solid, mp 141-142 °C, 5 % yield. 1H NMR (300 MHz, CDCl3) δ 8.41 (2H, s), 8.02 (2H, d, J = 8.4 Hz), 7.87 (2H, d, J = 8.1 Hz), 7.77 (2H, dt, J = 6.9, 1.2 Hz), 7.62 (2H, d, J = 9.0 Hz), 7.59 (2H, dd, J = 15.6, 10.8 Hz), 7.48 (2H, d, J = 15.6 Hz), 7.11 (2H, dd, J = 15.6, 11.1 Hz), 6.72 (2H, d, J = 15.0 Hz). 13C NMR (75 MHz, CDCl3) δ 189.3, 150.6, 148.0, 143.0, 136.3, 135.3, 131.7, 131.5, 131.1, 129.5, 129.1, 128.5, 128.3, 127.9. IR (film) νmax: 3071, 1640, 1603, 1575, 1493, 1387 cm−1. HR-MS (ESI) m/z: calcd for C27H19N2OCl2 [M+H]: 457.0874; found 457.0868; 459.0845; found 459.0837; 461.0815; found 461.0808.
4.3.20. (1E,3E,6E,8E)-1,9-Di(quinolin-4-yl)nona-1,3,6,8-tetraen-5-one (78)
Yellow solid, mp 189-190 °C, 16% yield. 1H NMR (300 MHz, CDCl3) δ 8.93 (d, J = 4.5 Hz, 2H), 8.15 (dd, J = 8.3, 4.0 Hz, 4H), 7.76 (dd, J = 14.9, 5.1 Hz, 2H), 7.75 (d, J = 14.9 Hz, 2H), 7.69–7.56 (m, 6H), 7.20 (dd, J = 15.4, 11.1 Hz, 2H), 6.74 (d, J = 15.2 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 188.7, 150.3, 149.0, 142.41 141.3, 135.5, 132.9, 131.2, 130.5, 129.8, 127.3, 126.0, 123.2, 117.5. IR (film) νmax: 1644, 1610, 1596, 1577, 1505, 1263, 1073 cm−1; HRMS (ESI): m/z calcd for C27H20N2O [M+H]+: 389.1654; found: 389.1652.
4.3.21. (1E,3E,6E,8E)-1,9-Bis(1-methyl-1H-benzo[d]imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (79)
brown solid, mp 198 °C (decomposed), 28 % yield. 1H NMR (300 MHz, CDCl3) δ 7.86-7.77 (4H, overlapped), 7.54 (2H, dd, J = 15.0, 11.4 Hz), 7.39-7.32 (6H, overlapped), 7.03 (2H, d, J = 15.0 Hz), 6.77 (2H, d, J = 15.0 Hz), 3.87 (6H, s). 13C NMR (75 MHz, CDCl3) δ 189.6, 149.8, 142.2, 136.5, 136.1, 133.2, 124.8, 124.7, 124.5, 124.2, 119.6, 110.4, 30.3. IR (film) νmax: 3058, 2925, 1644, 1600, 1460, 1322 cm−1. HR-MS (ESI) m/z: calcd for C25H23N4O [M+H]: 395.1872; found 395.1872.
4.3.22. (1E,3E,6E,8E)-1,9-Bis(1-ethyl-1H-benzo[d]imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (80)
yellow solid, mp 139-141 °C, 52 % yield. 1H NMR (300 MHz, CDCl3) δ 7.81-7.72 (4H, overlapped), 7.52 (2H, dd, J = 15.0, 11.7 Hz), 7.36-7.26 (6H, overlapped), 6.96 (2H, d, J = 14.4 Hz), 6.73 (2H, d, J = 15.0 Hz), 4.29 (4H, q, J = 7.2 Hz), 1.46 (6H, t, J = 7.2 Hz). 13C NMR (75 MHz, CDCl3) δ 189.2, 149.4, 144.0, 142.2, 135.7, 135.1, 132.5, 125.3, 124.1, 123.8, 120.5, 110.2, 39.0, 16.3. IR (film) νmax: 3059, 2977, 2928, 1643, 1600, 1580, 1470, 1447, 1409, 1382 cm−1. HR-MS (ESI) m/z: calcd for C27H27N4O [M+H]: 423.2185; found 423.2181.
4.3.23. (1E,3E,6E,8E)-1,9-Bis(1-propyl-1H-benzo[d]imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (81)
yellow solid, mp 205 °C (decomposed), 44 % yield. 1H NMR (300 MHz, CDCl3) δ 7.81 (2H, dd, J = 12.7, 4.5 Hz), 7.77 (2H, d, J = 12.8 Hz), 7.53 (2H, dd, J = 15.0, 11.4 Hz), 7.36-7.28 (6H, overlapped), 6.98 (2H, d, J = 15.0 Hz), 6.74 (2H, d, J = 15.3 Hz). 13C NMR (75 MHz, CDCl3) δ 189.1, 149.9, 143.9, 142.3, 136.2, 135.0, 132.4, 125.5, 124.1, 123.7, 120.4, 110.4, 45.7, 24.4, 12.1. IR (film) νmax: 3054, 2965, 2934, 1643, 1600, 1461, 1449, 1408 cm−1. HR-MS (ESI) m/z: calcd for C29H31N4O [M+H]: 451.2498; found 451.2492.
4.3.24. (1E,3E,6E,8E)-1,9-Bis(1-isopropyl-1H-benzo[d]imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (82)
deep brown solid, mp 113-115 °C, 68 % yield. 1H NMR (300 MHz, CDCl3) δ 7.88-7.79 (4H, overlapped), 7.58-7.49 (4H, overlapped), 7.34-7.29 (4H, overlapped), 7.08 (2H, d, J = 14.7 Hz), 6.77 (2H, d, J = 15.0 Hz), 4.90 (2H, hept, J = 6.9 Hz), 1.72 (12H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ 189.2, 149.3, 143.9, 142.2, 135.8, 134.6, 132.6, 125.8, 123.9, 123.6, 120.6, 112.4, 48.6, 22.5. IR (film) νmax: 3055, 2978, 2934, 1643, 1598, 1489, 1446, 1408 cm−1. HR-MS (ESI) m/z: calcd for C29H31N4O [M+H]: 451.2498; found 451.2493.
4.3.25. (1E,3E,6E,8E)-1,9-Bis(1-isobutyl-1H-benzo[d]imidazol-2-yl)nona-1,3,6,8-tetraen-5-one (83)
deep brown solid, mp 104-105 °C, 81 % yield. 1H NMR (300 MHz, CDCl3) δ 7.43 (2H, dd, J = 7.8, 3.6 Hz), 7.75 (2H, d, J = 14.4 Hz), 7.52 (2H, dd, J = 15.0, 11.7 Hz), 7.35-7.25 (6H, overlapped), 6.95 (2H, d, J = 14.7 Hz), 6.73 (2H, d, J = 15.0 Hz), 4.00 (4H, d, J = 7.5 Hz), 2.26-2.10 (2H, m), 0.97 (12H, d, J = 6.6 Hz). 13C NMR (75 MHz, CDCl3) δ 189.2, 150.1, 143.8, 142.3, 136.5, 135.0, 132.4, 125.7, 124.1, 123.7, 120.4, 110.7, 51.6, 30.6, 20.9. IR (film) νmax: 3060, 2960, 2929, 1646, 1600, 1467, 1324 cm−1. HR-MS (ESI) m/z: calcd for C31H35N4O [M+H]: 479.2811; found 479.2805.
4.4. Cell culture
All cell lines were initially purchased from American Type Culture Collection (ATCC™). The PC-3 and LNCaP prostate cancer cell lines were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cultures were maintained in a high humidity environment supplemented with 5% carbon dioxide at a temperature of 37°C. The DU-145 prostate cancer cells were routinely cultured in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS and 1% penicillin/ streptomycin.
4.5. WST-1 cell proliferation assay
PC-3, DU-145, or LNCaP cells were plated in 96-well plates at a density of 3,200 each well in 200 μL of culture medium. The cells were then treated with curcumin, or synthesized molecules separately at different doses for 3 days, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in a CO2 incubator at 37 °C for three days. 10 μL of the premixed WST-1 cell proliferation reagent (Clontech) was added to each well. After mixing gently for one minute on an orbital shaker, the cells were incubated for additional 3 hours at 37 °C. To ensure homogeneous distribution of color, it is important to mix gently on an orbital shaker for one minute. The absorbance of each well was measured using a microplate-reader (Synergy HT, BioTek) at a wavelength of 430 nm. The IC50 value is the concentration of each compound that inhibits cell proliferation by 50% under the experimental conditions and is the average from at least triplicate determinations that were reproducible and statistically significant. For calculating the IC50 values, a linear proliferative inhibition was made based on at least five dosages for each compound.
4.6. Cell cycle analysis
PC-3 cells were plated in 24-well plates at a density of 200,000 each well in 400 μL of culture medium. After 3 h of cell attachment, the cells were then treated with compound 75 and 68 at 20 μM, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in CO2 incubator at 37 °C for 16 h. Both attached and floating cells were collected in a centrifuge tube by centrifugation at rcf 450 g for 5 min. After discarding the supernatant, the collected cells were re-suspended with 500 μL 80% cold ethanol to fix for 30 min in 4 °C. The fixed cells could be stored at −20 °C for one week. After fixation, the ethanol was removed after centrifuging and the cells were washed with PBS. The cells were then re-suspended with 100 μL of 100 mg/mL ribonuclease and were cultured at 37 °C for 30 min to degrade all RNA. The cells were stained with 200 μL of 50 μg/mL propidium iodide (PI) stock solution for 30 min at −20 °C, and then the fluorescence intensity of PI was detected in individual PC-3 cells using an Attune flow cytometer (Life Technologies) within 0.5-1 h after staining.
4.7. F2H12S and CYTOX AADvanced double staining assay
PC-3 cells were plated in 24-well plates at a density of 200,000 each well in 400 μL of culture medium. After 3 h of cell attachment, the cells were then treated with each test compound at different concentration for 15 h, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in CO2 incubator at 37 °C for 16 h. Both attached and floating cells were collected in a centrifuge tube by centrifugation at rcf value of 450 g for 5 min. The collected cells were re-suspended with 500 μL HBSS to remove proteins which may affect flow signal and centrifuged again. After discarding the supernatant, the collected cells were re-suspended with 300 μL HBSS and stained with 0.3 μL of F2N12S for 3-5 min followed by 0.3 μL SYTOX AADvanced for an additional 5 min. The fluorescence intensity of the two probes was further measured in individual PC-3 cells using an Attune flow cytometer (Life Technologies) within 0.5-1 h after staining.
4.8. Statistical analysis
All data are represented as the mean ± standard deviation (S.D.) for the number of experiments indicated. Other differences between treated and control groups were analyzed using the Student’s t-test. A p-value < 0.05 was considered statistically significant.
Supplementary Material
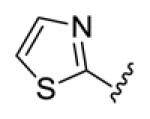
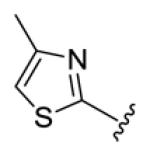
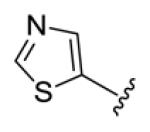
Table 1.
Structures of basic nitrogen-containing heteroaromatic rings (BHR) in 1,9-diarylnona-1,3,6,8-tetraen-5-ones (59-83)
| BHR |
|
|
|
|
|
| compds | 59 | 60 | 61 | 62 | 63 |
| BHR |
|
|
|
|
|
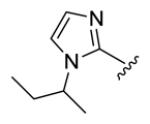
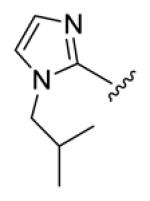
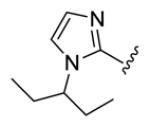
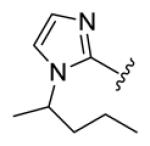
| compds | 64 | 65 | 66 | 67 | 68 |
| BHR |
|
|
|
|
|
| compds | 69 | 70 | 71 | 72 | 73 |
| BHR |
|
|
|
|
|
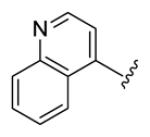
| compds | 74 | 75 | 76 | 77 | 78 |
| BHR |
|
|
|
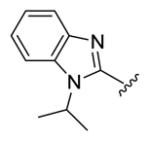
|
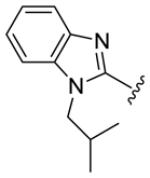
|
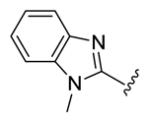
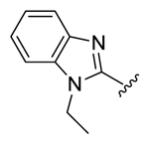
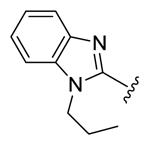
| compds | 79 | 80 | 81 | 82 | 83 |
Acknowledgments
This study was financially supported by California State University (CSU)-Fresno and CSU Program for Education and Research in Biotechnology (CSUPERB) New Investigator Award and Research Development Award. The HRMS data were supported by NIH RCMI program at Xavier University of Louisiana through Grant 2G12MD007595 (G.W.) and NIH-NIGMS through Grant 1U54GM104940 (G.W.). We are also grateful to the ASI and the Graduate Net Initiative at CSU-Fresno for Graduate Research Grants (to X.Z.), and to the Undergraduate Studies at CSU-Fresno for Undergraduate Research Grants (to G.R.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data (comprised of copies of 1H and 13C NMR spectra for the final products) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2016.
References and notes
- 1.American Cancer Society What are the key statistics about prostate cancer? Available at: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics (Accessed June 12, 2016)
- 2.Feldman BJ, Feldman D. Nat. Rev. Cancer. 2001;1:34. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O'Driscoll L. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teiten MH, Gaascht F, Eifes S, Dicato M, Diederich M. Genes Nutr. 2010;5:61. doi: 10.1007/s12263-009-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q-H. Anti-Cancer Agents Med. Chem. 2015;15:138. doi: 10.2174/1871520615666150116102442. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Kumar A, Bharti AC. Anticancer Res. 2003;23:363. [PubMed] [Google Scholar]
- 7.Agrawal DK, Mishra PK. Med. Res. Rev. 2010;30:818. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 8.Basnet P, Skalko-Basnet N. Molecules. 2011;16:4567. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonn GA, Aronson W, Litwin MS. Prostate Cancer Prost. Dis. 2005;8:304. doi: 10.1038/sj.pcan.4500825. [DOI] [PubMed] [Google Scholar]
- 10.Mimeault M, Batra SK. Chin. Med. 2011;6:31. doi: 10.1186/1749-8546-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nambiar D, Singh RP. Nutr. Cancer. 2013;65(Suppl. 1):12. doi: 10.1080/01635581.2013.785006. [DOI] [PubMed] [Google Scholar]
- 12.Hajare RA, Bajad SA, Parwani KS, Chandekar NA, Chandewar AV. Pharma Rev. 2009;7:123. [Google Scholar]
- 13.Kurien BT, Scofield RH. Mol. Nutr. Food Res. 2009;53:939. doi: 10.1002/mnfr.200990022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal BB. Cancer Biol. Ther. 2008;7:1436. doi: 10.4161/cbt.7.9.6659. [DOI] [PubMed] [Google Scholar]
- 15.Gupta SC, Patchva S, Aggarwal BB. AAPS J. 2013;15:195. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cretu E, Trifan A, Vasincu A, Miron A. Rev. Med.-Chir. Soc. Med. Nat. Isai. 2012;116:1223. [PubMed] [Google Scholar]
- 17.Thiyagarajan M, Sharma SS. Life Sci. 2004;74:969. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Goel A, Kunnumakkara AB, Aggarwal BB. Biochem. Pharmacol. 2008;75:787. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Chen C, Zhang X, Zhang C, Zhong Q, Chen G, Zhang Q, Zheng S, Wang G, Chen Q-H. J. Med. Chem. 2015;58:4713. doi: 10.1021/acs.jmedchem.5b00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R, Zhang X, Chen C, Chen G, Zhong Q, Zhang Q, Zheng S, Wang G, Chen Q-H. Eur. J. Med. Chem. 2016;110:164. doi: 10.1016/j.ejmech.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, harakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biochem. Pharmacol. 2008;76:1590. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Akiba M, Kawamata J. Jpn. Tokkyo Koho. 2003 JP 2003075961 A 20030312. [Google Scholar]
- 23.Goto Y, Nakayama M. Jpn. Tokkyo Koho. 1988 JP 63265901 A 19881102. [Google Scholar]
- 24.Samaan N, Zhong Q, Fernandez J, Chen G, Hussain AM, Zheng S, Wang G, Chen Q-H. Eur. J. Med. Chem. 2014;75:123. doi: 10.1016/j.ejmech.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seto M, Miyamoto N, Aikawa K, Aramaki Y, Kanzaki N, Lizawa Y, Baba M, Shiraishi M. Bioorg. Med. Chem. 2005;13:363. doi: 10.1016/j.bmc.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Corbel B, Medinger L, Haelters JP, Sturtz G. Synthesis. 1985:1048. [Google Scholar]
- 27.Wichitnithad W, Nimmannit U, Wacharasindu S, Rojsitthisak P. Molecules. 2011;16:1888. doi: 10.3390/molecules16021888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorai T, Gehani N, Katz A. Prostate Cancer Prostatic Dis. 2000;3:84. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 29.Hilchie AL, Furlong SJ, Sutton K, Richardson A, Robichaud MRJ, Giacomantonia CA, Ridgway ND, Hoskin DW. Nutr. Cancer. 2010;62:379. doi: 10.1080/01635580903441238. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Sanchez-Rosello M, Acena JL, del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H. Chem. Rev. 2014;114:2432. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 31.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. J. Med. Chem. 2015;58:8315. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 32.van Loevezijn A, Venhorst J, Iwema Bakker WI, de Korte CG, de Looff W, Verhoog S, van Wees J, van Hoeve M, van de Woestijne RP, van der Neut MAW, Borst AJM, van Dongen MJP, de Bruin NMWJ, Keizer HG, Kruse CG. J. Med. Chem. 2011;54:7030. doi: 10.1021/jm200466r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.