Abstract
Background
IgG4-related disease (IgG4-RD) is a systemic condition of unknown etiology, characterized by highly fibrotic lesions with dense lymphoplasmacytic infiltrates. CD4+ T cells constitute the major inflammatory cell population in IgG4-RD lesions.
Objective
We used an unbiased approach to characterize CD4+ T cell subsets in IgG4-RD subjects based on their clonal expansion and their ability to infiltrate affected tissue sites.
Methods
We used flow cytometry to identify CD4+ effector/memory T cells (TEM) in a cohort of 101 IgG4-related disease (IgG4-RD) patients. These expanded cells were characterized by gene expression analysis and flow cytometry. Next-generation sequencing of the T cell receptor β chain gene was performed on CD4+SLAMF7+ CTLs and CD4+GATA3+ TH2 cells in a subset of patients to identify their clonality. Tissue infiltration by specific T cells was examined using quantitative multi-color imaging.
Results
CD4+ effector/memory T cells with a cytolytic phenotype were expanded in IgG4-RD patients. Next-generation sequencing revealed prominent clonal expansions of these CD4+CTLs but not CD4+GATA3+ memory TH2 cells in subjects with IgG4-RD. The dominant T cells infiltrating a range of inflamed IgG4-RD tissue sites were clonally-expanded CD4+CTLs that expressed SLAMF7, granzyme A, IL-1β, and TGF-β1. Clinical remission induced by rituximab-mediated B cell depletion was associated with a reduction in disease-associated CD4+ CTLs
Conclusions
IgG4-RD is prominently linked to clonally-expanded, IL-1β, and TGF- β1 secreting, CD4+ CTLs in peripheral blood as well as in inflammatory tissue lesions. These active, terminally-differentiated, cytokine-secreting effector CD4+ T cells are now linked to a human disease characterized by chronic inflammation and fibrosis.
Keywords: Fibrosis, IgG4-related disease, IgG4, CD4+ cytotoxic T cells, TH2 cells, rituximab, IL-1β
Introduction
IgG4-related disease (IgG4-RD) is a chronic inflammatory syndrome whose pathogenesis is poorly understood. This disease can affect virtually every organ system of the body and is characterized by tumefactive lesions, storiform fibrosis, obliterative phlebitis and the presence of IgG4 secreting plasma cells in affected tissues 1–3. IgG4 itself is generally considered to be a non-inflammatory immunoglobulin due to its limited ability to fix complement and bind activating Fc receptors 4, 5. There is very limited evidence that the autoantibodies described so far are of the IgG4 subclass and it is unclear whether they are involved in disease pathogenesis 6. On the other hand, T cells are the most abundant cells in the lymphoplasmacytic infiltrate in IgG4-RD lesions and are thought to be the drivers of IgG4-RD pathogenesis2, 3. The analysis of circulating TH1 and TH2 cells has led to conflicting results in IgG4-RD subjects. One study reported a TH1 skew in peripheral blood T cells in autoimmune pancreatitis while other studies on IgG4-related sialoadenitis showed an increase in cells expressing Th2 cytokines in peripheral blood 7–10. Some TH2 type cytokines as well as M2 macrophages have been found in IgG4-RD lesions suggesting that this disease may be caused by TH2 cells 11. We have recently reported that atopic manifestations are seen in about 40% of IgG4-RD subjects, a proportion that is within the range seen in the population at large 12. We also performed studies on GATA-3 expressing circulating TH2 cells in IgG4-RD subjects. Circulating GATA-3+ IL-4, IL-5 and IL-13 secreting TH2 cells were only found in IgG4-RD subjects with a history of atopy 13.
Fibrosis is a prominent feature of many chronic inflammatory disorders, including rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus and IgG4-related disease among others. Many distinct triggers are known to contribute to fibrosis, but a detailed understanding of this pathological process has proved elusive 14. Both innate and adaptive immune mechanisms may drive fibrotic responses 15 but it is unclear what constitutes the tipping point between physiological wound healing and pathological fibrosis. Activated macrophages secrete cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) that activate fibroblasts and induce the overproduction of extracellular matrix (ECM) proteins 15. In the murine model of bleomycin-induced pulmonary fibrosis, inflammasome activation and IL-1R/MyD88 signaling are critical aspects of the profibrotic activity of IL-1β 16. Transient expression of IL-1β alone in the rat lung has been shown to result in tissue damage and progressive fibrosis 17. Transgenic overexpression of IL-1β in the murine pancreas also results in a fibrotic pancreatitis 18.
Numerous studies have implicated the type 2 cytokines, IL-4, IL-5 and IL-13, in driving progressive fibrosis 19. IL-4 can directly induce mouse and human fibroblasts to synthesize ECM proteins 19–21. IL-13, secreted by TH2 cells, mediates fibrotic remodeling in a TGF-β1 dependent or independent manner in experimental lung fibrosis, and may contribute to the pathogenesis of idiopathic pulmonary fibrosis, systemic sclerosis, dermatitis induced skin fibrosis and liver fibrosis associated with persistent infections 22–27. M2 macrophages that have been triggered by IL-4 and IL-13 can induce other cells to produce IL-4, IL-10, IL-13 and TGF-β1 and thus contribute to fibrosis. M2 macrophages involved in wound healing also secrete large amounts of TGF-β1 28, a cytokine that has also been linked to the genesis of fibrosis. Although fibrosis is generally linked to what is presumably the uncontrolled activity of TH2 cells, M2 macrophages and fibroblasts, the prominent role in fibrosis of IL-1β, a cytokine typically made by M1 macrophages, suggests that such TH2 biased models may be applicable to a subset of fibrotic diseases. The presumption that a number of fibrotic diseases have an underlying TH2 origin may be an oversimplification of an otherwise complex and poorly understood pathogenic processes.
Because expansions of circulating TH2 cells were not observed in IgG4-RD subjects without atopy, we undertook an unbiased approach using next-generation sequencing to study the clonality of effector CD4+ T cells in patients with active untreated IgG4-RD. Our goal was to identify any specific CD4+ effector sub-population that is clonally expanded in subjects with this disease. We report here that CD4+ T cells with a cytotoxic T lymphoid phenotype are clonally expanded in IgG4-RD subjects. Further these unusual CD4+ T cells can synthesize and secrete IFN-γ, IL-1β and TGF- β1 following TCR or TLR triggering. In addition to these cells being expanded in the blood, they are also found as the dominant CD4+ T cell population within diseased tissue sites where they also synthesize cytokines. Their numbers decline concomitant with a clinical response to rituximab therapy, suggesting a contributory role for these CD4+SLAMF7+ cytotoxic T cells in the pathogenesis of this systemic fibrotic disease. We have also observed CD4+ CTL expansions in a smaller cohort with systemic sclerosis. Our data therefore suggest that IFN-γ, IL-1β and TGF-β1 secreting CD4+ CTLs contribute to the pathogenesis of IgG4-RD and are likely to be of broader relevance in other inflammatory fibrotic disorders.
Materials and Methods
Patients
We evaluated the peripheral blood from 101 IgG4-RD patients and selected tissue samples from a subset of those followed at the Massachusetts General Hospital (MGH). This study was approved by the Partners Institutional Review Board. All subjects provided written informed consent. Data pertaining to demographics, prior treatment, and laboratory findings at baseline evaluations were derived from MGH Autoimmune Disease Center of Excellence for IgG4-RD and the medical record. All patients had biopsies from at least one organ that were reviewed and confirmed at our center. For certain analyses, only patients with active, untreated disease at sampling were included. Confirmation of the IgG4-RD diagnosis was predicated upon both specific histopathologic features and an increased number of IgG4+ plasma cells (or increased IgG4+/IgG+ ratio) in affected tissues 29. Data from IgG4-RD patients were compared with 35 healthy controls (age 32–70 years).
Tissue sections from 5 IgG4-related dacryoadenitis and sialadenitis (i.e., IgG4-related Mikulicz disease) subjects (mean age = 68.6 ±4.12) and 5 Sjögren's syndrome patients (mean age = 50.8 ± 9.14) obtained from Department of Oral and Maxillofacial Surgery, Kyushu University Hospital, Fukuoka, were also studied.
A subset of 74 IgG4-RD patients that met the definitions of the European Academy of Allergy and Clinical Immunology30 were categorized based on their atopic history (Supplementary table 1). Seventeen patients with systemic sclerosis (age 24–85 yrs, median = 51 yrs; Supplementary table 2) were also chosen for this study.
Flow cytometry methods
Please see Appendix for detailed laboratory methods. Briefly, flow cytometry was performed by incubating cells in staining buffer (Biolegend) containing optimized concentrations of fluorochrome-conjugated antibodies. Surface staining was performed at 4°C for 30 minutes. For intracellular staining of transcription factors (T-bet, GATA-3, and Foxp3), cytokines (IFNγ and IL-4) as well as cytolytic molecules (granzyme B and perforin) cells were fixed and permeabilized with the Foxp3-staining kit (eBioscience) according to the manufacturer's guidelines. Cells were then stained in permeabilization buffer at 4°C for 45 minutes. Stained samples were analyzed on a BD LSR II and sorted on a BD FACSAria II (BD Biosciences).
Methods for next generation sequencing
Next-generation sequencing analysis of the TCR Vβ repertoire was undertaken using the ImmunoSeq® platform (Adaptive Biotechnologies), designed to target an output of 200,000 assembled output sequences 31, 32.
Gene expression analysis
The nCounter® human immunology panel (NanoString Technologies) was used to quantify the gene expression of effector/memory CD4+ T cells. Targets were reverse-transcribed and pre-amplified for 14 cycles using the standard multiplexed target enrichment protocol for 458 immunology-related target genes, previously validated to yield a linear response. The amplified products were hybridized in solution to color-coded nCounter probes, captured on an nCounter Cartridge for high-resolution digital scanning, and analyzed on the GEN2 Digital Analyzer. Detailed methodology is included in Supplementary Appendix 1 at www.jacionline.org
Results
Clinical data
Clinical data on the 101 patients with IgG4-RD are shown in Table 1. Of the 101 patients, 61.4% were male and 75% were of European descent. The average age of disease onset was 51.63 years (± 15.3). About 53.5% patients had elevated serum IgG4 levels at baseline. Our IgG4-RD patient cohort represented untreated patients with diverse presentation of the disease with some patients showing a localized single organ disease (like salivary gland involvement and lung disease) while others showed a more systemic multi-organ disease (pancreas, kidney, lung, skin etc). As reported earlier, around 45% of IgG4-RD patients had a history of atopy33 (Supplementary table 1).
Table I.
Overview of IgG4-RD subjects studied
| Mean age at initial evaluation (mean +/− SD) | 56.31 years (+/− 13.9) |
| Mean age at disease onset (mean +/− SD) | 51.63 years (+/− 15.3) |
| % Male | 61.4% |
| Race/Ethnicity | |
| % White | 75% |
| % Black | 6% |
| % Asian | 11% |
| % Hispanic | 6% |
| % Middle Eastern | 2% |
| Serum IgG4 (Median, IQR)* | 147.5 mg/dL (60.3 – 387) |
| % with Elevated Serum IgG4 at Baseline* | 53.5% |
| Number of organs affected (mean +/− SD) | 2.3 (+/− 1.4) |
| Organ Involvement | |
| % with type 1 IgG4-related pancreatitis | 18.8% |
| % with Submandibular/Parotid Gland Disease | 38.6% |
| % with orbital disease*** | 19.8% |
| % with lymphadenopathy | 34.7% |
| % with retroperitoneal fibrosis | 14.9% |
| % with tubulointerstitial nephritis | 11.9% |
| % with pulmonary disease | 19.8% |
Among those with active disease
Including lacrimal gland involvement (dacryoadenitis)
Effector memory T cells with cytolytic and myeloid features are expanded in IgG4-RD
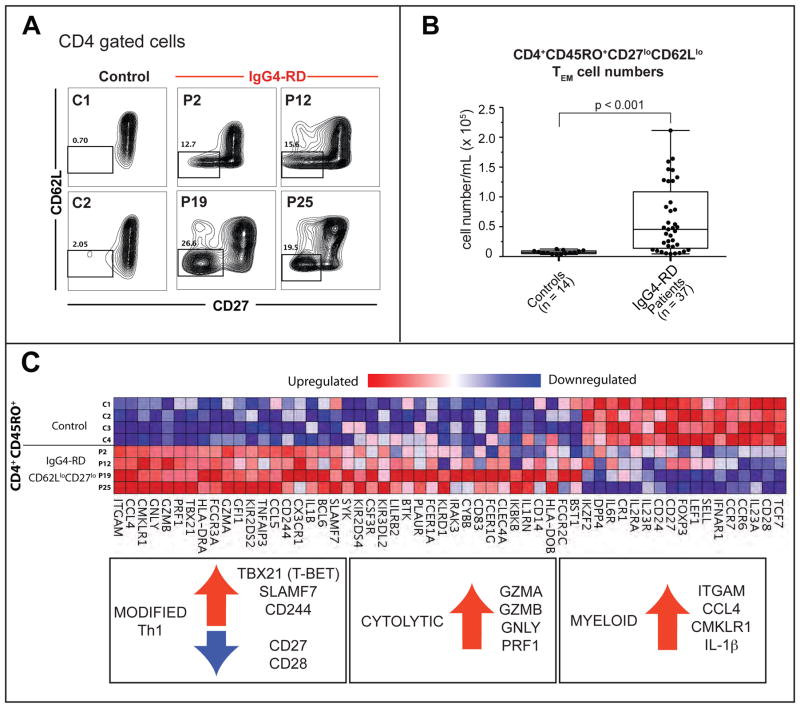
We have previously reported that Th2 cells are seen in the circulation only in a subset of IgG4-RD patients with concomitant apotic disease13. We corroborated these findings on atopic and non-atopic IgG4-RD subjects in this larger cohort of IgG4-RD patients (Fig E1). We sought to investigate IgG4-RD subjects with active disease in an unbiased way by looking for the presence of CD4+ T-cell populations that have an effector/memory phenotype presumably resulting from persistent antigenic stimulation 34, 35. We initially performed in-depth studies on four active, untreated IgG4-RD subjects. Although all four had biopsy-proven IgG4-RD, their clinical features differed substantially. P2 had lung and kidney involvement, P12 presented with submandibular gland disease with lymph node involvement, P19 had lesions in the pancreas and submandibular glands and also had retroperitoneal fibrosis, and P25 had widespread disease with involvement of the lacrimal glands, the parotid, sublingual and submaxillary glands, the kidney, lungs, skin and prostate. We observed a striking increase in the proportion and number of CD4+ CD27loCD62Llo T cells in all four patients (Fig 1, A). CD4+ CD27loCD62Llo cells are all CD45RO+ (Fig E2) and represent CD4+ T effector/memory (TEM) cells, which presumably arise from persistent exposure to auto-antigens or environmental antigens34, 36. The presence of regulatory T cells in tissue lesions has been documented in subjects with IgG4-related pancreatitis, sialadenitis and cholangitis 7, 9, 36. However, we observed only a marginal increase in circulating regulatory T cells that were identified as CD4+CD45RO+CD39+CD25+ Foxp3+ cells in our cohort of IgG4-RD subjects; this difference was not statistically significant when compared to controls (Fig E3). Given the significant increase of CD4+ TEM cells in IgG4-RD patients compared to healthy controls (Fig 1, B), we decided to characterize these cells further and investigate their abundance and potential pathogenic role in IgG4-RD.
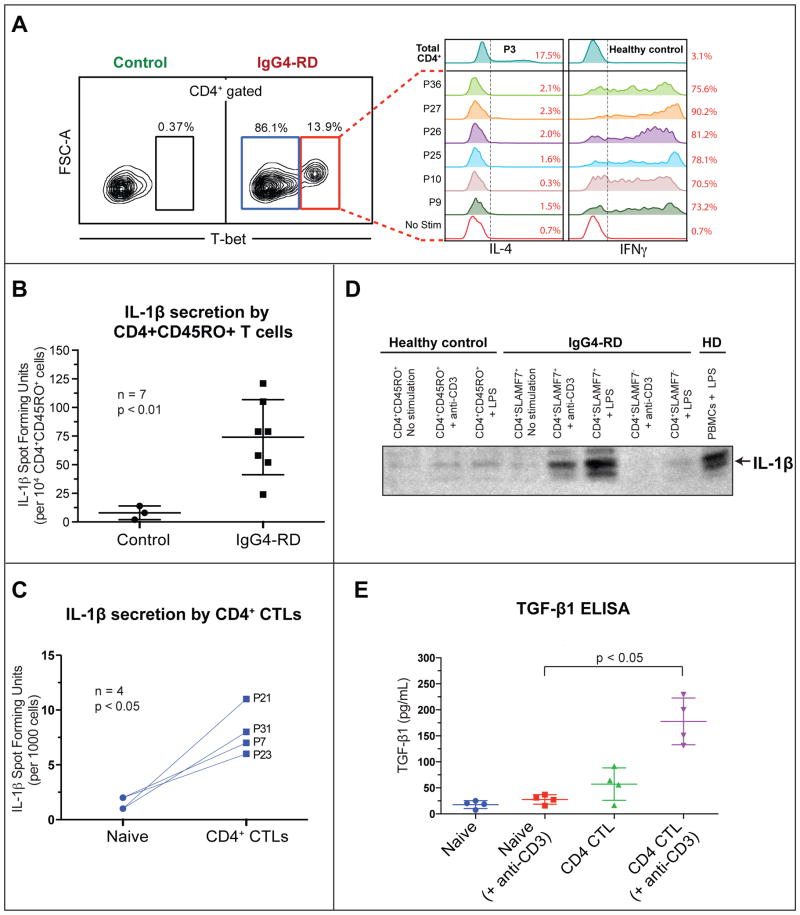
Figure 1. Expansions of TEM cells with a cytolytic phenotype in IgG4-RD.
(A) CD4+CD62LloCD27lo TEM cells in two non-atopic (P2 & P19) two atopic (P12 & P25) IgG4-RD patients and 2 representative healthy controls.
(B) CD4+CD45RO+CD62LloCD27lo TEM cells in peripheral blood of IgG4-RD subjects. Boxplot displays the 25th to 75th percentiles. P values are based on the Mann-Whitney test.
(C) Heat map depicting differentially expressed immune-related genes in TEM cells from four IgG4-RD patients compared to CD4+CD45RO+ T cells from four healthy controls. Upregulated genes representing modified Th1, cytolytic and myeloid signatures are highlighted.
We profiled the expression of a selected panel of genes in this expanded pool of CD4+ TEM cells in the four patients described above. Similar gene expression profiles were seen in all 4 patients (Fig 1, C). A modified Th1 gene signature was observed, with increased expression of the transcription factor T-bet along with co-expression of genes encoding two SLAM family members, SLAMF7 and 2B4. A number of genes encoding cytolytic proteins including perforin, granzyme A, granzyme B, and granulysin were also expressed at high levels. Moreover, these T cells exhibited a distinct myeloid signature with increased expression of genes encoding CD11b, CMKLR1, IL-1β, and CCL4.
Disease-related TEM cells are functional cytolytic cells
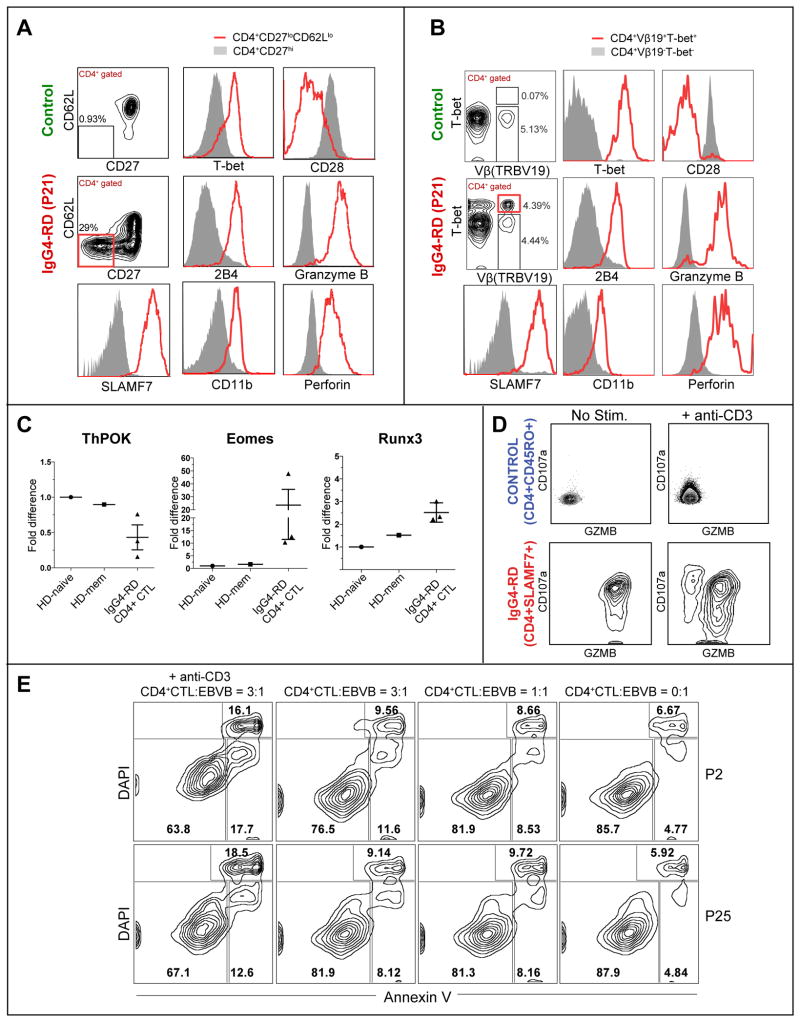
The expression of many of the differentially expressed genes in TEM cells was validated at the protein level using flow cytometry and a large proportion of the expanded CD4+ TEM cells in PBMCs of disease subjects co-expressed SLAMF7, perforin, granzyme B and T-bet (Fig 2, A). The above markers were also found to be co-expressed in a single expanded clone identified using the corresponding TCR Vβ-specific antibody, suggesting that the expanded clones represent a uniformly differentiated sub-population that can be tracked by the expression of SLAMF7 and T-bet (Fig 2, B). These cells appear to be phenotypically identical to previously reported CD4+CTLs seen in chronic viral infections11, 37. These clonally expanded CD4+CTLs maintained their phenotype in vitro when cultured in the presence of anti-CD3/anti-CD28 beads and recombinant human IL-2 (20 ng/mL) (Fig E4).
Figure 2. Expanded TEM cells in IgG4-RD subjects exhibit a functional cytolytic profile.
(A & B) Key hits from the gene expression analysis from Fig. 1 were validated using flow cytometry in TEM cells (A) and using TCR Vβ-specific antibodies (B).
(C) Levels of ThPOK and Runx3 in CD4+SLAMF7+ CTLs from IgG4-RD subjects and CD4+CD45RO+ T cells in healthy controls. Error bars show SEM. P values are based on the Mann-Whitney test.
(D) Granzyme B and CD107a staining on CD4+ CTLs from an IgG4-RD patient, before and after stimulation with anti-CD3 .
(E) Cytotoxicity of in vitro expanded CD4+ CTLs derived from two subjects (P2 and P25) against an allogeneic EBV-transformed B cell target cell line, measured after 12 hours of co-culture with or without anti-CD3 (10 μg/mL) at varying CD4+ CTL: target ratios. The data are representative of two separate experiments done on 4 patients.
The CD4+ CTLs seen in IgG4-RD subjects bear a striking resemblance to cytotoxic CD4+ T lymphocytes reported in mice in the context of chronic antigenic stimulation 38. The development of these cells in mice is associated with the gain of Eomes and Runx3 and the loss of ThPOK expression 38. Like the murine CD4+ CTLs, CD4+SLAMF7+ T cells from IgG4-RD patients exhibited decreased levels of ThPOK and increased expression of Runx3 when compared to CD4+CD45RA+ naïve cells and CD4+CD45RO+ memory cells from healthy controls (Fig 2, C). These cells also express surface CD8α as has been reported in mice (Fig E5) 38.
These CD4+ SLAMF7+ T cells cells have potent cytolytic function; upon in vitro stimulation with anti-CD3, these cells undergo degranulation as inferred from the surface expression of CD107a (Fig 2, D) and exhibit cytotoxic activity against allogeneic EBV-transformed B cell targets (Fig 2, E and Fig E6).
Circulating CD4+SLAMF7+ CTLs exhibit large clonal expansions while CD4+GATA3+ TH2 cells in IgG4-RD subjects are clonally diverse
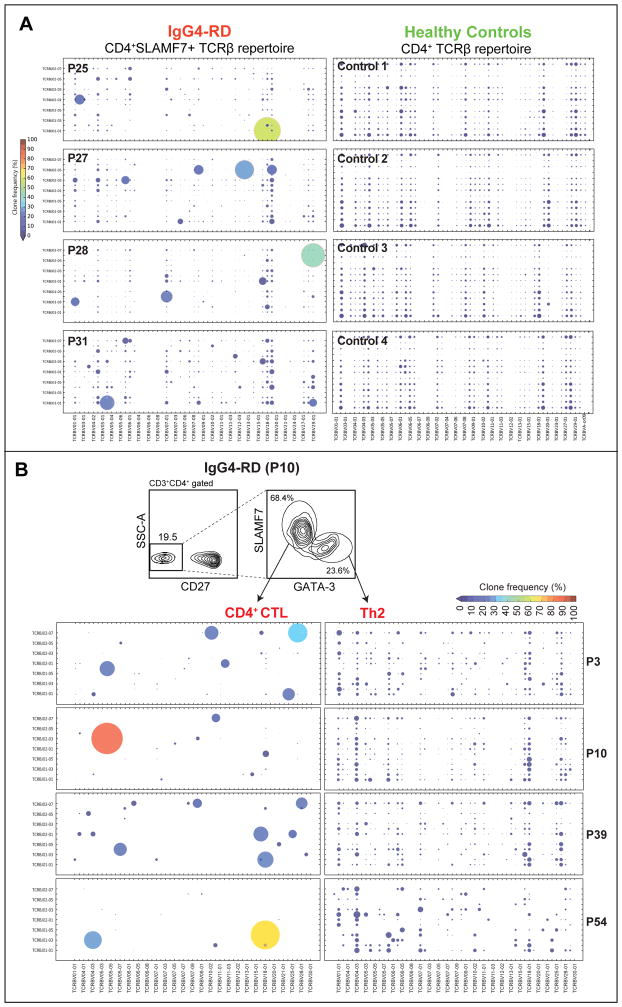
In order to identify potentially pathogenic subsets of CD4+ TEM cells that were clonally expanded in response to a potential antigen, we analyzed their TCRβ chain gene rearrangements using next-generation sequencing. We initially analyzed the TCRβ chain gene repertoire of CD4+ CTLs from four untreated subjects with active, biopsy-proven IgG4-RD with distinct clinical features. We found that CD4+ CTLs were oligoclonally expanded, typically with a single dominant clone representing 15% to 50% of all the in-frame V-D-J rearrangements detected (Figs 3, A and B). Several minor clones were also seen in each patient. In contrast, the repertoire of CD4+ T cells from 4 healthy controls analyzed was highly diverse and no dominant clones were observed in these individuals (Fig 3, A and Fig E7, A). The Vβ-Jβ gene segment usage of the most expanded clones was not identical across IgG4-RD subjects and there were no clones with shared CDR3 sequences across individuals; disease subjects have diverse MHC class II alleles (data not shown). The TCR Vβ gene usage in a subset of expanded CD4+ CTL clones as determined by next-generation sequencing was validated using TCR Vβ-specific antibodies in all 3 subjects for whom Vβ-specific antibodies could be obtained (Fig E7, B and C).
Figure 3. The TCR Vβ repertoire of expanded TEM cells in IgG4-RD.
(A) TCRβ repertoire of the expanded circulating TEM subset in 4 IgG4-RD subjects (P31, P28, P27 and P25) and from total CD4+ T cells from four healthy controls, represented as bubble charts, where the size and color corresponds to the frequency of the observed Vβ-Jβ rearrangements.
(B) TCRβ repertoire of expanded circulating CD4+GATA-3+ TH2 cells in four IgG4-RD subjects with an atopic history (P3, P10, P39 & P54), contrasted with the expanded CD4+SLAMF7+ CTLs from the same individuals. See legend to Fig.3A for details.
In four atopic IgG4-RD subjects (P3, P10, P39 and P54), in whom circulating CD4+GATA3+ memory Th2 cells as well as CD4+SLAMF7+ CTLs were expanded, we performed massively parallel sequencing of the T cell receptor (TCR)-Vβ chain to investigate clonal diversity of these CD4+ T cell subsets. CD4+SLAMF7+ CTLs were highly oligoclonal with one or a few expanded clones representing up to ~80% of the productive sequencing reads (Fig 3, B). In contrast, when analyzing simultaneously expanded GATA-3+ CD4+ T cells, even the most expanded clones represented < 2% of the sequencing reads in all four subjects. These data strongly imply that CD4+SLAMF7+ CTLs have expanded in response to a specific causal antigen, and may thus have a direct role in disease pathogenesis. In contrast, the repertoire of TH2 phenotype cells may reflect the accumulated T cell memory acquired over time against a wide range of environmental allergens.
CD4+SLAMF7+ CTLs can be identified both in the circulation and in fibrotic lesions
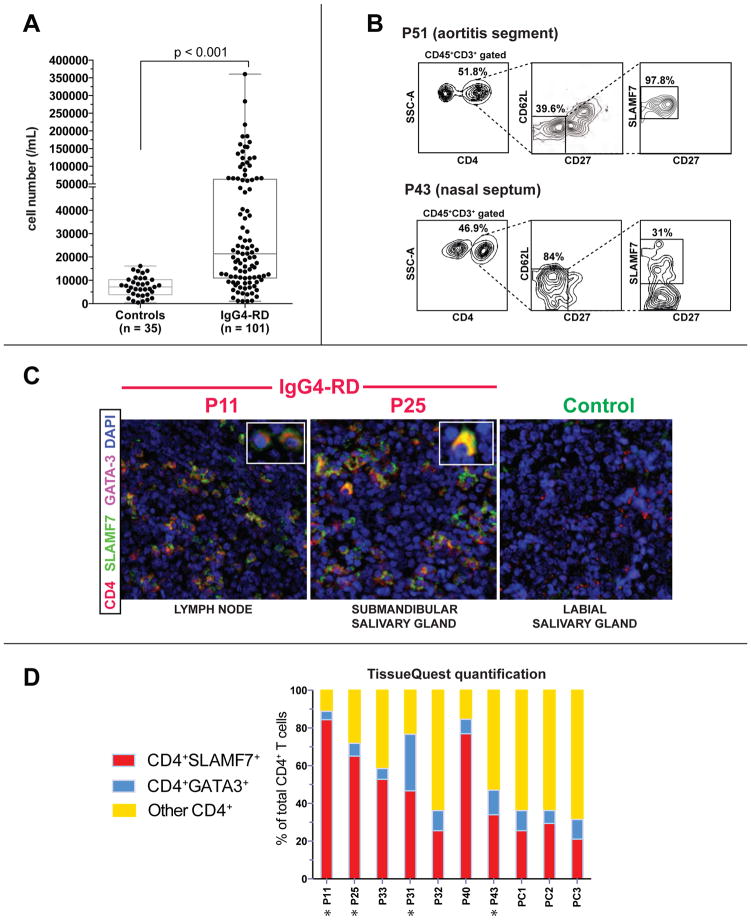
CD4+SLAMF7+ CTLs cells were elevated in the peripheral blood of 101 IgG4-RD subjects studied compared to healthy controls (p < 0.01) (Fig 4, A & Fig E8). Unlike circulating GATA-3+ TH2 memory cells, CD4+ SLAMF7+ CTLs were abundant both in patients with and without atopy (Fig E9). A segment of diseased aorta obtained at autopsy from a previously described patient (P51), who succumbed to IgG4-RD showed the presence of CD4+SLAMF7+ cells in the vessel wall by flow cytometric analysis (Fig 4, B) 39. Similarly, CD4+ SLAMF7+ cells were also detected in the biopsy of the involved nasal septum from a patient (P43) with IgG4-related midline destructive lesion (Fig 5, B)40.
Figure 4. Expansion of CD4+SLAMF7+ CTLs in tissue lesions of IgG4-RD subjects.
(A) Expansion of CD4+SLAMF7+ CTLs in 101 IgG4-RD subjects.
(B) CD4+CD62LloCD27loSLAMF7+ CTLs in the aortic wall of a subject with IgG4-related aortitis (P51) and the involved nasal septum of a subject with IgG4-RD (P43).
(C) Immunofluorescence staining of CD4+SLAMF7+ CTLs and CD4+GATA-3+ Th2 cells in the affected tissues of IgG4-RD subjects (lymph node biopsy from P11 and salivary gland biopsy from P25). CD4 (red), DAPI (blue), GATA-3 (magenta) and SLAMF7 (green) staining are shown.
(D) Quantification of CD4+SLAMF7+, CD4+GATA-3+ and CD4+SLAMF7-GATA-3- cells in tissue biopsies from 10 IgG4-RD patients (atopic patients are marked with asterisks).
Figure 5. Disease associated CD4+ CTLs secrete IFNγ, IL-1β and TGF-β1 upon in vitro restimulation.
(A) Intracellular staining for IFN-γ and IL-4 in restimulated CD4+ CTLs from seven IgG4-RD patients. The empty histogram (red) depicts an unstimulated control.
(B & C) ELISPOT detection of the frequency of IL-1β producers among re-stimulated CD4+CD45RO+ T cells from seven IgG4-RD subjects compared to healthy donors (error bars show SEM, unpaired t-test) (A) and CD4+ CTLs from four IgG4-RD subjects compared to naïve CD4+CD45RA+ T cells from the same individuals (p< 0.05, paired t-test) (B).
(D) Western Blot detection of IL-1β from culture supernatants of in vitro expanded T cells, maintained in IL-2 for two weeks (10 ng/mL). Supernatants from CD4+CD45RO+ T cells from a healthy donor and CD4+CD45RO+SLAMF7+ or CD4+CD45RO+SLAMF7− T cells from an IgG4-RD subject were used without any stimulation (US), with 5 μg/mL LPS or 3μg/mL anti-CD3. LPS stimulated PBMCs from a healthy donor were used as a positive control.
(E) Detection of TGF-β1 production among naïve CD4+ cells and CD4+SLAMF7+ CTLs from four IgG4-RD subjects by ELISA, with or without in vitro re-stimulation (with 3 μg/mL anti-CD3). Error bars show SEM. P values are based on the Mann-Whitney test.
We quantitatively analyzed tissue sections from the affected organs of 10 IgG4-RD patients for whom well-preserved biopsies were available, for the presence of GATA3+ TH2 cells and SLAMF7+ CD4+ CTLs. The organs studied included submandibular glands (2 patients), lymph nodes (3 patients), and nasal palate/septum, retroperitoneal masses, kidney and lung (1 patient each). In these affected organs, infiltrating CD4+SLAMF7+ CTLs vastly out-numbered the sparse CD4+GATA3+ TH2 T cells in all the IgG4-RD subjects studied, including both atopic and non-atopic individuals (Fig 4, C and D). These data suggest that TH2 cells are unlikely to be the primary drivers of pathogenicity in IgG4-RD while CD4+ CTLs appear, by all criteria examined, to be intimately linked to the pathogenesis of this disease. Abundance of CD4+SLAMF7+ CTLs in IgG4-RD lesions was also confirmed in the affected organs of 20 IgG4-RD subjects (Fig E10, A). In one subject with IgG4-RD (P25) the expanded Vβ17+ TEM cell clone that was dominant in the circulation (Fig 2, A) was also found to be abundant in an inflamed tissue site (Fig E10, B). CD4+SLAMF7+ CTLs also expressed granzyme A in IgG4-RD disease lesions (Fig E11). These data suggest that expanded CD4+ CTLs are abundant in involved tissues and may be directly involved in the pathogenesis of IgG4-RD.
CD4+SLAMF7+ CTLs secrete profibrotic cytokines: IFNγ, IL-1β and TGF-β1
In order to understand the functional behavior of the disease-related CD4+ CTLs, peripheral blood mononuclear cells were briefly re-stimulated with PMA and ionomycin to mimic TCR signaling, followed by intracellular staining for T-bet, IFN-γ and IL-4. Since T-bet is a lineage-determining transcription factor that is exclusively expressed in all the clonally expanded CD4+ CTLs in IgG4-RD subjects (Fig 2, A and B), we identified re-stimulated CD4+ CTLs among total CD4+ lymphocytes using T-bet staining. We did not identify any T-bet+ cells that lacked the expression of perforin or granzyme B, and which could therefore represent bona fide Th1 cells, in these patients (Fig 2, A and B). Re-stimulated CD4+ CTLs cells from all IgG4-RD patients made large amounts of IFN-γ but no IL-4 (Fig. 5A). Clonally expanded CD4+ CTLs, identified using specific TCR Vβ antibody staining, consistently expressed IFN-γ but not IL-4 upon re-stimulation (Fig E12).
IL-1β was the most prominent cytokine at the mRNA level in the expanded TEM population. We also analyzed IL-1β at the protein level along with other cytokines more typically seen in effector T cells. Human CD4+ T cells are known to express pro-IL-1β that gets processed and secreted as bioactive IL-1β upon LPS stimulation 41. We sought to determine if CD4+SLAMF7+ cells release the processed form of IL-1β following stimulation. Upon in vitro re-stimulation with PMA and ionomycin, IL-1β producing cells were more abundant in flow-sorted CD4+CD45RO+ T cells from IgG4-RD subjects as detected by ELISPOT analysis compared to CD4+CD45RO+ T cells from healthy controls (Fig 5, B). Flow-sorted CD4+ CTLs cells contained more IL-1β producing cells compared to naïve CD4+CD45RA+ T cells from four IgG4-RD subjects tested (Fig 5, C). These cells could be expanded in vitro and maintained in IL-2 with intermittent TCR stimulation without a loss of phenotype (Fig E4). The expanded CD4+ CTLs retained their SLAMF7 expression and cytotoxic markers and were found to secrete the processed form (17 kDa) of IL-1β upon re-stimulation with either anti-CD3 or LPS as determined by Western blot analysis of culture supernatants (Fig 5, D). The amount of IL-1β secreted was comparable to the amount secreted by myeloid cells exposed to LPS.
Given the role of TGF-β1 in many fibrotic diseases, we examined the ability of CD4+ CTLs to make TGF-β1 ex vivo as well as at sites of disease. Upon in vitro stimulation, flow sorted CD4+ CTLs secreted large amounts of TGF-β1 compared to the naïve CD4+ cells from the same patients (Fig 5, E).
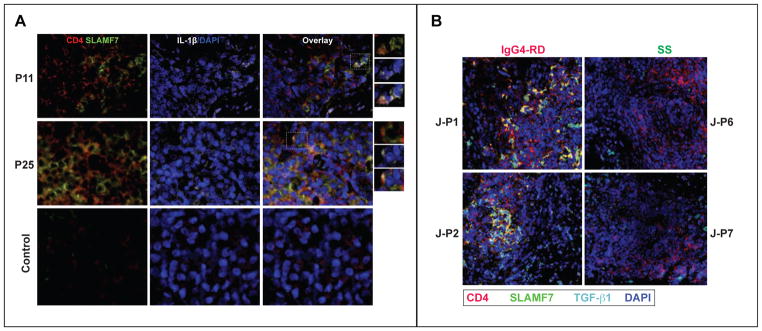
Multi-color immunofluorescence staining of the affected organs of 22 IgG4-RD subjects showed high expression of IL-1β in a large proportion of CD4+ SLAMF7+ cells (Fig 6, A, and Fig E13). Furthermore, a large number of CD4+ TGF-β1+ T cells were detected in biopsies at the site of IgG4-RD lesions (Fig 6, B and Fig E14). These data suggest that CD4+ CTLs have a pro-fibrotic potential and may be the drivers of fibrosis in the IgG4-RD lesions.
Figure 6. Expansion of IL-1β and TGF-β1 secreting CD4+ CTLs in IgG4-RD lesions.
(A) Immunofluorescence staining of IL-1β-producing CD4+ SLAMF7+ cells in the tissues of 2 IgG4-RD subjects (lymph node biopsy from P11 and salivary gland biopsy from P25) and a healthy control subject (labial salivary gland biopsy). CD4 (red), SLAMF7 (green) DAPI (blue) and IL-1β (white).
(B) Immunofluorescence staining of TGF-β1-producing CD4+ SLAMF7+ cells in the tissues of 2 IgG4-DS and 2 Sjögren’s syndrome subjects (submandibular salivary gland biopsies). CD4 (red), Granzyme A (green) DAPI (blue) and IL-1β (cyan) staining are shown.
Circulating CD4+SLAMF7+ CTLs diminish in IgG4-RD subjects following rituximab therapy
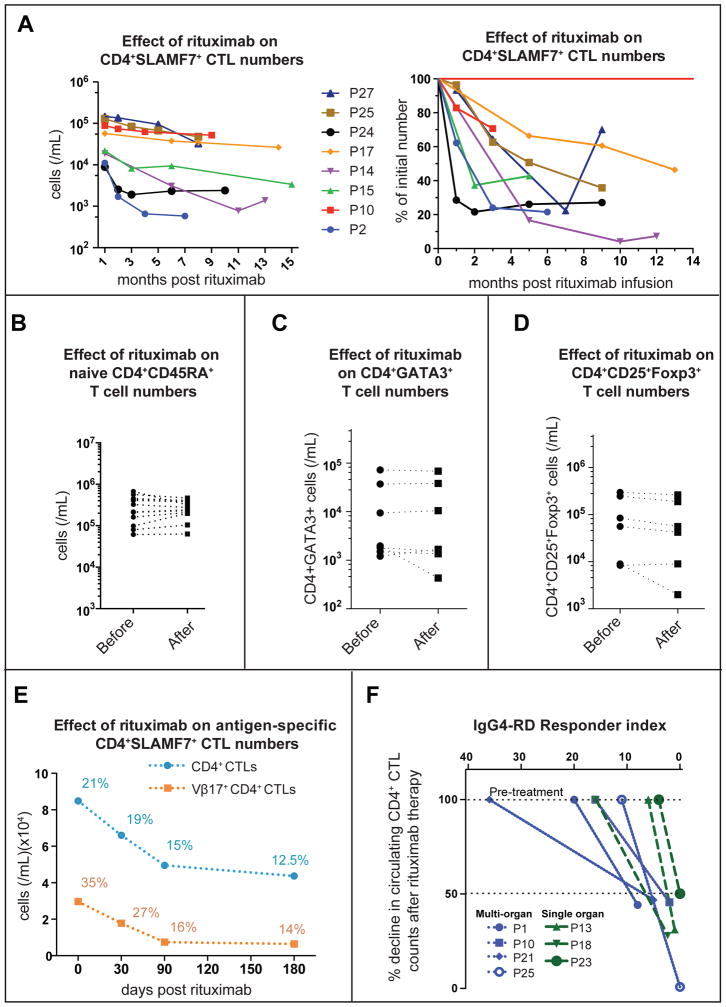
Therapeutic B-cell depletion with rituximab (an anti-CD20 monoclonal antibody) generally results in clinical improvement in IgG4-RD 42, 43. CD19+ B cells decline dramatically after rituximab therapy, followed by a clinically apparent reduction in disease activity as measured using the IgG4-RD Responder Index 44. Interestingly, a significant decrease in the percentages and numbers of CD4+SLAMF7+ CTLs was also observed up to 12 months after rituximab treatment while the number of naive CD4+CD45RA+ T cells remained stable (Fig 7, A and B).
Figure 7. Rituximab-mediated depletion of CD4+ CTLs.
(A) Decline in CD4+ SLAMF7+ CTLs (n = 8 subjects) over 6–14 months following rituximab infusion shown as a change in cell counts and proportion.
(B) Effect of rituximab on CD4+ CD45RA+ naïve T cell counts in the peripheral blood of IgG4-RD patients.
(C & D) Effect of rituximab on CD4+GATA-3+ TH2 cells and CD4+CD25+Foxp3+ cells in the peripheral blood of IgG4-RD patients 90–120 days after rituximab therapy.
(E) Decline in the number and proportion of an expanded CD4+ CTL clone tracked using a TCR-Vβ specific antibody following rituximab therapy in an IgG4-RD subject (P25).
(F) Decline in circulating CD4+ CTL numbers at day 70–95 following rituximab therapy (normalized to the pretreatment levels) is plotted against the IgG4-RD Responder Index, a clinical measure of disease activity.
Rituximab therapy had minimal or no impact on the frequency and number of CD4+GATA-3+ Th2 phenotype cells, or CD4+CD25+Foxp3+ T regulatory cells in peripheral blood (Fig 7, C and D). The CD4+SLAMF7+ CTL numbers decreased despite the fact that these cells do not express any detectable CD20 on their cell surface (Fig E15). In one serially monitored subject, for whom a TCR Vβ-specific antibody was available, rituximab treatment induced a decline in the Vβ17+ CTL clone as well as of total CD4+SLAMF7+ CTLs (Fig 7, E). A positive clinical response to rituximab therapy was accompanied by a greater than 50% reduction in circulating CD4+SLAMF7+ CTL numbers (Fig 7, F). These data provide additional circumstantial evidence for a pathogenic role for IL-1β secreting CD4+ CTLs in IgG4-RD.
Discussion
One of the challenges in human immunology is the difficulty in advancing from correlation to causation. Our studies reveal that a poorly-studied human CD4+ T cell population that secretes pro-fibrotic cytokines is expanded in subjects with IgG4-RD. The demonstration of the clonal expansion of these T cells by next generation sequencing, their localization and in situ re-activation at sites of disease, and their decline following successful therapy provide strong evidence for a likely causal role for IL-1β, IFN-γ and TGF-β1 secreting CD4+ cytotoxic T cells in IgG4-related disease, and likely other fibrotic inflammatory disorders. Such an approach, determining which lymphocytes are clonally expanded and infiltrate tissue sites of disease, may serve as a template for implicating specific adaptive immune cells in disease processes.
This study illustrates several novel and clinically relevant findings. Oligoclonal expansions of circulating CD4+SLAMF7+ CTLs are seen in IgG4-RD patients, especially during active disease. These T-cell clones are unusual in terms of their ability to make IL-1β, TGF- β1 and IFN-γ; IL-1β is a cytokine typically thought to be produced by non-lymphoid cells 45. These expanded T cells were also found in affected tissues providing strong evidence for their potential role in disease pathogenesis. The production of large amounts of IL-1β by these T cells following brief re-stimulation, and the expression of pre-formed cytotoxic mediators suggests that the CD4+SLAMF7+ CTL population represents an unusual and not easily categorized subset of human CD4+ effector T cells that potentially drives the disease process, perhaps in collaboration with other T cells. The secretion of processed IL-1β suggests that inflammasome activation occurs in activated CD4+ CTLs. The mechanism of TGF-β1 induction in CD4+ CTLs and the presumed processing of secreted TGF-β1 in disease tissues remains to be investigated.
Fibrosis is an essential feature of the histopathology of IgG4-RD 46, but the etiology of the fibrosis in this disease has not been clear. IgG4 antibodies are generally considered to be non-inflammatory because they do not efficiently engage activating Fc receptors or complement and may be functionally monovalent in vivo 47. We have observed active disease in a number of subjects with relatively low levels of plasma IgG4 but a clear expansion of the CD4+SLAMF7+ CTL population. The contribution of IgG4 antibodies to disease pathogenesis is therefore unclear. It is possible that the IgG4 response seen in disease represents an exaggerated but ineffectual attempt to sequester antigens and dampen inflammation.
Our observations show that clonally-expanded CD4+ CTLs that express granzyme B, perforin, IFN-γ, IL-1β and TGF-β1 are prominent in this disease and a number of prior observations suggest that they may induce the fibrotic pathology seen in IgG4-RD. Transient expression of IL-1β alone in the rat lung has been shown to result in tissue damage and progressive fibrosis 17. Transgenic overexpression of IL-1β in the murine pancreas results in a fibrotic pancreatitis 18. IL-1R1/MyD88 signaling and the inflammasome are essential drivers of the fibrotic process in the widely studied murine model of pulmonary fibrosis induced by bleomycin 16. IFN-γ has been shown to contribute to fibrosis in a murine thyroiditis model 48. Indeed IL-1β induced fibrosis in mice has been shown to be dependent on IL-17A and IFN-γ. These CD4+ CTLs observed in IgG4-RD represent an abundant source of IL-1β and TGF-β1 in the tissue lesions of IgG4-RD, thereby potentially driving fibrosis.
In addition to our studies on IgG4-RD, studies on smaller numbers of subjects with systemic sclerosis suggest that CD4+Tbet+SLAMF7+ CTLs contribute to the pathogenesis of a broader range of fibrotic inflammatory diseases (Fig E16). The abundance of these cells in subjects with systemic sclerosis, for instance, is similar to that seen in IgG4-RD. CD4+ CD28lo cells, which have been shown to express cytotoxic mediators and IFN-γ in some studies, have previously been identified in idiopathic pulmonary fibrosis, severe rheumatoid arthritis, multiple sclerosis, granulomatosis with polyangiitis (formerly Wegener’s granulomatosis) 49–51 and a small proportion of healthy elderly subjects. The CD4+ SLAMF7+ CTLs that we have identified in IgG4-RD subjects also express reduced levels of CD28 and may be related to these previously described CD4+CD28lo cells. A survey of SLAM family protein expression in systemic lupus erythematosus revealed higher levels of SLAMF7 in some B and T cells, but the cell phenotypes were not characterized further 52.
Many types of fibrosis including murine models of schistosomiasis and bleomycin-induced pulmonary fibrosis have been linked to TH2 cells and TH2 cytokines 19. Although TH2 cells have been implicated in IgG4-RD in some studies 7–9, we have recently demonstrated that TH2 cell expansions are highly correlated with the allergic history of patients. We have not seen a consistent expansion of TH2 effector cells in active IgG4-RD. Instead, we find that IgG4-RD subjects with chronic allergies in addition to fibrotic tumescent lesions have an expansion of both TH2 cells as well as IL-1β producing CD4+ CTLs and clonal expansions of Th2 cells are not seen even in subjects with concomitant allergic disease (Mattoo et al. submitted; accompanying manuscript). CD4+ CTLs in IgG4-RD subjects synthesize IL-1β and TGF-β1 in affected fibrotic tissues, suggesting that fibrotic disease mechanisms that have been elucidated downstream of IL-1β 30 and TGF-β1 3 are likely of importance in IgG4-RD.
We have recently demonstrated marked expansions of IgG4+ plasmablasts in IgG4-RD subjects with active disease 53. Since these plasmablasts express high levels of MHC class II molecules, are depleted by rituximab, and are also present in disease lesions, it is possible that they play an important role in the reactivation of the CD4+CTLs and induce them to either make cytokines including IL-1β and TGFβ1, or to kill parenchymal cells.
The selective decline in CD4+ CTLs, including the expanded clones after rituximab-mediated B-cell depletion, may have broad relevance. These observations imply that in humans, as in rodents, B cells play an important role in the maintenance of effector/memory CD4+ T cells, including disease-associated, “rogue” T-cell clones 54, 55. These findings may explain why rituximab is effective in diseases such as relapsing-remitting multiple sclerosis in which end-organ damage is primarily mediated by autoreactive T cells. B cells that have the unique ability to bind specific auto-antigens can act as potent antigen-presenting cells at low antigen concentrations 56, and may provide efficient access to T-cell epitopes. Thus, it is possible that in T-cell mediated autoimmune disorders that are responsive to rituximab therapy, the effector/memory CD4+ T cell response is maintained by B cells. Alternatively, pathogenic T cells may be dependent upon B cell derived growth factors 54. These possibilities are not mutually exclusive.
The SLAM family is a subset of the immunoglobulin superfamily of receptors. Members of the SLAM family can be involved in both homotypic as well as heterotypic interactions 57, 58. Given the presence of immune-receptor tyrosine based switch motifs (ITSMs) in its cytoplasmic tail, SLAMF7 can lead to activating or inhibitory signaling within the cell, depending on its association with a SLAM associated protein (SAP) family adaptor called EAT2. Both plasmablasts and CD4+ CTLs in diseased tissue express SLAMF7, and it is unclear at this time whether SLAMF7 in CD4+ CTLs participates in homotypic or heterotypic interactions, or whether it transduces activating or inhibitory signals in these cells.
From a therapeutic standpoint, depleting SLAMF7-expressing cells as well as neutralizing IL-1β may represent rational strategies in a range of immune-mediated conditions associated with severe tissue damage and fibrosis. A few biologic agents that target SLAMF7 or IL-1β are already in clinical use or in advanced stages of drug development. A humanized monoclonal antibody directed against the human SLAMF7, elotuzumab has shown promise in patients with advanced multiple myeloma 59. Anakinra, a non-glycosylated recombinant form of the naturally occurring IL-1 receptor antagonist that blocks inflammasome dependent IL-1β signaling has been successfully used in type 2 diabetes, asbestosis and other conditions 60. Canakinumab is a monoclonal antibody that binds to and antagonizes IL-1β and is being studied in a number of clinical trials 61.
In summary, our studies suggest that CD4+ CTLs with a unique hitherto undescribed phenotype expand clonally in the circulation and tissue sites and probably mediate the pathological changes seen in IgG4-RD. These cells elaborate a unique combination of cytokines, some of which are known to contribute to fibrosis in animal models, and the numbers of these cells correlate well with clinical disease activity. Furthermore, therapeutic improvement in IgG4-RD mediated by B cell depletion is linked to a specific reduction of these CD4+ CTLs and not of naive T cells, regulatory T cells or memory T follicular helper cells. Examining subjects with newly diagnosed, untreated and active disease has allowed the identification and characterization of clonally expanded effector T cells linked to disease and to the observation of their attenuation by rituximab. These studies suggest a mechanism by which rituximab mediates clinical improvement in inflammatory disorders that are primarily linked to T cells. Based on our studies, we believe that B cell depletion will also likely prove effective in other immune-mediated disorders involving pathogenic effector T cell subsets that depend upon B cells for antigen presentation or maintenance.
Rituximab infusion of IgG4-RD patients
This study was approved by the institutional review board and informed, written consent was obtained from all subjects referred to or presenting at the rheumatology clinic of the Massachusetts General Hospital. Twelve IgG4-RD patients with active disease were treated with two 1000 mg doses of rituximab, 15 days apart. 15 mL of peripheral blood was collected at initial presentation and each subsequent clinical visit. These patients were longitudinally followed for 9–15 months at the rheumatology clinic. Peripheral blood was collected in EDTA or ACD tubes (BD Vacutainer) and transported to the laboratory for cell isolation on the same day.
Isolation of mononuclear cells
Mononuclear cells were isolated from peripheral blood of IgG4-RD subjects and healthy controls by Ficoll-Paque Plus (GE Healthcare) density-gradient centrifugation following the manufacturer's protocol. To facilitate subsequent analysis of cells in batches, PBMCs were resuspended in fetal bovine serum containing 10% dimethyl sulfoxide and cryopreserved in vapor phase liquid nitrogen.
Immunofluorescence
Anti-human TCRVβ17-PE (clone E17.5F3.15.13, Beckman-Coulter), anti-human CD4-biotin and mouse anti-biotin Alexa fluor 647 (Life technologies), mouse anti-human CD319 (clone 162.1, Biolegend) and goat anti-mouse Alexa fluor 488 (Life technologies), rabbit polyclonal anti-human IL-1β (Clone 3553, Biovision) and goat anti-rabbit Alexa fluor 488 (Life technologies) were used for immunofluorescence detection of CD4+SLAMF7+ cells and IL-1β producing CD4+ cells in deparaffinized sections of tissue biopsies using established protocols.
In addition, three multi-color immunofluorescence staining panels CD4/SLAMF7/GATA-3, CD4/SLAMF7/IL-1β and CD4/ TGFβ1 were devised to measure the frequency and in vivo function CD4+SLAMF7+ CTLs. Detection was performed using mouse or rabbit Superpicture polymer detection kits (HRP; Life Technologies) followed by tyramide signal amplification by Opal 3-plex kit (Perkin Elmer) by following the manufacturer’s protocol. The slides were counterstained with DAPI (Thermo Scientific) and mounted using Vectashield mounting medium (Vector labs). Stained tissues were scanned on TissueFAX (Tissuegnostics) and the frequency for CD4+ cells co-expressing SLAMF7 and IL-1β was calculated using the proprietary TissueQuest software. The anti-IL-1β antibody used recognizes primarily the processed mature form of IL-1β.
Flow cytometric analysis
Fluorescence labeling for flow cytometry was performed by incubating cells in staining buffer (Biolegend) containing optimized concentrations of fluorochrome-conjugated antibodies. Except where indicated, all antibodies were procured from Biolegend. The following monoclonal antibodies were used in this study: anti-human CD19-Pacific Blue (clone HIB19), anti-human CD27-APC (clone O323), anti-human CD38-FITC (clone HIT2), anti-human IgG4 (clone 6025, Southern Biotech), anti-human CD4-PE-Cy7 (clone OKT4), anti-human CD8α-PE, anti-human CXCR5-PE (clone J252D4), CD39 (Clone A1), anti-human CD45RA-PE (clone HI100), anti-human CD45RO-APC (clone UCHL1), anti-human CD62L-FITC (clone DREG56), anti-human CD244-biotin (clone C1.7, eBioscience), anti-human CD20-APC, anti-human CD319 (SLAMF7)-PE (clone 162.1), anti-human CD11b-APC Cy7 (clone ICRF44), anti-human CD28-PerCP Cy5.5 (clone CD28.2), anti-human TCR Vβ23 FITC (clone αHUT7), anti-human TCR Vβ17-PE (clone E17.5F3.15.13, Beckman-Coulter), anti-human TCR Vβ2-PE (clone MPB2D5, Beckman-Coulter), CD39-PE Cy7 (Clone A1, Biolegend) and CD25-APC Cy7 (Clone BC96, Biolegend). A table of concordance between the TCR Vβ gene nomenclatures and IMGT gene names, which is available on the IMGT website (http://www.imgt.org), was used to verify that the appropriate Vβ-specific antibody clones were selected to detect clonally-expanded T-cell clones identified by next-generation sequencing 56.
PBMCs and single cell suspensions from tissue biopsies were reacted with Fc blocking agent (Human FcX, Biolegend). Surface staining was performed using appropriate dilution of antibodies in 12x75mm round bottomed polystyrene tubes in the dark at 4°C. For intracellular staining (T-bet, GATA-3, Foxp3, granzyme B and perforin) cells were fixed and permeabilized with the Foxp3-staining kit (eBioscience) according to manufacturer's guidelines. Cells were then stained in permeabilization buffer with anti-human T-bet PECy7 (clone 4B10, eBioscience), GATA-3 PE-Cy7 (clone L50-823, BD Biosciences), anti-human FOXP3 Alexa Fluor 488 (Clone 206D, Biolegend), anti-human Perforin PE (clone B-D48) and anti-human granzyme B FITC (clone GB11). For the degranulation assay, CD4+SLAMF7+ CTLs were stimulated with 3 μg/mL anti-human CD3 (OKT3) for 4 hours and surface staining for anti-human CD107a (Biolegend) was performed followed by permeabilization and intra-cellular staining for Granzyme B as discussed above.
EBV-transformed B cell lines from an IgG4-RD patient (P46) were used as targets in the allogeneic CTL assay. 5 x 104 EBV-transformed B cells were co-cultured for 12 hours with CD4+ CTLs from two patients at different ratios in presence or absence of anti-CD3. Cells were harvested and surface stained for anti-human CD4 as described above followed by staining with Annexin V-APC in Annexin V binding buffer (15 minutes at room temperature). DAPI was added to the cells at a final concentration of 1 μg/ml. Target cells were gated as CD3-negative and percentage of apoptotic/dead cells were estimated by Annexin V+/DAPI+ gates.
Intracellular staining for cytokines in restimulated T cells
For detecting intracellular levels of cytokines, mononuclear cells were stimulated with 100 ng/mL of phorbol-myristoyl acetate (Sigma-Aldrich) and 100 ng/mL of ionomycin (Invitrogen) in the presence of Brefeldin A (Sigma-Aldrich) for 4 hrs at 37°C. They were subsequently labeled with the LIVE/DEAD* fixable violet viability dye (Invitrogen) in phosphate-buffered saline for 20 minutes and stained for cell surface markers. Cells were then fixed/permeabilized and stained with anti-human IFN-γ APC (clone 4S.B3), anti-human IL-4 Alexa Fluor® 488 (clone 8D4-8) for 45 minutes on ice. Cells were washed two times with permeabilization buffer and once with PBS and acquired/analyzed on a BD LSR II (BD Biosciences). The .fcs files were analyzed using FlowJo software (version 9.6.3, Treestar).
Cell sorting
To preserve cell viability mononuclear cells were stained with relevant cell surface markers in DMEM (Gibco) with ITS+ Universal Culture Supplement (BD Biosciences). For batch sorts, antibody-stained cells were resuspended at ~20 million/mL in HBSS (Gibco) plus 10 mM glucose and sorted on a BD FACSAria II (BD Biosciences). Sorted cells were collected in 5 mL tubes containing 1 mL collection medium (DMEM with 30% FBS) and re-analyzed on the sorter to ensure > 99% purity in defined gates.
Deep sequencing analysis of TCR Vβ repertoire
Next-generation sequencing analysis of the TCR Vβ repertoire was undertaken using the ImmunoSeq® platform at Adaptive Biotechnologies Inc. at the “survey” level of sequencing depth, that is designed to target an output of 200,000 assembled output sequences after preprocessing filters 57, 58. Briefly, genomic DNA was isolated from flow-sorted CD4+ T cell subsets (TEM, CD4+SLAMF7+ CTLs and GATA-3+ TH2 cells) from individual IgG4-RD subjects. Sorted cell numbers ranged from ~5,000 to ~40,000, assuring at least 5-fold depth of sequencing. Genomic rearrangements of V-D-J gene segments at the TCRβ locus were amplified using multiplex PCR with forward and reverse primers specific for Vβ and Jβ gene segments respectively, and analyzed by paired-end Illumina sequencing. Use of barcoded primers allowed multiplexing of next-generation sequencing samples on an Illumina Hi-Seq instrument. The sequences were assembled in silico, and V-D-J regions were reconstructed, following standard IMGT gene nomenclature for TCR Vβ gene segments 56. Non-productive rearrangements were excluded from the analysis.
Gene-expression analysis
The nCounter® human immunology panel (NanoString Technologies), comprising ~500 immunology-related genes was used to quantify the gene expression of effector/memory T cells in IgG4-RD. RNA was extracted from 10,000–50,000 flow-sorted T cells using the Qiagen RNAEasy Micro kit according to manufacturer's protocol. Targets were reverse-transcribed and pre-amplified for 14 cycles using the standard multiplexed target enrichment (MTE) protocol (NanoString Technologies) for 458 target genes, which has been previously validated to yield a linear response. The amplified products were hybridized in solution to color-coded nCounter capture and reporter probes and captured on an nCounter Cartridge for high-resolution digital scanning and analysis on the GEN2 Digital Analyzer at NanoString Technologies. The raw gene expression data was normalized to the mean of the spiked-in internal positive control probes to correct for technical assay variation and subsequently normalized to the mean of 15 housekeeping genes included in the nCounter codeset to correct for differences in sample input or variation in reverse-transcription/pre-amplification. Biological replicates were evaluated for consistency and differential expression analysis of gene expression was undertaken using the ComparativeMarkerSelection module in the GenePattern pipeline (version 3.5.0) 59, 60.
Quantitative real time PCR
Total RNA was extracted from ~10,000 – 40,000 cells using the RNAeasy plus Micro kit (Qiagen). cDNA was synthesized using a RT2 first-strand kit (Qiagen) followed by quantitative real time PCR analysis (SYBR green; Life technologies). GAPDH mRNA expression was used as the normalizing control. The primers used were:
ThPOK (F: 5’-gtctgccacaagatcatcca-3’, R: 5’-tcgtagctgtgcaggaagc-3’),
Runx3 (F:5’cagaagctggaggaccagac-3’, R: 5’-gtcggagaatgggttcagtt-3’) &
GAPDH (F: 5’-atgttcgtcatgggtgtgaa-3’, R: 5’-gtcttctgggtggcagtgat-3’)
ELISpot and western blotting for IL-1β
Prior to coating, 96-well polyvinylidene difluoride (PVDF) membrane plates (Millipore) were prewet with 50ul 70% ethanol/well for 2 minutes and washed three times with 200ul sterile filtered water. ELISpot assay for IL-1β was performed using human IL-1β ELISpot Ready-SET-Go kit (eBioscience) according to the manufacturer's recommendations. In brief, plates were coated overnight at 4°C with the anti-human IL-1β antibody provided, followed by gentle washing with ELISpot wash buffer (1X PBS plus 0.05% Tween-20) and blocking with complete medium (RPMI plus 10% fetal bovine serum) for 2 hours at room temperature. 10000 sorted CD4+CD45RO+ cells from healthy controls and IgG4-RD patients were rested on the plate in complete medium for 4 hours post-sorting, and stimulated with PMA (100ng/ml) and ionomycin (100ng/ml) overnight at 37°C. Cells were decanted gently using wash buffer to detach any adherent cells and plates were washed three times with wash buffer followed by incubation with the recommended dilution of biotinylated detection antibody for 2 hours at room temperature. After two additional washes, plates were incubated with horse-radish peroxidase conjugated with streptavidin for 45 minutes at room temperature. The plates were washed extensively 4 times with wash buffer followed by two additional washes with PBS to remove any traces of tween-20. 100 μL of TMB substrate (MabTech) was added and spots were allowed to develop for upto 20 minutes. Counting and visual analysis of the spots were done using a computer-operated CTL ELISpot reader and the fraction of IL-1β secreting cells was quantified as the number of spots detected per 10,000 cells applied to the well.
For Western blot analysis of secreted IL-1β, CD4+CD45RO+ cells from healthy donors, CD4+CD27− gated SLAMF7+ and SLAMF7− cells from an IgG4-RD subject were sorted and expanded in vitro in a U-bottomed 96-well plate (BD Falcon) with weekly anti-CD3 stimulation (Biolegend; OKT3, 3μg/ml) in media supplemented with 10ng/mL of rhIL-2. 150,000 cells were re-stimulated with anti-human CD3 (Biolegend; OKT3, 3μg/ml) or Lipopolysaccharide (LPS; Sigma, 5μg/ml) and the culture supernatants were harvested at 60hrs. These supernatants were run on an SDS-PAGE gel and transferred onto Immobilon-P membranes. IL-1β was detected using a rabbit anti-human IL-1β antibody (Biovision) followed by goat anti-rabbit Ig-HRP (Thermo scientific) and developed using SuperSignal West Pico Chemiluminescent Substrate (Biorad). LPS stimulated PBMCs were used as positive controls.
ELISA for TGFβ1
CD4+ CTLs and CD4+CD45RA+ naïve cells from IgG4-RD patients were stimulated in vitro in 96 well plates (50,000 cells/well) with anti-human CD3 (Biolegend; OKT3, 3μg/ml) in media supplemented with 10ng/mL of rhIL-2 and the culture supernatants were harvested at 48hrs. ELISA was performed on these culture supernatants using a Human TGFβ1 Quantikine ELISA kit using the manufacturer’s protocol. Standards provided in the kit were used to quantify TGFβ1 in culture supernatants.
Supplementary Material
Key messages.
CD4+ T effector memory cells with cytolytic and myeloid features are expanded in IgG4-RD patients.
CD4+SLAMF7+ CTLs are clonally expanded while CD4+GATA3+ TH2 memory cells in atopic IgG4-RD patients are clonally diverse. GATA3+ TH2 cells represent a minor proportion of CD4+ cells in affected tissues.
In IgG4-RD patients CD4+SLAMF7+ CTLs infiltrate the diseased tissues and secrete the pro-fibrotic cytokines IL-1β and TGF-β1.
The decline in CD4+SLAMF7+ CTL numbers upon rituximab therapy is associated with clinical remission of IgG4-RD patients
Acknowledgments
This study was funded by grants U19-AI110495 and AI 113163 from the NIH. The MGH flow core is supported by grant S10RR023440 from the NIH.
Abbreviations
- IgG4-RD
IgG4 related disease
- CD4+ CTL
CD4+ cytotoxic T lymphocyte
- ECM
Extra-cellular matrix
- IgG4-DS
IgG4-related dacryoadenitis and sialadenitis
- SS
Sjögren’s syndrome
- ANA
Anti-nuclear antibodies
- PBMCs
Peripheral blood mono-nuclear cells
- TCR
T-cell receptor
- PMA
Phorbol 12-myristate 13-acetate
- LPS
Lipo-polysaccharide
- ELISPOT
Enzyme-Linked ImmunoSpot
Footnotes
Conflict of Interest
The authors declare that they have no relevant conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-Related Disease. Annu Rev Pathol. 2014;9:315–47. doi: 10.1146/annurev-pathol-012513-104708. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–71. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 4.Bindon CI, Hale G, Bruggemann M, Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988;168:127–42. doi: 10.1084/jem.168.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–25. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 6.Aoki S, Nakazawa T, Ohara H, Sano H, Nakao H, Joh T, et al. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients' sera. Histopathology. 2005;47:147–58. doi: 10.1111/j.1365-2559.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64:254–63. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 8.Kanari H, Kagami S, Kashiwakuma D, Oya Y, Furuta S, Ikeda K, et al. Role of Th2 cells in IgG4-related lacrimal gland enlargement. Int Arch Allergy Immunol. 2010;152(Suppl 1):47–53. doi: 10.1159/000312125. [DOI] [PubMed] [Google Scholar]
- 9.Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–46. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–81. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 11.Johnson S, Eller M, Teigler JE, Maloveste SM, Schultz BT, Soghoian DZ, et al. Cooperativity of HIV-Specific Cytolytic CD4 T Cells and CD8 T Cells in Control of HIV Viremia. J Virol. 2015;89:7494–505. doi: 10.1128/JVI.00438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. 2014;69:269–72. doi: 10.1111/all.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattoo H, Della-Torre E, Mahajan VS, Stone JH, Pillai S. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy. 2014;69:399–402. doi: 10.1111/all.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010;31:110–9. doi: 10.1016/j.it.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–99. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–36. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrache F, Tu SP, Bhagat G, Pendyala S, Osterreicher CH, Gordon S, et al. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology. 2008;135:1277–87. doi: 10.1053/j.gastro.2008.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest. 1998;101:2129–39. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606–14. [PubMed] [Google Scholar]
- 22.Fuschiotti P. Role of IL-13 in systemic sclerosis. Cytokine. 2011;56:544–9. doi: 10.1016/j.cyto.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172:4068–76. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 24.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–21. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40:2174–82. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–42. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng HL, Liu Y, Chen JL, Huang T, Xu LJ, Godoy P, et al. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology. 2009;50:230–43. doi: 10.1002/hep.22934. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande V. The pathology of IgG4-related disease: critical issues and challenges. Semin Diagn Pathol. 2012;29:191–6. doi: 10.1053/j.semdp.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–24. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 31.Larimore K, McCormick MW, Robins HS, Greenberg PD. Shaping of human germline IgH repertoires revealed by deep sequencing. J Immunol. 2012;189:3221–30. doi: 10.4049/jimmunol.1201303. [DOI] [PubMed] [Google Scholar]
- 32.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. 2013 doi: 10.1111/all.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jong R, Brouwer M, Hooibrink B, Van der Pouw-Kraan T, Miedema F, Van Lier RA. The CD27- subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992;22:993–9. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Koyabu M, Uchida K, Miyoshi H, Sakaguchi Y, Fukui T, Ikeda H, et al. Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol. 2010;45:732–41. doi: 10.1007/s00535-010-0199-3. [DOI] [PubMed] [Google Scholar]
- 37.Soghoian DZ, Streeck H. Cytolytic CD4(+) T cells in viral immunity. Expert Rev Vaccines. 2010;9:1453–63. doi: 10.1586/erv.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14:281–9. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone JH, Khosroshahi A, Hilgenberg A, Spooner A, Isselbacher EM, Stone JR. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis Rheum. 2009;60:3139–45. doi: 10.1002/art.24798. [DOI] [PubMed] [Google Scholar]
- 40.Della-Torre E, Mattoo H, Mahajan VS, Deshpande V, Krause D, Song P, et al. IgG4-related midline destructive lesion. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita M, Ukai-Tadenuma M, Miyamoto T, Sugaya K, Hosokawa H, Hasegawa A, et al. Essential role of GATA3 for the maintenance of type 2 helper T (Th2) cytokine production and chromatin remodeling at the Th2 cytokine gene loci. J Biol Chem. 2004;279:26983–90. doi: 10.1074/jbc.M403688200. [DOI] [PubMed] [Google Scholar]
- 42.Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 2012;91:57–66. doi: 10.1097/MD.0b013e3182431ef6. [DOI] [PubMed] [Google Scholar]
- 43.Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755–62. doi: 10.1002/art.27435. [DOI] [PubMed] [Google Scholar]
- 44.Carruthers MN, Stone JH, Deshpande V, Khosroshahi A. Development of an IgG4-RD Responder Index. Int J Rheumatol. 2012;2012:259408. doi: 10.1155/2012/259408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 46.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–92. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 47.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Balance of proliferation and cell death between thyrocytes and myofibroblasts regulates thyroid fibrosis in granulomatous experimental autoimmune thyroiditis (G-EAT) J Leukoc Biol. 2005;77:166–72. doi: 10.1189/jlb.0904538. [DOI] [PubMed] [Google Scholar]
- 49.Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–14. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 50.Komocsi A, Lamprecht P, Csernok E, Mueller A, Holl-Ulrich K, Seitzer U, et al. Peripheral blood and granuloma CD4(+)CD28(−) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener's granulomatosis. Am J Pathol. 2002;160:1717–24. doi: 10.1016/s0002-9440(10)61118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 2010;5:e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JR, Mathew SO, Patel RK, Pertusi RM, Mathew PA. Altered expression of signalling lymphocyte activation molecule (SLAM) family receptors CS1 (CD319) and 2B4 (CD244) in patients with systemic lupus erythematosus. Clin Exp Immunol. 2010;160:348–58. doi: 10.1111/j.1365-2249.2010.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.03.034. provisionally accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–10. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 56.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veillette A, Guo H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol. 2013;88:168–77. doi: 10.1016/j.critrevonc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 58.De Calisto J, Wang N, Wang G, Yigit B, Engel P, Terhorst C. SAP-Dependent and -Independent Regulation of Innate T Cell Development Involving SLAMF Receptors. Front Immunol. 2014;5:186. doi: 10.3389/fimmu.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120:552–9. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011;166:1–15. doi: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1beta monoclonal antibody. Clin Pharmacokinet. 2012;51:e1–18. doi: 10.2165/11599820-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.