Summary
BACKGROUND
Twelve months of oral cyclophosphamide (CYC) has been shown to alter the progression of scleroderma-related interstitial lung disease (SSc-ILD) when compared to placebo. However, toxicity was a concern and without continued treatment the efficacy disappeared by 24 months. We hypothesized that a two-year course of mycophenolate mofetil (MMF) would be safer, better tolerated and produce longer lasting improvements than CYC.
METHODS
Patients with SSc-ILD meeting defined dyspnea, pulmonary function and high-resolution computed tomography (HRCT) criteria were randomized in a double-blind, two-arm trial at 14 medical centers. MMF (target dose 1500 mg twice daily) was administered for 24 months in one arm and oral CYC (target dose 2·0 mg/kg/day) administered for 12 months followed by placebo for 12 months in the other arm. The primary endpoint, change in forced vital capacity as a percent of the predicted normal value (FVC %) over the course of 24 months, was assessed in a modified intention-to-treat analysis using an inferential joint model combining a mixed effects model for longitudinal outcomes and a survival model to handle non-ignorable missing data. The study was registered with ClinicalTrials.gov, number NCT00883129, and is closed.
RESULTS
Between November, 2009, and January, 2013, 142 patients were randomized. 126 patients (63 MMF; 63 CYC) with acceptable baseline HRCT studies and at least one outcome measure were included in the analysis. The adjusted FVC % (primary endpoint) improved from baseline to 24 months by 2.17 in the MMF arm (95% CI, 0.53–3.84) and 2·86 in the CYC arm (95% confidence interval 1·19–4·58) with no significant between-treatment difference (p=0·24), indicating that the trial was negative for the primary endpoint. However, in a post-hoc analysis of the primary endpoint, within-treatment improvements from baseline to 24 months were noted in both the CYC and MMF arms. A greater number of patients on CYC than on MMF prematurely withdrew from study drug (32 vs 20) and failed treatment (2 vs 0), and the time to stopping treatment was significantly shorter in the CYC arm (p=0·019). Sixteen deaths occurred (11 CYC; 5 MMF) with most due to progressive ILD. Leukopenia (30 vs 4 patients) and thrombocytopenia (4 vs 0 patients) occurred more often in patients treated with CYC. In post-hoc analyses, within- (but not between-) treatment improvements were also noted in defined secondary outcomes including skin score, dyspnea and whole-lung HRCT scores.
INTERPRETATION
Treatment of SSc-ILD with MMF for two years or CYC for one year both resulted in significant improvements in pre-specified measures of lung function, dyspnea, lung imaging, and skin disease over the 2-year course of the study. While MMF was better tolerated and associated with less toxicity, the hypothesis that it would have greater efficacy at 24 months than CYC was not confirmed. These findings support the potential clinical impact of both CYC and MMF for progressive SSc-ILD, as well as the current preference for MMF due to its better tolerability and toxicity profile.
FUNDING
National Heart, Lung and Blood Institute/National Institutes of Health with drug supply provided by Hoffmann-La Roche/Genentech.
Keywords: Scleroderma-related interstitial lung disease, mycophenolate mofetil, oral cyclophosphamide, lung function
INTRODUCTION
Progressive interstitial lung disease (ILD) is the leading cause of death attributed to systemic sclerosis (SSc)1,2 and treatment options are limited. In Scleroderma Lung Study (SLS) I, oral cyclophosphamide (CYC) for one year was associated with better pulmonary function, dyspnea, cutaneous sclerosis and health-related quality of life (HRQoL),3 as well as improved scores for lung fibrosis on high-resolution computed tomography (HRCT).4,5 However, when SLS I subjects were followed for an additional year off-treatment, the beneficial effects of CYC waned, and by 24 months, no significant differences from placebo were identified for most outcomes, including pulmonary function.6 Moreover, in SLS I, CYC was associated with acute toxicity,3,7 and its long-term administration is limited by the risk for developing treatment-related malignancies.8
Because of its immunosuppressive properties and favorable safety profile, mycophenolate mofetil (MMF) is used post-solid organ transplantation9 and in the treatment of many autoimmune conditions.10 Moreover, uncontrolled studies suggest that MMF may be an effective immunosuppressive agent for treating SSc-ILD.11–16 SLS II was designed to compare the efficacy and safety of MMF (administered for 2 years) and oral CYC (given for 1 year followed by placebo for another year) in symptomatic SSc-ILD.
METHODS
Study design and participants
SLS II was a double-blind, parallel group, randomised trial performed between November, 2009, and January, 2015, at 14 US medical centers. The protocol was approved by a Data and Safety Monitoring Board (DSMB) constituted by the National Heart, Lung and Blood Institute, National Institutes of Health, and by the institutional review boards at each participating site. All participants gave written informed consent. A study flow diagram (Supplementary Figure 1, pp12) and the complete protocol (pp 27–115) appear in the web appendix.
Randomization and masking
Consecutive patients who met the following inclusion criteria were enrolled: defined SSc17 with either limited (lcSSC) or diffuse (dcSSc) cutaneous involvement; age 18–75 years; screening forced vital capacity (FVC) <80% but ≥ 45% of the predicted value and reproducible within 10% at the baseline visit (but ≤85% predicted); exertional dyspnea ≥Grade 2 on the Magnitude of Task component of the Mahler Baseline Dyspnea Index (BDI);18 any ground glass opacity (GGO) on HRCT whether associated with reticulations (fibrosis) or not;4 and the onset of their first non-Raynaud’s symptom of SSc within the previous 7 years. Exclusion criteria included FVC<45% predicted, FEV1/FVC ratio <65%, pulmonary hypertension according to echocardiograpy (ECHO) or right heart catheterization (RHC) and judged by the investigator to be clinically significant and warranting drug therapy; a single-breath diffusing capacity of the lung for carbon monoxide (DLCO) <40% predicted (30–39% predicted allowed if echocardiography and/or RHC failed to show evidence of pulmonary hypertension); clinically significant abnormalities on HRCT not attributable to SSc; smoking within the past 6 months; evidence of significant airflow obstruction; persistent unexplained hematuria (>10 RBCs/hpf); persistent leukopenia (WBC <4.0 ×103/μl) or thromboyctopenia (platelet count <150 ×103/μl); clinically significant anemia (<10.0 g/dl); baseline liver function test (ALT, AST) or bilirubin >1.5 × upper normal limit; concomitant and present use of captopril; serum creatinine >2.0mg/dl; uncontrolled congestive heart failure; pregnancy (documented by urine pregnancy test) and/or breast feeding; prior use of oral CYC or MMF for more than 8 weeks or the receipt of more than two intravenous doses of CYC in the past; use of CYC and/or MMF in the 30 days prior to randomization; active infection (lung or elsewhere) whose management would be compromised by CYC or MMF; other serious concomitant medical illness (e.g., cancer), chronic debilitating illness (other than SSc), unreliability or drug abuse that might compromise the patient’s participation in the trial; if of child bearing potential (a female participant < 55 years of age who has not been postmenopausal for ≥ 5 years and who has not had a hysterectomy and/or oophorectomy), failure to employ two reliable means of contraception; use of contraindicated medications (see web appendix, pp. 55–58); and use of medications with putative disease-modifying properties within the past month (e.g., D-penicillamine, azathioprine, methotrexate, Potaba);
The data coordinating center at UCLA confirmed all entry criteria and randomly assigned patients using a double-blind, double-dummy, center-blocked design to receive either once-daily oral CYC during the initial 12 months followed by placebo for the second 12 months or twice daily MMF administered for the entire 24 months. Masking was carried out by the UCLA Pharmacy Core, which formulated all study drugs (25 mg of CYC, 250 mg of MMF or placebo) into matching 250 mg gel-capsules. Patients received medications as single dose packages containing either 6 or 8 capsules, depending upon patient weight, with the composition of the capsules (active vs placebo) adjusted by the pharmacist to administer the required daily dose while maintaining the blind.
Procedures
CYC (Roxanne Laboratories) was administered once daily (morning dose active drug, evening dose placebo) for 12 months starting with an initial dose of 50 to 150 mg (based on weight) and titrated up to a maximum dose of 1·8 to 2·3 mg/kg according to a defined protocol (web appendix pp 47–49). During the second 12 months, patients assigned to the CYC arm received only placebo. MMF (CellCept, Roche Pharmaceuticals, or a generic after July 2014) was initiated at a dose of 500 mg twice daily and increased over time to a maximum dose of 1·5 g twice daily according to a defined protocol (web appendix pp 49–51) and continued for a total of 2 years. Drug safety was assessed by laboratory testing every 2 weeks for the first 2 months and then monthly for the remainder of the 24-month study. Study drugs were withheld or down-titrated for safety and/or tolerability reasons and resumed with defined dose and titration schedules according to pre-specified protocol criteria (web appendix pp 72–75).
Baseline assessments included spirometry, subdivisions of lung volume, DLCO (corrected for hemoglobin), DL/VA ratio, skin thickness score using the modified Rodnan measurement method (mRSS)19 and various self-reported questionnaires as described in the web appendix (pp 60–61). All assessments were repeated at 3-month intervals except for the lung volume measurements, which were repeated every 6 months, and the BDI, which was replaced by the Transition Dyspnea Index (TDI)20 at follow-up assessments. In addition, volumetric thoracic HRCT was performed at baseline and 24 months. The extent of lung involvement by fibrosis (reticulations), GGO and honeycombing (HC) were measured on thoracic HRCT using a validated, computer-aided scoring method.21 (web appendix for additional details regarding these assessments, pp 59–60).
Outcomes
Our primary hypothesis was that MMF would have a significantly greater effect on % predicted FVC than CYC, when measured over the entire 24 months, with an expected effect size at 24 months of 4% after adjustment for baseline FVC and HRCT-measured fibrosis score. This hypothesis was based on the assumption that MMF would be as effective as CYC during the first 18 months (point of maximal CYC improvement in %-predicted FVC in SLS I),6,22 and that continued treatment with MMF would at least maintain FVC at this value, while the 1-year treatment with CYC would be associated with a fall in %-predicted FVC back to untreated values by 24 months.6 The primary outcome variable addressed all of these assumptions simultaneously by focusing on the course of the FVC % over time from 3 to 24 months. Finally, it was also hypothesized that MMF would be safer and better tolerated than CYC as measured by the frequency of protocol-defined adverse events (AEs), all patient-reported AEs and serious AE (SAEs), study withdrawals and treatment failures. Treatment failure, mandating withdrawal from active study treatment, was defined as an absolute decrease from baseline FVC of ≥15% of predicted occurring ≥3 months after randomization and lasting for ≥1 month.
Secondary outcome variables included the following: the course from 3 to 24 months of the DLCO %-predicted, TDI and mRSS scores, and the change from baseline in quantitative HRCT scores for lung fibrosis and total ILD at 24 months.
An independent three-person Morbidity and Mortality Committee reviewed the causes of all SAEs and deaths to determine relatedness (to treatment, underlying disease or unrelated cause)..
Statistical analysis
A sample size of 150, assuming a 30% drop-out rate, was calculated to provide 80% power at the 5% alpha level (2-tailed) to detect a 4% difference between treatment arms at 24 months. A modified intention-to-treat principle was applied to all analyses using an inferential joint model consisting of a mixed effects model for longitudinal outcomes and a survival model to handle non-ignorable missing data due to study dropout, treatment failure or death (i.e. likely related to disease or treatment and therefore not random).23,24 Consistent with the intention-to-treat principle, treatment failures and others who prematurely withdrew from the double-blind treatment phase were encouraged to return for outcome monitoring at the 12, 18 and 24 month visits and their outcomes included in the analysis. Pre-specified covariates for the primary outcome included baseline FVC %-predicted, the HRCT-measured extent of lung fibrosis in the lobe of maximal involvement, a time trend, treatment assignment, and treatment-time trend interactions (see web appendix pp 113–115 for statistical details). Secondary efficacy endpoints were also analyzed using a joint model approach (no adjustment for multiple comparisons). For safety and tolerability analyses, Kaplan Meier survival curves were generated for each treatment group with significance determined using a log-rank test. Chi-square or Fisher’s exact tests were employed to compare the incidence of AEs and SAEs between treatment arms..
The statistical analyses were performed with SAS software (version 9·2, SAS Institute, Cary, NC) where appropriate. The study was registered with ClinicalTrials.gov (NCT00883129)
Role of the funding source
Roche, the manufacturer of MMF used in the study, donated the drug but had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
RESULTS
Participants
Between November 2009 and January 2013, 198 patients were screened and 142 were deemed eligible (56 ineligible) and randomized (73 to CYC and 69 to MMF). A CONSORT diagram summarizes the disposition of all randomized patients (Figure 1). 126 (63 MMF; 63 CYC) had acceptable baseline HRCT studies and at least one outcome measure and were included in the primary analysis.
Figure 1.
Disposition of study participants
In the CYC arm, 36 patients prematurely stopped drug treatment (2 deaths, 2 treatment failures, and 32 other withdrawals), while only 20 patients in the MMF arm prematurely stopped drug treatment (1 death, 0 treatment failures, 19 other withdrawals). Reasons for premature discontinuation of study treatment are listed in Supplementary Table 1 (web appendix p 19). There were an additional 9 deaths in the CYC arm (11 total) and 4 in the MMF arm that occurred in subjects who had already withdrawn for other reasons.
Baseline characteristics
Participants averaged 52 years (range 28–79), were predominantly women (73·9%), had onset of their first SSc symptoms an average of 2·6 years prior to entering the study (range 0·3–7·1 years) and 58·5% met the definition for diffuse skin involvement. Mean FVC was 66·5%-predicted (SD ±9·1), TLC was 65·8%-predicted (SD ±11·1%) and DLCO was 54·0 %-predicted (SD ±12·7%). Complete baseline characteristics appear in Table 1 with no apparent differences noted between treatment groups.
Table 1.
Baseline Characteristics of SLS II Participants*
| Characteristics | N | All Patients (N=142) | Cyclophosphamide (N=73) | Mycophenolate (N=69) |
|---|---|---|---|---|
| Age (yr) | 142 | |||
| Mean | 52.3±9.7 | 52.0±9.8 | 52.6±9.7 | |
| Range | 28–79 | 28–71 | 34–79 | |
| Female sex (% of patients) | 142 | 73.9 | 78.1 | 69.6 |
| Duration of scleroderma (yr) | 139 | |||
| Mean | 2.6±1.8 | 2.5±1.8 | 2.6±1.7 | |
| Range | 0.3–7.13 | 0.4–7.13 | 0.3–6.5 | |
| Limited/Diffuse (% of patients) | 142 | 41.5/58.5 | 45.2/54.8 | 37.7/62.3 |
| FVC (% of predicted) | 142 | 66.5±9.1 | 66.5±9.9 | 66.5±8.3 |
| FEV1:FVC (% of predicted) | 142 | 82.6±5.6 | 83.3±5.6 | 81.0±5.5 |
| Total lung capacity (% of predicted) | 142 | 65.8±11.1 | 65.5±12.0 | 66.3±10.0 |
| DLCO (% of predicted)† | 142 | 54.0±12.7 | 54.1±14.1 | 54.0±11.1 |
| DL/VA (% of predicted) | 142 | 60.9±12.8 | 61.0±13.7 | 60.9±11.8 |
| Mahler Dyspnea Index (Focal score) | 139 | 7.2±2.2 | 7.1±2.3 | 7.3±2.1 |
| Visual-analogue score for breathing | 139 | 24.5±28.1 | 24.4±28.9 | 24.5±27.4 |
| Leicester Cough Questionnaire | 139 | 16.7±4.0 | 16.7±4.0 | 16.8±4.0 |
| SF-36 score | ||||
| Physical component | 142 | 35.8±9.9 | 35.6±9.8 | 36.0±10.0 |
| Mental component | 142 | 49.4±9.0 | 49.8±10.0 | 49.1±7.9 |
| Skin-thickening score (mRSS) | ||||
| All patients | ||||
| Mean | 142 | 14.7±10.5 | 14.0±10.6 | 15.3±10.4 |
| Range | 1–46 | 2–46 | 1–41 | |
| Patients with dcSSc | ||||
| Mean | 83 | 20.8±9.4 | 20.6±9.9 | 21.0±9.0 |
| Range | 3–46 | 3–46 | 4–41 | |
| Patients with lcSSc | ||||
| Mean | 59 | 6.1±3.8 | 6.2±4.3 | 5.9±3.3 |
| Range | 1–18 | 2–18 | 1–14 | |
| HAQ disability index (0–3) | 142 | 0.7±0.7 | 0.7±0.7 | 0.7±0.6 |
| QLF-WL | 137 | 8.6±6.9 | 8.9±7.0 | 8.3±6.9 |
| QLF-LM | 137 | 22.8±19.6 | 22.6±19.3 | 23.0±20.2 |
| QILD-WL | 137 | 29.5±14.0 | 31.6±14.4 | 27.2±13.2 |
| QILD-LM | 137 | 51.2±20.3 | 52.3±19.9 | 50.0±20.9 |
| Auto-antibody (% positive in patients tested) | ||||
| ANA | 134 | 94.8 | 93.0 | 96.8 |
| Topoisomerase-1 | 134 | 45.5 | 45.1 | 46.0 |
| RNA polymerase III | 134 | 13.4 | 12.7 | 14.3 |
| Centromere | 134 | 2.2 | 2.8 | 1.6 |
| Th\to | 127 | 6.3 | 7.5 | 5.0 |
| ro52 | 127 | 17.3 | 16.4 | 18.3 |
Values are mean ± standard deviation, unless otherwise noted.
Adjusted for hemoglobin
Definitions of abbreviations: FVC = forced vital capacity; DLCO = diffusing capacity of the lung for carbon monoxide; DL/VA = ratio of DLCO to alveolar volume; TDI = Mahler’s transition dyspnea index; mRSS = modified Rodnan skin score; QLF = quantitative extent of lung fibrosis on HRCT; QILD = quantitative extent of total interstitial lung disease (including fibrosis, honeycomb and ground glass opacity); WL = whole lung; LM = lobe of maximal involvement. Scores for the Mahler Baseline Dyspnea Index can range from 0 to 12, with lower scores indicating worse dyspnea. Scores for the visual analogue scale for breathing can range from 1 to 100, with higher numbers indicating increasing difficulty breathing. Scores for the Leicester Cough Questionnaire (total scores) can range from 3 to 21, with lower scores representing worse cough-specific quality of life. Scores for the Medical Outcomes Study 36-item Short-Form General Health Survey (SF-36) can range from 0 to 100, with lower scores indicating worse health status. Scores for skin thickening (modified Rodnan Skin Scores, mRSS) can range from 0 to 51, with higher scores indicating more severe thickening. Scores for HAQ Disability Index can range from 1 to 3, with higher numbers indicating greater disability.
Primary outcome (primary analysis)
The primary hypothesis was that patients receiving 24 months of MMF would experience a sustained improvement in lung function, while those treated with 12 months of CYC would exhibit a significant loss of efficacy after 18 months. However, this pattern was not observed and no significant difference was found between the two treatment groups in the course of the FVC % over the entire 24 months (p=0·24) (Figure 2A, Table 2). In post-hoc analyses based on the joint model, each treatment group showed significant increases from baseline in FVC % not only at 12 and 18 months, but also at 21 and 24 months. The improvement from baseline was maximal at 21 months in both groups (Figure 2A), and at 24 months the improvement averaged ±2·88 %-predicted for the CYC arm (95% CI 1·19–4·58) and ±2·19% for the MMF arm (95% CI 1·53–3·84).
Figure 2.
Figure 2A. Primary Outcome; The course of %-predicted FVC from 3 through 24 months by treatment arm based on the joint model†
†Adjustments for baseline FVC%-predicted, baseline HRCT lung fibrosis score (QLF) and non-ignorable missing data (time to premature discontinuation of study drug, deaths and treatment failure). The horizontal dotted line represents the average baseline FVC%-predicted for both treatment arms based on the joint model (baseline values did not differ between the two treatments). The vertical lines (dashed CYC; solid MMF) represent 95% confidence intervals. Of the 142 randomized participants, 126 (63 - CYC; 63- MMF) were included in the primary analysis. The 16 randomized subjects excluded from analysis did not have outcome assessments at ≥3 months or baseline HRCT scans that were suitable for quantitative assessment of the extent of lung fibrosis (a covariate in the joint model). Three and two participants assigned to CYC and MMF, respectively, who did not have a 3-month visit did have assessments at ≥6 month visits and were therefore included in the analysis. On the other hand, two and three participants assigned to CYC and MMF, respectively, who had a 3-month visit did not have a baseline HRCT scan that was suitable for quantitative assessment of the extent of fibrosis and were therefore excluded from the analysis.
Figure 2B. Frequency distribution of changes from baseline to 24 months in FVC%-predicted by treatment arm and by whether participants completed the entire study treatment or not (all observed data, ITT).
Figure 2C. Tolerability and Toxicity Assessment; Time to premature withdrawal from study medication or treatment failure by treatment arm. The protocol definition of treatment failure was an absolute decline in FVC% predicted of ≥15% that persisted for at least 1 month.
Table 2.
Mean changes from baseline to 24 months for study outcomes (in absolute values) with 95% confidence intervals (CI); by treatment group with between-treatment differences based on post-hoc estimates from the joint model.
| CYC | MMF | ΔMMF - ΔCYC | ||||||
|---|---|---|---|---|---|---|---|---|
| N | change | 95% CI | N | change | 95% CI | Δ | 95% CI | |
| %-predicted FVC | 51 | 2.88 | 1.19 to 4.58 | 53 | 2.19 | 0.53 to 3.84 | −0.70 | −3.1 to 1.7 |
| %-predicted TLC | 51 | 0.45 | −1.43 to 2.32 | 53 | 1.24 | −0.68 to 3.18 | 0.80 | −2.0 to 3.6 |
| %predicted DLCO | 48 | −2.14 | −4.59 to 0.31 | 52 | −0.40 | −2.81 to 2.01 | 1.74 | −1.6 to 5.1 |
| %-predicted DL/VA | 51 | −3.43 | −5.7 to −1.2 | 52 | −2.46 | −4.7 to −0.2 | 0.96 | −2.2 to 4.1 |
| TDI | 39 | 2.16 | 1.14 to 3.18* | 40 | 1.77 | 0.75 to 2.79 | −0.39 | −1.8 to 1.0 |
| mRSS (including lcSSc & dcSSc) | 53 | −5.35 | −6.9 to −3.8 | 53 | −4.90 | −6.4 to −3.4 | 0.45 | −1.7 to 2.6 |
| QLF-WL | 47 | 1.13 | −1.71 to 3.98 | 51 | 2.15 | −0.72 to 5.03 | 1.02 | −2.99 to 5.03 |
| QLF-LM | 47 | −0.27 | −1.43 to 1.69 | 51 | 0.12 | −1.02 to 1.26 | 0.39 | −1.27 to 2.05 |
| QILD-WL | 47 | −1.84 | −5.16 to 1.46 | 51 | −0.95 | −4.1 to 2.2 | 0.89 | −3.58 to 5.36 |
| QILD-LM | 47 | −2.78 | −5.17 to −0.40 | 51 | −2.51 | −4.9 to −0.15 | 0.27 | −3.09 to 3.67 |
Primary outcome (post-hoc secondary analyses)
To understand the frequency and magnitude of individual changes in pulmonary function over time, a post-hoc frequency distribution was constructed for the subjects returning for a 24-month measurement who experienced different magnitudes (in 5% intervals) of improvement or decrement in FVC % values from baseline to 24 months (Figure 2B; Supplementary Table 2, web appendix p 20). Similar to the joint model, the overall change (±SE) averaged 3·0±1·2 for the CYC arm and 3·3±1·1 for the MMF arm. The majority of subjects in each arm had improving FVC % values over time (CYC: 64·7%; MMF: 71·7%) and no significant difference was identified between treatments (p=0·55; Fisher’s exact test). Of the 34 patients in the CYC arm and the 38 patients in the MMF arm with a positive change in FVC % over time, the average (±SE) improvement was 7·1±0·7 and 7·5±0·9, respectively. We also noted that the majority of subjects who experienced decrements in lung function represented participants who had stopped drug treatment prematurely but returned for the 24-month outcome measurement. Stratifying the frequency distribution of outcomes by duration on the study protocol (Figure 2B) confirmed that 80·6 % and 75·5% of those who completed the entire treatment period experienced an overall improvement in their FVC % (CYC and MMF arms, respectively), while 66·6% and 75·0% of those who prematurely withdrew from treatment but returned for the 24 month outcome (CYC and MMF arms, respectively) experienced an overall worsening in their FVC %. The time course of observed changes in FVC % from baseline to 24 months, without modeling, appears in Supplementary Figure 2 (web appendix p 13). These curves suggest an earlier increase in FVC % at 6–9 months in subjects receiving MMF compared to those on CYC, but with both treatment arms eventually producing a similar overall maximal response.
Secondary outcomes
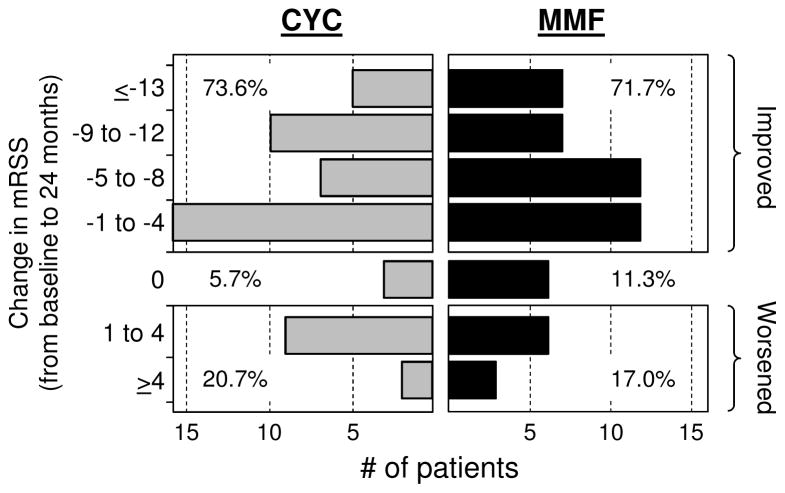
Applying the primary analysis approach (i.e. the longitudinal joint model), no significant between-treatment differences were identified for the course of mRSS (diffuse and limited cutaneous SSc patients combined) (Figure 3A) or TDI (Table 2) over the 24 months of the study. However, as with the primary outcome, in post-hoc analyses the joint model identified improvements from baseline to 24 months in each individual arm for both the mRSS (CYC: −5·35; MMF: −4·90) and the TDI (CYC: 2·16; MMF: 1·77). In addition, in secondary analyses, the frequency distribution of individual subject responses demonstrated that the majority of subjects improved (Figure 3B; Supplementary Tables 3–5, web appendix pp 21–24). Values for the mRSS decreased (i.e. improved) in 73·6% of subjects on CYC and 71·7 % of subjects on MMF, and in the majority (78% and 64%, respectively), the improvements were ≥5 units. TDI values increased (i.e. improved) by at least 1 unit in 59% of subjects on CYC and 47·5% of subjects on MMF, with 78% and 84% of these improvements, respectively, being ≥3 units (Supplementary Table 4, web appendix p 22). As such, in most cases the improvements in skin and dyspnea exceeded what are considered minimal clinically important changes.22,23 In contrast, DLCO and DL/VA did not change in the MMF arm (except for a decrease in DL/VA at 24 months) or decreased (worsened) at most time points in the CYC arm. (Table 2 & Supplementary Figure 3A and 3B, web appendix p 14–15; Supplementary Table 5, web appendix p 23–24).
Figure 3.
Figure 3A. Absolute change in modified Rodnan Skin Score (mRSS) from baseline by treatment arm based on the joint model†
†Adjustments for baseline skin score (mRSS), baseline HRCT lung fibrosis score and non-ignorable missing data (time to premature discontinuation of study drug, deaths and treatment failure). Vertical lines represent 95% confidence intervals. Dotted horizontal line represents the average baseline skin score for both treatment arms based on the joint model (baseline values did not differ between the two treatments).
Figure 3B. Frequency distribution of observed changes at 24 months from baseline in modified Rodnan Skin Score (mRSS). (observed data)
Both limited and diffuse cutaneous SSc patients (N= 52 CYC; 53 MMF)
Treatment with MMF or CYC was not associated with a change in the HRCT-measured quantitative lung fibrosis (QLF) scores in the lobe of most involvement (QLF-LM) or in the whole lung (QLF-WL), nor was a difference between the two treatment arms identified (Table 2 & Supplementary Table 5, web appendix p 23–24). The same was true for the HRCT quantitative interstitial lung disease (QILD) score in the lobe of maximal involvement (QILD-LM). On the other hand, QILD in the whole lung (QILD-WL) was reduced (improved) to a small degree in both the CYC arm (−2·78; 95% CI: −5·17 to −0·40) and the MMF arm (−2·51; 95% CI: −4·8 to −0·15).
Adverse Events
The predominant causes of protocol-defined AEs were anemia and leukopenia (Table 3), and the latter occurred in significantly more patients in the CYC arm (30 vs 4 patients; p<0·05). Thrombocytopenia, while infrequent, occurred exclusively in the CYC arm (4 vs 0 patients; p<0·05). There was no difference in anemia or pneumonia between treatment arms. (See Supplementary Figure 4 for AE results, web appendix p 16). SAEs occurred slightly more frequently in the MMF arm (N=42) than the CYC arm (N=36), while numerically more SAEs in the CYC arm (N=8), as compared to the MMF arm (N=3), were deemed by the Morbidity and Mortality committee to be related to the study drug. Relatively equal numbers of SAEs were attributed to SSc itself (16 in each arm) or to other causes (11 CYC; 12 MMF) (Table 3).
Table 3.
Adverse Events and Serious Adverse Events
| CYC | MMF | |||
|---|---|---|---|---|
| No. of AEs | No. of patients | No. of AEs | No. of patients | |
| Adverse event (AE)* | ||||
| Leukopenia† | 51 | 30 | 5 | 4 |
| Neutropenia | 7 | 5 | 3 | 3 |
| Anemia | 26 | 13 | 18 | 8 |
| Thrombocytopenia | 7 | 4 | 0 | 0 |
| Hematuria | 2 | 2 | 3 | 3 |
| Pneumonia | 4 | 4 | 6 | 5 |
| Serious adverse event (SAE) | ||||
| Number of patients with SAEs | 22 | 27 | ||
| Total number of SAEs | 36 | 42 | ||
| Related to treatment‡ | 8 | 3 | ||
| Related to underlying disease‡ | 16 | 16 | ||
| Due to other causes‡,§ | 11 | 22 | ||
| Death | 11 | 5 | ||
pre-defined by protocol as likely to be related to study drug and to warrant protocol-defined management (except for pneumonia): anemia = Hgb <10 gm/dl or <9 for those with Hgb <11 at enrollment; leukopenia = WBC <2500; neutropenia = neutrophils <1000; thrombocytopenia = platelets <100,000; hematuria = >25 red blood cells (or 10–15 red blood cells on more than one urinalysis) in absence of urinary tract infection or menses
p<0.05; Fisher’s exact test comparing the number of patients experiencing AE between the two groups
according to consensus classification by Morbidity and Mortality Committee
Other causes included cancer (n=3); renal/bladder (n=2); syncope/seizures (n=3); hematologic (including anemia and blood clot; n=3); GI (dysphagia/vomiting, gastroenteritis, bile duct obstruction, SSc bowel disorder; n=7); musculoskeletal (n=7); respiratory infection (n=9); cardiac (including heart failure, palpitation, arrhythmias,, chest pain, pericarditis, ischemic heart disease; n=20); miscellaneous (including weight loss, sulfa allergy and elective surgery; n=6)
Time to withdrawal from the study medication or treatment failure was significantly shorter in the CYC arm (p=0·019, Figure 2C). Of subjects who prematurely stopped treatment in the CYC arm, 16 returned for at least one additional outcome visit at 12, 18, or 24 months. In the MMF arm, 5 of those who prematurely stopped treatment returned for at least one of these visits. Of the subjects who prematurely stopped treatment but returned for the final study visit, 12 of 16 in the CYC arm and 2 of 4 in the MMF arm reported that they had started an alternative therapy.
Sixteen deaths (11·3% of randomized patients) occurred during the 2-year course of the trial (11 CYC; 5 MMF) with all but one deemed probably due to underlying SSc and not the treatment. Three of the deaths occurred while subjects were still on study drug; the remainder occurred 1–17 months after patients had withdrawn from treatment.
DISCUSSION
Employing a double-blind, randomized controlled clinical trial approach, SLS II compared a continuous 24-month course of MMF with a 12-month course of oral CYC (followed by 12 months of placebo) for the treatment of symptomatic SSc-ILD. Contrary to the central component of the primary study hypothesis, no significant difference was observed between the two treatment arms in the course of FVC % over 24 months. If considered in isolation, one would consider this as a “negative study”. However, the failure to identify a difference between treatments was not due to a lack of efficacy. As hypothesized, both treatments produced improvements in lung function, making this the first prospective randomized controlled trial to document a positive response in patients with SSc-ILD after treatment with MMF. The average peak improvement from baseline occurred at 18–21 months in both treatment arms (Figure 2A), similar to findings with CYC from the SLS I study. What did not occur as originally hypothesized was the nearly-complete loss of efficacy beyond 18 months (i.e., beyond 6 months after the study drug was discontinued) in those patients assigned to the CYC arm. While the average FVC % did decline between 21 and 24 months, this occurred in both arms and was modest in magnitude.
It is important to consider alternative explanations, other than loss of efficacy, for the observed decline in lung function between 18–24 months. One consideration is that the values between 18 and 24 months reflect a weighted mean effect – i.e. the impact of having subjects who prematurely withdrew from treatment (and did worse) return for the final outcome measures. Indeed, at the 24 month time-point there were 15 such patients in the CYC arm and 4 in the MMF arm. Furthermore, in a secondary analysis we observed that the change in FVC % from baseline to 24 months improved over time in the overwhelming majority of patients who completed therapy (CYC = 80·6%; MMF = 75·5%) whereas the majority of subjects who had prematurely withdrawn from treatment but returned for the primary outcome measure experienced a loss of lung function over time (Figure 2B). The net impact of including data from all subjects at the 24-month time point would therefore appear as a terminal decline in lung function.
Applying this explanation, the observed differences in treatment responses between SLS I and SLS II might represent the recent trend of using alternative types of disease modifying therapy in those doing poorly with CYC. Of the 15 subjects in SLS II who withdrew from or failed treatment but returned for final study outcome measures, 12 indicated that they were switched to potentially disease-modifying therapy (MMF, intravenous CYC, rituximab, tocilizumab, intravenous immunoglobulin, or azathioprine) and these alternative therapies could have influenced the results.
Another possible explanation for the outcome difference between SLS I and II is that the SLS II patient population might have been more responsive to treatment. In a post-hoc analysis of SLS I data we had identified three independent baseline variables that appeared to predict responsiveness to CYC therapy.22 As previously reported,25 the subjects enrolled into SLS II exhibited significantly more dyspnea at baseline (BDI score, p<0·0001) and more extensive lung fibrosis (QLF-LM score, p<0·04) than did those enrolled into SLS I. These features represent two of the three baseline characteristics that may predict a superior response to CYC.22 If so, superior responsiveness could have contributed to a better course of disease between 18–24 months.
While improvements from baseline in % predicted FVC of 3.0±1.2% and 3.3±1.1% were observed within the CYC and MMF treatment arms, respectively, one may question the clinical relevance of these relatively modest changes. However, the same response patterns observed for the change over time in FVC % (the primary outcome) occurred for a number of pre-defined secondary outcomes. Both treatments resulted in improvements from baseline to 24 months in skin thickness, self-reported dyspnea, and the quantitative extent of ILD (QILD) in the whole lung, even though no between-treatment differences were found for these outcome measures. In most cases, improvements in the mRSS and TDI exceeded pre-defined minimal clinically important changes.26,27 Collectively, the secondary outcomes reinforce the impact of both CYC and MMF on lung function and suggest a clinically relevant effect. Other secondary outcomes, including cough and general and scleroderma-specific health-related quality of life measures, are currently being analyzed and will be reported separately. As already noted, the absence of a true placebo is an important limitation and the clinical significance of all findings should be considered inferential rather than direct.
The only significant between-treatment differences in pre-specified efficacy outcomes were that the DLCO %-predicted and DL/VA %-predicted decreased less during the course of treatment in the MMF arm than in the CYC arm (Table 2 & Supplementary Figure 3A & 3B, web appendix pp 14–15), suggesting a possible benefit of MMF in moderating the destructive effects of SSc-ILD on gas transfer. A positive effect of MMF on pulmonary vascular remodeling has been suggested by its protective effects in a rat model of pulmonary arterial hypertension.28 In an observational study in patients with scleroderma-related pulmonary hypertension, treatment with MMF also resulted in a survival advantage.29
Since scleroderma-associated pulmonary vasculopathy could affect several outcomes including dyspnea, DLCO and early withdrawal, patients known to have clinically significant resting pulmonary hypertension requiring treatment were specifically excluded from the trial. While an ECHO was not required per-protocol, 121 of the 142 patients (85%) had an ECHO and an additional 14 (10%) had undergone RHC prior to enrollment, only 3 of whom had values (2 of the 3 only slightly) exceeding the investigators’ agreed-on thresholds for pulmonary hypertension warranting therapy (estimated pulmonary artery pressure (PAP) of >45 mm Hg on ECHO or a mean PAP >30 mm Hg on RHC). Furthermore, of the 20 patients with a DLCO <40% predicted, cases that required an ECHO or RHC to exclude pulmonary hypertension, only one patient (who was assigned to CYC) had a mild elevation of mean PAP on RHC and was inadvertently randomized. During the course of the trial only 2 cases (1 CYC and 1 MMF) were diagnosed with new onset of pulmonary hypertension. While ECHO estimates of PAP are not always reliable, these data make it unlikely that pulmonary hypertension was prevalent or a significant factor in the overall outcome,
In addition to predicting a differential effect of treatment on FVC % over time, part of our primary hypothesis was that MMF would be less toxic and better tolerated. Consistent with this, hematopoietic suppression (specifically leukopenia and thrombocytopenia) occurred in a greater percentage of patients treated with CYC. However, this did not translate into a greater number of pneumonias, infections, SAEs or deaths. In terms of tolerability, a greater proportion of subjects in the CYC arm discontinued the drug prematurely and assignment to CYC was associated with a significantly shorter time to premature withdrawal from study drug. Moreover, most patients in the MMF arm achieved and maintained close to the target dose of MMF, whereas the tolerated dose of CYC declined over time to approximately 75% of the target dose (Supplementary Figure 6, web appendix p 18). The latter findings might have accounted for the apparently slower onset of improvement in FVC % predicted among patients in the CYC arm.
While deaths occurred in approximately twice as many participants in the CYC arm than in the MMF arm, neither the difference in mortality nor the time to death between the two treatments was statistically significant. However, the study was not powered to detect a mortality difference between the two treatment arms.
It is important to consider the particular strengths and weaknesses associated with this trial. As the second large multi-center clinical trial conducted by the SLS Investigators, it builds upon their past experience, extensive training and methods for standardization. We also employed a placebo-controlled RCT design using a unique drug administration protocol that allowed maintenance of blinding while administering two active drugs with different dosing and administration schedules.
On the other hand, potential limitations included the premature withdrawal of a significant proportion of subjects over the 24 month treatment phase (CYC 32/73, 43·8%; MMF 20/69 = 28·9%). However, this was partially mitigated by anticipating a 30% drop-out in the study design and employing a modified joint model in which patients were encouraged to return for study outcome measures even after premature withdrawal from treatment. Indeed, 15 patients in the CYC arm and 4 in the MMF arm who had discontinued their study medication prematurely returned for one or more of the 18–24 month visits and these data were included in the primary analysis. However, as already noted, the majority of these patients received potentially disease-modifying therapy that could have influenced the results. While the joint model was specifically intended to minimize such biases it does not eliminate them.23,2
Another limitation was that baseline HRCT scans from 5 patients did not meet minimal image acquisition criteria and these subjects had to be excluded from the primary analysis as they lacked the requisite CT image score as a pre-specified covariate. However, in the absence of a placebo arm (as was present in SLS I), baseline fibrosis score was not identified as a statistically significant covariate in SLS II. As a result, we performed a secondary analysis using a version of the joint model that did not require a baseline HRCT fibrosis score. However, adding back these 5 missing patients had no identifiable impact on the primary outcome. There were also 11 subjects who did not have at least 3-month outcome data and who were excluded from the analysis. However, a comparison of the baseline characteristics for all 16 excluded subjects (those without baseline CT or at least a 3 month follow-up) with the 126 subjects who were included in the analysis did not reveal any consistent or significant differences except for the presence of a higher skin score of borderline significance (p=0.042) in the subjects without outcome data.
Some might also consider our choice of oral CYC over IV CYC as a limitation given evidence suggesting that IV CYC might be safer and better tolerated. However, IV CYC was not considered logistically or financially feasible for a large blinded study compared to MMF and we are left to speculate regarding its relatively efficacy, tolerability and durability compared to MMF. Finally, given that gastrointestinal (GI) involvement is a common feature of SSc and GI toxicity is commonly associated with the administration of both MMF and oral CYC, it is possible that absorption of MMF and/or CYC might have been impaired, potentially influencing the study outcomes. Indeed, GI AE’s were the second most frequently reported category of AE’s recorded during the course of the study (see Supplementary Figure 4, web appendix p 16) although a significant between-treatment difference in the frequency of patients with GI AEs was not identified. While serum levels of MMF might have provided some insight, they were not obtained.
In summary, in patients with symptomatic SSc-ILD, we failed to find a significant difference in the primary endpoint (course of the FVC % over the 24-month trial) when comparing treatment with MMF for 24 months to CYC for 12 months followed by placebo. However, in post-hoc analyses of the primary and secondary outcomes, we found that treatment with either MMF or CYC resulted in improvements over 24 months in FVC % predicted, TDI and mRSS without any significant between-treatment differences. These responses occurred primarily between 9 and 24 months, despite the discontinuation of CYC treatment at 12 months, and they support the slow onset but potentially disease-modifying effects associated with immunosuppression in SSc-ILD. As hypothesized, MMF appeared to be better tolerated than CYC based on the time to patient withdrawal, number of treatment failures, and incidence of leukopenia and thrombocytopenia, although the latter was not associated with any difference in the incidence of infections or bleeding. These findings further substantiate the potential value of CYC or MMF in treating SSc-ILD.
Supplementary Material
Research in context.
Evidence before this study
Cyclophosphamide (CYC) was the first agent found to stabilize the progression of SSc-ILD in RCTs. Two high quality RCTs demonstrated efficacy of oral or pulse CYC compared with placebo for SSc-ILD. These findings were consistent with prior uncontrolled studies designed to assess the efficacy of CYC for SSc-ILD. However, the benefits of CYC in SSc-ILD came at the expense of a high degree of adverse effects, and an analysis of SLS I data one year after treatment cessation revealed the loss of CYC-related benefits.
Because the benefit to risk ratio of CYC for SSc-ILD is somewhat inconclusive, researchers have been actively pursuing the study of alternative agents for SSc-ILD. Mycophenolate mofetil (MMF) is an immunosuppressive agent with both anti-fibrotic and immunomodulatory effects and appears to be well tolerated in SSc. In a study of 172 SSc patients with or without ILD investigators found a lower frequency of clinically significant pulmonary fibrosis, as well as improved survival, in the MMF-treated subgroup (N=109), compared with the subgroup receiving other immunosuppressive medications (N=63). In another prospective trial of 13 patients with SSc-ILD, all of whom presented with a significant decrease in forced vital capacity (FVC) in the 12 months preceding trial entry, investigators observed a significant improvement in FVC after 12 months of treatment with MMF. Additional prospective, observational studies have affirmed that MMF is safe, well-tolerated and may prevent pulmonary function deterioration in SSc-ILD.
Given the favorable effects of MMF on SSc-ILD in observational studies, we designed Scleroderma Lung Study (SLS) II, the first randomized controlled trial to compare MMF and CYC for the treatment of symptomatic SSc-ILD. CYC was included as an active comparator since it is the only agent that has been found in previous RCTs to demonstrate efficacy in SSc-ILD.
Added value of this study
As the only randomized controlled trial to date to assess the benefits and risks of MMF in patients with SSc-ILD, this study avoids the pitfalls and biases that limit the interpretation of observational and retrospective studies.
Implications of all the available evidence
The findings from SLS II are consistent with the evidence from previous (mostly uncontrolled) studies demonstrating the efficacy of both CYC and MMF in improving lung function in patients with progressive SSc-ILD. Moreover, MMF was shown to be less toxic and better tolerated than CYC with significantly fewer withdrawals from the study medication. Concurrent improvements in skin scores suggest an overall systemic benefit and improvements in dyspnea reveal improvements in patient-centered outcomes. Taken together, these findings support the increasingly common practice of prescribing MMF for patients with symptomatic SSc-ILD.
Acknowledgments
This work was supported by grants from the NHLBI/NIH: R01 HL089758 and R01 HL089901. Study drug (mycophenolate) and matching placebo were supplied at no charge through Drug Supply Grant # CEL539 from Hoffmann-La Roche/Genentech.
The Scleroderma Lung Study II Research Group includes the authors listed in the mast head and the following support staff: E. Kissin, F.Y. Cheong (Boston University); G, Marlis, J. Mason-Berry, P. Saffold, M. Rodriguez, L. Guzman, J. Brook, G. Ibrahim, K. Largaespada (UCLA); C. Fridley, M. Zulmastashvili, A. Manu, S. Moore (Georgetown University); L. Hummers, G. Leatherman (Johns Hopkins University); F.N. Hant, K. Gibson (Medical University of South Carolina); M. Morrison (National Jewish Health); H. Donnelly, C. Marlin, J. Gangar (Northwestern University); D.A. McCloskey (Rutgers University); A. Eller, D. Leong, M. Lalosh, J. Obata (UCSF); S. Arami, D. Franklin (University of Illinois); E. Schiopu, M. Benedict-Blue, V. Leone, J. Shaw (University of Michigan); F. Tan, M. Perry, J. Anderson, A Saulino (University of Texas, Houston); P. Carey, M. Esplin (Univesity of Utah); P. Carlson (University of Minnesota).
We also acknowledge the NHLBI-appointed Data Safety and Monitoring Board (DSMB) and the members of the Mortality and Morbidty Review Committee (M&M Committee).: H. Paulus, N.S. Wenger, S. Levy (David Geffen School of Medicine at UCLA)
Declaration of interests
Philip J. Clements, MD, Eric C. Kleerup, MD, Johnathan Goldin, MD, Edgar Arriola, PharmD, Suzanne Kafaja, MD, Richard Silver, MD, Charlie Strange, MD, Fredrick Wigley, MD, David J. Riley, MD, Sabiha Hussain, MD, Vivien M Hsu, MD, Bela Patel, MD, Kristine Philips, MD, Jeffery Golden, MD, M. Kari Connolly, MD, John Varga, MD, Jane Dematte Damico, MD, Monique E. Hinchcliff, MD, Arthur Theodore, MD, Robert Simms, MD, Suncica Volkov, MD, Dean E Schraugnagel, MD, Tracy Frech, MD, Kristin Highland, MD, Charles A. Read, MD, Chi-Hong Tseng, PhD, and Robert M Elashoff, PhD report grant support from the National Heart, Lung and Blood Institute/National Institutes of Health subcontracted through the David Geffen School of Medicine during the conduct of the study.
Elizabeth R. Volkmann, MD and Marvin J. Fitzler, MD report nothing to disclose.
Donald P. Tashkin, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; personal fees from EMD Serono, non-financial support from Genentech, outside the submitted work.
Michael D. Roth, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, non-financial support from Hoffmann-La Roche/Genentech, during the conduct of the study.
Daniel E. Furst reports grants from National Heart, Lung and Blood Institute/National Institutes of Health during the conduct of the study; and Grant/Research support AbbVie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB Consultant AbbVie, Amgen, BMS, Cytori, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB Speaker’s Bureau (CME ONLY) AbbVie, Actelion, UCB.
Dinesh Khanna, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; personal fees from Bayer, grants from Bristol-Myer Squibb, personal fees from Cytori, personal fees from EMD Serono (Merck), personal fees from Forward, grants and personal fees from Genentech/Roche (InterMune), personal fees from Lycera, grants from NIH/NIAID, grants from NIH/NIAMS, grants from NIH/NIAMS, grants from PCORI, grants from Scleroderma Foundation, personal fees from Seattle Genetics, outside the submitted work.
Virginia Steen, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; grants from Intermune, grants from Boehlinger Ingelheim, grants from Genetec, grants from Sanofi, other from Bristol Myers Squib, grants from Bayer, outside the submitted work.
Robert Wise, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; grants and personal fees from AstraZeneca/Medimmune/Pearl, grants and personal fees from Boehringer Ingelheim, grants, personal fees and non-financial support from GlaxoSmithKline, personal fees from Novartis, personal fees from Vertex, personal fees and non-financial support from Merck, personal fees from Pfizer, personal fees from Sunovion, personal fees from Roche Genentech, personal fees from Janssen, personal fees from Bristol Myers Squibb, personal fees from Verona, personal fees from Takeda, outside the submitted work.
Maureen Mayes, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; grants from Intermune, grants from Roche/Genentech, grants from Bayer, grants from University of Michigan, grants from Boehringer-Ingelheim, grants from NIH/NHLBI, grants from NIH/NIAID, grants from DOD, grants from Corbus, grants from Cytori, outside the submitted work;.
Shervin Assassi, MD reports grants from National Institute of Health, during the conduct of the study; grants and personal fees from Boehringer Ingelheim, grants from Genentech, grants and personal fees from Biogen Idec, grants from Bayer HealthCare, outside the submitted work.
Fernando Martinez reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, grants from National Institutes of Health, non-financial support from Bayer, non-financial support from Centocor, non-financial support from Gilead, non-financial support from Promedior, personal fees from Ikaria, personal fees from Genentech, personal fees from Nycomed/Takeda, personal fees from Pfizer, personal fees from Vertex, personal fees from American Thoracic Society, personal fees from Inova Health System, personal fees from MedScape, personal fees from Spectrum Health System, personal fees from University of Texas Southwestern, personal fees from Stromedix/Biogen, personal fees from Axon Communications, personal fees from Johnson & Johnson, personal fees from Genzyme, personal fees from National Association for Continuing Education, personal fees from Boehringer Ingelheim, personal fees from Veracyte, personal fees from AcademicCME, grants from Boehringer Ingelheim, grants from Roche/Genentech, personal fees from Falco, personal fees from Kadman, during the conduct of the study; personal fees from Forest, personal fees from Janssens, personal fees from GSK, personal fees from Nycomed/Takeda, personal fees from Amgen, personal fees from Astra Zeneca, personal fees from CSA Medical, personal fees from Ikaria/Bellerophon, personal fees from Forest, personal fees from Genentech, personal fees from GSK, personal fees from Janssens, personal fees from Merck, personal fees from Pearl, personal fees from Nycomed/Takeda, personal fees from Pfizer, personal fees from Roche, personal fees from CME Incite, personal fees from Inova Health System, personal fees from Miller Medical, personal fees from National Association for Continuing Education, personal fees from Paradigm, personal fees from Peer Voice, personal fees from St. John’s Hospital, personal fees from St. Mary’s Hospital, personal fees from UpToDate, personal fees from GSK, personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees from Nycomed/Takeda, personal fees from Informa, personal fees from Annenberg, personal fees from California Society for Allergy and Immunology, personal fees from Haymarket Communications, personal fees from Integritas, personal fees from InThought, personal fees from Western Society of Allergy and Immunology, personal fees from AstraZeneca, personal fees from Theravance, personal fees from Boehringer ingelheim, personal fees from Carden Jennings, personal fees from Novartis, personal fees from Sunovion, personal fees from Novartis, personal fees from Axon, outside the submitted work.
Aryeh Fischer, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; personal fees from Actelion, from Bayer, from Boehringer-Ingelheim, from Genentech, from Glaxo Smith Kline, from Bristol Meyer Squibb, from Seattle Genetics, outside the submitted work;
Richard Meehan MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; grants from DOD grant W81XWH-11-1-0216 Development of a Morphometric Approach to quantification of Small Airways Disease and a Particulate Matter Exposure in lung Biopsies of Deployed US Military Personnel, outside the submitted work.
Jeffery Swigris, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; personal fees from Genetech, personal fees from Boehringer-Ingelheim, outside the submitted work.
Mary Beth Scholand, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; personal fees and other from Genentech, personal fees and other from Boehringer Ingelheim, other from MedImmune, other from Firbogen, personal fees from Mallinckrodt, other from Gilead, outside the submitted work.
Jerry A Molitor, MD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; other from Actelion, outside the submitted work.
Grace Hyun J Kim, PhD reports grants from National Heart, Lung and Blood Institute/National Institutes of Health, during the conduct of the study; In addition, Dr. Kim has a patent AUTOMATED IMAGE SYSTEM FOR SCORING CHANGES IN QUANTITATIVE INTERSTITIAL LUNG DISEASE issued.
Footnotes
Authors and contributors: All authors met contribution criteria consistent with the ICMJE recommendations. DPT, MDR and RME had primary responsibility for the design, conduct, interpretation and reporting of the study with C-HT and RME as the principal study biostatisticians. Primary writing of the manuscript was carried out by DPT, MDR, ERV, and C-HT with significant contributions to the final manuscript by PJC, DEF, DK, GHJK, and JG. All authors contributed to the repeated review, revision and final approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 2.Nihtyanova S, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66:1625–35. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Elashoff R, Clements PJ, et al. Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 4.Goldin J, Elashoff R, Kim HJ, et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest. 2009;136:1333–40. doi: 10.1378/chest.09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HJ, Brown MS, Elashoff R, et al. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosophamide. Eur Radiol. 2011;21:2455–65. doi: 10.1007/s00330-011-2223-2. [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP, Elashoff R, Clements PJ, et al. Scleroderma Lung Study Research Group. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furst DE, Tseng CH, Clements PJ, Strange C, Tashkin DP, Roth MD, Khanna D, Li N, Elashoff R, Schraufnagel DE Scleroderma Lung Study. Adverse events during the Scleroderma Lung Study. Am J Med. 2011;124:459–67. doi: 10.1016/j.amjmed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FJ, McCune JW. Cyclophosphamide for scleroderma lung disease. N Engl J Med. 2006;354:2707–9. doi: 10.1056/NEJMe068095. [DOI] [PubMed] [Google Scholar]
- 9.Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88:1351–89. doi: 10.1007/s00204-014-1247-1. [DOI] [PubMed] [Google Scholar]
- 10.Hahn BH, McMahon MA, Wilkinson A, et al. American college of rheumatology guidelines for screening, treatment and management of lupus nephritis. Arthritis Care Res. 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nihtyanova SI, Brough GM, Black CM, Denton CP. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis–a retrospective analysis. Rheumatology (Oxford) 2007;46:442–5. doi: 10.1093/rheumatology/kel244. [DOI] [PubMed] [Google Scholar]
- 12.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133:455–60. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 13.Swigris JJ, Olson AL, Fischer A, Lynch DA, Cosgrove GP, Frankel SK, Meehan RT, Brown KK. Mycophenolate mofetil is safe, well tolerated and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130:30–6. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 14.Zamora AC, Wolters PJ, Collard HR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008;102:150–55. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, et al. Effect of mycophenolate sodium in scleroderma-related interstitial lung disease. Clin Rheumatol. 2011;30:1393. doi: 10.1007/s10067-011-1823-1. [DOI] [PubMed] [Google Scholar]
- 16.Fischer A, Brown KK, DuBois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40:640–6. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masi T Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheumatol. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 19.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis (SSc): An assessment of inter-observer variability in three independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 20.Mahler DA, Ward J, Fierro-Carrion G, et al. Development of self-administered versions of modified baseline and transition dyspnea indexes in COPD. COPD: J of COPD. 2004;1:1–8. doi: 10.1081/copd-120030829. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Tashkin DP, Clements PJ, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol. 2010;5:S26–35. [PMC free article] [PubMed] [Google Scholar]
- 22.Roth MD, Tseng CH, Clements PJ, et al. Scleroderma Lung Study Research Group. Predicting Lung function and treatment outcomes in scleroderma-related interstitial lung disease. Arthritis Rheumatism. 2011;63:2797–808. doi: 10.1002/art.30438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Elashoff RM, Li G, Tseng CH. Joint analysis of bivariate longitudinal ordinal outcomes and competing risks survival times with nonparametric distributions for random effects. Stat Med. 2012;31:1707–21. doi: 10.1002/sim.4507. [DOI] [PubMed] [Google Scholar]
- 24.Elashoff RM, Li G, Li N. A joint model for longitudinal measurements and survival data in the presence of multiple failure types. Biometrics. 2008;64:762–771. doi: 10.1111/j.1541-0420.2007.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashkin DP, Volkmann ER, Tseng CH, et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann Rheum Dis. 2016;75:374–81. doi: 10.1136/annrheumdis-2014-206076. [DOI] [PubMed] [Google Scholar]
- 26.Khanna D, Furst DE, Hays RD, et al. Minimally important difference in diffuse systemic sclerosis: results of the D-penicillamine study. Ann Rheum Dis. 2006;65:1325–9. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna D, Tseng CH, Furst DE, et al. Minimally important differences in the Mahler’s Transition Dyspnoea Index in a large randomized controlled trial—results from the Scleroderma Lung Study. Rheumatology (Oxford) 2009;48:1537–40. doi: 10.1093/rheumatology/kep284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Li M, Zhang Y, Shi X, Li L, Jin M. The effects and mechanisms of mycophenolate mofetil on pulmonary arterial hypertension in rats. Rheumatol Int. 2010;30:341–8. doi: 10.1007/s00296-009-0966-8. [DOI] [PubMed] [Google Scholar]
- 29.Saketkoo LA, Lammi MR, Fischer A, Molitor J, Steen VD on behalf of the PHAROS Investigators. Observations from the pulmonary hypertension recognition and outcomes in scleroderma (Pharos) Cohort [Abstract] Ann Rheum Dis. 2015;74:820. [Google Scholar]
- 30.Khanna D, Hayes RD, Maranian P, et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 2009;61:1257–63. doi: 10.1002/art.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae S, Allanor Y, Furst DE, et al. Associations between a scleroderma-specific gastrointestinal instrument and objective tests of upper gastrointestinal involvements in systemic sclerosis. Clin Exp Rheumatol. 2013;31:57–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.