To the Editor
A recent comprehensive and systematic review of worldwide traffic emissions and health science by a Special Panel convened by the Health Effects Institute (HEI) found sufficient evidence that exposure to traffic-related air pollution (TRAP) causes asthma exacerbation in children1. Diesel exhaust particles (DEP) represent the major component of TRAP particulate matter and the main contributor to TRAP-related asthma exacerbations in children. We have previously shown that in children with allergic asthma, TRAP exposure is associated with earlier sensitization, and increased asthma prevalence and severity2, 3. Asthma severity, defined as more frequent weekly symptoms, was associated with increased IL17A but not IL4, IL5 or IL13 blood levels2. Indeed, although asthma has long been described as a disease resulting from an abnormal TH2 immune response to environmental allergens, accumulating evidence suggests a role for TH17 cells, especially in severe asthma4. A recent study demonstrated that dual-positive TH2/TH17 cells and IL17A were present at a higher frequency in the bronchoalveolar lavage fluid (BALF) of patients with severe asthma5. Further, they found these TH2/TH17 cells were resistant to dexamethasone-induced cell death. We recently reported that DEP co-exposure augmented allergen-induced airway hyper-responsiveness (AHR), eosinophilia, TH2 and TH17 cytokines levels, and resulted in increased numbers of TH2/TH17 cells in the BALF2. Collectively, these data suggest that a subgroup of asthmatics with high DEP exposure and mixed TH2/TH17 responses may benefit from anti-IL17A therapy alone or in combination with steroids. Although inhibition of IL17 receptor A did not result in significant improvement among subjects with moderate to severe asthma in a recent randomized control trial6, targeted anti-IL17 therapy in a subset of severe asthmatic patients with high DEP exposure may be beneficial alone or in combination with steroids.
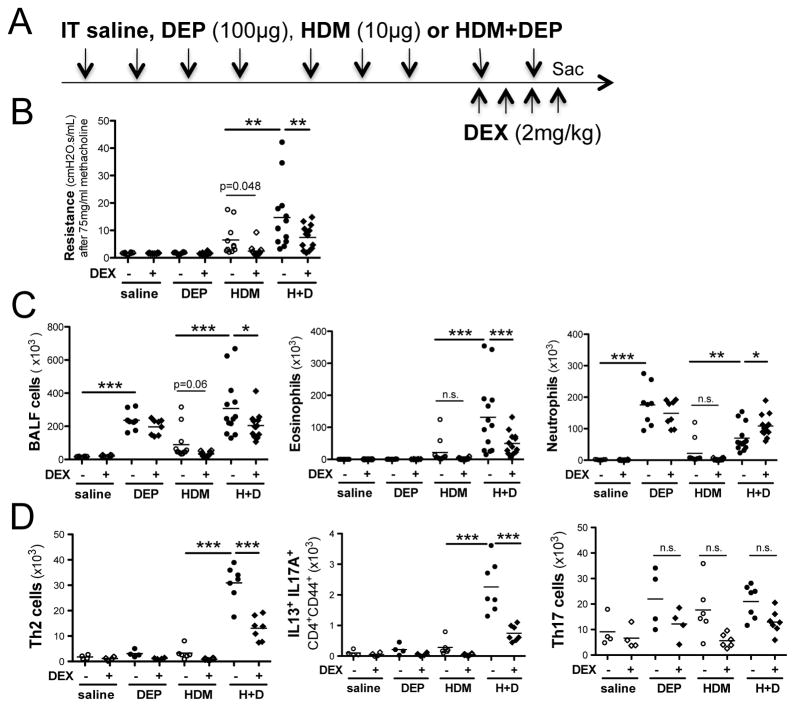
A significant proportion of patients with asthma remain symptomatic despite treatment with high-dose inhaled corticosteroids (ICS). This group has been defined as having severe asthma, refractory asthma, or treatment-resistant asthma. This group of patients comprises about 15% of asthmatics but accounts for >50% of health care utilization related to this disease7. Although it is evident that DEP exposure results in increased asthma severity in children and in mouse models2, 3, the impact of DEP exposure on steroid responsiveness in the context of asthma is largely unknown. Hence, our goal was to directly examine the impact of DEP exposure on the steroid sensitivity of allergen-induced asthma. Specifically, we exposed BALB/c mice intratracheally to either saline, DEP(100ug), house dust mite extract (HDM; Greer; 10ug) or HDM+DEP 9 times over a 3-week period as previously described2, 3. Once asthma was established, mice were treated with once daily dexamethasone (2mg/kg) or a vehicle control as outlined in Figure 1A. This dose of dexamethasone was effective and resulted in weight loss, decreased spleen size, and decreased lung cells in all animals (supplemental Figure S1A–B). As we have previously shown2, co-exposure to DEP significantly increased HDM-induced AHR compared to exposure to HDM alone (Figure 1B). Dexamethasone treatment abrogated HDM-induced AHR. Interestingly, one mouse in this group did not respond. This mouse was treated with a 25% lower dexamethasone dose due to its small size suggesting that perhaps there is a threshold dose that is required for optimal responsiveness. In contrast, 4 days of systemic steroid treatment only partially reduced AHR in mice co-exposed to HDM+DEP (Figure 1B). Dexamethasone abrogated cell recruitment, including eosinophils into the airways in HDM-exposed mice (Figures 1C). HDM-exposed mice did not exhibit a significant neutrophilic recruitment into the airways, in contrast to DEP-exposed mice, which had a strong neutrophilic response. However, the neutrophilia remained unchanged following treatment with dexamethasone (Figure 1C). HDM+DEP-exposed mice had a mixed eosinophilic and neutrophilic response as we previously reported2. Dexamethasone treatment of co-exposed mice resulted in a partial decrease in BALF eosinophil levels and no change in neutrophil levels resulting in a shift towards predominant neutrophilic BALF inflammation following dexamethasone treatment (Figure 1C).
Figure 1. Impact of steroid treatments on HDM+DEP induced asthma exacerbations.
(A) Protocol (it, intratracheal. ip: intraperitoneal). (B) Airway resistance to methacholine challenges was assess using FlexiVent (n=8–13 mice/group from 2 separate experiments) (C) Total BALF cell numbers, BALF eosinophil and neutrophil counts. (D) Lung TH2 and TH17 cells were identified by their expression of CD3, CD4, CD44 and IL13 or IL17A respectively following acquisition on a FACS Canto III (Becton Dickinson, Mountain View, Calif) and analyzed with FlowJo software (Tree Star, Ashland, Ore). n=4–7 mice/group; n.s.: not significant, *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Bonferroni multiple comparison test.
Next, we examined the T cell populations present in the lungs of asthmatic mice following dexamethasone treatment. As mentioned above, dexamethasone decreased the total number of lung cells regardless of exposure (supplemental Figure S1B). Exposure to HDM induced a modest increase in lung IL13+TH2 cells, which was completely abrogated following dexamethasone treatment (supplemental Figure S1C). In contrast, HDM+DEP exposure resulted in a marked increase in lung IL13+CD4+TH2 cells, which was only partially abrogated by dexamethasone (supplemental Figure S1C). Dexamethasone treatment decreased lung T cell levels, but the decrease in TH17 cells did not reach significance while the TH2 cells were markedly decreased following dexamethasone (Figure 1D).
While TH17 cells and dual-positive TH2/TH17 cells are thought to be steroid resistant4, 8, 4 days of dexamethasone treatment did significantly decrease IL13/IL17A double producers in HDM+DEP co-exposed mice (Figure 1D). One potential explanation is that the treatment regimen we utilized was high and may have had a profound systemic impact. Indeed, dexamethasone treated mice lost about 5–10% of their body weight and we observed a dramatic decrease in their spleen size and weight (supplemental Figure S1A). Thus, the dexamethasone treatment regimen that we utilized appears to be more immunosuppressive than “comparable doses” of corticosteroid used in humans, as previously suggested9. Even if dexamethasone did not directly impact TH17 cell survival, it may affect the generation of cytokines important in their induction or maintenance. Indeed, dexamethasone decreased not only lung mRNA levels of IL4 but also TH17-related cytokines including IL1β, IL6 and IL17A in mice co-exposed to HDM+DEP (supplemented Figure S2). Nevertheless, dexamethasone treatment only partially reduced the number of TH17 and IL13/IL17A double producing cells in the lungs of mice co-exposed to HDM+DEP. Since we previously demonstrated a role for IL17A in HDM+DEP–induced asthma exacerbations2, we investigated the impact of anti-IL17A treatment alone or in combination with dexamethasone on HDM+DEP-induced AHR. Briefly, we delivered anti-IL17A (200 μg) or an IgG1 control antibody intra-tracheally as depicted in Figure 2A. In contrast to our prior published work where prophylactic treatment with similar doses of anti-IL17A significantly alleviated HDM+DEP-induced AHR exacerbations2, blocking IL17A once disease is established failed to significantly decrease AHR (Figure 2B). Dexamethasone treatment alone had only a modest impact on AHR (Figure 2B). Anti-IL17A treatment, in combination with dexamethasone, significantly reduced AHR compared to either treatment alone (Figure 2B), supporting the notion that IL17A contributes to steroid resistance. Dexamethasone treatment decreased BALF eosinophilia and promoted BALF neutrophilia as expected (Figure 2C). Similar to our previous observation2, anti-IL17A treatment did not alter BALF neutrophil levels (Figure 2C), most likely due to the fact that DEP can directly stimulate epithelial cells to secrete neutrophil chemokines.
Figure 2. Combination treatment abrogates HDM+DEP induced AHR.
(A) Protocol: 4 days of treatment. (B) Airway resistance following treatment with dexamethasone (2mg/kg) and/or anti-IL17A (200 mg) or an IgG1 control antibody (n=3–6 mice/group) (C) BALF eosinophil and neutrophil counts. (D) Protocol: 1 week of treatment. (E) Airway resistance and (F) BALF eosinophil and neutrophil counts. (S: saline, H: HDM, HD: HDM+DEP; n=4–8 mice/group; 2-way ANOVA, *P < .05, **P < .01, ***P < .001).
Although the combination treatment with dexamethasone and anti-IL17A was effective, the combination did not fully abrogate AHR in HDM+DEP exposed mice, thus we increased the total dose and duration dexamethasone treatment as indicated in Figure 2D. Increasing the magnitude of the dexamethasone treatment significantly but incompletely reduced AHR compared to untreated mice exposed to HDM+DEP (Figure 2E). Adding anti-IL17A treatment to the enhanced dexamethasone regimen resulted in complete abrogation of AHR in all mice (Figure 2E). While these results suggest that increasing steroid dosage and/or duration of treatment can reduce DEP-induced asthma exacerbations, hard-to-treat patients respond poorly to high doses of steroids and more aggressive steroid doses would be associated with unacceptable side effects. Thus, patients with allergic asthma who are exposed to high levels of DEP may benefit from combination treatment with ICS and anti-IL17A.
Recently, a randomized double-blind placebo-controlled study of an anti-IL17 receptor monoclonal antibody (Brodalumab) demonstrated positive results only in a subgroup of moderate to severe asthmatics6, underscoring the need to identify specific asthma endotypes that would benefit from blocking the IL17 pathway and/or DEP-associated neutrophilia. Our data suggest that patients with exposure to high levels of TRAP/DEP characterized by elevated levels of serum IL17A and/or increased numbers of TH2/TH17 double producers may represent this treatment responding endotype.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences (NIEHS) T32 ES010957 (EBB) and NHLBI R01HL097135 (GKKH).
The DEP was kindly provided by Ian Gilmour (EPA, Research Triangle Park, NC) and the anti-IL17A (M210) and control IgG1 (4D2) antibody by Amgen (Seattle, WA). We thank Paige Bolcas, Brandy Ruff, and Stacey Bass for technical assistance and Cynthia Chappell for editorial assistance.
Footnotes
Conflict of interest: The other authors have declared that there are no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.HEI Panel on the Health Effects of Traffic-Related Air Pollution. HEI Special Report 17. Boston, MA: Health Effects Institute; 2010. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. [Google Scholar]
- 2.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–204. e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol. 2015;136:295–303. e7. doi: 10.1016/j.jaci.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 5.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–86. e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 7.Bell MC, Busse WW. Severe asthma: an expanding and mounting clinical challenge. J Allergy Clin Immunol Pract. 2013;1:110–21. doi: 10.1016/j.jaip.2013.01.005. quiz 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin RA, Hodgkins SR, Dixon AE, Poynter ME. Aligning mouse models of asthma to human endotypes of disease. Respirology. 2014;19:823–33. doi: 10.1111/resp.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.