Abstract
Gut barrier disruption is often implicated in pathogenesis associated with burn and other traumatic injuries. In this study, we examined whether therapeutic intervention with mesalamine (5-ASA), a common anti-inflammatory treatment for patients with inflammatory bowel disease, reduces intestinal inflammation and maintains normal barrier integrity after burn injury. Male C57BL/6 mice were administered an ~20% total body surface area dorsal scald burn and resuscitated with either 1mL normal saline or 100mg/kg of 5-ASA dissolved in saline. We examined intestinal transit and permeability along with levels of small intestine epithelial cell pro-inflammatory cytokines and tight junction protein expression one day after burn injury in the presence or absence of 5-ASA. A significant decrease in intestinal transit was observed one day after burn injury, which accompanied a significant increase in gut permeability. We found a substantial increase in the levels of IL-6 (by ~1.5 fold) and IL-18 (by ~2.5 fold) in small intestine epithelial cells one day after injury. Furthermore, burn injury decreases expression of the tight junction proteins claudin-4, claudin-8, and occludin. Treatment with 5-ASA after burn injury prevented the burn induced increase in permeability, partially restored normal intestinal transit, normalized levels of the pro-inflammatory cytokines IL-6 and IL-18, and restored tight junction protein expression of claudin-4 and occludin to that of sham levels. Together these findings suggest that 5-ASA can potentially be used as treatment to decrease intestinal inflammation and normalize intestinal function after burn injury.
Keywords: Burn, Mesalamine (5-ASA), Gut Barrier, Transit, Inflammation, Tight Junction Proteins
Introduction
Over 450,000 individuals suffer from burn injury every year, and the American Burn Association estimates that 40,000 of those individuals consequently require hospitalization1. Burn injury remains a prominent medical issue to be resolved, not only due to the sheer number of injuries each year, but also due to the fact that burn trauma results in patients with increased risk of sepsis, progressing to septic shock, and ending in multiple organ dysfunction2, 3. Sepsis and multiple organ dysfunction syndrome (MODS) continue to be the leading causes of burn related mortality4.
Immediately after the initial burn injury, systemic inflammation ensues affecting organs such as the lungs, liver, and the intestines. Intestinal inflammation has been shown to contribute to adverse consequences of cell death and stress, which can then lead to breakdowns in gut barrier integrity5-9. Stalled intestinal peristalsis has been suggested to be connected to increases in intestinal inflammation which could potentiate increases in intestinal permeability10-12. The inability to effectively move luminal content down the GI tract could dramatically change the luminal microenvironment creating more favorable environments for opportunistic pathogens10, 11, 13-16. With over 100 trillion microbes present in the gastrointestinal tract, the integrity of the gut barrier is of utmost importance, and under normal homeostatic conditions, tolerance exists between the resident intestinal microbes and defenses of the gastrointestinal tract17-19. However, our lab has recently demonstrated that burn injury leads to drastic alterations in the intestinal microbiome, which correlated with increases in intestinal permeability and inflammation20, 21.
These burn related symptoms of intestinal inflammation, microbial dysbiosis, and permeability compounded with inhibitions in normal intestinal peristalsis post injury could be potentiating increased risk of sepsis in burn patients. It is of interest to note the parallels between intestinal symptoms post burn trauma to that of those present in patients with Inflammatory Bowel Disease (IBD). As IBD mimics many of the inflammatory symptoms seen in the intestine after burn injury, applying common therapeutics currently used in IBD treatment and prevention, such as mesalamine, for the treatment of burn injury could yield promising results. Mesalamine is known to reduce intestinal inflammation through the inhibition of NF-κB. Others have suggested that it activates the heat shock protein (HSP) cytoprotective response22-27. Although its benefits in the treatment of ulcerative colitis and Crohn's disease have been extensively studied, mesalamine's potential for therapeutic intervention has yet to be applied in the context of burn injury. Therefore, we asked whether 5-ASA could potentially be used as a novel therapy to inhibit burn related breakdowns in gut barrier integrity. Reducing intestinal permeability and inflammation after burn via therapeutics could potentially help in alleviating the risk of sepsis and other post burn complications.
Materials and Methods
Animals
Male C57BL/6 mice, 8-9 week old, weighing 22-25g, were obtained from Charles River Laboratories and used in all experiments. Animals were allowed to acclimate to the facility for 7-10 days before being used for the experiments. All experiments were conducted in accordance with the guidelines set forth by the Animal Welfare Act and were approved by the Institution Animal Care and Use Committee at the Loyola University Chicago Health Sciences Division.
Murine Model of Burn Injury
Mice were anesthetized with xylazine and ketamine, their dorsal surface shaved, and placed in a template exposing ~20% total body surface area (TBSA) as calculated by the Meeh formula as described by Walker and Mason28. The mice were divided into two treatment groups, those receiving burn injuries or sham injuries. The burn group was then submerged in a water bath set to 85-95°C for 7-9 seconds while the sham group was submerged in a water bath set to 37°C. Following burn or sham burn, mice were resuscitated with 1ml of normal saline. This procedure models a severe ~20% TBSA full thickness third degree burn.
Mesalamine Treatment
Mice were divided into four groups: sham plus saline, sham plus 5-ASA, burn plus saline, and burn plus 5-ASA. The burn or sham burn proceeded as described above. Immediately after burn or sham injury, mice were given an intraperitoneal injection of either 1 mL of normal saline as described above or 100mg/kg of 5-ASA dissolved in 1 mL of saline used for the normal resuscitation. The dose for 5-ASA was selected from a previous study, and it most closely mimics the dosage used to treat IBD patients29, 30
FITC-dextran Assay for the Assessment of Intestinal Transit and Permeability
Intestinal permeability and transit were assessed as described in a previous study. In short, one day after burn or sham injury mice were gavaged with 0.4ml of 22mg/ml FITC-dextran in PBS. After 3 hours, blood was drawn, and the mice were sacrificed. The blood was centrifuged at 8000rpm for 10min at 4°C, plasma isolated, and read spectrophotometrically at 480nm excitation and 520nm emission wavelengths for intestinal permeability
For the measurement of intestinal transit, various parts of the GI tract (starting from stomach to the colon) were harvested. The small intestine was divided into three equal parts with section #1 being proximal and section #3 being distal to the stomach. The luminal contents of stomach, small intestinal section #1, #2, and #3, and large intestine were collected, suspended in PBS (weight/volume) and sonicated (XL-2000 Misonix) until the solution was homogenous. Homogenates were centrifuged at 8000rpm for 10min at 4°C, supernatants were collected, and read spectrophotometrically at 480nm excitation and 520nm emission wavelengths for intestinal transit.
Intestinal Epithelial Cell Isolation
Isolation of intestinal epithelial cell was performed as described previously by Weigmann et al31. In short, the distal 10cm of the small intestine was separated, cut longitudinally, and placed in ice cold PBS + 1% penicillin/streptomycin (pen/strep) cocktail. Following two washes in PBS + pen/strep, tissues were placed in a digestion solution containing 5% heat-inactivated fetal bovine serum (FBS), 1% HEPES, 1% pen/strep, 0.5% gentamicin, 5mM EDTA, and 1mM dithiothreitol (D.T.T.) in Hank's Balanced Salt Solution (HBSS) at 37°C. Tissues were placed in a 37°C incubator and shaken on a rotator at 250rpm for 20 minutes. Tissues were vortexed to separate the epithelial cells from the tissue and passed through a 100μm filter. Cells were counted on a hemocytometer to determine epithelial cell purity (≥90%). Intestinal epithelial cells were then processed for downstream applications.
RNA Isolation and cDNA synthesis
RNA isolation was performed using a RNeasy Mini Kit (Qiagen, Valencia, CA) as described by the manufacturer. Genomic DNA was removed by DNase digestion using an RNase-free-DNase Set (Qiagen). Isolated RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Bannockburn, IL). Only samples with a 260/280 ratio of ≥2.0 were used for cDNA synthesis. cDNA synthesis was performed using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA) and reactions were run on a Veriti 96-well Fast Thermocycler (Life Technologies) per the manufacturer's instructions.
Real-Time PCR
Expression of claudin-4, claudin-8, occludin, HSP25, and HSP72 mRNA levels were analyzed by qPCR using TaqMan primer probes and TaqMan Fast Advanced Master Mix (Life Technologies). Target gene Ct cycle values were normalized to housekeeping control GAPDH or β-actin Ct values. Data were calculated using the ΔΔCt method, and all groups were expressed relative to the sham group.
Cytokine quantification
IECs were isolated from the distal 10cm of the small intestine and allowed to incubate in 500uL of 1X cell lysis buffer (Cell Signaling Technology) containing 1mM PMSF, 1X Protease inhibitor, and 1X Phosphatase Inhibitor (Cell Signaling Technology). The homogenates were centrifuged at 10,000 RPM for 5min and the supernatant was removed, aliquoted, and stored in −80°C for IL-18 (eBioscience), IL-6 (BD), or KC (R&D) ELISAs. Protein measurements of the same samples were done from Bio-Rad protein assay kit. Data were normalized as amount of cytokine/mg protein.
Statistics
The data, wherever applicable, are presented as means + SEM and were analyzed using analysis of variance (ANOVA) with Tukey's post-hoc test or Student t test (GraphPad Prism6). Unless otherwise noted, p < 0.05 between two groups is considered statistically significant.
Results
Decreases in intestinal transit one day after burn injury
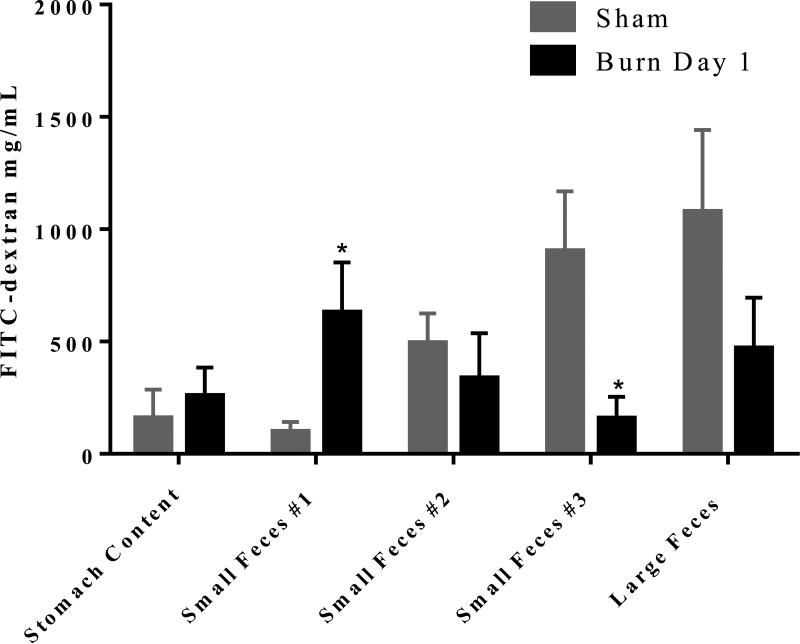
As mentioned above, we recently observed dramatic dysbioses of the intestinal microbiome and increases in intestinal permeability one day following burn injury20. These two burn related consequences compounded with stalled intestinal peristalsis could contribute to intestinal bacterial translocation to extraintestinal sites as observed in many previous studies20, 21, 32, 33. Therefore, it is critical to determine whether burn injury inhibits intestinal peristalsis. Hence, we performed a FITC-dextran transit assay. Mice were gavaged with FITC-dextran one day after burn injury. Three hours later plasma, luminal contents of the stomach, small intestine sections #1, #2, and #3, and large intestine were collected and analyzed for the presence of FITC spectrophotometrically. We found a significant decrease in normal intestinal transit in burn injured mice as evidenced by an accumulation of FITC-dextran in the stomach content and proximal part of the small intestine (Figure 1). This accumulation of FITC-dextran in the first section of the small intestine of burn animals was found to be significantly higher compared to that of sham animals. In sham injured mice most of the dye accumulation was observed in distal ileum (intestine section #3) or in colon. Together, these results suggest a decrease in intestinal transit in mice receiving burn injury compared to shams (Figure 1).
Figure 1. Decreases in intestinal transit one day after burn injury.
Mice were gavaged with FITC-dextran one day after burn or sham injury. Three hours after FITC-dextran gavage stomach content, small intestine luminal content divided into three equal lengths (#1 being proximal to stomach and #3 being distal), and large intestine feces were collected and visualized spectrophotometrically for presence of FITC-dextran. Values are mean ± SEM of 6-8 animals per group. *, p<0.05 burn day 1 compared to sham by Student's t-test at each intestinal section.
Partial restoration of intestinal transit with 5-ASA treatment after burn injury
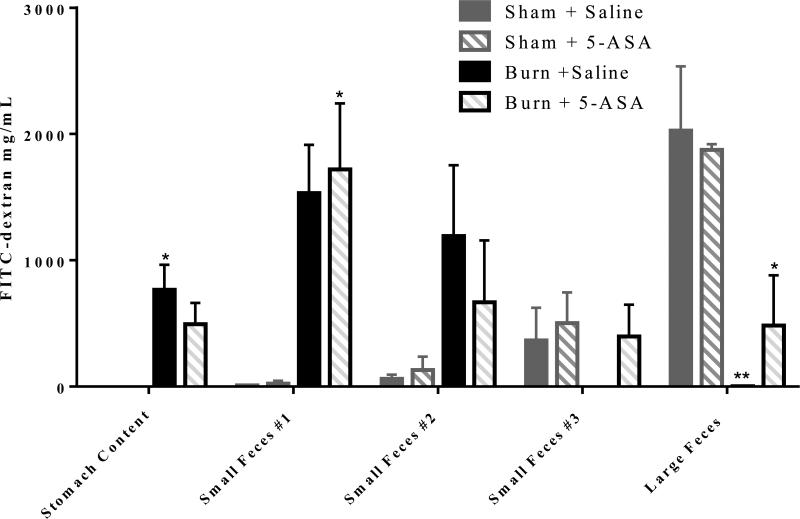
We further examined whether treatment of mice with 5-ASA re-establishes normal intestinal transit in mice after burn injury. As shown in Figure 2, treatment with 5-ASA following burn injury prevented the decrease in intestinal transit. The results further demonstrate that most of the FITC accumulation in untreated and 5-ASA treated sham animals is in the colon. By contrast, the FITC-dextran transit is halted in burned animals. As shown in Figure 2, burn mice have very little FITC-dextran in the colon and the vast majority of FITC-dextran remains in the stomach and small intestinal content #1. Treatment with 5-ASA post burn allows for more efficient transit of FITC-dextran as more FITC-dextran can be detected in large feces with treatment (Figure 2). It is not, however, back to sham control levels.
Figure 2. 5-ASA treatment partially restores normal intestinal transit one day after burn injury.
Mice were gavaged with FITC-dextran one day after burn or sham injury. Three hours after FITC-dextran gavage stomach content, small intestine luminal content divided into three equal lengths (#1 being proximal to stomach and #3 being distal), and large intestine feces were collected and visualized spectrophotometrically for presence of FITC-dextran. Values are mean ± SEM of 4-6 animals per group. *, p<0.05, **, p<0.01 all groups compared to sham saline by ANOVA and Tukey multiple comparison tests.
5-ASA treatment significantly reduces observed increases in intestinal permeability post burn injury
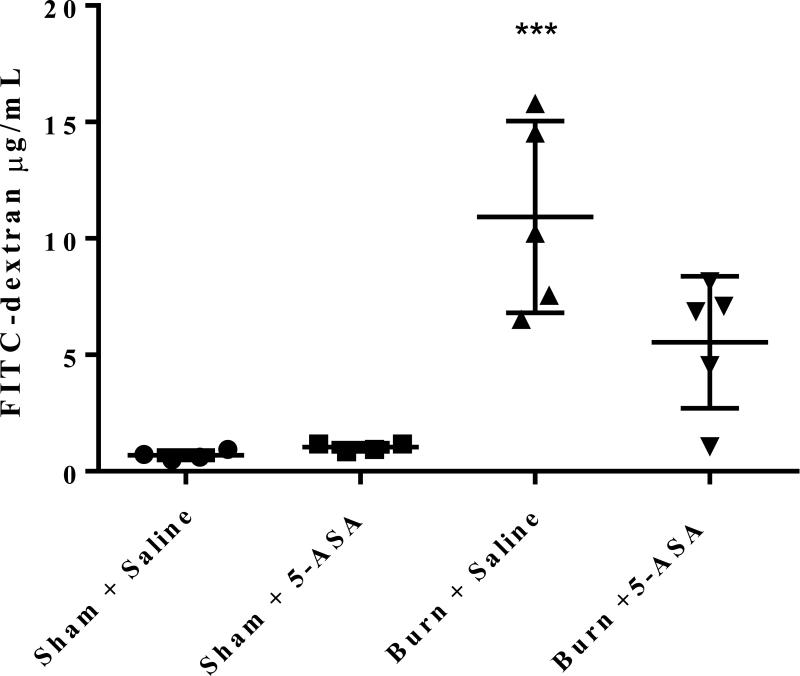
To further determine whether 5-ASA treatment restores burn related increases in intestinal permeability, one day after injury mice were gavaged with FITC-dextran and three hours later plasma was collected and analyzed for the presence of FITC spectrophotometrically. As reported previously, the concentration of FITC-dextran in the plasma was significantly increased in mice receiving burn injury alone compared to the sham saline group20 (Figure 3). However, upon treatment with 5-ASA following burn, the increase in intestinal permeability was restored to that of sham levels as there is no statistically significant difference in FITC-dextran concentrations in the plasma between sham controls and 5-ASA treated burn animals (Figure 3).
Figure 3. Treatment with 5-ASA following burn injury significantly reduces intestinal permeability to that of sham levels one day after injury.
Mice were gavaged with FITC-dextran one day after either burn or sham injury. Three hours later FITC-dextran was measured in plasma spectrophotometrically. Values are mean ± SEM of 4-6 animals per group. ***, p<0.0001 burn saline compared to sham control by ANOVA and Tukey multiple comparison tests.
Tight junction expression following 5-ASA treatment
Tight junction (TJ) proteins uphold the physical intestinal barrier by joining two adjacent intestinal epithelial cells restricting the large number of bacteria present in the GI tract to the luminal space34-36. Any change in expression of tight junction proteins after burn injury could potentially break this selective barrier allowing an increase in bacterial translocation. Our lab has previously seen decreases in TJ mRNA expression in small intestinal total tissue homogenates after burn12, 20. Yet, it was critical to understand whether burn injury results in decreased TJ expression in small IECs, and more specifically whether 5-ASA treatment could restore potential decreases in IEC TJ proteins after burn injury.
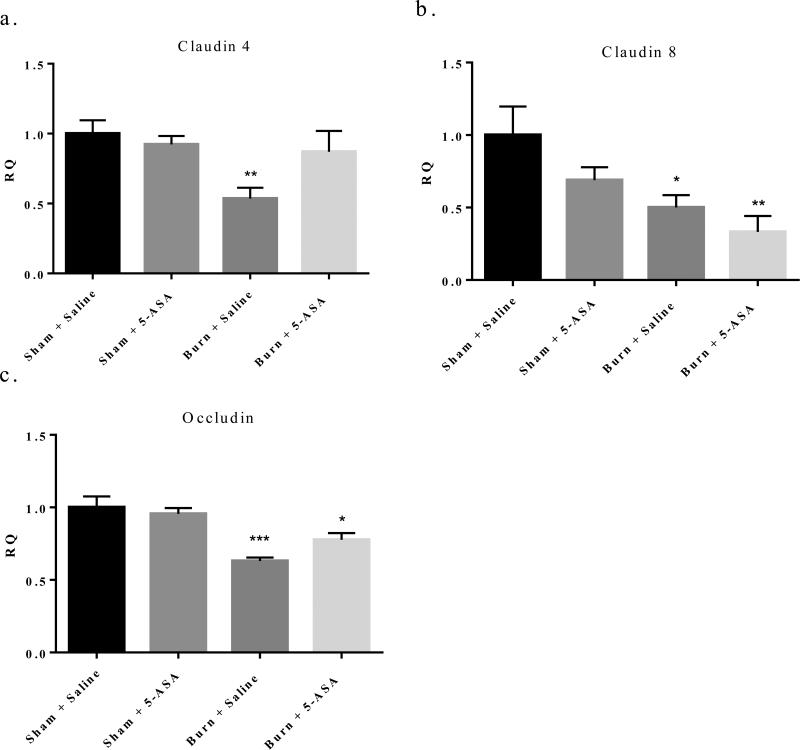
Therefore, we profiled the mRNA expression of several key TJ proteins, claudin-4, claudin-8, and occludin, in small IECs one day following either burn or sham injury and with or without 5-ASA treatment. We observed a 54% decrease in claudin-4, a 49% decrease in claudin-8, and a 38% decrease in occludin expression relative to sham levels one day post burn injury as can be seen in Figure 4a, b, and c respectively. Treatment with 5-ASA after burn injury was able to restore claudin-4 expression back to that of sham levels one day following injury (Figure 4a). 5-ASA treatment partially restored occludin expression in burn injured mice, but it was not back to sham levels (Figure 4c). Conversely, similar treatment of mice with 5-ASA did not influence claudin-8 expression after burn injury (Figure 4b).
Figure 4. Treatment with 5-ASA at time of burn injury significantly restores small intestine IEC claudin-4 expression to that of sham levels one day after injury, while a trend toward restoration exists of occludin expression in small intestine IECs one day post injury.
q-RTPCR of claudin-4, claudin-8, and occludin mRNA expression relative to β-actin. Values are mean ± SEM of 4-7 animals per group expressed relative to sham. **, p<0.01 burn day one claudin-4 compared to sham saline by Student's t-test. *, p<0.05, **, p<0.01, ***, p<0.001 burn day one claudin-8 and occludin compared to sham saline by ANOVA and Tukey post hoc test.
Reductions in post burn induced IEC inflammation following 5-ASA treatment
Burn injury is associated with high levels of inflammation in the gastrointestinal tract, which can potentiate the increase in intestinal permeability. We broadened our analysis of 5-ASA treatment to determine whether 5-ASA could be deemed beneficial in reducing the high levels of intestinal pro-inflammatory cytokines and chemokines after burn injury. We, and others, have previously seen elevations of IL-18, IL-6, and KC post burn injury37-41. Yet, treatment with 5-ASA significantly reduced the small intestine IEC pro-inflammatory cytokine IL-18, which increased 62% one day post burn, back to that of sham levels after treatment (Figure 5a). Additionally, IL-6 levels experienced a 34% increase one day after injury in small intestine IECS, but these levels returned to normal after treatment with 5-ASA (Figure 5b). Of the pro-inflammatory chemokines, KC levels seemed to increase in small intestine IECs one day following burn, but this did not reach significance. Of note, there is a trend toward that of a decrease in the elevated levels of KC with 5-ASA treatment one day post burn (Figure 5c).
Figure 5. Treatment with 5-ASA after burn injury significantly reduces the observed increases in the pro-inflammatory cytokines IL-18 and IL-6 back to that of sham levels one day after injury in small intestine IECs, with a trend toward reduction in the pro-inflammatory chemokine, KC, one day after injury.
IECs of the small intestine were harvested and cells were lysed for protein extraction. ELISAs on IL-18, IL-6, and KC were performed on the protein homogenate one day after injury and expressed as pg/mg protein. Values are mean ± SEM of 4-7 animals per group. *, p<0.05, **, p<0.001, all groups compared to sham saline by ANOVA and Tukey multiple comparison tests.
Mechanism of 5-ASA treatment after burn injury
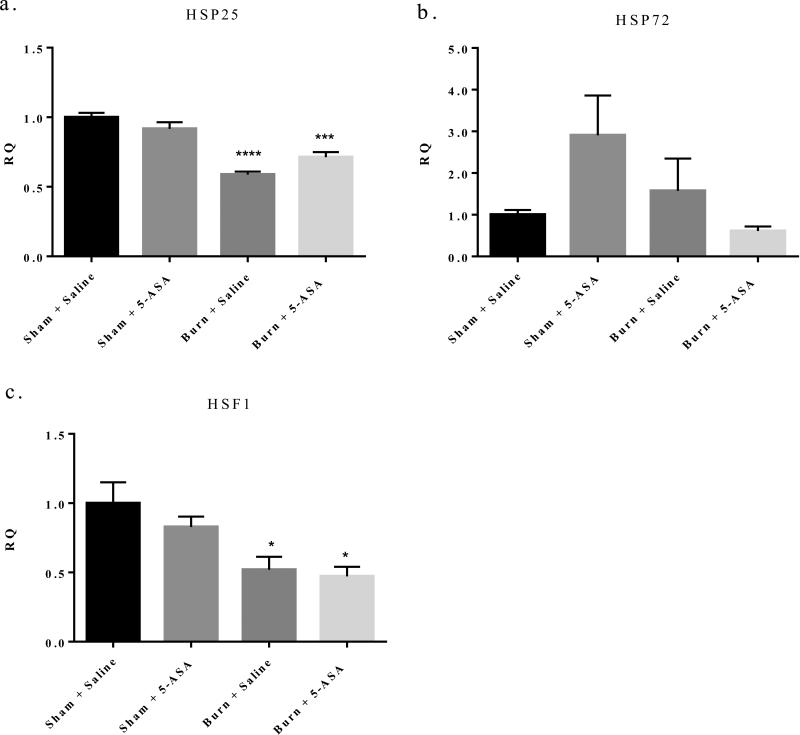
To elucidate the mechanism by which 5-ASA could potentially be used as treatment in burn related gut barrier breakdown and inflammation, we turned to a previous study which found 5-ASA could be acting as an anti-inflammatory agent by up-regulating the heat shock protein HSP72 in rat intestinal epithelial cells27. HSPs are classically defined as small cytoprotective proteins, which become upregulated in times of cellular stress42-44. Burn injury induces drastic cellular stress in the intestinal epithelium. Therefore, induction of HSPs could allow for an alleviation of the inflammation and cellular stress we observe in intestinal tissue following burn injury. However, we found that mice treated with 100mg/kg 5-ASA at time of resuscitation, did not up-regulate HSP25 or 72 in small intestine IECs as seen by mRNA expression (Figure 6a and b). Furthermore, HSF1, a master regulator of many HSPs, was also significantly decreased one day after burn injury, and the expression of HSF1 was not affected in burn mice treated with 5-ASA45 (Figure 6c). These data suggest that the beneficial effects we have observed after treatment with 5-ASA were not a direct result of 5-ASA upregulating HSPs in small IECs after burn.
Figure 6. 5-ASA treatment after burn injury does not significantly induce mRNA expression of HSF1 or HSP25 and HSP72 following injury.
q-RTPCR of HSF1, HSP25 and HSP72 mRNA expression in small intestine IECs relative to β-actin. Values are mean ± SEM of 4-7 animals per group expressed relative to sham. ***, p<0.001, ****, p<0.0001 all groups relative to sham saline by ANOVA and Tukey post hoc test.
Discussion
In this series of experiments, we show burn injury results in stalled intestinal peristalsis, which presents as an accumulation of the FITC-dextran dye in the small intestine sections most proximal to the stomach after gavage. This inhibition of normal intestinal transit compounded with increased intestinal permeability, inflammation, and decreases in IEC TJ expression after burn injury could potentiate bacterial translocation to extraintestinal sites. Of patients who survive the initial burn injury, increases in bacterial translocation have been implicated in the severe burn related complications of sepsis and MODS. Therefore, identifying a therapeutic regime, which could potentially protect against these adverse burn induced complications, would be of great benefit.
We were able to show that the use of 5-ASA treatment after burn injury aids in the restoration of burn induced inhibition of intestinal transit, increases in both intestinal permeability and inflammation, and decreases in tight junction proteins of small IECs. After applying 5-ASA treatment in our measure of intestinal transit after burn injury, we found that 5-ASA partially restored normal intestinal peristalsis. The sheer fact that 5-ASA treatment could moderately aid in reversing the inhibition in normal intestinal peristalsis we observe after burn injury was promising, as it is well known that a combination of multiple factors must be disrupted to give rise to the debilitated state of burn patients, not simply inhibited intestinal transit. For this reason, our finding that treatment with 5-ASA after burn injury completely restores the drastic increases in intestinal permeability we have seen after burn injury was of particular interest. Other studies have suggested that the source of systemic infection after burn injury originates in the gut, and the leakiness we observe in the intestine post burn injury gives support to our previous findings of increased bacterial translocation after injury. The ability for a post burn treatment to restore intestinal permeability back to sham levels provides convincing evidence for 5-ASA as a potential therapeutic intervention in decreasing the risk of sepsis after burn injury.
These restorations in burn induced physiologic conditions by 5-ASA treatment were coupled with restorations of decreases in TJ protein expression and rises in inflammation we observe following burn injury. Our results were consistent with others who have observed decreases in TJ protein expression after burn in that claudin-4, claudin-8, and occludin expression were significantly decreased following burn12, 20, 21. 5-ASA treatment was able to bring back the expression of both claudin-4 and occludin to the levels of sham injured animals one day after burn injury, but 5-ASA was not able to restore the expression claudin-8 post burn. The family of tight junction proteins contains a vast number of proteins including claudin-1, -2 -3, -4, -5, -8, zonal occludin-1 and -2, and more. Therefore, the fact that 5-ASA was not able to restore claudin-8 expression is not alarming, but does emphasize the need for understanding whether 5-ASA's treatment benefits expand into the other members of the tight junction protein family.
Additionally, we found that administration of 5-ASA after burn injury significantly reduces levels of the pro-inflammatory cytokines IL-18 and IL-6 and trends toward a reduction in the pro-inflammatory chemokine KC in small intestine epithelial cells one day after injury. It is well known that burn injury leads to altered TJ protein expression and dramatic increases in intestinal inflammation, but our findings highlight a potential option for resolution of those burn related complications with treatment of 5-ASA after burn injury.
The exact mechanism behind which 5-ASA produces its beneficial effects after burn injury remains elusive as we were unable to show that 5-ASA up-regulates the heat shock protein response. Jiang et al showed that 5-ASA acts as a PPARγ agonist25. In other studies, PPARγ agonists have been shown to inhibit the production of the pro-inflammatory cytokines of TNF-α, IL-6, and IL-1β, which are commonly elevated after burn injury20, 24, 25, 46-48. The second way in which 5-ASA has been shown to act is through the inhibition of NF-κB, which would halt the transcriptional messages required for the increased inflammation22, 25, 29.
While more studies are needed to investigate the mechanism underlying the protective effects of 5-ASA, our findings presented in this manuscript clearly suggest that 5-ASA can potentially be used as treatment to decrease intestinal inflammation and normalize intestinal function after burn injury.
References
- 1.American Burn Association [July 15, 2014]; Available at: http://www.ameriburn.org/resources_factsheet.php.
- 2.Deitch EA. Gut lymph and lymphatics: A source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207(Suppl 1):E103–11. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 3.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- 4.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Critical Care [serial online] 2009 doi: 10.1186/cc8170. 2014-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayala A, Wang P, Ba ZF, Perrin MM, Ertel W, Chaudry IH. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991;260:R167–71. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- 6.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): Are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Calum H, Moser C, Jensen PO, et al. Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin Exp Immunol. 2009;156:102–110. doi: 10.1111/j.1365-2249.2008.03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of toll-like receptor responses. J Leukoc Biol. 2004;75:400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 10.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(Suppl 1):i41–4. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Akhtar S, Choudhry M. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochimica et biophysica acta. 2012:196–203. doi: 10.1016/j.bbadis.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg R. The indigenous gastrointestinal microflora. Trends Microbiology. 1996:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 14.Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: Effects of glucose transport. Gastroenterology. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 15.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. The Journal of Burn Care and Research. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 16.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 17.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 19.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 20.Earley Z, Akhtar S, Green S, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10:e0129996. doi: 10.1371/journal.pone.0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: A cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Egan LJ, Mays DC, Huntoon CJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 23.Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: A review of medical therapy. World J Gastroenterol. 2008;14:354–377. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602–609. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gisbert JP, Gomollon F, Mate J, Pajares JM. Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: A systematic review. Dig Dis Sci. 2002;47:471–488. doi: 10.1023/a:1017987229718. [DOI] [PubMed] [Google Scholar]
- 26.Allgayer H. Review article: Mechanisms of action of mesalazine in preventing colorectal carcinoma in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):10–14. doi: 10.1046/j.1365-2036.18.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 27.Burress GC, Musch MW, Jurivich DA, Welk J, Chang EB. Effects of mesalamine on the hsp72 stress response in rat IEC-18 intestinal epithelial cells. Gastroenterology. 1997;113:1474–1479. doi: 10.1053/gast.1997.v113.pm9352849. [DOI] [PubMed] [Google Scholar]
- 28.Walker HL, Mason AD., Jr. A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Rousseaux C, Lefebvre B, Dubuquoy L, et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland L, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2003;(3):CD000543. doi: 10.1002/14651858.CD000543. [DOI] [PubMed] [Google Scholar]
- 31.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 32.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–47. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 33.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411–416. [PubMed] [Google Scholar]
- 34.Will C, Fromm M, Muller D. Claudin tight junction proteins: Novel aspects in paracellular transport. Perit Dial Int. 2008:577–84. [PubMed] [Google Scholar]
- 35.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 36.Van Itallie CM, Holmes J, Bridges A, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 37.Deitch E, Xu D, Kaise V. Role of the gut in the development of injury- and shock induced SIRS and MODS: The gut-lymph hypothesis, a review. Europe PubMed Central. 2006;11:520–8. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 38.Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: A cytokine-generating organ in systemic inflammation? Shock. 1995;4:193–199. [PubMed] [Google Scholar]
- 39.Shankar R, Melstrom KA, Jr, Gamelli RL. Inflammation and sepsis: Past, present, and the future. J Burn Care Res. 2007;28:566–571. doi: 10.1097/BCR.0B013E318093DF16. [DOI] [PubMed] [Google Scholar]
- 40.Vindenes HA, Ulvestad E, Bjerknes R. Concentrations of cytokines in plasma of patients with large burns: Their relation to time after injury, burn size, inflammatory variables, infection, and outcome. Eur J Surg. 1998;164:647–656. doi: 10.1080/110241598750005525. [DOI] [PubMed] [Google Scholar]
- 41.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Morse D, Choi A. Heat-shock proteins: New keys to the development of cytoprotective therapies. Expert Opin Ther Targets. 2006;10:759–69. doi: 10.1517/14728222.10.5.759. [DOI] [PubMed] [Google Scholar]
- 44.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 45.Xiao X, Zuo X, Davis AA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 47.Yeh FL, Lin WL, Shen HD, Fang RH. Changes in serum tumour necrosis factor-alpha in burned patients. Burns. 1997;23:6–10. doi: 10.1016/s0305-4179(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 48.Yeh FL, Lin WL, Shen HD, Fang RH. Changes in circulating levels of interleukin 6 in burned patients. Burns. 1999;25:131–136. doi: 10.1016/s0305-4179(98)00150-8. [DOI] [PubMed] [Google Scholar]