Abstract
Purpose
In Healthy Control subjects (HCs) certain brain regions of interest (ROIs) demonstrate increased regional cerebral blood flow (rCBF) in response to painful stimuli. The effect of bladder distension on arterial spin label-functional MRI (ASL-fMRI) measures of rCBF within ROIs was examined in subjects with Interstitial Cystitis (ICs).
Methods and Materials
Female ICs (n= 11) and HCs (n=11) underwent three brain perfusion scan studies using ASL-fMRI: with a full bladder; with an empty bladder; and while experiencing heat pain. rCBF was calculated using custom software and individual scans were spatially normalized to the MNI template. An analysis was performed of ROI-based absolute rCBF in each condition and of the within group/within subject rCBF distribution changes induced by each condition.
Results
Bladder distension was associated with robust increases in rCBF in ICs greater than that of HCs in multiple ROIs including the Supplemental Motor Area (mainly Brodmann’s Area 6), motor and sensory cortex, the insula bilaterally, hippocampal structures bilaterally and the middle and posterior cingulate areas bilaterally. During heat pain, HCs had more robust rCBF increases in the amygdala bilaterally. At baseline with an empty bladder, there was a lower rCBF level in the insula and mid- and posterior cingulate cortex bilaterally of ICs.
Conclusions
Compared to HCs, ICs have limited differences in rCBF in baseline (empty bladder) conditions as well as during heat pain, but robust rCBF increases in the full bladder state in ROIs typically associated with pain, emotion and/or motor control indicating altered processing of bladder-related sensations.
Keywords: Arterial Spin Labelling, fMRI, interstitial cystitis
INTRODUCTION
Studies have demonstrated that multiple central nervous system (CNS) structures are activated by bladder filling by using changes in regional cerebral blood flow (rCBF) as measures of this activation. Studied mainly in healthy men and women1,2 or in women suffering from non-painful bladder disorders 3,4 these studies have observed activation of multiple cortical and subcortical brain structures which are important for sensation and autonomic function. Studies of pain sensation have employed similar perfusion methodologies5–7, but none have examined painful bladder disorders.
The functional magnetic resonance imaging (fMRI) technique called continuous arterial spin labelling (CASL) quantifies rCBF non-invasively and avoids use of radioactive nucleotides6. CASL is accomplished by continuously inverting proton spins in blood upstream of the imaging slice to be measured so that the inverted magnetization of the blood water acts as a tracer. In order to determine whether the CNS processing of sensory information from the bladder differed between healthy controls (HCs) and subjects with the diagnosis of interstitial cystitis (ICs) we used CASL-fMRI to measure rCBF in these two groups. We used natural bladder filling induced by oral fluid consumption as a stimulus and measured rCBF in subjects when they were experiencing a full bladder (in the case of the ICs, a painfully full bladder) and compared it with rCBF associated with an empty bladder. We also examined the effects of heat pain, to contrast bladder pain with an experimentally induced somatic pain.
METHODS AND MATERIALS
Study Summary
These studies were approved by the University of Alabama at Birmingham’s Institutional Review Board for Human Studies. 22 female subjects (11 subjects meeting criteria for IC8,9 and 11 HCs were recruited as part of the multicenter Multidisciplinary Approach to Chronic Pelvic Pain (MAPP) Research Network10–12. Only tests which were site-specific are described. Subjects underwent a high resolution MRI image and three different ASL-fMRI measures: once in the “Full Bladder” condition, once in the “Empty Bladder” condition and once in the “Heat Pain” condition.
Full and Empty Bladder Conditions
During the pre-scan period, subjects were encouraged to consume non-caffeinated fluids (typically cold water up to 750 ml). In most subjects the “Full Bladder” CASL-fMRI scan measures were obtained as the initial scans, followed by complete voiding and then the “Empty Bladder” and “Heat Pain” measures obtained. An anatomical scout MRI scan allowing for realignment was performed each time the patient was moved in the scanner. Pain/discomfort ratings on a scale of 0–10 (0=no pain/sensation; 10=most intense/worst pain) were solicited before/after each scan.
Hot Thermal Stimulus
Thermal testing of the right volar forearm was performed using a thermal stimulator (Medoc PATHWAY ATS- fMRI compatible unit). Similar to our previous studies5 a sinusoidal thermal stimulus was adjusted to evoke a pain intensity rating of 7–9/10 by adjusting the peak target temperature to 43–49°C and alternating between a temperature 2°C lower for an 8 second period begun every 20 seconds and lasting through the duration of the CASL-fMRI scan.
Imaging Parameters and Preprocessing
Scans were obtained using a 3T MR Phillips Achieva MRI scanner. Structural imaging was performed with a T1-weighted 3D MPRAGE sequence with TR/TI/TE=1620/950/3msec, flip angle=30°, matrix=192×256×160, and voxel size=0.98×0.98×1mm3. ASL images were acquired using a continuous ASL sequence we have previously reported5 with the following parameters: 1600 Hanning window shaped pulses for a total labeling time = 2.4 sec, post-labeling delay = 1.4 sec, FOV=230mm, matrix=128×128, bandwidth=3kHz/pixel, TR/TE=5000/56 ms, 13 slices with thickness of 7 mm plus 1.25 mm gap, mean Gz of 0.6mT/m, labeling was placed at 80mm below the center of imaging region. 42 tag/control image pairs were acquired during the resting state, and 30 tag/control image pairs were acquired at each pain stimulation condition. The following preprocessing steps were used: motion correction, residual motion effect removing, denoising, registration, spatial smoothing, CBF calculation, and CBF map spatial normalization. Both the labeled and control images were realigned together using the six parameters-based rigid body transformation.
Calculation of rCBF
Motion-corrected ASL scans were compartmentalized into groups of control or labeled images. Quantitative CBF maps were calculated from the control-tag perfusion signal difference using a modified two-compartment ASL perfusion model13: f = ΔMλR1a exp(ωR1a) / 2Moα × [1 − exp(−τ R1a)]−1 where f is CBF, ΔM is the difference signal between the control and label acquisitions, R1a is the longitudinal relaxation rate of blood, τ is the labeling time, ω is the post labeling delay time, α is the labeling efficiency, λ is blood/tissue water partition coefficient, and Mo is approximated by the control image intensity. The parameters used in this study were R1a = 1/1664 sec, α=0.85, λ=0.09g/ml, τ =2.4 sec, ω=1.4 sec. rCBF maps so generated were normalized to the Montreal Neurology Institute template and smoothed using a 8×8×8 mm Gaussian kernel.
Selection of Regions of Interest
Based on the existing literature on pain imaging5,14,15 we selected 16 bilateral cortical and subcortical regions of interest (ROIs; listed in Table 1) as well as an ROI centered on peri-aqueductal gray matter. These ROIs were selected with the Wake Forest University pick atlas, which uses the Talairach Daemon database16. There is some partial redundancy/overlap between some of the Brodmann areas we chose and the “pre-central” and “post central” cortical ROIs of this atlas, but felt that would help interpretation and comparison with other studies.
Table 1.
States in Selected Regions of Interest Associated With Pain Sensation
| rCBF in ROI as % of total gray matter cerebral blood flowa (with standard deviations) | ||||
|---|---|---|---|---|
|
| ||||
| Region of Interest | Healthy Control Subjects | Interstitial Cystitis Subjects | ||
| Empty | Full | Empty | Full | |
| rAmygdala | 0.855+0.13 | 0.940+0.18 | 0.890+0.14 | 0.997+0.14 |
| lAmygdala | 0.871+0.16 | 0.923+0.19 | 0.935+0.17 | 1.000+0.16 |
| Premotor | ||||
| rBA6 | 0.785+0.09 | 0.756+0.11 | 0.822+0.10 | 0.912+0.11** |
| lBA6 | 0.820+0.07 | 0.771+0.13 | 0.844+0.13 | 0.918+0.13* |
| rSuppMotor | 0.737+0.15 | 0.727+0.15 | 0.766+0.14 | 0.911+0.13 |
| lSuppMotor | 0.725+0.13 | 0.706+0.13 | 0.803+0.12 | 0.859+0.14* |
| Motor | ||||
| rPreCentral | 0.789+0.14 | 0.747+0.14 | 0.837+0.14 | 0.896+0.09** |
| lPreCentral | 0.809+0.11 | 0.772+0.14 | 0.808+0.13 | 0.912+0.13* |
| rBA4 | 0.760+0.09 | 0.702+0.11 | 0.793+0.12 | 0.871+0.10** |
| lBA4 | 0.753+0.11 | 0.715+0.12 | 0.815+0.16 | 0.909+0.13** |
| Somatosensory | ||||
| rPostCentral | 0.750+0.08 | 0.699+0.10 | 0.755+0.12 | 0.871+0.11*** |
| lPostCentral | 0.763+0.11 | 0.729+0.11 | 0.813+0.15 | 0.908+0.13** |
| rBA3 | 0.719+0.10 | 0.664+0.10 | 0.765+0.14 | 0.891+0.12*** |
| lBA3 | 0.736+0.13 | 0.707+0.11 | 0.784+0.15 | 0.888+0.12** |
| rBA2 | 0.704+0.13 | 0.630+0.10 | 0.759+0.14 | 0.870+0.12*** |
| lBA2 | 0.721+0.13 | 0.680+0.12 | 0.771+0.16 | 0.883+0.13** |
| rInsula | 0.991+ 0.11 | 0.995+0.09 | 0.885 +0.14 | 0.978+0.06 |
| lInsula | 0.997+ 0.11 | 0.956+0.08 | 0.895 +0.11* | 0.960+0.07 |
| rCerebellum | 1.13+0.22 | 1.140+0.19 | 1.039+0.15 | 1.050+0.09 |
| lCerebellum | 1.11+0.20 | 1.118+0.22 | 1.05+0.14 | 1.090+0.11 |
| rThalamus | 0.847+0.10 | 0.905+0.14 | 0.865+0.15 | 0.918+0.09 |
| lThalamus | 0.906+0.11 | 0.909+0.12 | 0.846+0.15 | 0.945+0.12 |
| rHippocampus | 0.931+0.10 | 0.924+0.14 | 0.862+0.13 | 0.985+0.12 |
| lHippocampus | 0.940+0.10 | 0.909+0.08 | 0.919+0.13 | 0.986+0.13 |
| rAntCingulate | 0.896+0.12 | 0.938+0.11 | 0.862+0.17 | 0.964+0.06 |
| lAntCingulate | 0.957+0.11 | 0.997+0.10 | 0.924+0.17 | 0.961+0.09 |
| rMidCingulate | 0.874+0.12 | 0.872+0.13 | 0.746+0.18 | 0.895+0.09 |
| lMidCingulate | 0.829+0.13 | 0.847+0.13 | 0.709+0.18 | 0.865+0.10 |
| rPostCingulate | 0.949+0.11 | 0.822+0.17 | 0.774+0.18* | 0.893+0.10 |
| lPostCingulate | 0.971+0.12 | 0.864+0.15 | 0.761+0.20** | 0.896+0.10 |
| rPreFrontal | 0.937+0.08 | 0.951+0.07 | 0.955+0.12 | 0.954+0.05 |
| lPreFrontal | 0.934+0.07 | 0.912+0.05 | 0.993+0.08 | 0.962+0.09 |
| PeriAquaGray | 0.774+0.09 | 0.892+0.16 ++ | 0.847+0.19 | 0.908+0.14 |
normalized in each subject to their mean gray matter rCBF
indicate statistically different from measure in Healthy Control Subjects with p<0.05, <0.01,<0.001
indicates statistically different from Empty Bladder (rest baseline) measure (paired t-test analysis)
Post-hoc tests after ANOVA main effects of condition (df=2; F=5.0; p=.012), of ROIs (df=32; F=19.28; p=0.000), ROI x group interaction (df=32; F=3.32; p=0.000) and a conditions x ROI x group interaction (df=64; F=1.45; p=0.013).
Statistical Analysis
Presented data represent means ± SD unless otherwise stated. A mixed design ANOVA was performed on absolute rCBF values using a two groups (IC, HC) by three conditions (Empty Bladder, Full Bladder, Heat Pain) analysis in the 33 selected ROIs. This was followed by post hoc t-tests where appropriate to identify specific effects. Absolute rCBF values (ml/100g/min) were utilized in the analysis and creating most data tables. In addition, we also examined rCBF patterns or “relative distribution” in the three conditions by dividing all ROI values in each subject by that subject’s total gray matter rCBF calculated from a global mask that is part of the Wake Forest Pick Atlas. We performed supplementary whole brain image analysis and subtractions using absolute mean rCBF values for each condition and group using FSL. SPM was also used to evaluate normalized rCBF pattern changes: SPM analysis was intended as a check of areas activated in a whole brain analysis without regard to defined ROIs and any specific hypotheses. It was added as a way to evaluate if the quantitative ROI analysis missed some important changes and to determine whether our strongest findings using ROIs also appear on SPM’s more whole-brain, “data-driven” approach.
RESULTS
Demographics and Physiological Measures
The two groups were of equivalent age (ICs 46.3±11.9 y.o.; HCs 42.6±11.2 y.o.; n.s. p=0.464). 19 were Non-Hispanic Whites (10 ICs, 9 HCs). 3 were African-American (1 ICs, 2 HCs) and one HC identified herself as Hispanic. Most ICs were on chronic daily opioids (8 of 11). Use of other medications (e.g., antidepressants, anxiolytics, antispastic agents) was also common in ICs (10 of 11). Medicines were not used by HCs. Co-morbidities in the ICs, as identified by the methods of the broader study10,11, included fibromyalgia (5), irritable bowel syndrome (4), chronic fatigue syndrome (3) and vulvodynia (5). Overlap of co-morbid disorders was common but not universal – only 4 ICs had no co-morbid disorders.
Overall rCBF analysis
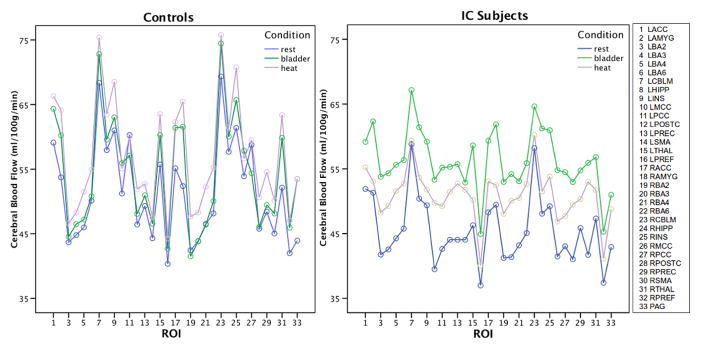
The condition x group x ROIs ANOVA revealed a significant main effect of condition (df=2; F=5.0; p= .012), a main effect of ROIs (df=32; F=19.28; p=0.000), an “ROI” x “group” interaction (df=32; F=3.32; p=0.000) and a “conditions” x “ROI” x “group” interaction (df=64; F=1.45; p=0.013). A within-subjects Full Bladder vs Empty Bladder condition analysis demonstrated a condition effect (df=1; F=10.256; p=0.004) similar to Heat Pain vs Empty Bladder analysis (df=1; F=5.8; p=.025). Figure 1 graphically illustrates the differences in rCBF based on ROI, condition and subject group that lead to the main effects and interactions shown by ANOVA. Based on the significant ANOVA results we conducted simple post hoc t-tests to identify the specific ROI effects by group and condition (summary in Tables 1 & 2).
Figure 1.
ROI by condition by groups analysis. Mean rCBF in each ROI for each condition for Healthy Control subjects (HCs; n=11) and Interstitial Cystitis subjects (ICs; n=11). Note how the rCBF in HCs does not vary as much across conditions as the ICs, especially during bladder pain. ANOVA was based on these data and showed significant main effects of condition (p=.012) and ROI (p.=000) and an ROI*Condition*Group interaction (p=.013).
Table 2.
States Compared with Baseline State in Selected Regions of Interest Associated With Pain
| rCBF in ROI as change in absolute rCBF (ml/100g/min) (with standard deviations) | ||||
|---|---|---|---|---|
|
| ||||
| Region of Interest | Healthy Control Subjects | Interstitial Cystitis Subjects | ||
| Δ rCBF Heat | Δ rCBF Bladder | Δ rCBF Heat | Δ rCBF Bladder | |
| rAmygdala | 13.1+8.8++ | 9.2+6.5++ | 3.1+6.6*** | 12.5+12.6 |
| lAmygdala | 10.4+7.8++ | 6.5+8.6 | 1.8+9.2* | 11.0+11.2 |
| Premotor | ||||
| rBA6 | 7.1+8.5 | 2.0+4.8 | 7.6+8.1+ | 10.8+7.9**++ |
| lBA6 | 4.9+8.2 | 1.6+6.3 | 7.0+7.2+ | 10.7+8.8**++ |
| rSuppMotor | 5.2+10.2 | −0.1+4.3 | 11.3+8.3++ | 14.2+8.7**++ |
| lSuppMotor | 3.3+10.3 | 1.2+5.5 | 7.7+7.7+ | 8.9+7.7*+ |
| Motor | ||||
| rPreCentral | 6.1+8.9 | 0.3+4.2 | 4.4+7.3 | 8.9+9.2*+ |
| lPreCentral | 3.4+8.2 | 1.7+4.9 | 8.7+7.6+ | 11.7+9.4**++ |
| rBA4 | 5.8+10.8 | −0.1+4.3 | 7.3+8.9 | 9.9+6.7**++ |
| lBA4 | 5.5+10.7 | 1.7+5.8 | 7.3+8.3+ | 11.3+10.2**+ |
| Somatosensory | ||||
| rPostCentral | 4.8+10.3 | 0.3+4.2 | 8.5+7.2+ | 12.0+6.4***++ |
| lPostCentral | 5.5+9.4 | 1.7+4.9 | 7.5+7.9+ | 11.3+8.9**++ |
| rBA3 | 4.4+10.5 | −0.1+4.3 | 8.6+7.6+ | 12.8+7.1***++ |
| lBA3 | 3.5+9.1 | 1.7+5.8 | 6.8+7.9 | 11.8+8.8**++ |
| rBA2 | 5.2+9.2 | −1.0+4.5 | 6.8+7.8+ | 11.8+8.1***++ |
| lBA2 | 2.9+9.0 | 0.8+5.6 | 6.6+8.0 | 12.1+7.7***++ |
| rInsula | 9.3+6.7+ | 4.3+4.1+ | 4.6+7.0 | 11.7+9.9*+ |
| lInsula | 7.5+9.2 | 2.0+4.8 | 2.4+4.0 | 9.8+8.2*++ |
| rCerebellum | 6.4+7.5 | 5.2+7.4 | 2.0+7.6 | 6.4+8.9 |
| lCerebellum | 7.0+10.9 | 4.4+8.3 | 0.5+5.1 | 8.4+8.8+ |
| rThalamus | 11.3+9.3++ | 7.8+8.0+ | 4.4+8.0 | 9.5+10+ |
| lThalamus | 7.8+8.8+ | 4.6+7.3 | 3.9+11.0 | 12.3+12.3+ |
| rHippocampus | 3.8+6.1 | 2.3+6.2 | 3.4+4.2 | 13.2+10**++ |
| lHippocampus | 5.6+5.8+ | 1.7+5.9 | 3.3+6.3 | 11.0+11.4*+ |
| rAntCingulate | 7.2+8.2+ | 6.3+5.8+ | 4.8+8.3 | 11.0+9.8+ |
| lAntCinguate | 7.2+7.6+ | 5.2+7.6 | 3.3+9.8 | 7.3+9.2 |
| rMidCingulate | 2.7+7.3 | 4.0+5.7 | 5.4+6.3 | 13.3+10.7*++ |
| lMidCingulate | 3.9+8.3 | 4.7+4.9+ | 10.2+11.2+ | 13.8+11.1*++ |
| rPostCingulate | 0.7+8.3 | −4.4+8.3 | 4.7+8.1 | 11.5+9.0**++ |
| lPostCingulate | −0.23+8.2 | −3.2+6.9 | 6.7+10.1 | 12.6+10.5***++ |
| rPreFrontal | 4.74+5.6 | 3.75+2.9 ++ | 3.51+5.8 | 7.3 +7.0 ++ |
| lPreFrontal | 3.66+5.1 | 2.73+5.5 | 2.91+4.3 | 7.5 +6.3 ++ |
| PeriAquaGray | 9.2+8.7 + | 9.16+6.3++ | 4.41+9.3 | 7.6+11.8 |
indicate statistically different from measure in Healthy Control Subjects with p<0.05, <0.01,<0.001
indicate statistically different from Empty Bladder (resting baseline) measure with p<0.05 and p<0.01 (in paired t-test analysis of pain-rest within subjects)
Post-hoc tests after ANOVA main effects of condition (df=2; F=5.0; p=.012), of ROIs (df=32; F=19.28; p=0.000), ROI x group interaction (df=32; F=3.32; p=0.000) and a conditions x ROI x group interaction (df=64; F=1.45; p=0.013)
Baseline rCBF Measures
In the Empty Bladder condition there were differences in rCBFs in pain-related ROIs (Table 1); sites with a robust statistically significant difference between ICs and HCs were the left insula and the posterior cingulate cortex bilaterally. Figure 2a is a subtraction image using FSL, highlighting where activity differs between HCs and ICs during the Empty Bladder condition: the insular cortex has lower rCBF in the IC group. Baseline pain ratings with an empty bladder were statistically greater in the ICs when compared with HCs (6.0±1.6 vs. 0.9±2.0; p<0.01).
Figure 2.
FSL subtraction images showing rCBF differences between Interstitial Cystitis subjects (ICs) and Healthy control subjects (HCs) at rest (panel A); and changes in rCBF due to bladder filling (“full bladder” minus “empty bladder” states) in ICs (panel B) and in HCs (panel C). Color scale shows absolute rCBF difference (panel A) or change (panels B, C) in ml/100g/min.
In panel A it is seen that the insular cortex has lower rCBF at rest in the ICs than in the HCs. (Cross hairs are centered on coordinates x = −40.7, y= −4.9, z= 1.3, where the mean rCBF was 12.7 ml/100g/min lower in the ICs).
In panel B it is seen that ICs have rCBF increases or “activation” of the SMA bilaterally and of more posterior sections of the cingulate cortex. (Cross hairs in Fig 2b are centered on coordinates x= 11.3, y= −0.6, z= 62.6 where rCBF increased by 15.2 ml/100g/min). In addition some midbrain regions, including the periaquaductal gray matter, are also activated.
In panel C it is seen that there is less extensive activation in HCs but definite involvement of the cerebellum and amygdala (which is also activated in ICs).
Effects of Bladder Fullness on rCBFs
Most selected ROIs demonstrated increased rCBF in ICs in the Full Bladder condition when compared to the Empty Bladder condition (Tables 1 & 2). When rCBF measures from all ROIs are combined, bladder filling resulted in a greater change in ICs than in HCs (10.8±2.0 versus 2.7±3.1 ml/100g/min; diff p=0.0001). Only a few of the same ROIs selected demonstrated similarly increased rCBF when examined in HCs. Comparison of the rCBF in ROIs which had increases during the Full Bladder condition in HCs (amygdala, insula, thalamus, anterior cingulate mid-cingulate, pre-frontal cortex, and PAG sites), with those in ICs demonstrated greater and more bilateral increases in the ICs in most of these sites (Table 2). Figure 2b and 2c illustrate some of the main rCBF changes seen in the brains of each group during the Full Bladder condition. The FSL subtraction image of absolute rCBF in Fig 2b shows that ICs have activation of the SMA bilaterally and of more posterior sections of the cingulate cortex, as also seen in our ROI analysis and tables. This is further validated by the whole brain SPM analysis of normalized rCBF in Fig 3, which also showed activation of the SMA and cingulate cortex. As expected, ratings of pain/discomfort intensity due to a full bladder were statistically greater in ICs than in HCs (6.8±2.0 vs 5.3±2.0; p=0.04). Although not measured in this study, our previous studies have demonstrated that ICs tolerate less volume/pressure of urine in their bladders before reaching their pain tolerance level17 and so it is likely that the ICs had a lesser mechanical stimulus which produced a greater sensory report and changes in rCBF.
Figure 3. SPM results of bladder pain activation in IC subjects.

SPM of whole brain data was conducted as a secondary assessment to determine whether unidentified ROIs were apparent and whether strongest findings using focused ROI analysis also appear on SPM’s more whole brain, “data driven” approach. Color scale shows values of t in SPM analysis of full bladder-induced rCBF changes including the SMA and posterior cingulate cortex. Significance level of the brightest spots is approximately p=.0002 and of the darkest spots is approximately p=.03. Cross hairs are centered on coordinates x= −6.0, y=0.0, z= 54, in the SMA. Thus, there was substantial overlap between our most significant ROI findings and the SPM activation map. Specifically, both analyses point to the SMA and several regions of the cingulate cortex as areas of activation.
Effects of Thermal Pain on rCBFs
Similar to our previous studies5 the Heat Pain condition was associated with increases in rCBF in multiple ROIs in HCs and ICs (Table 2). When rCBF measures from all ROIs are combined, a similar increase in rCBF was observed in both ICs and HCs (5.5±2.6 vs. 5.7±2.8 ml/100g/min respectively). The only ROIs with a change in rCBF that differed in magnitude between the HCs and ICs was the left and right amygdala with greater increases in rCBF due to heating in the HCs when compared with the ICs (p=0.0073 for right; p=0.0283 for left). Ratings of heat pain intensity were similar in the ICs and HCs (7.8±1.0 vs 7.7±1.3; p=0.844). The maximal temperature tolerated was statistically similar between the two groups (ICs 46.6±2.0 vs HCs 47.7±1.3; p=0.143).
DISCUSSION
We all know that we should believe our patients when they tell us they are in pain, but when symptoms appear out-of-proportion to observed pathology, there is always doubt to their report. Therefore, it is comforting to have corroborative evidence that a stimulus which is reported as painful to ICs but not to HCs produces a high level of activation in ICs of CNS structures that have been traditionally associated with pain. The “pain neuromatrix” is a concept that was put forward by Melzack18 in an attempt to explain the widespread activation of CNS structures that serve the multiple sensory and affective/motivational aspects of pain. It requires an interactive balance between multiple cortical and subcortical structures. Neuroimaging studies have given more precise structure to this pain neuromatrix14, and identified sites of importance not previously recognized. It is notable that the increases in rCBF present in the Full Bladder state in ICs are almost all pain neuromatrix sites. Many of these same sites are activated by bladder fullness in HCs as noted by the present and previous studies but generally, activation is more widespread and statistically more robust in ICs1–4,19,20. Of particular note are activation sites in the SMA (Brodmann’s Area 6) which are sites commonly activated by bladder sensations3 and multiple other painful stimuli5.
Baseline (Empty Bladder condition) rCBF pattern differences between ICs and HCs were limited and so it is less likely that “empty bladder” fMRI scans will prove to have diagnostic value. In contrast, ASL-fMRI scans obtained during the Full Bladder condition could have diagnostic value, particularly if coupled with imaging of the Empty Bladder condition so that reactivity can be assessed. Additional study of patients suffering from other chronic pain disorders including other pelvic pain disorders, vulvar vestibulitis21 and other visceral pain disorders such as irritable bowel syndrome22,23 would allow for a determination of specificity of the measure. These studies allow us to glimpse the differences in central nervous system processing that are present in ICs. It is notable that the increased reactivity of ICs is only in relation to the stimulus of bladder filling and not a global alteration in reactivity: their rCBF responses to a heat pain stimulus are similar, or in some cases less robust, than those of HCs.
The method of ASL-fMRI is non-invasive, temporally brief in acquisition and requires limited instrumentation over standardly available MRI software. This means it could serve as an additional piece of data that informs clinical decisions related to the treatment of IC. Other methods of imaging have allowed for a “functional connectivity” assessment of areas of the brain associated with bladder sensation, but perfusion-type methods which examine rCBF have typically required the use of radioactive nucleotides (positron emission tomography [PET]; single photon emission computed tomography [SPECT]) and a separate co-registration with an anatomical MRI. Perfusion methods are experimentally powerful because they allow an absolute comparison of subjects in different clinical conditions allowing observation of how these subjects may differ when in their “baseline” clinical state. Current functional MRI methods have the advantage that they are able to employ only one imaging system which allows for easier alignment and interpretation of data. The most common fMRI methods assess changes in Blood Oxygen Level Dependent [BOLD] measures of subjects, but unfortunately these methods only measure changes in BOLD levels which may occur spontaneously12 or in response to a brief evocative stimulus such as pelvic floor contractions or small intravesical changes in pressure19,20,24. As a consequence, BOLD methodology, as currently employed, only allows for an assessment of relative changes and differential responses to brief evocative stimuli. This can be quite useful for sensations related to the surface of our body, sites which can be readily reached and transiently stimulated, but examination of deeper tissues has proven more challenging.
Our choice to use an ASL-fMRI method was based on a desire to observe absolute differences between HCs and ICs, as well as patterns of change produced by a natural stimulus. We also desired to utilize methods that would be easy to use clinically. Industry generated ASL software is available for most high resolution MRI units so it would be possible to translate the findings of the present study to clinical practice with relative ease. Future research with sample sizes sufficient to establish precise normative and disease-specific values may demonstrate a correlation between precise sites and magnitude of activation that prove diagnostic for IC. The present study suggests some ROIs which are robustly different in ICs and so worth additional investigation. Large enough samples might even suggest potential subpopulations of the ICs as has been noted in other visceral pain disorders25. Painful visceral disorders may be a special case of pain due to the diffuse and bilateral nature of their sensory input to the CNS. Other painful disorders have also demonstrated altered rCBF including irritable bowel syndrome, fibromyalgia and vulvodynia21–23, 26. Some changes observed in rCBF in the ICs could also be secondary to the existence of co-morbidity in this population. A larger sampling of the ICs could allow for a more complete evaluation of this separate question.
Limitations associated with this study include the fact that only female subjects were studied. Whether similar differences in rCBF are noted in males with the diagnosis of IC is yet to be determined. This study utilized the more stringent NIDDK consensus panel criteria for IC9 in that all subjects had cystoscopic findings of glomerulations or a Hunner’s patch, and so it is not known whether these results can be extrapolated to patients assigned the IC diagnosis using the more symptom-based criteria proposed by the AUA8. One must presume that a population characterized using symptom-based criteria will be more heterogenous in nature and may have less symptom chronicity. The precise importance of increased rCBF in specific ROIs is a topic of debate as it may represent functional alterations of CNS processing of sensory inputs or may simply represent the increased importance, or salience, of the particular stimulus to the subject. Given their symptomatology, one must assume that the sensation of bladder fullness is of high importance to IC subjects and so the results of the present study may simply reflect the mental state of the IC patient. Other studies performed by our group also give evidence of differences between ICs and HCs when connectivity and structural differences are measured12, 27–30. Coupled with the present study, these converging lines of evidence give strong support for the assertion that CNS function differs in these two populations.
Conclusions
Activation of brain sites associated with the stimulus of a full bladder differs between ICs and HCs. When ROIs typically associated with the pain neuromatrix are examined, sensation of a full bladder produced greater activation in ICs than in HCs consistent with this patient group reporting pain produced by the same stimulus. The sites of increased activation correspond to the portion of the visceral sensory cortex (the insula) but also in sites known to be associated with emotional and motivational components of pain sensation. Identification of greater reactivity of these sites to bladder fullness could be useful clinically to stratify patient groups and to track effectiveness of therapeutic interventions.
Acknowledgments
The MAPP Research Network acknowledges support through NIH grants: U01 DK82315. This article outlines independent research commissioned by the National Institute for Health (NIH). The views expressed in this article are those of the author(s) and are not necessarily those of the NIH, the NIDDK, or the Department of Health.
LIST OF ABBREVIATIONS
- AUA
American Urological Association
- BA
Brodmann’s Area
- CASL
continuous arterial spin labelling
- CNS
central nervous system
- FSL
FMRIB software Library
- fMRI
functional magnetic resonance imaging
- HCs
healthy control subjects
- IC
interstitial cystitis
- ICs
interstitial cystitis subjects
- NIDDK
National Institute for Diabetes, Digestive Disease and Kidney
- SMA
Supplemental Motor Area
- SPM
Statistical parametric mapping
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Georg Deutsch, Department of Radiology, University of Alabama at Birmingham, Birmingham, AL.
Hrishikesh Deshpande, Department of Radiology, University of Alabama at Birmingham, Birmingham, AL.
Michael A. Frölich, Department of Anesthesiology, University of Alabama at Birmingham, Birmingham, AL.
H. Henry Lai, Division of Urologic Surgery, Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Timothy J. Ness, Department of Anesthesiology, University of Alabama at Birmingham, Birmingham, AL.
References
- 1.Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- 2.Blok BFM, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- 3.Kuhtz-Buschbeck JP, Gilster R, van der Horst C, et al. Control of bladder sensations: an fMRI study of brain activity and effective connectivity. Neuroimage. 2009;47:18–27. doi: 10.1016/j.neuroimage.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths D, Tadic SD. Bladder control, urgency and urge incontinence: evidence form functional brain imaging. Neurourology and Urodynamics. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 5.Frolich MA, Deshpande H, Ness TJ, et al. Quantitative changes in regional cerebral blood flow induced by cold, heat and ischemic pain: a continuous arterial spin labeling study. Anesthesiology. 2012;117:857–867. doi: 10.1097/ALN.0b013e31826a8a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MC, Tracey I. Imaging pain: a potent means for investigating pain mechanisms in patients. Br J Anaesthesia. 2013;111:64–72. doi: 10.1093/bja/aet174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37:S80–88. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Hanno PM, Burks DA, Clemens JQ, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185:2162–2170. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontari MA, Hanno PM, Wein AJ. Logical and systematic approach to the evaluation and management of patients suspected of having interstitial cystitis. Urology suppl. 1997;49:114. doi: 10.1016/s0090-4295(97)00184-2. [DOI] [PubMed] [Google Scholar]
- 10.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urology. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urology. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. J Urol. 2014;192:947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkes LM. Quantification of cerebral perfusion using arterial spin labeling: two compartment models. J Magn Reson Imaging. 2005;22:732–736. doi: 10.1002/jmri.20456. [DOI] [PubMed] [Google Scholar]
- 14.Tracey I, Johns E. The pain matrix: Reloaded or reborn as we image tonic pain using arterial spin labelling. Pain. 2010;148:359–60. doi: 10.1016/j.pain.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Duerden EG, Albanse M-C. Localization of pain-related brain activation: a meta-analysios of neuroimaging data. Human Brain Mapping. 2013;34:109–149. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ness TJ, Powell-Boone T, Cannon R, et al. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983–1987. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 18.Melzack R. Evolution of the neuromatrix theory of pain. Pain Practice. 2005;5:85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- 19.Nardos R, Gregory WT, Krisky C, et al. Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI. Neurourol Urodyn. 2013;33:1–9. doi: 10.1002/nau.22458. [DOI] [PubMed] [Google Scholar]
- 20.Krhut J, Holy P, Tintera J, et al. Brain activity during bladder filling and pelvic floor muscle contractions: a study using functional magnetic resonance imaging and synchronous urodynamics. Int J Urology. 2014;21:169–174. doi: 10.1111/iju.12211. [DOI] [PubMed] [Google Scholar]
- 21.Pukall CF, Strigo IA, Binik YM, et al. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115:118–127. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Rapps N, van Oudenhove L, Enck O, et al. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosomatic Research. 2008;64:599–604. doi: 10.1016/j.jpsychores.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Tadic SD, Tannenbaum C, Resnick NM, et al. Brain responses to bladder filling in older women without urgency incontinence. Neurourology and Urodynamics. 2013;32:435–440. doi: 10.1002/nau.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Berman S, Mayer EA, et al. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol. 2003;98:1354–1361. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- 26.Derbyshire SWG. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol. 2003;98:12–20. doi: 10.1111/j.1572-0241.2003.07168.x. [DOI] [PubMed] [Google Scholar]
- 27.Martucci KT, Shirer WR, Bagarinao E, et al. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network - a resting-state study from the MAPP Research Network. Pain. 2015;156:1755–1764. doi: 10.1097/j.pain.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagarinao E, Johnson KA, Martucci KT, et al. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014;155:2502–9. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kairys AE, Schmidt-Wilcke T, Puiu T, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2014;193:131–7. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutch JJ, Yani MS, Asavasopon S, et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP Research Network neuroimaging study. Neuroimage Clin. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]