Abstract
Purpose
Little is known about the effects of physical activity behavior change interventions on health outcomes such as lower extremity dysfunction and SF-36 physical health (predictor of mortality) in breast cancer survivors. Furthermore, effect moderators are rarely reported. Therefore, we report the effects of the 3-month BEAT Cancer physical activity behavior change intervention on global health status and health indicators along with moderators of intervention outcomes.
Methods
Post-primary treatment breast cancer survivors (N=222) were randomized to BEAT Cancer or usual care (UC). SF-36, muscle strength, body mass index, lower extremity dysfunction (WOMAC) and life satisfaction were measured at 3 months (M3) and 6 months (M6).
Results
At M3, adjusted linear mixed-model analyses demonstrated statistically significant effects of BEAT Cancer versus UC on SF-36 physical health [mean between group difference (M)=2.1; 95% confidence interval (CI)=0.3 to 3.9; P=.023], SF-36 mental health (M=5.2; CI=2.8 to 7.6; P <.001), and all SF-36 subscores. Intervention benefits occurred for lower extremity physical dysfunction (M =−2.7; CI=−5.0 to −0.5; P=0.018), WOMAC total (M=−3.7; CI=−6.7 to −0.6; P=0.018), and life satisfaction (M=2.4; CI=0.9 to 3.9; P=0.001). Statistically significant effects persisted at M6 for mental health and vitality. Baseline value, income, marital status, cancer treatment, cancer stage, and months since diagnosis moderated one or more outcomes.
Conclusions
BEAT Cancer improves SF-36, WOMAC, and life satisfaction outcomes with improvements in vitality and mental well-being continuing 3 months post-intervention. Several moderators with potential to guide targeting individuals for optimal intervention benefit warrant further study.
Keywords: oncology, survivorship, psychosocial, exercise, WOMAC
Introduction
Due to early detection and current treatment modalities, the 5-year breast cancer survival rate is 89% in the United States with over 3 million breast cancer survivors living into and beyond middle age [1, 13]. The combination of a breast cancer diagnosis with advancing age contributes to progressive loss in muscle strength, unfavorable changes in body composition, and lower extremity dysfunction all of which negatively affect physical function and quality of life in breast cancer survivors [35, 37]. Recently, Petrick et al. [28] reported that functional status trajectory declines dramatically within one year after cancer diagnosis compared to non-cancer controls. Furthermore, breast cancer survivors who report poorer physical health (measured by the SF-36 composite score) are 42% more likely to experience additional breast cancer events and 37% more likely to die from any cause [34]. Interventions that improve or reverse this negative sequela experienced by breast cancer survivors are needed.
Exercise training can improve quality of life, muscle strength, and body composition after cancer diagnosis [5, 26]. However, only two randomized controlled trials have reported that exercise training can favorably influence arthralgias (i.e., joint pain) among breast cancer survivors [7, 18]. Moreover, few randomized trials have reported the effects of exercise on health status measured with the SF-36, of particular importance given its association with breast cancer events and mortality [25, 34].
Physical activity is the primary outcome when testing physical activity behavior change interventions. In contrast to exercise training trials, behavior change trials include various health parameters as secondary measures to determine if the increases in physical activity adherence are sufficient for improved health status [9]. Few randomized controlled physical activity behavior change trials in breast cancer survivors have reported intervention effects on muscle strength or lower extremity dysfunction [4, 32]. Also, little is known about the factors that moderate the health status response to physical activity behavior change interventions. Identifying moderators can be used for cost-effective targeting of the intervention to cancer survivors more likely to benefit while also providing information for intervention refinements [23]. Therefore, this report presents the effects of the Better Exercise Adherence after Treatment for Cancer (BEAT Cancer) physical activity behavior change intervention on health status outcomes and examines the moderators of these effects.
Our group has previously reported that BEAT Cancer significantly improved physical activity behavior, cardiorespiratory fitness, and quality of life measured using the Functional Assessment of Cancer Therapy (FACT)-Breast scale [31, 33]. Specifically, a statistically significant mean between group difference favoring BEAT Cancer was noted for self-report weekly minutes of moderate-to-vigorous physical activity immediately post-intervention (+93 minutes) and 3 months later (+74 minutes) [31]. Accordingly, the primary purpose of the current report was to compare the effects of BEAT Cancer to usual care (UC; written materials) on health status as measured by the composite SF-36 scores (primary outcomes for this report). We also report the effects on the SF-36 subscores and the health status outcomes of muscle strength, body mass index (BMI), lower extremity dysfunction, and satisfaction with life. We hypothesized that, when compared with UC, BEAT Cancer would result in significant improvements in all outcomes immediately post-intervention (month 3; M3) and 3 months post-intervention (month 6; M6). The secondary aim was to determine moderators of BEAT Cancer compared to UC on the aforementioned outcomes. Demographic (age, income, marital status) and medical (cancer stage, months since diagnosis, cancer treatment) were chosen based on plausibility and literature review [6, 8, 10-12, 20, 27, 29].
Methods
Study design
The methods for this multicenter randomized controlled trial have been previously described [31, 33]. In brief, women (ages 18 to 70) with history of ductal carcinoma in situ (DCIS) or stage I-IIIA breast cancer who self-reported engaging in ≤30 minutes of vigorous or ≤60 minutes of moderate intensity physical activity per week on average over the past 6 months were enrolled. Participants had to be post-primary treatment, ≥8 weeks post-surgery, English speaking, and medically cleared by their physician. Exclusion criteria included conditions that would interfere with assessment or intervention completion (e.g., dementia, inability to ambulate, physical activity contraindication, surgery or travel planned for during the intervention) and current participation in another exercise study (see Rogers et al. [33] for additional details regarding study criteria). Institutional Review Board (IRB) approval was obtained and all participants provided written informed consent. As described [33], 222 breast cancer survivors were randomized (in the order of baseline assessment completion) using computer generated numbers in blocks of 4 within each recruiting site. Research personnel were blinded to the order of randomization until allocation was revealed following completion of the baseline assessment.
Better Exercise Adherence after Treatment for Cancer (BEAT Cancer) intervention
Details regarding the 3-month social cognitive theory-based BEAT Cancer intervention have been published elsewhere [33]. This intervention includes 12 supervised exercise sessions tapered over six weeks followed by three face-to-face update counseling sessions every two weeks with a trained exercise specialist. Six group sessions led by trained facilitators provided additional behavioral counseling (e.g., time management, stress management, behavioral modification strategies, etc.). The exercise prescription gradually increased participants to 150 weekly minutes of moderate-intensity exercise using a progression as previously reported [33]. An educational notebook and personal heart rate monitor were provided as part of the intervention. Intervention participants also received the same written materials given to the usual care group. Quality control for fidelity and participant adherence has been previously described [31, 33].
Measures
Outcomes were measured at baseline, M3 (immediately post-intervention), and M6 (3 months after intervention completion) [33]. Staff members completing physical assessments and data entry were blinded to the participant’s study group allocation. A self-administered survey assessed global health status using the 36-item SF-36 health-related quality of life survey [38]. The 8 subscores (i.e., physical functioning, social functioning, role-physical, role-emotional, mental health, vitality, pain, and general health) and two composite scores (physical and mental) were calculated according to published protocol and reported as scores transformed to a 0 to 100 scale [38]. Muscle strength was measured using a portable back/leg dynamometer (best of three efforts) (Takei Back Strength Dynamometer TKK5002). For anthropometrics, height and weight were measured in light clothing without shoes using a stadiometer and scale; BMI was calculated from the following equation [weight (kg)/height (m2)].
The self-administered survey also included the Western Ontario and McMaster Universities Arthritis index (WOMAC) to assess lower extremity pain (5 items), stiffness (2 items), and physical dysfunction (17 items) using a 5-point Likert scale (i.e., 1=none to 5=extreme) [30]. Items were summed for the 3 subscores and the subscores were summed for the overall score (higher score indicates greater dysfunction). Life satisfaction was measured with the 5-item Satisfaction with Life Scale (SWLS; 7-point Likert scale from 1 = strongly disagree to 7 = strongly agree) [14].
In addition to the baseline value of the outcome, the following potential moderators were self-reported at baseline: age, annual household income, marital status, cancer stage, months since breast cancer diagnosis, history of chemotherapy, history of radiation therapy, and months on hormonal therapy [16]. Moderators were dichotomized as follows: age (<55 versus ≥55), annual income (<$50,000 versus ≥$50,000), marital status (married or living with significant other versus other), cancer stage (DCIS or stage I versus stage II or III), months since diagnosis (≤12 versus >12) [15], time on hormonal therapy (none versus ≤ 1 year versus >1 year), and BMI (<30 versus ≥30).
Statistical analyses
Statistical analyses for our primary study purpose used adjusted linear mixed models incorporating an unstructured covariance matrix and SAS® statistical software (Cary, NC). Previously identified covariates were included in the model (i.e., baseline value of the outcome, study site, breast cancer stage, history of chemotherapy, history of radiation, time on hormonal therapy, comorbidities, and marital status) [31]. Our retained sample size of 213 participants at M6 allowed detection of a small to medium effect size = 0.39 (p < 0.05, power of 0.80). For our secondary study aim, the change over time across the intervention-moderator interaction term was modeled. All analyses were intention-to-treat with all data available being used. Only 162 participants had back/leg dynamometer data due to equipment failure. Statistical significance was defined by two-sided p-value < 0.05.
Results
Overview
Participant enrollment took place from 2010 to 2013 during which 222 participants were randomized to either the BEAT Cancer (n = 110) or UC (n = 112) groups. Mean data from both groups indicated participants were aged 54 ± 9 years and had 16 ± 3 years of education while 84% self-identified as White. Cancer stages were as follows: DCIS (11%), stage I (42%), stage II (35%), and stage III (12%). On average, the time since cancer diagnosis was 54 ± 55 months with 58% and 68% reporting a history of chemotherapy and radiation therapy, respectively. Nearly half (49%) of all participants reported current hormonal therapy [31].
BEAT Cancer effects on health status outcomes at month 3 (M3) and month 6 (M6)
As shown in Table 1, significant between-group differences favoring BEAT Cancer occurred for both SF-36 composite scores and all 8 SF-36 subscores at M3. Notable mean between group differences in a beneficial direction for the intervention group were as follows: mental health composite score (5.2; CI = 2.8 to 7.6; p < 0.001), vitality (12.5; CI = 8.0 to 17.0; p < 0.001), and role-emotional (9.8; CI = 4.7 to 15.0; p < 0.001). Additional statistically significant between-group differences were observed at M6, indicating a continued positive effect on mental health composite (3.0; CI = 0.5 to 5.4; p = 0.017), vitality (7.8; CI = 3.3 to 12.4; P = 0.001), and mental health subscore (4.3; CI = 0.2 to 8.5; p = 0.038) for the intervention. As shown in Table 2, no between-group differences were observed at M3 or M6 for muscle strength, BMI, and lower extremity joint pain or stiffness. In contrast, statistically significant between group differences favoring the intervention were found at M3 for lower extremity physical dysfunction (−2.7; CI = −5.0 to −0.5; p = 0.018), WOMAC total (−3.7; CI = −6.7 to −0.6; p = 0.018), and satisfaction with life (2.4; CI = 0.9 to 3.9, p = .001).
Table 1.
Effects of the BEAT Cancer intervention on global health status (i.e., SF-36 composite and subscores) post-intervention (month 3) and 3 months after intervention completion (month 6) in breast cancer survivors
| Unadjusted means | Adjusteda between-group differences Estimated least square mean with (95% CIb); p value |
||||
|---|---|---|---|---|---|
|
| |||||
|
Outcome |
Baseline mean (SDc) |
Month 3 mean (SD) |
Month 6 mean (SD) |
BEAT Cancer vs usual care at month 3 (post-intervention) |
BEAT Cancer vs usual care at month 6 (3 months post- intervention) |
| SF-36 Physical health composite score |
2.1 (0.3 to 3.9); .023 | 0.1 (−1.8 to 1.9); .93 | |||
| BEAT Cancer | 47.9 (8.3) | 49.2 (8.7) | 47.6 (10.9) | ||
| Usual care | 47.9 (9.8) | 47.4 (9.8) | 47.6 (9.9) | ||
|
| |||||
| SF-36 Mental health composite score |
5.2 (2.8 to 7.6); <.001 | 3.0 (0.5 to 5.4); .017 | |||
| BEAT Cancer | 48.1 (10.2) | 52.0 (8.8) | 50.2 (10.7) | ||
| Usual care | 49.0 (9.8) | 47.7 11.3) | 47.8 (11.2) | ||
|
| |||||
| SF-36 subscores | |||||
|
| |||||
| Physical functioning | 6.4 (1.9 to 11.0); .006 | 2.7 (−1.9 to 7.3); .25 | |||
| BEAT Cancer | 75.8 (19.6) | 82.6 (19.1) | 78.4 (23.5) | ||
| Usual care | 75.6 (23.7) | 76.6 (23.6) | 75.5 (23.9) | ||
|
| |||||
| Role-physical | 8.6 (3.0 to 14.2); .003 | 1.0 (−4.7 to 6.7); .72 | |||
| BEAT Cancer | 78.8 (22.9) | 83.3 (21.6) | 79.2 (26.6) | ||
| Usual care | 76.5 (24.9) | 74.1 (29.6) | 77.1 (26.0) | ||
|
| |||||
| Bodily pain | 6.2 (1.4 to 11.0); .012 | 2.4 (−2.5 to 7.3); .34 | |||
| BEAT Cancer | 69.6 (21.5) | 71.3 (21.1) | 66.2 (25.0) | ||
| Usual care | 72.8 (23.1) | 68.3 (24.1) | 66.8 (25.1) | ||
|
| |||||
| General health | 5.8 (2.2 to 9.4); .002 | 0.5 (−3.1 to 4.2); .78 | |||
| BEAT Cancer | 66.7 (12.1) | 71.1 (20.1) | 68.7 (23.2) | ||
| Usual care | 68.2 (23.1) | 66.9 (23.9) | 68.9 (24.8) | ||
| Vitality | 12.5 (8.0 to 17.0); <.001 | 7.8 (3.3 to 12.4); .001 | |||
| BEAT Cancer | 50.7 (18.9) | 62.5 (16.3) | 57.3 (19.4) | ||
| Usual care | 51.3 (21.8) | 51.1 (23.9) | 49.8 (22.1) | ||
|
| |||||
| Social functioning | 9.8 (4.1 to 15.4); .001 | 3.1 (−2.6 to 8.9); .29 | |||
| BEAT Cancer | 81.5 (21.1) | 87.4 (20.5) | 81.7 (24.9) | ||
| Usual care | 82.8 (21.6) | 79.8 (25.6) | 80.2 (26.9) | ||
| Role-emotional | 9.8 (4.7 to 15.0); <.001 | 4.6 (−0.6 to 9.8); .09 | |||
| BEAT Cancer | 82.0 (21.3) | 89.3 (17.4) | 85.8 (21.7) | ||
| Usual care | 84.1 (20.2) | 81.3 (25.6) | 82.6 (23.7) | ||
|
| |||||
| Mental health | 7.1 (3.1 to 11.2); .001 | 4.3 (0.2 to 8.5); .038 | |||
| BEAT Cancer | 73.5 (17.7) | 79.1 (15.0) | 76.4 (18.1) | ||
| Usual care | 74.4 (18.0) | 72.7 (18.0) | 72.4 (19.1) | ||
Adjusted for baseline value, study site, breast cancer stage, history of chemotherapy, history of radiation therapy, current hormonal therapy, comorbidities, and marital status
Confidence intervals
Standard deviation
Table 2.
Effects of the BEAT Cancer intervention on health status indicators post-intervention (month 3) and 3 months after intervention completion (month 6) in breast cancer survivors
| Unadjusted means | Adjusteda between-group differences Estimated least square mean with (95% CIb); p value |
||||
|---|---|---|---|---|---|
|
| |||||
| Outcome | Baseline mean (SDc) |
Month 3 mean (SD) |
Month 6 mean (SD) |
BEAT Cancer vs usual care at month 3 (post-intervention) |
BEAT Cancer vs usual care at month 6 (3 months post- intervention) |
| Back/leg muscle strength (kg)d |
2.0 (−3.1 to 7.2); .44 | 1.0 (−4.2 to 6.2); .70 | |||
| BEAT Cancer | 55.4 (25.5) | 62.2 (24.7) | 63.2 (26.7) | ||
| Usual care | 53.7 (20.7) | 58.9 (20.6) | 60.8 (20.7) | ||
|
| |||||
| BMI | −0.08 (−0.44 to 0.27); .65 | −0.05 (−0.41 to 0.31); .77 | |||
| BEAT Cancer | 30.8 (6.9) | 30.5 (7.0) | 30.3 (7.1) | ||
| Usual care | 30.5 (6.8) | 30.5 (6.8) | 30.5 (7.0) | ||
|
| |||||
| Lower extremity dysfunction (WOMAC) |
|||||
|
| |||||
| Joint pain | −0.7 (−1.5 to 0.1); .08 | −0.2 (−1.0 to 0.6); .68 | |||
| BEAT Cancer | 3.6 (3.4) | 3.5 (3.3) | 3.7 (4.3) | ||
| Usual care | 3.6 (3.4) | 4.0 (3.8) | 3.9 (3.7) | ||
|
| |||||
| Joint stiffness | −0.2 (−0.6 to 0.2); .24 | −0.0 (−0.4 to 0.4); .87 | |||
| BEAT Cancer | 2.6 (1.6) | 2.2 (1.6) | 2.3 (1.6) | ||
| Usual care | 2.4 (1.7) | 2.4 (1.8) | 2.3 (1.8) | ||
|
| |||||
| Physical dysfunction | −2.7 (−5.0 to -0.5); .018 | −1.2 (−3.4 to 1.1); .31 | |||
| BEAT Cancer | 10.1 (9.6) | 8.1 (8.9) | 9.2 (12.3) | ||
| Usual care | 9.7 (11.0) | 10.3 (12.1) | 10.3 (12.3) | ||
|
| |||||
| WOMAC total | −3.7 (−6.7 to -0.6); .018 | −1.4 (−4.4 to 1.7); .38 | |||
| BEAT Cancer | 16.4 (13.6) | 13.9 (12.6) | 15.2 (17.3) | ||
| Usual care | 15.6 (15.3) | 16.6 (16.6) | 16.5 (16.9) | ||
|
| |||||
| Satisfaction with life | 2.4 (0.9 to 3.9); .001 | 1.3 (−0.2 to 2.8); .08 | |||
| BEAT Cancer | 24.9 (7.1) | 26.4 (6.7) | 25.8 (7.5) | ||
| Usual care | 24.3 (7.5) | 23.6 (8.2) | 23.9 (8.1) | ||
Adjusted for baseline value, study site, breast cancer stage, history of chemotherapy, history of radiation therapy, current hormonal therapy, comorbidities, and marital status
Confidence intervals
Standard deviation
Sample size = 162 due to equipment malfunction
Moderator results
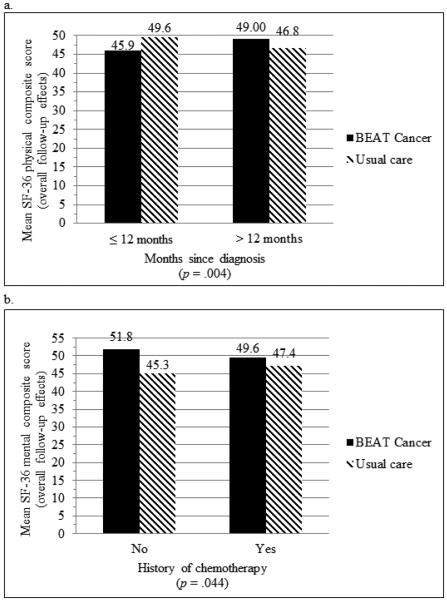
Neither age nor time on hormonal therapy moderated any of the outcomes. Improvements in the physical composite score and multiple SF-36 subscores favored participants who were >12 months compared with those ≤12 months since diagnosis (physical composite score = +2.23 versus −3.61, p = 0.004; physical functioning = +7.40 versus −7.11, p = 0.005; role-physical = +7.59 versus −6.78, p = 0.018; pain = +6.84 versus −6.42, p = 0.012; general health = +5.18 versus −5.18; p = 0.009; vitality = +12.27 versus +1.31, P = 0.031). Intervention effects on physical functioning also favored participants with <$50,000 annual income compared to ≥$50,000 (+15.05 versus +0.79, p = 0.002) and marital status categorized as other compared with married/living with significant other (+14.07 versus +0.74, p =.003). Participants with a history of chemotherapy were less likely to experience intervention improvements in mental health composite score (+2.24 versus +6.57, p = 0.044), role-emotional (+3.12 versus +12.62, p = .032), and mental health subscore (+2.53 versus +10.02, p = 0.037). The intervention effects on mental health composite score, physical functioning, vitality, social functioning, role-emotional, and mental health subscore were more pronounced in participants with lower baseline scores (all p values < 0.05). The moderators of the composite scores other than baseline value are provided in Fig 1.
Fig 1.
Months since diagnosis as a moderator of overall BEAT Cancer intervention effects on SF-36 physical composite score (a) and history of chemotherapy as a moderator of overall BEAT Cancer intervention effects on SF-36 mental health composite score (b) at follow-up
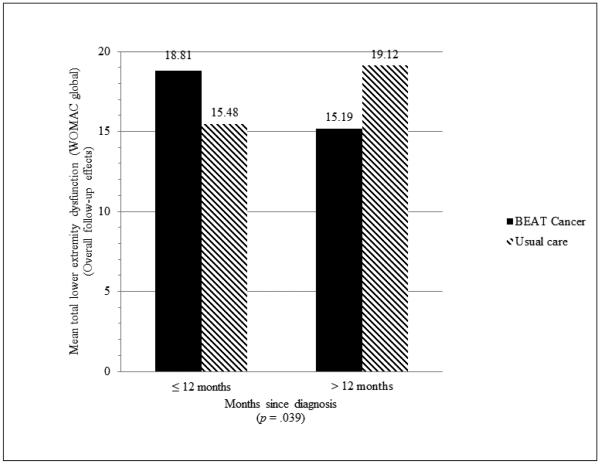
No statistically significant moderators were noted for muscle strength. Intervention effects on BMI favored participants with prior history of radiation (−0.36 for prior radiation versus 0.54 for no prior radiation, p = 0.012) and higher baseline BMI (−0.95 for BMI ≥ 30 versus 0.47 for BMI < 30, p = 0.035). Intervention effects on lower extremity pain (WOMAC pain subscore) favored participants with >12 months since diagnosis (−0.77 versus +1.00 for participants ≤12 months, p = 0.041) and lower breast cancer stage (−1.22 for DCIS/stage I versus 0.48 for stage II/III, p = 0.011). Effects on lower extremity physical dysfunction also favored participants >12 months since diagnosis (−3.00 versus +2.50 for participants ≤12 months, p = 0.033) and lower breast cancer stage (−3.79 for DCIS/stage I versus 0.17 for Stage II/III, P = 0.047). Only months since diagnosis moderated total WOMAC score (−3.92 for >12 months since diagnosis versus +3.33 for participants ≤12 months; p = 0.039) (Fig 2). Baseline scores moderated the intervention effects on satisfaction with life with lower baseline scores reporting greater intervention benefit (4.08 if < 26 and 0.32 if ≥ 26, p = 0.016).
Fig 2.
Months since diagnosis as a moderator of overall BEAT Cancer intervention effects on lower extremity dysfunction (WOMAC global) at follow-up (lower score indicates less dysfunction)
Discussion
When compared to UC (i.e., printed physical activity materials), BEAT Cancer demonstrated significant beneficial effects on all components of the SF-36 health-related quality of life scale, lower extremity dysfunction, and satisfaction with life. Statistically significant benefit favoring BEAT Cancer continued 3 months after intervention completion for SF-36 vitality subscore and mental health (subscore and composite score). No statistically significant intervention effects on back/leg muscle strength, BMI, lower extremity pain, and lower extremity stiffness were found at 3 or 6 months. Along with baseline values, demographic characteristics (income, marital status) and cancer-related factors (cancer treatment, cancer stage, and time since diagnosis) moderated one or more of the outcomes.
Intervention effects on SF-36 outcomes exceeded the minimally important difference (MID) for general health, vitality, social functioning, mental health subscore, and mental health composite score at month 3 and continued to reach or exceed the MID at month 6 for vitality and mental health [22]. The intervention’s effect on vitality is of substantial clinical relevance given that fatigue is a frequent and persistent side effect of cancer treatment that causes disability and negatively impacts quality of life [3, 19]. Similarly, the significant between group difference in the physical health composite score is of clinical importance given that breast cancer survivors with higher scores are at lower risk for breast cancer events and all-cause mortality [34]. The attenuation of SF-36 physical health benefits at month 6 is most probably related to recidivism in physical activity behavior. Hence, intervention refinements (e.g., ongoing booster sessions or contacts) are needed to maintain the increases in physical activity over a longer period of time.
Importantly, BEAT Cancer’s effects on lower extremity physical dysfunction and total WOMAC exceed the minimally important clinical difference (MCID) of an improvement of ≥17% of baseline [2]. Given the prevalence of joint symptoms post-breast cancer treatment and in older patient populations, these improvements are clinically noteworthy and consistent with exercise training trials [7, 18, 36]. It is conceivable that the individualized attention provided by the exercise specialists in our intervention facilitated effective tailoring of the exercise prescription to better cope with joint complaints. However, further research is needed to test this possibility.
Several moderators warrant discussion. First, additional study is needed to determine why a history of chemotherapy blunted the intervention benefits on mental health measured by SF-36, including but not limited to the possible role of long term effects on cognitive function and related poor quality of life [36]. Further research is also needed to determine why a shorter time since diagnosis blunted the intervention benefits on SF-36 and WOMAC outcomes. It is possible that cancer survivors who are within a year of their diagnosis suffer greater limitations that would benefit from a longer intervention period.
Although not a weight loss intervention and no significant between group difference with regard to BMI for all participants combined was noted, baseline BMI as a moderator (i.e., participants with a BMI >30 were more likely to lose weight with the intervention) is noteworthy because reducing obesity after a cancer diagnosis can reduce recurrence and death [24]. Also, the greater intervention benefit in those reporting lower annual incomes is significant because lower socioeconomic status is associated with less physical activity and participation in physical activity behavior change interventions [21, 39]. Further research to replicate this finding and identify potential key intervention components responsible for this benefit is warranted. Lastly, the greater benefit in participants who were unmarried is consistent with two previous reports by Courneya et al. [11, 12]. and the greater improvements in physical functioning seen with psychosocial interventions among individuals with less support at home [17].
Our study limitations include lack of generalizability to survivors with other cancer types and exercise without behavioral support. Also, our moderator testing can only be considered exploratory because our study was not originally powered for nor we were able to adjust for multiple secondary comparisons. Nevertheless, support for the potential moderating role of factors such as time since cancer diagnosis and history of chemotherapy is strengthened by their moderation of multiple outcomes.
Our study strengths include its randomized design, inclusion of understudied health-related outcomes (e.g., lower extremity dysfunction, SF-36 physical health associated with cancer and mortality risk), and exploration of moderating factors. Our study also identifies several areas warranting further study including but not limited to why a history of chemotherapy and shorter time since diagnosis moderated the intervention effects and how the blunting effect of these factors can be overcome. Furthermore, these results suggest additional research is needed to determine how to best tailor physical activity interventions based on cancer-related moderating factors so that optimal improvements in health, well-being, and risk of cancer outcomes can be achieved after a breast cancer diagnosis.
Acknowledgements and Funding Information
The authors acknowledge the contributions of Robert Mocharnuk, MD, Karen Hoelzer, MD, Sara Mansfield, MS, Amanda Fogleman, BS, Ruth Sosnoff, PhD, Southern Illinois University School of Medicine Center for Clinical Research, University of Illinois at Urbana Champaign kinesiology graduate students and assistants, and the University of Alabama at Birmingham Nutrition Obesity Research Center Physical Activity core. This project was supported by a grant from the National Cancer Institute R01CA136859, P30DK056336, and R25CA47888. Kerry S. Courneya is supported by the Canada Research Chairs Program.
Footnotes
ClinicalTrials.gov identifier: NCT00929617
Conflict of Interest: All contributing authors declare they have no personal or professional relationships that may represent a potential conflict of interest.
Ethical approval: All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.ACS . Cancer Facts and Figures 2015. American Cancer Society; Atlanta, GA: 2015. [Google Scholar]
- 2.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- 3.Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107:2496–2503. doi: 10.1002/cncr.22274. doi: 10.1002/cncr.22274. [DOI] [PubMed] [Google Scholar]
- 4.Basen-Engquist K, Taylor CL, Rosenblum C, Smith MA, Shinn EH, Greisinger A, Gregg X, Massey P, Valero V, Rivera E. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Battaglini CL, Mills RC, Phillips BL, Lee JT, Story CE, Nascimento MG, Hackney AC. Twenty-five years of research on the effects of exercise training in breast cancer survivors: A systematic review of the literature. World J Clin Oncol. 2014;5:177–190. doi: 10.5306/wjco.v5.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffart LM, Newton RU, Chinapaw MJ, Taaffe DR, Spry NA, Denham JW, Joseph DJ, Lamb DS, Brug J, Galvao DA. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer. 2015;121:2821–2830. doi: 10.1002/cncr.29406. doi: 10.1002/cncr.29406. [DOI] [PubMed] [Google Scholar]
- 7.Cantarero-Villanueva I, Fernandez-Lao C, Caro-Moran E, Morillas-Ruiz J, Galiano-Castillo N, Diaz-Rodriguez L, Arroyo-Morales M. Aquatic exercise in a chest-high pool for hormone therapy-induced arthralgia in breast cancer survivors: a pragmatic controlled trial. Clin Rehabil. 2013;27:123–132. doi: 10.1177/0269215512448256. doi: 10.1177/0269215512448256. [DOI] [PubMed] [Google Scholar]
- 8.Carmack Taylor CL, de Moor C, Basen-Engquist K, Smith MA, Dunn AL, Badr H, Pettaway C, Gritz ER. Moderator analyses of participants in the Active for Life after cancer trial: implications for physical activity group intervention studies. Ann Behav Med. 2007;33:99–104. doi: 10.1207/s15324796abm3301_11. doi: 10.1207/s15324796abm3301_11. [DOI] [PubMed] [Google Scholar]
- 9.Courneya KS. Efficacy, effectiveness, and behavior change trials in exercise research. Int J Behav Nutr Phys Act. 2010;7:81. doi: 10.1186/1479-5868-7-81. doi: 1479-5868-7-81 [pii] 10.1186/1479-5868-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courneya KS, McKenzie DC, Gelmon K, Mackey JR, Reid RD, Yasui Y, Friedenreich CM, Forbes CC, Trinh L, Jespersen D, Cook D, Proulx C, Wooding E, Dolan LB, Segal RJ. A multicenter randomized trial of the effects of exercise dose and type on psychosocial distress in breast cancer patients undergoing chemotherapy. Cancer Epidemiol Biomarkers Prev. 2014;23:857–864. doi: 10.1158/1055-9965.EPI-13-1163. doi: 10.1158/1055-9965.EPI-13-1163. [DOI] [PubMed] [Google Scholar]
- 11.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, Segal RJ. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy. Cancer. 2008;112:1845–1853. doi: 10.1002/cncr.23379. [DOI] [PubMed] [Google Scholar]
- 12.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Friedenreich CM, Peddle CJ, Basi S, Chua N, Tankel K, Mazurek A, Reiman T. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2009;18:2600–2607. doi: 10.1158/1055-9965.EPI-09-0504. doi: 10.1158/1055-9965.EPI-09-0504. [DOI] [PubMed] [Google Scholar]
- 13.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 14.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein D, Bennett BK, Webber K, Boyle F, de Souza PL, Wilcken NR, Scott EM, Toppler R, Murie P, O'Malley L, McCourt J, Friedlander M, Hickie IB, Lloyd AR. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol. 2012;30:1805–1812. doi: 10.1200/JCO.2011.34.6148. doi: 10.1200/JCO.2011.34.6148. [DOI] [PubMed] [Google Scholar]
- 16.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Helgeson VS, Cohen S, Schulz R, Yasko J. Group support interventions for women with breast cancer: who benefits from what? Health Psychol. 2000;19:107–114. doi: 10.1037//0278-6133.19.2.107. [DOI] [PubMed] [Google Scholar]
- 18.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y, Harrigan M, Sanft T, Schmitz K, Neogi T, Hershman D, Ligibel J. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–1111. doi: 10.1200/JCO.2014.57.1547. doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JM, Olson K, Catton P, Catton CN, Fleshner NE, Krzyzanowska MK, McCready DR, Wong RK, Jiang H, Howell D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2015 doi: 10.1007/s11764-015-0450-2. doi: 10.1007/s11764-015-0450-2. [DOI] [PubMed] [Google Scholar]
- 20.Kalter J, Buffart LM, Korstjens I, van Weert E, Brug J, Verdonck-de Leeuw IM, Mesters I, van den Borne B, Hoekstra-Weebers JE, Ros WJ, May AM. Moderators of the effects of group-based physical exercise on cancer survivors' quality of life. Support Care Cancer. 2015;23:2623–2631. doi: 10.1007/s00520-015-2622-z. doi: 10.1007/s00520-015-2622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao MC, Jarosz R, Goldin M, Patel A, Smuck M. Determinants of physical activity in America: a first characterization of physical activity profile using the National Health and Nutrition Examination Survey (NHANES) PM R. 2014;6:882–892. doi: 10.1016/j.pmrj.2014.03.004. doi: 10.1016/j.pmrj.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43:1478–1487. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of general psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 24.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, Fabian CJ, Gucalp A, Hershman DL, Hudson MM, Jones LW, Kakarala M, Ness KK, Merrill JK, Wollins DS, Hudis CA. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra SI, Scherer RW, Snyder C, Geigle P, Gotay C. Are exercise programs effective for improving health-related quality of life among cancer survivors? A systematic review and meta-analysis. Oncol Nurs Forum. 2014;41:E326–342. doi: 10.1188/14.ONF.E326-E342. doi: 10.1188/14.ONF.E326-E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol. 2013;24:1443–1449. doi: 10.1093/annonc/mdt037. doi: 10.1093/annonc/mdt037. [DOI] [PubMed] [Google Scholar]
- 28.Petrick JL, Reeve BB, Kucharska-Newton AM, Foraker RE, Platz EA, Stearns SC, Han X, Windham BG, Irwin DE. Functional status declines among cancer survivors: trajectory and contributing factors. J Geriatr Oncol. 2014;5:359–367. doi: 10.1016/j.jgo.2014.06.002. doi: 10.1016/j.jgo.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto B, Stein K, Dunsiger S. Peer mentorship to promote physical activity among cancer survivors: effects on quality of life. Psychooncology. 2015 doi: 10.1002/pon.3884. doi: 10.1002/pon.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers JC, Irrang JJ. Measures of adult lower extremity function. Arthritis & Rheumatism. 2003;49:S67–S84. [Google Scholar]
- 31.Rogers LQ, Courneya KS, Anton PM, Hopkins-Price P, Verhulst S, Vicari SK, Robbs RS, Mocharnuk R, McAuley E. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015;149:109–119. doi: 10.1007/s10549-014-3216-z. doi: 10.1007/s10549-014-3216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, Dunnington G, Lanzotti V, Wynstra J, Shah L, Edson B, Graff A, Lowy M. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 33.Rogers LQ, McAuley E, Anton PM, Courneya KS, Vicari S, Hopkins-Price P, Verhulst S, Mocharnuk R, Hoelzer K. Better exercise adherence after treatment for cancer (BEAT Cancer) study: rationale, design, and methods. Contemporary clinical trials. 2012;33:124–137. doi: 10.1016/j.cct.2011.09.004. doi: 10.1016/j.cct.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saquib N, Pierce JP, Saquib J, Flatt SW, Natarajan L, Bardwell WA, Patterson RE, Stefanick ML, Thomson CA, Rock CL, Jones LA, Gold EB, Karanja N, Parker BA. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. 2011;20:252–259. doi: 10.1002/pon.1742. doi: 10.1002/pon.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schootman M, Aft R, Jeffe DB. An evaluation of lower-body functional limitations among long-term survivors of 11 different types of cancers. Cancer. 2009;115:5329–5338. doi: 10.1002/cncr.24606. doi: 10.1002/cncr.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–2592. doi: 10.1002/cncr.23448. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villasenor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, Neuhouser ML. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6:398–406. doi: 10.1007/s11764-012-0234-x. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36® Health Survey. Quality Metric Incorporated; Lincoln, RI: 2000. [Google Scholar]
- 39.Waters LA, Galichet B, Owen N, Eakin E. Who participates in physical activity intervention trials? J Phys Act Health. 2011;8:85–103. doi: 10.1123/jpah.8.1.85. [DOI] [PubMed] [Google Scholar]