Abstract
Purpose
To pool data across multiple institutions internationally and report on the cumulative experience of brainstem stereotactic radiosurgery (SRS).
Methods and Materials
Data on patients with brainstem metastases treated with SRS were collected through the International Gamma Knife Research Foundation. Clinical, radiographic, and dosimetric characteristics were compared for factors prognostic for local control (LC) and overall survival (OS) using univariate and multivariate analyses.
Results
Of 547 patients with 596 brainstem metastases treated with SRS, treatment of 7.4% of tumors resulted in severe SRS-induced toxicity (grade ≥3, increased odds with increasing tumor volume, margin dose, and whole-brain irradiation). Local control at 12 months after SRS was 81.8% and was improved with increasing margin dose and maximum dose. Overall survival at 12 months after SRS was 32.7% and impacted by age, gender, number of metastases, tumor histology, and performance score.
Conclusions
Our study provides additional evidence that SRS has become an option for patients with brainstem metastases, with an excellent benefit-to-risk ratio in the hands of experienced clinicians. Prior whole-brain irradiation increases the risk of severe toxicity in brainstem metastasis patients undergoing SRS.
Introduction
Stereotactic radiosurgery (SRS) has repeatedly demonstrated its safety and effectiveness when used to treat patients with brain metastases (1-3). As a result of several large trials, SRS has gained popularity by demonstrating excellent local control and uncommon toxicity (4).
One caveat is that the studies validating SRS (5-7), as well as recent protocols (8), did not include patients with tumors involving or abutting the brainstem (ie, the midbrain, pons, and medulla oblongata). This is based on concerns that SRS dose, even at the tumor margin, exceeds brainstem goal dose tolerance for a single fraction and that SRS-induced injury to this location could be severe and potentially life-threatening (9, 10).
Metastatic tumors within the brainstem pose many unique and difficult therapy decisions. Local progression of disease within the brainstem is associated with an acute and severe neurologic decline. Access by craniotomy is not indicated because of the risk associated with the approach corridors needed to resect the metastasis even if it is exophytic. Systemic chemotherapy has little demonstrated effectiveness.
Several small, single-institution series have reported the outcomes of brainstem SRS, usually with fewer than 60 patients included (11-19). Early studies had limited ability to draw conclusions because of their small sample size and heterogeneity across patients. Moreover, some reports have established conflicting recommendations regarding several prognostic factors, including optimum SRS margin dose(17, 18, 20-22).
Our proposed aims include to: (1) define the safety of SRS for brainstem metastases; (2) define the efficacy of SRS for brainstem metastases; and (3) evaluate clinical and treatment factors that might affect survival after radiosurgery.
Methods and Materials
Data collection
We proposed the following analysis to the International Gamma Knife Research Foundation. After approval by the steering committee all participating institutions were invited to provide their deidentified institutional data of all patients treated with SRS for brainstem metastases at their institution with follow-up data available. Patients with brainstem metastases diagnosed at initial cancer presentation or anytime thereafter were included. Clinical data collection included age, gender, Karnofsky performance score, tumor histology (as determined by the primary site), time from primary diagnosis to SRS, total intracranial metastases, active extracranial disease presence and location, whole-brain radiation therapy (WBRT) dose and timing, and chemotherapy use before SRS. Tumor location was divided into midbrain, pons, medulla oblongata, or overlapping. Tumors of the cerebellopontine angle were included when the tumor invaded the brainstem proper, resulting in at least prescription dose to the brainstem parenchyma. Patients without radiographic or clinical follow-up were excluded. Collection and sharing of data was approved through each participating center’s institutional review board.
The SRS parameters included brainstem tumor volume, margin dose, isodose, and maximum dose. Follow-up data collected included radiographic local control and evidence of severe clinical toxicity (grade ≥3 according to the Common Terminology Criteria for Adverse Events, version 4.03) (23), and overall survival after brainstem SRS. To allow for subtle differences in MRI technique and patient positioning, tumor volume changes of 10% or less from baseline were termed unchanged in size (24).
Statistical analysis
Tumors were said to be controlled locally if they were decreased or unchanged in size, and to have failed locally if they increased in size (as determined by a volume increase of >10%) over the follow-up period (24). Collected data were analyzed via Kaplan-Meier plots of overall and progression-free survival after SRS. Log-rank tests were used to investigate possible differences between these curves after stratification by various prognostic factors. Potential prognostic factors were analyzed for an association with clinical outcomes via univariate and multivariate logistic and/or Cox regression analysis. Differences were considered statistically significant if P<.05, and statistics were calculated using commercially available statistics software (SPSS version 20.0; SPSS, Chicago, IL).
Participating centers
Ten centers from 4 countries provided data meeting inclusion criteria on 547 patients with 596 brainstem tumors treated with Gamma Knife SRS (Elekta AB, Stockholm, Sweden). Table 1 provides the clinical characteristics of the patients included in our series. The median age of patients at the time of SRS was 61 years. Twenty-six percent underwent SRS for a single brain metastasis located in the brainstem; 76% had additional brain lesions (16% with 1 other lesion, 11% with 2 other lesions, and 48% with >2 other lesions). Brainstem tumor volume varied from 0.01 mL to 21 mL (median 0.8 mL). Forty-nine percent of patients underwent WBRT before brainstem SRS. Although SRS prescription dose varied between patients and institutions, the median dose was 16 Gy prescribed to the 50% isodose line and a median maximum dose of 30 Gy.
Table 1.
Clinical characteristics of 596 brainstem metastatic tumors in 547 patients
| Characteristic | Value | Percentage or range |
|---|---|---|
| Sex (male:female) | 266:281 | |
| Median age (y) | 61 | 23-96 |
| Brainstem tumor size (mL) | 0.8 | 0.01-21.0 |
| Total intracranial metastases (n=547) |
||
| 1 (single lesion) | 142 | 26 |
| 2 | 85 | 16 |
| 3 | 58 | 11 |
| >3 | 253 | 48 |
| Location of tumor (n=596) | ||
| Midbrain | 126 | 21 |
| Pons | 345 | 58 |
| Medulla | 45 | 8 |
| CP angle | 14 | 2 |
| Midbrain-pons | 44 | 7 |
| Pons-medulla | 22 | 4 |
| KPS (median) | 90 | 30-100 |
| Extracranial metastasis (n=547) |
||
| Yes | 370 | 68 |
| No | 177 | 32 |
| Primary tumor (n=547) | ||
| NSCLC | 227 | 41 |
| Breast cancer | 140 | 26 |
| Melanoma | 75 | 14 |
| Renal cell | 58 | 11 |
| GI tract origin | 17 | 3 |
| SCLC | 16 | 3 |
| Other* | 7 | 1 |
| Unknown | 7 | 1 |
| Neurologic symptoms/signs (n=547, may be multiple) |
||
| Long tract signs | 281 | 51 |
| Cerebellar signs | 75 | 14 |
| Cranial nerve palsy | 151 | 28 |
| Asymptomatic | 179 | 33 |
| Median image follow-up (mo) |
5.5 | 0.1-244 |
| Median clinical follow-up (mo) |
5.6 | 0.1-237 |
| Prior WBRT (n=547) | 266 | 49 |
| Prior chemotherapy (nZ547) | 412 | 75 |
| SRS† | ||
| Margin dose (Gy) | 16 | 8-25 |
| Isodose level (%) | 50 | 26-98 |
| Maximum dose (Gy) | 30 | 13-67 |
| Median survival (mo) | 5.6 | 0.1-237 |
Abbreviations: CP = cerebellopontine; GI = gastrointestinal; KPS = Karnofsky performance status; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer; SRS = stereotactic radiosurgery; WBRT = whole-brain radiation therapy.
“Other” includes bladder cancer, lymphoma, oral cavity cancer, malignant peripheral nerve sheath tumor, nasopharyngeal cancer, Ewing’s sarcoma, salivary gland cancer, skin cancer, thyroid cancer, fibrohistiocytoma, and carcinoma of unknown origin.
Parameters of SRS are reported as median values for each brainstem metastasis, not the entire treatment volume.
Results
Local tumor control
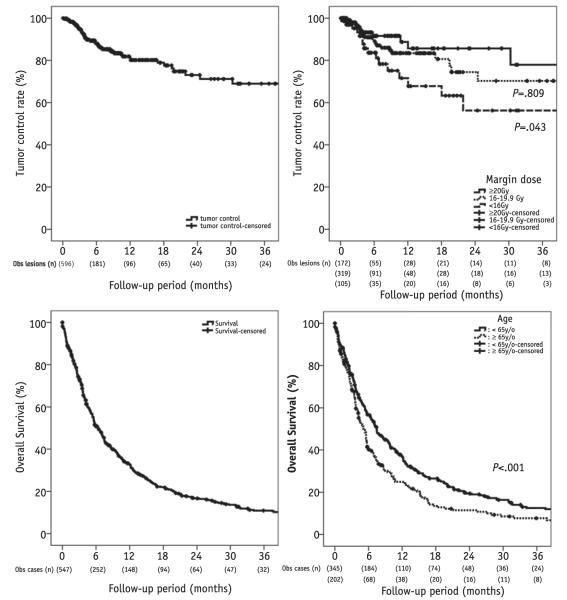
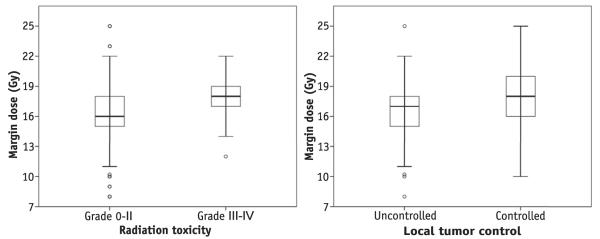
Local tumor control (brainstem tumors only) among all patients in the series is depicted in Figure 1. At 1 year after brainstem SRS, local control was 81.8%. Table 2 provides an analysis of factors associated with increased risk of local tumor failure. As demonstrated, age, margin dose of <16 Gy (compared with margin dose of ≥20 Gy), and maximum dose were the only factors associated with increased risk of local failure on multivariate analysis (P=.007, P=.039, and P=.020, respectively). The multivariate analysis of local failure was reanalyzed using margin dose as a continuous variable, and increased margin dose trended toward, but did not meet, statistical significance (hazard ratio 0.572, P=.091). When stratifying according to the receipt of WBRT, there was no difference in margin dose selection or ultimate brainstem local control (P=.405 and .686, respectively). Tumor histology did not impact the rate of local failure (Table 2). Figure 2 (right) displays the ranges of margin doses used in patients stratified by whether they went on to develop local tumor control after SRS.
Fig. 1.
Kaplan-Meier curves for local tumor control among all tumors (upper left) and among tumors stratified by margin dose (upper right, P values compare with ≥20-Gy margin dose), as well as for overall survival after brainstem stereotactic radiosurgery among all patients (lower left) and among patients stratified by age (lower right).
Table 2.
Prognostic factors associated with local failure in 596 brainstem metastases (presented by Cox regression)
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Factor | n | P | Hazard ratio* | 95% CI | P | Hazard ratio* | 95% CI |
| Age (y) | .090 | 007 | |||||
| <65 | 366 | Ref | Ref | ||||
| ≥65 | 230 | 1.588 | 0.930-2.711 | 2.377 | 1.261-4.482 | ||
| Gender | .064 | .575 | |||||
| Male | 286 | Ref | Ref | ||||
| Female | 310 | 0.607 | 0.358-1.029 | 0.815 | 0.398-1.668 | ||
| No. of lesions | .694 | ||||||
| Single | 142 | Ref | |||||
| Multiple | 454 | 0.896 | 0.517-1.551 | ||||
| Active systemic metastases | .035 | .143 | |||||
| No | 197 | Ref | Ref | ||||
| Yes | 399 | 1.957 | 1.049-3.653 | 1.683 | 0.838-3.380 | ||
| Tumor volume (mL) | .482 | ||||||
| <1 | 271 | Ref | |||||
| 1-2 | 68 | .283 | 1.551 | 0.696-3.458 | |||
| >2 | 201 | .376 | 1.308 | 0.722-2.369 | |||
| Primary tumory† | .076 | .568 | |||||
| Breast cancer | 159 | Ref | Ref | ||||
| NSCLC | 246 | .212 | 1.642 | 0.754-3.576 | .756 | 1.155 | 0.466-2.859 |
| SCLC | 16 | .984 | 1.034 | 0.812-1.188 | .985 | 1.036 | 0.814-1.189 |
| RCC | 60 | .498 | 1.431 | 0.507-4.035 | .954 | 0.964 | 0.279-3.338 |
| Melanoma | 84 | .003 | 3.881 | 1.599-9.417 | .135 | 2.450 | 0.757-7.926 |
| GI tract origin | 17 | .983 | 1.022 | 0.972-1.029 | .985 | 1.031 | 0.988-1.021 |
| Tumor location | .591 | ||||||
| Midbrain | 126 | Ref | |||||
| Pons | 345 | .416 | 0.491 | 0.088-2.725 | |||
| Medulla | 45 | .718 | 0.858 | 0.373-1.972 | |||
| CP angle | 14 | .256 | 1.465 | 0.758-2.832 | |||
| Midbrain-pons | 44 | .994 | 1.005 | 0.332-3.043 | |||
| Pons-medulla | 22 | .728 | 0.738 | 0.133-4.095 | |||
| Margin dose (Gy) | .110 | .096 | |||||
| <16 | 172 | Ref | Ref | ||||
| 16-19.9 | 319 | .061 | 0.496 | 0.238-1.032 | .809 | 0.883 | 0.322-2.422 |
| ≥20 | 105 | .074 | 0.562 | 0.298-1.058 | .043 | 0.478 | 0.234-0.977 |
| Maximum dose (Gy) | .036 | .039 | |||||
| <32 | 300 | Ref | Ref | ||||
| 32-40 | 211 | .073 | 0.469 | 0.205-1.073 | .020 | 0.290 | 0.102-0.823 |
| >40 | 85 | .966 | 0.983 | 0.442-2.185 | .389 | 0.660 | 0.256-1.700 |
| Prior WBRT | .686 | ||||||
| No | 301 | Ref | |||||
| Yes | 295 | 1.124 | 0.639-1.977 | ||||
| Prior chemotherapy | .995 | ||||||
| No | 155 | Ref | |||||
| Yes | 441 | 1.002 | 0.570-1.760 | ||||
Abbreviation: CI = confidence interval. Other abbreviations as in Table 1.
Higher hazard ratio, higher relative risk to local treatment failure.
Excludes unknown and other primary histology.
Fig. 2.
Ranges of margin doses used in patients stratified by whether they went on to develop severe toxicity (left) and local tumor control (right) after stereotactic radiosurgery.
Overall survival
Figure 1 demonstrates the overall survival of all patients after brainstem SRS, as well as overall survival among patients stratified by age. Median survival was 5.6 months, and the 1-year survival was 32.7%. The 2-year survival was 16.7%, and 10.9% of patients remained alive 3 years after brainstem SRS. A total of 143 patients died of systemic disease progression (26%), 88 patients died of non-brainstem intracranial disease progression (16%), 4 died of brainstem disease progression (0.7%), and the cause of death could not be determined in 312 (57%).
Table 3 provides an analysis of factors prognostic for overall survival. As demonstrated, several factors were associated with longer survival, including younger age, single metastases (brainstem metastasis—only patients), and non-melanoma histology (P<.001, P<.001, and P=.039, respectively) favorably impacting overall survival.
Table 3.
Prognostic factors associated with overall survival in 547 patients with brainstem metastases (presented by Cox regression)
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Factor | n | P | Hazard ratio* | 95% CI | P | Hazard ratio* | 95% CI |
| Age (y) | .002 | <.001 | |||||
| <65 | 346 | Ref | Ref | ||||
| ≥65 | 201 | 1.363 | 1.122-1.675 | 1.573 | 1.258-1.966 | ||
| Gender | <.001 | .031 | |||||
| Male | 266 | Ref | Ref | ||||
| Female | 281 | 0.704 | 0.584-0.849 | 0.769 | 0.605-0.976 | ||
| No. of lesions | .003 | <.001 | |||||
| Single | 142 | Ref | Ref | ||||
| Multiple | 405 | 1.400 | 1.124-1.744 | 1.636 | 1.274-2.099 | ||
| Extracranial metastasis | .024 | .059 | |||||
| No | 177 | Ref | Ref | ||||
| Yes | 370 | 1.264 | 1.031-1.548 | 1.237 | 0.992-1.542 | ||
| Total tumor volume (mL) | .917 | ||||||
| <1 | 251 | Ref | |||||
| 1-2 | 59 | .820 | 1.036 | 0.763-1.408 | |||
| >2 | 181 | .788 | 0.972 | 0.791-1.195 | |||
| Primary tumor† | .007 | .022 | |||||
| Breast cancer | 140 | Ref | Ref | ||||
| NSCLC | 227 | .286 | 1.146 | 0.892-1.471 | .610 | 0.928 | 0.695-1 .238 |
| SCLC | 16 | .811 | 0.916 | 0.445-1.884 | .557 | 0.801 | 0.383-1.678 |
| RCC | 58 | .540 | 1.113 | 0.790-1.568 | .347 | 0.826 | 0.554-1.231 |
| Melanoma | 75 | <.001 | 1.825 | 1.335-2.496 | .039 | 1.473 | 1.020-2.126 |
| GI origin | 17 | .858 | 1.061 | 0.553-2.036 | .219 | 0.645 | 0.320-1.299 |
| KPS | 547 | <.001 | 0.979 | 0.972-0.986 | <.001 | 0.971 | 0.962-0.979 |
| Margin dose (Gy) | .135 | .235 | |||||
| <16 | 156 | Ref | Ref | ||||
| 16-19.9 | 289 | .088 | 1.215 | 0.971-1.520 | .170 | 1.192 | 0.927-1.532 |
| ≥20 | 102 | .072 | 1.299 | 0.977-1.726 | .105 | 1.311 | 0.945-1.820 |
| Maximum dose (Gy) | .539 | ||||||
| <32 | 274 | Ref | |||||
| 32-40 | 198 | .270 | 1.120 | 0.916-1.370 | |||
| >40 | 75 | .857 | 1.031 | 0.744-1.428 | |||
| Prior WBRT | .185 | ||||||
| No | 281 | Ref | |||||
| Yes | 266 | 1.136 | 0.941-1.371 | ||||
| Prior chemotherapy | .718 | ||||||
| No | 135 | Ref | |||||
| Yes | 412 | 1.037 | 0.851-1.263 | ||||
Radiation toxicity
Severe toxicity after brainstem SRS was rare. It was defined by established criteria. We used the following grading: severe (grade ≥3) toxicity was defined as local edema, hemorrhage, or radionecrosis requiring intervention (medical or surgical), or any treatment-related death (grade 5). Severe toxicity also included any neuropathy, encephalopathy, seizure, syncope, memory loss, or ataxia with severe symptoms limiting activities of daily living. Forty-four patients (7.4%) developed a grade 3 to 4 toxicity as a result of brainstem SRS at any time point in follow-up. There were 2 grade 4 toxicities (0.3%; intrapontine hemorrhage and hemibody paralysis); the remaining toxicities were grade 3 or less (severe extremity motor weakness in 5 patients, severe ataxia in 3 patients, cranial nerve paralysis in 2 patients, severe headaches in 1 patient, otherwise grade 3 with unknown details). There were no neurologic deaths attributable to brainstem SRS (0%).
Of the 266 patients (48.6%) who received WBRT before brainstem SRS, the median interval between WBRT and SRS was 4.5 months (range 0-46.1 months). Of the 44 patients who developed a severe toxicity, 84% had undergone WBRT before brainstem SRS (compared with 47% in the subgroup that did not go on to develop a severe toxicity, P<.001). Among patients who received prior WBRT, an increased interval from WBRT to brainstem SRS was also found to predict for decreased risk of radiation toxicity (odds ratio 0.116 for ≥4.5 months compared with <4.5 months, P<.001). The median WBRT total dose was 30 Gy (range, 20-57.5 Gy). No severe toxicity was noted in brainstem tumors smaller than 0.1 mL or in tumors treated to a margin dose of <12 Gy. There was no effect of brainstem tumor location or volume of tissue receiving 12 Gy (V12) on severe toxicity (P=.30 and .06, respectively).
Table 4 provides an analysis of factors associated with increased odds of a severe toxicity. As demonstrated, increasing tumor volume, increasing margin dose, and pre-SRS WBRT were each associated with increased odds of severe toxicity after SRS (P<.001, P=.049, and P=.002, respectively). Figure 2 (left) displays the ranges of margin doses used in patients stratified by whether they went on to develop a severe SRS toxicity.
Table 4.
Prognostic factors associated with severe radiation toxicity in 596 brainstem metastases (presented by logistic regression)
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Factor | n | P | Odds ratio | 95% CI | P | Odds ratio | 95% CI |
| Age (y) | .900 | ||||||
| <65 | 366 | Ref | |||||
| ≥65 | 230 | 0.960 | 0.507-1.817 | ||||
| Gender | .172 | ||||||
| Male | 286 | Ref | |||||
| Female | 310 | 0.651 | 0.351-1.205 | ||||
| Tumor volume (mL) | <.001 | <.001 | |||||
| <1 | 271 | Ref | Ref | ||||
| 1-2 | 68 | <.001 | 12.842 | 4.357-37.851 | <.001 | 14.407 | 4.355-47.662 |
| >2 | 201 | <.001 | 9.992 | 3.751-26.246 | <.001 | 11.736 | 3.876-35.540 |
| Primary tumor* | .008 | .606 | |||||
| Breast cancer | 159 | Ref | Ref | ||||
| NSCLC | 246 | .352 | 1.438 | 0.621-3.808 | .640 | 1.269 | 0.467-3.452 |
| SCLC | 16 | .830 | 1.267 | 0.146-11.008 | .463 | 2.522 | 0.214-29.775 |
| RCC | 60 | .002 | 4.957 | 1.841-13.347 | .446 | 1.545 | 0.505-4.730 |
| Melanoma | 84 | .423 | 0.521 | 0.105-2.571 | .255 | 0.374 | 0.069-2.035 |
| GI origin | 17 | .830 | 1.267 | 0.146-11.008 | .967 | 0.951 | 0.089-10.114 |
| Margin dose*(Gy) | .012 | .049 | |||||
| <16 | 172 | Ref | Ref | ||||
| 16-19.9 | 319 | .009 | 3.654 | 1.386-9.634 | .041 | 3.786 | 1.054-13.598 |
| ≥20 | 105 | .004 | 4.998 | 1.682-14.848 | .015 | 5.787 | 1.416-23.653 |
| Maximum dose (Gy) | <.001 | .956 | |||||
| <32 | 300 | Ref | Ref | ||||
| 32-40 | 211 | <.001 | 4.921 | 2.352-10.295 | .949 | 0.966 | 0.335-2.783 |
| >40 | 85 | .105 | 2.696 | 0.812-8.951 | .778 | 0.811 | 0.190-3.472 |
| Prior WBRT | <.001 | .002 | |||||
| No | 301 | Ref | Ref | ||||
| Yes | 295 | 4.688 | 2.055-10.698 | 4.488 | 1.768-11.396 | ||
| Prior chemotherapy | .393 | ||||||
| No | 155 | Ref | |||||
| Yes | 441 | 0.757 | 0.399-1.436 | ||||
Discussion
The therapeutic window
In the largest series reported on the topic, our results support SRS as an effective treatment option for many patients with brainstem metastases. Unlike other locations within the brain, brainstem metastases are seldom resected given the surgical risks associated with their location in regions of critical brain function. Before the widespread use of SRS, WBRT was the only palliative treatment option (25). We found that SRS provided a generally low risk of toxicity and a favorable rate of local control. Our results are novel for several reasons. First, they evaluated a large series of patients treated across a variety of institutions. Second, our results demonstrate the increased risk of toxicity with brainstem SRS after WBRT, and that this risk is lessened with increased interval from WBRT to SRS. Third, our results provide data on the therapeutic ratio of radiosurgery (a dose-response and dose-toxicity relationship), and this can assist the development of clinically appropriate dose guidelines for patients with brainstem metastases treated with SRS.
Tumor margin dose selection is particularly important in patients with brainstem metastases because the tissue just outside of the tumor often serves important neurologic functions. Poor selectivity of the radiation dose beyond the tumor margin can lead to an increased risk of radiation-related neurologic toxicity. The literature notes conflicting recommendations regarding the optimum margin dose to maximize the therapeutic ratio (17, 18, 20, 21). Although some small series demonstrated improve local control with the use of doses of at least 15 to 20 Gy (20-22), other series recommend tumor margin doses as low as 12 Gy(18, 26). Additionally, the radiosurgical technology used may affect the rate of fall-off of radiation beyond the tumor margin and impact toxicity rate. Although the impact of hypofractionated radiosurgery on brainstem metastasis local control and toxicity is unknown, it has the potential to further improve the therapeutic ratio in selected patients.
Edema, hemorrhage, or radionecrosis in the brainstem after SRS can clinically manifest as cranial nerve deficits, hemiplegia, loss of consciousness, or death. This realization has led some to use lower doses that are potentially ineffective for tumor control. Some reports of severe toxicity after brainstem SRS margin doses >16 Gy have led some authors to recommend avoiding doses this high(18, 26). Because of the small sample sizes, there was limited statistical power to associate SRS brainstem toxicity with tumor volume, location, margin dose, or previous WBRT. As a result, a dose threshold, volume threshold, or other prognostic factor that can estimate radiation-related toxicity was not developed. Although the existing studies demonstrated statistically significant results and recommendations regarding brainstem SRS, other reports have failed to demonstrate a dose-response relationship (11-18, 27-30).
Dose optimization
Our results demonstrate that, depending on tumor volume, margin doses in the 16- to 24-Gy range may provide an appropriate balance of the risks of severe toxicity while maintaining tumor control (Fig. 2 and Tables 2 and 4). We found that prior WBRT significantly increased the risk of delayed severe radiation-related toxicity. If WBRT has already been administered and SRS is required to gain tumor control, a further reduction in the SRS tumor margin dose may be needed. In the present study we did not find that the 12-Gy volume (which includes the tumor and the surrounding brain outside of the tumor margin) was associated with detection of delayed toxicity. This is perhaps because of the smaller anatomic volume of the brainstem and tendency for brainstem metastases themselves to be smaller at the time of recognition. In this study we found that the median survival was 5.6 months, and the 1-year survival was 32.7%. The 2-year survival was 16.7%.
We believe that this analysis confirms that SRS is an ideal option for patients with brainstem metastases otherwise eligible for SRS. Although metastatic disease in the brainstem in the past has portended a generally poor prognosis (31), future advances in systemic therapies, including targeted and immune therapies, may improve systemic control, and the development of novel radio-sensitizers or radio-protectors may further change the effective dose that SRS can provide.
Study limitations
These data were obtained through a retrospective chart review of prospectively collected data. Although a phase 3 randomized trial that evaluates efficacy and toxicity in this patient population would be desirable, such a study is unlikely. The present study is a retrospective analysis of a heterogeneous patient population, and it is possible that other unmeasured confounding variables may affect our results. For example, targetable genetic mutations, and associated other medical comorbidities, may influence both local and systemic disease control. Likewise, steroid therapy concurrent with SRS may impact radiation-induced toxicity. On the basis of the presented survival data, follow-up time is generally limited in this patient population, and it is possible that our reported toxicity rate may increase in the small volume of long-term survivors. It is noteworthy that survival was measured from the time of brainstem SRS. This means that some patients had already survived months (or years) after their initial systemic cancer or brain metastasis presentation. However, to inform on survival of these patients in a practical way that could be extended to patients in clinic, we limited survival analyses to the methods described.
All patients in this series underwent Gamma Knife SRS at centers with extensive SRS expertise. Brainstem SRS using other treatment platforms is not described here, but can likely be extrapolated to other validated platforms where appropriate.
Conclusion
This study supports our view that SRS delivered at centers with advanced technology and experienced practitioners is an appropriate treatment option for patients with brainstem metastases. It provides favorable local control and relatively rare toxicity. In the setting of brainstem SRS after WBRT, particularly when a short interval exists between the two (ie, <4.5 months), dose reduction should be considered to lessen the risk of adverse radiation effects. Further study through prospective trials and clinical registries can be performed to ascertain the generalizability of the present findings.
Summary.
Stereotactic radiosurgery (SRS) within the brainstem exceeds historically identified dose tolerance guidelines. In the largest series to be reported, our results demonstrate that SRS for brainstem metastases resulted in a local control rate of 82% at 1 year and an overall severe toxicity rate of 7.4%. Margin doses of at least 20 Gy were associated with improved local control but increased toxicity.
Footnotes
Conflict of interest: I.S.G. has stock interests and is on the Board of Directors for Greater Michigan Gamma Knife; and has an institutional research grant held with Elekta as part of the Elekta Collaborative Lung Research Group and is the Principle investigator. R.L. is a consultant for Elekta. L.D.L. is a consultant for and shareholder in Elekta.
References
- 1.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 3.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 4.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169–1176. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 7.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown PD. [Accessed February 19, 2015];N0574: Stereotactic radiation therapy with or without whole-brain radiation therapy in treating patients with brain metastases. Available at: http://meetinglibrary.asco.org/content/146056-156.
- 9.Sharma MS, Kondziolka D, Khan A, et al. Radiation tolerance limits of the brainstem. Neurosurgery. 2008;63:728–732. doi: 10.1227/01.NEU.0000325726.72815.22. [discussion 732-723] [DOI] [PubMed] [Google Scholar]
- 10.Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76:S36–S41. doi: 10.1016/j.ijrobp.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kased N, Huang K, Nakamura JL, et al. Gamma knife radiosurgery for brainstem metastases: The UCSF experience. J Neurooncol. 2008;86:195–205. doi: 10.1007/s11060-007-9458-4. [DOI] [PubMed] [Google Scholar]
- 12.Koyfman SA, Tendulkar RD, Chao ST, et al. Stereotactic radiosurgery for single brainstem metastases: The Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2010;78:409–414. doi: 10.1016/j.ijrobp.2009.07.1750. [DOI] [PubMed] [Google Scholar]
- 13.Hatiboglu MA, Chang EL, Suki D, et al. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011;69:796–806. doi: 10.1227/NEU.0b013e31821d31de. [discussion 806] [DOI] [PubMed] [Google Scholar]
- 14.Kawabe T, Yamamoto M, Sato Y, et al. Gamma Knife surgery for patients with brainstem metastases. J Neurosurg. 2012;117(Suppl.):23–30. doi: 10.3171/2012.7.GKS12977. [DOI] [PubMed] [Google Scholar]
- 15.Sengoz M, Kabalay IA, Tezcanli E, et al. Treatment of brainstem metastases with gamma-knife radiosurgery. J Neurooncol. 2013;113:33–38. doi: 10.1007/s11060-013-1086-6. [DOI] [PubMed] [Google Scholar]
- 16.Peterson HE, Larson EW, Fairbanks RK, et al. Gamma knife treatment of brainstem metastases. Int J Mol Sci. 2014;15:9748–9761. doi: 10.3390/ijms15069748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilburn JM, Ellis TL, Lovato JF, et al. Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: Is there a volume threshold for toxicity? J Neurooncol. 2014;117:167–174. doi: 10.1007/s11060-014-1373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CS, Selch MT, Lee SP, et al. Accelerator-based stereotactic radiosurgery for brainstem metastases. Neurosurgery. 2012;70:953–958. doi: 10.1227/NEU.0b013e31823c40fe. [discussion 958] [DOI] [PubMed] [Google Scholar]
- 19.Yen CP, Sheehan J, Patterson G, et al. Gamma knife surgery for metastatic brainstem tumors. J Neurosurg. 2006;105:213–219. doi: 10.3171/jns.2006.105.2.213. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzoni JG, Devriendt D, Massager N, et al. Brain stem metastases treated with radiosurgery: Prognostic factors of survival and life expectancy estimation. Surg Neurol. 2009;71:188–195. doi: 10.1016/j.surneu.2008.01.029. [discussion 195-186] [DOI] [PubMed] [Google Scholar]
- 21.Leeman JE, Clump DA, Wegner RE, et al. Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol. 2012;7:107. doi: 10.1186/1748-717X-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuto T, Fujino H, Asada H, et al. Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien) 2003;145:755–760. doi: 10.1007/s00701-003-0034-1. [DOI] [PubMed] [Google Scholar]
- 23.National Insitutes of Health. National Cancer Institute [Accessed July 22, 2015];Common Terminology Criteria for Adverse Events (CTCAE) (Version 4.0). Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 24.Snell JW, Sheehan J, Stroila M, et al. Assessment of imaging studies used with radiosurgery: A volumetric algorithm and an estimation of its error. Technical note. J Neurosurg. 2006;104:157–162. doi: 10.3171/jns.2006.104.1.157. [DOI] [PubMed] [Google Scholar]
- 25.Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Valery CA, Boskos C, Boisserie G, et al. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phys. 2011;80:362–368. doi: 10.1016/j.ijrobp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Huang CF, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91:563–568. doi: 10.3171/jns.1999.91.4.0563. [DOI] [PubMed] [Google Scholar]
- 28.Yoo TW, Park ES, Kwon do H, et al. Gamma knife radiosurgery for brainstem metastasis. J Korean Neurosurg Soc. 2011;50:299–303. doi: 10.3340/jkns.2011.50.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly PJ, Lin YB, Yu AY, et al. Linear accelerator-based stereotactic radiosurgery for brainstem metastases: The Dana-Farber/Brigham and Women’s Cancer Center experience. J Neurooncol. 2011;104:553–557. doi: 10.1007/s11060-010-0514-0. [DOI] [PubMed] [Google Scholar]
- 30.Jung EW, Rakowski JT, Delly F, et al. Gamma Knife radiosurgery in the management of brainstem metastases. Clin Neurol Neurosurg. 2013;115:2023–2028. doi: 10.1016/j.clineuro.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Trifiletti DM, Lee CC, Shah N, et al. How does brainstem involvement affect prognosis in patients with limited brain metastases? Results of a matched-cohort analysis. World Neurosurg. 2016;88:563–568. doi: 10.1016/j.wneu.2015.10.089. [DOI] [PubMed] [Google Scholar]