Abstract
Neuroimaging data has shown that activity in the lateral posterior parietal cortex (PPC) correlates with item recognition and source recollection, but there is considerable debate about its specific contributions. Performance on both item and source memory tasks were compared between participants who were given bilateral transcranial direct current stimulation (tDCS) over the parietal cortex to those given prefrontal or sham tDCS. The parietal tDCS group, but not the prefrontal group, showed decreased false recognition, and less bias in item and source discrimination tasks compared to sham stimulation. These results are consistent with a causal role of the PPC in item and source memory retrieval, likely based on attentional and decision-making biases.
Keywords: parietal cortex, tDCS, decision bias, false recognition, item memory, source memory
1. Introduction
Neuroimaging studies have consistently shown activity in the lateral posterior parietal cortex (PPC) during episodic memory retrieval (Kahn et al., 2004; Shannon and Buckner, 2004; Wagner et al., 2005). Specifically, the PPC was more active during retrieval of studied than unstudied items (Cansino et al., 2002; Henson et al., 2005; Wagner et al., 2005), and source memory judgments compared to item memory judgments (Dobbins et al., 2003; Hayes et al., 2011; Hutchinson et al., 2012). Lesions to the PPC, however, did not produce amnesia (e.g. Haramati et al., 2008; for review see, Corbetta and Shulman, 2002); PPC damage primarily resulted in deficits in attention (Bays et al., 2010; Steinmetz and Constantinidis, 1995). Thus, the contributions of the PPC to memory accuracy is likely to reflect attentional processes (Cabeza, 2008; Ciaramelli et al., 2008; Wagner et al., 2005). Because lesion studies are limited in that behavior can also reflect encoding deficits or functional recovery (Corbetta et al., 2005; Kolb et al., 2001) and neuroimaging studies are correlational, the goal of this experiment was to test the role of the PPC in memory retrieval by directly manipulating cortical activity in intact neurological populations using transcranial Direct Current Stimulation (tDCS). We compared performance on item and source memory tasks with active tDCS over the PPC to both sham tDCS and tDCS over the prefrontal cortex (PFC), another brain region that has been implicated in memory (Cansino et al., 2002; Rugg et al., 1999; Shimamura et al., 1995).
TDCS is a technique by which weak electrical currents are applied at the scalp by means of two electrodes, one stimulating electrode, often referred to as the “anode”, and one return electrode, typically referred to as the “cathode” (DaSilva et al., 2011; Reato et al., 2010). Application of tDCS has been shown to alter the likelihood of neuronal excitation in the cortex of non-human animals (Bikson et al., 2004; Reato et al., 2010) and humans (Antal et al., 2004; Nitsche and Paulus, 2000). Notably, the effects of tDCS can be modulated by the charge of the overlying electrode, such that excitability under the anode increases while the excitability under the cathode decreases, at least in the case of primary visual and motor cortices (Antal et al., 2004; Nitsche and Paulus, 2000). Interestingly, bilateral montages, which place the anode over the region of interest of one hemisphere and the cathode over the region of interest in the contralateral hemisphere, have been shown to have enhanced effects on behavior compared to unilateral montages, likely by attenuating interhemispheric inhibition (Vines et al., 2008). An additional benefit of a bilateral montage is that the current flow of tDCS is more restricted to the cortical regions of interest compared to unilateral montages (Vines et al., 2008), which is important because tDCS brings about network changes even in regions that are not stimulated (Keeser et al., 2011; Lang et al., 2005), suggesting that unilateral stimulation can modulate the contralateral hemisphere and lead to behavioral effects. Bilateral tDCS can therefore oppose such modulation and, in the case of the PPC, may be a better model for mnemonic contributions because changes to memory have been primarily noted in patients with bilateral lesions (Berryhill et al., 2007; Berryhill and Olson, 2008; Drowos et al., 2010; Simons et al., 2010). Thus, in our study we used bilateral montages, placing the anode over the left hemisphere and cathode over the right hemisphere, to determine the nature of the causal role of the PPC in item and source memory retrieval.
Previous research has shown that bilateral tDCS effectively alters attentional processes when applied over the PPC (Benwell et al., 2015; Giglia et al., 2011; Sparing et al., 2009), executive control processes when applied over the PFC (Leite et al., 2013; Nelson et al., 2014; Nozari and Thompson-Schill, 2013), which we used as a control site, and has been used to dissociate the PPC and the PFC (Iuculano and Cohen Kadosh, 2013), suggesting the possibility that tDCS could manipulate these underlying processes during memory retrieval. It is worth noting, however, that the efficacy of tDCS has been questioned in recent meta-analyses (Horvath et al., 2014a; Horvath et al., 2015), but these meta-analyses were limited by available published data, and did not have enough data to account for parameters known to effect tDCS such as stimulation duration (Antal et al., 2015) or task difficulty (Berryhill et al., 2014). Thus, behavioral changes may be selective to the combination of tDCS and task parameters used, and should be interpreted as such.
The PPC has been argued to support memory retrieval by way of attentional mechanisms that influence what mnemonic information is sought after (Cabeza et al., 2008; Ciaramelli et al., 2008; Wagner et al., 2005), or decision-related mechanisms that influence criterion setting (Aminoff et al., 2015; Dobbins et al., 2012; Donaldson et al., 2010; Pisoni et al., 2015; Sestieri et al., 2013; Wagner et al., 2005). Patients with PPC damage have shown deficits in orienting attention to external stimuli (Corbetta and Shulman, 2002), leading to the hypothesis that the contents of retrieval, as relevant internal stimuli, reorient attention in a manner that enhances the processing of task-relevant information, and this is mediated by the parietal cortex (Cabeza et al., 2008; Ciaramelli et al., 2008; Wagner et al., 2005). Consistent with this hypothesis, patients with parietal lesions had less confidence in their memories (Davidson et al., 2008; Simons et al., 2010), were less likely to report detailed memories either through spontaneous recall (Berryhill et al., 2007; Berryhill et al., 2010) or subjective “remember” responses (Davidson et al., 2008; Drowos et al., 2010), had less false memories for associated words (Drowos, et al., 2010), and were less likely to use memory cues to support retrieval (Ciaramelli et al., 2010). Thus, attention may support the ability to select what information will be retrieved, and the experience associated with recovering such selected information. Correspondingly, evidence from fMRI and event-related potentials (ERPs) studies converged to implicate the PPC in memory processes that were supported by attentional functions. For example, because “new” items are unstudied, they should not elicit retrieval-related activity but may elicit attentional processing, and studies have shown greater activation in the PPC for falsely recognized new (unstudied) items compared to correctly rejected new items (Kahn et al., 2004; Wheeler and Buckner, 2004), high confidence compared to low confidence false recognition of strongly related lures (Kim and Cabeza, 2007), high confidence correct compared to low confidence correct old and new judgments (Kuchinke et al., 2013), and invalidly cued compared to validly cued correct old and new judgments (Jaeger et al., 2013; O’Connor et al., 2010). Also consistent with the idea that the parietal cortex is involved in attentional selection of mnemonic information, ERPs over parietal areas were increased for specific recollections such as when a probe was self-generated compared to imagined (Leynes, 2012), was endorsed as accompanied with greater details (Vilberg and Rugg, 2009), and was identical to what was studied compared to when changed (Ally et al., 2008).
In a related hypothesis, the PPC may subserve decision-making aspects of memory tasks. Evidence for a role of the PPC in decision-making can be seen from work showing that, during a sensory task, neurons in the primate parietal cortex responded based on the accumulation of attentional sensory information that formed the basis for a response decision (Platt and Glimcher, 1999; Shadlen and Newsome, 1996). Memory researchers have hypothesized that the human parietal cortex may play a similar role in memory; parietal neurons may modulate based on the accumulation of mnemonic (old and new) information as a basis for a goal directed response (Donaldson et al., 2010) or that attention serves to establish a decision bias (Dobbins, et al., 2012). From this perspective, the reduced confidence, recollected detail, and associative false recognition in patients with PPC damage (Berryhill et al., 2007; Davidson et al., 2008; Drowos et al., 2010; Simons et al., 2010) reflects an inability to incorporate attended information as a basis for a decision. In line with a decision-making role for the parietal cortex in retrieval, one study used a paradigm that yields high rates of false alarms and showed tDCS over the parietal cortex increased false recognition (Pergolizzi and Chua, 2015), whereas another study used a standard item recognition paradigm and showed decreased false recognition (Pisoni et al., 2015). These opposing effects on false recognition when different paradigms were used are consistent with decisional aspects of retrieval and the idea that task demands can differentially influence or bias item recognition judgments. Thus, if the role of the parietal cortex in memory is via attention and/or decision processes, then, in a combined item and source memory task, the parietal cortex may play a role in prioritizing attention to source recollection (because source information is the most task-relevant), which could lead to improved source recollection or biased responding based on criterion setting toward features that are weighted more or less importantly according to task demands.
We compared the effects of tDCS over the PPC to effects of sham tDCS and tDCS over the PFC because the PFC has also been implicated in memory tasks (for review see; Mitchell and Johnson, 2009; Preston and Eichenbaum, 2013; Rugg et al., 2002; Simons and Spiers, 2003), and potentially dissociating the roles of the PPC vs. PFC is of interest. The PFC has been argued to support memory retrieval under conditions when retrieval is difficult and demands executive control, such as establishing strategies to search for specific information (Nolde et al., 1998), monitoring and evaluating memories (Rugg et al., 1999), or inhibiting irrelevant or competing memories (Shimamura et al., 1995). In general, source memory retrieval is considered to be more difficult, due to requiring the recovery of greater information than item memory retrieval, which is a justification as to why activity in the left or bilateral PFC is consistently shown to be increased during source memory judgments compared to item recognition judgments (e.g. Cansino et al., 2002; Dobbins and Han, 2006; Hayes et al., 2011; Slotnick et al., 2003). Furthermore, patients with PFC damage have deficits accurately recovering source information (Ciaramelli and Spaniol, 2009; Duarte et al., 2005; Janowsky et al., 1989b; Schacter et al., 1984; Simons et al., 2002), increased false recognition (Curran et al., 1997; Schacter et al., 1996a), increased susceptibility to interference (Shimamura et al., 1995), and in some cases confabulation, a disorder where patients confidently remember things that did not happen (Ciaramelli and Ghetti, 2007; Kan et al., 2010; Moscovitch and Melo, 1997), suggesting general issues when attributing or distinguishing the source of retrieved memories. Neuroimaging and neuropsychological literature taken together, therefore, suggest tDCS over the PFC should alter the ability to discriminate source information and reject false information, with the caveat that retrieval must be difficult for such processes to alter behavior. This is important as one experiment has shown that tDCS over the PFC only altered accurate recollection on a task that was more cognitively demanding compared to other less demanding tasks (Gray et al., 2015), suggesting PFC contributions can be unnecessary or obscured when the task is not sufficiently difficult.
In this experiment, we applied active tDCS over the PPC or the PFC, or sham tDCS, during an item recognition task followed by source judgment. If the role of the PPC in retrieval is related to attentional shifts towards recollected information or decision biases (Cabeza et al., 2008; Ciaramelli et al., 2008; Dobbins et al., 2012; Donaldson et al., 2010), then tDCS over the PPC should lead to reduced false recognition and changes in bias, as indexed by models of memory processing. Bias in item memory tasks could be indicated by favoring a “new” response compared to an “old” response, and bias in source memory tasks could be indicated by favoring one source over the other. If the role of the PFC in retrieval is related to retrieval difficulty, then tDCS over the PFC may enhance source accuracy because of the increased demand to recollect details (e.g. Janowsky et al., 1989b; Schacter et al., 1984; Schacter et al., 1996a), but only if source retrieval is sufficiently difficult.
2. Materials and Methods
2.1 Participants
Participants were 58 undergraduate students (34 female) from Brooklyn College of the City University of New York. Eligibility was determined by a self-report questionnaire to rule out use of psychoactive medications, chronic skin conditions, pregnancy, metallic implants or history of neuropsychiatric disorder or seizures. All subjects had normal or corrected-to-normal vision and learned English before age 5. Two participants withdrew due to minor discomfort from the effects of stimulation. Two additional participants were excluded from the analyses, one due to poor performance, resulting in near 100% false recognition, another due to experimenter error while administering stimulation. The results reported are from the 54 remaining participants (31 female). The remaining participants were 18 to 31 years of age (mean age 19.6 years, SD 3.06 years). Each participant received course credit and gave written, informed consent in a manner approved by the Human Research Protection Program (HRPP) of the City University of New York.
2.2 Transcranial direct current stimulation protocol
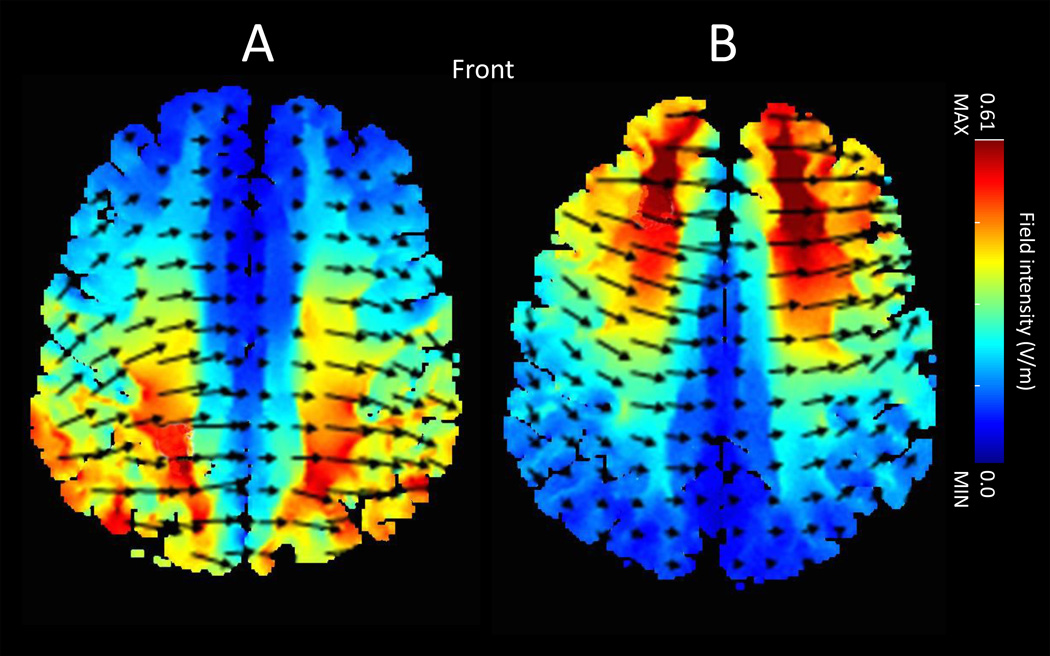
Stimulation was applied via one anode and one cathode rubber electrode each encased in a 35 cm2 saline-soaked sponge pocket. Direct current was delivered through a battery-driven constant current stimulator with a maximum output of 2mA (1×1 Transcranial Direct Current (tDCS) Low-Intensity Stimulator Model 1224-B, Soterix Medical, USA). To target the PPC, we used a bilateral montage, placing the anode over site CP3 and cathode over site CP4 of the International 10–20 System for EEG electrode placement (Pergolizzi and Chua, 2015). To target the PFC, we again used a bilateral montage, placing the anode over site F3 and cathode over site F4 according to the International 10–20 System for EEG electrode placement. A bilateral montage was chosen because it has been shown to produce additive effects on performance compared to unilateral stimulation (Vines et al., 2008), and to avoid distributing stimulation through distal brain regions. Computer simulations were conducted using tDCS Explore software (Soterix Medical, New York, NY) (Kempe et al., 2014) which confirmed that the CP3/CP4 and F3/F4 montages targeted the posterior parietal and prefrontal cortex, respectively, and that there was minimal overlap in stimulation (Figure 1).
Figure 1.
Computational models of cortical currents during tDCS using A) montage CP3/CP4 (parietal) and B) montage F3/F4 (prefrontal). Arrows indicate the direction of current flow. The model depicts current flow from the anode placed on the left hemisphere to the cathode placed on the right hemisphere, as used in the current study. Active stimulation was administered for twenty minutes at 2mA.
Participants were randomized to receive 20 minutes of prefrontal (N=18), parietal (N=18) or sham (N=18) stimulation. During prefrontal and parietal stimulation, the Soterix device applied a constant current of 2mA (current density of 0.06 mA/cm2) for 20 minutes, with an additional 1 minute for ramp up at the beginning and ramp down at the end of stimulation. For sham stimulation, the Soterix device has a built in switch to initiate a 1 minute ramp up to the set current of 2 mA immediately followed by ramp down to 0.1 mA. The device repeats this 1-minute ramp up/ramp down at the end of the stimulation period. This allows participants to experience similar sensory effects of active stimulation (Gandiga et al., 2006). Our subjects were also provided a questionnaire following stimulation to report sensations and judge whether the believed they were in the active or sham condition. Twelve of 18 sham participants incorrectly guessed they were receiving actual stimulation, compared to 11 of 18 parietal participants and 13 of 18 prefrontal participants correctly guessing that they received active stimulation.
2.3 Materials and Stimuli
The experiment was conducted on a Dell Optiplex 980 PC connected to a 22” VGA monitor running Psychopy v.1.74.02 (Peirce, 2007) . Stimuli consisted of 300 nouns selected from the MRC Psycholinguistic Database (Coltheart, 1981) with a mean length of 5.8 letters. Nouns were of moderate concreteness (M=577.3), familiarity (M=529.02) and imageability (M=600.6). Stimuli were divided into two lists of 150 words, further subdivided into three sets of 50 words matched for concreteness, familiarity and imageability. These were counterbalanced to three possible item types: 1) old/living, 2) old/bigger, or 3) new. All participants received 100 old words (words that were presented at study) and 50 new words at test.
2.4 Procedure
The experimental session began with a study session of 100 words. Participants were instructed to try to remember the words for later testing, and also to make a decision about the word. Participants were presented with the cue word “living” or “bigger” for 0.5s, followed by a 4s presentation of the word to be studied. Cues appeared at the top of the screen and study words were presented in the center of the screen. All stimuli were presented in white letters on grey background presented in Arial font. During “living” trials, participants were instructed to decide if the word represented something that was living. During “bigger” trials they were instructed to decide if the word was bigger than a shoebox (size judgment). All participants indicated their decisions by pressing keys “1” for yes or “2” for no. Trials were presented in random order. Following study, the electrodes were placed on participants’ heads for administering tDCS (∼15min). Stimulation was administered for five minutes before beginning the recognition test. The period of five minutes was chosen based on evidence that approximately 3–5 minutes of stimulation is necessary to observe measurable changes in performance (Nitsche and Paulus, 2000).
During the recognition test, participants were presented with all 100 studied (“old”) words randomly intermixed with 50 unstudied (“new”) words. To assess item recognition and source recollection, we used a sequential response method; participants first made old/new item judgments on a 4-point confidence scale pressing buttons “1”, “2”, “3”, or “4” as follows: 1= Definitely Old, 2= Probably Old, 3= Probably New, 4= Definitely New. Each item judgment was followed by a source judgment on a 4-point confidence scale: 1= Definitely Living, 2=Probably Living, 3= Probably Bigger, 4= Definitely Bigger. If participants recognized the item as new, they were instructed to guess the source. All responses were self-paced.
2.5 Data Analysis
Memory performance for words studied under the bigger and living cues, and overall, were initially analyzed using conventional item and source memory measures, by examining hits and false alarms in the case of item memory and source accuracy in the case of source memory. Hits were calculated as the proportion correct out of: 1) items encoded under “bigger” judgments, 2) items encoded under or “living” judgments, and 3) out of all old items irrespective of encoding judgment. Because false alarms were not encoded, and therefore couldn’t be attributed to a specific source task, false alarms by source were calculated as the proportion of new items called: 1) “old” and “bigger”, and 2) called “old” and “living”; false alarm rate was also calculated overall as the proportion of new items called “old” irrespective of subsequent source attribution out of all new items.” Analysis of source trials were conditionalized on item memory; “source correct” trials represented when the participant correctly recognized an “old” item (i.e., a hit) and subsequently correctly attributed the source to the “bigger” or “living” study cue, whereas “source incorrect” trials represented when the participant correctly recognized an “old” item (i.e., a hit) and subsequently inaccurately attributed the source to the “bigger” or “living” study cue. One-way ANOVAs were performed to test for effects of stimulation group (PPC, PFC, and sham), followed by Bonferroni adjusted post-hoc tests when significant. Two-way ANOVAs were performed to test the effects of source (“bigger” or “living”) and stimulation group (PPC, PFC, and sham) on performance. In some cases, assumptions of normality were violated and are reported in the results. For such cases Kruskal-Wallis tests were performed followed by Mann-Whitney U tests for the following comparisons: PPC vs. sham, PPC vs. PFC, PFC vs. sham. Comparisons were considered significant at p<0.05.
Our main analyses used multinomial processing models to provide separate estimates of item and source memory in a manner that does not assume process-pure tasks (Batchelder and Riefer, 1990). These multinomial models assume that multiple cognitive processes can result in the same response. For example, a participant could make a correct source “bigger” judgment because: 1) the item was experienced as old and recollected as “bigger”, 2) the item was experienced as old but lacked recollection and was guessed to be “bigger”, or 3) the item was guessed to be old and guessed to be “bigger”. Conventional analyses used above, which calculate “bigger” responses conditional on “old” responses, do not disentangle these possible experiences and decision processes, therefore, confounding item memory, source memory, and response bias. The family of multinomial processing tree models proposed by Batchelder and Riefer (1990), which we applied to our data, include parameters that are allowed to differ for: 1) probabilities of item detection of the “bigger” old items (Dbigger) or “living” old items (Dliving); 2) probabilities of source discrimination for items from the “bigger” source (dbigger) or the “living” source living (dliving); and 3) responses biases as indicated by the probability of responding “old” to a undetected old items and new items (b), the probability of guessing that an item belongs to the source “bigger” when the item is detected (a), and the probability of guessing that an item belongs to the source “bigger” when the subject has guessed that the item is old (g). Thus, Dbigger, Dliving, dbigger, dliving, b, a and g provide seven possible parameters to estimate when modeling underlying cognitive processes in our data.
Batchelder and Riefer (1990) proposed several nested models that restrict parameters based on different psychological assumptions. Using a data-driven approach, we first tested which of Batchelder and Riefer’s (1990) models best fit the data while allowing for parameter estimates to vary between stimulation groups. For different models, we tested whether inclusion of different factors contributed to a model’s goodness-of-fit assessed by G2 – the log-likelihood ratio statistic that is compared to the critical value of a chi-square distribution (Batchelder and Riefer, 1990; Riefer and Batchelder, 1988). For simplicity, we report data from the best fitting model (Model 5c, see Batchelder and Riefer, 1990), which allowed detection for “bigger” items to differ from detection of “living” items (Dbigger does not equal Dliving), set source discrimination for “bigger” and “living” as equal (dbigger, dliving), source guessing biases (g, a) as equal, and allowed item guessing bias (b) to freely vary [G2(3) = 1.40, p> 0.05]. This resulted in five parameters – correct item detection for items studied with the bigger source (Dbigger) or the living source (Dliving); correct source discrimination (d); and guessing during item detection (b) or source discrimination (g) – which were estimated for each participant individually. We compared the individual estimates of each parameter using one-way ANOVAs, followed by Bonferroni adjusted post-hoc tests when significant. Modeling, individual parameter estimates, and model fits (G2 derivations) were done using Microsoft Excel Solver (Dodson et al., 1998; Dodson and Shimamura, 2000).
3. Results
3.1 Item and Source memory performance
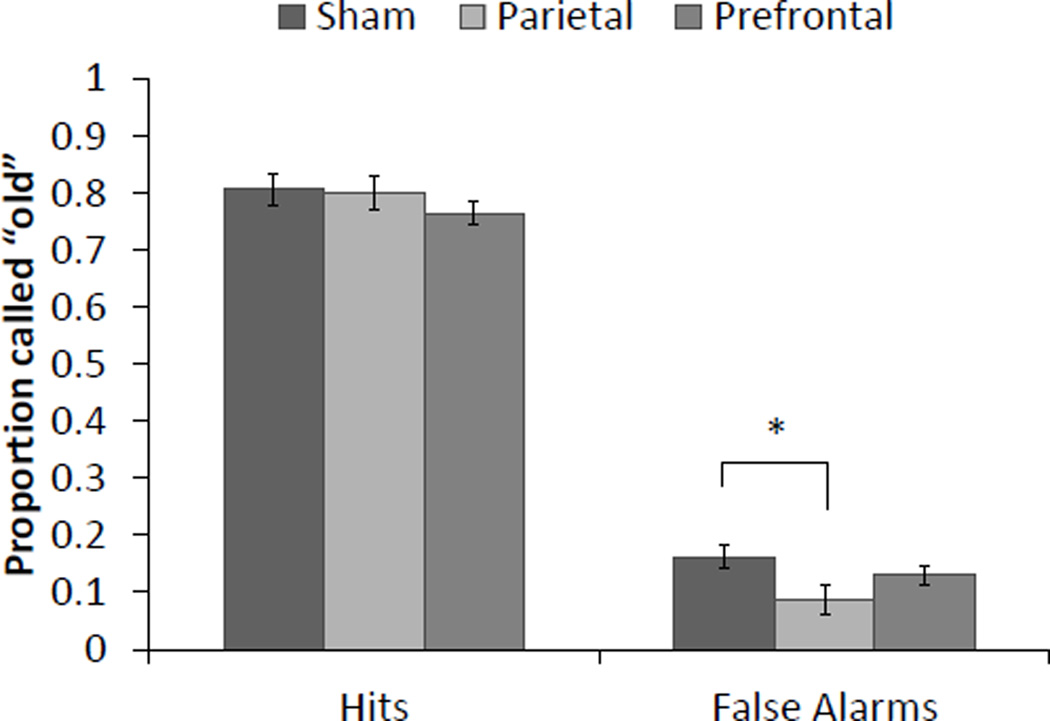
Overall, participants performed well on the item detection and source discrimination tasks (Table 1). For item memory, there were no differences in overall hit rate between groups, F(2,51) = 0.788, p< 0.46, partial η2 = 0.030 (Figure 2). Data from the false alarm rate did not meet the assumptions of ANOVA and violated homogeneity of variance (p< 0.015); therefore, we performed a Kruskal-Wallis test and showed a significant difference in false alarms between the stimulation groups, χ2(2) = 7.483, p < 0.024 (Figure 2). Post-hoc Mann-Whitney U tests indicated the distributions for PPC and sham stimulation differed significantly (mean ranks of PPC and sham stimulation were 23.69 and 13.31, respectively; U = 68.50, Z = −2.962, p< 0.002). However, PFC stimulation did not significantly differ from PPC (U = 141.50, Z = −0.65, p< 0.521) or sham stimulation (U = 115.50, Z = −1.472, p< 0.143). For source discrimination, there were no differences in conditionalized source accuracy between groups, F(2,51) = 1.396, p> 0.257, partial η2 = 0.052.
Table 1.
Mean (SEM) Proportion Responses for Item and Source Memory Performance for Each Stimulation Group.
| tDCS Group | Memory Measure | Bigger | Living | All |
|---|---|---|---|---|
| Sham | ||||
| Item Hits | 0.84 (0.03) | 0.77 (0.03) | 0.81 (0.03) | |
| Item False Alarms | 0.10 (0.01) | 0.06 (0.01) | 0.16 (0.02) | |
| Source Correct | 0.78 (0.02) | 0.67 (0.04) | 0.73 (0.03) | |
|
Parietal (PPC) |
||||
| Item Hits | 0.84 (0.02) | 0.76 (0.02) | 0.80 (0.02) | |
| Item False Alarms | 0.04 (0.01)* | 0.04 (0.01) | 0.09 (0.02)* | |
| Source Correct | 0.74 (0.03) | 0.78 (0.03) | 0.76 (0.02) | |
|
Prefrontal (PFC) |
||||
| Item Hits | 0.79 (0.03) | 0.73 (0.04) | 0.76 (0.03) | |
| Item False Alarms | 0.06 (0.01) | 0.07 (0.01) | 0.13 (0.03) | |
| Source Correct | 0.71 (0.03) | 0.69 (0.04) | 0.70 (0.03) |
Item Hits reflect proportion correct out of all items encoded under “bigger” or “living” judgments, or out of “all” old items irrespective of encoding judgment. Item False Alarms reflect proportion called “old” and “bigger”, called “old” and “living”, or called “old” irrespective of subsequent source attribution out of all new items. Source Correct reflect item hits encoded as “bigger” and subsequently correctly attributed as” bigger”, item hits encoded as “living” and subsequently correctly attributed as “living”, or all item hits subsequently correctly attributed to their respective source.
indicates a significant difference between parietal and sham stimulation (p<0.05).
Figure 2.
Mean proportion of hits and false alarms for item recognition. There was a significant main effect for false alarms, driven by a significant difference between sham and parietal stimulation. Errors bars represent SEM. *p<0.05
Turning to trials studied under different sources, items studied under the “bigger” cue had a greater hit rate than items studied under the “living” cue, F(2,51) = 22.174, p< 0.0001, partial η2 = 0.303, but there were no differences based on stimulation group (Table 1). False alarms were attributed to the “bigger” source more often for sham stimulation than the other stimulation groups, as indicated by a source by stimulation group interaction, F(2,51) = 5.140, p< 0.009, partial η2 = 0.168 (Table 1). Bonferroni adjusted post-hoc comparisons indicated sham and PPC stimulation significantly differed for false alarms called “bigger”, with the sham stimulation group falsely endorsing more items as “bigger”; p< 0.005, 95% CI of the difference [0.014, 0.097], but not false alarms called “living”, p< 0.841, 95% CI of the difference [−0.024, 0.061]; and neither the PPC or sham stimulation groups differed from the PFC stimulation group.
Furthermore, during correct source attributions there was a significant interaction between stimulation group and source “bigger” or source “living” judgments, F(2,51) = 3.354, p< 0.043, partial η2 = 0.116, driven by within-group differences such that the sham group showed better source accuracy for bigger than living items, t(17) = 2.655, p< 0.017, 95% CI of the difference [0.022, 0.197], whereas the PFC, t(17) = 0.441, p< 0.665, 95% CI of the difference [−0.077, 0.118], and PPC groups; t(17) = −1.148, p< 0.267, 95% CI of the difference [−0.113, 0.033], were equivalently accurate for either source. Thus, there appeared to be a general response bias toward “bigger” source attributions in the sham group (Table 1).
3.2 Multinomial processing models
To separately estimate item detection, source discrimination and response biases without assuming that item and source tasks are process-pure, we took a multinomial processing model approach (Batchelder and Riefer, 1990). Table 2 shows the mean of the parameter estimates for an individual’s model based on stimulation group. Comparisons of stimulation groups revealed similar item detection and source discrimination between groups. Item detection did not differ between groups for either bigger items (Dbigger), F(2,51) = 1.020, p< 0.368, partial η2 = 0.038, or living items (Dliving), F(2,51) = 0.444, p< 0.644, partial η2 = 0.017. Source discrimination (d) did not differ between groups either; F(2,51) = 1.193, p< 0.312, partial η2 = 0.045.
Table 2.
Mean (SEM) Multinomial Model Parameter Estimates for Each Stimulation Group.
|
Parameter |
|||||
|---|---|---|---|---|---|
| tDCS Group | Dbigger | Dliving | d | B | g |
| Sham | 0.80 (0.03) | 0.73 (0.04) | 0.47 (0.05) | 0.16 (0.02) | 0.59 (0.03) |
|
Parietal (PPC) |
0.81 (0.02) | 0.73 (0.02) | 0.53 (0.05) | 0.09 (0.02)* | 0.45 (0.03)* |
|
Prefrontal (PFC) |
0.76 (0.03) | 0.69 (0.05) | 0.41 (0.05) | 0.13 (0.03) | 0.50 (0.03) |
Dbigger: probability for correct detection of items studied with source bigger; Dliving: probability for correct detection of items studied with source living; d: probability of discriminating the source given that the item was correctly detected; b: probability of guessing an undetected item is “old”, i.e. a false alarm guessing bias; g: probability a false alarm is guessed to belong to a source.
indicates a significant difference between parietal and sham stimulation, Bonferroni corrected for multiple comparisons (p<0.05).
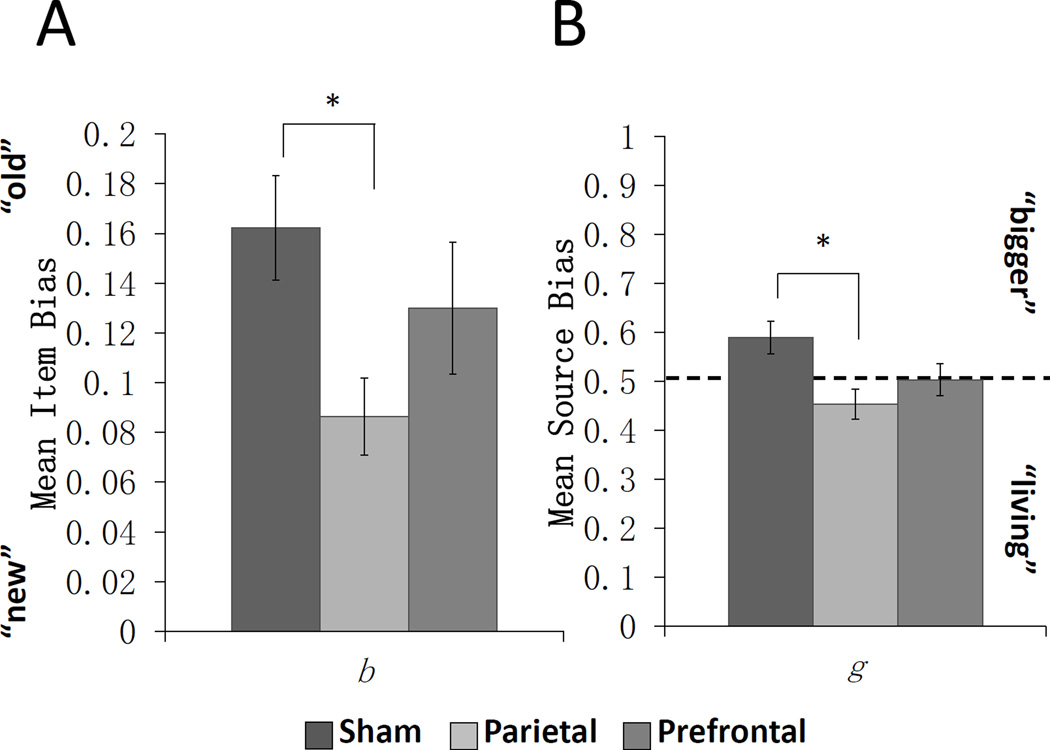
Both bias parameters showed significant effects of PPC stimulation (Figure 3). The data for the parameter for item guesswork (b) did not meet the assumptions of ANOVA and violated homogeneity of variance, p< 0.023; consequently, the Kruskal-Wallis test showed a significant between group difference in parameter b; χ2(2) = 7.307, p < 0.026; which resulted from a significant difference between parietal and sham stimulation; U = 72.00, Z = −2.847, p< 0.004, with the parietal group being less likely to endorse an undetected item as “old.” The ANOVA for source guesswork (g) was also significant; F(2,51) = 4.530, p< 0.015 partial η2 = 0.151, with Bonferroni adjusted post-hoc tests indicating parietal stimulation was significantly different from sham; p< 0.013, 95% CI of the difference [−0.249, −0.023], but no differences between prefrontal and sham stimulation; p< 0.195, 95% CI of the difference [−0.199, 0.027]. Parameter g, ranged from 0–1, where 0.5 indicated no bias and greater values indicated a bias toward attributing the source to the “bigger” cue. As can be seen in Table 2, the parietal group showed a bias toward “living” whereas the sham group was biased toward “bigger”, and the prefrontal group showed no evidence of bias with values at 0.5.
Figure 3.
Mean bias parameters estimated individually using Multinomial Model Parameter Estimates. A) Item bias estimated by parameter b. Higher values indicate a bias toward calling items “old”. The sham and parietal group significantly differed from one another. B) Source bias estimated by parameter g. A value of 0.5 represents a neutral bias. Values greater than 0.5 indicate a bias toward attributing the source as “bigger”. The sham and parietal group significantly differed from one another. Errors bars represent SEM, *p<0.05
4. Discussion
This experiment tested contributions of the PPC to memory performance by applying tDCS over the PPC and comparing it to tDCS over the PFC and sham during an item and source memory paradigm. There were no differences between stimulation groups for item and source accuracy. However, tDCS over the PPC altered item and source biases compared to sham tDCS, with decreased false recognition and less of a bias towards identifying items as studied under the “bigger” context. There was no detectable effect of tDCS over the PFC on item and source memory or bias compared to sham tDCS or tDCS over the PPC. We interpret these findings in support of theorized functional contributions of the parietal cortices to memory via attentional and decision-making processes.
Manipulating activity of the PPC was specifically associated with response bias effects compared to the sham group, and this occurred during both item and source memory judgments as evidenced by differences in parameter estimates for item (b) and source biases (g) from multinomial processing models and a decrease in false alarm rate to new items. In particular, the sham group was more likely to guess that an item was “old” (b, an item bias) and also to attribute both correctly detected items and guesses to the “bigger” source (g, a source bias), and stimulation over the PPC comparatively lessened these biases. Retrieval orientation toward recollection has been shown to decrease false alarms and alter source bias without effecting item or source accuracy (Budson et al., 2005; Dodson and Schacter, 2002; Gallo et al., 2004; Israel and Schacter, 1997). In terms of brain activity, orienting toward source recollections compared to item detection has shown a greater fMRI signal in the PPC (Dobbins et al., 2003). Thus in our experiment, it is likely that tDCS over the PPC led to more orienting towards recollection, and resulted in decreased false alarms and decreased source bias. A role for the parietal cortex in retrieval orientation is consistent with broader theories of parietal function, in that the parietal cortex is believed to play a role in the attentional control of memory and support top-down selection of relevant features based on retrieval goals (Cabeza et al., 2008; Ciaramelli et al., 2008; Jaeger et al., 2013), in this case resulting in orientation towards recollection.
There is some evidence in our experiment that participants in the PPC group oriented attention toward distinctive recollections, not just to recollection in general, which led to changes in our bias measures. It has been shown that individuals can use a heuristic to remember distinctive information and reduce false recognition (without effecting true recognition; McDonough and Gallo, 2008), a metacognitive strategy called the distinctiveness heuristic (Budson et al., 2005; Dodson and Schacter, 2002; Israel and Schacter, 1997). In the source memory literature, source attributions can reflect biases about the expected memorability derived from certain events (Johnson and Raye, 1981), such as experiencing that words spoken by oneself are more vividly remembered than those spoken by an other (e.g., Johnson et al., 1981). Consequently, when falsely recognizing items as old, subjects attribute the source to the “other” weaker source, referred to as the “it-had-to-be-you” effect (Johnson et al., 1981). In other words, when using the distinctiveness heuristic, items that lack source information are attributed to the less distinctive source. Turning to the current findings, “bigger” items were better remembered (greater hits) than “living” items across all groups, suggesting “bigger” was the more distinctive source, and “living” was the weaker source. One possibility is that parietal tDCS may have resulted in more use of the “it-had-to-be-you” strategy compared to sham. The sham group showed a bias towards the “bigger” source, which is inconsistent with the “it-had-to-be-you” effect, but with parietal tDCS we showed that the bias towards the “bigger” source that had been evident in the sham group was removed and shifted towards the “living” source. This may have been based in a variant of the “it-had-to-be-you” effect in the parietal group, whereby the less distinctive “living” source became more often endorsed for new items that inherently lacked source information. Thus, the distinctiveness heuristic can account for the opposing source bias between the PPC (more toward “living”) and sham groups (more toward “bigger), and the reduction in false recognition in the PPC group. This is similar to results from Gallo et al. (2004) that showed use of the distinctiveness heuristic for pictures over words decreased false recognition and biased source attributions following false alarms toward the weaker source (words). Indeed, the distinctiveness heuristic has been related to increased electrical activity over lateral parietal cortex (Budson et al., 2005). In our experiment, it is possible that PPC stimulation facilitated retrieval orientation toward recollection of distinctive source details, which in turn decreased false recognition and shifted source bias toward the weaker source, by invoking something akin to the distinctiveness heuristic.
Alternatively, instead of shifting retrieval orientation towards recollection, it is possible that tDCS over the parietal cortex altered memory-related decision-making processes. An extension to the attention to memory model proposes a decision-biasing framework in which parietal regions engage top-down attentional modulation in service of decision criteria (Dobbins et al., 2012), which could account for our findings of shifts in item and source bias. The source memory task demands may have directed attention toward specific mnemonic features, leading to an actual decision bias (criterion). This parallels work in visual attention which defines top-down processes specifically as biases generated by task demands that originate in the PPC and spatially direct attention to specific stimulus features (Beck and Kastner, 2009). In the domain of memory, patients with parietal lesions have shown deficits in subjective aspects of memory (Davidson et al., 2008; Drowos et al., 2010; Simons et al., 2010), which may reflect issues with forming criteria or experiencing a bias about recollection requirements. Consistent with this idea, neuroimaging has suggested parietal activity tracks response bias more than memory strength (Aminoff et al., 2015) and transcranial magnetic stimulation of the PPC altered source bias without altering source accuracy (Sestieri et al., 2013).
The decision-biasing framework may explain differences between previously reported tDCS studies of the parietal cortex in memory. In a prior experiment, we showed that tDCS over the parietal cortex increased false recognition (Pergolizzi & Chua, 2015), whereas in this experiment, and in another experiment (Pisoni et al., 2015), tDCS over the parietal cortex led to decreased false recognition. These seemingly opposing results may be consistent with a parietally-mediated decision-biasing mechanism. Bias is characterized as being influenced by task demands, and the demands and biases in our previous experiment were quite different from the current experiment (Pergolizzi and Chua, 2015). We previously used the Deese-Roediger-McDermott (DRM) task, in which subjects study lists of semantically related words that converge on a critical lure, and then are tested on old and new words, as well as the critical lure (Deese, 1959; Roediger and McDermott, 1995). This paradigm typically produces high rates of false recognition and false recall (Gallo, 2010), and results in a liberal bias (Miller and Wolford, 1999). One hypothesis underlying increased false recognition in the DRM paradigm is reliance on gist processing at encoding (e.g., Brainerd et al., 1998; Schacter et al., 1996b). If tDCS over the PPC biases retrieval orientation based on task demands, it can be argued that the DRM paradigm can lead to a bias toward reinstating gist processing at retrieval, causing more words to appear similar and thereby increase false recognition. This is the opposite of source tasks, which bias towards reinstating verbatim recollection. Given this interpretation, future work comparing the influence of different encoding/retrieval tasks (e.g. DRM and source memory procedures) on retrieval performance following stimulation of the PPC would be especially informative.
There were differences between the PPC and sham groups for both false alarms and responses biases, but there was no difference when compared to the PFC group. Because our results were significant when comparing tDCS over the PPC to a passive control group (i.e., sham), but not an active control group (i.e., PFC), this raises the possibility that the effects could be due to participants’ expectations about the effects of stimulation or the sensations of the stimulation. Although this is a possibility, it seems unlikely because a similar proportion of participants in each group believed they were receiving stimulation. It is more likely that increased variability with active tDCS stimulation requires additional power to detect differences (Datta et al., 2012). This may also explain why we failed to detect differences in the PFC group compared to the sham group. Future work with larger sample sizes could help address this issue.
Limited power may play a role in why we failed to see effects of tDCS over the PFC in item and source accuracy, but the lack of effects may also relate to the nature of the task. One experiment showed increased recollection accuracy with tDCS over the PFC at retrieval, but this was only true in the most demanding retrieval task and not in less demanding tasks (Gray et al., 2015). A related finding reported that anodal tDCS over the PFC selectively improved performance on a more demanding working memory task compared to a less demanding task and to sham (Gill et al., 2015). Given that source accuracy in the current study was relatively high, it may be that task demands were not sufficient when combined with tDCS over the prefrontal cortex to produce effects.
Recent meta-analyses of tDCS work has raised the question about whether tDCS leads to replicable effects in healthy, adult populations, and have suggested that the effects of single sessions of tDCS are close to zero in cognitive tasks (Horvath et al., 2014, 2015; Tremblay et al., 2014). Although we report data from a single study, a study by Pisoni et al. (2015) reported decreased false alarms with tDCS over the parietal cortex compared to over the temporal cortex, demonstrating that decreased false alarms on a standard item recognition paradigm replicate across labs and stimulation conditions. Notably, they used a slightly more posterior montage, yet the effects on behavior were similar. Although a read of the meta-analyses may leave one feeling pessimistic about the effects of tDCS, the meta-analyses are limited by the lack of comparable data, and there were often 2–3 studies in each domain examined (Horvath et al., 2015). It seems likely that tDCS can have consistent effects, and that inconsistent effects represent the need to identify additional variables that may determine the direction of the effects of tDCS. Indeed, in our own lab, we have shown both increased (Pergolizzi and Chua, 2015) and decreased false recognition with tDCS over the PPC. As outlined above, consideration of the task structure and the retrieval orientation set by the task, led to an alternative explanation; the parietal cortex serves to bias attention in a task-relevant manner. Future work should test this hypothesis more directly.
It is worth noting that there are some general limitations to the scope of our findings based on the use of tDCS. First, tDCS has limited spatial resolution in its delivery of current to the cortex, and current is thought to flow throughout multiple subregions of the lateral parietal and prefrontal cortex (Figure 1). Although we applied tDCS over the PPC and PFC and made inferences about these broad areas of cortex, we must acknowledge that the each area consists of several subregions. Indeed, several studies have shown dissociable memory processes in ventral and dorsal aspects of the parietal and the prefrontal lobe (Ciaramelli et al., 2008; Dobbins et al., 2002; Dobbins and Wagner, 2005; Herron et al., 2004; Johnson et al., 2013; Yu et al., 2012), and our interpretations are more consistent with the proposed functions of the dorsal PPC (e.g. Cabeza et al., 2011; Hutchinson et al., 2009; but see King and Miller, 2014). Second, the use of unilateral or bilateral montages can result in different patterns of behavioral performance over a given region (Tremblay et al., 2014). We chose a bilateral montage based on its usefulness in eliciting behavioral changes specific to attentional and executive processes implicated in the PPC and PFC, respectively (PPC: Benwell et al., 2015; Giglia et al., 2011; Sparing et al., 2009; PFC: Leite et al., 2013; Nelson et al., 2014; Nozari and Thompson-Schill, 2013). As a result our results are best understood as the consequence of simultaneous stimulation of the left hemisphere and right hemisphere, and correspondingly downstream monitoring and attention processes.
5. Conclusions
This experiment demonstrates that manipulating brain activity in the PPC has a causal effect on item recognition and source memory performance, primarily through attentional and decision processes. By administering an item and source memory paradigm while delivering tDCS to the PPC or PFC, we showed that parietal regions alter item and source memory biases based on task demands. Future work should further test the consequences of tDCS over the parietal cortex under conditions that lead to different biases. This would clarify the role of the parietal cortex and help illuminate the nature of differences across various tDCS studies.
Highlights.
We examined the role of prefrontal and parietal cortices in item and source memory
Transcranial Direct Current Stimulation (tDCS) was applied during memory retrieval
TDCS over the parietal cortex decreased false alarms compared to sham
TDCS over the parietal cortex altered item and source biases compared to sham
The parietal cortex directly contributes to decision biases in memory
Acknowledgments
EFC was supported by the National Institute of General Medical Sciences and the National Institute on Aging under award number SC2AG046910. The content is solely responsible of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff EM, Freeman S, Clewett D, Tipper C, Frithsen A, Johnson A, Grafton ST, Miller MB. Maintaining a cautious state of mind during a recognition test: A large-scale fMRI study. Neuropsychologia. 2015;67:132–147. doi: 10.1016/j.neuropsychologia.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Antal A, Keeser D, Priori A, Padberg F, Nitsche MA. Conceptual and Procedural Shortcomings of the Systematic Review “Evidence That Transcranial Direct Current Stimulation (tDCS) Generates Little-to-no Reliable Neurophysiologic Effect Beyond MEP Amplitude Modulation in Healthy Human Subjects: A Systematic R. Brain Stimul. 2015;8:846–849. doi: 10.1016/j.brs.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability Changes Induced in the Human Primary Visual Cortex by Transcranial Direct Current Stimulation: Direct Electrophysiological Evidence. Investig. Opthalmology Vis. Sci. 2004;45:702. doi: 10.1167/iovs.03-0688. [DOI] [PubMed] [Google Scholar]
- Batchelder WH, Riefer DM. Multinomial processing models of source monitoring. Psychol. Rev. 1990;97:548–564. [Google Scholar]
- Bays PM, Singh-Curry V, Gorgoraptis N, Driver J, Husain M. Integration of Goal-and Stimulus-Related Visual Signals Revealed by Damage to Human Parietal Cortex. J Neurosci. 2010;30:5968–5978. doi: 10.1523/JNEUROSCI.0997-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Res. 2009;49:1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell CSY, Learmonth G, Miniussi C, Harvey M, Thut G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex. 2015;69:152–165. doi: 10.1016/j.cortex.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia. 2008;46:1775–1786. doi: 10.1016/j.neuropsychologia.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: leveraging tDCS to advance cognitive research. Front. Psychol. 2014;5:800. doi: 10.3389/fpsyg.2014.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal Lobe and Episodic Memory: Bilateral Damage Causes Impaired Free Recall of Autobiographical Memory. J. Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Picasso L, Arnold R, Drowos D, Olson IR. Similarities and differences between parietal and frontal patients in autobiographical and constructed experience tasks. Neuropsychologia. 2010;48:1385–1393. doi: 10.1016/j.neuropsychologia.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JGR. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Stein LM, Reyna VF. On the development of conscious and unconscious memory. Dev. Psychol. 1998;34:342–357. doi: 10.1037//0012-1649.34.2.342. [DOI] [PubMed] [Google Scholar]
- Budson AE, Droller DBJ, Dodson CS, Schacter DL, Rugg MD, Holcomb PJ, Daffner KR. Electrophysiological Dissociation of Picture Versus Word Encoding: The Distinctiveness Heuristic as a Retrieval Orientation. J. Cogn. Neurosci. 2005;17:1181–1193. doi: 10.1162/0898929055002517. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Mazuz Y, Stokes J. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. J. Cogn. Neurosci. 2011;23:3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S. What are confabulators’ memories made of? A study of subjective and objective measures of recollection in confabulation. Neuropsychologia. 2007;45:1489–1500. doi: 10.1016/j.neuropsychologia.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Levine B, Ween J, Moscovitch M. Top-down and bottom-up attention to memory are dissociated in posterior parietal cortex: neuroimagingand and neuropsychological evidence. J. Neurosci. 2010;30:4943–4956. doi: 10.1523/JNEUROSCI.1209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Spaniol J. Ventromedial prefrontal damage and memory for context: Perceptual versus semantic features. Neuropsychology. 2009;23:649–657. doi: 10.1037/a0015937. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Q. J. Exp. Psychol. 1981;33:497–505. [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Curran T, Schacter DL, Norman KA, Galluccio L. False recognition after a right frontal lobe infarction: memory for general and specific information. Neuropsychologia. 1997;35:1035–1049. doi: 10.1016/s0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp. 2011:1–10. doi: 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-Individual Variation during Transcranial Direct Current Stimulation and Normalization of Dose Using MRI-Derived Computational Models. Front. Psychiatry. 2012;3:91. doi: 10.3389/fpsyt.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. J. Exp. Psychol. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Jaeger A, Studer B, Simons J. Use of explicit memory cues following parietal lobe lesions. Neuropsychologia. 2012;50:2992–3003. doi: 10.1016/j.neuropsychologia.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb. Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Isolating rule- versus evidence-based prefrontal activity during episodic and lexical discrimination: A functional magnetic resonance imaging investigation of detection theory distinctions. Cereb. Cortex. 2006;16:1614–1622. doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Prinzmetal W, Shimamura AP. Using Excel to estimate parameters from observed data: An example from source memory data. Behav. Res. Methods, Instruments, Comput. 1998;30:517–526. [Google Scholar]
- Dodson CS, Schacter DL. When False Recognition Meets Metacognition: The Distinctiveness Heuristic. J. Mem. Lang. 2002;46:782–803. [Google Scholar]
- Dodson CS, Shimamura AP. Differential effects of cue dependency on item and source memory. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:1023–1044. doi: 10.1037//0278-7393.26.4.1023. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Wheeler ME, Petersen SE. Remember the source: dissociating frontal and parietal contributions to episodic memory. J. Cogn. Neurosci. 2010;22:377–391. doi: 10.1162/jocn.2009.21242. [DOI] [PubMed] [Google Scholar]
- Drowos DB, Berryhill M, André JM, Olson IR. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24:465–475. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of Unilateral Prefrontal Lesions on Familiarity, Recollection, and Source Memory. J. Neurosci. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA. False memories and fantastic beliefs: 15 years of the DRM illusion. Mem. Cognit. 2010;38:833–848. doi: 10.3758/MC.38.7.833. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Weiss JA, Schacter DL. Reducing false recognition with criterial recollection tests: Distinctiveness heuristic versus criterion shifts. J. Mem. Lang. 2004;51:473–493. [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Giglia G, Mattaliano P, Puma A, Rizzo S, Fierro B, Brighina F. Neglect-like effects induced by tDCS modulation of posterior parietal cortices in healthy subjects. Brain Stimul. 2011;4:294–299. doi: 10.1016/j.brs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Gill J, Shah-Basak PP, Hamilton R. It’s the Thought That Counts: Examining the Task-dependent Effects of Transcranial Direct Current Stimulation on Executive Function. Brain Stimul. 2015;8:253–259. doi: 10.1016/j.brs.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Brookshire G, Casasanto D, Gallo DA. Electrically stimulating prefrontal cortex at retrieval improves recollection accuracy. Cortex. 2015;73:188–194. doi: 10.1016/j.cortex.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2008;46:1756–1766. doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Buchler N, Stokes J, Kragel J, Cabeza R. Neural correlates of confidence during item recognition and source memory retrieval: evidence for both dual-process and strength memory theories. J. Cogn. Neurosci. 2011;23:3959–3971. doi: 10.1162/jocn_a_00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: an FMRI study. J. Cogn. Neurosci. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Herron JE, Henson RNA, Rugg MD. Probability effects on the neural correlates of retrieval success: An fMRI study. Neuroimage. 2004;21:302–310. doi: 10.1016/j.neuroimage.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be) Front. Syst. Neurosci. 2014a;8:2. doi: 10.3389/fnsys.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS) Brain Stimul. 2015;8:535–550. doi: 10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) Generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy Human subjects: A systematic review. Neuropsychologia. 2014b;66:213–236. doi: 10.1016/j.neuropsychologia.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: Convergent and divergent effects of attention and memory. Learn. Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Weiner KS, Bressler DW, Silver MA, Preston AR, Wagner AD. Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb. Cortex. 2012;24:49–66. doi: 10.1093/cercor/bhs278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel L, Schacter DL. Pictorial encoding reduces false recognition of semantic associates. Psychon. Bull. Rev. 1997;4:577–581. [Google Scholar]
- Iuculano T, Cohen Kadosh R. The Mental Cost of Cognitive Enhancement. J. Neurosci. 2013;33:4482–4486. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger A, Konkel A, Dobbins IG. Unexpected novelty and familiarity orienting responses in lateral parietal cortex during recognition judgment. Neuropsychologia. 2013;51:1061–1076. doi: 10.1016/j.neuropsychologia.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Suzuki M, Rugg MD. Recollection, familiarity, and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study. Front. Hum. Neurosci. 2013;7:219. doi: 10.3389/fnhum.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL. Reality monitoring. Psychol. Rev. 1981;88:67–85. [Google Scholar]
- Johnson MK, Raye CL, Foley HJ, Foley MA. Cognitive Operations and Decision Bias in Reality Monitoring. Am. J. Psychol. 1981;94:37. [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J. Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Larocque KF, Lafleche G, Coslett HB, Verfaellie M. Memory monitoring failure in confabulation: evidence from the semantic illusion paradigm. J. Int. Neuropsychol. Soc. 2010;16:1006–1017. doi: 10.1017/S1355617710000536. [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Moller H-J, Reiser M, Padberg F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI. J. Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe R, Huang Y, Parra LC. Simulating pad-electrodes with high-definition arrays in transcranial electric stimulation. J. Neural. Eng. 2014;11:026003. doi: 10.1088/1741-2560/11/2/026003. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J. Neurosci. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DR, Miller MB. Lateral posterior parietal activity during source memory judgments of perceived and imagined events. Neuropsychologia. 2014;53:122–136. doi: 10.1016/j.neuropsychologia.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Kolb B, Brown R, Witt-Lajeunesse A, Gibb R. Neural compensations after lesion of the cerebral cortex. Neural. Plast. 2001;8:1–16. doi: 10.1155/NP.2001.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinke L, Fritzemeier S, Hofmann MJ, Jacobs AM. Neural correlates of episodic memory: Associative memory and confidence drive hippocampus activations. Behav. Brain Res. 2013;254:92–101. doi: 10.1016/j.bbr.2013.04.035. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Boggio PS, Gonçalves ÓF. The Effects of Cross-Hemispheric Dorsolateral Prefrontal Cortex Transcranial Direct Current Stimulation (tDCS) on Task Switching. Brain Stimul. 2013;6:660–667. doi: 10.1016/j.brs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Leynes PA. Event-related potential (ERP) evidence for source-monitoring based on the absence of information. Int. J. Psychophysiol. 2012;84:284–295. doi: 10.1016/j.ijpsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- McDonough IM, Gallo DA. Autobiographical elaboration reduces memory distortion: Cognitive operations and the distinctiveness heuristic. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:1430–1445. doi: 10.1037/a0013013. [DOI] [PubMed] [Google Scholar]
- Miller MB, Wolford GL. Theoretical commentary: The role of criterion shift in false memory. Psychol. Rev. 1999;106:398–405. [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol. Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Melo B. Strategic retrieval and the frontal lobes: Evidence from confabulation and amnesia. Neuropsychologia. 1997;35:1017–1034. doi: 10.1016/s0028-3932(97)00028-6. [DOI] [PubMed] [Google Scholar]
- Nelson JT, McKinley RA, Golob EJ, Warm JS, Parasuraman R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS) Neuroimage. 2014;85:909–917. doi: 10.1016/j.neuroimage.2012.11.061. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;527(Pt. 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, Raye CL. The role of prefrontal cortex during tests of episodic memory. Trends Cogn. Sci. 1998;2:399–406. doi: 10.1016/s1364-6613(98)01233-9. [DOI] [PubMed] [Google Scholar]
- Nozari N, Thompson-Schill SL. More attention when speaking: Does it help or does it hurt? Neuropsychologia. 2013;51:2770–2780. doi: 10.1016/j.neuropsychologia.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. The Inferior Parietal Lobule and Recognition Memory: Expectancy violation or successful retrieval. J. Neurosci. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy-Psychophysics software in Python. J. Neurosci. Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi D, Chua EF. Transcranial direct current stimulation (tDCS) of the parietal cortex leads to increased false recognition. Neuropsychologia. 2015;66:88–98. doi: 10.1016/j.neuropsychologia.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Pisoni A, Turi Z, Raithel A, Ambrus GG, Alekseichuk I, Schacht A, Paulus W, Antal A. Separating Recognition Processes of Declarative Memory via Anodal tDCS: Boosting Old Item Recognition by Temporal and New Item Detection by Parietal Stimulation. PLoS One. 2015;10:e0123085. doi: 10.1371/journal.pone.0123085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci. 2010;30:15067–15079. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefer DM, Batchelder WH. Multinomial modeling and the measurement of cognitive processes. Psychol. Rev. 1988;95:318–339. [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. J. Exp. Psychol. Learn. Mem. Cogn. 1995;21:803–814. [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002;357:1097–110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Harbluk JL, McLachlan DR. Retrieval without recollection: An experimental analysis of source amnesia. J. Verbal Learning Verbal Behav. 1984;23:593–611. [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: A case study. Neuropsychologia. 1996a;34:793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Pradere D. The Neuropsychology of Memory Illusions: False Recall and Recognition in Amnesic Patients. J. Mem. Lang. 1996b;35:319–334. [Google Scholar]
- Sestieri C, Capotosto P, Tosoni A, Luca Romani G, Corbetta M. Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia. 2013;51:900–906. doi: 10.1016/j.neuropsychologia.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Motion perception: seeing and deciding. Proc. Natl. Acad. Sci. 1996;93:628–633. doi: 10.1073/pnas.93.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB, Knight RT. Susceptibility to Memory Interference Effects following Frontal Lobe Damage: Findings from Tests of Paired-Associate Learning. J. Cogn. Neurosci. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb. Cortex. 2010;20:479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS. Recollection-based memory in frontotemporal dementia: implications for theories of long-term memory. Brain. 2002;125:2523–2536. doi: 10.1093/brain/awf247. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn. Brain. Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Küst J, Karbe H, Fink GR. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- Steinmetz MA, Constantinidis C. Neurophysiological evidence for a role of posterior parietal cortex in redirecting visual attention. Cereb. Cortex. 1995;5:448–456. doi: 10.1093/cercor/5.5.448. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Lepage J-F, Latulipe-Loiselle A, Fregni F, Pascual-Leone A, Théoret H. The uncertain outcome of prefrontal tDCS. Brain Stimul. 2014;7:773–783. doi: 10.1016/j.brs.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg M. Functional significance of retrieval-related activity in lateral parietal cortex: Evidence from fMRI and ERPs. Hum. Brain. Mapp. 2009;30:1490–1501. doi: 10.1002/hbm.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Yu SS, Johnson JD, Rugg MD. Dissociation of recollection-related neural activity in ventral lateral parietal cortex. Cogn. Neurosci. 2012;3:142–149. doi: 10.1080/17588928.2012.669363. [DOI] [PMC free article] [PubMed] [Google Scholar]