Abstract
Prenatal exposure to maternal Toxoplasma gondii (T. gondii) IgG antibody titer has been associated previously with an increased risk of offspring schizophrenia (SZ) and cognitive impairment. We examined maternal T. gondii, offspring bipolar disorder (BP) and childhood cognition using a population-based birth cohort. Maternal sera, drawn in the third trimester, were analyzed for T. gondii IgG antibody titer, and offspring cognition at ages 5 and 9–11 was measured with the Peabody Picture Vocabulary Test (PPVT) and the Raven Matrices (Raven). Raw scores were standardized and the ages combined. Potential cases with BP from the cohort were identified by database linkages. This protocol identified 85 cases who were matched 1:2 to controls. Maternal T. gondii IgG was not associated with the risk of BP in offspring. Neither moderate [HR = 1.43 (CI: 0.49, 4.17)] nor high IgG titer [HR = 1.6 [CI: 0.74, 3.48)] were associated with offspring BP. Associations were not observed between maternal T. gondii and BP with psychotic features or BP type 1. In addition, maternal T. gondii was not associated with childhood cognition. Our study suggests that T. gondii may be specific to SZ among major psychotic disorders, though further studies with larger sample sizes are required.
Keywords: Psychotic disorders, Toxoplasma gondii, Neurodevelopment, Cognition, Birth cohort, Raven Matrices, PPVT
1. Introduction
Prenatal exposure to maternal Toxoplasma gondii (T. gondii), a ubiquitous intracellular parasite (Remington, 2011; Scheld et al., 2004), has been associated previously with an increased risk of offspring schizophrenia (SZ) and cognitive impairment (Mwaniki et al., 2012; Sever et al., 1988; Torrey et al., 2012). In the present investigation, we examined the relationship between this exposure during pregnancy and the occurrence of offspring bipolar disorder (BP) and childhood cognitive functioning. Only primary infection with T. gondii crosses the placental barrier to cause congenital malformations (Sullivan and Jeffers, 2012), and enables its own spread throughout the central nervous system, causing neuropsychiatric disorders, including psychiatric illnesses (Carruthers and Suzuki, 2007; Fekadu et al., 2010). Prospective studies which followed offspring of exposed mothers into adulthood found an association between serologically documented maternal T. gondii IgG antibody during pregnancy and an increased risk of offspring schizophrenia spectrum disorder (Brown et al., 2005). Brown et al found a 2.6-fold increased risk for SZ among offspring of mothers with high T. gondii IgG antibody titers in archived maternal sera from the Child Health and Development Study (CHDS). That finding has been replicated in other cohorts (Blomstrom et al., 2012; Mortensen et al., 2007a) and confirmed in two meta-analyses (Sutterland et al., 2015; Torrey et al., 2012).
However, only three previous studies have examined maternal T. gondii and affective psychosis or bipolar disorder (BP) in offspring (Simanek and Meier, 2015). No association was observed between maternal T. gondii IgG antibody titers and offspring BP in a population based study from Denmark (Mortensen et al., 2011). In that study, IgG antibody levels were based on dried filter paper blood spots obtained from the infant rather than maternal sera obtained during pregnancy. This approach to determining exposure could differ from direct measurement of maternal sera if placental transfer of IgG diluted the measurable effect. A second study, with a smaller Danish sample, also found no association between maternal T. gondii IgG antibody levels and offspring BP (Mortensen et al., 2007b). However, a third study, based on the Collaborative Perinatal Project, reported a significant association between maternal exposure to the type I strain of T. gondii and an increased risk of offspring affective psychoses (Xiao et al., 2009). The finding was strong for cases with affective psychoses, although BP was not examined specifically. Because that study did not examine BP and the finding was specific to a particular strain of T. gondii, it is not directly comparable to the other studies. A recent review suggested T. gondii may have potential as a biomarker for SZ but not BP because the weight of evidence strongly supports an association between maternal T. gondii and SZ, but seemingly not between maternal T. gondii and BP (Brown, 2015).
In a previous study, Brown et al reported an association of maternal infections, including T. gondii and impaired executive functioning in adults with SZ (Brown et al., 2009). Both animal models and observational studies suggest an association between exposure to T. gondii and cognitive impairment (Kannan and Pletnikov, 2012). One prior study found that children exposed prenatally to T. gondii and treated with pyrimethamine and sulfadiazine for approximately one year had significant neurologic and cognitive impairment through childhood (Roizen et al., 1995), and evidence indicates that T. gondii continues to cause damage to the fetal brain once the maternal immune system responds to the infection (Ferguson et al., 2013). A large, prospectively followed cohort of children found that those born to mothers who tested positive for T. gondii antibodies during pregnancy had increased risks for microcephaly and intellectual disability at age seven (Sever et al., 1988). A recent international meta-analysis found an association between T. gondii and learning difficulties, developmental delays, impaired cognition, and visual deficits in children with congenital exposure (Mwaniki et al., 2012).
A number of meta-analyses and reviews have reported domain specific cognitive impairment in patients with BP (Bearden et al., 2001; Bearden et al., 2010; Daban et al., 2006; Goodwin et al., 2008; Harvey et al., 2010; Lim et al., 2013; Quraishi and Frangou, 2002; Savitz et al., 2005; Stefanopoulou et al., 2009). The cognitive domains in which deficits were observed are executive functioning, verbal learning, verbal memory, sustained attention, and psychomotor speed. The effect sizes were moderate and large in these domains, but also tend to be less severe than those observed in SZ (Consortium, 2013; Keshavan et al., 2004; Mesholam-Gately et al., 2009; Olvet et al., 2013; Reichenberg and Harvey, 2007; Woodberry et al., 2008). In SZ, cognitive impairments are observed in nearly all domains and are consistently worse than in BP (Arts et al., 2008; Seidman et al., 2013; Urfer-Parnas et al., 2010; Zanelli et al., 2010).
On average, individuals who are later diagnosed with BP have cognitive impairment during all phases of illness, including during the premorbid period of development (Bearden et al., 2010; Daban et al., 2006; Goodwin et al., 2008; Harvey et al., 2010; Hill et al., 2013; Kurtz and Gerraty, 2009; Martinez-Aran et al., 2004; Pol et al., 2012; Reichenberg et al., 2009; Reichenberg et al., 2002). A review of population based studies, however, concluded that the evidence did not yet support premorbid cognitive impairment as a trait of later BP (Kravariti et al., 2009). Draft board studies which examined premorbid functioning in those who later develop BP reported conflicting findings (Sorensen et al., 2012; Tiihonen et al., 2005). However, an analysis of four birth cohorts found that the subjects who later developed BP performed better than the general population on verbal, spatial, and inductive reasoning (MacCabe et al., 2013). Similarly, our group previously reported that childhood cognition was also not associated with BP in the CHDS birth cohort in a study of perinatally administered oxytocin and BP (Freedman et al., 2015).
In this paper we addressed the following: 1) the association between maternal T. gondii and offspring BP; and 2) the association between maternal T. gondii and offspring cognitive performance in childhood. With regard to the latter aim, we sought to improve upon prior studies which used school performance as a proxy for cognition with direct measures of cognitive ability.
2. Method
BP cases and matched controls were drawn from the Child Health and Development Study (CHDS) birth cohort. The CHDS recruited nearly all pregnant women receiving obstetric care (N=19,004) from the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) in Alameda County, California between 1959 and 1966 and followed them prospectively (van den Berg et al., 1988). Comprehensive data were collected from maternal medical records and interviews, child assessments, and other sources. KPNC enrolled approximately 30% of the population of the Bay Area of California at the time. This birth cohort has been extensively studied for prenatal and other early developmental risk factors for SZ (Brown et al., 2004; Brown et al., 2005; Brown et al., 2009; Freedman et al., 2011; Perrin et al., 2007).
2.1 Case Identification
Ascertainment of cases and controls has been described previously (Brown et al., 2013; Canetta et al., 2014; Freedman et al., 2015). Subjects with potential DSM-IV-TR BP, which included BP I, BP II, BP NOS, and BP with psychotic features, were ascertained from three sources: the KPNC electronic medical records database, the Alameda County Behavioral Health Care Services (ABHCS) database, and a mailed survey to the entire living CHDS birth cohort (mothers and children). This ascertainment would have reached all CHDS cohort members who belonged to KPNC when first treated and subjects who left KPNC prior to the first treatment of BP, but who resided in Alameda County and did not have other health insurance. The mailed survey would have ascertained subjects not obtained otherwise. Letters were mailed to all living mothers (N=6,971) and cohort members (N=13,009) with known addresses in the entire CHDS cohort (excluding families in which potential cases had already been identified), along with a questionnaire on mental and physical health. This was conducted from 2009–11.
Subjects identified by these methods were invited to participate in the study, receiving a letter to the most recent address. Those who did not refuse contact by returning a postcard, were contacted to arrange a diagnostic interview. Several repeat appointments were scheduled for subjects who failed to attend the interview. Extensive efforts were made to locate individuals who were no longer living at the most recent listed address, including using Department of Motor Vehicles (DMV) records, telephone directories, and contacting the subjects’ parents or siblings from CHDS or KPNC files. Mortality records, reverse directories, jail searches, and visits to previous addresses were also used as necessary.
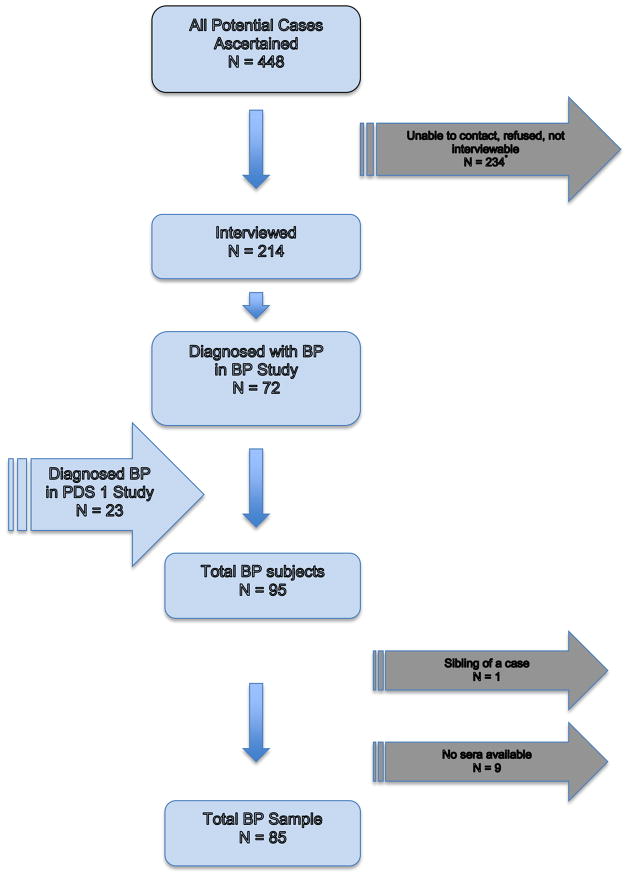
The ascertainment process identified 448 subjects who potentially met the criteria for BP and/or other psychotic disorders.
2.2 Diagnostic protocol
A total of 214 subjects (48% of those ascertained) were interviewed using the Structured Clinical Interview for DSM-IV-TR (SCID). The reasons that some subjects were not interviewed were: 100 could not be contacted, 80 refused or failed to keep the appointment, and 54 could not be interviewed because he/she had died, was incarcerated, was too psychotic or mentally disabled, or permission from the physician could not be obtained. Comparing the interviewed to those not interviewed demonstrated few differences (Table 1). Although both maternal and paternal age differ, the amount of difference in mean age is 2 years for fathers and 1 year for mothers. Similarly, gestational age differs by 4 days. These differences are not considered to be significant clinically. No differences exist on the exposures of interest for this study. Nevertheless, as with all longitudinal studies, loss to follow-up may be a potential bias for this study. The ascertainment process was conducted to capture as many potential cases as possible and every effort was made to locate and interview each. While few meaningful demographic differences were observed, it is not possible to calculate the extent to which bias from loss to follow-up might be having an effect or the direction of that hypothetical effect. It is important to note, however, that the prevalence of BP cases identified in this study comports with national and international rates (Merikangas et al., 2007; Merikangas et al., 2011), providing some confidence that few cases have been missed.
Table 1.
Characteristics of potential BP disorder case subjects interviewed and not interviewed.
| Characteristics | Potential cases interviewed (N=214) | Potential cases not interviewed (N=234) | p value* |
|---|---|---|---|
| Maternal age, mean years (SD) | 27.7 (6.7) | 26.5 (6.5) | 0.06 |
| Paternal age, mean years (SD) | 32.2 (7.7) | 30.1 (7.5) | 0.01 |
| Maternal race, N (%) | 0.48a | ||
| White | 119 (55.9) | 118 (51.1) | |
| Black | 76 (35.7) | 87 (37.7) | |
| Other | 18 (8.4) | 26 (11.2) | |
| Maternal education, N (%) | 0.75a | ||
| Less than high school | 47 (24.2) | 52 (25.2) | |
| High school graduate | 76 (39.2) | 86 (41.8) | |
| Some college/college graduate | 71 (36.6) | 68 (33.0) | |
| Gestational age, mean days (SD) | 282.5 (17.7) | 278.6 (19.5) | 0.03 |
| Any maternal psychiatric history, N (%) | 0.73 | ||
| Yes | 21 (10.1) | 25 (11.1) | |
| No | 188 (90.0) | 201 (88.9) | |
| Childhood Raven number tested (mean) | 105 (−0.2) | 97 (−0.11) | 0.5 |
| Childhood Peabody number tested (mean) | 107 (96.68) | 97 (97.79) | 0.62 |
Reported p value indicates the level of statistical significance from Chi Square comparisons of the difference between groups.
Maternal race and maternal education p values indicates Chi square comparison between the groupings, testing for overall difference between groups.
Study interviewers had a minimum of a master’s degree in a mental health field and were trained to reliably administer the SCID. DSM-IV-TR diagnoses including diagnostic qualifiers representing subtypes of BP were systematically assigned by consensus of three experienced clinicians (psychiatrists/Ph.D. psychologist), based on review of the SCID and medical records. This yielded 72 total BP cases. Among those interviewed, consensus diagnoses of non-BP disorders included SZ (n=61); major depressive disorder (n=62); and other diagnoses (n=19). These non-BP categories were not included in the present study. Although unipolar major depressive disorder was not included in the screening procedure, the diagnostic protocol enabled us to exclude subjects with database diagnoses of BP and/or psychotic disorders who were found instead to have unipolar depressive disorder in accord with structured research criteria.
In addition, some cases of BP had been ascertained through KPNC records by an earlier study (Prenatal Determinants of Schizophrenia I, PDS I) and were included in the present study (Susser et al., 2000). The PDS I enrolled people with SZ, but identified some people with BP during the diagnostic interview in that study. The protocol for the PDS I included the same electronic linkages with the KPNC inpatient, outpatient, and pharmacy databases, and utilized the same ICD-9 diagnostic codes (295–298). Ascertainment covered the period from 1981–1998. The only other differences in the screening methods are that the PDS I did not include review of pharmacy records for treatment with mood stabilizers, and the PDS I included a second screening step, which involved psychiatrist review of abstracted data from inpatient/outpatient records for symptoms of psychosis. The Diagnostic Interview for Genetic Studies (DIGS), rather than the SCID, was used for interviewing potential subjects in the PDS I. There were 23 BP cases diagnosed in the PDS I study.
In total, then, 95 cases with BP were diagnosed following ascertainment from all sources and clinical interview (Figure 1).
Figure 1.
Case Ascertainment Flow Chart
*Includes deceased, incarcerated, and those who repeatedly failed to keep appointments.
2.3 Control Selection
In order to ensure that controls would have been equally likely (as the cases to whom they are matched) to be ascertained if they had been treated for BP in KPNC or ABHCS, controls were matched to cases on membership in KPNC (for cases ascertained through KPNC records) or residence in Alameda County (for cases ascertained through ABHCS or by CHDS mailing survey) in the year the case was first treated as reported in the SCID. For KPNC, membership in the plan at first treatment of the case was used for control matching, since they would have been in KPNC databases if they sought care for BP. For ABHCS and mailed survey cases, DMV records indicating residence in Alameda County at the time of case diagnosis was used, since these subjects would have represented the population at risk for treatment at same time.
Birth cohort members who screened positive for potential BP or psychotic disorders but did not have BP (N=376) were excluded as potential control subjects. Siblings of selected controls were excluded from further control selection, so that controls were independent observations, each representing a single family or pregnant woman. Control matching criteria included: date of birth (+/− 30 days), sex, and availability of maternal archived sera. A ratio of 1:8 case to controls was achieved.
This protocol yielded 754 matched controls. Of the potential matched controls for each case, two were randomly selected to derive the matched case-control sets, resulting in 170 control subjects.
2.4 Analytic Sample
Two of the initial 95 bipolar disorder case subjects were siblings and one of these siblings was excluded at random, resulting in 94 case subjects. Eighty-five of these subjects had maternal archived sera available for the present study. We compared those with sera available to those without and found no significant differences [chi sq group comparison of maternal race (p = 0.74); maternal education (p = 0.77); offspring sex (p = 0.91); and family psychiatric history (p = 0.69). Case subjects were matched with comparison subjects at a 1:2 ratio as described above. Thus, 85 case subjects and 170 comparison subjects comprised the analytic sample for this study. These 85 cases of bipolar disorder included 36 with psychotic features. The majority had bipolar disorder I (N = 71), 10 had bipolar disorder II, and 4 had bipolar disorder not otherwise specified.
After a complete description of the study was provided to the subjects, written informed consent was obtained. The study protocol was approved by the Institutional Review Boards of the New York State Psychiatric Institute and KPNC.
2.5 Measurement of T. gondii antibody titers
The CHDS aimed to obtain maternal sera for each pregnancy during multiple time points. Maternal sera were drawn and stored frozen for almost all pregnancies. For the current analyses of T. gondii, serum samples drawn in the third trimester were analyzed. Three assays were used in accord with a standard protocol. The first two concern the assessment of T. gondii IgG antibody titer. Samples were first screened for the presence of IgG antibody titer. Following this step, the Sabin-Feldman dye test (Sabin and Feldman, 1948) was performed in the samples that screened positive. T. gondii IgM antibody, which is indicative of recent infection, was also assayed, using the double sandwich enzyme-linked immunosorbent test (IgM-ELISA). The dye test IgG titers were classified into three groups: negative (<1:16) (reference), moderate titer (1:16–1:64), and high titer (≥1:128) in accord with our previous publication on T. gondii and SZ (Brown et al., 2005).
2.6 Cognitive measures
Cognitive testing of randomly selected, large subsets of the CHDS birth cohort was conducted at age 5 and ages 9–11. Random sampling from the birth cohort reduces the chance of bias. Childhood cognitive measures included the Peabody Picture Vocabulary Test (PPVT) and the Raven Progressive Matrices and Raven Coloured Matrices (Raven), and both tests were given at each time period. For the subjects in the present study, fifty cases and 215 matched controls received the cognitive tests in childhood. Cognitive testing was available at only one time point for each subject as none of the subjects tested at age 5 was also tested at ages 9–11. Because subjects were not all tested at the same age, but no subject was tested at both periods, the results on the Raven and the PPVT were converted to standardized scores by age; then, the standardized Raven scores for age 5 were combined with the standardized Raven scores for ages 9–11, and standardized PPVT scores for age 5 with standardized PPVT scores for ages 9–11.
The PPVT is a well-known, commonly used test which estimates receptive verbal ability (Dunn, 1965; Strauss et al., 2006). The examinee is shown a plate with four pictures, the examiner speaks a word describing one of the four, and the examinee selects the correct picture either by speaking or pointing. In the CHDS, the PPVT was given to 3,413 children at age 5 and 3,737 at ages 9–11. Standardization of the PPVT for this sample was performed by converting raw scores to standard scores (z scores) by using the mean and standard deviation observed in each tested sample by age group. For the PPVT, the mean was set at 100, standard deviation of 15, as is the common practice for measures which estimate IQ. Once standardized, testing for ages 5 and 9–11 were combined to increase statistical power and analyzed as a continuous variable.
The Raven instruments assess visual-spatial processing, inductive reasoning, relational reasoning and problem solving (Raven, 1956, 1960; Strauss et al., 2006). Each question displays a pattern with a block missing, and four choices, one of which accurately completes the pattern. In the CHDS, at age 5 children were administered the Raven Coloured Matrices, which consists of 21 plates. Twenty-one plates were shown to each child, and they were asked to choose among the four options to complete the pattern in the picture. At age 9–11, the age appropriate Raven Progressive Matrices were given. Children were shown 60 plates and asked, for each, to select the pattern that completes the image from among four options. A total of 3,412 five year olds, and 3,737 children at ages 9–11, were assessed with the Raven. For these analyses, cohort norms for the Raven have been calculated using a mean set at 0 and a standard deviation of 1. As with the PPVT, after converting the scores into standard units based on the proportion correct, the results of the Raven were combined for ages 5 and 9–11 to gain statistical power and analyzed as a continuous variable.
As a result of standardizing the scoring for both the PPVT and the Raven, differences between groups are measured and reported in standard deviation units. Fifty youth who later developed BP were cognitively tested. We compared those case subjects with the 44 who were not tested, finding no significant differences [chi sq group comparison of maternal race (p = 0.94); maternal education (p = 0.41); offspring sex (p = 0.16); and family psychiatric history (p = 0.52).
2.7 Statistical analyses and covariates
Cases and controls were compared on potential confounders selected from the literature based on associations with T. gondii and/or BP (Brown et al., 2005; Jones et al., 2001; Mortensen et al., 2011). These included maternal age (<35 [reference], ≥35), maternal and paternal ethnicity (Caucasian [reference], African American, other), maternal and paternal educational achievement (defined as maternal education: <high school, high school only [reference], some college/college graduate), parity, maternal psychiatric history, birth weight and gestational age of the serum sample (in days after last menstrual period). Each of these potential confounders was tested for its possible association with both the exposure (T. gondii) and the outcomes (BP and cognitive performance).
Conditional logistic regression models, utilizing proportional hazards regression which maintained the integrity of the matching scheme described above, were used for the matched case-control data with BP and cognition as outcomes. This included analyses for relationships between T. gondii and BP, with BP as the outcome, and T. gondii and childhood cognition, with cognition as the outcome. Point and interval estimates of hazard ratios were obtained. Statistical significance was judged at α=0.05.
3. Results
3.1 Maternal T. gondii and offspring bipolar disorder
With respect to potential confounding, cases and controls in the analytic sample did not differ on any of the demographic covariates (Table 2). Therefore, none of the covariates was associated with case status and none were included in the statistical models as confounders affecting the potential association between T. gondii and BP. The seroprevalence of IgG antibody was 33/170 (19.4%) in controls. This value is similar to the 17.5% seroprevalence found in a large previous study of T. gondii in reproductive-aged women (Jones et al., 2001). None of the subjects tested positive for IgM. Screen agglutination which compared BP cases and matched controls with regard to seropositivity revealed no significant difference [HR = 1.55 (CI: 0.89, 2.3) p = 0.12]. As shown in Table 3, compared to the reference titer, neither moderate IgG titer [HR = 1.43 (CI: 0.491, 4.17) p = 0.51] nor high IgG titer [HR = 1.6 [CI: 0.74, 3.48) p = 0.23] were significantly associated with BP.
Table 2.
Demographic comparison of the analytic sample
| Bipolar Cases (N = 85) | Comparison Subjects (N = 170) | p value* | |
|---|---|---|---|
| Maternal age at child’s birth, Mean (SD)a | 27.5 (6.6) | 28.2 (6.0) | 0.37 |
| Maternal education, N (%)b | 0.641 | ||
| < High school | 16 (20.2) | 26 (16.1) | |
| High school graduate | 30 (38.0) | 59 (36.7) | |
| Some college or college graduate | 33 (41.8) | 76 (47.2) | |
| Maternal race, N (%)a | 0.201 | ||
| white | 58 (69.0) | 109 (64.1) | |
| African-American | 22 (26.2) | 41 (24.1) | |
| other | 4 (4.8) | 20 (11.8) | |
| Maternal psychiatric history, N (%)c,d | 22 (26.2) | 35 (20.7) | 0.33 |
| Gestational age in days, Mean (SD)a | 281.4 (16.3) | 280.5 (16.5) | 0.68 |
Data missing for one case subject
Data missing for six case subjects and nine comparison subjects
Data missing for one case subject and one comparison subject
Maternal psychiatric history was defined as psychoses, schizophrenia, affective disorder, anxiety, alcohol/substance abuse, mental deficiency, or other mental disorder
Reported p value indicates the level of statistical significance from Chi Square comparisons of the difference between groups.
Maternal race and maternal education p values indicates Chi square comparison between the groupings, testing for overall difference between groups.
Table 3.
Maternal T. gondii titer levels and risk of BP disorder in offspring
| Risk of BP (N = 85) | Risk of BP 1 (N = 26) | Risk of BP with psychotic features (N = 14) | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI)* | p value | Hazard Ratio (95% CI) | p value | Hazard Ratio (95% CI) | p value | |
| Low Titer (N = 200: Cases = 63 Controls = 137) | Reference group | -- | Reference group | -- | Reference group | -- |
| Moderate Titer (N = 20: Cases = 7 Controls = 13) | 1.43 (0.49, 4.17) | p = 0.51 | 0.99 (0.33, 2.94) | 0.99 | 0.32 (0.04, 2.70) | 0.29 |
| High Titer (N = 35: Cases = 15 Controls = 20) | 1.6 (0.74, 3.48) | p = 0.23 | 1.89 (0.9, 3.98) | 0.09 | 2.16 (0.84, 5.6) | 0.11 |
Hazard Ratio’s and p values indicate the comparison of the titer group compared to the reference group (Low Titer)
For BP with psychotic features, neither the moderate T. gondii IgG antibody titer [HR = 0.32 (CI: 0.04, 2.70) p = 0.29] nor the high titer [HR = 2.16 (CI: 0.84, 5.6) p = 0.11] were associated with the outcome. Similarly, for BP type I, neither the moderate titer [HR = 0.99 (CI:0.33, 2.94) p = 0.99] nor the high titer [HR = 1.89 (CI: 0.9, 3.98) p = 0.09] were associated with the outcome.
3.2 Premorbid cognition and T. gondii
Childhood cognition was not significantly associated with maternal T. gondii IgG antibody titer. As shown in Table 4, neither the moderate T. gondii IgG antibody titer (p = 0.8) nor the high titer (p = 0.66) were associated with performance on the PPVT. Similarly, there were no associations between the moderate titer (p = 0.63) and high titer (p = 0.81) and performance on the Raven.
Table 4.
Maternal T. gondii IgG titer levels and childhood cognition
| Compared to Reference Titer (<1:16) | Parameter Estimate | HR | 95% Confidence Interval | p value* | |
|---|---|---|---|---|---|
| PPVT (N = 250) | Moderate (1:16–1:64) | 0.1 | 1.1 | (0.52, 2.35) | 0.8 |
| High (1:128–1:512) | 0.3 | 0.88 | (0.49, 1.56) | 0.66 | |
| Raven (N = 146) | Moderate (1:16–1:64 | 0.37 | 1.45 | (0.32, 6.60) | 0.63 |
| High (1:128–1:512) | −0.1 | 0.9 | (0.39, 2.08) | 0.81 |
P value reports titer level compared to the reference which is Low Titer.
4. Discussion
Overall, no statistically significant association was observed between exposure to maternal T. gondii IgG antibody and offspring BP. Separate examination of T. gondii IgG antibody titer and BP subtypes including BP I and BP with psychotic features also did not reveal significant associations. Although not statistically significant, a possible trend towards significance was observed for BP with psychosis, suggesting the possibility that there may also be a relationship between T. gondii and BP with psychosis. The findings concerning T. gondii in relation to specific subtypes of BP have not been reported previously. Our result for BP is consistent with that reported by Mortensen et al (Mortensen et al., 2011) using an independent birth cohort and a different methodology for assessing exposure to T. gondii. That study, as noted above, analyzed dried blood spots on filter paper obtained from the infant while the present study was based on maternal sera obtained during late pregnancy.
The significant association reported in the study based on the Collaborative Perinatal Project (Xiao et al., 2009) may have resulted in part from the broader range of psychotic disorders considered. This heterogeneity may limit comparability with our study, in which the diagnostic outcome was limited to BP. Additionally, the positive association in that study was specific to one strain of T. gondii while our study did not differentiate between strains.
T. gondii IgG antibody titer has been mostly found to increase the risk for SZ (Blomstrom et al., 2012; Brown et al., 2005; Brown et al., 2009; Mortensen et al., 2007a), although not in the Xiao et al study (Xiao et al., 2009). While there are several similarities between BP and SZ with regard to potential etiologies, neurodevelopmental trajectories and psychotic symptoms (Demjaha et al., 2012; Hall et al., 2012; Howes et al., 2011; Murray et al., 2004; Sanches et al., 2008; Skjelstad et al., 2010), there are also major differences including affective symptomatology, course of illness, severity of neurocognitive dysfunction, and morphologic and pathophysiologic alterations (Arnone et al., 2009; Baumann and Bogerts, 1999; Bearden et al., 2001; Goodwin et al., 2007; Goodwin et al., 2008; Kasai et al., 2003; Strakowski et al., 2000). The identification of risk factors specific for each of these disorders offers the potential for unique preventive, early intervention, and treatment approaches (Brown, 2015). Our study suggests the possibility that T. gondii may be one exposure that is specific to SZ among major psychotic disorders, though further studies with larger sample sizes are required given that the relative number of studies of T. gondii and BP is considerably fewer than those for SZ. As the field moves toward elucidating the mechanisms that underlie neurodevelopmental illnesses (Insel, 2014), the specificity of etiologies could become critical in that they may help to elucidate unique biological mechanisms between disorders despite similarities in symptomatic manifestations.
Our second finding is that maternal T. gondii IgG antibody exposure was not associated with childhood cognitive performance, at least as measured with the Raven and PPVT. The cognitive domains covered by these instruments include visual-spatial processing, inductive reasoning, relational reasoning, problem solving, and receptive verbal ability. This differs from some of the previous, albeit limited, research on T. gondii and cognitive outcomes in offspring. As noted, however, only a few studies have documented cognitive deficits in offspring following perinatal exposure to T. gondii (Brown et al., 2009; Mwaniki et al., 2012). A recent review, which included studies of latent maternal T. gondii exposure and offspring cognition and development, suggested that T. gondii’s effect on dopamine systems in offspring may explain why offspring had delayed motor development, an increased prevalence of mental retardation and cognitive delays as well as increased risks of some neuropsychiatric illnesses (Abdoli et al., 2014).
Another study, which prospectively followed a large cohort of children born to mothers tested for acute T. gondii infection during pregnancy, reported increased risks for microcephaly and intellectual disability at age seven (Sever et al., 1988). The authors reported that T. gondii infection increased the number of offspring with IQ’s below 70 by 30% and increased the risk of microcephaly by 60%. Additionally, Mwaniki et al’s meta-analysis of prenatal exposure to T. gondii included five studies (N = 512) which assessed maternal exposure to T. gondii and offspring cognition (Mwaniki et al., 2012). They reported that congenital T. gondii was related to cognitive difficulties or developmental delay in 33% of subjects. However, specific measures of cognition were not reported. Inclusion criteria for maternal exposure in that study were broad and considered several markers of congenital T. gondii infection. The differences in the identification of maternal exposure (elevated IgG titer versus congenital infection) and in assessment of cognitive functioning (specific versus general measures of cognitive performance) may explain the differences in results between these studies and our own.
As noted, our group previously reported that childhood cognitive performance was not associated with later BP in this birth cohort (Freedman et al., 2015), though cases had a higher mean PPVT score compared to controls (103.1 versus 100.1) and a lower mean score on the Raven (0.0237 versus 0.0254), the significance levels were as follows (PPVT: p = 0.19; Raven: p = 0.93). Although prior research reported that premorbid cognitive impairments are observed in BP, they are domain-specific rather than generalized (Bearden et al., 2001; Bearden et al., 2010; Daban et al., 2006; Goodwin et al., 2008; Harvey et al., 2010; Lim et al., 2013; Quraishi and Frangou, 2002; Savitz et al., 2005; Stefanopoulou et al., 2009). The PPVT and Raven do not fully measure the breadth of cognitive domains which have been found to be impaired during the premorbid period in BP. The PPVT assesses receptive verbal ability. One previous study reported receptive language to be low at ages 3 and 9, but high at ages 5 and 7 in 20 people who later developed mania (Cannon et al., 2002). Few other studies have studied premorbid receptive language performance in BP, perhaps because it is not considered a domain that is impaired in BP (Goodwin et al., 2008; Olvet et al., 2010). A few studies have examined executive functioning during the premorbid period in BP (Martino et al., 2015; Meyer et al., 2004; Tiihonen et al., 2005). The Raven captures some aspects of executive functioning, similar to the visuospatial component testing assessed at military induction by Tiihonen et al, which found deficits among those who later developed BP (Tiihonen et al., 2005). A second conscript study reported that those who later developed BP had the same performance as controls on the visuospatial portion of the conscript battery (Reichenberg et al., 2002). While the Raven captures some aspects of executive functioning, the evidence for deficits during the premorbid period remains inconclusive. Our findings suggest that for visual-spatial processing, inductive reasoning, relational reasoning and problem solving during childhood, those who later develop BP do not perform worse than controls.
This study has a number of strengths. First, T. gondii antibody was measured during the third trimester and prospective data were collected on offspring bipolar disorder and cognition. This direct measure of maternal T. gondii infection in mothers during pregnancy eliminates temporal ambiguity in the association. Second, research diagnoses of BP derived from systematic ascertainment and consensus diagnoses from clinical interview, which reduces the risk of diagnostic misclassification. Finally, performance on cognitive testing in childhood is not affected by social and environmental factors which occur later in life, for instance premorbid and prodromal disease course, or duration and quality of education acquired subsequent to this early assessment.
The main limitation was the modest sample size for both the analysis of T. gondii as a risk factor for BP and cognitive deficits. This may have explained the non-significant results. This study had 80% power to detect a moderate association (OR = 2.7, p = .05), similar to the effect size previously observed in the study of maternal T. gondii IgG and SZ in this cohort (Brown et al., 2005). However, though our findings were not significant, there was evidence of a trend relationship between T. gondii and BP. In addition, there was 80% power to detect a moderate association between maternal T. gondii IgG and cognitive performance (OR = 2.8, p = .05). On standardized cognitive measures, this difference is quite large, requiring differences in performance significantly larger than those observed for any domain in BP. Larger samples sizes are therefore needed to obtain sufficient power to observe differences in cognitive functioning. Additionally, analyzed maternal sera for T. gondii IgG, which provides a measure of infection at a point in the past but is unlikely to identify infection with this parasite given the rarity of its occurrence over the course of a pregnancy. Consequently, we are unable to determine when the infection occurred in relation to the pregnancy or birth.
Despite these limitations, the findings of this study suggest that future efforts are necessary to delineate the effects of particular exposures on specific psychiatric and neurocognitive outcomes. Our study only considered the latent maternal exposure and effects on offspring. We postulate that latent exposure may have consequences for maternal immune activation during gestation on offspring odds of BP, though further work on immune mechanisms relevant to this phenomenon is necessary. Further research in large, prospectively followed birth cohorts is necessary to realize this goal. Such research would best be conducted in birth cohorts which assess risk factors for both BP and SZ for direct comparison. Analyses which combined existing cohorts to increase statistical power would also provide needed additional evidence. T. gondii is an established risk factor for SZ and as such merits further consideration in studies of other psychiatric illnesses.
Highlights.
Exposure to maternal T. gondii does not increase the risk of offspring BP
Exposure to maternal T. gondii was not associated with BP subtypes
Exposure to maternal T. gondii may be a risk factor specific to SZ and not BP
Exposure to maternal T. gondii is not associated with offspring cognitive performance
Acknowledgments
Funding Sources: The research was supported by NIMH grants 2T32-MH-13043 to DF; 5R01-MH073080 and 5K02-MH65422 to ASB; 5R01 MH069819 to CS; and National Institute on Child Health and Development grants N01-HD-1-3334 and NO1-HD-6-3258. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; nor in the decision to submit the article for publication.
The authors wish to acknowledge the assistance with data analysis of Keely Cheslack-Postava.
Footnotes
Disclosures:
The authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdoli A, Dalimi A, Arbabi M, Ghaffarifar F. Neuropsychiatric manifestations of latent toxoplasmosis on mothers and their offspring. Journal of Maternal-Fetal & Neonatal Medicine. 2014;27:1368–1374. doi: 10.3109/14767058.2013.858685. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Baumann B, Bogerts B. The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophrenia Research. 1999;39:141–148. doi: 10.1016/s0920-9964(99)00113-9. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151–103. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woogen M, Glahn DC. Neurocognitive and neuroimaging predictors of clinical outcome in bipolar disorder. Current psychiatry reports. 2010;12:499–504. doi: 10.1007/s11920-010-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrom A, Karlsson H, Wicks S, Yang S, Yolken RH, Dalman C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring-a matched case-control study. Schizophrenia Research. 2012;140:25–30. doi: 10.1016/j.schres.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Brown A, Bao Y, McKeague I, Shen L, Schaefer C. Parental age and risk of bipolar disorder in offspring. Psychiatry research. 2013;208:225–231. doi: 10.1016/j.psychres.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. The Kraepelinian Dichotomy From the Perspective of Prenatal Infectious and Immunologic Insults. Schizophr Bull. 2015;41:786–791. doi: 10.1093/schbul/sbv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Liu LY, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. The American journal of psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta SE, Bao Y, Co MD, Ennis FA, Cruz J, Terajima M, Shen L, Kellendonk C, Schaefer CA, Brown AS. Serological Documentation of Maternal Influenza Exposure and Bipolar Disorder in Adult Offspring. The American journal of psychiatry. 2014 doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophrenia Bulletin. 2007;33:745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium C.-D.G.o.t.P.G. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Balanza-Martinez V, Salazar-Fraile JS, Selva-Vera G, Vieta E. Specificity of cognitive deficits in bipolar disorder versus schizophrenia - A systematic review. Psychotherapy and Psychosomatics. 2006;75:72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- Demjaha A, MacCabe JH, Murray RM. How Genes and Environmental Factors Determine the Different Neurodevelopmental Trajectories of Schizophrenia and Bipolar Disorder. Schizophrenia Bulletin. 2012;38:209–214. doi: 10.1093/schbul/sbr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM. Peabody Picture Vocabulary Test - Manual. American Guidance Service, Inc; Circle Pines, Minnesota: 1965. [Google Scholar]
- Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behaviour disorders--overview of evidence and mechanisms. Folia Parasitol (Praha) 2010;57:105–113. doi: 10.14411/fp.2010.013. [DOI] [PubMed] [Google Scholar]
- Ferguson DJP, Bowker C, Jeffery KJM, Chamberlain P, Squier W. Congenital Toxoplasmosis: Continued Parasite Proliferation in the Fetal Brain Despite Maternal Immunological Control in Other Tissues. Clinical Infectious Diseases. 2013;56:204–208. doi: 10.1093/cid/cis882. [DOI] [PubMed] [Google Scholar]
- Freedman D, Brown AS, Shen L, Schaefer CA. Perinatal oxytocin increases the risk of offspring bipolar disorder and childhood cognitive impairment. Journal of affective disorders. 2015;173:65–72. doi: 10.1016/j.jad.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, Deicken R, Kegeles LS, Vinogradov S, Bao Y, Brown AS. Maternal-fetal blood incompatibility and neuromorphologic anomalies in schizophrenia: Preliminary findings. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1525–1529. doi: 10.1016/j.pnpbp.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR, Ghaemi SN. Manic-depressive illness : bipolar disorders and recurrent depression. 2. Oxford University Press; New York, N.Y: 2007. [Google Scholar]
- Goodwin GM, Martinez-Aran A, Glahn DC, Vieta E. Cognitive impairment in bipolar disorder: Neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharmacol. 2008;18:787–793. doi: 10.1016/j.euroneuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hall MH, Smoller JW, Cook NR, Schulze K, Hyoun Lee P, Taylor G, Bramon E, Coleman MJ, Murray RM, Salisbury DF, Levy DL. Patterns of deficits in brain function in bipolar disorder and schizophrenia: A cluster analytic study. Psychiatry research. 2012;200:272–280. doi: 10.1016/j.psychres.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Wingo AP, Burdick KE, Baldessarini RJ. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord. 2010;12:364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological Impairments in Schizophrenia and Psychotic Bipolar Disorder: Findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. The American journal of psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed]
- Howes OD, Lim S, Theologos G, Yung AR, Goodwin GM, McGuire P. A comprehensive review and model of putative prodromal features of bipolar affective disorder. Psychological Medicine. 2011;41:1567–1577. doi: 10.1017/S0033291710001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. American Journal of Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. American journal of epidemiology. 2001;154:357–365. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- Kannan G, Pletnikov MV. Toxoplasma gondii and cognitive deficits in schizophrenia: an animal model perspective. Schizophr Bull. 2012;38:1155–1161. doi: 10.1093/schbul/sbs079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–1077. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Kennedy JL, Murray R. Neurodevelopment and schizophrenia. Cambridge University Press; Cambridge, U.K.; New York, NY, USA: 2004. [Google Scholar]
- Kravariti E, Kane F, Murray RM. Neurocognitive Endophenotypes for Bipolar Disorder: Evidence from Case-Control, Family and Twin Studies. Springer; Dordrecht: 2009. [Google Scholar]
- Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23:551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Baldessarini RJ, Vieta E, Yucel M, Bora E, Sim K. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: Review of the evidence. Neuroscience and biobehavioral reviews. 2013;37:418–435. doi: 10.1016/j.neubiorev.2013.01.003. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, Allebeck P, Dalman C. Decline in Cognitive Performance Between Ages 13 and 18 Years and the Risk for Psychosis in Adulthood A Swedish Longitudinal Cohort Study in Males. Jama Psychiatry. 2013;70:261–270. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. The American journal of psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Samame C, Ibanez A, Strejilevich SA. Neurocognitive functioning in the premorbid stage and in the first episode of bipolar disorder: A systematic review. Psychiatry Research. 2015;226:23–30. doi: 10.1016/j.psychres.2014.12.044. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Carlson GA, Wiggs EA, Martinez PE, Ronsaville DS, Klimes-Dougan B, Gold PW, Radke-Yarrow M. A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Development and Psychopathology. 2004;16:461–476. doi: 10.1017/s095457940404461x. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Torrey EF, Yolken RH. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biological Psychiatry. 2007a;61:688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophrenia Bulletin. 2007b;33:741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, McGrath JJ, Hougaard DM, Norgaard-Petersen B, Mors O, Borglum AD, Yolken RH. Neonatal antibodies to infectious agents and risk of bipolar disorder: a population-based case-control study. Bipolar Disorders. 2011;13:624–629. doi: 10.1111/j.1399-5618.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Mwaniki MK, Atieno M, Lawn JE, Newton CRJC. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Burdick KE, Cornblatt BA. Assessing the potential to use neurocognition to predict who is at risk for developing bipolar disorder: a review of the literature. Cogn Neuropsychiatry. 2013;18:129–145. doi: 10.1080/13546805.2012.724193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Stearns WH, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Comparing clinical and neurocognitive features of the schizophrenia prodrome to the bipolar prodrome. Schizophrenia Research. 2010;123:59–63. doi: 10.1016/j.schres.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MA, Chen H, Sandberg DE, Malaspina D, Brown AS. Growth trajectory during early life and risk of adult schizophrenia. British Journal of Psychiatry. 2007;191:512–520. doi: 10.1192/bjp.bp.106.034694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol HEH, van Baal GCM, Schnack HG, Brans RGH, van der Schot AC, Brouwer RM, van Haren NEM, Lepage C, Collins DL, Evans AC, Boomsma DI, Nolen W, Kahn RS. Overlapping and Segregating Structural Brain Abnormalities in Twins With Schizophrenia or Bipolar Disorder. Archives of General Psychiatry. 2012;69:349–359. doi: 10.1001/archgenpsychiatry.2011.1615. [DOI] [PubMed] [Google Scholar]
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. Journal of affective disorders. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Raven JC. Guide to using the Coloured Progressive Matrices. Dumfries; Scotland: 1956. [Google Scholar]
- Raven JC. Guide to the Standard Progressive Matrices. Williams Grieve and Sons; Dumfries, Scotland: 1960. [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychological bulletin. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. American Journal of Psychiatry. 2002;159:2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Remington JS. Infectious diseases of the fetus and newborn infant. 7. Saunders/Elsevier; Philadelphia, PA: 2011. [Google Scholar]
- Roizen N, Swisher CN, Stein MA, Hopkins J, Boyer KM, Holfels E, Mets MB, Stein L, Patel D, Meier P, Withers S, Remington J, Mack D, Heydemann PT, Patton D, McLeod R. NEUROLOGIC AND DEVELOPMENTAL OUTCOME IN TREATED CONGENITAL TOXOPLASMOSIS. Pediatrics. 1995;95:11–20. [PubMed] [Google Scholar]
- Sabin AB, Feldman HA. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma) Science. 1948;108:660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Sanches M, Keshavan MS, Brambilla P, Soares JC. Neurodevelopmental basis of bipolar disorder: a critical appraisal. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:1617–1627. doi: 10.1016/j.pnpbp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disorders. 2005;7:216–235. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Scheld WM, Whitley RJ, Marra CM. Infections of the central nervous system. 3. Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychological Medicine. 2013;43:119–131. doi: 10.1017/S0033291712000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever JL, Ellenberg JH, Ley AC, Madden DL, Fuccillo DA, Tzan NR, Edmonds DM. TOXOPLASMOSIS - MATERNAL AND PEDIATRIC FINDINGS IN 23,000 PREGNANCIES. Pediatrics. 1988;82:181–192. [PubMed] [Google Scholar]
- Simanek AM, Meier HCS. Association Between Prenatal Exposure to Maternal Infection and Offspring Mood Disorders: A Review of the Literature. Current Problems in Pediatric and Adolescent Health Care. 2015;45:325–364. doi: 10.1016/j.cppeds.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder A systematic review. Journal of affective disorders. 2010;126:1–13. doi: 10.1016/j.jad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sorensen HJ, Saebye D, Urfer-Parnas A, Mortensen EL, Parnas J. Premorbid intelligence and educational level in bipolar and unipolar disorders: a Danish draft board study. Journal of affective disorders. 2012;136:1188–1191. doi: 10.1016/j.jad.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. International review of psychiatry (Abingdon, England) 2009;21:336–356. doi: 10.1080/09540260902962149. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests : administration, norms, and commentary. 3. Oxford University Press; New York: 2006. [Google Scholar]
- Sullivan WJ, Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS microbiology reviews. 2012;36:717–733. doi: 10.1111/j.1574-6976.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophrenia Bulletin. 2000;26:257–273. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- Sutterland AL, Fond G, Kuin A, Koeter MWJ, Lutter R, van Gool T, Yolken R, Szoke A, Leboyer M, de Haan L. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatrica Scandinavica. 2015;132:161–179. doi: 10.1111/acps.12423. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Henriksson M, Cannon M, Kieseppa T, Laaksonen I, Sinivuo J, Lonnqvist J. Premorbid intellectual functioning in bipolar disorder and schizophrenia: Results from a cohort study of male conscripts. American Journal of Psychiatry. 2005;162:1904–1910. doi: 10.1176/appi.ajp.162.10.1904. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and Other Risk Factors for Schizophrenia: An Update. Schizophrenia Bulletin. 2012;38:642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer-Parnas A, Mortensen EL, Saebye D, Parnas J. Pre-morbid IQ in mental disorders: a Danish draft-board study of 7486 psychiatric patients. Psychological Medicine. 2010;40:547–556. doi: 10.1017/S0033291709990754. [DOI] [PubMed] [Google Scholar]
- van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatric and perinatal epidemiology. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta-analytic review. American Journal of Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Xiao JC, Buka SL, Cannon TD, Suzuki Y, Viscidi RP, Torrey EF, Yolken RH. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes and Infection. 2009;11:1011–1018. doi: 10.1016/j.micinf.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A, Jones PB, Doody GA, Kapur S, Murray RM. Specific and Generalized Neuropsychological Deficits: A Comparison of Patients With Various First-Episode Psychosis Presentations. American Journal of Psychiatry. 2010;167:78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]