To the Editor
Colony stimulating factor 2 receptor beta (CSF2RB) is the shared beta-chain receptor and essential signaling component for IL-3, IL-5 and GM-CSF receptor activation. In the context of GM-CSF signaling, the ligand-specific alpha chain (CSF2RA) complexes with CSF2RB and GM-CSF ligand, forming a dodecameric complex in which the proximity of CSF2RB subunits allows associated JAK2 kinases to trans-phosphorylate1. CSF2RB signals through several pathways including JAK2/STAT5, PI3K/mTOR and MEK/ERK to promote survival, proliferation and differentiation (reviewed in2). Both spontaneous transformation3, 4 and random mutagenesis4, 5 screens have shown that CSF2RB mutations can result in ligand-independent activation in vitro. However, these predicted oncogenic mutations have never been observed clinically. In fact, the only phenotypic CSF2RB mutations reported are recessive, loss-of-function, germline mutations in pulmonary alveolar proteinosis6. As oncology moves toward an age of personalized and molecularly-targeted treatment it will require the identification and in vitro characterization of targetable genetic lesions. Here we describe the first case of a leukemia patient harboring a germline, CSF2RB-activating point mutation (R461C) and identify small molecule inhibitors with therapeutic potential against this mutation.
We obtained a pediatric T-cell acute lymphoblastic leukemia (T-ALL, Supplemental Figure 1A) with informed consent approved by the Institutional Review Boards of Oregon Health & Science University and Erasmus University Medical Center - Sophia Children’s Hospital. We isolated mononuclear cells and performed deep sequencing as previously described7, uncovering the CSF2RB R461C mutation at a 54% allele burden (Supplemental Figure 1B and C). The patient also presented with somatic NOTCH1-truncation, NOTCH1 missense, and PTEN point mutations, all recurrent and leukemia-associated mutations8 (other observed mutations in Supplemental Table 1). R461C was later confirmed by Sanger sequencing as a heterozygous, germline variant using a minimal residual disease day 79 sample for normal DNA (Supplemental Figure 1D). CSF2RB R461C has never been previously described in cancer patients (including public databases) but is listed as a SNP in the 1000 Genomes database9 (rs371045078) at a low allele frequency (MAF=0.000998, 5 observations in 1000 Genomes9; MAF=0.000272, 33 observations in the Exome Aggregation Consortium10) in exclusively heterozygous cases. Prior work has established that cancer-predisposing mutations exist in the germline of healthy individuals at frequencies as high as 1.1%, but those frequencies elevate to over 8% in children and adolescents with cancer, most of which demonstrate no family history of cancer11. Congenital mutations in CSF3R have also been observed to precede the development of acute myeloid leukemia12. In addition, there are numerous reports of families with gain-of-function germline mutations in receptors with similar biology as CSF2RB (e.g., CSF3R13, EPOR14, MPL15) and in many of these pedigrees the affected family members do not develop overt leukemia, though they sometimes exhibit elevated blood counts.
The location of this variant, within the putative membrane-spanning portion of the receptor, is of particular interest as this region is a hot spot for activating mutations in vitro5. Previously, mutations altering the number of membrane-spanning or membrane-adjacent unpaired cysteines have been shown to activate the IL-7 receptor in T-ALL16 and RET in medullary thyroid carcinoma17. CSF2RB has been shown to be partially activated in vitro by replacement of the extracellular domain with a short, cysteine-containing sequence4. Lastly, multiple sequence alignment of CSF2RB homologues demonstrated that arginine 461 is conserved across most mammals (Supplemental Figure 2) increasing the potential that R461C could have functional relevance and cooperate with canonical leukemogenic mutations. Accordingly, we hypothesized that R461C could activate CSF2RB-signaling and contribute to the patient’s leukemia.
We cloned CSF2RB R461C into the pMXs-IRES-Puro plasmid and transfected into IL-3-dependent murine Ba/F3 cells by electroporation. Stably transfected cells were selected using two weeks of continual 2µg/ml puromycin selection prior to performing IL-3 withdrawal transformation assays as previously described7. R461C conferred factor-independent growth while wild-type (WT) CSF2RB did not (Figure 1A, Supplemental Figure 3). The full CSF2RB transgene was sequence confirmed in two biologically replicate, IL-3 independent cell lines and used for further studies.
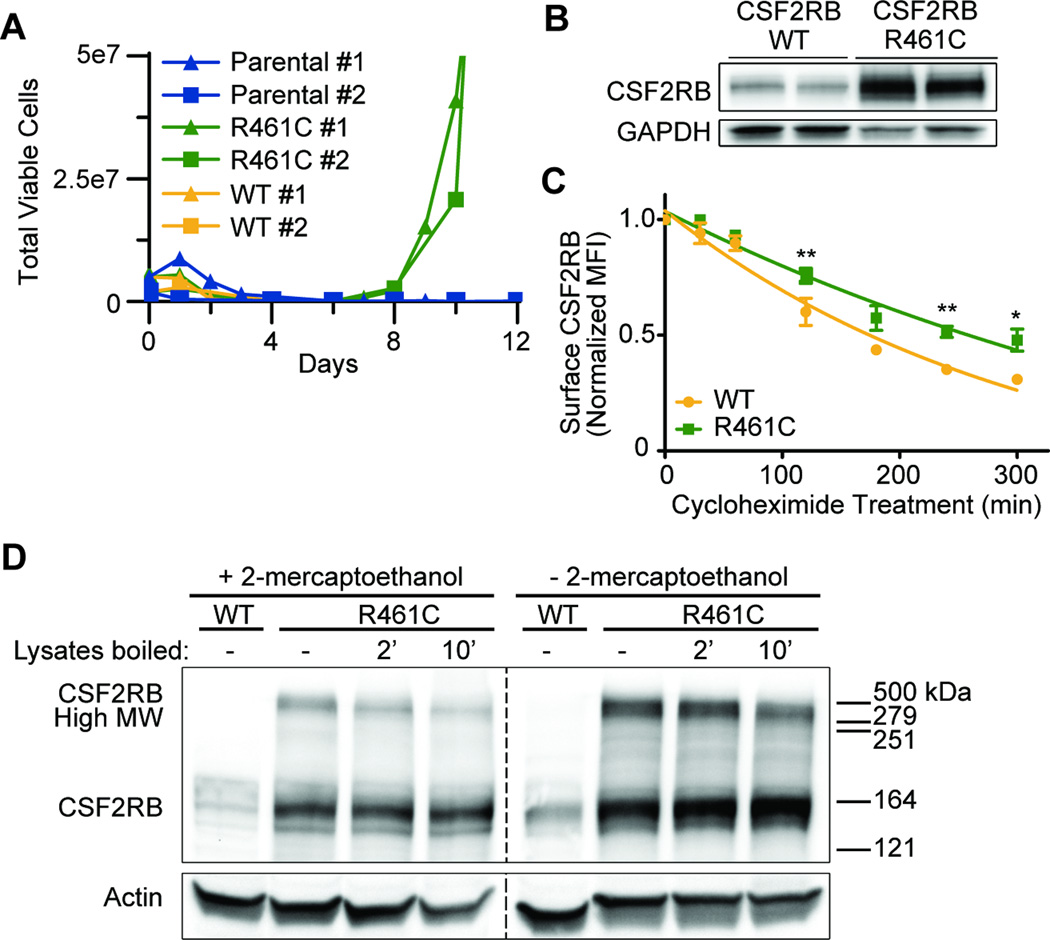
Figure 1. CSF2RB R461C is a transforming mutation which results in receptor stabilization and formation of higher molecular weight complexes.
(A) Biologically replicate Ba/F3 lines expressing CSF2RB WT or R461C were monitored for growth in the absence of IL-3. Validation experiments in Supplemental Figure 3. (B) CSF2RB immunoblot of transformed Ba/F3 lines. Prior to lysis the cells were starved overnight in 0.1% FBS. WT cells were grown in IL-3 supplemented media prior to overnight starvation. (C) Ba/F3 cells expressing CSF2RB WT or R461C were treated with cycloheximide and stained with PE-conjugated anti-CSF2RB antibody and analyzed by flow cytometry (BD FACSAria IIIu and BD LSR II). Normalized mean fluorescence intensity (MFI) is shown over time (*p<0.05, **p<0.01). (D) Protein lysates were analyzed by immunoblot to determine the presence of high molecular weight, CSF2RB-containing complexes. Treatment with a reducing agent (2-mercaptoethanol) is compared with lysate boiling at 95°C. WT cells were grown in IL-3 supplemented media and prior to lysis all lines were starved overnight in 0.1% FBS.
We identified that CSF2RB R461C expression resulted in an accumulation of surface CSF2RB protein, relative to WT, prior to IL-3 withdrawal when assayed by flow cytometry (Supplemental Figure 4). Furthermore, immunoblot analysis showed that the R461C protein is significantly increased in IL-3-independent cells relative to WT cells grown in IL-3 (Figure 1B, details and all antibodies included Supplemental methods and Supplemental Table 2). As both exogenous versions of CSF2RB were expressed by identical promoters we rationalized that the accumulation was due to changes in CSF2RB protein stability. We performed cycloheximide time course experiments using 100ug/ml cycloheximide in DMSO followed by staining with PE-conjugated anti-CSF2RB to determine alterations in surface protein stability. The R461C mutant possessed a prolonged surface half-life relative to WT CSF2RB (6.5 vs 3.6 hours; Figure 1C). Given the presence of a novel cysteine and increased stability in the R461C mutant, we investigated disulfide-linked receptor oligomerization by immunoblot under non-reducing conditions. Comparing the WT- and R461C-expressing cells, we observed CSF2RB-containing complexes of ~500 kDa that showed a reduced intensity when 2-mercaptoethanol was added to the lysate (normally CSF2RB runs at 125 kDa, Figure 1D), indicating R461C enables novel disulfide interactions with additional CSF2RB monomers or other endogenous receptors.
Previous studies have shown that mutations within receptor transmembrane domains can result in constitutive receptor oligomerization and activation15–17. The role of the CSF2RB transmembrane domain in receptor stability and signal transduction has not been characterized1. Even the boundaries of the membrane-spanning region have not been empirically studied, though it is commonly assumed to span residues 444–460 based on early domain modeling5. Various modern models consistently predict a much larger domain (Supplemental Figure 5), including residue 461, suggesting R461C might be within the transmembrane domain and alter receptor-receptor interactions. Another possibility is that the loss of a charged residue near the membrane boundary could result in shifting or fluidity of the transmembrane domain of the mutant receptor.
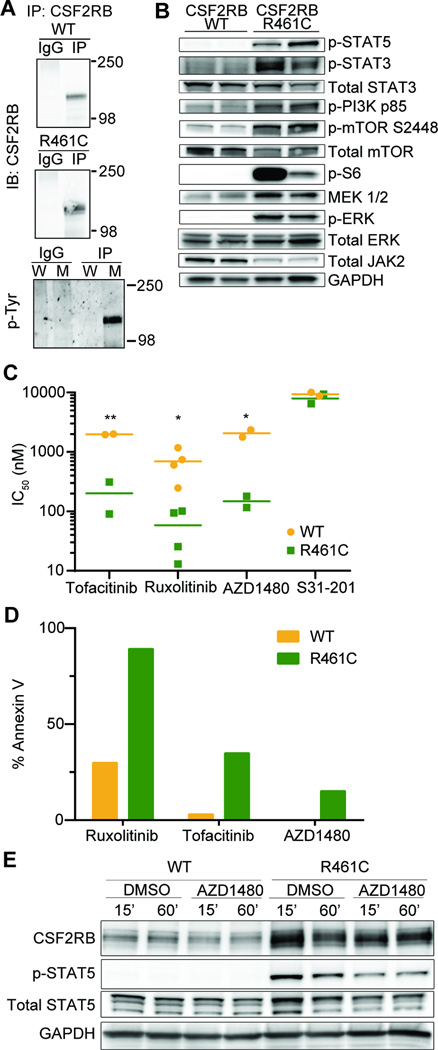
Using immunoblot and co-immunoprecipitation analysis, we showed that transformed Ba/F3 cells expressing CSF2RB R461C exhibited constitutive receptor phosphorylation and signaled through several pathways, including STAT5, PI3K/mTOR1, and MEK/ERK (Figure 2A and B). To identify therapeutically targetable pathways we utilized a 104 small molecule inhibitor library screen, as previously described7, 18, and compared IC50 values for R461C-expressing cells to WT-expressing cells. JAK inhibitors (Tofacitinib, Ruxolitinib, and AZD1480) constituted the three top hits in the screen (Figure 2C, Supplemental Figure 6A–C, full screen Supplemental Table 3). These treatments were specific and cytotoxic, inducing apoptosis in R461C-expressing cells (Figure 2D and Supplemental Figure 6D). While these inhibitors have a differential specificity for targeting JAK kinases (Tofacitinib: JAK2/3, Ruxolitinib: JAK1/2, AZD1480: JAK2), the strongest target is JAK2. We also observed reduced levels of total JAK2 in R461C cells (Figure 2B), which is consistent with JAK2 activation and then degradation through a STAT5/SOCS-1 negative feedback loop19. These results suggest a dependency on the JAK/STAT pathway that is consistent with ligand-independent activation of CSF2RB in R461C-expressing cells. Targeted inhibition of JAK2 using AZD1480 quickly reduced levels of phosphorylated STAT5, validating the specific nature of the inhibition and highlighting JAK2 as the key mediator of CSF2RB R461C signaling (Figure 2E).
Figure 2. CSF2RB R461C is constitutively phosphorylated and activates canonical downstream pathways that are sensitive to JAK2 inhibition.
(A) CSF2RB was immunoprecipitated from transformed Ba/F3 lysates starved overnight in 0.1% FBS and probed for CSF2RB or phosphorylated tyrosine residues on replicate blots (W – WT lysate, M – R461C mutant lysate). WT cells were grown in IL-3 supplemented media. (B) Immunoblot analysis for activation of canonical CSF2RB signaling members across biologically replicate lysates. WT cells were grown in IL-3 supplemented media and all lines were starved overnight in 0.1% FBS. (C) Biologically replicate WT and R461C lines were screened on a small molecule inhibitor library and the top three hits (determined by % IC50 difference between the two lines) were the JAK-inhibitors Tofacitinib, Ruxolitinib and AZD1480. The STAT3 inhibitor S31-201 was equally ineffective on both WT and R461C (*p<0.05, **p<0.01). WT cells were grown and tested in IL-3 supplemented media. Key inhibitors are validated in Supplemental Figure 6A–C, full inhibitor panel results are found in Supplemental Table 3. (D) Lines were treated with indicated JAK-inhibitors (Ruxolitinib 350nM, Tofacitinib 350nM, AZD1480 1µM) for 48 hours and analyzed for apoptotic induction using Annexin V staining and flow cytometry (Guava PCA). 24 hour reads are shown in Supplemental Figure 6D. (E) Cells were starved overnight in 0.1% FBS and then treated with the JAK2 specific inhibitor AZD1480 (500nM) for the indicated time and immunoblotted for CSF2RB downstream signaling. WT cells were maintained in IL-3 supplemented media prior to starvation and treatment.
In sequencing 449 primary hematopoietic malignancies, we found 7 additional CSF2RB mutations (Supplemental Table 4) but none were membrane spanning and all 7 were incapable of transforming Ba/F3 cells (data not shown). Several other CSF2RB-activating mutations have been observed in vitro, but have not been observed in primary patient samples. CSF2RB R461C is a rare germline variant and recent studies of germline cancer-predisposing mutations have not included CSF2RB11, possibly because of the lack of previous evidence showing the oncogenic potential of CSF2RB. Our data would support the inclusion of CSF2RB in future studies. CSF2RB R461C is a transforming mutation in vitro, and further investigation is necessary to determine its status as a predisposing or cooperative congenital mutation.
CSF2RB has previously been shown to become factor-independent through in vitro mutagenesis screens. One such screen successfully predicted R461C to be an active variant5 (though insufficient to transform Ba/F3 cells in a short-term withdrawal assay), demonstrating the potential for predictive mutagenesis screens. In spite of this in vitro evidence, there has never before been a reported case of cancer with a CSF2RB-activating mutation. We have demonstrated for the first time that a targetable CSF2RB variant found in a human leukemia confers factor-independent growth, receptor phosphorylation and accumulation, and constitutive JAK/STAT pathway activation. Our findings contribute to ongoing efforts to identify potential germline predisposition mutations in pediatric cancers, and are consistent with observations that genetic alterations activating cytokine receptor pathways are common in leukemia. Our research highlights the need for basic research to investigate and characterize the functional role of the CSF2RB transmembrane domain in signaling and recycling of the receptor. Finally, JAK inhibitors blocked the growth of R461C-transformed cells, thereby providing a therapeutic rationale to consider JAK inhibitors in any future cases of CSF2RB-activated leukemias.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Caroline Enns and Dr. William Skach, for guidance on the non-reducing PAGE experiments. We would like to thank David K. Edwards V (NSF GRFP DGE-1448072) and Marilynn Chow (Knight Cancer Institute) for critical review of the manuscript. This work was funded by a Howard Hughes Medical Institute Investigator award and NIH/NCI MERIT award (R37CA065823) to B.J.D., and NIH/NCI R00 award (5R00CA151670-04) to A.A. K.W.S. was supported by a T32 program training grant (5T32GM071338-08), Knight Cancer Institute stipend award and the Allan Price memorial ARCS scholar award. B.J.D. and J.T. are also supported by Leukemia Lymphoma Society (LLS) Beat AML program. J.P.P.M. was supported by KiKa foundation grants (2010-082, 2013-123) and the Dutch Cancer society (EMCR-KWF-20110-4691).
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHORSHIP CONTRIBUTIONS
K.W.S. designed and performed research, interpreted data, and wrote the manuscript. C.T. and A.A. designed research and edited the manuscript. J.P.P.M. supplied essential samples, provided critical feedback and reviewed the manuscript. B.J.D. and J.W.T. provided critical feedback and reviewed the manuscript.
Supplementary information is available at Leukemia’s website
REFERENCES
- 1.Broughton SE, Nero TL, Dhagat U, Kan WL, Hercus TR, Tvorogov D, et al. The betac receptor family - Structural insights and their functional implications. Cytokine. 2015 May 14; doi: 10.1016/j.cyto.2015.02.005. PubMed PMID: 25982846. Epub 2015/05/20. Eng. [DOI] [PubMed] [Google Scholar]
- 2.Hercus TR, Dhagat U, Kan WL, Broughton SE, Nero TL, Perugini M, et al. Signalling by the betac family of cytokines. Cytokine & growth factor reviews. 2013 Jun;24(3):189–201. doi: 10.1016/j.cytogfr.2013.03.002. PubMed PMID: 23535386. Epub 2013/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 3.D'Andrea R, Rayner J, Moretti P, Lopez A, Goodall GJ, Gonda TJ, et al. A mutation of the common receptor subunit for interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor, and IL-5 that leads to ligand independence and tumorigenicity. Blood. 1994 May 15;83(10):2802–2808. PubMed PMID: 8180376. Epub 1994/05/15. eng. [PubMed] [Google Scholar]
- 4.Hannemann J, Hara T, Kawai M, Miyajima A, Ostertag W, Stocking C. Sequential mutations in the interleukin-3 (IL3)/granulocyte-macrophage colony-stimulating factor/IL5 receptor beta-subunit genes are necessary for the complete conversion to growth autonomy mediated by a truncated beta C subunit. Molecular and cellular biology. 1995 May;15(5):2402–2412. doi: 10.1128/mcb.15.5.2402. PubMed PMID: 7739524. Pubmed Central PMCID: PMC230469. Epub 1995/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins BJ, Blake TJ, Gonda TJ. Saturation mutagenesis of the beta subunit of the human granulocyte-macrophage colony-stimulating factor receptor shows clustering of constitutive mutations, activation of ERK MAP kinase and STAT pathways, and differential beta subunit tyrosine phosphorylation. Blood. 1998 Sep 15;92(6):1989–2002. PubMed PMID: 9731057. Epub 1998/09/10. eng. [PubMed] [Google Scholar]
- 6.Tanaka T, Motoi N, Tsuchihashi Y, Tazawa R, Kaneko C, Nei T, et al. Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. Journal of medical genetics. 2011 Mar;48(3):205–209. doi: 10.1136/jmg.2010.082586. PubMed PMID: 21075760. Epub 2010/11/16. eng. [DOI] [PubMed] [Google Scholar]
- 7.Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. The New England journal of medicine. 2013 May 9;368(19):1781–1790. doi: 10.1056/NEJMoa1214514. PubMed PMID: 23656643. Pubmed Central PMCID: PMC3730275. Epub 2013/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic acids research. 2015 Jan;43(Database issue):D805–D811. doi: 10.1093/nar/gku1075. PubMed PMID: 25355519. Pubmed Central PMCID: Pmc4383913. Epub 2014/10/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Genomes Project C. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. 10/01/print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lek M, Karczewski K, Minikel E, Samocha K, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. bioRxiv. 2015 doi: 10.1038/nature19057. 2015-01-01 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. The New England journal of medicine. 2015 Dec 10;373(24):2336–2346. doi: 10.1056/NEJMoa1508054. PubMed PMID: 26580448. Pubmed Central PMCID: Pmc4734119. Epub 2015/11/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong F, Brynes RK, Tidow N, Welte K, Lowenberg B, Touw IP. Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. The New England journal of medicine. 1995 Aug 24;333(8):487–493. doi: 10.1056/NEJM199508243330804. PubMed PMID: 7542747. Epub 1995/08/24. eng. [DOI] [PubMed] [Google Scholar]
- 13.McMahon DP, Lance A, Price AE, Gerber JM, Baxter SA, Druhan LJ, et al. Congenital Neutrophilia Arising from a T618I Germline Mutation in the CSF3R Gene. Blood. 2015;126(23):1623. 2015-12-03 241 00:00:00. [Google Scholar]
- 14.Sokol L, Luhovy M, Guan Y, Prchal JF, Semenza GL, Prchal JT. Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood. 1995 Jul 1;86(1):15–22. PubMed PMID: 7795221. Epub 1995/07/01. eng. [PubMed] [Google Scholar]
- 15.Ding J, Komatsu H, Wakita A, Kato-Uranishi M, Ito M, Satoh A, et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood. 2004 Jun 1;103(11):4198–4200. doi: 10.1182/blood-2003-10-3471. PubMed PMID: 14764528. Epub 2004/02/07. eng. [DOI] [PubMed] [Google Scholar]
- 16.Zenatti PP, Ribeiro D, Li W, Zuurbier L, Silva MC, Paganin M, et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nature genetics. 2011 Oct;43(10):932–939. doi: 10.1038/ng.924. PubMed PMID: 21892159. Epub 2011/09/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjaer S, Kurokawa K, Perrinjaquet M, Abrescia C, Ibanez CF. Self-association of the transmembrane domain of RET underlies oncogenic activation by MEN2A mutations. Oncogene. 2006 Nov 9;25(53):7086–7095. doi: 10.1038/sj.onc.1209698. PubMed PMID: 16732321. Epub 2006/05/30. eng. [DOI] [PubMed] [Google Scholar]
- 18.Tyner JW, Yang WF, Bankhead A, 3rd, Fan G, Fletcher LB, Bryant J, et al. Kinase pathway dependence in primary human leukemias determined by rapid inhibitor screening. Cancer research. 2013 Jan 1;73(1):285–296. doi: 10.1158/0008-5472.CAN-12-1906. PubMed PMID: 23087056. Pubmed Central PMCID: PMC3537897. Epub 2012/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. Journal of cell science. 2004 Mar 15;117(Pt 8):1281–1283. doi: 10.1242/jcs.00963. PubMed PMID: 15020666. Epub 2004/03/17. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.