To the Editor
Type 2 immune responses are critically dependent on the canonical cytokines, IL-4 and IL-13, two related cytokines that both utilize IL-4Rα for signalling. These mediators have both overlapping and independent functions with IL-4 involved in the initiation of Th2 differentiation and immunoglobulin class switching and IL-13 in Th2 inflammatory responses (iTh2). Hallmarks of infections associated with type 2 immune responses are the infiltration of affected tissues by helper T cells, eosinophils and basophils, activation of macrophages, smooth muscle and tissue remodelling, and elevated levels of IgE. 1
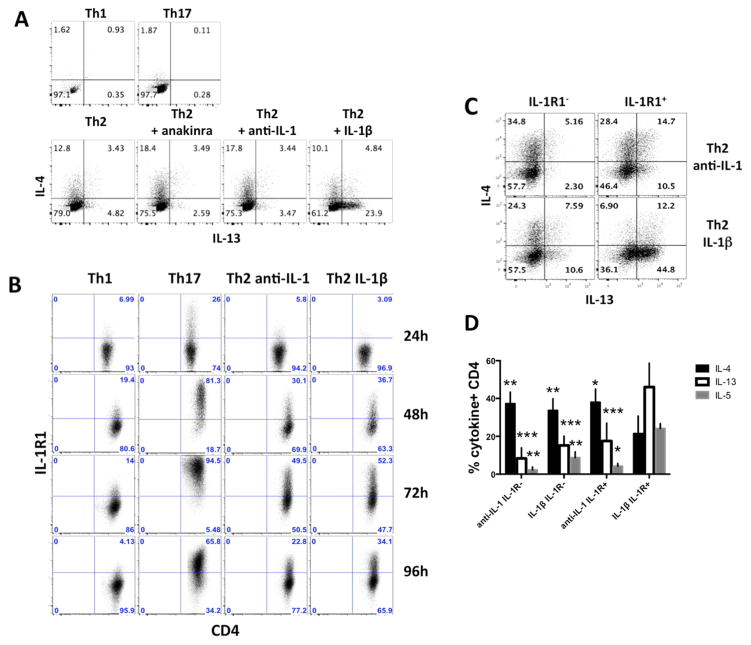
We have previously reported that IL-1β strikingly enhances CD4 T cell survival, antigen-driven expansion, differentiation and cytokine in vivo production. 2, 3 To investigate the impact of IL-1β on Th2 differentiation in greater detail, pigeon cytochrome c (PCC)-specific 5C.C7 CD4 T cells were cultured under Th2 polarizing conditions. Anti-IL-1α/β or IL-1Ra (anakinra) were included in control cultures to generate Th2 cells in the absence of IL-1 signalling for comparison to those exposed to IL-1β. IL-4 production was reduced in Th2 cells primed with IL-1β compared to the anti-IL-1 and anakinra groups; by contrast, IL-13 production was dramatically increased among cells primed with IL-1β (Fig. 1A). IL-1R1 was also detected on over 50% of Th2 cells at 72h and maintained at higher levels on Th2 IL-1β cells as compared to anti-IL-1 treated Th2 cells at 96h (Fig. 1B). To assess the contribution of IL-1R expression to this pattern of cytokine production, T cells were cultured for 2 days with anti-IL-1α/β or IL-1β, sorted for IL-1R1, and then placed back in their original culture conditions for 2 days before analysis (Fig. 1C). The data suggest that cells acquiring expression of IL-1R during early Th2 differentiation are especially susceptible to adopting a dominant IL-13 producing phenotype when exposed to IL-1 at this time. IL-5 expression showed similar enhancement in the IL-1R+, IL-1 group (Fig. 1D). Similar results were obtained using wild type and other TCR-Tg CD4+T cells suggesting that the phenomenon was not restricted to a single antigen or TcR (data not shown).
FIG. 1. IL-13 is increased and IL-4 decreased when Th2 cells are differentiated in presence of IL-1β.
A, IL-13 and IL-4 expression on Th1, Th17 and Th2 cells differentiated in the presence of IL-1β, anti-IL-1α/β or anakinra. B, Kinetic analysis of IL-1R1 expression. C, D, IL-13, IL-4 and IL-5 expression on Th2 groups sorted based on IL-1R1 expression. Statistical analyses were compared to the IL-1β IL-1R+ group.
To assess their phenotypic stability, both Th2 groups differentiated in vitro were sorted for IL-1R1 and either reprimed in vitro under neutral, Th1 or Th2 conditions or transferred in vivo into mice rechallenged intranasally (IN): Fig. E1A and B show that independently of the secondary condition, the original phenotypes were stably maintained.
Myd88 and NF-κB are involved in the IL-1R1 canonical signalling pathway. To analyze their contributions in our system, we cultured OT-II, OT-II IL-1R1−/− or OT-II Myd88−/− T cells together with wild type APCs. Fig. E1C shows that IL-1β-dependent enhancement of IL-13 production required both IL-1R1 and Myd88 expression on the responding CD4 T cells but not on APCs (data not shown). To test the contribution of NF-κB, Th2 cells ± anti-IL-1α/β were treated with an NF-κB activation inhibitor. No effect was observed with anti-IL-1, but IL-13 expression was significantly diminished and IL-4 production enhanced in the IL-1β group indicating a contribution of IL-1R/NF-κB pathway to the cytokine production phenotype we observe after IL-1β exposure (Fig. E1D).
Chromatin immunoprecipitation analysis of Il13, Il4 and Ifnγ promoter regions was carried out on both Th2 groups using antibody to the activating AcH3K27 modification (Fig. E2A). 4 Il13 HSII and Il13 HSIII, but not Il13 or Il4 HSIII showed a clear enhanced immunoprecipitation in the “IL-1β, IL-1R+” group. Thus, Th2 priming in the presence of IL-1β causes the promoter and second intron of Il13 to become more transcriptionally active.
Microarray (not shown) and RT-PCR analysis of both Th2 groups showed that Pth, IL-13, IL-5, Ccl17, Timd2, Slc15a3, Slc2a6 and Nts were more highly expressed in the “IL-1β, IL-1R+” group and IL-4, IL-10 and Myo6 were more highly expressed in the “anti-IL-1, IL-1R-” group. Parathyroid hormone (Pth) showed the greatest enhancement in the “IL-1β IL-1R+” group. Its expression has previously been reported in activated Th2 cells but not in Th1 cells (Fig. E2B). 5 We confirmed PTH secretion by ELISA and measured biologically active PTH via cAMP functional assay (Fig. E2C, D).
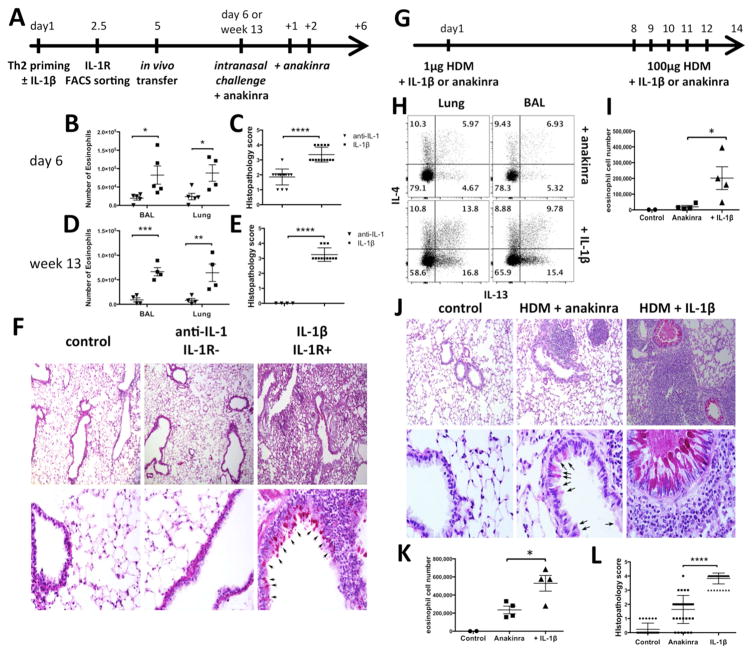
Th2 differentiation is central to cell recruitment, induction of inflammation and mucus production in the lungs. 6 Using PCC-specific 5C.C7 CD4+T cells, lymphocytes from the anti-IL-1 treated and the IL-1β exposed groups were sorted for IL-1R1, adoptively transferred into wild type mice and the mice challenged IN with antigen + anakinra only - to avoid any potential secondary effect of endogenous IL-1β (Fig. 2A). The number of eosinophils in the bronchoalveolar lavage (BAL) and in the lungs were significantly greater in mice that received IL-1R+cells primed with IL-1β. These differences were maintained when the mice were challenged 13 weeks after transfer (Fig. 2B, D). PAS staining of lung sections shows greater peribronchial infiltration of inflammatory cells, goblet cells metaplasia and striking enhancement of mucus production (magenta) in the “IL-1β, IL-1R+” group with an average histopathology score significantly higher than the controls (Fig. 2C, E, F).
FIG. 2. iTh2 cells induce lung hyper eosinophilia and goblet cell metaplasia.
A–F, Wild type mice were transferred with 5C.C7 Th2 cells sorted as in Fig. 1C and rechallenged IN. B, D, I, K, Eosinophils count after IN challenge. C, E, F, J, L, Lung sections stained with PAS, and scored for inflammation as described in Methods. Arrows indicate mucus-producing cells (magenta). Wild type (G–I) and RORγt−/− mice (J–L) were sensitized with HDM + anakinra or IL-1β and rechallenged IN. H, CD44hi CD4+ T cells were analyzed for IL-13 and IL-4 expression.
To examine the possible role of IL-1β during an in vivo house dust mite Th2 allergic response, wild type mice were sensitized IN with HDM + anakinra or IL-1β, and rechallenged 7 days later for 5 consecutive days (Fig. 2G). 7 IL-13 expression by CD44hi effector CD4+ T cells in the lung and in the BAL was dramatically increased when HDM priming was accompanied by IL-1β (Fig. 2H). In this model, IL-4 production was also slightly enhanced. Additionally, as anticipated from the increased IL-13 production, the number of infiltrating eosinophils was increased in the BAL of the HDM + IL-1β group (Fig. 2I). To exclude possible IL-1 enhancement of IL-17 priming, RORγt−/− mice were used 8: histological analysis revealed striking peribronchial inflammatory cell infiltrates, an increase of mucus production (magenta) and goblet cell metaplasia in IL-1β-treated mice and an average score of inflammation higher compared to the anakinra control (Fig. 2J–L). These results indicate that IL-1β present during in vivo Th2 priming causes increased allergic effector responses. Subsequently, IL-1β blockade maintains low levels of IL-13, limiting the infiltration and mucus production.
Based on these findings we propose that natural Th2 priming that occurs in inflammatory settings where IL-1β is present gives rise to iTh2 cells that are specialized to induce allergic inflammatory responses, while those primed in its absence are more important as regulatory cells, i.e. amplifiers of Th2 cells and antibody response by B cells. Signalling through the IL-1 receptor during priming is the major determinant of the distinctive phenotypes of these two types of Th2 cells and translates into differences in in vivo inflammatory responses.
Material & Methods
Mice
C57BL/6 and B6 RORγt−/− mice were obtained from Jackson Laboratory, C57BL/6 OT-II Rag2−/− CD45.1, B6 OT-II Rag2−/− IL-1R1−/−, B10.A, B10.A 5C.C7 Rag2−/− CD45.1, BALB/c DO11.10 Rag2−/− CD45.1 and BALB/c mice were obtained from Taconic Farms Germantown, NY. B6 OTII-Myd88−/− mice were obtained from Ryoji Yagi. All mice were housed under specific pathogen-free animal conditions at the National Institute of Allergy and Infectious Diseases (NIAID), and used between 6 and 12 weeks of age in accordance with guidelines provided by the Institutional Animal Care and Use Committee of the NIAID.
In vitro T cell differentiation
cell differentiation was performed as previously described9. Briefly, naive CD4 T cells were cultured with irradiated T-depleted splenocytes or DCs in presence of their cognate peptide with combinations of antibodies and cytokines for 4–5 days: IL-2, IL-12 and anti-IL-4 for Th1 differentiation; IL-2, IL-4, anti-IFN-γ and anti-IL-12 for Th2 and anti-IL-4, anti-IFN-γ, anti-IL-12, IL-6, TGFβ, and IL-21 for Th17 differentiation. Th2 cells were rested in IL-2 supplemented medium prior in vivo transfer when required. When specified a combination of 1μg/ml anti-IL-1αβ + 1μg/ml anti-IL-1β (R&D Systems) or IL-1β 10ng/ml Peprotech) was used.
In some experiments, 6-Amino-4-(4-phenoxyphenylethylamino) quinazoline (Calbiochem), NF-κB activation inhibitor was also added. To generate IL-1R- and IL-1R+ cells, Th2 cells primed for 2 days in the presence of anti-IL-1α/β or IL-1β were washed and resuspended with fresh Th2 medium to remove IL-1β bound to the IL-1R. 14h later, activated TCR-Tg CD4 T cells were harvested, sorted for IL-1R1 expression amplified with PE FASER kit Miltenyi) and put back in the original culture medium.
Secondary culture
Th2 cells primed for 5 days in the presence of anti-IL-1α/β or IL-1β were reprimed under neutral (IL-2 + 1μM PCC with T-depleted irradiated APC), Th1, or Th2 conditions.
Cell transfer and in vivo immunization
0.5 to 1 million cells were transferred intravenously and mice were immunized intranasally with 100μg ovalbumin or pigeon cytochrome c (Sigma) + 25μg lipopolysaccharide InVivogen). For cytokine instillation, mice were given 1μg of recombinant IL-1β Peprotech) or 500μg IL-1Ra (anakinra, Kineret) intranasally. For induction of allergic asthma, mice were sensitized intranasally with 1μg HDM + IL-1β or anakinra, and rechallenged with 100μg HDM + IL-1β or anakinra for 5 consecutive days 6 days.
Isolation of Lung cells
Lungs were extensively perfused with PBS before being harvested. Lungs were minced with gentleMACS dissociator (Miltenyi Biotec) and digested in Liberase TM with DNase I Invitrogen) for 30 minutes at 37°C. The digested tissue was processed on a 40μm cell strainer (BD Biosciences), and a single cell suspension was enriched on a 40/60% Percoll gradient centrifugation (GE Healthcare). Broncho alveolar fluid was collected by 5 consecutive washes with PBS+BSA.
Flow Cytometry
Lymph nodes, BAL, spleen and lungs were harvested and single-cell suspensions were prepared. For intracellular staining, cells were stimulated with 1μM ionomycin and 10ng/ml PMA for 4–6h and 5μM of monensin. Cell were stained with live/dead (In vitrogen), CD4 (RM4.5), CD44 (IM7), CD45.1 (A20), CD45.2 (104), IL-2 (JES6-5H4), IL-4 11B11), IL-5 (TRFK5), IL-17A (TC11-18H10), IFN-γ (XMG1.2) (BD Bioscience), IL-13 eBio13A) (eBioscience) antibodies. IL-1R1 (JAMA 174) antibody was purchased from BioLegend, and stained using Faser Kit-PE amplification system (Miltenyi). Data were collected with BDLSRII and analyzed with FlowJo (TreeStar).
Immunohistochemistry and Histopathology
Lung lobes were perfused and immersed in 5% Formalin. Samples were prepared and stained with periodic acid schiff (HistoServe, Germantown, MD; American Histolab, Gaithersburg, MD). Images were acquired using a Leica Episcope inverted microscope, and processed with Leica LASX software. Siglec-F+ CD11b+ CD11c-expressions were used to identify eosinophils.
Histopathologic scoring system was developed as follows: (0) normal lungs; (1) minor perivascular inflammation around large blood vessels; (2) moderate perivascular and peribronchial inflammation, minimal evidence of goblet cell hyperplasia; (3) increased perivascular and peribronchial inflammation with increased goblet cell hyperplasia beginning in smaller airways; (4) severe formation of perivascular, peribronchial, and interstitial inflammation as well as goblet cell hyperplasia in small and large airways. Grading was performed blinded on unidentified sections.
PTH ELISA
Th1, Th2, Th17, iTreg CD4 T cells primed for 4 days were stimulated with plate bound anti-CD3 (10μg/ml)/anti-CD28 (10μg/ml). Supernatants were collected 24h later and tested for PTH production by ELISA kit according to manufacturer’s recommendation (Immutopics).
cAMP fluorescence resonance energy transfer (FRET) assay
Th1 or Th2 supernatants were mixed with serum-free DMEM containing IBMX and Ro 20–1724 (Sigma, MO). hPTH1R-transfected HEKP7 cells were treated with 30μl of this mix at 37C for 1h. cAMP-d2 and cAMP-kryptate were added for 1h. cAMP was measured using FRET at 665nm and 620nm (cisbio, MA).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as previously described10. In brief, cells were extensively washed with 0.5% BSA/PBS and then resuspended in digestion buffer (Roche). Cells were incubated with 5U of Micrococcal Nuclease (MNase, New England BioLabs) and sonicated to break chromatin to about 200–500bp. The chromatin preparation was dialyzed against RIPA buffer, incubated with anti-acetylated histone 3 H3K27 or control IgG (Abcam), and then precipitated with Dynabeads protein A beads (Life Technologies). The immunoprecipitated DNA was purified and quantified using real-time PCR with SYBRR Green reagents (ABI).
Primers Il13 HSI: 5′-GCC-CCT-CAA-GAC-AAG-CAG-AA-3′ and 5′-ATC-GAC-CCC-ATC-TCC-CGT-TA-3′. Il13 HSII: 5′-CCC-CTG-GTC-TCT-GCT-TTG-TT-3′ and 5′-CTG-GAA-ACC-CTG-TCC-CAG-AC-3′. Il13 HSIII: 5′-GCC-TAG-AAT-GTC-GGG-GCT-TT-3′ and 5′-GTA-GCC-TAG-GCC-AGC-CAA-AA-3′. Ifnγ promoter: 5′-CGA-GGA-GCC-TTC-GAT-CAG-GT-3′ and 5′-GGT-CAG-CCG-ATG-GCA-GCT-A-3′.
Microarray and Real-time PCR
For microarray analysis, RNA was prepared from Th2 anti-IL-1α/β, IL-1R- and Th2 IL-1β, IL-1R+ cells using the Qiagen RNeasy Mini kit. Total RNA was sent to microarray research facility at NIAID Research Technologies Branch for hybridization using the Illumina BeadChip platform.
For RT-PCR, total RNA was purified from in vitro cultures according to the manufacturer’s protocol (Life Technologies). Reverse transcription was performed with oligo(dT)20 primers. TaqMan probes were used for measurement of various gene’s expression, and mRNA relative expression was adjusted to Gapdh (Mm03302249_g1; Life Technologies). Primers: mPth (Mm01271501_m1 Mm00451600_g1), mNts (Mm00481140_m1), mIl13 (Mm00434204_m1), mIl5 (Mm00439646_m1), m Slc15a3 (Mm00491666_m1), mSlc2a6 (Mm00554217_m1), mCcl17 (Mm00516136_m1), mTimd2 (Mm00506693_m1), mIl10 (Mm00439614_m1), mIl4 (Mm00445259_m1), mMyo6 (Mm00500651_m1), mIl1r1 (Mm00434237_m1), mgata3 (Mm00484683_m1).
Statistical analysis
Sample sizes were determined empirically. Differences between data sets of similar variance were analyzed by unpaired two-tailed Student’s t-test. A p value <0.05 was considered significant. ns: p> 0.05; *: p ≤ 0.05; **: p≤ 0.01; ***: p≤ 0.001; ****: p≤ 0.0001
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoruts A, Osness RE, Jenkins MK. IL-1 acts on antigen-presenting cells to enhance the in vivo proliferation of antigen-stimulated naive CD4 T cells via a CD28-dependent mechanism that does not involve increased expression of CD28 ligands. Eur J Immunol. 2004;34:1085–90. doi: 10.1002/eji.200324170. [DOI] [PubMed] [Google Scholar]
- 4.Guo L, Hu-Li J, Paul WE. Probabilistic regulation of IL-4 production in Th2 cells: accessibility at the Il4 locus. Immunity. 2004;20:193–203. doi: 10.1016/s1074-7613(04)00025-1. [DOI] [PubMed] [Google Scholar]
- 5.Young N, Mikhalkevich N, Yan Y, Chen D, Zheng WP. Differential regulation of osteoblast activity by Th cell subsets mediated by parathyroid hormone and IFN-gamma. J Immunol. 2005;175:8287–95. doi: 10.4049/jimmunol.175.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–47. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Fitch FW, Gajewski TF, Hu-Li J. Production of TH1 and TH2 cell lines and clones. Curr Protoc Immunol. 2006;Chapter 3(Unit 3):13. doi: 10.1002/0471142735.im0313s72. [DOI] [PubMed] [Google Scholar]
- 10.Cuddapah S, Barski A, Cui K, Schones DE, Wang Z, Wei G, et al. Native chromatin preparation and Illumina/Solexa library construction. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.prot5237. pdb prot5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.