Alterations in genes encoding epigenetic regulators are common in myeloid malignancies, and several recent studies have demonstrated that these mutations are present at high frequencies within peripheral blood cells in approximately 10% of individuals over 60 years of age. Although the presence of these mutations carries an increased risk of subsequent hematologic malignancies, the vast majority of individuals do not progress clinically and the natural history of clonal hematopoiesis is unclear.1-3 Thus, the term Clonal Hematopoiesis of Indeterminate Potential (CHIP) was proposed.4
Allogeneic bone marrow transplantation (alloBMT) remains the only curative therapy for many patients with hematologic malignancies. Over the past decade, advances in alloBMT have permitted older patients to successfully undergo the procedure. Accordingly, older matched sibling donors are being utilized. Moreover, the development of safe and effective haploidentical BMT allows parents to serve as related donors for their children.5 We hypothesized that clinically silent “premalignant” clones (which do not result in overt malignancy under homeostatic conditions within the donor) may be subjected to substantial proliferative and self-renewal stress during the engraftment process and could undergo transformation. We report 2 cases of donor cell leukemia (DCL) arising from CHIP marked by somatic mutations in leukemia-related genes in donors over age 60.
The study was approved by the Institutional Review Board at the Johns Hopkins University. Clonality was detected by a custom leukemia DNA sequencing panel covering 637 genes important in oncogenesis (Supplementary Methods).
From 2011-2014, we used 61 bone marrow donors >60 years-of-age at our institution (Supplementary Table 1). Median and maximum follow-up were 389 and 1481 days, respectively. Two recipients developed DCL for a cumulative incidence of 6.3% (95% CI: 0.94%, 19.6%). Donors and recipients characteristics are in Supplementary Tables 1 and 2. Patient 1 was diagnosed with therapy-related AML and received two rounds of induction chemotherapy followed by myeloablative, haploidentical allo-BMT from his 68-year-old mother. The patient's course was complicated by primary graft failure and he subsequently underwent salvage nonmyeloablative haploidentical peripheral blood stem cell transplantation from the same donor. The bone marrow biopsy at 2.5 years showed focal erythroid dysplasia and no increased blasts (Supplementary Figure 1a). His routine 3-year post-BMT marrow revealed erythroid dysplasia and a rare (1%) population of abnormal myeloblasts identified by flow cytometry (Supplementary Figure 1b). The bone marrow biopsy 1 month later revealed >30% myeloid blasts, a normal female karyotype (Supplementary Figure 1c) and full donor chimerism, consistent with DCL (Figure 1d).
Figure 1.
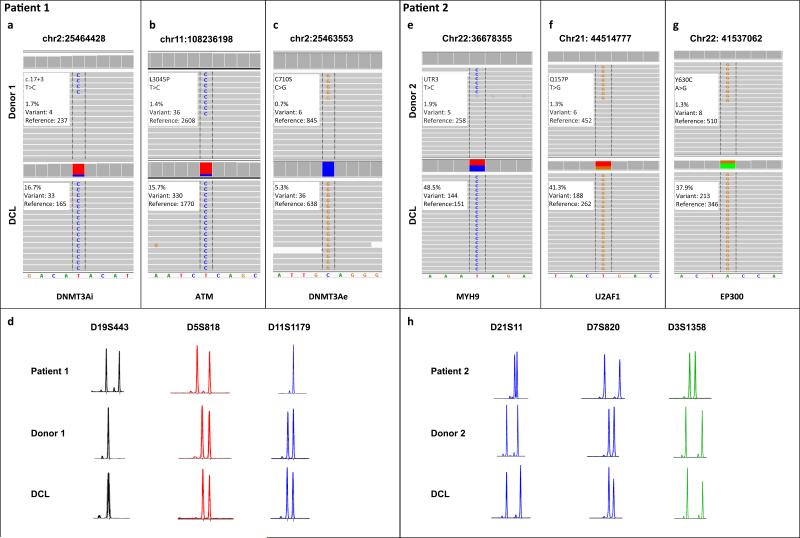
Somatic mutations in donor cells prior to alloBMT and in DCL detected by targeted sequencing. (a) Patient/donor 1; the mutation in DNMT3A chr2:25464428T>C (DNMT3Ai) was present in donor cells pre-transplant and at a 10 fold higher frequency in the DCL sample. (b) An ATM L3045P (ATM) mutation was also detected in donor cells and the DCL at a similar frequency to DNMT3Ai. (c) Another mutation in DNMT3A C710S (DNMT3Ae) was found in both samples at a frequency lower than the previous 2 mutations suggesting the presence of a second, lower frequency clone. (d) Short tandem repeat (STR) analysis of donor and patient DNA prior to transplant along with DNA extracted from the DCL confirmed that leukemia originated from the donor. STR markers used are shown. (e-g) Patient/donor 2; somatic mutations in MYH9, U2AF1 and EP300 identified at low frequencies in donor's peripheral blood and at 25 fold higher frequencies in the recipient at the time of DCL diagnosis. (h) STR analysis confirmed the donor origin of leukemia. Representative images from Integrative Genomics Viewer (Broad Institute) as well as a description of mutations and the clone frequencies are shown.
Patient 2 was diagnosed with a stage IIIB B-cell lymphoma. The patient eventually achieved a complete remission and underwent a nonmyeloablative, haploidentical allo-BMT with 8 cycles of post-BMT rituximab maintenance. Patient's 61-year-old mother was utilized as a donor. His post-transplant course was complicated by protracted late-onset neutropenia and thrombocytopenia. Bone marrow biopsy at 13 months was unremarkable (Supplementary Figure 1d). Cytopenias persisted; repeat bone marrow biopsy 18 months post-BMT showed significant erythroid dysplasia, monosomy 7 in one third of the cells (Supplementary Figure 1e) and full donor chimerism consistent with myelodysplastic syndrome (MDS) of donor origin (Figure 1h).
To identify the presence of clonal hematopoiesis in donors, which may have contributed to DCL, we compared next-generation DNA sequencing (NGS) datasets from donors and paired DCL samples in order to detect variants that expanded >3 fold from donor to DCL. Using this approach we identified low frequency clones marked by somatic mutations in both older donors. In donor 1 two mutations affected DNMT3A were found: a missense mutation in exon 18, codon 710 (chr2:25463553C>G p.C710S, referred to as DNMT3Ae; variant allele frequency (VAF)=0.7%) and an intronic mutation (chr2:25464428T>C, referred to as DNMT3Ai) (VAF=1.7%). The location of the latter within the conserved splice donor site raises the possibility of aberrant splicing and altered protein function. The third mutation was found in ATM (chr11:108236198T>C, p.L3045P, VAF=1.4%). In donor 2, three mutations were identified: a missense mutation in U2AF1 (chr21; 44514777T>G, p.Q157P; VAF=1.3%) and EP300 (chr22; 41537062A>G, p.Y630C; VAF= 1.3%), along with a mutation in the 3’ untranslated region of MYH9 (chr22; 36678355T>G; VAF=1.9%). In donor 1, clonal expansion was likely driven by alterations in the DNMT3A gene. The further dominance of one of the clones was likely facilitated by the acquisition of a cooperating ATM mutation. In donor 2, similar frequencies of the mutations suggest a close temporal acquisition of all 3 mutations (Figure 1e-g and Supplementary Table 3). Despite the retrospective identification of CHIP, donors 1 and 2 have not developed any clinically evident hematological diseases during nearly 5 years and 2 years of follow up, respectively.
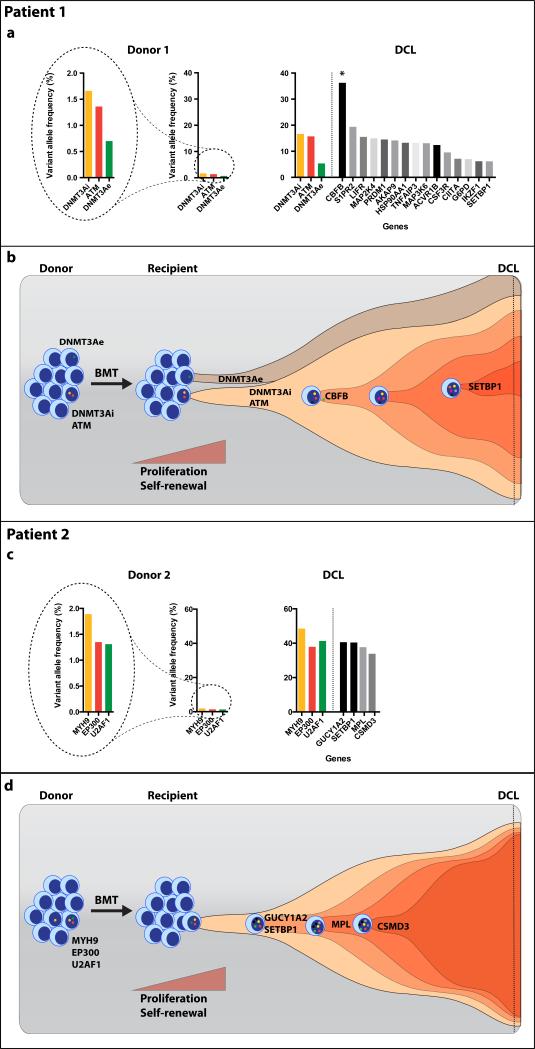
Patient 1 was diagnosed with DCL 3.5 years post-alloBMT. NGS analysis of DCL identified 18 somatic mutations in 17 genes including 3 mutations present in donor pre-alloBMT (Supplementary Table 3). Although both donor clones significantly expanded in the recipient, the VAF of the clone carrying DNMT3Ai and ATM mutations increased more than 10 fold (VAF=17% and 16% respectively) compared to 7 fold for the minor clone with DNMT3e mutation (VAF=5%) (Figure 1a-b and Figure 2a). Moreover, the leukemic blast percentage of about 30% was consistent with the AML arising from the premalignant clone with heterozygous mutations in DNMT3Ai (VAF=17%) and ATM (VAF=16%). The blasts appeared to have acquired an additional mutation in CBFB (VAF=36%). Further clonal evolution was marked by acquisition of cooperating mutations involving CSF3R, S1PR2, and SETBP1 among others (Figure 2b). The VAF of the CBFB mutation was nearly double the VAF of the DNMT3Ai/ATM clone suggesting either deletion of the wild-type CBFB or biallelic mutation. Metaphase karyotyping revealed a normal female karytope both in the donor cells prior to alloBMT and at DCL diagnosis. Single nucleotide polymorphism array (SNP-A) revealed acquired copy-neutral loss of heterozygosity (CN-LOH) spanning chromosome 16q, the location of CBFB (Supplementary Figure 2). This explains the biallelic CBFB mutation and suggests it is in the same clone as the antecedent DNMT3Ai and ATM mutations.
Figure 2.
Evolution of premalignant clones identified in the donors. (a) Patient/donor 1; VAFs of the mutations found in donor's peripheral blood and the DCL The star indicates biallelic mutation in CBFB gene due to CN-LOH of chromosome 16q. The scale was adjusted to better visualize the difference in VAF in donor cells pre-alloBMT (left). (b) Two clones were present in the donor pre-alloBMT. One clone carried ATM and DNMT3Ai while the other, lower frequency clone, harbored a sole DNMT3Ae mutation. Cell extrinsic stress related to BMT and hematopoietic reconstitution resulted in the expansion of both clones. Further malignant transformation of the clones carrying DNMT3Ai and ATM mutations via acquisition of CBFB mutation followed by cooperating mutations resulted in DCL. (c) Patient/donor 2; VAFs of the mutated genes in donor's blood and the recipient's bone marrow at the time of DCL. (d) Low-frequency clone marked by MYH9, EP300 and U2AF1 mutations, present in donor's bone marrow, acquired additional, cooperating somatic changes allowing for its expansion that manifested clinically as DCL.
Patient's 2 post-alloBMT course was complicated by protracted pancytopenia and bone marrow at 18 months showed significant erythroid dysplasia and monosomy 7. NGS analysis revealed 7 somatic mutations including 3 mutations present in donor pre-alloBMT (Supplementary Table 3). Three mutations identified in the donor expanded more than 25 fold (Figure 1e-g and Figure 2c). The evolution of DCL was also associated with the acquisition of cooperating mutations in GUCY1A2, CSMD3, MPL and SETBP1. Our data suggest that premalignant donor-derived clones expanded within the recipient, and acquired additional mutations causing malignant transformation (Figure 2d). The donor origin of leukemia was confirmed by STR polymorphism analysis (Figure 1d and 1h).
DCL was first described in 1971, and for years was considered rare.6 The pathogenesis of DCL has been unclear; some cases may have unrecognized clonal disorders in the bone marrow donors7, 8 or inherited defects when cord blood is used.9 The frequency of DCL appears to be steadily increasing, perhaps in part a result of routine and sensitive post-BMT chimerism testing.10 However, the utilization of older donors who are more likely to carry clonal hematopoiesis may also account for the increase. Our study describes the molecular evolution of CHIP within the donors into DCL. Somatic mutations in MDS-associated genes like U2AF1 and DNMT3A along with ATM, MYH9 and EP300 resulted in their clonal expansion. Previous studies suggest that alterations in DNMT3A and U2AF1 are the first genetic events to arise within clonal hematopoiesis and likely represent true drivers/predisposing mutations in the oncogenic process.11 Animal studies have shown that the loss of DNMT3A impaired differentiation and up-regulation of multipotency genes in hematopoietic stem and progenitor cells (HSPC).12 Similarly, mutated U2AF1 resulted in cytopenias and the expansion of HSPC in mice.13 Although necessary for initial clonal expansion, these changes were insufficient for oncogenic transformation as the donors remain hematologically normal nearly 5 and 2 years since transplantation. In the recipients, the change from homeostatic to regenerative hematopoiesis after alloBMT may have driven further clonal expansion and eventually oncogenic transformation. The use of alkylating agents like post-transplant cyclophosphamide (PTCy) as GvHD prophylaxis may have contributed to genotoxic stress and acquisition of additional mutations and structural chromosomal changes like deletion 7 or CN-LOH of 16q. However, we do not believe PTCy is necessary for development of DCL since Patient 1 did not receive it after his 2nd transplant, and because the incidence of DCL is similar in our cohort who received PTCy to those who did not.5 The transformation appeared to be associated with acquisition of cooperating mutations followed by a series of subclonal events such as SETBP1 mutations that are late progression events in myeloid malignancies.14
The acquisition of genetic abnormalities leading to clonal dominance has been demonstrated in the transformation of MDS to secondary AML.15 Our study tracks changes from subclinical clonal hematopoiesis to malignancy and confirms the propensity of specific mutated clones to evolve to MDS and leukemia. Similar to other premalignant conditions the latency period may span years or even decades, such that the majority of patients are unlikely to develop overt disease. This time frame may radically shorten under cell-extrinsic stress uniformly associated with the post-BMT reconstitution of donor-derived hematopoiesis. Thus, meticulous assessment of older donors beyond routine hemograms, bone marrow morphology and traditional cytogenetics should be considered. Moreover, formal studies in alloBMT patients and their donors are needed to further understand the incidence of clonal donor hematopoiesis leading to DCL.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Health (P01 CA015396) (R.J.J) and (P30 CA006973), Edward P. Evans Foundation (L.P.G) and the Commonwealth Foundation. Next generation sequencing was performed with the support of the Next Generation Sequencing Center in the Sidney Kimmel Comprehensive Cancer Center.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Authorship contributions
L.P.G., G.Z., C.D.G., G.G., W.M. and R.J. designed the study. A.E.D. and M.L. helped with regulatory oversight and data collection. L.P.G. and N.T. were responsible for clinical data collection. G.Z., J.E. and C.D.G. analyzed the bone marrow morphology, flow cytometric, cytogenetic and bone marrow engraftment data. V.Y. performed next-generation DNA sequencing. R.V., G.Z. and M.L. performed the statistical analysis. L.P.G., G.Z., W.M., C.D.G. and R.J. wrote the manuscript which was edited and approved by all authors.
Supplementary Information is available at the Leukemia's website (http://www.nature.com/leu)
References
- 1.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014 Dec 25;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014 Dec 25;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014 Dec;20(12):1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015 Jul 2;126(1):9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasamon YL, Bolanos-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of Nonmyeloablative HLA-Haploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. J Clin Oncol. 2015 Oct 1;33(28):3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fialkow PJ, Thomas ED, Bryant JI, Neiman PE. Leukaemic transformation of engrafted human marrow cells in vivo. Lancet. 1971 Feb 6;1(7693):251–255. doi: 10.1016/s0140-6736(71)90998-6. [DOI] [PubMed] [Google Scholar]
- 7.Tilson MP, Jones RJ, Sexauer A, Griffin CA, Morsberger LA, Batista DA, et al. Targeted pathologic evaluation of bone marrow donors identifies previously undiagnosed marrow abnormalities. Biol Blood Marrow Transplant. 2013 Aug;19(8):1254–1259. doi: 10.1016/j.bbmt.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda T, Ueno T, Fukumura K, Yamato A, Ando M, Yamaguchi H, et al. Leukemic evolution of donor-derived cells harboring IDH2 and DNMT3A mutations after allogeneic stem cell transplantation. Leukemia. 2014 Feb;28(2):426–428. doi: 10.1038/leu.2013.278. [DOI] [PubMed] [Google Scholar]
- 9.Shiozaki H, Yoshinaga K, Kondo T, Imai Y, Shiseki M, Mori N, et al. Donor cell-derived leukemia after cord blood transplantation and a review of the literature: differences between cord blood and BM as the transplant source. Bone Marrow Transplant. 2014 Jan;49(1):102–109. doi: 10.1038/bmt.2013.127. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman DH. Donor cell leukemia: a review. Biol Blood Marrow Transplant. 2011 Jun;17(6):771–789. doi: 10.1016/j.bbmt.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012 Jul 20;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012 Jan;44(1):23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirai CL, Ley JN, White BS, Kim S, Tibbitts J, Shao J, et al. Mutant U2AF1 Expression Alters Hematopoiesis and Pre-mRNA Splicing In Vivo. Cancer Cell. 2015 May 11;27(5):631–643. doi: 10.1016/j.ccell.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thol F, Suchanek KJ, Koenecke C, Stadler M, Platzbecker U, Thiede C, et al. SETBP1 mutation analysis in 944 patients with MDS and AML. Leukemia. 2013 Oct;27(10):2072–2075. doi: 10.1038/leu.2013.145. [DOI] [PubMed] [Google Scholar]
- 15.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012 Mar 22;366(12):1090–1098. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.