Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset neurodegenerative disorder associated with premutation alleles of the FMR1 gene. Iron is essential for many facets of cell metabolism in the brain but when altered is likely to contribute to the development of neurodegenerative diseases. We previously reported that iron accumulates in the choroid plexus and the putamen in FXTAS, and that the level and distribution of key iron-binding proteins are also altered, suggesting a potential alteration of iron metabolism in the brain. Here we hypothesize that iron metabolism is also altered in the FXTAS cerebellum. To test this hypothesis we used cerebellum samples collected from FXTAS and control subjects and measured the amount of iron contained within the cerebellar cortex and dentate nucleus. We found that the number of iron deposits increased in the cerebellum of a subset of cases of FXTAS. This accumulation is likely to be mediated by factors other than – or in addition to – CGG-repeat coupled pathology. Thus, iron deposition in the cerebellum cannot be used as a hallmark of FXTAS pathogenesis.
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset neurodegenerative disorder associated with premutation alleles (55–200 CGG repeats) of the FMR1 gene. Expansions of more than 200 CGG repeats give rise to fragile X syndrome, the most common inherited form of cognitive impairment (1, 2). FXTAS is characterized by the presence of ubiquitin-positive inclusions in neurons and astrocytes. FXTAS symptoms include cerebellar ataxia, tremor, cognitive deficits, peripheral neuropathy, autonomic dysfunction, and psychiatric involvement.
Iron is essential for many facets of cell metabolism in the brain. For example, iron is required for the synthesis of myelin and neurotransmitters (3). On the other hand, uncomplexed iron reacts with molecular oxygen to generate the reactive oxygen species that lead to oxidative stress (4). Thus, altered brain iron metabolism is likely to initiate or contribute to the development of neurodegenerative diseases, such as Parkinson and Alzheimer diseases, amyotrophic lateral sclerosis, restless leg syndrome, and prion diseases (5). We previously reported that iron accumulates in the choroid plexus and the putamen in FXTAS, and that the level and distribution of key iron-binding proteins are also altered, suggesting a potential alteration of iron metabolism in the brain (6). FXTAS cases present with moderate cerebellar atrophy, white matter changes, Purkinje cell loss and Bergmann gliosis (7, 8). In addition, we recently described the presence of single and twin ubiquitin-positive inclusions in the Purkinje cells of a majority of FXTAS cases (in this volume). Based on this evidence, we hypothesize that iron metabolism is altered in the cerebellum in FXTAS cases. To test this hypothesis we used cerebellum samples collected from FXTAS and control subjects to measure the number of iron deposits contained within the cerebellar cortex and dentate nucleus.
Materials and Methods
Sample collection
Samples from 12 FXTAS subjects and 13 control subjects were obtained from the FXTAS brain repository at the University of California, Davis, School of Medicine. Additional control tissue was obtained from the Pathology Department at the University of California, Davis, Health System. Control tissue was obtained from subjects who did not have any significant neurological history. Tissue specimens were obtained through consented autopsies with institutional review board approval.
Perl’s staining
A block of cerebellar tissue (2 × 2 × 0.25 μm2), containing cortex and dentate nucleus for each case, was immersed in 20% sucrose (Fisher, USA) and embedded in OCT (Fisher). Blocks were cut into 12 μm sections using a cryostat. Sections were left to dry for 10–30 min at 37° C before being washed with deionized water. Slides were submerged in a solution containing a 1:1 ratio of 20% hydrochloric acid (Fisher) and 10% potassium ferrocyanide (Fisher) for 20 minutes at room temperature. The presence of blue deposits (iron bound to hemosiderin) was confirmed using a microscope (Nikon Eclipse E200). Slices were counterstained with nuclear fast red (Ricca Chemical Company, USA) for 5 min. Slides were washed with water, dehydrated with ethanol, and cleared with xylene before being coverslipped with Permount (Fisher).
Analysis
A 0.5 cm2 area of both the dentate nucleus and the cortex was imaged using a Keyence BZ9000 microscope. Each 0.5 cm2 image was a merge of 36 single 20x images. Analysis was conducted using the NIH program ImageJ. The desired blue color (iron) was measured and the percentage of area occupied by iron was compared between FXTAS and control subjects using correlation analysis. A p value of 0.05 was used for statistical significance.
Results
The age of FXTAS subjects ranged from 52 to 87 years with an average of 73.8 ± 3.0 (sem) years of age. Control subjects ranged from age 54 to 91 with an average of 73.4 ± 2.9 years of age. FXTAS cases encompassed 8 males and 4 females, while control cases included 5 males and 8 females. The number of CGG repetitions in the FXTAS group was between 59 and 113 with an average of 83.1 ±4.7 repetitions.
Iron deposits are present in the cerebellar cortex of a subset of FXTAS cases
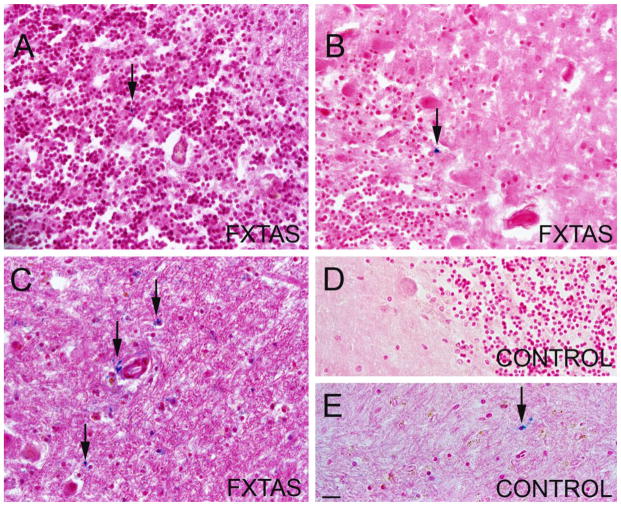
We used tissue blocks obtained from random samples of cerebellar cortex (containing molecular, Purkinje and granular layers) to study iron localization using Perl’s method for iron staining, which detects iron bound to hemosiderin (Figure 1), (9). We considered a case to have a significant amount of iron deposits when at least 0.01 % of tissue stained for iron (blue). We did not find iron deposits in the cerebellar cortex of any of the control case. Of the FXTAS cases evaluated, 33.3 % presented with a significant accumulation of iron deposits (Figure 1A,B). In these cases the iron deposits where occupying and average area of 0.08 % of tissue. One of the cases contained a particularly high amount of iron when compared to the rest of FXTAS cases, specifically 20 fold the amount of iron. The rest of the FXTAS cases were similar to control cases in that they did not contain a significant amount of iron deposits. We performed a correlation analysis between the presence of iron deposits and diagnosis and found a significant correlation between the presence of iron deposition and the presence of FXTAS in the cases, but not with control cases (p = 0.007). We did not find any correlation between FXTAS cases with iron deposits and age (p = 0.5), gender (p = 0.4), or number of CGG repetitions (p = 0.9). Iron was localized intracellularly in cells of the granular layer (Figure 1) and Purkinje cell layer (Figure 1B). No Purkinje cells were observed containing iron. No extracellular iron was detected.
Figure 1.
Perl’s (iron in blue) and H&E staining of cerebellar tissue. Arrows indicate iron deposits (blue). A. Molecular layer of a FXTAS case. A small iron deposit is indicated with an arrow. B. Molecular, granular, and Purkinje layers in a FXTAS case. The Purkinje layer presents with an iron deposit. C. Dentate nucleus in one of the FXTAS cases that present with an increased iron deposition. D. Molecular, granular, and Purkinje layers in a control cases. No iron deposition is present. E. Dentate nucleus in a control case with an average amount of iron deposits. Scale bar: 30 μm.
Iron accumulates in the dentate nucleus in a minority of FXTAS cases
We obtained (as described above) sections of cerebellar tissue containing dentate nucleus and stained with the Perl’s method for iron (Figure 1). We found that all FXTAS and control cases contained iron deposits in the dentate nucleus, and there was no significant difference in the amount of iron in FXTAS vs. control cases (FXTAS: 0.32 ± 0.18; CT: 0.059 ± 0.01; p = 0.12). However, we found that three of the 12 FXTAS cases presented with a greater amount of iron when compared to the rest of the FXTAS and control cases. These three cases contained, on average, 10 (0.5%), 22 (1.1%), and 44 fold (2.21 %) increase in iron accumulation when compared to the rest of the FXTAS cases, respectively. The incidence of increased iron accumulation in these three FXTAS cases was not correlated with age (p = 0.7), gender (p = 0.5), or number of repetitions (p = 0.9). The dentate nucleus is the major exit tract of the cerebellum and when lesion occurs leads to postural and action tremor. We correlated iron deposition increase within the cerebellar dentate nucleus with the presence or absence of tremor. Of the 12 cases included in this study we only had access to the clinical history of 7 of them. Of these 7 cases 3 presented with tremor and 4 did not. We did not find a significant correlation between increased iron deposition and tremor (p = 0.1). However, the clinical histories available included 2 of the 3 cases with a greater amount of iron accumulation and both presented with tremor.
Discussion
We previously demonstrated an increase of iron deposits in the choroid plexus and the putamen in FXTAS (6). Here we report mild alterations in iron levels in the cerebellar cortex in less than half of the of FXTAS cases we examined. We found that while control cases and most of FXTAS cases did not present with abnormal iron deposition, around 33% of FXTAS cases presented with a mild increase in the amount of iron deposits within the cerebellar cortex. The number and size of these iron deposits was very small when compared to those found in choroid plexus and putamen in FXTAS. All control and most FXTAS cases presented with a small amount of iron deposits in the dentate nucleus of the cerebellum, however a quarter of the FXTAS cases presented with a large increase in the quantity of these deposits. These cases were not advanced in age, nor did they present with a significant difference in the amount of CGG repetitions when compared to the remaining cases, however their clinical history indicated that they presented with tremor, while most of the remaining cases did not. These cases may represent a subpopulation of patients with FXTAS that are more susceptible than others to iron metabolism alterations in the cerebellum.
While it is possible that the expanded CGG transcripts sequester proteins that are important for regulating the production of iron-sequestering and iron-binding proteins, we have demonstrated that iron accumulation in the cortex of the cerebellum only occurs in a minority of the cases of FXTAS, unlike iron accumulation in the choroid plexus. Thus, the abnormal cerebellar iron deposition in these cases is likely to be influenced by other genetic or cellular mechanisms that are not directly linked to FXTAS pathogenesis (i.e., not linked to the expanded CGG repeat).
Diseases stemming from improper iron accumulation have been linked to neurotoxic events (i.e. oxidative stress) that trigger apoptosis, resulting in massive cell death. This phenomenon has been widely demonstrated in cases of neurodegenerative diseases, as in the substantia nigra in Parkinson’s disease (10). However, except for Purkinje cells, no loss of a specific cell type has been described in the FXTAS cerebellum, which is in agreement with our finding of a mild cerebellar accumulation of iron among the FXTAS cases. Neurodegenerative diseases that present with increased iron accumulation within the cerebellum do not present with iron deposits within Purkinje cells, consistent with the idea that this cell population is not thought to be target of redox metal accumulation, as is seen in Alzheimer disease (11). Therefore, the current results suggest that Purkinje cell death in FXTAS and other neurodegenerative diseases may be caused by factors other than those arising through iron accumulation.
Conclusion
This preliminary study indicates that, whereas iron deposits accumulate in the cerebellum of a subset of cases of FXTAS, such accumulation is likely to be mediated by factors other than–or in addition to–CGG-repeat coupled pathology. Thus, iron deposition in the cerebellum cannot be used as a hallmark of FXTAS pathogenesis.
Acknowledgments
This work was supported by the National Institute of Health grants MH094681 (Dr. Martínez-Cerdeño), HD040661 (Dr. P Hagerman), HD036071 (covered brain collection), and by the Department of Pathology and Laboratory Medicine at UC Davis and the Shriners Hospitals.
Footnotes
Conflict of interest: PJH holds patents for assays of CGG-repeat expansion and for FMRP ELISA; he is also in non-remunerative collaborations with Pacific Biosciences, Inc. and Roche Diagnostics. Authors otherwise declare no conflict of interest.
References
- 1.Hagerman PJ. Current Gaps in Understanding the Molecular Basis of FXTAS. Tremor Other Hyperkinet Mov (N Y) 2012:2. doi: 10.7916/D80C4TH0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagerman RJ, Hall DA, Coffey S, Leehey M, Bourgeois J, Gould J, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3(2):251–62. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard JL, Connor JR. Iron status and neural functioning. Annual review of nutrition. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 4.Beard J. Iron deficiency alters brain development and functioning. The Journal of nutrition. 2003;133(5 Suppl 1):1468S–72S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 5.Batista-Nascimento L, Pimentel C, Menezes RA, Rodrigues-Pousada C. Iron and neurodegeneration: from cellular homeostasis to disease. Oxidative medicine and cellular longevity. 2012;2012:128647. doi: 10.1155/2012/128647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariza J, Steward C, Rueckert F, Widdison M, Coffman R, Afjei A, et al. Dysregulated iron metabolism in the choroid plexus in fragile X-associated tremor/ataxia syndrome. Brain research. 2015;1598:88–96. doi: 10.1016/j.brainres.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain : a journal of neurology. 2006;129(Pt 1):243–55. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 8.Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain : a journal of neurology. 2002;125(Pt 8):1760–71. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 9.McManus Mowry. Staining methods: histological and histochemical. Medical Division of Harper and Brothers; New York, NY: 1960. [Google Scholar]
- 10.Ayton S, Lei P. Nigral iron elevation is an invariable feature of Parkinson’s disease and is a sufficient cause of neurodegeneration. BioMed research international. 2014;2014:581256. doi: 10.1155/2014/581256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. Journal of Alzheimer’s disease : JAD. 2010;19(1):363–72. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]