Abstract
Background
While urine based testing for human papillomavirus (HPV) is being explored as a practical approach for cervical cancer screening, whether the results differ by age, race and indicators of excess body weight or in populations exposed to HPV vaccines have not been documented by previous studies. The purpose of the study was to determine the accuracy of urinary HPV testing for presence of cervical HPVs and high grade cervical intraepithelial lesions (CIN 2 and 3) by the above stated population characteristics.
Methods
Study population consisted of 502 women diagnosed with different grades of CIN. HPV testing was performed with paired urine and cervical cell DNA using Roche Diagnostics Linear Array. Agreement coefficient 1 (AC1) and probabilities were calculated to determine the accuracy of urinary HPV testing for the presence of cervical HPVs and CIN lesions.
Results
We observed a substantial to almost perfect agreement (0.66–0.83) in the detection of any HPV genotype in urine compared to cervical specimens irrespective of population characteristics. Although the PPV for detection of CIN lesions was relatively low, the NPV for CIN 3 was high (≥ 90%) among women positive for any of the urinary or cervical HR-HPV genotypes or HPV genotypes not included in currently available HPV vaccines.
Conclusions
Our results demonstrated that urine HPV testing provides highly satisfactory results for excluding the possibility of having any cervical HPV infections, including HPV types not included in vaccines and CIN lesions associated with any HR-HPV regardless of woman’s age, race and excess body weight.
Keywords: cervix, urine, HPV, CIN, accuracy
Precis
The purpose of the study was to determine the accuracy of urinary HPV testing for presence of cervical HPVs and higher grade cervical intraepithelial lesions. Our results demonstrated that urine HPV testing provides highly satisfactory results for excluding the possibility of having any cervical HPV infections, including HPV types not included in vaccines and CIN lesions associated with any HR-HPV regardless of woman’s age, race and excess body weight.
Background
Infection with carcinogenic or high risk human papillomaviruses (HR-HPVs), which are sexually transmitted, represent the most important risk factors for the development of cervical cancer (CC) as well as cervical intraepithelial neoplasia (CIN), precursor lesions for developing CC.1,2,3 More than 90% of CCs are associated with HR-HPV DNA.4 Studies that used improved HPV testing procedures have established HPV as a causative agent for CIN as well.5 Therefore, identification of women infected with HR-HPVs or with HR-HPV associated CIN lesions is of critical importance for preventing CC. Currently, CC is largely prevalent in the developing world where women are rarely screened for this disease. Testing for HPVs in samples other than cervical cells such as urine, a noninvasive and easy to-collect sample, could be more attractive to women because it bypasses medical examination, a significant barrier for women in the developing world because of socio-cultural or religious reasons. Further, urine HPV testing would offer a more cost effective option in lower-income countries that lack infrastructure for medical care. In developed countries such as in the USA, nearly 50% of CCs are found in women never screened, and an additional 10% of CCs occur among women not screened within the past five years.6,7 These observations suggest that it is important to understand the reasons for not receiving screening in this group of women. In developed countries, the body image disturbances may negatively affect preventive medical care such as screening for CC, not only because of avoiding screening care but also because of the difficulties in obtaining a proper sample such as pap smear if appropriate instruments such as a large vaginal specula is unavailable.8,9 Even though, testing for HPV in urine may be more acceptable in this situation, the possibility of this approach has never been evaluated. Since studies have documented that African American (AA) women are more satisfied with their bodies than are their Caucasian American (CA) counterparts10, it is also important to understand whether the accuracy of urinary HPV testing for the presence of cervical HPVs differ by race.
Currently there are three main ways to prevent CC, namely, HPV vaccination to prevent infection, continue to monitor vaccinated women for risk associated with non-vaccine HPV genotypes and screening of women already infected with HPVs to detect the disease at an earlier stage, when it is easier to prevent the progression of lesions. Since currently available prophylactic HPV vaccines (bivalent, quadrivalent and 9-valent) do not provide complete protection against HPVs, HPV-based screening tests are still needed in the management of vaccinated individuals who may get exposed to HPV genotypes not included in vaccines and develop CIN lesions associated with those HPV genotypes. A urine HPV test rather than a cervical HPV test would be more acceptable and cost-effective for this population as well.
A recent meta-analysis reported that the detection of HPV DNA in urine has good accuracy for the presence of cervical HPVs.11 This meta-analysis was unable to derive the accuracy of CIN prediction as only two studies reported relevant data.12 None of these previous studies have adequately evaluated whether the accuracy of urinary HPV testing for the presence of cervical HPVs differ by race or by indicators of excess body weight. Further, there are no reports on whether the prevalence of CIN lesions differ based on the presence of HPV genotypes detected in cervical vs. urine in a non-vaccinated or a vaccinated population. In addition, even though HPV testing has been approved by the FDA for primary screening of ≥25 year old females, no studies have evaluated whether the accuracy of urinary HPV testing for the presence of cervical HPVs differs by this age cut point.
Based on this background, the purpose of the study was to 1) determine HPV genotypes in paired specimens of cervical and urinary cell DNA and test the overall accuracy of urinary HPV testing for the presence of cervical HPVs and evaluate whether the accuracy differs by age, race, indicators of excess body weight (body mass index [BMI] and % body fat [%BF]) and CIN status; 2) determine whether the positive predictive value (PPV) and negative predictive value (NPV) of detecting CIN lesions differ based on the presence of HPV genotypes detected in cervical vs. urine and evaluate whether the results differ by age, race and by indicators of excess body weight; 3) determine whether the concordance between urine and cervical HPV genotypes differ if the currently available HPV prophylactic vaccines are effective in preventing those infections and 4) determine whether PPV and NPV of detecting CIN lesions differ based on the presence of HPV genotypes detected in cervical vs. urine if the currently available HPV prophylactic vaccines are effective in preventing those infections.
Methods
Study Population
The study population (N=502) included women enrolled by two studies funded by the National Cancer Institute (NCI) (R01CA105448 and R01CA102489). All women were diagnosed with abnormal cervical cells in clinics of the Health Departments in Jefferson County and surrounding counties in Alabama and were referred to the University of Alabama at Birmingham (UAB) for further examination by colposcopy. Women were 19–50 years old, had no history of CC or other cancer of the lower genital tract, no history of hysterectomy or destructive therapy of the cervix; were not pregnant, and were not using anti-folate medications. Based on Roche Diagnostics Linear Array, 63% and 37% were positive and negative for any HR-HPV in cervical samples, respectively. The distribution of CIN diagnosis of the population is the following: 106 women were diagnosed with CIN 2+ (cases, including CIN 2 [n=72], CIN 3 [n=34]) and 396 women were diagnosed with ≤ CIN 1 (non-cases, including normal cervical epithelium [n=23], HPV cytopathic effect [HCE, n=40], reactive nuclear enlargement [RNE, n=73] or CIN 1 [n=260]). The BMI was calculated using the height and weight measurements (weight kg/[height m]2). Height and weight measurements were obtained using standard protocols by the study personnel, reducing the potential for BMI misclassification compared with self-report. The % BF was measured using a TANITA-bioelectrical impedance analysis instrument (Model TBF-300A) and summarized as two categories, ≤ 33% or > 33%. The study protocols and procedures were approved by the UAB Institutional Review Board. Urine samples were collected before the collection of cervical cells. Exfoliated cervical cells collected with a cervical brush and immediately rinsed in 10 mL of PBS were kept cold until transported to the laboratory on ice within 2 hours of collection. In the laboratory, both cervical cell and urine samples were centrifuged and the resulting pellets were stored at −20°C until HPV detection assays were performed.
Detection and determination of HPV genotypes
DNA was extracted from cervical and urine cell pellets using the QIAamp MiniElute Media kit (Qiagen, Inc.) following the manufacturer’s instructions. HPV genotyping test (Linear array; Roche Diagnostics) was performed according to the manufacturer's instructions by a research associate trained by personnel from Roche Diagnostics. Briefly, target DNA was amplified by PCR using the PGMY09/11 L1 consensus primer system that included co-amplification of a human cellular target, β-globin that served as an internal control for adequate sample cellularity and extraction.
Detection and HPV genotyping were achieved using a reverse line-blot method, and this test included probes for 37 anogenital HPV genotypes [6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39, and CP6108]. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 were considered as HR-HPV genotypes and all other types as low risk HPV genotypes in this analysis.
Data Analysis
The differences in the demographic characteristics between cases and non-cases were tested using Chi-square test. We calculated the detection rate of HPV genotypes (any HPV, any HR-HPV and HPV 16 or 18) by diagnosis in paired specimens of cervical and urinary cell DNA and used McNemar’s test to determine the differences between paired proportions. The overall accuracy of urinary HPV testing for the presence of cervical HPVs was evaluated by using agreement statistics which included crude concordance, and the agreement coefficient 1 (AC1)13. AC1 rather than Cohen’s kappa, a commonly used measure of agreement, was used in our analysis because kappa is sensitive to uneven distributions of the categories. The presence of uneven category distributions, often the case with observational data such as ours can potentially result in a so-called kappa “paradox”14 where high crude agreement is associated to low or even negative kappa values. The AC1 statistic is an alternative that differs from kappa in the way chance agreement is computed and may be a more robust indicator of chance-corrected agreement with uneven distributions of categories. In our analysis, we have defined the degree of agreement based on the categorization as proposed by Landis and Koch15 < 0 no agreement, 0.01 to 0.2 slight agreement, 0.21 to 0.40 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 to 0.80 substantial agreement and 0.8 to 1.0 almost perfect agreement. All analyses were performed in the entire population and by categories of age (< 25 years and ≥ 25 years), race (CA and AA), indicators of excess body weight (BMI, < 25 kg/m2 and ≥ 25 kg/m2; % BF, < 33% and ≥ 33%) and case status (non-cases [≤ CIN 1] and cases [CIN 2+]). We determined the PPV, the probability of the presence of higher grade CIN lesions (CIN 2 or 3) when positive for any HR-HPV and the negative predictive value (NPV), the probability of the absence of CIN 2 or 3 when negative for any HR-HPV using HR-HPV genotype results of urine and cervical specimens in the entire population and by categories of age, race and indicators of excess body weight. The interpretation of PPV and NPV was based on a preset categorization scheme (≥ 90 high, ≥ 80-<90 moderately high, ≥ 70-<80 Moderate, < 70 relatively low).
Assuming that the currently available HPV vaccines have a 100% efficacy in preventing infections of HPV genotypes included in those vaccines, we assessed the accuracy of urine HPV testing for the presence of HPV genotypes in the cervix that are not included in the bivalent vaccine (negative for HPV 16 and 18), quadrivalent HPV vaccine (negative for HPV 6, 11,16 and 18) and the 9-valent vaccine (negative for HPV 6, 11,16,18, 31, 33, 45, 52 and 58) between paired specimens of urine and cervical using the crude concordance and AC1 agreement. We also calculated the detection rate of HPV genotypes by diagnosis in paired specimens of cervical and urine in these subsets of the population and also assessed the PPV and NPV of HR-HPV genotypes in detecting CIN lesions.
Results
The tabulation of demographic characteristics of study participants with respect to CIN status is shown in Table 1. We observed that the proportion of CIN 2 compared to ≤ CIN 1 did not differ by the categories of age, race and indicators of excess body weight. However, the proportion of CIN 3 compared to ≤ CIN 1 differed by race and indicators of excess body weight. The prevalence of CIN 3 was significantly higher among CAs compared to AAs and among women with BMI < 25 kg/m2 and % BF < 33 compared to BMI ≥ 25 kg/m2 or % BF ≥ 33.
Table 1.
Description of the study population (n=502)
| Demographic | ≤ CIN1c n=396 |
CIN 2d n=72 |
P-valuee | ≤ CIN1c n=396 |
CIN 3d n=34 |
P-valuee |
|---|---|---|---|---|---|---|
| Age ≥ 25 | 167 (85%) | 30 (15%) | 0.9363 | 167 (93%) | 13 (7%) | 0.6539 |
| < 25 | 229 (84%) | 42 (16%) | 229 (92%) | 21 (8%) | ||
| Race: African American | 254 (85%) | 44 (15%) | 0.6229 | 254 (95%) | 13 (5%) | 0.0028 |
| Caucasian American | 142 (84%) | 28 (16%) | 142 (87%) | 21 (13%) | ||
| BMI a: ≥ 25 kg/m2 | 246 (86%) | 41 (14%) | 0.5412 | 246 (94%) | 15 (6%) | 0.0356 |
| < 25 kg/m2 | 148 (84%) | 29 (16%) | 148 (87%) | 19 (11%) | ||
| BF b: ≥ 33% | 247 (85%) | 45 (15%) | 0.8186 | 247 (94%) | 15 (6%) | 0.0314 |
| < 33% | 146 (85%) | 25 (15%) | 146 (85%) | 19 (12%) |
BMI- body mass index
BF-Body fat
Non-cases;
Cases
Chi-square test
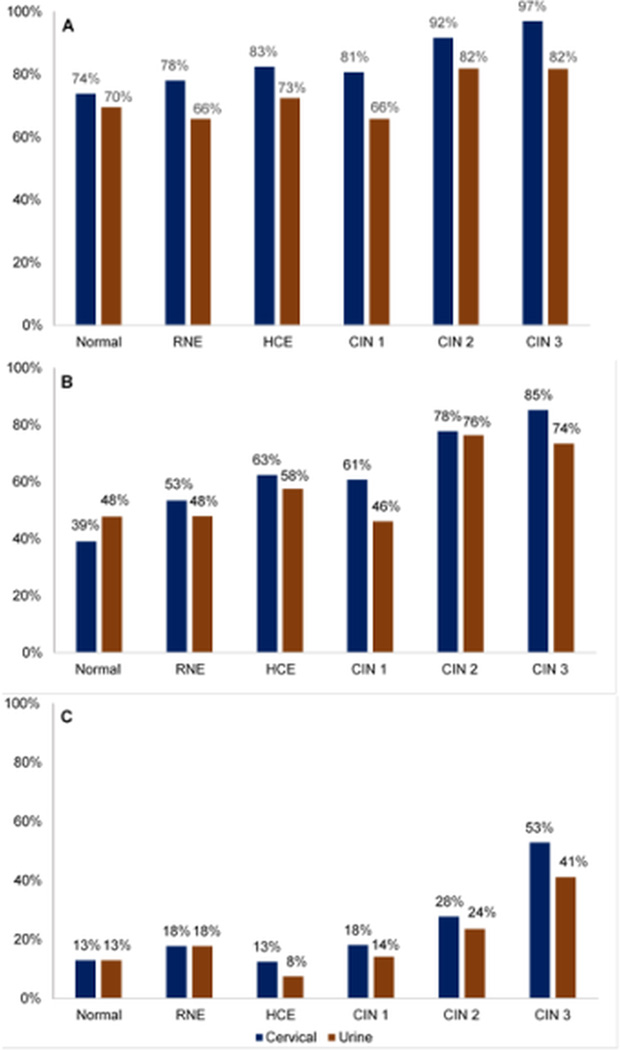
The detection rate of any HPV, any HR-HPV and HPV 16 or 18 types in paired cervical and urine specimens by diagnosis is shown in Figure 1. The detection rates for any HPV, any HR-HPV and HPV 16 or 18 types were lower in specimens of urine compared to cervical in women diagnosed with CIN 1, CIN 2 or CIN 3. The detection rates of HR-HPVs in urine was slightly higher than in cervical among women with normal cervical epithelium. When we assumed that this population has been given the currently available HPV vaccines and those have 100% efficacy, the results showed a similar pattern of detection rates for any HPV or any HR-HPV in urine and cervical specimens (Supplemental Figure 1). The accuracy of urinary HPV testing for the presence of cervical HPVs is shown in Table 2. We observed an almost perfect agreement (0.84 and 0.83, respectively) as per crude concordance and a substantial agreement (0.75 and 0.67, respectively) as per the AC1 agreement measure in the detection of any HPV/any HR-HPV genotypes in urine compared to the cervical specimens in the entire population. There was an almost perfect agreement with both crude concordance (0.94) and AC1 agreement (0.91) measure in the detection of HPV 16 or 18 genotypes in the urine compared to cervical specimen. When the population was stratified by age, race, indicators of excess body weight and case-status, the degree of agreement did not differ by categories of age, race, indicators of excess body weight and case-status (Supplemental Table 1).
Figure 1.
Detection rates of A) any HPV B) any HR-HPV and C) HPV 16 or 18 genotypes by diagnosis in paired cervical and urine specimens.
Table 2.
The accuracy of urinary HPV testing for the presence of cervical HPVs
| HPV status | Count | Number of + or− for urine and cervical HPV results |
Crude concordance* |
AC1 Agreement* (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| +/+ | −/+ | +/− | −/− | ||||
| Positive for any HPV | 502 | 344 | 72 | 7 | 79 | 0.84 (0.81–0.88) | 0.75 (0.70–0.81) |
| Positive for any HR-HPV | 502 | 250 | 66 | 19 | 167 | 0.83 (0.80–0.86) | 0.67 (0.61–0.74) |
| Positive for HPV 16 or 18 | 502 | 81 | 25 | 6 | 390 | 0.94 (0.92–0.96) | 0.91 (0.88–0.94) |
Degree of agreement: < 0 – no agreement, 0.01 to 0.2 slight agreement, 0.21 to 0.40 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 to 0.80 substantial agreement and 0.8 to1.0 almost perfect agreement
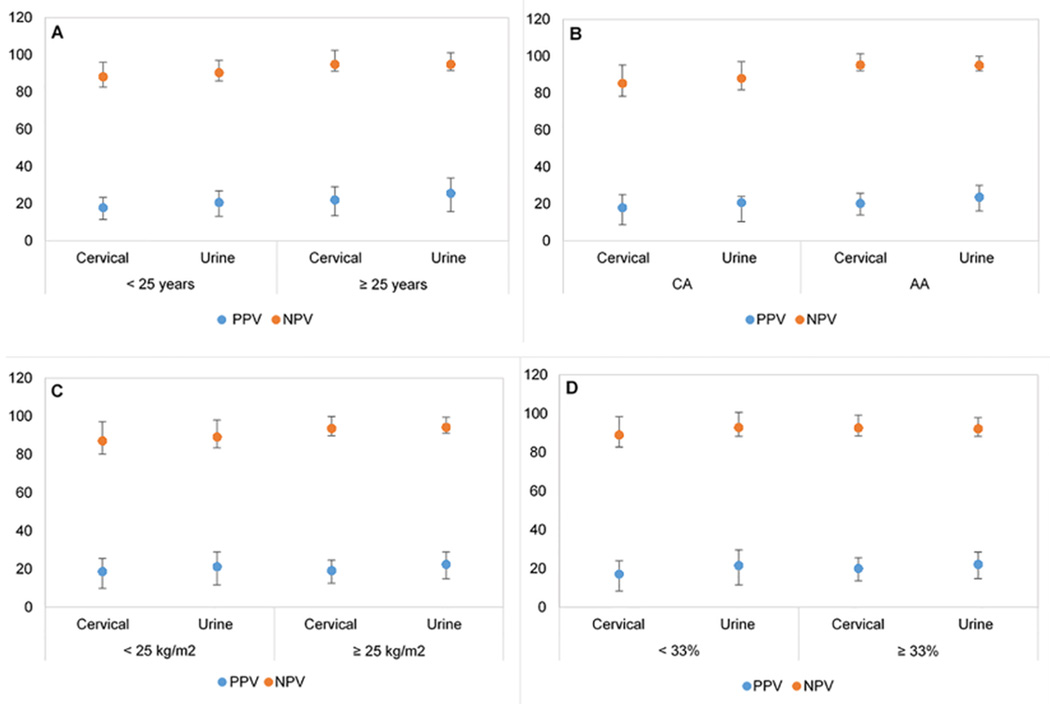
Next we analyzed whether the PPV and NPV of detecting CIN lesions differed based on the presence of HR-HPV genotypes detected in the urine vs. cervical specimens (Table 3). As expected, the PPV for detecting CIN 2 or CIN 3 lesions was relatively low with both cervical (19.5% and 11.2%, respectively) and urine (22.5% and 11.7%, respectively) HR-HPV results and the NPV for detecting CIN 2 or 3 lesions was high with both cervical (91.2% and 97.1%, respectively) and urine (92.4% and 95.8%, respectively) HR-HPV results. We observed an overall similar pattern in the PPV and NPV when the population was categorized by age, race, BMI and % BF. (Supplemental Table 2, Figure 2 and Figure 3). The PPV for detection of CIN 2 and CIN 3 was low (< 26%) in categories of age, race, BMI and % BF, especially for CIN 3. In contrast, we observed that the NPV for detecting CIN 2 was high (≥ 90%) in most categories with urine HR-HPV results with very few exceptions of being slightly < 90% (CA women, 88%; BMI <25 kg/m2, 89%). Similarly, we observed that the NPV for detecting CIN 2 was high (≥ 90%) in most categories with cervical HR-HPV results with very few exceptions of being slightly < 90% (< 25 years, 88%; CA women, 85%; BMI <25 kg/m2, 87%; % BF < 33%, 89%) However, the NPV for detection of CIN 3 was high (≥ 90%) in categories of age, race, BMI and % BF with both cervical and urine HR-HPV results.
Table 3.
Comparison of PPV and NPV of cervical and urine HR-HPV for detection of histology- confirmed CIN 2 and CIN 3 lesions
| Lesion status |
Specimen | Number of specimens | PPVg (95% CI) |
NPVh (95% CI) |
||||
|---|---|---|---|---|---|---|---|---|
| Count | Disease present |
Disease absent |
||||||
| TPc | FNd | FPe | TNf | |||||
| CIN 2a | Cervical | 468 | 56 | 16 | 231 | 165 | 19.5 (15.1–24.6) | 91.2 (86.0–94.9) |
| Urine | 468 | 55 | 17 | 189 | 207 | 22.5 (17.5–28.3) | 92.4 (88.1–95.5) | |
| CIN 3b | Cervical | 430 | 29 | 5 | 231 | 165 | 11.2 (7.6–15.6) | 97.1 (93.3–99.0) |
| Urine | 430 | 25 | 9 | 189 | 207 | 11.7 (7.7–16.8) | 95.8 (92.2–98.1) | |
CIN 2 vs ≤ CIN 1 (excluded CIN 3)
CIN 3 vs ≤ CIN 1 (excluded CIN 2)
TP-True positive
FN-False negative
FP-False positive
TN-True negative
PPV-Positive predictive value
NPV-Negative predictive value
Figure 2.
PPV and NPV for the detection of CIN 2 with cervical and urine HR-HPV results by A) age, B) race C) BMI and D) % BF
Figure 3.
PPV and NPV for the detection of CIN 3 with cervical and urine HR-HPV results by A) age, B) race C) BMI and D) % BF
Supplemental Table 3 shows the concordance between urine and cervical HPV genotypes assuming that this population has been given the currently available HPV vaccines and those have 100% efficacy. For example, those given the bivalent vaccine should be negative for HPV 16 and 18, those given the quadrivalent HPV vaccine should be negative for HPV 6,11,16 and18, those given the 9-valent vaccine should be negative for HPV 6,11,16,18, 31, 33, 45,52 and 58. The agreement was assessed between cervical and urine HPV results for the presence of any HPV or any HR-HPV. We observed that the crude concordance between urine and cervical specimens for detecting any HPV or any HR-HPV in the groups vaccinated with different vaccine types was substantial to almost perfect (0.79–0.85) while the AC1 agreement was moderate to substantial (0.60–0.74).
Assuming that the currently available HPV vaccines have 100% efficacy in preventing CIN lesions associated with those HPV genotypes, we determined the PPV and NPV for detecting CIN 2 and 3 lesions associated with non-vaccine HR-HPV types. The results showed that the PPV for detecting CIN 2 and CIN 3 lesions was low with both urine and cervical HPV results. However, the NPV for detecting CIN 2 and CIN 3 lesions, was high (> 90%) with both urine and cervical HPV results (Supplemental Table 4).
Discussion
Even though the incidence of and mortality from CC in the USA have declined over time as a result of progress in primary prevention programs, there are still opportunities to improve the preventive care of women as 12,042 women were diagnosed with CC and 4,074 women died from the disease in 2012.16 Studies have documented that some of the frequently endorsed barriers to screening were ethnicity, embarrassment, fear or pain associated with the collection of cervical cells and bad experience in the past.17, 18, 19 Recent studies have documented that HPV-based screening provides 60–70% greater protection against CCs compared with cytology.20 Therefore, a non-invasive and acceptable way of testing for HPV infections will revolutionize CC prevention and control around the globe as it will address several barriers to CC screening attendance.
Despite the fact that the progression of HR-HPV infections to CC could be successfully preventable with regular screening and treatment of precancerous lesions associated with those infections, screening coverage remains poor in developing countries and a notable proportion of females even in developed countries also are not screened according to current guidelines.21, 22 In the US, it has been estimated that 56% of incident CC is explained by insufficient screening and approximately 11% of 21–65 year old US females reported no history of screening within the preceding five years in 2012.23,24 Currently available screening strategies (pap testing, testing for HR-HPVs) not only require a pelvic examination that needs a medical setting, but also a significant proportion of women are reluctant to subject themselves to an examination of that nature. Testing for HPVs in urine may overcome these barriers for screening and this may lead to better population coverage in screening programs. Payan et al showed that the response rate of women having been invited for a cervical smear examination substantially increased when they were asked to provide a self-sampled urine specimen via mail.25 Urine samples have also been successfully used in the post-treatment follow-up of CC patients.26 Further, Ducancelle et al documented that urine HPV testing may be an appropriate method for providing screening to underprivileged women who do not have access to medical care or for women who refuse pap smear testing.27 Since HR-HPV testing was approved by the FDA in 2014 for primary screening of women ≥25 years, further evaluation of urine-based HR-HPV testing is increasingly recognized as important.28
Some studies have shown a higher HR-HPV DNA detection rate in the initial stream than in mid-stream urine samples and in pellet29, but others have shown no difference in HR-HPV detection rates in the first void, initial stream, mid-stream and pellet fractions.30 These differences are likely due to variations in the study populations, sample storage conditions, DNA extraction procedures or HPV detection methods used. All urine samples in our study were collected in the morning, but we did not gather information on sampling time or the collection method. Both urine and cervical samples were collected within few minutes apart, urine sampling followed by cervical sampling, and were processed and stored under similar conditions. Since we observed substantial to almost perfect AC1 agreement (0.67–0.91) between cervical and urine samples in our overall population, we believe that our sample collection method is appropriate for studies of this nature. We also observed a similar agreement (substantial to almost perfect AC1 agreement of 0.65–0.92) in the detection of HPV genotypes between urine and cervical specimens by categories of age, race, BMI, % BF and case status, demonstrating that urine HPV test has highly satisfactory accuracy for detecting cervical HPVs irrespective of women’s age, race, BMI, % BF and CIN status. These results show that urine HPV testing can overcome barriers for screening among women with excess body weight and women with different stages of HR-HPV associated CIN lesions, irrespective of whether their age is over or under 25 years. We also noted that the agreement was almost perfect for HPV 16 or 18 for all categories (AC1 agreement 0.81–0.92), indicating that urine HPV testing can be utilized for identifying the two most carcinogenic HPV genotypes in younger or older women, women with varying degrees of excess body weight and varying severity of CIN lesions. Further, a substantial agreement (AC1 agreement 0.60–0.74) observed in populations with HPV genotypes likely to be present after HPV vaccines are given indicated that urine testing may be useful in monitoring such populations for HPV genotypes not included in the currently available HPV vaccines.
With regard to detecting CIN 2 or 3 lesions among women tested positive for HR-HPVs, overall, our results suggested that both cervical and urine HPV testing has low PPV similar to previously published studies30, 31 and PPV remained low irrespective of HPV genotypes present, age, race and excess body weight indicating that urine HPV testing is not useful for identifying women with underlying CIN lesions in vaccinated or non-vaccinated populations. These observations indicate the need for developing next generation molecular tests with higher PPV for this purpose.
With regard to excluding the possibility of having underlying CIN lesions, however, both cervical and urine showed more than 90% NPV value for both CIN 2 or 3 lesions, indicating that urine HPV testing is highly appropriate for identifying low risk women and making recommendations regarding the frequency of testing for HPV-related CIN risk. Other studies have reported similar results31, but we document that urine HPV testing has ≥ 90% NPV for CIN 3 lesions irrespective of women’s age, race, BMI and % BF. More importantly, our results also showed that urine HPV testing has more than 90% NPV for CIN lesions associated with HPV genotypes not included in HPV vaccines, including the 9-valent vaccine indicating its use in monitoring the risk in vaccinated populations.
In summary, we demonstrated that urine HPV testing, a non-invasive approach, provides highly satisfactory results for excluding the possibility of having any cervical HPV infection, including HPV types not included in vaccines and CIN lesions associated with any HR-HPV regardless of woman’s age, race and excess body weight and will overcome barriers for screening for CC in developed as well as in developing countries.
Supplementary Material
Acknowledgments
Partially supported by R01 CA102489 and R01 CA105448 (C. Piyathilake) funded by the National Cancer Institute
Footnotes
Conflict of interest: None
Author’s contribution:
Formulation of research goals and aims-CJP
Generation of data and verification-MMC, CJP, SB
Data analysis and interpretation-CJP, SB, IB
Writing original draft and presentation of results-CJP, SB
Review and editing of the final manuscript-CJP, SB, IB, MMC, RM, EEP
References
- 1.Franco EL, Rohan TE, Villa LL. Epidemiologic evidence and human papillomavirus infection as a necessary cause of cervical cancer. J Natl Cancer Inst. 1999;91:506–511. doi: 10.1093/jnci/91.6.506. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Human papillomaviruses. IARC Monographs on the evaluation of carcinogenic risks to humans. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 6.Freeman H, Wingrove B. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. Rockville, MD: National Cancer Institute; 2005. [Google Scholar]
- 7.Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007;45:93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Ridolfi DR, Crowther JH. The link between women's body image disturbances and body-focused cancer screening behaviors: a critical review of the literature and a new integrated model for women. Body Image. 2013;10:149–162. doi: 10.1016/j.bodyim.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 9.National Task Force on the Prevention and Treatment of Obesity. Medical Care for Obese Patients: Advice for Health Care Professionals. Am Fam Physician. 2002;65:81–88. [PubMed] [Google Scholar]
- 10.Greenberg DR, LaPorte DJ. Racial differences in body type preference of men for women. Int J Eat Disord. 1996;19:275–278. doi: 10.1002/(SICI)1098-108X(199604)19:3<275::AID-EAT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Pathak N, Dodds J, Zamora J, et al. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264. doi: 10.1136/bmj.g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farnbrini M, Penna C, Pieralli A, et al. PCR detection rates of high risk human papillomavirus DNA in paired self-collected urine and cervical scrapes after laser CO2 conization for high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2008;109:59–64. doi: 10.1016/j.ygyno.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(Pt1):29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 14.Cook RJ. Kappa and Its Dependence on Marginal Rates. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2nd. Wiley; 2005. [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 16.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2015. Available at: www.cdc.gov/uscs. [Google Scholar]
- 17.Waller J, Bartoszek M, Marlow L, et al. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. 2009;16:199–204. doi: 10.1258/jms.2009.009073. [DOI] [PubMed] [Google Scholar]
- 18.Tangka FK, Howard DH, Royalty J, Dalzell LP, Miller J, O'Hara BJ, Sabatino SA, Joseph K, Kenney K, Guy GP, Jr, Hall IJ. Cervical cancer screening of underserved women in the United States: results from the National Breast and Cervical Cancer Early Detection Program, 1997–2012. Cancer Causes Control. 2015;26:671–686. doi: 10.1007/s10552-015-0524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd TL, Chavez R, Wilson KM. Barriers and facilitators of cervical cancer screening among Hispanic women. See comment in PubMed Commons below Ethn Dis. 2007;17:129–134. [PubMed] [Google Scholar]
- 20.Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson L, Pontén J, Zack M, et al. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 22.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 24.Benard VB, Thomas CC, King J, et al. Vital signs: cervical cancer incidence, mortality, and screening—United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2014;63:1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 25.Payan C, Tran A, Foll Y, et al. Evaluation of a new strategy for cervix cancer screening in women who do not access to pap smear screening in West Brittany, using a urine test for human papilloma- virus (HPV) detection on a large scale plateformcombining EasyMag extractor and real-time PCR LightCycler system (the PAPU29 PHASE 1 study) J Clin Virol. 2009;46(Suppl 1):S12. [Google Scholar]
- 26.D’Hauwers K, Depuydt C, Bogers JP, et al. Urine versus brushed samples in human papillomavirus screening: study in both genders. Asian J Androl. 2007;9:705–710. doi: 10.1111/j.1745-7262.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 27.Ducancelle A, Reiser J, Pivert A, et al. Home-based urinary HPV DNA testing in women who do not attend cervical cancer screening clinics. J Infect. 2015;71:377–384. doi: 10.1016/j.jinf.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Huh WK, Ault KA, Chelmow D, Davey DD, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Vorsters A, Van den Bergh J, Micalessi I, et al. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol Infect Dis. 2014;33:2005–2014. doi: 10.1007/s10096-014-2147-2. [DOI] [PubMed] [Google Scholar]
- 30.Senkomago V, Des Marais AC, Rahangdale L, et al. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J Clin Virol. 2016;74:26–31. doi: 10.1016/j.jcv.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Sahasrabuddhe VV, Gravitt PE, Dunn ST, et al. Comparison of human papillomavirus detections in urine, vulvar, and cervical samples from women attending a colposcopy clinic. J Clin Microbiol. 2014;52:187–192. doi: 10.1128/JCM.01623-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.