Abstract
Background
Efavirenz frequently causes central nervous system (CNS) symptoms. We evaluated genetic associations with efavirenz discontinuation for CNS symptoms within 12 months of treatment initiation.
Methods
Patients had initiated efavirenz-containing regimens at an HIV primary care clinic in the southeastern United States, and had at least 12 months of follow-up data. Polymorphisms in CYP2B6 and CYP2A6 defined efavirenz metabolizer categories. Genome-wide genotyping allowed adjustment for population stratification.
Results
Among 563 evaluable patients, 99 (17.5%) discontinued efavirenz within 12 months, 29 (5.1%) for CNS symptoms. The hazard ratio (HR) for efavirenz discontinuation for CNS symptoms in slow versus extensive metabolizers was 4.9 (95% C.I. 1.9 to 12.4; p = 0.001). This HR in Whites was 6.5 (95% CI: 2.3 to 18.8; p = 0.001), and in Blacks was 2.6 (95% C.I. 0.5 to 14.1; p = 0.27). Considering only slow metabolizers, the HR in Whites versus Blacks was 3.1 (95% CI: 0.9 to 11.0; p = 0.081). The positive predictive value of slow metabolizer genotypes for efavirenz discontinuation was 27% in Whites and 11% in Blacks.
Conclusions
Slow metabolizer genotypes were significantly associated with efavirenz discontinuation for reported CNS symptoms. This association was considerably stronger in Whites than in Blacks.
Keywords: HIV, efavirenz, pharmacogenomics, pharmacokinetics, CYP2B6
Introduction
Efavirenz is one of the most frequently prescribed antiretrovirals worldwide in first-line regimens for human immunodeficiency virus (HIV)-1 infection. Its efficacy has been demonstrated in multiple prospective, randomized clinical trials [1-6]. However, some patients who initiate efavirenz experience central nervous system symptoms [7]. A retrospective analysis of data from prospective clinical trials showed that randomization to efavirenz-containing arms was associated with a 2-fold increased hazard of suicidality [8], although such an association was not found in the Food and Drug Administration Adverse Event Reporting System (FAERS) [9] or in the United States administrative claims data for commercially and Medicaid-insured individuals [10]. In April 2015, United States prescribing guidelines were updated to move efavirenz-containing regimens from recommended status to an alternative option as initial therapy for HIV-1 infection [11].
Efavirenz is metabolized primarily by cytochrome P450 (CYP) 2B6 [12], with minor metabolism by CYP2A6 [12, 13] and direct N-glucuronidation by UDP-glucuronosyltransferase (UGT) 2B7 [14]. Three CYP2B6 single nucleotide polymorphisms (SNPs) predict increased plasma efavirenz exposure and explain approximately 35% of interindividual variability in plasma efavirenz exposure [15]. Of greatest impact is CYP2B6 516G→T (rs3745274) [15-20], which is more frequent among Africans than among Caucasians [21]. A less frequent SNP, CYP2B6 983T→C (rs28399499), also predicts increased plasma efavirenz exposure [15, 22-24] and appears to be present only with African ancestry [21]. The per allele effect of CYP2B6 983T→C on efavirenz exposure is somewhat greater than that of 516G→T [15]. A third SNP, CYP2B6 15582C→T (rs4803419), predicts modestly increased plasma efavirenz exposure with both Caucasian and African ancestry, and is most frequent in Caucasians [15]. Slow metabolizer genotypes comprise either homozygosity for CYP2B6 516 T/T, homozygosity for 983 C/C, or dual heterozygosity for 516 G/T-983 C/T [15]. The above three CYP2B6 are not in linkage disequilibrium, and in fact appear to reside on mutually exclusive haplotypes. Among CYP2B6 slow metabolizers, plasma efavirenz exposure is further increased when loss-of-function CYP2A6 SNPs are also present (e.g., −48T→G, rs28399433) [25-27] and possibly UGT2B7 (735A→G, rs28365062) [26, 27].
In adults, efavirenz is typically prescribed at a dose of 600 mg once daily, and higher plasma efavirenz concentrations have been reported to correlate with increased central nervous system symptoms [19, 24, 28-31]. Among participants from AIDS Clinical Trials Group (ACTG) protocols 384 and A5095, CYP2B6 slow metabolizer genotypes were associated with increased central nervous system adverse events among 276 White participants (p=0.04) but not among 217 Black participants (p=0.58) [24]. In a subsequent evaluation of all-cause (i.e., not just central nervous system-related) discontinuation of efavirenz treatment among 272 efavirenz recipients in the Swiss HIV Cohort Study, likelihood of discontinuation was increased in 13 individuals with a genetic risk score of 6 (calculated as the total number of variant alleles at six loci in CYP2B6, CYP2A6 and CYP3A4) [32]. The same genetic score approach was applied to 758 participants from the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) and the Community Programs for Clinical Research on AIDS (CPCRA) studies, and similarly showed increased likelihood of treatment discontinuation among 20 individuals with a genetic risk score of 6 [33]. The latter two studies may have been confounded by population stratification, as homozygosity for CYP3A4 rs4646437 T/T is found in approximately 72% of Africans but only 1% of Caucasians [21]. In the Swiss HIV Cohort Study, 77% of individuals with a genetic risk score of 6 were Black, while in INSIGHT, 65% of individuals with a score of 6 were Black in [33]. In addition, CYP3A4 rs4646437 may not predict plasma efavirenz exposure [15].
Smoking is prevalent among HIV-infected adults in the United States [34], and metabolic pathways for nicotine and efavirenz overlap somewhat. The predominant metabolite of nicotine, cotinine, is metabolized by CYP2A6 [35, 36], while evidence from an animal model suggests that nicotine administration can induce CYP2B6 expression in the brain [37]. These shared pathways suggest that smoking status might in some way influence pharmacogenetic associations between efavirenz and central nervous system symptoms.
The present study examined genetic associations with efavirenz discontinuation for central nervous system symptoms among clients of a large HIV primary care clinic in the Southeastern United States. We focused on SNPs that have been definitively associated with efavirenz exposure, and used genome-wide genotype data to minimize confounding by population stratification. We also assessed relationships between cigarette tobacco smoking and efavirenz discontinuation.
Methods
Study Participants
Eligible participants had initiated efavirenz-containing antiretroviral therapy (ART) regimens while receiving primary care at the Vanderbilt Comprehensive Care Clinic (formerly the Comprehensive Care Center) in Nashville, Tennessee, had at least 12 months of follow-up data, and had provided written informed consent and DNA for genetic research. Clinical data were manually entered into electronic medical records by providers at the time of patient encounter, while clinical laboratory data were automatically uploaded. Laboratory and ART data, including medication start and stop dates, were validated by systematic chart review. All treatment discontinuation events were reviewed and confirmed by study investigators (PL and DWH), without knowledge of genotype results.
Beginning in March 2011, self-reported data on packs of tobacco cigarettes smoked in the prior week were collected at every clinic visit as part of routine clinical care. We assumed that smoking reported after March 14, 2011 reflected the period before such data were consistently collected. We defined smokers as individuals with any cigarette smoking reported at any clinic visit, and also stratified smoking status based on average number of packs of cigarettes reported at each visit.
This study was approved by the Vanderbilt University Institutional Review Board.
Genotyping
Genotyping was done on stored DNA. Genotyping of CYP2B6 983T→C (rs28399499) and 15582C→T (rs4803419) in 592 individuals was done using a custom designed, 24-SNP MassARRAY® iPLEX Gold (Sequenom, Inc.) assay at Vanderbilt Technologies for Advanced Genomics (VANTAGE) essentially as described elsewhere [15]. Genotyping of CYP2B6 516G→T (rs3745274) and CYP2A6 −48T→G (rs28399433) was done by TaqMan™. Each of the four SNPs was in Hardy Weinberg equilibrium within each race/ethnicity group (p > 0.05). Stored DNA from a total of 837 patients who had initiated either efavirenz-containing regimens (including patients in the present association analysis) or atazanavir-containing regimens was genotyped for 535,543 SNPs by Illumina HumanCore Exome assay. Genotype call rates exceeded 99% on 833 samples. Laboratory personnel with no knowledge of clinical data performed genotyping.
After the initial quality control (QC), 582 of the 592 individuals were retained for analysis. Then two individuals in parent/offspring pairs, and one in a half-sibling pair, based on identity by descend (IBD) coefficients, were removed from the analysis. Four individuals with genotype efficiency of less than 75% for CYP2B6 were also removed. For sub-analyses performed separately among Whites, Blacks and Hispanics, seven individuals for whom self-reported race disagreed with clustering based on MDS coordinates were censored as well as five additional individuals whose MDS coordinates fell outside the primary clusters. At the end of the QC process, 563 individuals were included in the analysis.
Genetic variants
The four CYP2B6 and CYP2A6 SNPs were used to assign each participant to 1 of 12 metabolizer categories that are associated with progressively greater plasma efavirenz exposure, as previously described [15, 27]. In addition, composite CYP2B6 genotypes were collapsed into three categories as follows: extensive metabolizer genotypes (15582CC-516GG-983TT or 15582CT-516GG-983TT); intermediate metabolizer genotypes (15582TT-516GG-983TT, 15582CC-516GT-983TT, 15582CC-516GG-983CT, 15582CT-516GT-983TT, or 15582CT-516GG-983CT); and slow metabolizer genotypes (15582CC-516TT-983TT, 15582CC-516GT-983CT, or 15582CC-516GG-983CC). With slow metabolizer genotypes, the presence of CYP2A6 −48GT or −48GG predicts even greater plasma efavirenz exposure.
Multifactor dimensionality scaling
To control for possible population stratification, whole genome data were used for multidimensional scaling (MDS) in PLINK [38]. Over 500,000 SNPs available from 582 of the study participants, as well as 224 additional patients not in this efavirenz analysis, were used to generate MDS coordinates. Individuals with genotyping efficiency less than 99% were censored, as were SNPs with genotyping efficiency less than 99%, minor allele frequencies less than 10%, and Hardy-Weinberg values greater than 0.00001. Pruning produced a set of SNPs using a window of 50, shifting at each 5 SNPs, and removing SNPs with linkage disequilibrium (R2) values greater than 0.2. The first two MDS coordinates were used to generate scatter plots using R version 2.6.2, to allow comparison of self-reported race/ancestry with genetic ancestry.
Statistical analyses
Baseline characteristics of study participants are presented as median and interquartile ranges [IQR]. Log-rank test (chi2) was used to assess the correlation between genotype and binomial variables. Cox proportional hazard regression model, adjusted for population stratification based on MDS coordinates, was used to examine associations between genotype and efavirenz discontinuation. Primary analyses considered three metabolizer groups (slow, intermediate, and extensive). Post hoc analyses considered 12 ordinal strata of plasma efavirenz exposure (defined by various combinations of the four CYP2B6 and CYP2A6 SNPs). Analyses were also performed separately in subgroups stratified for self-reported race/ethnicity, excluding individuals in whom self-reported race disagreed with clustering based on MDS coordinates. All analyses used a 5% two-sided significance level and were performed using Stata/IC version 10.0.
Results
Study cohort
From 1998 to 2012, a total of 563 individuals initiated efavirenz-containing ART regimens, had at least 12 months of follow-up data, provided DNA for genetic analysis, and had genotype data that passed quality control. Of these individuals, 99 (17.4%) permanently discontinued efavirenz within the first year of ART, while 464 continued to receive efavirenz for at least 12 months. Baseline demographics of study subjects are shown in Table 1, and generally reflect the demographics of the Vanderbilt Comprehensive Care Clinic during the study period. Among the 563 individuals on whom MDS data were available, MDS clustering agreed closely with self-reported race/ethnicity in 335 Whites (59.5%), 198 Blacks (35%), 25 Hispanics (4.5%), and 5 Asians (1%). Individuals who lacked ethnicity data were classified based on MDS clustering, since such individuals did not have direct evidence of disagreement. Scatter plots of MDS coordinates are provided in Supplemental Figure. Concomitant nucleos(t)ide reverse transcriptase inhibitors (NRTIs) were similar between cases and controls, with tenofovir and emtricitabine being most frequent. Of the 99 cases who discontinued efavirenz, the primary cause was central nervous system symptoms in 29 (29.3% of discontinuations, 5.1% of all individuals), which included dizziness, nightmares, hallucinations, insomnia and depression (Table 2). Of these 29 individuals, 20 were White and 9 were Black.
Table 1. Baseline demographics of study participants.
| Characteristic | Controls n = 464 |
Cases n = 99 |
All N = 563 |
|---|---|---|---|
| Male, n (%) | 408 (88) | 78 (79) | 486 (86) |
| Female, n (%) | 56 (12) | 21 (21) | 77 (14) |
| Age in years, median (IQR) | 38.1 (31.8 – 44.7) | 37.2 (29.7 – 46.2) | 37.9 (31.5 – 45.4) |
| Self-identified race/ethnicity, n (%) | |||
| White | 283 (61) | 49 (49.5) | 332 (59) |
| Black | 144 (31) | 39 (39.5) | 183 (32.5) |
| Hispanic | 18 (4) | 6 (6) | 24 (4) |
| Asian | 5 (1) | - | 5 (1) |
| Not reported | 14 (3) | 5 (5) | 19 (3.5) |
| Concomitant NRTIs, n (%) | |||
| Tenofovir | 52 (53) | 243 (52) | 295 (52) |
| Emtricitabine | 50 (51) | 233 (50) | 283 (50) |
| Lamivudine | 40 (40) | 204 (44) | 244 (43) |
| Zidovudine | 33 (33) | 158 (34) | 191 (34) |
| Abacavir | 5 (5) | 32 (7) | 37 (7) |
| Stavudine | 2 (2) | 22 (5) | 24 (4) |
| Didanosine | 0 (0) | 7 (2) | 7 (1) |
Table 2. Primary reason for discontinuation of efavirenza.
| Cause | Number of cases (n = 99) |
Percentage |
|---|---|---|
| Central nervous system symptoms | 29 | 29.3 |
| Rash | 23 | 23.2. |
| Viral drug resistance mutations | 16 | 16.2 |
| Non-adherence | 9 | 9.1 |
| Gastrointestinal side effects | 6 | 6.1 |
| Drug-drug interaction | 2 | 2.0 |
| Hypercholesterolemia | 2 | 2.0 |
| Other | 2 | 2.0 |
| Unspecified | 10 | 10.1 |
Based on review of provider notes in the electronic medical record.
Genetic associations with efavirenz discontinuation
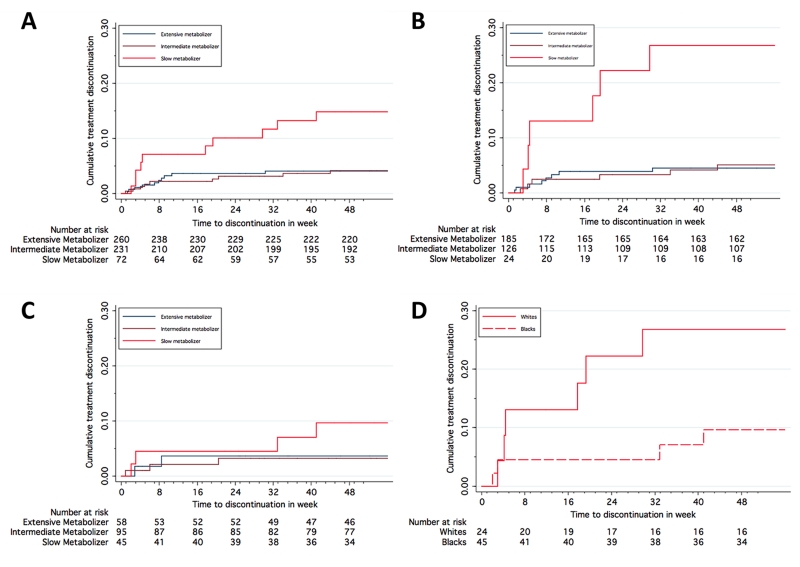
Among all 563 participants, adjusted hazard ratios (adjusted for two MDS coordinates) for efavirenz discontinuation for central nervous system symptoms were 4.86 (95% CI: 1.91 to 12.39; p = 0.001) for slow and 1.09 (95% CI: 0.44 to 2.73; p=0.84) for intermediate metabolizers. Time to efavirenz discontinuation for central nervous system symptoms stratified by genotype is shown in Figure 1, panel A. Among 335 Whites, unadjusted hazard ratios for discontinuation with slow versus extensive, and with intermediate versus extensive metabolizer genotypes were 6.50 (95% CI: 2.25 to 18.75; p = 0.001) and 1.11 (95% CI: 0.38 to 3.20; p=0.85), respectively (Figure 1, panel B). Among 198 Blacks, unadjusted hazard ratios with slow versus extensive, and with intermediate versus extensive metabolizer genotypes were 2.59 (95% C.I. 0.47 to 14.13; p = 0.27) and 0.90 (95% CI: 0.15 to 5.39; p=0.90), respectively (Figure 1, panel C). Among the 25 Hispanics in this analysis, none discontinued for central nervous system symptoms (data not shown).
Figure 1. Time to discontinuation of efavirenz for CNS side effects, stratified by genotype.
Panel A: all participants stratified by CYP2B6 metabolizer genotype; Panel B: White participants stratified by CYP2B6 metabolizer genotype; Panel C: Black participants stratified by CYP2B6 metabolizer genotype; Panel D: Participants with slow metabolizer genotype, stratified by race.
The above analyses only considered discontinuation for central nervous system symptoms. We repeated analyses based on all-cause efavirenz discontinuation (i.e., not just central nervous system symptoms) within the first 12 months of ART. Among all 563 participants, adjusted hazard ratios (adjusted for two MDS coordinates) for all-cause efavirenz discontinuation in slow versus extensive, and in intermediate versus extensive metabolizers were 1.69 (95% C.I. 0.96 to 2.99; p = 0.069) and 1.03 (95% C.I. 0.66 to 1.63; p=0.87), respectively. Among 335, Whites, unadjusted hazard ratios in slow versus extensive, and in intermediate versus extensive metabolizers were 2.96 (95% C.I. 1.32 to 6.61; p = 0.008) and 1.22 (95% C.I. 0.66 to 2.24; p=0.52), respectively. Among 198 Blacks, unadjusted hazard ratios in slow versus extensive, and in intermediate versus extensive metabolizers were 1.31 (95% CI: 0.58 to 2.91; p = 0.51) and 0.89 (95% CI: 0.43 to 1.86; p=0.76), respectively. Among the 25 Hispanics in this analysis, 5 discontinued efavirenz for skin rash, 1 for treatment failure, and 1 for unspecified reasons.
We analyzed post hoc whether, within each CYP2B6 metabolizer group, likelihood of efavirenz discontinuation for central nervous system symptoms differed by race. In 69 slow metabolizers, the unadjusted hazard ratio for efavirenz discontinuation in Blacks (n = 45) versus Whites (n = 24 was 0.33 (95% C.I. 0.09 to 1.15; p = 0.081) (Figure 1, panel D). In 221 intermediate metabolizers this hazard ratio was 0.66 (95% C.I. 0.16 to 2.63; p = 0.56), and in 243 extensive metabolizers it was 0.81 (95% C.I. 0.17 to 3.83; p = 0.79).
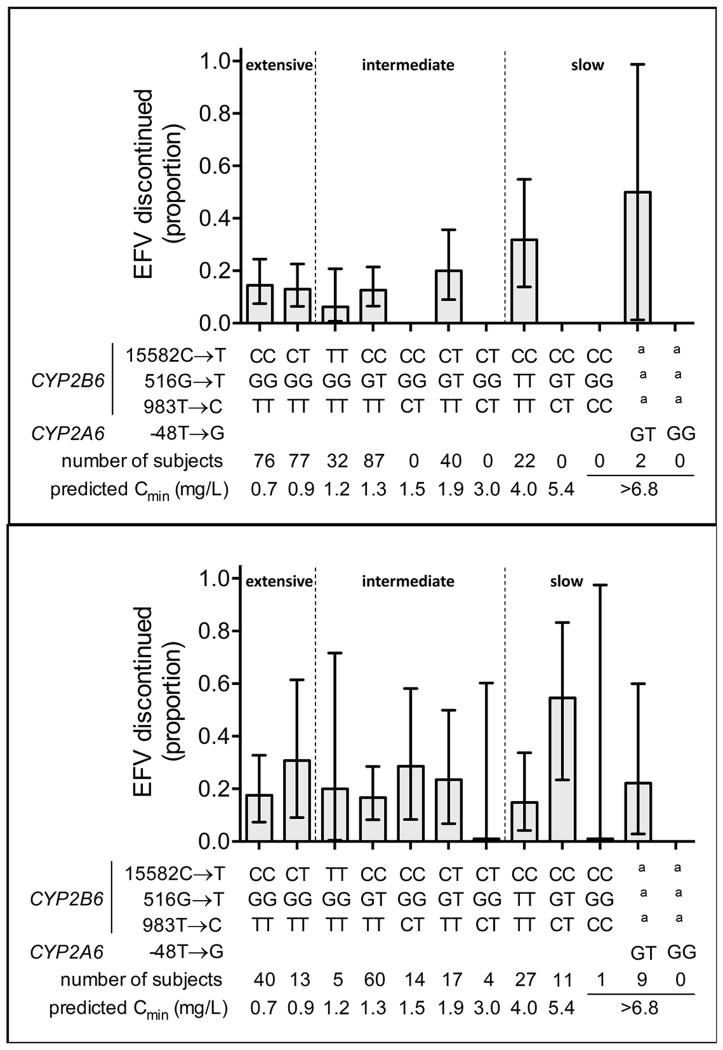
The four CYP2B6 and CYP2A6 SNPs in various combinations define 12 ordinal strata of plasma efavirenz exposure. The proportion of individuals that discontinued efavirenz for central nervous system symptoms within each stratum, among Whites and Blacks analyzed separately, are presented in Figure 2. Among 69 CYP2B6 slow metabolizers, a subset of 11 individuals (9 Black and 2 White) were predicted to have even greater plasma efavirenz concentrations based on heterozygosity for CYP2A6 −48GT. Of these 11 individuals, 3 (27.2%) discontinued efavirenz for central nervous system symptoms, including 2 (22.2%) of 9 Blacks and 1 (50%) of 2 Whites.
Figure 2. Proportions of patients who discontinued efavirenz within the first 12 months of therapy, stratified by composite CYP2B6/CYP2A6 genotype.
Graphs represent subjects who initiated efavirenz 600 mg once daily, had no change in their antiretroviral therapy regimen before week 48, and had HIV-1 RNA data available at both week 0 and week 48. Panel A: Self-identified White participants. Panel B: Self-identified Black participants. The 12 strata are defined by CYP2B6 15582C→T, 516G→T, 983T→C, and CYP2A6 −48T→G as indicated. Plasma efavirenz concentrations were not measured in the present study. Predicted median efavirenz Cmin concentrations are from the study by Holzinger et al, in which median plasma efavirenz exposure within each genotype stratum were very similar among Blacks and Whites [15]. Error bars represent 95% confidence intervals by the modified Wald method. a Indicates that the CYP2A6 polymorphism is present together with any of the 3 CYP2B6 strata furthest to the right. The top of each indicated relationships between metabolizer groups (extensive, intermediate, and slow) and the 12 genotype categories.
Smoking and efavirenz discontinuation
Among the 563 evaluable study participants, smoking data were available on 453 (80%), of whom 205 (36%) were smokers and 248 (44%) were non-smokers. Among smokers, 43 (21%) smoked less than 1 pack per week, 85 (41.5%) smoked 1 to 3.5 packs per week, and 77 (37.5%) smoked greater than 3.5 packs per week. The hazard ratio for efavirenz discontinuation for central nervous system symptoms in smokers versus non-smokers was 1.36 (95% C.I: 0.61 to 3.04; P = 0.45). Among smokers, the hazard ratio for efavirenz discontinuation for central nervous system symptoms in patients who smoked 1 to 3.5 packs per week versus non-smokers was 0.84 (95% C.I: 0.29 to 2.42; P=0.75). In patients who smoked more than 3.5 packs per week this hazard ratio was 2.25 (95% C.I: 0.89 to 5.69; P = 0.087. Among 136 Whites who smoked greater than 3.5 packs per week, hazard ratios for efavirenz discontinuation in slow versus extensive, and in intermediate versus extensive metabolizers were 18.3 (95% C.I: 4.03 to 82.7; P < 0.001) and 2.33 (95% C.I: 0.52 to 10.4; P=0.27), respectively. Among 48 Blacks who smoked greater than 3.5 packs per week, the hazard ratio for efavirenz discontinuation in slow versus extensive metabolizers was 2.0 (95% C.I: 0.12 to 32.0; p = 0.62). There were no efavirenz discontinuations among Blacks who were intermediate metabolizers and who also smoked greater than 3.5 packs per week.
Positive and negative predictive values
The positive predictive value (PPV) of slow metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 16.1% (95% C.I. 8.0% to 27.7%). Among 326 Whites, the PPV of slow metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 27.2% (95% C.I. 10.7% to 50.2%). Among 191 Blacks, the PPV of slow metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 10.8% (95% C.I: 3.0% to 25.4%).
The negative predictive values (NPV) of either extensive or intermediate metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 95.6% (95% C.I. 93.2% to 97.3%). Among 326 Whites, the NPV of either extensive or intermediate metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 95.0% (95% C.I. 91.8% to 97.3%). Among 191 Blacks, the NPV of slow metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 96.1% (95% C.I. 91.1% to 98.7%).
Discussion
Efavirenz is widely prescribed for HIV-1 infection, but central nervous system symptoms with efavirenz are frequent. The present study showed that, among 563 patients who had initiated efavirenz-containing regimens at an HIV primary care clinic in the Southeastern United States, CYP2B6 slow metabolizer genotypes were significantly associated with increased likelihood of discontinuing efavirenz for central nervous system symptoms during the first 12 months of therapy. The association was significant in Whites (hazard ratio = 6.50, p = 0.001) but not in Blacks (hazard ratio = 2.59, p = 0.27), this despite slow metabolizer genotypes being more frequent in Blacks. A difference by race was also reflected in all-cause discontinuation of efavirenz, with slow metabolizer genotypes again significantly associated with all-cause discontinuation in Whites (hazard ratio = 2.96, p = 0.008) but not in Blacks (hazard ratio = 1.31, p = 0.51). Use of genome-wide genotype data to minimize confounding by population stratification strengthened this study.
The present study replicates an apparent difference by race that was first reported in an analysis of data from ACTG clinical trials 384 and A5095 [24]. That analysis found a significant association between CYP2B6 slow metabolizer genotypes and increased central nervous system adverse events among 276 Whites (p=0.04) but not among 217 Blacks (p=0.58). A similar difference by race was seen in a recent pharmacogenetic analysis of suicidality involving patients who had been randomized to efavirenz-containing regimens in ACTG protocols A5095, A5142, A5175, and A5202 [8]. In that analysis, CYP2B6/CYP2A6 loss-of-function genotypes were associated with increased suicidality among 716 Whites but not among 589 Blacks. These three studies strongly suggest that, among patients with slow metabolizer genotypes who are prescribed efavirenz in the United States, central nervous system side effects are substantially less likely to be reported among Blacks than among Whites, this despite strong evidence that plasma efavirenz exposure is very similar among Blacks and Whites with CYP2B6 slow metabolizer genotypes [15].
Two other analyses that examined pharmacogenetic associations with efavirenz discontinuation used a very different analytical approach. Analyses of both the Swiss HIV Cohort Study [32] and INSIGHT and CPCRA studies [33] derived a genetic risk score defined as the number of variant alleles at six loci in CYP2A6, CYP2B6 and CYP3A4. Inclusion of CYP3A4 rs4646437 in the risk score calculation may have introduced confounding by population stratification, since rs4646437 is primarily a marker of ancestry [21] and may not predict plasma efavirenz exposure [15]. While both cohorts were predominantly of European ancestry, those with high genetic risk scores were predominantly of African descent. In addition, both studied only considered all-cause discontinuation. It is thus difficult to reconcile those two prior studies with the present study.
There are several possible explanations for the attenuated association of CYP2B6 slow metabolizer genotypes with efavirenz discontinuation in Blacks compared to Whites. It is possible that Blacks with central nervous system symptoms are less likely to report such symptoms than Whites with similar symptoms, resulting in misclassification of reasons for discontinuation. It is possible that Blacks are less likely than Whites to discontinue efavirenz despite similar central nervous system symptoms with high plasma efavirenz exposures. While it is possible that providers queried Blacks less than Whites regarding reasons for discontinuing efavirenz, this is unlikely since all patients received care at a single HIV primary care clinic where, since 1997 every ART treatment change is presented and thoroughly discussed at a multidisciplinary Antiretroviral Therapy Conference. As noted above, plasma efavirenz exposure is known to be very similar among Blacks and Whites with CYP2B6 slow metabolizer genotypes [15], so differences in plasma efavirenz exposure are unlikely to explain the attenuated genetic association with efavirenz discontinuation in Blacks compared to Whites.
The overlapping metabolic pathways for nicotine and efavirenz prompted us to explore whether smoking might influence pharmacogenetic associations. We found no significant association between smoking and likelihood of efavirenz discontinuation for central nervous system symptoms among all patients, and among Whites and Blacks analyzed separately. In a post hoc analysis involving Whites who smoked at least 3.5 packs of tobacco cigarettes per week, CYP2B6 slow metabolizers appeared to be at particularly high risk of discontinuing efavirenz for central nervous system symptoms (hazard ratio = 18.26 (95% C.I: 4.03 to 82.77; P < 0.001). This association, if true, might reflect a drug-drug interaction in the brain whereby individuals with impaired efavirenz clearance due to slow metabolizer genotypes experience even a further reduction in efavirenz clearance in the presence of nicotine. This is analogous to the increased plasma efavirenz exposure that occurs when CYP2B6 slow metabolizers are prescribed isoniazid, which interferes with efavirenz metabolism by CYP2A6 [39-41]. Alternatively, efavirenz appears to be a partial agonist of 5-HT2A receptors, a ligand for 5-HT2C, and inhibits serotonin reuptake transporters (SERT), dopamine transporters (DAT), and monoamine vesicular transporters (VMAT2) [42]. If these interactions explain central nervous system symptoms, it is conceivable that nicotine and/or other molecules from tobacco affect interactions between efavirenz and these off-target molecules that in some way increases symptoms.
Prior reports suggest that efavirenz dose reduction among individuals with slow metabolizer genotypes would reduce central nervous system symptoms [43] and would be cost effective [44]. The present study further supports that concept. However, the PPV of slow metabolizer genotypes for efavirenz discontinuation for central nervous system symptoms was 27.2% in Whites and 10.8% in Blacks, suggesting that genotype alone would not effectively identify individuals at very high likelihood of discontinuing efavirenz for central nervous system symptoms. Conversely, while the NPV was approximately 95% regardless of race, this does not differ substantially from the 5% of patients overall who discontinued for central nervous system symptoms, regardless of genotype, suggesting that genotype alone would not effectively identify individuals at very low likelihood of discontinuing efavirenz for central nervous system symptoms.
In summary, among patients who had initiated efavirenz-containing regimens, CYP2B6 slow metabolizer genotypes were significantly associated with increased likelihood of discontinuing efavirenz for central nervous system symptoms. This association was highly significant in Whites but was markedly attenuated in Blacks. The reason for this apparent difference by race is not known.
Supplementary Material
Acknowledgments
Grant support included:
This work was supported in part by the National Institute of Allergy and Infectious Diseases grants R01 AI077505, P30 AI110527, and UL1 000445 (DWH).
Footnotes
Potential conflicts:
There are no potential conflicts.
References
- 1.van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363(9417):1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 2.Gulick RM, Ribaudo HJ, Shikuma CM, Lalama C, Schackman BR, Meyer WA, III, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296(7):769–81. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 3.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358(20):2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DA, Heera J, Goodrich J, Tawadrous M, Saag M, Dejesus E, et al. Maraviroc versus Efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis. 2010;201(6):803–13. doi: 10.1086/650697. [DOI] [PubMed] [Google Scholar]
- 6.Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Budhathoki C, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154(7):445–56. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford DB, Evans S, Yang Y, Acosta EP, Goodkin K, Tashima K, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143(10):714–21. doi: 10.7326/0003-4819-143-10-200511150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Mollan KR, Tierney C, Eron JJ, Hudgens M, Gulick RM, Haubrich R, et al. Composite CYP2B6/CYP2A6 genotype and risk for suicidality among HIV-infected individuals randomly assigned to initiate efavirenz-containing regimens in AIDS Clinical Trials Group studies; Presented at the 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Vancouver, British Columbia, Canada. 19-22 July 2015; abstract TUPEB273. [Google Scholar]
- 9.Napoli AA, Wood JJ, Coumbis JJ, Soitkar AM, Seekins DW, Tilson HH. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J Int AIDS Soc. 2014;17:19214. doi: 10.7448/IAS.17.1.19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkhoma ET, Coumbis J, Farr AM, Johnston SS, Chu BC, Rosenblatt LC, et al. No Evidence of an Association Between Efavirenz Exposure and Suicidality Among HIV Patients Initiating Antiretroviral Therapy in a Retrospective Cohort Study of Real World Data. Medicine (Baltimore) 2016;95(3):e2480. doi: 10.1097/MD.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Panel on Clinical Practices for Treatment of HIV Infection [Accessed April 28, 2016];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015 Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Updated January 28, 2016.
- 12.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306(1):287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 13.Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8(6):547–58. doi: 10.2217/14622416.8.6.547. [DOI] [PubMed] [Google Scholar]
- 14.Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine. Drug Metab Dispos. 2009;37(9):1793–6. doi: 10.1124/dmd.109.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzinger ER, Grady B, Ritchie MD, Ribaudo HJ, Acosta EP, Morse GD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genom. 2012;22(12):858–67. doi: 10.1097/FPC.0b013e32835a450b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18(18):2391–400. [PubMed] [Google Scholar]
- 17.Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M, et al. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319(4):1322–6. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 18.Haas DW, Smeaton LM, Shafer RW, Robbins GK, Morse GD, Labbe L, et al. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an Adult AIDS Clinical Trials Group Study. J Infect Dis. 2005;192(11):1931–42. doi: 10.1086/497610. [DOI] [PubMed] [Google Scholar]
- 19.Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genom. 2005;15(1):1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Novoa S, Barreiro P, Rendon A, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005;40(9):1358–61. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 21.dbSNP - Short Genetic Variations. National Center for Bioinformatics; [Accessed April 28, 2016]. 2015. Available at: http://www.ncbi.nlm.nih.gov/projects/SNP/ [Google Scholar]
- 22.Wang J, Sonnerborg A, Rane A, Josephson F, Lundgren S, Stahle L, et al. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genom. 2006;16(3):191–8. doi: 10.1097/01.fpc.0000189797.03845.90. [DOI] [PubMed] [Google Scholar]
- 23.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, et al. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemo. 2008;61(4):914–8. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribaudo HJ, Liu H, Schwab M, Schaeffeler E, Eichelbaum M, Motsinger-Reif AA, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis. 2010;202(5):717–22. doi: 10.1086/655470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genom. 2009;19(4):300–9. doi: 10.1097/FPC.0b013e328328d577. [DOI] [PubMed] [Google Scholar]
- 26.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009;67(4):427–36. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas DW, Kwara A, Richardson DM, Baker P, Papageorgiou I, Acosta EP, et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J Antimicrob Chemother. 2014;69(8):2175–82. doi: 10.1093/jac/dku110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15(1):71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 29.Gallego L, Barreiro P, del Rio R, Gonzalez de Requena D, Rodriguez-Albarino A, Gonzalez-Lahoz J, et al. Analyzing sleep abnormalities in HIV-infected patients treated with Efavirenz. Clin Infect Dis. 2004;38(3):430–2. doi: 10.1086/380791. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez F, Navarro A, Padilla S, Anton R, Masia M, Borras J, et al. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis. 2005;41(11):1648–53. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- 31.Puls R, Amin J, Losso M, Phanuphak P, Nwizu C, Orrell C, et al. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet. 2014;383(9927):1474–82. doi: 10.1016/S0140-6736(13)62187-X. [DOI] [PubMed] [Google Scholar]
- 32.Lubomirov R, Colombo S, di Iulio J, Ledergerber B, Martinez R, Cavassini M, et al. Association of pharmacogenetic markers with premature discontinuation of first-line anti-HIV therapy: an observational cohort study. J Infect Dis. 2011;203(2):246–57. doi: 10.1093/infdis/jiq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummins NW, Neuhaus J, Chu H, Neaton J, Wyen C, Rockstroh JK, et al. Investigation of Efavirenz Discontinuation in Multi-ethnic Populations of HIV-positive Individuals by Genetic Analysis. EBioMedicine. 2015;2(7):706–12. doi: 10.1016/j.ebiom.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhtar-Khaleel WZ, Cook RL, Shoptaw S, Surkan PJ, Teplin LA, Stall R, et al. Long-Term Cigarette Smoking Trajectories Among HIV-Seropositive and Seronegative MSM in the Multicenter AIDS Cohort Study. AIDS Behav. 2016 doi: 10.1007/s10461-016-1343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki H, Inoue K, Hashimoto M, Shimada T. Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch Toxicol. 1999;73(2):65–70. doi: 10.1007/s002040050588. [DOI] [PubMed] [Google Scholar]
- 36.Ring HZ, Valdes AM, Nishita DM, Prasad S, Jacob P, 3rd, Tyndale RF, et al. Gene-gene interactions between CYP2B6 and CYP2A6 in nicotine metabolism. Pharmacogenet Genomics. 2007;17(12):1007–15. doi: 10.1097/01.fpc.0000220560.59972.33. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson CS1, Miksys S, Palmour RM, Tyndale RF. Ethanol self-administration and nicotine treatment induce brain levels of CYP2B6 and CYP2E1 in African green monkeys. Neuropharmacology. 2013;72:74–81. doi: 10.1016/j.neuropharm.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS. 2011;25(3):388–90. doi: 10.1097/QAD.0b013e3283427e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dooley KE, Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, et al. Pharmacokinetics of Efavirenz and Treatment of HIV-1 Among Pregnant Women With and Without Tuberculosis Coinfection. J Infect Dis. 2015;211(2):197–205. doi: 10.1093/infdis/jiu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luetkemeyer AF, Rosenkranz SL, Lu D, Grinsztejn B, Sanchez J, Ssemmanda M, et al. Combined effect of CYP2B6 and NAT2 genotype on plasma efavirenz exposure during rifampin-based antituberculosis therapy in the STRIDE study. Clin Infect Dis. 2015;60(12):1860–3. doi: 10.1093/cid/civ155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatch MB, Kozlenkov A, Huang RQ, Yang W, Nguyen JD, Gonzalez-Maeso J, et al. The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology. 2013;38(12):2373–84. doi: 10.1038/npp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, et al. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis. 2007;45(9):1230–7. doi: 10.1086/522175. [DOI] [PubMed] [Google Scholar]
- 44.Schackman BR, Haas DW, Park SS, Li XC, Freedberg KA. Cost-effectiveness of CYP2B6 genotyping to optimize efavirenz dosing in HIV clinical practice. Pharmacogenomics. 2015;16(18):2007–18. doi: 10.2217/pgs.15.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.