Abstract
BACKGROUND
The use of surgery and radiation therapy in treating ductal carcinoma in situ (DCIS) is directed by treatment guidelines and evidence from research. We sought to investigate recent patterns in DCIS treatment by demographic factors.
METHODS
Data for women diagnosed with DCIS between 1998 and 2011 (n = 416,232) in the National Cancer Data Base were assessed for trends in treatment patterns by age group, calendar year, ancestral/ethnic group and geographic region. The likelihood of receiving specific treatment modalities was analyzed using multivariable logistic regression.
RESULTS
DCIS cases were most frequently treated with breast conserving surgery (BCS) and adjuvant radiation (45.6%). After an initial rise, the use of adjuvant radiation following BCS plateaued at around 70% after 2007, with increasing utilization of mastectomy beyond 2005. Additionally, there was an increasing trend in post-mastectomy reconstruction over time, and women of African ancestry (odds ratio, 0.69; 95% confidence interval,0.66–0.72) and Hispanic women were less likely to undergo reconstruction (odds ratio, 0.83; 95% confidence interval, 0.78–0.89) compared to women of European ancestry. A similar trend was observed in contralateral risk reducing mastectomy utilization, with women of European ancestry having a more rapid rise in the utilization of contralateral risk reducing mastectomy among all ancestral/ethnic groups.
CONCLUSION
Recent trends demonstrate a plateau in radiation therapy administration following BCS, with increasing utilization of mastectomy, reconstruction and contralateral risk reducing mastectomy. There are substantial differences in treatment utilization according to ancestry/ethnicity and geographical region. Further studies examining patient-physician decision making surrounding DCIS treatment are warranted.
Keywords: Breast cancer, ductal carcinoma in situ, mastectomy, reconstruction, radiation
INTRODUCTION
Ductal carcinoma in situ (DCIS) is a pre-invasive breast lesion, with one woman diagnosed with DCIS for every four women diagnosed with invasive breast cancer.1 Prior to routine mammography, DCIS lesions accounted for less than 5% of breast cancer cases.2 However, widespread screening mammography caused a rise in the detection of DCIS lesions.3 The incidence of DCIS in the US increased from 1.87 per 100,000 women in 1973–1975 to 32.5 in 2004.4
Various treatment options to lower the risk of recurrence and prevent invasive breast cancer are available for patients with DCIS. The DCIS 5-year mortality rate is <2%.5 Surgical excision with or without adjuvant therapy is the primary approach for DCIS treatment. Surgical options include breast conserving surgery (BCS) with or without radiotherapy, or mastectomy.2, 6 Adjuvant tamoxifen may also be utilized, especially among women with estrogen receptor (ER) positive disease.7
Variations in the utilization of treatment modalities for DCIS treatment likely result in under-treatment in some cases or overly aggressive surgical therapy for others.8, 9 Avoidance of adjuvant radiation therapy following BCS may increase the utilization of mastectomy despite the lack of overall survival benefit.10, 11 Geographic and temporal variations have been observed in the treatment of DCIS, with the Midwest and south-central states having higher rates of mastectomies compared to Northeastern states.8 Breast reconstruction following mastectomy is associated with geographical/regional location, institutional practice pattern, age and race/ethnicity.8, 10
The utilization of contralateral mastectomy (i.e. surgical removal of the uninvolved breast), particularly among high-risk women, is controversial. Factors associated with contralateral mastectomy include younger age, family history, genetic predisposition, tumor size and higher grade.12, 13
Given the historical variation in treatment of DCIS, we sought to examine recent trends using the National Cancer Data Base (NCDB) including the association of demographic factors with local DCIS treatment.
MATERIALS AND METHODS
Study Population
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. Over 1500 cancer care institutions contribute data to the NCDB, including 70% of all newly diagnosed cancers in the United States. Further details about the NCDB have been reported elsewhere.14, 15 We obtained data from the NCDB for women ≥20 years diagnosed with DCIS between 1998 and 2011. The study was approved by the University of Wisconsin-Madison institutional review board. Women diagnosed with DCIS were identified using International Classification of Diseases for Oncology third edition (behavior code 2 and morphology codes 8050, 8201, 8210, 8230, 8401, 8500, 8501, 8503, 8504, 8507, 8522, 8523, 8540 and 8543), and were coded as stage 0 according to the American Joint Committee on Cancer seventh edition guidelines.16, 17 A total of 434,695 cases met these criteria. Patients with no treatment data (n= 4,248), who had an unspecified mastectomy type with no information on receipt of reconstruction or contralateral mastectomy (n=1,562), extended radical mastectomy (n=87) or did not receive any treatment (n=12,566) were excluded.
Variables of Interest
Treatments were categorized as BCS, BCS with radiation, and mastectomy (i.e. total mastectomy). Women undergoing mastectomy were sub-classified based on whether they received contralateral mastectomy and/or breast reconstruction. Ancestry/ethnicity was classified as Non-Hispanic European, Non-Hispanic African, Hispanic, and other. Region of residence was categorized as Northeast, Midwest, West and South. Facility type was classified into community cancer program, comprehensive community cancer program, academic/research program (including NCI-designated comprehensive cancer centers) and other. Treatment facilities were divided into patient volume tertiles based on the number of women treated for DCIS.
Statistical Analysis
We estimated the odds ratios (OR) and 95% confidence intervals (CI) of receiving adjuvant radiation therapy following BCS and the utilization of BCS (with or without radiation therapy) compared to mastectomy using multivariable logistic regression models. Additionally, we evaluated breast reconstruction following mastectomy and contralateral breast removal following therapeutic mastectomy. In all models, covariates included age of diagnosis, ancestry/ethnicity, year of diagnosis and geographic region. We also adjusted for comorbidity, health insurance, tumor size and grade, treatment facility and institutional volume. Two sided P-values <0.05 were considered to be statistically significant. Interaction between ancestry/ethnicity and year of diagnosis were examined. Age-adjusted rates of surgeries following therapeutic mastectomy (i.e. breast reconstruction and contralateral risk-reducing mastectomy) by ancestral/ethnic groups were calculated using the 2000 U.S. standard million population.18 Analyses were performed using SAS®, version 9.3.
RESULTS
We identified 416,232 women diagnosed with DCIS between 1998 and 2011 (Table 1). Women in the 45–54 and 55–64 age groups accounted for most cases (over 26% each). Women of non-Hispanic European ancestry comprised most cases (80.4%). Over 95% had health insurance. 46% were treated with adjuvant radiation therapy and 29% received adjuvant endocrine therapy.
Table 1.
Characteristics of Women Diagnosed with Ductal Carcinoma in situ in the National Cancer Data Base, 1998–2011
| Characteristic | N | % |
|---|---|---|
| Total | 416,232 | |
| Age group, y | ||
| <45 | 47,567 | 11.4 |
| 45–54 | 108,907 | 26.2 |
| 55–64 | 109,767 | 26.4 |
| 65–74 | 89,712 | 21.5 |
| ≥75 | 60,285 | 14.5 |
| Year of diagnosis | ||
| 1998–1999 | 48,002 | 11.5 |
| 2000–2001 | 54,101 | 13.0 |
| 2002–2003 | 56,418 | 13.5 |
| 2004–2005 | 56,421 | 13.6 |
| 2006–2007 | 61,994 | 14.9 |
| 2008–2009 | 70,605 | 17.0 |
| 2010–2011 | 68,691 | 16.5 |
| Ancestry/ethnicity | ||
| Non-Hispanic, European | 334,757 | 80.4 |
| Non-Hispanic, African | 42,648 | 10.2 |
| Hispanic | 16,354 | 3.9 |
| Other | 22,473 | 5.4 |
| Geographic region | ||
| Northeast | 103,564 | 25.0 |
| Midwest | 102,289 | 24.5 |
| South | 139,354 | 33.5 |
| West | 71,025 | 17.0 |
| Health insurance | ||
| Private | 250,004 | 60.1 |
| Government | 151,069 | 36.3 |
| Uninsured | 6,173 | 1.5 |
| Unknown | 8,986 | 2.2 |
| Primary treatment | ||
| Breast conserving surgery without adjuvant radiation | 95,076 | 22.8 |
| Breast conserving surgery with adjuvant radiation | 189,847 | 45.6 |
| Mastectomy | 131,309 | 31.5 |
| Adjuvant endocrine therapy | ||
| Yes | 120,607 | 29.0 |
| No | 270,859 | 65.1 |
| Unknown | 24,766 | 5.9 |
| Facility type | ||
| Community cancer program | 40,832 | 9.8 |
| Comprehensive community cancer program | 247,915 | 59.5 |
| Academic/research program | 118,025 | 28.4 |
| Other specified types of cancer programs | 9,460 | 2.3 |
BCS and Mastectomy
Women ≥45 years were more likely to undergo BCS (Table 2). Compared to 1998–1999, women diagnosed since 1999 were more likely to undergo BCS, peaking during 2006–2007 (OR, 1.23; 95% CI, 1.16–1.31) and subsequently declining. Ancestry/ethnicity was associated with BCS treatment, as women of African and Hispanic ancestry were more likely to undergo BCS. Surgery patterns changed over time according to ancestry/ethnicity with BCS rates for women of African ancestry being lowest in 1998, while women of European ancestry had the lowest rates in 2011 (data not shown). Women outside the Northeast had lower odds of undergoing BCS.
Table 2.
Demographics of Breast Conserving Surgery among Women Diagnosed with Ductal Carcinoma in situ, National Cancer Data Base, 1998–2011
| Variable | Mastectomy (N= 131,309) Row % |
BCS (N= 284,923) Row % |
ORa (95% CI) |
|---|---|---|---|
| Age group, y | |||
| <45 | 43.5 | 56.5 | 1 |
| 45–54 | 32.9 | 67.1 | 1.60 (1.54–1.65) |
| 55–64 | 29.2 | 70.8 | 1.92 (1.85–1.99) |
| 65–74 | 28.4 | 71.6 | 2.14 (2.05–2.23) |
| ≥75 | 28.6 | 71.4 | 2.11 (2.02–2.21) |
| Year of diagnosis | |||
| 1998–1999 | 33.4 | 66.6 | 1 |
| 2000–2001 | 31.9 | 68.1 | 1.12 (1.08–1.16) |
| 2002–2003 | 29.9 | 70.1 | 1.21 (1.15–1.26) |
| 2004–2005 | 29.3 | 70.7 | 1.21 (1.13–1.29) |
| 2006–2007 | 30.2 | 69.8 | 1.23 (1.16–1.31) |
| 2008–2009 | 32.8 | 67.2 | 1.12 (1.05–1.19) |
| 2010–2011 | 33.1 | 66.9 | 1.12 (1.05–1.20) |
| Ancestry/ethnicity | |||
| Non-Hispanic, European | 31.4 | 68.6 | 1 |
| Non-Hispanic, African | 32.2 | 67.8 | 1.05 (1.01–1.08) |
| Hispanic | 31.6 | 68.4 | 1.14 (1.08–1.21) |
| Other | 32.3 | 67.7 | 1.00 (0.94–1.06) |
| Geographic region | |||
| Northeast | 25.9 | 74.1 | 1 |
| Midwest | 31.7 | 68.3 | 0.75 (0.73–0.77) |
| South | 35.2 | 64.8 | 0.64 (0.62–0.66) |
| West | 32.4 | 67.6 | 0.70 (0.68–0.73) |
Adjusted for comorbidity index, health insurance, facility type, DCIS patient volume, tumor size and grade
Test of interaction between year of diagnosis and ancestry/ethnicity: X2=42.70, df=18, P< 0.01
BCS with Adjuvant Radiation Therapy
Age was associated with the likelihood of undergoing adjuvant radiation therapy following BCS (Table 3). There was an increase in the proportion of women undergoing adjuvant radiation therapy following BCS from 58.5% in 1998–1999 to 70% during 2006–2011. Women of European ancestry were more likely to undergo adjuvant radiation therapy following BCS than other ancestral/ethnic groups. Women in the Midwest were more likely to receive adjuvant radiation therapy following BCS.
Table 3.
Demographics of Radiation Treatment Following Breast Conserving Surgery for Ductal Carcinoma in situ, National Cancer Data Base, 1998–2011
| Variable | BCS Only (N=95,076) Row % |
BCS with Adjuvant Radiation (N=189,847) Row % |
ORa (95% CI) |
|---|---|---|---|
| Age group, y | |||
| <45 | 31.0 | 69.0 | 1 |
| 45–54 | 29.1 | 70.9 | 1.07 (1.04–1.11) |
| 55–64 | 28.3 | 71.8 | 1.10 (1.07–1.14) |
| 65–74 | 32.7 | 67.3 | 0.95 (0.92–0.98) |
| ≥75 | 52.2 | 47.8 | 0.41 (0.39–0.43) |
| Year of diagnosis | |||
| 1998–1999 | 41.4 | 58.6 | 1 |
| 2000–2001 | 39.1 | 60.9 | 1.07 (1.04–1.11) |
| 2002–2003 | 36.2 | 63.8 | 1.12 (1.08–1.16) |
| 2004–2005 | 32.4 | 67.6 | 1.19 (1.13–1.25) |
| 2006–2007 | 29.2 | 70.8 | 1.38 (1.31–1.46) |
| 2008–2009 | 29.1 | 70.9 | 1.40 (1.32–1.47) |
| 2010–2011 | 29.9 | 70.1 | 1.32 (1.25–1.39) |
| Ancestry/ethnicity | |||
| Non-Hispanic, European | 32.9 | 67.1 | 1 |
| Non-Hispanic, African | 34.2 | 65.8 | 0.92 (0.90–0.95) |
| Hispanic | 36.8 | 63.2 | 0.86 (0.83–0.90) |
| Other | 35.6 | 64.4 | 0.89 (0.86–0.93) |
| Geographic region | |||
| Northeast | 36.2 | 63.8 | 1 |
| Midwest | 25.8 | 74.2 | 1.62 (1.58–1.65) |
| South | 35.0 | 65.0 | 0.99 (0.97–1.01) |
| West | 36.9 | 63.1 | 0.83 (0.81–0.85) |
Adjusted for comorbidity index, health insurance, facility type, DCIS patient volume, tumor size and grade
Test of interaction between year of diagnosis and ancestry/ethnicity: X2=21.03, df=18, P= 0.28
Breast Reconstruction following Mastectomy
Younger age at diagnosis was associated with undergoing breast reconstruction (Table 4). Women diagnosed in 2010–2011 were more likely to undergo reconstruction following mastectomy compared to women in 1998–1999 (OR, 3.57; 95% CI, 3.27–3.91). Breast reconstruction rates have been increasing among the three racial/ancestral groups with women of European ancestry having the highest rates (Figure 1A). Women in the Northeast were more likely to undergo breast reconstruction following mastectomy.
Table 4.
Demographics of Reconstruction and Contralateral Risk Reduction Mastectomy among Women Diagnosed with Ductal Carcinoma in situ, National Cancer Data Base, 1998–2011
| Variable | Mastectomy alone (N=87,130) Row % |
Mastectomy with Reconstruction (N=44,179) Row % |
ORa (95% CI) | Unilateral Mastectomy (N=104,970) Row % |
Contralateral Mastectomy (N=26,339) Row % |
ORa (95% CI) |
|---|---|---|---|---|---|---|
| Age group, y | ||||||
| <45 | 45.5 | 54.5 | 1 | 67.1 | 32.9 | 1 |
| 45–54 | 52.0 | 48.0 | 0.75 (0.72–0.79) | 73.6 | 26.4 | 0.67 (0.65–0.70) |
| 55–64 | 66.0 | 34.0 | 0.42 (0.41–0.44) | 80.3 | 19.7 | 0.45 (0.43–0.47) |
| 65–74 | 83.9 | 16.1 | 0.24 (0.23–0.25) | 88.8 | 11.2 | 0.29 (0.27–0.31) |
| ≥75 | 95.9 | 4.1 | 0.06 (0.05–0.06) | 94.8 | 5.2 | 0.13 (0.12–0.14) |
| Year of diagnosis | ||||||
| 1998–1999 | 78.7 | 21.3 | 1 | 91.4 | 8.6 | 1 |
| 2000–2001 | 74.5 | 25.5 | 1.31 (1.24–1.38) | 88.4 | 11.6 | 1.43 (1.33–1.54) |
| 2002–2003 | 72.9 | 27.1 | 1.40 (1.31–1.49) | 85.0 | 15.0 | 1.85 (1.70–2.01) |
| 2004–2005 | 69.9 | 30.1 | 1.57 (1.43–1.72) | 82.0 | 18.0 | 2.12 (1.93–2.41) |
| 2006–2007 | 64.5 | 35.5 | 2.04 (1.86–2.23) | 77.3 | 22.7 | 2.95 (2.64–3.29) |
| 2008–2009 | 58.5 | 41.5 | 2.76 (2.52–3.02) | 72.8 | 27.2 | 3.79 (3.40–4.23) |
| 2010–2011 | 53.6 | 46.4 | 3.57 (3.27–3.91) | 69.7 | 30.3 | 4.56 (4.09–5.08) |
| Ancestry/ethnicity | ||||||
| Non-Hispanic, European | 65.5 | 34.5 | 1 | 78.5 | 21.5 | 1 |
| Non-Hispanic, African | 72.0 | 28.0 | 0.69 (0.66–0.72) | 88.5 | 11.5 | 0.43 (0.41–0.45) |
| Hispanic | 66.3 | 33.7 | 0.83 (0.78–0.89) | 83.2 | 16.8 | 0.57 (0.53–0.62) |
| Other | 68.0 | 32.0 | 0.66 (0.63–0.70) | 82.9 | 17.1 | 0.56 (0.52–0.60) |
| Geographic region | ||||||
| Northeast | 60.6 | 39.5 | 1 | 81.2 | 18.8 | 1 |
| Midwest | 66.0 | 34.0 | 0.88 (0.85–0.92) | 80.6 | 19.5 | 1.14 (1.10–1.20) |
| South | 68.5 | 31.5 | 0.81 (0.78–0.84) | 80.0 | 20.0 | 1.29 (1.24–1.34) |
| West | 69.0 | 31.0 | 0.72 (0.68–0.75) | 77.4 | 22.6 | 1.49 (1.42–1.56) |
Adjusted for comorbidity index, health insurance, facility type, DCIS patient volume, tumor size and grade
Reconstruction: Test of interaction between year of diagnosis and ancestry/ethnicity: X2=25.90, df=18, P=0.10
Contralateral risk reducing mastectomy: Test of interaction between year of diagnosis and ancestry/ethnicity: X2=27.63, df=18, P=0.07
Figure 1.
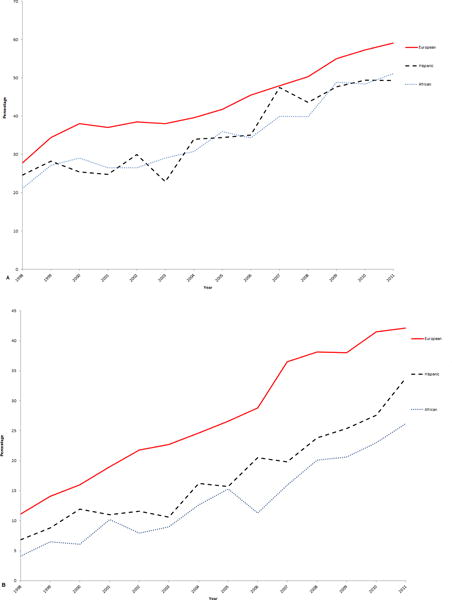
Age-adjusted annual proportion of patients undergoing (A) reconstruction and (B) risk reducing contralateral mastectomy among women with mastectomy for ductal carcinoma in situ according to European, African, and Hispanic ancestry, National Cancer Data Base, 1998–2011.
Contralateral Risk Reducing Mastectomy
Rates of contralateral risk reducing mastectomy decreased with increasing age at diagnosis (Table 4). Women diagnosed in 2010 were more likely to undergo contralateral mastectomy than women diagnosed in 1998–1999 (OR, 4.56; 95% CI, 4.09–5.08). The annual proportion of women undergoing contralateral mastectomy increased in all 3 racial/ancestral groups (Figure 1B). Women outside the Northeast were more likely to undergo contralateral mastectomy.
DISCUSSION
In analyzing the patterns of care for DCIS among women using a large nationwide clinical database, we observed an increase in BCS among women diagnosed with DCIS between 1998 and 2005. This was followed by a decline in BCS through 2011, with a corresponding rise in mastectomy utilization. This is consistent with previous observations of increasing mastectomy rates among women with early stage breast cancer.19, 20 Unlike previous studies which included small invasive node negative cancers and in situ cancer, we observed these findings specifically among DCIS patients.
Using the NCDB, we observed an increase in adjuvant radiation therapy utilization following BCS until 2007. BCS and adjuvant radiation treatment is beneficial in preventing localized ipsilateral breast cancer recurrence compared to BCS alone, with similar survival benefit to mastectomy.11, 21 Although most women were treated with BCS and adjuvant radiation therapy (46%), the proportion of women undergoing adjuvant radiation therapy following BCS has plateaued at 70% after 2007. The increasing trend in the proportion of women undergoing adjuvant radiation therapy following DCIS diagnosis has been previously shown.8, 22 However, our findings suggest adjuvant radiation therapy utilization may be at a saturation level. Not all women diagnosed with DCIS undergoing BCS are ideal candidates for adjuvant radiation therapy and women may have concerns regarding adverse effects of radiation. Social factors such as cultural beliefs, marital status and social support may be related to choice of undergoing radiation therapy following BCS.23, 24 In terms of population density/metro area, previous research has demonstrated differences in receipt of radiotherapy among breast cancer patients.25 Specifically, a greater proportion of women dwelling in urban areas receive adjuvant radiation treatment compared to women with rural residence locations. Additionally, women living at an increased distance from a hospital with a radiotherapy facility were less likely to undergo BCS.26
Since 2005, the proportion of women undergoing mastectomy following DCIS has increased, despite BCS with adjuvant radiation therapy generally being an appropriate and less extensive treatment option. Apart from concerns about the effects of radiation therapy, some women may be dissatisfied with their cosmetic outcome following BCS.27 Breast reconstruction following mastectomy may be favored for cosmetic and psychological reasons.28, 29 Legislative mandates such as the Women’s Health and Cancer Rights Act (WHCRA) requiring coverage for breast reconstruction following mastectomy by most insurance plans may have influenced the increase. A recent study observed 2-to-4-fold increases in reconstruction following the enactment of the legislation.30
Throughout the study period, women of European ancestry consistently had higher proportions undergoing breast reconstruction following mastectomy. However, women of African ancestry and Hispanic women showed an increasing trend in post-mastectomy reconstruction, almost parallel to that observed among women of European ancestry. Lack of insurance coverage, lack of knowledge about post-mastectomy reconstruction, cultural issues and socioeconomic status have been previously associated with observed differences in post-mastectomy reconstruction by ancestry/ethnicity.31, 32
We observed an increasing trend in the utilization of contralateral risk reducing mastectomy among women undergoing mastectomy and a more rapid rise among women of European ancestry compared to other racial/ancestral groups. This trend has been observed previously among woman <45 years of age diagnosed with early stage breast cancer.33 Previous research has also shown similar prevalence of BRCA1/2 mutations among breast cancer patients of European, African, and Hispanic ancestry.34 Mammography screening rates appear to be higher among women of European ancestry.35, 36 Ancestral/ethnic differences in screening may be lead to differences in diagnosis and treatment. Furthermore, previous research has shown that women of European ancestry are less likely to delegate treatment decisions to their physicians.37 This may be related to higher educational attainment.38 Women with higher levels of educational attainment have increased participation in surgical decision making and are more likely to undergo mastectomy.39, 40
Breast cancer diagnosed in younger women is associated with a higher risk of recurrence following breast conserving surgery.41 Undergoing lifelong surveillance may be disruptive and anxiety provoking for some. Hence, younger women may prefer to undergo mastectomy including the removal of the uninvolved breast. The decision to undergo mastectomy may be influenced by multifocal or widespread disease, positive margins, age, physician’s preference, access to radiation facilities, fear of recurrence and insurance coverage.19, 20, 42 For many women, bilateral mastectomy may be considered aggressive treatment given the generally low absolute risk of a future invasive carcinoma. There is no overall survival benefit for contralateral risk reducing mastectomy in early stage breast cancer among ER-negative patients.43 Survival benefits seen in some studies may be due to selection bias.44 Among BRCA1/2 mutation carriers, contralateral mastectomy may confer a survival advantage.45 Despite comparable overall survival to BCS with adjuvant radiation therapy, mastectomy in some instances may be a preferred treatment option among women diagnosed with DCIS without any deleterious BRCA mutations (such as in multifocal disease).6, 11The role of contralateral mastectomy for DCIS treatment in general, is debatable.
Geographical variations in the utilization of surgical treatments including post-mastectomy reconstruction among women diagnosed with DCIS have been documented previously.8 We observed persistent geographic variations in the utilization of DCIS treatment options. For instance, the Northeast had the greatest odds of undergoing breast conserving surgery and reconstruction following mastectomy, and the smallest odds of undergoing contralateral mastectomy. This may suggest a preference towards aesthetic preservation in the Northeast. Regional variations may reflect practice differences among institutions and available surgical expertise. In our study, the West and South compared to Northeast had the highest odds ratios for contralateral mastectomy and the least odds ratios for breast conserving surgery alone and with adjuvant radiation therapy. The variations observed in the utilization of contralateral mastectomy may be related to physician preferences including institutional practice patterns, and access to radiation treatment facilities.26, 46 The presence of more surgeons with reconstruction expertise in treatment facilities is associated with increased utilization of these procedures following mastectomy.10
The NCDB is a rich resource for examining patterns of DCIS treatment, but it does have limitations. Cancer cases are from only Commission on Cancer accredited hospitals. Hence, the NCDB may represent selected cases. The inability to differentiate between immediate and delayed reconstruction was another limitation. The absence of data on hormone receptor status and human epidermal growth factor receptor 2 (HER2/neu) for most patients and lack of information on some genetic markers such as BRCA gene status precluded the assessment of treatment variation according DCIS molecular subtypes and genetic risk. Finally, we lacked information on patients’ preferences and physician’s characteristics including variations in the geographic distribution of reconstructive surgeons and radiation oncologists. However, our study findings corroborate findings from population based cancer registry data such as SEER.19, 20 The NCDB has the added advantage of being the largest national cancer registry, with data from over 70% of new cancer cases, from health facilities ranging from academic to community based cancer facilities. With this resource, we have been able to provide updated information regarding trends in local therapies for DCIS treatment with the discovery of some new findings.
CONCLUSION
In assessing patterns of care for women diagnosed with DCIS, substantial variation exists in all four major local treatment decisions. Significant differences between treatment types were observed according to ancestry/ethnicity and geographical region. There was increasing utilization of adjuvant radiation treatment following breast conserving surgery and breast reconstruction following mastectomy since 1998. These increases coincided with the introduction of policies and clinical guidelines that favored their utilization. The study period mostly encompassed the years prior to the passage of the Patient Protection and Affordable Care Act of 2010. It will be interesting to examine trends in DCIS treatment following the implementation of this legislation. Finally, the impact of treatment variation on cancer recurrence and progression to invasive cancer warrants further investigation.
Acknowledgments
We would like to thank Julie McGregor, Kathy Peck, Laura Stephenson, Berta Geller, Dawn Pelkey, Kathleen Howe, and John Mace for study support and data collection. Additionally, we thank Caprice Greenberg MD, MPH for her suggestions while preparing the manuscript. All data utilized in our analysis were de-identified and derived from the NCDB. The authors bear sole responsibility for reporting and interpretation of the analyzed data. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
FUNDING SOURCE: NIH/NCI grants U54 CA163303, R01 CA067264, and P30 CA014520.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
AUTHOR CONTRIBUTION
Conception and design: Amy Trentham-Dietz, Oyewale O. Shiyanbola, Brian L. Sprague, Donald L. Weaver
Data analysis and interpretation: Oyewale O. Shiyanbola, John M. Hampton, Amy Trentham-Dietz, Brian L. Sprague, Donald L. Weaver, Kim Dittus, Ted A. James, Sally Herschorn, Ronald E. Gangnon
Manuscript writing: Oyewale O. Shiyanbola, Amy Trentham-Dietz, Ronald E. Gangnon, Brian L. Sprague, Kim Dittus, Ted A. James, Sally Herschorn, Donald L. Weaver
Final approval: Oyewale O. Shiyanbola, Amy Trentham-Dietz, Ronald E. Gangnon, Brian L. Sprague, Donald L. Weaver, Kim Dittus, John M. Hampton Ted A. James, Sally Herschorn
References
- 1.Allegra CJ, Aberle DR, Ganschow P, et al. NIH state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ (DCIS) NIH Consens State Sci Statements. 2009;26:1–27. [PubMed] [Google Scholar]
- 2.Arpino G, Laucirica R, Elledge RM. Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005;143:446–457. doi: 10.7326/0003-4819-143-6-200509200-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ernster VL, Barclay J. Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: a dilemma. JNCI Monographs. 1997;1997:151–156. doi: 10.1093/jncimono/1997.22.151. [DOI] [PubMed] [Google Scholar]
- 4.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 5.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160:953–958. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Breast Cancer: NCCN Guidelines for Patients Version 2. National Comprehensive Cancer Network. 2011 Available from URL: http://www.nccn.org/patients/guidelines/breast/index.html#1/z [accessed April 30, 2014]
- 7.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 9.Joslyn SA. Ductal carcinoma in situ: trends in geographic, temporal, and demographic patterns of care and survival. Breast J. 2006;12:20–27. doi: 10.1111/j.1075-122X.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg CC, Lipsitz SR, Hughes ME, et al. Institutional variation in the surgical treatment of breast cancer: a study of the NCCN. Ann Surg. 2011;254:339–345. doi: 10.1097/SLA.0b013e3182263bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 12.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29:2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 13.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 14.Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–78. doi: 10.1016/j.juro.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen ME, Mallin K, Weaver MA, et al. The Association of Hospital Volume With Conditional 90-day Mortality After Cystectomy: An Analysis of the National Cancer Database. BJU Int. 2013 doi: 10.1111/bju.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Geneva: World Health Organization; 2000. [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York, NY: Springer; 2010. [Google Scholar]
- 18.Surveillance Epidemiology and End Results Program. Standard Populations-19 Age Groups: U.S. Standard Population 1940–2000. Available from URL: http://seer.cancer.gov/stdpopulations/stdpop.19ages.html [accessed June 2, 2015]
- 19.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013;20:1436–1443. doi: 10.1245/s10434-012-2732-5. [DOI] [PubMed] [Google Scholar]
- 20.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16:2682–2690. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 21.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 22.Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst. 2015;107:djv263. doi: 10.1093/jnci/djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25:2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 24.Fontana V, Castro T, Polynice A. Preferences of healthy inner city women and the surgical treatment of early stage breast cancer. Am Surg. 2007;73:215–221. [PubMed] [Google Scholar]
- 25.Baldwin LM, Patel S, Andrilla CH, Rosenblatt RA, Doescher MP. Receipt of recommended radiation therapy among rural and urban cancer patients. Cancer. 2012;118:5100–5109. doi: 10.1002/cncr.27488. [DOI] [PubMed] [Google Scholar]
- 26.Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–1346. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 27.Wang HT, Barone CM, Steigelman MB, et al. Aesthetic outcomes in breast conservation therapy. Aesthet Surg J. 2008;28:165–170. doi: 10.1016/j.asj.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Duggal CS, Metcalfe D, Sackeyfio R, Carlson GW, Losken A. Patient motivations for choosing postmastectomy breast reconstruction. Ann Plast Surg. 2013;70:574–580. doi: 10.1097/SAP.0b013e3182851052. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106:1014–1025. doi: 10.1097/00006534-200010000-00010. discussion 1026–1017. [DOI] [PubMed] [Google Scholar]
- 30.Yang RL, Newman AS, Lin IC, et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119:2462–2468. doi: 10.1002/cncr.28050. [DOI] [PubMed] [Google Scholar]
- 31.Morrow M, Mujahid M, Lantz PM, et al. Correlates of breast reconstruction: results from a population-based study. Cancer. 2005;104:2340–2346. doi: 10.1002/cncr.21444. [DOI] [PubMed] [Google Scholar]
- 32.Kruper L, Xu X, Henderson K, Bernstein L. Disparities in reconstruction rates after mastectomy for ductal carcinoma in situ (DCIS): patterns of care and factors associated with the use of breast reconstruction for DCIS compared with invasive cancer. Ann Surg Oncol. 2011;18:3210–3219. doi: 10.1245/s10434-011-2010-y. [DOI] [PubMed] [Google Scholar]
- 33.Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in Contralateral Prophylactic Mastectomy Rates According to Racial Groups in Young Women with Breast Cancer, 1998 to 2011: A Report from the National Cancer Data Base. J Am Coll Surg. 2015;221:187–196. doi: 10.1016/j.jamcollsurg.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72–78. doi: 10.1097/GCO.0b013e328332dca3. [DOI] [PubMed] [Google Scholar]
- 35.Rao SR, Breen N, Graubard BI. Trends in Black-White Disparities in Breast and Colorectal Cancer Screening Rates in a Changing Screening Environment: The Peters-Belson Approach Using United States National Health Interview Surveys 2000–2010. Med Care. 2015 doi: 10.1097/MLR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 36.Wharam JF, Landon BE, Xu X, Zhang F, Ross-Degnan D. National Trends and Disparities in Mammography Among Commercially Insured Women, 2001–2010. J Public Health Manag Pract. 2015;21:426–432. doi: 10.1097/PHH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 37.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Center for Education Statistics. The Condition of Education : Educational Attainment. Available from URL: https://nces.ed.gov/programs/coe/indicator_caa.asp [accessed November 29, 2015]
- 39.Hawley ST, Lantz PM, Janz NK, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007;65:387–395. doi: 10.1016/j.pec.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101:1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neff PT, Bear HD, Pierce CV, et al. Long-term results of breast conservation therapy for breast cancer. Ann Surg. 1996;223:709–716. doi: 10.1097/00000658-199606000-00009. discussion 716–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruper L, Xu XX, Henderson K, Bernstein L, Chen SL. Utilization of mastectomy and reconstruction in the outpatient setting. Ann Surg Oncol. 2013;20:828–835. doi: 10.1245/s10434-012-2661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol. 2014;21:3231–3239. doi: 10.1245/s10434-014-3956-3. [DOI] [PubMed] [Google Scholar]
- 44.Jatoi I, Parsons HM. Contralateral prophylactic mastectomy and its association with reduced mortality: evidence for selection bias. Breast Cancer Res Treat. 2014;148:389–396. doi: 10.1007/s10549-014-3160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans DG, Ingham SL, Baildam A, et al. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat. 2013;140:135–142. doi: 10.1007/s10549-013-2583-1. [DOI] [PubMed] [Google Scholar]
- 46.Feinstein AJ, Soulos PR, Long JB, et al. Variation in receipt of radiation therapy after breast-conserving surgery: assessing the impact of physicians and geographic regions. Med Care. 2013;51:330–338. doi: 10.1097/MLR.0b013e31827631b0. [DOI] [PMC free article] [PubMed] [Google Scholar]


