Abstract
Classic studies of object-based attention have utilized keypress responses as the main dependent measure. However, people typically make saccades to fixate important objects. Recent work has shown that attention may act differently when deployed covertly versus in advance of a saccade. We further investigated the link between saccades and attention by examining whether object-based effects can be observed for saccades. We adapted the classical double-rectangle cueing paradigm of Egly et al., (1994), and measured both the first saccade latency and keypress reaction time (RT) to a target that appeared at the end of one of the two rectangles. Our results showed that saccade latency exhibited higher sensitivity than RT in detecting effects of attention. We also assessed the generality of the attention effects by testing three types of cues: hybrid (predictive and peripheral), exogenous (non-predictive and peripheral), and endogenous (predictive and central). We found that both RT and saccade latency exhibited effects of both space-based and object-based attentional selection. However, saccade latency showed a more robust attentional modulation than RTs. For the exogenous cue, we observed a spatial inhibition-of-return along with an object-based effect, implying that object-based attention is independent of space-based attention. Overall, our results reveal an oculomotor correlate of object-based attention, suggesting that, in addition to spatial priority, object-level priority also affects saccade planning.
Introduction
The amount of visual information available at any moment is far too sizable for our nervous system to handle. This requires some prioritization of information so as not to overwhelm the system. Enhancement of relevant stimuli and suppression of irrelevant stimuli are, thus, crucial for survival. Attention serves to prioritize important stimuli by increasing neural responses to attended ones and decreasing neural responses to unattended ones (see Kanwisher & Wojciulik, 2000 for review). In certain circumstances, attention is able to select information on the basis of objectness (see Chen, 2012 for review). Attending to an object enhances properties of that object, such that (after reporting one object feature) it becomes easier to report a second feature of the attended object, versus reporting a feature of an unattended object (Duncan, 1984). The focus of the current study is to broaden our knowledge of the conditions under which attention is object-based.
In a seminal study, Egly, Driver, and Rafal (1994) investigated the effects of both space-based and object-based attention within a single paradigm. Two rectangles were shown on each trial. One end of a rectangle was cued, followed by a target at one of three locations: (1) the cued end of the rectangle (valid location), (2) the uncued end of the cued rectangle (same-object location), or (3) the equidistant end of the uncued rectangle (different-object location). Results showed that less time was required to detect the target at the cued location than any other location; evidence of spatial cueing. Critically, target detection was also faster at the same-object location compared to the different-object location. This performance difference, often referred to as the “same-object advantage”, cannot be explained by spatial attention, as the two invalid locations were equidistant from the cue. Hence, the same-object advantage reflects the attentional priority assigned to the cued object, i.e., object-based attention. Further studies using the double-rectangle paradigm found that both detection (Atchley & Kramer, 2001; He, Fan, Zhou, & Chen, 2004; Lamy & Egeth, 2002) and discrimination (Drummond & Shomstein, 2010; Macquistan, 1997; Moore, Yantis, & Vaughan, 1998; Shomstein & Behrmann, 2008; Shomstein & Yantis, 2004) performance exhibited a same object advantage.
Nearly all object-based attention studies have investigated covert attention, i.e., attention in the absence of eye movements. In everyday life, however, people tend to fixate objects of interest via saccades (overt attention). Thus, in the interest of ecological validity, it is important to know whether object-based selection occurs for overt attention; in other words, whether saccades are affected by deployment of attention to the whole object once a part of the object has been selected.
The relationship between saccades and space-based covert attention has been of great interest to researchers. In particular, many studies have investigated how space-based attention affects the latency to initiate saccades, and have found that attention can have a small, but significant, effect on saccade latency. For example, Crawford and Müller (1992) presented exogenous cues followed by simple visual targets in a detection task and instructed participants to either execute an eye movement to the target or a keypress. They found that both saccade latency and keypress reaction time (RT) to targets at the cued location were faster than to other locations. These results suggest that exogenously driven space-based attention accelerates saccade preparation. Further studies have also found that endogenous space-based attention, as manipulated by reward and target predictability, influences saccade latency. For example, differentially rewarding saccades to a specific location led to faster latencies at higher-rewarded locations compared to other target locations (Kawagoe, Takikawa, & Hikosaka, 1998; Rothkirch, Ostendorf, Sax, & Sterzer, 2013, Takikawa, Kawagoe, Itoh, Nakahara, & Hikosaka, 2002). Likewise, as the probability of target appearance increases at a location, saccade latency to that location becomes faster (Abrams & Jonides, 1988; Dorris & Munoz, 1998; Murray & Giggey, 2006). These studies establish that saccade latency is a useful metric to index the deployment of attention.
The abovementioned studies on saccade latencies are consistent with the general notion that covert and overt attention share largely similar mechanisms, which is supported by findings showing coupling between saccades and attention (Deubel & Schneider, 1996; Godijn & Pratt, 2002; Hoffman & Subramaniam, 1995; Kowler, Anderson, Dosher, & Blaser, 1995; Shepherd, Findlay, & Hockey, 1986). Indeed, the premotor theory of attention suggests that overt and covert attention are the same phenomena (see Rizzolatti, Riggio, Dascola, & Umiltá, 1987; Sheliga, Riggio, & Rizzolatti, 1994). The similarity between covert and overt attention was further supported by physiological findings showing that saccade preparation and covert attention are processed in the same brain regions (for a review see Awh, Armstrong, & Moore, 2006). Although the coupling between covert and overt attention is generally accepted, there is also counter-evidence that challenges the strong coupling between covert and overt spatial attention. For example, attention is not always deployed to the saccade-prepared location (Hunt & Kingstone, 2003; Klein, 1980), and attentional deployment does not necessarily engender saccade preparation (Juan, Shorter-Jacobi, & Schall, 2004). In addition, saccade preparation deploys attention to the saccade prepared location much faster than deployment of covert attention (Rolfs & Carrasco, 2012). Lastly, physiological studies suggest that different sub-populations of neurons process attention and saccades (Juan et al., 2004; Sato & Schall, 2003; Thompson, Bichot, & Schall, 1997; Thompson, Biscoe, & Sato, 2005). Thus, although covert and overt spatial attention are usually correlated, they do not have identical properties (see also Smith & Schenk, 2012). Given the observed dissociations between overt and covert attention, one might not predict that saccades will follow the same pattern as covert attention in observing object-based effects as measured in the two-rectangle paradigm.
These considerations thus led us to investigate whether object-based attention also influences saccade latency. Few studies have investigated the effect of object-based attention on eye movements. McCarley, Kramer and Peterson (2002) and Theeuwes, Mathôt, and Kingstone (2010) used the double-rectangle cueing paradigm to investigate how object-based attention affects eye movements. In their experiments, after the initial spatial cue disappeared, participants first made a saccade to the cued location, which was followed by the presentation of three distractor letters and one target, each located at one end of one rectangle. The task was to report the identity/orientation of a small target letter that required foveating. Participants, thus, made a second saccade to the target, which became the focus of the analyses. Both studies found that the second saccade tended to stay within the cued object. Furthermore, McCarley et al. (2002) found that latency for the second saccade to the same-object location was faster than to the different-object location, although such a difference was not observed in the Theeuwes et al. (2010), study. A more recent experiment with a similar design using real-world pictures also reported that the second saccade latency to the same-object location was faster than the different-object location (Malcolm & Shomstein, 2015).
These results imply that there is a relationship between object-based attention and eye movements, but they are inconclusive for several reasons. First, the effect of object-based attention on second saccade latency was not consistently observed. Second, there was a potential low-level confound in the results for the second saccade. In these studies, the second saccade always began at the cued location. However, once the eyes landed on the cued location, there were several retinal-level differences between the same and different object locations. One of these was the presence of obstructing object edges in the path to the different-object location (which could impede saccades to this location), but an absence of such edges for the same-object location. More importantly, the saccade path to the same-object location was delineated by elongated lines (those of the rectangle edges) that were absent on the saccade path to the different-object location. ‘Following elongated edges’ can produce the same effect as ‘obstructing object edges’—they both lead to faster RTs at the same object location than the different object location. Importantly, however, there is empirical evidence for the ‘following elongated edges’ idea, such that the presence of contour lines on the saccade path biases eye movements along the direction of the contours (Wismeijer & Gegenfurtner, 2012). Thus, the same-object advantage of eye movements in these studies might have been caused by the presence of guiding lines along the same-object location rather than the effects of object-based attention.
Notably, in these studies, researchers did not focus on the first saccade, which is arguably a better measure of the initial distribution of attention to the entire display. Furthermore, at the time of the first saccade, the visual scene is symmetric, so low-level retinal features are equated (which serves to avoid the confound of guiding contours and obstructing edges detailed above). Our aim here is to investigate the effect of object-based attention on first saccade latency in the double-rectangle paradigm. We also varied the nature of the initial cue, using exogenous, endogenous, and a hybrid spatial cue, to examine the generality of the coupling between object-based attention and saccade preparation. In three experiments, we first measured the effect of object-based attention via conventional keypress RT, which provided a baseline measure. The saccade experiments used identical visual stimuli but participants made a rapid eye-movement to the target. We measured the latency of the first saccade to the target as an index of the initial distribution of attention. To preview our results, we found strong evidence that object-based attention facilitated both keypress RT and saccade latency.
Experiment 1: Hybrid cue
The double-rectangle cueing paradigm first introduced by Egly et al. (1994) used a hybrid cue, i.e., a peripheral cue that is also predictive of the target location. Previous research using the double-rectangle paradigm with hybrid cues has yielded results supporting Egly’s original findings (Atchley & Kramer, 2001; Moore, Yantis, & Vaughan, 1998; Shomstein & Behrmann, 2008). Here, we also performed a replication of Egly et al.’s (1994) original finding under our experimental setup, which serves as a point of departure to explore object-based effects in a novel saccade task. In our first experiment with keypress responses (Experiment 1a), we predicted both space-based effects and object-based effects in keypress RTs. We then conducted an experiment to investigate whether the effect of object-based attention can be detected using saccade latency (Experiment 1b). If object-based attention affects eye movement preparation, we should find analogous effects in saccade latency: faster first saccade latencies for targets appearing at the invalid same-object location compared to the invalid different-object location.
Experiment 1a-Methods
Participants
Sixteen participants (age ~18–30 years) provided written informed consent and took part in the experiment: all were students from Michigan State University. One participants’ data were excluded due to excessive premature responses (61.25% of trials). Participants had normal, or corrected-to-normal, vision and did not have any neurological or psychiatric disorders. They were compensated with course extra credit or $10/hour, with a bonus of $5 after the completion of the experiment. The experimental protocol was approved by the Institutional Review Board at Michigan State University.
Apparatus and Visual Stimuli
The experiment was programmed in MATLAB (The Mathworks, Natick, MA) with MGL extensions (http://gru.stanford.edu/doku.php/mgl/overview). The stimuli were displayed on a 19-inch CRT monitor (resolution: 1024×768 pixels) at a vertical refresh rate of 100Hz. The viewing distance was 68 cm ensured via the use of a chinrest.
The background of the display remained black (luminance: 0.051 cd/m2) throughout the experiment. The fixation cross (line length: 0.05°×0.05°, line width: 0.03°×0.03°) was a gray (luminance: 16.19 cd/m2) “plus sign” located at the center of the screen. Following Egly et al. (1994), we used two rectangle outlines as objects. The inner areas of the rectangles matched the screen background and the rectangle edges were delineated by gray lines (thickness: 0.2°, luminance: 16.19 cd/m2). The distance from the centroid of the each rectangle to the fixation cross was 4.8°. In the vertical orientation, each object’s centroid fell on the screen’s horizontal meridian, while in the horizontal orientation, each object’s centroid fell on the screen’s vertical meridian.
The spatial cue was a white thickening (thickness: 0.4°, luminance: 64.78 cd/m2) at one end of a rectangle. All three sides of the cue were equal to the length of the short edge of the rectangle. The target was a light gray circle (size: 1°×1°, luminance: 35.71 cd/m2), located at the end of the rectangle, at an eccentricity of 6.79° (see Figure 1 for example stimuli).
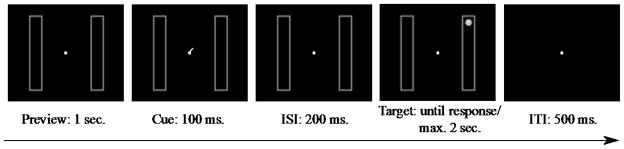
Figure 1.
The sequence of a trial in Experiment 1. The rectangles appeared in horizontal or vertical orientation (vertical is shown here). A peripheral cue flashed for 100 ms. After a 200ms cue-to-target interval, the target appeared at the cued location (60%), the invalid same-object location (10%), the invalid different-object location (10%), or it did not appear (20%).
Design and Procedure
The experiments took place in a dimly lit room. A fixation cross and two objects appeared on the screen at the beginning of each trial. The orientation of the objects (vertical vs. horizontal) was randomized across trials. One second after the onset of the initial display, the cue appeared randomly at one of the four corners of the two objects for 100 ms. After a 200 ms interval, the target appeared at the cued location on 60% of trials, at the uncued end of the cued object (same object) on 10% of trials, and at the equidistant corner of the uncued object on 10% of trials (different object). The remaining trials (20%) were “catch trials” in which the target did not appear. There were 10 blocks, each consisting of 80 trials.
Subjects were instructed to keep their eyes on the fixation cross throughout the experiment and quickly press the spacebar with their dominant hand as soon as the target appeared. In the absence of a correct keypress response (and on target-absent trials), the trial was terminated 2 seconds after the target appeared. The subsequent trial began 500 ms after the termination of the previous trial (Figure 1). Eye position was not recorded.
Experiment 1a-Results
For all statistical tests, we report the Greenhouse-Geisser corrected values when necessitated by Mauchly’s test of sphericity.
The Eligible Trials
We excluded all trials in which the reaction time was shorter than 200 ms or longer than 1500 ms. The proportion of eligible trials in each condition was submitted to a 2 (orientation: vertical, horizontal) × 3 (target location: cued location, same object, different object locations) repeated-measures ANOVA. None of the effects reached significance (all p > 0.1). On average, 0.96 of trials were eligible for analysis.
Reaction Time
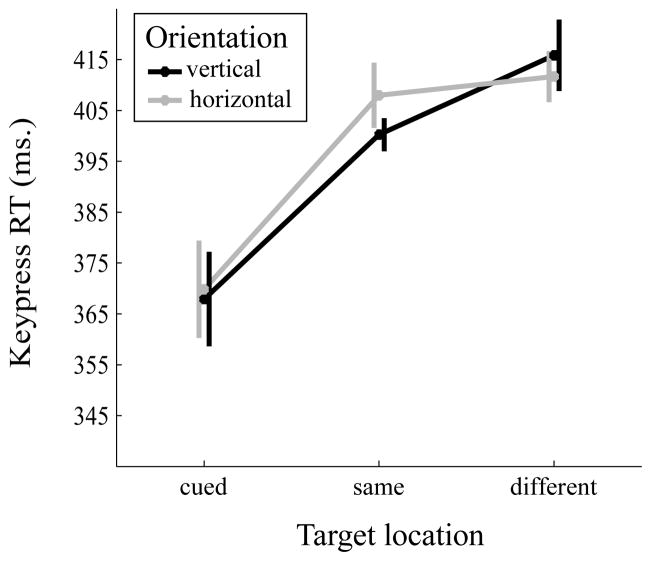
A 2 (orientation: vertical, horizontal) × 3 (target location: cued location, same object, different object locations) repeated-measures ANOVA was performed on the mean keypress RTs (Figure 2). The main effect of target location was significant (F(2,28)=8.38, MSE=3651.12, p=0.01, partial η2=0.37). Keypress RT was faster for cued location (M=368.89, SE=18.83) than both same object (M=404.14, SE=27.94, Cohen’s d=0.69) and different object (M=413.77, SE=29.65, Cohen’s d=0.8) locations (p=0.010 and p=0.008, respectively). Importantly, RT was significantly faster for the same object than different object location (p=0.021, Cohen’s d=0.67). The effects of orientation (F(1,14)=0.51, MSE=148.69, p=0.488, partial η2=0.04) and the interaction (F(2,28)=0.94, MSE=285.12, p=0.404, partial η2=0.06) were not significant.
Figure 2.
Mean reaction time as a function of object orientation (vertical vs. horizontal) and target location (cued, same object, different object). Error bars are within-subject tandard errors suggested by Cousineau (2005).
As predicted, our results replicated Egly et al.’s (1994) findings using the double-rectangle cueing paradigm. First, target detection was fastest at the cued location, indicative of enhanced processing due to space-based attention. Furthermore, target detection at the same object location was also faster than at the different object location. Given that these two locations were equidistant from the cued location, the observed performance difference cannot be attributed to space-based attention but must be due to attention enhancing the representation of the cued object—an object-based attention effect (sometimes termed a “same-object advantage”). Given our successful replication of manual RT results, we next used the double-rectangle cueing paradigm in an experiment with eye movement responses as the dependent measure.
Experiment 1b-Methods
Participants
Fifteen participants (age ~18–30 years) provided written informed consent and took part in the experiment: all were students from Michigan State University. One author (GS) participated in the experiment; none of the subjects had participated in Experiment 1a. The participant recruitment criteria were the same as Experiment 1a.
Apparatus and Visual Stimuli
Both the display and stimuli were identical to Experiment 1a. Eye movements were recorded by an Eyelink 1000 eye tracker (SR Research, Ontario, Canada) at a sampling rate of 1 kHz.
Design and Procedure
We included a training session in this experiment to familiarize participants with the use of saccades to indicate their responses. The training session and the experiment took place on the same day in a dimly lit room. Participants who had participated in a previous eye movement study in our lab were not required to complete the training session. The equipment and stimulus characteristics were identical in the training session and the experiment.
The experimenter was in the room during the training session. Before the beginning of the training session, a 9-grid calibration and validation was performed. A fixation cross appeared first for 800 ms, followed by a target at one of four possible locations, corresponding to the corners of the rectangles in the main experiment (the rectangles were not displayed). The subjects were instructed to keep their gaze at the fixation cross and make a quick eye movement to the target when it appeared. The target disappeared when the gaze stayed within a 2° radius of the target for 200ms. In the absence of a correct eye movement, the target was removed from the screen after two seconds. The next trial started after a 500 ms interval. The training session consisted of three blocks, with 40 trials per block.
The experimenter was present in the room during the main experiment, and during calibration and validation of the eye tracker. The main experiment began after a successful calibration and validation procedure. The main experiment was run as explained in the Design and Procedure section of Experiment 1a with the following exception: participants moved their eyes to fixate the target instead of using key presses to indicate target detection. They were instructed to (a) fixate on the central cross until the target appeared, (b) make a quick eye movement to the target, (c) keep their gaze on the target until it disappeared, and (d) look back at the fixation cross afterwards. The target disappeared if a participant’s gaze stayed within 1.5° of target center for at least 200 ms, which was defined as a correct response. In the absence of a correct eye movement (and on target-absent trials), the trial was terminated 2 seconds after the target appeared. The subsequent trial began 500 ms after the termination of the previous trial.
Data Preprocessing
We used SR Research’s eye-movement parser, set at the cognitive orientation, to detect saccades. The velocity threshold was 30°/sec, the acceleration threshold was 8000°/sec2, and the motion threshold was 0.1°.
We performed offline drift correction of the eye-movement data. The mean eye position during the initial 100 ms of a trial (i.e., initial fixation period) was subtracted from eye position values on that trial. Saccade latency is the time difference between the target onset and the saccade onset. Saccade duration is the time difference between the saccade onset and saccade offset. We excluded the following types of trials from analysis of the eye movement data: 1) eye movement occurred within 75ms of target onset (i.e., premature saccades); 2) failure to move the eye on target-present trials within 500ms of the target onset; 3) failure of the first saccade to reach the target (i.e., gaze landing > 2° from target center); 4) saccade latencies outside the range of 3 standard deviations from that participant’s mean; 5) saccade duration outside the range of 3 standard deviations from that participant’s mean.
Experiment 1b-Results
The Eligible Trials
The proportion of eligible trials in each condition, after preprocessing, was submitted to a 2 (orientation: vertical, horizontal) × 3 (target location: cued, same object, different object locations) repeated-measures ANOVA. The main effect of target location was significant, F(2,28)=6.3, MSE=<0.01, p=0.006, partial η2=0.31. The proportion of eligible trials was higher when the target appeared at the cued location (M=0.87, SE=0.03) in comparison to when the target appeared at the same object location (M=0.82, SE=0.03), p=0.002. There were no further significant differences between target locations, all p’s >0.05. The effects of orientation and the interaction were not statistically significant (all p’s>0.1). On average, 0.84 of trials were eligible for analysis. The proportion of trials that were eliminated due to mistakenly saccading towards the cued location did not depend on the configuration, target location, or their interaction (Grand M=0.11, all p’s >0.05). Investigation of the proportion of trials eliminated due to making a wrong saccade followed by saccading at the target showed that no corrective saccade was made when the target appeared at the cued location. Among the invalidly cued locations, proportion of corrective saccades depended on neither orientation (horizontal vs. vertical) nor target location (same vs. different object locations), nor the interaction between orientation and target location, all p’s>.5 (Grand M=0.07, SE=0.02).
Saccade Latency
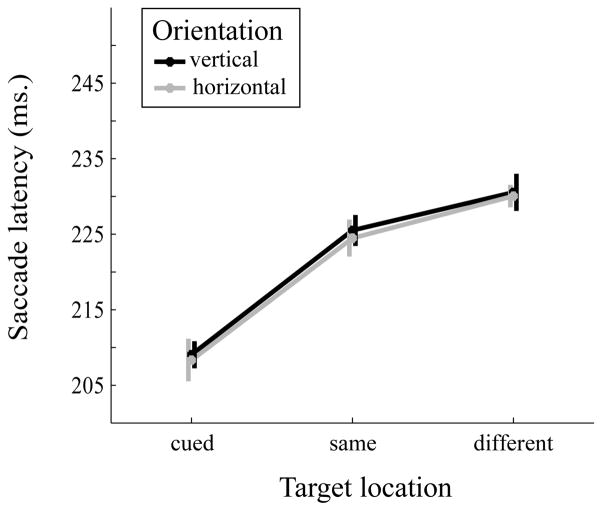
A 2 (orientation: vertical, horizontal) × 3 (target location: cued location, same object, different object) repeated-measures ANOVA was conducted on the mean saccade latency (Figure 3). The main effect of target location was significant (F(2,28)=28.54, MSE=185.99, p<0.001, partial η2=0.67). The mean saccade latency was faster at the cued location (M=208.68 ms, SE=5.68 ms) in comparison to same object (M=224.99 ms, SE=7.1 ms, Cohen’s d=1.29) and different object (M=230.29 ms, SE=7.77 ms, Cohen’s d=1.58) locations, all p’s<0.001. Importantly, saccade latency to the same object location was faster than to the different object location (p=0.012, Cohen’s d=0.74). The effects of orientation (F(1,14)=0.29, MSE=42.73, p=0.596, partial η2=0.02) and the interaction (F(2,28)<0.01, MSE=117.01, p=0.992, partial η2<0.01) were not statistically significant.
Figure 3.
Mean saccade latency as a function of object orientation (vertical vs. horizontal) and target location (cued, same object, different object). Error bars are within-subject standard errors suggested by Cousineau (2005).
Saccade Gain and Landing Distance
Endpoint accuracy of the saccades was measured to assess whether the observed differences in saccade latencies could be explained by a tradeoff between saccade speed and landing precision. There are two measures of landing precision. Saccade gain ratio is calculated by dividing the measured distance of the saccade by the minimum distance to the target (Crawford & Müller, 1992). Landing distance is the distance between saccade endpoint and the centroid of the target. Since saccade latencies were not influenced by orientation or the interaction between orientation and target location, we collapsed across orientations when calculating saccade gain ratios and landing distances. From experiment 1b onwards, paired-sample t-tests are employed to compare saccade gain ratios and landing distances between target locations.
The saccade gain ratio at the cued location (M=1.01, SE=0.01) was larger than the saccade gain ratio at the same object (M=0.99, SE=0.01) and different object (M=0.97, SE=0.01) locations. The saccade gain ratio at the same object location was higher than the saccade gain ratio at the different object location, all p<0.005. Landing distance did not differ between target locations (Grand M=.81, SE=.03), all p>0.2.1
Experiment 1-Discussion
We aimed to measure the effect of object-based attention on oculomotor programming. Our findings parallel those in the keypress condition. Here, saccade latency was fastest at the cued location compared to all other locations. This finding supports the previous literature (Abrams & Jonides, 1988; Dorris & Munoz, 1998; Kawagoe et al., 1998; Kustov & Robinson, 1996; Murray & Giggey, 2006; Rothkirch et al., 2013; Takikawa et al., 2002; Crawford & Müller, 1992) showing that deployment of space-based attention shortens the saccade latency at the attended location. With respect to object-based attention, saccade latency was faster for targets at the same object location than at the different object location. There was no effect of object orientation for either the saccade or keypress experiments, although the object-based effect was numerically smaller in the horizontal orientation than the vertical orientation with keypress responses (Expt 1a). A speed-endpoint accuracy tradeoff was not evident. Indeed, saccades to the different object locations slightly undershot the target, more so than saccades to the same object location. Taken together, these results suggest that object-based attentional selection is responsible for the observed saccade latency differences between same and different object locations.
Because the peripheral cue in this experiment was also predictive of target location (i.e., hybrid cue), the object-based effect could be driven by either the endogenous or exogenous properties of the cue. In the next two experiments, we further investigated whether purely exogenous or endogenous cues can drive object-based effects as measured by saccade latency.
Experiment 2: Exogenous cue
In this experiment, we used a purely exogenous cue to direct attention. The target could appear at any one of the four ends of the two rectangles with equal probability. Macquistan (1997) claimed that an exogenous cue is not only sufficient to direct object-based attention to the cued object, it is the only type of cue that can elicit object-based attention. The role of exogenous cues in evoking object-based attention is very well documented (Abrams & Law, 2000; Goldsmith & Yeari, 2003, 2012) which leads us to predict significant effects of space-based attention and object-based attention during keypress responses (Experiment 2a). In the eye movement experiment, we expect to find significant effects of space-based attention (Experiment 2b). Finding a correlate of object-based attention during eye-movements would indicate that exogenously driven object-based attention is sufficient to influence saccade latencies.
Experiment 2a-Methods
Participants
Fifteen participants (age ~18–30 years) provided written informed consent and took part in the experiment: all were students from Michigan State University. One participant participated in Experiment 1b. The participant recruitment criteria were the same as the Experiment 1a.
Apparatus
The apparatus was identical to Experiment 1a for seven participants that participated in the same room as Experiment 1a. For eight participants (who completed the experiment in a different room) the stimuli were displayed on a 19-inch CRT monitor (resolution: 1024×768 pixels) with a vertical refresh rate of 100 Hz; and the viewing distance was 85 cm ensured by use of a chinrest.
Stimuli and Procedure
The sizes and colors of the background, fixation cross, inner areas and outer edges of the rectangles, the cue and the target were the same as in Experiment 1a. Seven participants participated the experiment in the room of Experiment 1a. Eight participants participated in another room on a different CRT monitor, in which the luminance values of the stimuli were slightly different (background and inner areas of the rectangles: 0.01 cd/m2, edges of the rectangles and the fixation cross: 5 cd/m2, target: 13.5 cd/m2).
The sequence and duration of the stimuli, and the response modalities were the same as in Experiment 1a. The primary difference between the experiments was the cue validity during target-present trials (80 %). The target could appear at the cued, same object, different object, and the opposite (the farthest corner to the cue) locations with equal probability. Instructions were the same as in Experiment 1a. There were 10 blocks, each consisting of 80 trials. Eye movements were not recorded.
Experiment 2a-Results
The Eligible Trials
We excluded all trials in which the reaction time was shorter than 200 ms or longer than 1500 ms. The number of eligible trials in each condition was subjected to a 2 (orientation: vertical, horizontal) × 4 (target location: cued location, same object, different object, opposite location) repeated measures ANOVA. The proportion of eligible trials in each condition was submitted to a 2 (orientation: vertical, horizontal) × 4 (target location: cued location, same object, different object locations) repeated-measures ANOVA. None of the effects reached significance (all p > 0.1). On average, .94 were eligible for analysis.
Reaction Time2
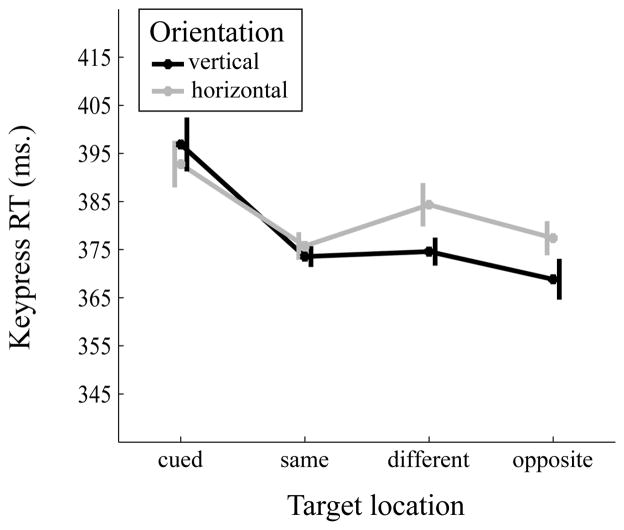
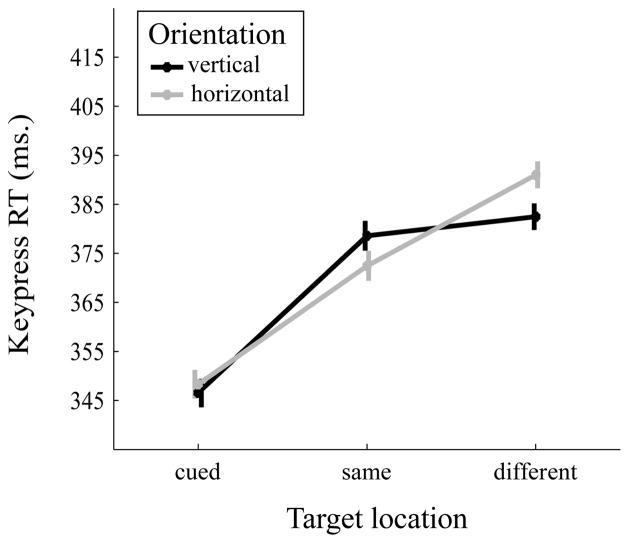
A 2 (orientation: vertical, horizontal) × 4 (target location: cued location, same object, different object, opposite location) repeated-measures ANOVA was run on the mean keypress RTs (Figure 4). The main effect of orientation was significant, F(1,14)=7.97, MSE=63.13, p=0.014, partial η2=0.36. Mean reaction time in vertical orientation trials (M=378.47, SE=21.68) was faster than in horizontal orientation trials (M=382.57, SE=22.06). The main effect of object location was significant, F(3,42)=6.81, MSE=833.7, p=0.008, partial η2=0.33. Keypress RT was slower for the cued location (M=394.83, SE=22.38) than the same object (M=374.66, SE=22.42, Cohen’s d=0.9), different object (M=379.47, SE=21.4, Cohen’s d=0.56), and opposite (M=373.12, SE=22.2, Cohen’s d=0.74) locations, all p’s<0.05. RTs for the remaining target location comparisons were not significantly different, all p’s>0.05. The interaction between target location and orientation was not significant, F(3,42)=1.71, MSE=267.37, p=0.199, partial η2=0.11.
Figure 4.
Mean reaction time (in ms) as a function of object orientation (vertical vs horizontal) and target location (cued, same object, different object, opposite). Error bars are within-subject standard errors suggested by Cousineau (2005).
Experiment 2b-Methods
Participants
Fifteen participants (age ~18–30 years) provided written informed consent and took part in the experiment: all were students from Michigan State University. Four subjects (including author GS) also participated in Experiment 1b, and one also participated in Experiment 1a. The participant recruitment criteria were the same as in Experiment 1a.
Apparatus and Visual Stimuli
Both the display and stimuli were identical to Experiment 1a. Eye movements were recorded by an Eyelink 1000 eye tracker (SR Research, Ontario, Canada) at a sampling rate of 1 kHz.
Design and Procedure
The training procedure was the same as in Experiment 1b except that we introduced an online drift correction procedure (drift correction was performed offline in Experiment 1b). The participants were instructed to fixate at the beginning of each trial. If their gaze stayed within 1° of the fixation cross for at least 500ms in a 2 sec period, the drift check was skipped and the trial began; otherwise, the experiment paused and a message instructed the participant to fixate and make a keypress that initiated the drift correction. After drift correction, the participant pressed a key to continue the trial. The trial continued as explained in Experiment 1b. This online drift correction procedure improved the eye tracking data quality, as slow drifts tend to occur during an experimental session.
The main experiment was identical to that of Experiment 1b except the addition of the drift correction procedure in the training phase (described above). The only other difference between Experiment 1b and 2b was the validity of the cue during target-present trials. In Experiment 2b, the target could appear at the cued, same-object, different-object, or opposite locations with equal probability. The correct eye movement criteria were the same as in Experiment 1b. When a participant failed to make a successful eye movement in a target-present trial for at least three trials in a block, a block was added to compensate for the number of trials with unsuccessful eye movements. There were 10 blocks initially, each consisting of 80 trials.
Data Preprocessing
Preprocessing of the data was the same as in Experiment 1b.
Experiment 2b-Results
The Eligible Trials
The number of eligible trials in each condition was subjected to a 2 (orientation: vertical, horizontal) × 4 (target location: cued, same, different, opposite location) repeated measures ANOVA. None of the effects reached significance (all p > 0.1). On average, .87 of trials were eligible for analysis. Only a small proportion of trials were eliminated due to mistakenly making a saccade towards the cued location, and the proportion of such trials did not depend on configuration, target location, or their interaction (M=0.05, all p’s >0.05). In terms of corrective saccades, none were needed when the target appeared at the cued location. Proportion of the corrective saccades for the targets at the invalidly cued locations did not depend on orientation (vertical vs. horizontal), object location (same, different, and opposite locations), and their interaction, all p’s>0.05 (Grand M=0.03, SE=0.01).
Saccade Latency
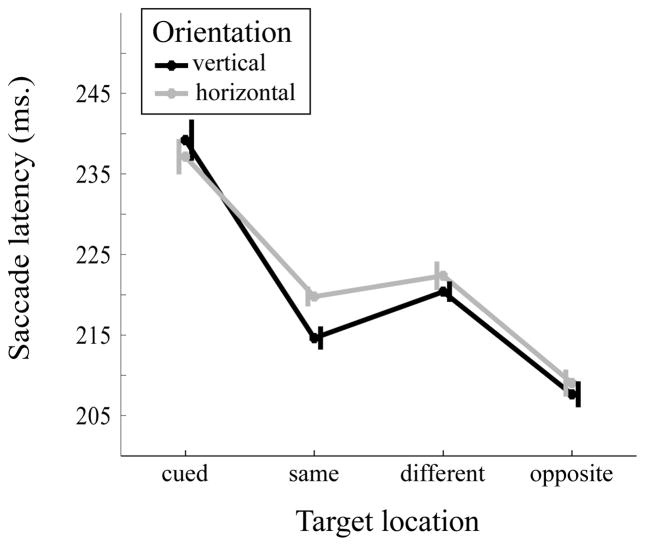
A 2 (orientation: vertical, horizontal) × 4 (target location: cued location, same object, different object, opposite location) repeated-measures ANOVA was conducted on the mean saccade latency (Figure 5). The main effect of target location was significant, F(3,42)=51.97, MSE=145.21, p=<001, partial η2=0.79. The mean eye movement latency was slower at the cued location (M=238.17 ms, SE=11.01 ms) compared to same object (M=217.21 ms, SE=9.63 ms, Cohen’s d=1.84), different object (M=221.37 ms, SE=9.35 ms, Cohen’s d=1.49), and opposite (M=208.35 ms, SE=9.17 ms, Cohen’s d=2.37), locations, all p’s<0.001. Saccade latency at the opposite location was faster than the cued (Cohen’s d=2.37), same object (Cohen’s d=1.24) and different object (Cohen’s d=1.96) locations, all p’s<0.001. Importantly, saccade latency to the same-object location was faster than to the different-object location, (p=0.011, Cohen’s d=0.75). The effects of orientation (F(1,14)=3.93, MSE=19.96, p=0.067, partial η2=0.22) and the interaction (F(3,42)=2.36, MSE=27.74, p=0.085, partial η2=0.14) were not statistically significant.
Figure 5.
Mean saccade latency (in ms) as a function of object orientation (vertical vs horizontal) and target location (cued, same object, different object, opposite). Error bars are within-subject standard errors suggested by Cousineau (2005).
Saccade Gain and Landing Distance to the Target
The saccade gain ratios did not differ between cued, same, different, and opposite locations, all p’s>0.095 (Grand M=1.01). The landing distance at the cued location (M=0.74, SE=0.03) was smaller than at the same object (M=0.77, SE=0.04), different object (M=0.77, SE=0.03), and opposite (M=0.78, SE=0.04) locations, respectively p’s=0.039, 0.009, and 0.009.The landing distances did not differ between targets at the same, different, and opposite locations, all p’s>0.49.3
Experiment 2-Discussion
In this experiment, we examined whether an exogenous cue was capable of eliciting an object-based effect in the double-rectangle paradigm. We found evidence of an object-based effect for saccade latency, as the same-object saccade latency was faster than the different-object latency; but this did not hold for keypress RTs. Somewhat unexpectedly, we also found that both saccade latency and keypress RTs were slowest when the target appeared at the cued location, indicative of inhibition of return (IOR). IOR is a decrease in attentional priority at a previously attended location, manifest as a slowing of target detection at that location on subsequent trials (for a review see Klein, 2000). The cue-target SOA in Experiment 2 was consistent with IOR effects under spatially exogenous cues (Klein, 2000). Previous studies that employed the two-rectangle paradigm found both spatial and object-based IOR at much longer SOAs (List & Robertson, 2007; Jordan & Tipper, 1999, Reppa & Leek, 2003). However, in one experiment, List and Robertson (2007; expt. 2) used a shorter SOA of 340 ms and detected both spatial IOR in the presence of a significant object-based effect. It is possible that spatial and object-based IOR effects can have different time courses, such that at short SOAs (e.g., 300 ms), only spatial IOR is present, but at longer SOAs, both spatial and object-based IOR are present.
Another interesting result of Experiments 2a and 2b is the performance benefit at the location opposite to the cued location. We think that such enhanced performance at the opposite location to the cue can be explained by attentional momentum, or the deployment of attentional resources to the opposite direction of the spatially inhibited location due to IOR. A number of studies not only found slower reaction time in detection tasks at the cued location due to IOR, they also found faster reaction times in detecting targets at the farthest location from the cue (Bennett & Pratt, 2001; Hubbard, 2014; Machado & Rafal, 2004; Pratt, Adam, & McAuliffe, 1998; Pratt, Spalek, & Bradshaw, 1999; Spalek & Hammad, 2004; Sumner, 2006). Our finding of inhibition at the cued location and enhancement at the farthest location is consistent with these studies and suggest that IOR and attentional momentum were operating in tandem.
Our findings suggest that object-based attention can operate even in the presence of spatial IOR. These data provide evidence for a dissociation between space-based and object-based attention (Duncan, 1984; He et al., 2004; He et al., 2008; Vecera & Farah, 1994), contrary to the idea that object-based attention is merely the spread of space-based attention within a cued object (Hollingworth, Maxcey-Richard, & Vecera, 2012; Martinez, Ramanathan, Foxe, Javitt, & Hillyard, 2007; Martínez, Teder-Salejarvi, & Hillyard, 2007; Müller & Kleinschmidt, 2003). Furthermore, these data suggest that saccade latency is a more sensitive measure of object-based attention than keypress RT. We conclude that an exogenous cue is sufficient to induce object-based attention, but it is only observed with a more sensitive measure such as saccades.
Experiment 3: Endogenous cue
Having established that both a hybrid and a purely exogenous cue can elicit object-based effects, we next investigated whether a purely endogenous cue can also drive object-based effects. Experiment 1 and 2 showed that saccade latencies are more sensitive to effects of attention compared to keypress RT. Thus, saccade latencies are well-suited to observation of small effects. In addition to our primary goal, we also planned to shed light on the controversy surrounding the role of central cues in object-based attention. On the one hand, Macquistan (1997) observed that only a peripheral cue was capable of eliciting object-based effects of attention. On the other hand, several studies (Abrams and Law, 2000; Chen & Cave, 2008; Law & Abrams, 2002; Greenberg, 2009) found that central cues could also elicit object-based effects. More recently, some researchers found that giving particular instructions to subjects may be necessary for central cues to elicit object-based attention (Goldsmith & Yeari, 2003, 2012). Our aim was to determine whether object-based attention can be elicited by central cues without instructing participants to use any specific strategies. If central cues can elicit object-based attention, saccade latency may be more capable of detecting the effect than keypress RT, as we found in Experiment 2.
Experiment 3a-Methods
Participants
Fifteen participants (age ~18–30 years) provided written informed consent and took part in the experiment; all were students from Michigan State University. Two subjects participated in Experiment 2a, one participated in both Experiments 2a and 1b, and another participated in both Experiments 2b and 1a. The participant recruitment criteria were the same as in Experiment 1a.
Apparatus
The apparatus details were identical to Experiment 2b.
Stimuli and Procedure
The sizes and colors of the background, inner areas and outer edges of the rectangles and the target were the same as in Experiment 1a, with the following exceptions: the luminance values of the stimuli were slightly altered because we used a different CRT monitor (as reported in Stimuli and Procedure section of Experiment 2a). The fixation cross in Experiment 1a was replaced with a fixation square in Experiment 3a. The fixation square was located at the center of the screen (white, 0.02×0.02°, luminance: 26 cd/m2). The peripheral cue in Experiment 1a was replaced by a central cue in Experiment 3a. The central cue was a white line (length: 0.5°, thickness: 0.015°, luminance: 26 cd/m2) extending from the centroid of the fixation square toward one of the four possible target locations (Figure 6).
Figure 6.
The sequence of a trial. The rectangles appeared in a horizontal or vertical orientation (vertical shown). A central cue pointed at one of the corners for 100 ms. After a 200ms interval, the target at the cued location (60%), at the far end of the cued object (10%), at the equidistant corner of the different object (10%), or it did not appear (20%).
The sequence and duration of the stimuli, target probability, and response modalities were the same as in Experiment 1a. The primary difference between the experiments was the replacement of the peripheral cue with a central cue in Experiment 3a. There were 10 blocks, each consisting of 80 trials. Eye movements were not recorded. Instructions and data preprocessing were the same as in Experiment 1a.
Experiment 3a-Results
The Eligible Trials
The proportion of eligible trials in each condition, after preprocessing, was submitted to a 2 (orientation: vertical, horizontal) × 3 (target location: cued location, same object, different object) repeated measures ANOVA. None of the effects reached significance (all p > 0.1). On average, 0.97 of trials were eligible for analysis.
Reaction Time
A 2 (orientation: vertical, horizontal) × 3 (target location: cued location, same object, different object) repeated-measures ANOVA was run on mean keypress RTs (Figure 7). The main effect of target location was significant, F(2,28)=58.2, MSE=211.56, p<0.001, partial η2=0.81. Keypress RT was faster for the cued location (M=347.41, SE=12.59) than the same object (M=375.51, SE=12.55, Cohen’s d=1.81) and different object (M=386.74, SE=13.95, Cohen’s d=2.7) locations, all p’s<0.001. Importantly, RT was faster for the same-object location than the different-object location, p=0.006, Cohen’s d=0.84. The effects of orientation (F(1,14)=0.79, MSE=55.86, p=0.388, partial η2=0.05) and the interaction (F(2,28)=2.46, MSE=205.91, p=0.118, partial η2=0.15) were not significant
Figure 7.
Mean reaction time (in ms) as a function of object orientation (vertical vs horizontal) and target location (cued, same object, different object). Error bars are within-subject standard errors suggested by Cousineau (2005).
Experiment 3b-Methods
Participants
Fifteen participants (age ~18–30 years) provided written informed consent and took part in the experiment: all were students from Michigan State University. Except for an author (GS) and one other participant (both participated in Experiment 2a), none had participated in previous experiments. The participant recruitment criteria were the same as in Experiment 1a.
Apparatus and Visual Stimuli
Both the display and stimuli were identical to Experiment 3a. Eye movements were recorded by an Eyelink 1000 eye tracker (SR Research, Ontario, Canada) with a sampling rate of 1 kHz.
Procedure
The training procedure was the same as in Experiment 2b. The main experiment procedure was identical to that of Experiment 2b: a drift check and correction phase preceded the main phase of the experiment on each trial. In experiment 3b, the peripheral cue was replaced with a central cue. The cue was informative of target location (60% valid, 10% same-object, 10% different-object, 20% catch). Criteria for correct eye movements were the same as in Experiment 1b. Instructions and data preprocessing were the same as in Experiment 1b.
Experiment 3b-Results
The Eligible Trials
The proportion of eligible trials in each condition, after preprocessing, was submitted to a 2 (orientation: vertical, horizontal) × 3 (target location: cued, same, different) repeated measures ANOVA. The main effect of target location was significant, F(2,28)=12.3, MSE=<0.01, p<0.001, partial η2=0.47. The proportion of eligible trials was higher when the target appeared at the cued location (M=0.86, SE=0.03) versus the same-object (M=0.81, SE=0.03) and different-object (M=0.81, SE=0.03) locations, p’s=0.001. There were no further significant differences between target locations (p=0.903). The effects of orientation and the interaction were not statistically significant (p>0.5). On average, 0.83 of trials were eligible for analysis. Only a small proportion of trials were eliminated due to mistakenly executing a saccade towards the cued location, and the proportion of such trials did not depend on configuration, target location, or their interaction (M=0.06, all p’s >0.05). In terms of corrective saccades, none were needed when the target appeared at the cued location. Proportion of the corrective saccades for the targets at the invalidly cued locations did not depend on orientation (vertical vs. horizontal), object location (same, different, and opposite locations), and their interaction, all p’s>0.05 (Grand M=0.04, SE=0.01).
Saccade Latency
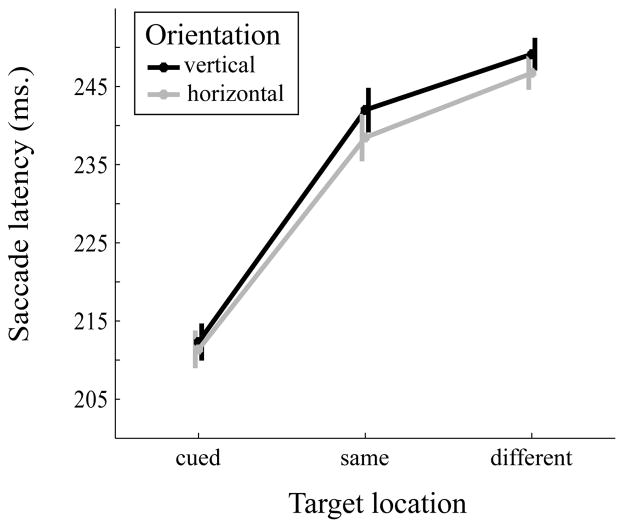
A 2 (orientation: vertical, horizontal) × 3 (target location: cued location, same object, different object) repeated-measures ANOVA was conducted on the mean saccade latency (Figure 8). The main effect of target location was significant, F(2,28)=66.31, MSE=210.68, p<0.001, partial η2=0.83. The mean eye movement latency was faster at the cued location (M=211.84 ms, SE=5.93 ms) versus the same object (M=240.23 ms, SE=7.07 ms, Cohen’s d=2.2) and different object (M=247.94 ms, SE=7.3 ms, Cohen’s d=2.34) locations, all p’s<0.001. Importantly, saccade latency to the same object location was faster than to the different object location, p=0.006, Cohen’s d=0.83. The effects of orientation (F(1,14)=1.87, MSE=63.16, p=0.193, partial η2=0.19) and the interaction (F(2,28)=0.14, MSE=131.35, p=0.787, partial η2=0.01) were not statistically significant.
Figure 8.
Mean saccade latency (in ms) as a function of object orientation (vertical vs horizontal) and target location (cued, same object, different object). Error bars are within-subject standard errors suggested by Cousineau (2005).
Saccade Gain and Landing Distance to the Target
The saccade gain ratio at the cued location (M=1, SE=0.01) was larger than the saccade gain ratio at the same object (M=0.98, SE=0.01) and different object (M=0.97, SE=0.01) locations, all p’s<0.001. The saccade gain ratio for the same object and different object locations were not significantly different, p=0.312.
Landing position at the cued location was closer to target centroid (M=0.77, SE=0.03) than to the same object (M=0.81, SE=0.04) or different object (M=0.82, SE=0.04) locations (p’s=0.016 and 0.044, respectively). There was no difference in landing position between the same object and different object locations, p=0.588.4
Experiment 3-Discussion
In Experiment 3 we investigated whether an endogenous cue is capable of affecting saccade latency and/or inducing object-based attention. Both saccade latency and keypress RT were fastest for targets appearing at the cued location (space-based attention). Unlike previous studies that claim endogenous cues are unable (or unlikely) to produce object-based selection, we found that both saccade latency and keypress RT yielded a same-object effect with endogenous cues. Pilz, Roggeveen, Creighton, Bennett, and Sekuler (2012) claim that object-based attention is sensitive to orientations of objects, such that object-based effects are more often observed for horizontally oriented objects. One explanation for Macquistan (1997)’s failure to find endogenously driven object-based attention is a possible interaction between object-based attention and orientation. Although Macquistan (1997) used both vertically and horizontally oriented rectangles as objects, he did not examine the effect of orientation in his experiment. If object-based attention is sensitive to orientation, as Pilz et al. (2012) concluded, Macquistan (1997) might have overlooked a partial endogenously driven object-based attention effect for a specific orientation. However, our results show that unlike Macquistan’s (1997) findings, object-based attention can be endogenously driven; and unlike Pilz et al.’s (2012) findings, we find no interaction between target location and orientation. Our results are consistent with recent reports showing that, indeed, orientation may not be the driving factor behind the differences Pilz et al. (2012) observed (Greenberg et al., 2014; Al-Janabi & Greenberg, this issue; Barnas & Greenberg, this issue).
General Discussion
In this study, we found that space-based attention, driven by endogenous or hybrid cues, shortens saccade latency to the target at the cued location. These findings are consistent with studies showing similar effects (Abrams & Jonides, 1988; Dorris & Munoz, 1998; Kawagoe et al., 1998; Kustov & Robinson, 1996; Murray & Giggey, 2006; Rothkirch et al., 2013; Takikawa et al., 2002; Crawford & Müller, 1992). More importantly, we examined how object-based attention influences saccade latency, which has not been systematically investigated. Regardless of the mode of attention (exogenous or endogenous), we show that first saccade latency to a target at the same-object location is faster than to the different-object location. We also show that this saccade latency difference between the same and different object targets is not due to a speed-accuracy tradeoff between saccade latency and accuracy (Wu, Kwon, & Kowler, 2010). These results reveal an oculomotor correlate of object-based selection.
First vs. second saccade for object-based effects
Three previous studies have investigated the role of object-based attention on saccades using Egly et al.’s (1994) double-rectangle cueing paradigm (Malcolm & Shomstein, 2015; McCarley et al., 2010; Theeuwes et al., 2010). In these experiments, participants made their first eye movement to the cued location and the main analysis focused on the second saccade (after fixation at the cued location). A potential confound of this approach is that it did not control for the possible guidance of saccades along object contours. Once the eye lands on the target, two elongated and highly salient contours (edges of the object) are the immediate neighbors of the target. Those lines always extend toward the same-object location, regardless of object orientation, whereas no such elongated contours exist between the target location and the different-object location. Given that saccades are likely to follow the path of a contour (Wismeijer & Gegenfurtner, 2012), faster eye movements to the same-object location can be explained by better guidance of saccades due to image contours, instead of a difference in attentional priority. To avoid this potential low-level visual confound, we investigated the first saccade, which, by definition, equated the retinal image for each target location. In other words, wherever the target appeared, the retinal image was identical at saccade initiation. Thus, our measure of the first saccade is not biased by low-level image features; and our paradigm allows us to isolate the effects of object-based attention on saccades.
The Role of Cue Type in Object-Based Attention
We tested the effectiveness of hybrid (predictive and peripheral), exogenous (nonpredictive and peripheral), and endogenous (predictive and central) cues on object-based attention by measuring both keypress RT and saccade latency. All three cue types were capable of producing object-based effects in saccade latency, though not in all keypress measures. In Experiment 2, where the cue was peripheral and non-predictive and the SOA was 300 ms, only saccade latency showed a significant modulation by object-based attention. Although our primary interest was in determining whether the object-based effect elicited by the hybrid cue in Experiment 1 was driven by its endogenous or exogenous component, our current experiments also shed some light on the controversy regarding the effectiveness of central cues. In an earlier study, Macquistan (1997) claimed that central cues do not evoke object-based selection; whereas others have found that object-based attention could be elicited by central cues (Abrams and Law, 2000; Chen & Cave, 2008; Law & Abrams, 2002; Greenberg, 2009; Al-Janabi & Greenberg, this issue). Some researchers claim that certain task demands (such as widening the focus of spatial attention) are necessary for central cues to elicit object-based attention (Goldsmith & Yeari, 2003, 2012). The participants in the current experiments were not instructed to employ a particular strategy, yet we found that central cues are as capable as peripheral cues in terms of eliciting object-based attention. Our findings, thus, support the view that central cues can elicit object-based attention.
Difference in the Timecourse Between Object- and Space-Based Attention
In Experiment 2, we observed that both saccade latency and keypress RT were much slower at the cued location in comparison to the other locations. However, both keypress RT and saccade latency for the same-object location were faster than for the different-object location, with this same-object advantage only reaching statistical significance for saccade latency. The observation of object-based facilitation in the presence of space-based inhibition has at least two plausible explanations. First, it is possible that the bracket cue masked the target at the cued location. However, such forward masking should also be present in Experiments 1a and 1b, where we observed a spatial facilitation effect. Moreover, forward masking operates in very short mask-to-target intervals (Enns & Di Lollo, 2000; Ramachandran & Cobb, 1995). Thus, the 200ms ISIs in the current experiments would be too long to allow for forward masking. We favor the alternative explanation based on inhibition of return (IOR). IOR was originally observed for keypress responses with non-informative cues at relatively long cue-target SOAs (for a review see Klein, 2000), as well as for saccade latency measures (Abrams & Dobkin, 1994; Briand, Larrison, & Sereno, 2000; Dorris, Klein, Everling, & Munoz, 2002; Rafal, Egly, & Rhodes, 1994). Some studies have since demonstrated that IOR does not only operate at the cued spatial location, it can also operate within cued objects (Gibson & Egeth, 1994; Jordan & Tipper, 1998, 1999; Leek, Reppa & Tipper, 2003; List & Robertson, 2007; Reppa & Leek, 2003; Tipper, Weaver, Jerreat, & Burak, 1994; Tipper, Driver, & Weaver, 1991). Our results in Experiment 2 are consistent with extant work investigating the time course of object-based IOR using the two-rectangle paradigm with non-predictive cues. When the cue-target SOA was relatively long (400ms to 1220ms), both spatial and object-based IOR are observed (Experiment 1 in List & Robertson, 2007; Jordan & Tipper, 1999, Reppa & Leek, 2003). However, when the SOA was short (340ms), space-based IOR was observed in the presence of an object-based facilitation (Experiment 2 in List & Robertson, 2007). Our results in Experiment 2 are thus consistent with previous studies and suggest that, at short SOAs, space-based inhibition had taken place without any object-based inhibition.
Our results in Experiment 2 provide more evidence for the possible independence, or dissociation, between space- and object-based attention. We found that object-based facilitation does not necessarily require the presence of space-based facilitation. This dissociation, i.e., object-based effects in the presence of spatial inhibition, can also inform the controversy regarding the role of space-based attention in object-based attention. Two classes of theories have attempted to explain how object- and space-based attention are inter-related: (1) Object-based attention is a spreading of space-based attention within the cued object (Hollingworth et al., 2012; Martinez, Ramanathan, Foxe, Javitt, & Hillyard, 2007; Martínez, Teder-Salejarvi, & Hillyard, 2007; Müller & Kleinschmidt, 2003) and (2) Object-based attention is fully independent of space-based attention (Duncan, 1984; Vecera & Farah, 1994; He et al., 2004; He at al., 2008). Our results support the latter view, as do the results of List & Robertson (2007). If space-based attention was a prerequisite of object-based attention, we should not have found an object-based effect in Experiment 2. However, we observed space-based IOR and object-based effects, concurrently. Therefore, we conclude that space-based attention and object-based attention do not share the same timecourse for IOR, and the presence of space-based facilitation is not a requirement for object-based attention.
Saccade Planning and the Object-Based Prioritization Map
Our results show that saccade planning (as measured by latency) is not only facilitated at the cued location, but is also facilitated at the far end of the attended object. Shorter saccade latency to the target that appears at the cued location has also been consistently observed in several previous studies (Abrams & Jonides, 1988; Dorris & Munoz, 1998; Kawagoe et al., 1998; Kustov & Robinson, 1996; Murray & Giggey, 2006; Rothkirch et al., 2013; Takikawa et al., 2002; Crawford & Müller, 1992). This space-based cueing effect can be accommodated within the theoretical framework of a priority map. Saccades are drawn to the highest priority location on the priority map (for a review, see Bisley & Goldberg, 2010; Zelinsky & Bisley, 2015). Our findings support a role of the priority map in saccade planning, because gaze to the highest priority location was faster than all other locations. However, the concept of a priority map cannot easily explain how attention is also modulated by the layout of objects and why saccades are also drawn to an attended object. This observation suggests an extension of the priority map concept. Indeed, a proto-object based saliency model has been proposed to explain object-based attention (Russell, Mihalaş, von der Heydt, Niebur, & Etienne-Cummings, 2014; Vecera, 2000). In this model, figure-ground segmentation is achieved by assigning border ownership, wherein the regularities in a scene are grouped together. Such a grouped array attracts attention, as well as saccades, because they become the winner of a competition between single, ungrouped features (Russell et al., 2014). Our empirical data provide support for this theory, which is based on model simulations. Even though the attended location has an advantage in saccade latency, a competition between objects seems to take place in addition to the competition between locations. Thus, our findings favor a role of both prioritized locations and objects in saccade preparation. It is worth noting here that this is also consistent with well-accepted theories positing voluntary control over object-based attention (Greenberg & Gmeindl, 2008; Greenberg, 2009; Drummond & Shomstein, 2010; Shomstein, 2012).
Comparison between Keypress Reaction Time and Saccade Latency
It is well established that saccades and attention are closely related (e.g., premotor theory of attention; Rizzolatti et al, 1987), suggesting strong ecological validity for measuring attention via saccade latency. When a potential target attracts our attention, we typically saccade to it (Herwig & Schneider, 2014). In general, we found parallel attention effects for keypress RT and saccade latency. Thus, saccade latency also exhibits standard object-based effects previously demonstrated with keypress RT. However, our data also show that saccade latency is a more sensitive measure for detecting effects of attention. Notably, in Experiment 2, an object-based effect was reliably observed via saccade latency, but only a small numeric trend was observed for keypress RT. The effect sizes of our reported statistical tests are another index of sensitivity. In Table 1, we list Cohen’s d calculated for both keypress and saccade measures in all experiments. Keypress RT effect sizes were generally smaller than those of saccade latency; this held for both space-based and object-based effects of attention. Presumably, the superior sensitivity of saccade latency in comparison to keypress RT stems from the ballistic nature of saccades: variability in saccades latency is smaller than that of keypress RT. This is also accordant with experimental results showing that, although the oculomotor and hand-movement systems are guided by shared attentional resources, the planning of eye- and hand-movements to an attended target are dominated by the oculomotor system (Khan et al., 2011). Given the ecological validity and superior sensitivity of saccades, future research in object-based attention may benefit from utilizing saccade latency as the dependent measure.
Table 1.
Cohen’s d measures for space-based effect (measured by comparing cued and same object locations) and object-based effect (measured by comparing same and different object locations) in each experiment.
| Experiment 1
|
Experiment 2
|
Experiment 3
|
||||
|---|---|---|---|---|---|---|
| a. RT | b. latency | a. RT | b. latency | a. RT | b. latency | |
|
|
|
|
||||
| Space-based effect size | 0.69 | 1.29 | 0.90* | 1.84* | 1.81 | 2.20 |
| Object-based effect size | 0.67 | 0.74 | 0.47 | 0.75 | 0.84 | 0.83 |
Note the direction of the space-based effect is reversed in Experiment 2.
Conclusion
We found that first saccade latency is facilitated by exogenously and endogenously driven object-based attention. Our observation of object-based effects of attention in the presence of space-based IOR suggests that object-based selection may be independent of space-based selection. Furthermore, compared to manual keypress RTs, saccade latency exhibited higher sensitivity in detecting effects of attention. Overall, the oculomotor correlates of object-based attention we demonstrated in this study are consistent with the idea that attentional priority maps not only encode (and are influenced by) spatial locations but also perceptual objects.
Acknowledgments
This research was supported by grants from the US-Israel Binational Science Foundation (2013400, A.S.G.) and the National Institutes of Health (R01EY022727, T.L.).
Footnotes
We also examined curvature of the initial saccade trajectories as a function of the target location (Van der Stigchel & Theeuwes, 2005). Initial trajectory deviation did not differ between same and different object locations (M=3° away from the cued location).
A 2 (orientation: vertical, horizontal) × 4 (target location: cued location, same object, different object, opposite location) × 2 (room: old, new) mixed-design ANOVA on the mean keypress RTs showed no indication of main effect of room or interaction of room with any other variables. Thus, we collapsed across the factor of room from the analysis.
We also examined curvature of the initial saccade trajectories as a function of the target location (Van der Stigchel & Theeuwes, 2005). Initial trajectory deviation did not differ between same and different object locations (M=2.3° away from the cued location).
We also examined curvature of the initial saccade trajectories as a function of the target location (Van der Stigchel & Theeuwes, 2005). Initial trajectory deviation did not differ between same and different object locations (M=2.7° away from the cued location).
References
- Abrams RA, Dobkin RS. Inhibition of return: Effects of attentional cuing on eye movement latencies. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(3):467–477. doi: 10.1037//0096-1523.20.3.467. [DOI] [PubMed] [Google Scholar]
- Abrams RA, Jonides J. Programming saccadic eye movements. Journal of Experimental Psychology: Human Perception and Performance. 1988;14(3):428–442. doi: 10.1037//0096-1523.14.3.428. [DOI] [PubMed] [Google Scholar]
- Abrams RA, Law MB. Object-based visual attention with endogenous orienting. Perception & Psychophysics. 2000;62(4):818–833. doi: 10.3758/bf03206925. [DOI] [PubMed] [Google Scholar]
- Al-Janabi S, Greenberg AS. Target-object integration, attention distribution, and object orientation interactively modulate object-based selection. Attention, Perception, and Psychophysics. doi: 10.3758/s13414-016-1126-3. current issue. [DOI] [PubMed] [Google Scholar]
- Atchley P, Kramer AF. Object and space-based attentional selection in three-dimensional space. Visual Cognition. 2001;8(1):1–32. [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: Links, causes and implications for spatial attention. Trends in Cognitive Sciences. 2006;10(3):124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Barnas A, Greenberg AS. Visual field meridians modulate the reallocation of object-based attention. Attention, Perception, and Psychophysics. doi: 10.3758/s13414-016-1116-5. current issue. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Pratt J. The spatial distribution of inhibition of return. Psychological Science. 2001;12(1):76–80. doi: 10.1111/1467-9280.00313. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annual Review of Neuroscience. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand KA, Larrison AL, Sereno AB. Inhibition of return in manual and saccadic response systems. Perception & Psychophysics. 2000;62(8):1512–1524. doi: 10.3758/bf03212152. [DOI] [PubMed] [Google Scholar]
- Chen Z. Object-based attention: A tutorial review. Attention, Perception, & Psychophysics. 2012;74(5):784–802. doi: 10.3758/s13414-012-0322-z. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cave KR. Object-based attention with endogenous cuing and positional certainty. Perception & Psychophysics. 2008;70(8):1435–1443. doi: 10.3758/PP.70.8.1435. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Müller HJ. Spatial and temporal effects of spatial attention on human saccadic eye movements. Vision Research. 1992;32(2):293–304. doi: 10.1016/0042-6989(92)90140-e. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1(1):42–45. [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research. 1996;36(12):1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Klein RM, Everling S, Munoz DP. Contribution of the primate superior colliculus to inhibition of return. Journal of Cognitive Neuroscience. 2002;14(8):1256–1263. doi: 10.1162/089892902760807249. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. The Journal of Neuroscience. 1998;18(17):7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond L, Shomstein S. Object-based attention: Shifting or uncertainty? Attention, Perception, & Psychophysics. 2010;72(7):1743–1755. doi: 10.3758/APP.72.7.1743. [DOI] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. Journal of Experimental Psychology: General. 1984;113(4):501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Egly R, Driver J, Rafal RD. Shifting visual attention between objects and locations: Evidence from normal and parietal lesion subjects. Journal of Experimental Psychology: General. 1994;123(2):161–177. doi: 10.1037//0096-3445.123.2.161. [DOI] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V. What’s new in visual masking? Trends in Cognitive Sciences. 2000;4(9):345–352. doi: 10.1016/s1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- Gibson BS, Egeth H. Inhibition of return to object-based and environment-based locations. Perception & Psychophysics. 1994;55(3):323–339. doi: 10.3758/bf03207603. [DOI] [PubMed] [Google Scholar]
- Godijn R, Pratt J. Endogenous saccades are preceded by shifts of visual attention: Evidence from cross-saccadic priming effects. Acta Psychologica. 2002;110(1):83–102. doi: 10.1016/s0001-6918(01)00071-3. [DOI] [PubMed] [Google Scholar]
- Goldsmith M, Yeari M. Modulation of object-based attention by spatial focus under endogenous and exogenous orienting. Journal of Experimental Psychology: Human Perception and Performance. 2003;29(5):897–918. doi: 10.1037/0096-1523.29.5.897. [DOI] [PubMed] [Google Scholar]
- Goldsmith M, Yeari M. Central-cue discriminability modulates object-based attention by influencing spatial attention. Experimental Psychology. 2012;59(3):132–137. doi: 10.1027/1618-3169/a000135. [DOI] [PubMed] [Google Scholar]
- Greenberg AS. PhD dissertation. Baltimore, MD: Johns Hopkins University; 2009. Uncertainty as a guiding principle in the strategic allocation of attention to objects. [Google Scholar]
- Greenberg AS, Gmeindl L. Strategic Control of Attention to Objects and Locations, Journal of Neuroscience. 2008;28(3):564–565. doi: 10.1523/JNEUROSCI.4386-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Hayes D, Roggeveen A, Creighton S, Bennett P, Sekuler A, Pilz K. Object-Based Attention is Modulated by Shifts Across the Meridians. Journal of Vision. 2014;14(10):1062. [Google Scholar]
- He X, Fan S, Zhou K, Chen L. Cue validity and object-based attention. Journal of Cognitive Neuroscience. 2004;16(6):1085–1097. doi: 10.1162/0898929041502689. [DOI] [PubMed] [Google Scholar]
- He X, Humphreys G, Fan S, Chen L, Han S. Differentiating spatial and object-based effects on attention: An event related brain potential study with peripheral cueing. Brain Research. 2008;1245:116–125. doi: 10.1016/j.brainres.2008.09.092. [DOI] [PubMed] [Google Scholar]
- Herwig A, Schneider WX. Predicting object features across saccades: Evidence from object recognition and visual search. Journal of Experimental Psychology: General. 2014;143(5):1903–1922. doi: 10.1037/a0036781. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Perception & Psychophysics. 1995;57(6):787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hollingworth A, Maxcey-Richard AM, Vecera SP. The spatial distribution of attention within and across objects. Journal of Experimental Psychology: Human Perception and Performance. 2012;38(1):135–151. doi: 10.1037/a0024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TL. Forms of momentum across space: Representational, operational, and attentional. Psychonomic Bulletin & Review. 2014;21(6):1371–1403. doi: 10.3758/s13423-014-0624-3. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Kingstone A. Covert and overt voluntary attention: linked or independent? Cognitive Brain Research. 2003;18(1):102–105. doi: 10.1016/j.cogbrainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Jordan H, Tipper SP. Object-based inhibition of return in static displays. Psychonomic Bulletin & Review. 1998;5(3):504–509. [Google Scholar]
- Jordan H, Tipper SP. Spread of inhibition across an object’s surface. British Journal of Psychology. 1999;90(4):495–507. [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(43):15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nature Reviews Neuroscience. 2000;1(2):91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience. 1998;1(5):411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Khan AZ, Song JH, McPeek RM. The eye dominates in guiding attention during simultaneous eye and hand movements. J Vis. 2011;11(1):9. doi: 10.1167/11.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Does oculomotor readiness mediate cognitive control of visual attention? In: Nickerson R, editor. Attention and Performance. Hillsdale: Erlbaum; 1980. pp. 259–276. [Google Scholar]
- Klein RM. Inhibition of Return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;35(13):1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lamy D, Egeth H. Object-based selection: The role of attentional shifts. Perception & Psychophysics. 2002;64(1):52–66. doi: 10.3758/bf03194557. [DOI] [PubMed] [Google Scholar]
- Law MB, Abrams RA. Object-based selection within and beyond the focus of spatial attention. Perception & Psychophysics. 2002;64(7):1017–1027. doi: 10.3758/bf03194753. [DOI] [PubMed] [Google Scholar]
- Leek EC, Reppa I, Tipper SP. Inhibition of return for objects and locations in static displays. Perception & Psychophysics. 2003;65(3):388–395. doi: 10.3758/bf03194570. [DOI] [PubMed] [Google Scholar]
- List A, Robertson LC. Inhibition of return and object-based attentional selection. Journal of Experimental Psychology: Human Perception and Performance. 2007;33(6):1322–1334. doi: 10.1037/0096-1523.33.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado L, Rafal RD. Inhibition of return generated by voluntary saccades is independent of attentional momentum. Quarterly Journal of Experimental Psychology Section A. 2004;57(5):789–796. doi: 10.1080/02724980343000486. [DOI] [PubMed] [Google Scholar]
- Macquistan AD. Object-based allocation of visual attention in response to exogenous, but not endogenous, spatial precues. Psychonomic Bulletin & Review. 1997;4(4):512–515. [Google Scholar]
- Malcolm GL, Shomstein S. Object-based attention in real-world scenes. Journal of Experimental Psychology: General. 2015;144(2):257–263. doi: 10.1037/xge0000060. [DOI] [PubMed] [Google Scholar]
- Martinez A, Ramanathan DS, Foxe JJ, Javitt DC, Hillyard SA. The role of spatial attention in the selection of real and illusory objects. The Journal of Neuroscience. 2007;27(30):7963–7973. doi: 10.1523/JNEUROSCI.0031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A, Teder-Salejarvi W, Hillyard SA. Spatial attention facilitates selection of illusory objects: Evidence from event-related brain potentials. Brain Research. 2007;1139:143–152. doi: 10.1016/j.brainres.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley JS, Kramer AF, Peterson MS. Overt and covert object-based attention. Psychonomic Bulletin & Review. 2002;9(4):751–758. doi: 10.3758/bf03196331. [DOI] [PubMed] [Google Scholar]
- Moore CM, Yantis S, Vaughan B. Object-based visual selection: Evidence from perceptual completion. Psychological Science. 1998;9(2):104–110. [Google Scholar]
- Murray NP, Giggey K. Saccadic latency and attentional control: Evidence for the premotor theory of attention. North American Journal of Psychology. 2006;8(2):383–396. [Google Scholar]
- Müller NG, Kleinschmidt A. Dynamic interaction of object-and space-based attention in retinotopic visual areas. The Journal of Neuroscience. 2003;23(30):9812–9816. doi: 10.1523/JNEUROSCI.23-30-09812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz KS, Roggeveen AB, Creighton SE, Bennett PJ, Sekuler AB. How prevalent is object-based attention? PLoS ONE. 2012;7(2):e30693. doi: 10.1371/journal.pone.0030693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J, Adam JJ, McAuliffe J. The spatial relationship between cues and targets mediates inhibition of return. Canadian Journal of Experimental Psychology. 1998;52(4):213–216. [Google Scholar]
- Pratt J, Spalek TM, Bradshaw F. The time to detect targets at inhibited and noninhibited locations: Preliminary evidence for attentional momentum. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(3):730–746. [Google Scholar]
- Rafal R, Egly R, Rhodes D. Effects of inhibition of return on voluntary and visually guided saccades. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale. 1994;48(2):284–300. doi: 10.1037/1196-1961.48.2.284. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Cobb S. Visual attention modulates metacontrast masking. Nature. 1995;373(6509):66–68. doi: 10.1038/373066a0. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Carrasco M. Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. The Journal of Neuroscience. 2012;32(40):13744–13752. doi: 10.1523/JNEUROSCI.2676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppa I, Leek EC. The modulation of inhibition of return by object-internal structure: Implications for theories of object-based attentional selection. Psychonomic Bulletin & Review. 2003;10(2):493–502. doi: 10.3758/bf03196512. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25(1A):31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rothkirch M, Ostendorf F, Sax AL, Sterzer P. The influence of motivational salience on saccade latencies. Experimental Brain Research. 2013;224(1):35–47. doi: 10.1007/s00221-012-3284-4. [DOI] [PubMed] [Google Scholar]
- Russell AF, Mihalaş S, von der Heydt R, Niebur E, Etienne-Cummings R. A model of proto-object based saliency. Vision research. 2014;94:1–15. doi: 10.1016/j.visres.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38(4):637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Experimental Brain Research. 1994;98(3):507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. The Quarterly Journal of Experimental Psychology. 1986;38(3):475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- Shomstein S. Object-based attention: Strategy versus automaticity. WIRE’s Cognitive Science. 2012;3:163–169. doi: 10.1002/wcs.1162. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Behrmann M. Object-based attention: Strength of object representation and attentional guidance. Perception & Psychophysics. 2008;70(1):132–144. doi: 10.3758/PP.70.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Configural and contextual prioritization in object-based attention. Psychonomic Bulletin & Review. 2004;11(2):247–253. doi: 10.3758/bf03196566. [DOI] [PubMed] [Google Scholar]
- Smith DT, Schenk T. The premotor theory of attention: time to move on? Neuropsychologia. 2012;50(6):1104–1114. doi: 10.1016/j.neuropsychologia.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Spalek TM, Hammad S. Supporting the attentional momentum view of IOR: Is attention biased to go right? Perception & Psychophysics. 2004;66(2):219–233. doi: 10.3758/bf03194874. [DOI] [PubMed] [Google Scholar]
- Sumner P. Inhibition versus attentional momentum in cortical and collicular mechanisms of IOR. Cognitive Neuropsychology. 2006;23(7):1035–1048. doi: 10.1080/02643290600588350. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Experimental Brain Research. 2002;142(2):284–291. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Mathôt S, Kingstone A. Object-based eye movements: The eyes prefer to stay within the same object. Attention, Perception, & Psychophysics. 2010;72(3):597–601. doi: 10.3758/APP.72.3.597. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. Journal of Neurophysiology. 1997;77(2):1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. Journal of Neuroscience. 2005;25(41):9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP, Driver J, Weaver B. Short report: Object-centred inhibition of return of visual attention. The Quarterly Journal of Experimental Psychology. 1991;43(2):289–298. doi: 10.1080/14640749108400971. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Weaver B, Jerreat LM, Burak AL. Object-based and environment-based inhibition of return of visual attention. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(3):478–499. [PubMed] [Google Scholar]
- Van der Stigchel S, Theeuwes J. Relation between saccade trajectories and spatial distractor locations. Cognitive Brain Research. 2005;25(2):579–582. doi: 10.1016/j.cogbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Vecera SP. Toward a biased competition account of object-based segregation and attention. Brain and Mind. 2000;1(3):353–384. [Google Scholar]
- Vecera SP, Farah MJ. Does visual attention select objects or locations? Journal of Experimental Psychology: General. 1994;123(2):146–160. doi: 10.1037//0096-3445.123.2.146. [DOI] [PubMed] [Google Scholar]
- Wismeijer DA, Gegenfurtner KR. Orientation of noisy texture affects saccade direction during free viewing. Vision Research. 2012;58:19–26. doi: 10.1016/j.visres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Wu CC, Kwon OS, Kowler E. Fitts’s Law and speed/accuracy trade-offs during sequences of saccades: Implications for strategies of saccadic planning. Vision Research. 2010;50(21):2142–2157. doi: 10.1016/j.visres.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinsky GJ, Bisley JW. The what, where, and why of priority maps and their interactions with visual working memory. Annals of the New York Academy of Sciences. 2015;1339(1):154–164. doi: 10.1111/nyas.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]