Abstract
In signaling, Rho-family GTPases bind effector proteins and alter their behavior. Here we present the crystal structure of Cdc42•GTP bound to the GTPase-activating protein (GAP)-related domain (GRD) of IQGAP2. Four molecules of Cdc42 are bound to two GRD molecules, which bind each other in a parallel dimer. Two Cdc42s bind very similarly to the Ras/RasGAP interaction, while the other two bind primarily to “extra domain” sequences from both GRDs –tying the GRDs together. Calorimetry confirms two-site binding of Cdc42•GTP for the GRDs of both IQGAP2 and IQGAP1. Mutation of important extra domain residues reduces binding to single-site and abrogates Cdc42 binding to a much larger IQGAP1 fragment. Importantly, Rac1•GTP displays only single-site binding to the GRDs, indicating that just Cdc42 promotes IQGAP dimerization. The structure identifies an unexpected role for Cdc42 in protein dimerization thus expanding the repertoire of interactions of Ras family proteins with their targets.
Graphical abstract
eTOC Blurb:
The three human IQGAPs are multi-domain scaffolds affecting many cellular processes including neurite outgrowth and carcinogenesis. Common binding partners include the active forms of Cdc42 and Rac1. Here, LeCour et al. show that Cdc42 stabilizes IQGAP dimers, enabling regulated dimer formation downstream of receptor signaling.
Introduction
Small GTPases of the Ras superfamily, including Cdc42 and Rac1, function as binary switches in signal transduction (Goitre et al., 2014). The switch is “on” when GTP is bound in their active sites and signaling is turned off once GTP is hydrolyzed to GDP. Cdc42 and Rac1 impact many cellular functions and influence the actin cytoskeleton, cell migration and invasion (Rathinam et al., 2011). IQGAPs are eukaryotic protein scaffolds that are found in species ranging from yeast to humans. The first human isoform identified and by far the most studied, IQGAP1, is a 1657 amino acid protein containing an amino-terminal calponin homology domain followed by six ~50 amino acid sequence repeats, a WW domain, four isoleucine- and glutamine (IQ) motifs, a RasGAP-related domain (GRD), and a RasGAP C-terminal domain (Figure 1). IQGAP1 is ubiquitously expressed and binds to a diverse range of proteins including calcium-binding proteins, growth factor and chemokine receptors, mitogen-activated protein kinase (MAPK) components, actin and actin-associated proteins, microtubule-associated proteins, small GTPases, and components of the adherens junctions (Weissbach et al., 1994; White et al., 2012). IQGAP1 is also known to form dimers and dimerization is required for its function (Ren et al., 2005). Because it is over-expressed in several cancers and over-expression in cultured mammalian cells promotes migration and invasion, IQGAP1 appears to be an oncoprotein (Jadeski et al., 2008; White et al., 2009). Less is known about IQGAP2 and IQGAP3. Each shares considerable overall sequence identity with IQGAP1 and with each other, but there is a more restricted tissue expression for these paralogs and some evidence that their functions are divergent (Hedman et al., 2015). In fact, IQGAP2, mainly expressed in the liver, kidney, and platelets, may be considered a tumor suppressor, because specific knockdown of IQGAP2 in mice results in early onset hepatocellular carcinoma that is dependent upon IQGAP1 expression (Schmidt et al., 2008).
Figure 1.
IQGAP1 schematic. CH; calponin homology domain, REPEATS; 6 ~50 amino acid sequence repeats, WW; tryptophan-containing polyproline-binding domain, IQ; 4 isoleucine- and glutamine-containing motifs, GRD; RasGAP-related domain, RGCT; RasGAP C-terminal domain found in IQGAPs. Underlined in green is the region previously shown to be important for mediating IQGAP1 dimerization (residues 763-863). Underlined in magenta are the equivalent Ex domain residues that participate in the GRD2-GRD2 interface in the crystal structure (IQGAP2 residues within 875-914 and 1204-1246). Indicated are the amino acids used for the GRD constructs. Note, all 3 human IQGAPs have a similar domain composition and layout.
All three human IQGAPs have been reported to bind to Cdc42 and Rac1 in their GTP-bound forms (Smith et al., 2015). There is good evidence that the binding site for these GTPases encompasses the RasGAP-related domains (GRDs) which display a high degree of sequence identity with each other. In fact, between IQGAP1 and IQGAP2, the 384 residue GRDs are over 75% identical in sequence. Ordinarily, a GTPase-activating protein (GAP) domain would enhance hydrolysis of GTP bound to a small GTPase by several orders of magnitude (Scheffzek et al., 1998b). However, there has been no report of an IQGAP GRD that functions in this respect, and instead of accelerating GTP hydrolysis, the IQGAP GRDs preserve the GTP-bound forms of the GTPases (Hart et al., 1996; Swart-Mataraza et al., 2002). The mechanism for this unusual interaction is unknown but speculation is that it is due to lack of a catalytic arginine “finger” seen in RasGAPs.
Over-expression of IQGAP1 increases levels of active Cdc42, augments filopodia formation and promotes cell motility in a Cdc42- and Rac1-dependent manner (Mataraza et al., 2003; Swart-Mataraza et al., 2002). Expression of an IQGAP1 GRD mutant that blocks Cdc42 binding results in cells with a close resemblance to a Cdc42−/− morphology. This same IQGAP1 mutant also blocks filopodia formation in response to the potent Cdc42 agonist, bradykinin (Swart-Mataraza et al., 2002). Binding of Cdc42 enhances F-actin crosslinking activity via the N-terminal calponin homology domain and affects interactions with both β-catenin and CLIP-170 which are mediated by C-terminal sequences, implying that binding of Cdc42 induces a considerable conformational change in IQGAP1 (Fukata et al., 1997; Fukata et al., 2002).
Prior to the current study, there were structures of four different RasGAP-related domains. The SynGAP, neurofibromin, and IQGAP1 GRD structures were determined in isolation while the p120RasGAP GRD structure was determined both in isolation and in complex with Ras (Kurella et al., 2009; Pena et al., 2008; Scheffzek et al., 1997; Scheffzek et al., 1998a; Scheffzek et al., 1996). All of these GRDs are entirely helical, and features a central domain (GAPc) which makes the majority of contacts with Ras in the Ras/RasGAP structure (Protein Databank ID 1WQ1), and a so-called extra sub-domain (Ex domain), composed of N- and C-terminal sequences, which makes few contacts with Ras in the Ras/RasGAP structure and has been postulated to primarily stabilize GAPc (Kurella et al., 2009; Pena et al., 2008; Scheffzek et al., 1997; Scheffzek et al., 1998a; Scheffzek et al., 1996).
To better understand how IQGAPs bind to GTPases, we crystallized the IQGAP2 GRD (GRD2) bound to GTP- loaded, catalytically-compromised Cdc42. Surprisingly, compared to the structure of the unbound IQGAP1 GRD (GRD1), interaction with Cdc42 has induced a significant conformational change in the Ex domain, creating two unanticipated Cdc42 binding surfaces on two closely associated GRD2s. Each binding site utilizes sequences from both GRD2s. These two Cdc42 molecules, in conjunction with the two Cdc42 molecules that bind very similarly to the Ras/RasGAP interaction, give an overall Cdc42-to-GRD2 stoichiometry of 2:1. Isothermal titration calorimetry (ITC) indicates that the GRDs of both IQGAP1 and IQGAP2 bind to two molecules of Cdc42 in a two-site mode of binding. Mutation of important Ex domain residues reduces Cdc42 binding to ‘one-site’ for both GRD1 and GRD2. The ~70% sequence identical Rac1, a known binding partner of IQGAPs, displays only one-site binding to all of our GRD constructs. The ITC data for Rac1 binding to the GRDs (wild-type and mutant) is endothermic in nature and very similar to that observed for Cdc42 binding to our Ex domain mutants; implying that Rac1 is binding to the RasGAP site and is not utilizing the Ex mode binding sites. Since the Ex mode binding sites are composed of sequences provided by two GRDs, apparently Cdc42, but not Rac1, promotes GRD dimerization.
Results
Overall structure
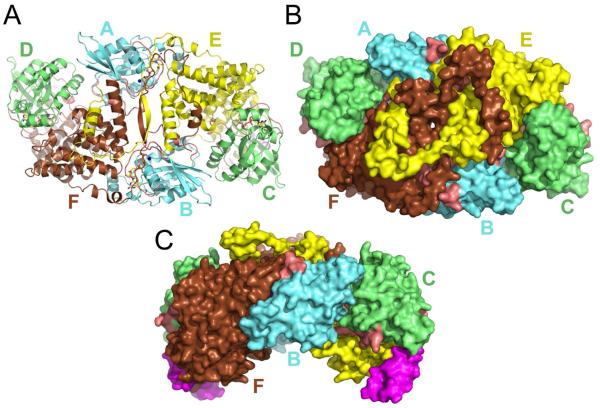
The crystal asymmetric unit contains four molecules of Cdc42 (molecules A-D) and two molecules of GRD2 (molecules E and F; Figure 2). Each Cdc42 is bound to a molecule of GTP and each GRD2 interacts with a net of two Cdc42 molecules. The entire complex, viewed from the “top” or “bottom” displays a high degree of 2-fold symmetry. However, there is some structural heterogeneity that breaks perfect two-fold symmetry, and because of this selective, light non-crystallographic symmetry restraints were used in refinement. Compared to the 80% sequence-identical Ex domain of the unbound GRD1 structure, there has been a large conformational change in the Ex domain of GRD2 that results in the positioning of the C-terminus of molecule E next to the N-terminus of molecule F, and vice versa, in an interaction reminiscent of a “Roman handshake” (Figures 2, 3 ,S1, S2). The E-F interaction involves exclusively Ex domain residues and buries over 3,850Å2 of solvent accessible surface area of which 70% is non-polar.
Figure 2.
The crystal asymmetric unit. Two IQGAP2 GRDs (brown, yellow) are bound to each other and each binds to two molecules of Cdc42 (cyan, green). The conformation-variable “switches” of Cdc42 are in light red and the 31 GRD residues with no structural equivalent in RasGAP, neurofibromin or SynGAP are colored magenta. The cyan Cdc42 molecules A and B (cyan letters) bind primarily to residues belonging to the Ex sub-domain of GRD molecules E and F, respectively (yellow and brown letters). Cdc42 molecules C and D (green letters) are binding in a mode very similar to the Ras/RasGAP interaction to GRD molecules E and F, respectively. Panels A and B are in the same orientation. Panel C has been rotated exactly 90° about the horizontal. See also Supplemental Figures S1 and S2.
Figure 3.
Comparison of the IQGAP1 and IQGAP2 GRDs. A, GRD2 Molecule E with Ex domain residues (875-934 and 1204-1246) and central domain residues (935-1203) in blue and yellow, respectively. Residues with no structural equivalent in RasGAPs are in magenta. B, The unbound GRD1 structure (Kurella et al., 2009)with Ex domain residues (962-1021 and 1291-1333) and central domain residues (1022-1290) colored similarly. Note the large structural differences between the two 80% identical Ex domains including the β-strand in GRD2 (black arrow). The two GRDs were aligned for the figure using LSQKAB (CCP4, 1994) and residues 939-1199 (GRD2 numbering). The r.m.s.d. for the 261 Cα atoms is 1.75Å.
Cdc42 molecules A and B are interacting primarily with Ex domain residues (residues 875-934 and 1204-1246) of molecules E and F, respectively. The A and B “Ex-mode” interactions bury an average of 2,910Å2 of solvent accessible surface area with E and F, respectively, of which 60.8% is non-polar. Molecules A and B also contact residues from the opposite GRD2 (A to F; B to E) securing the two GRDs together and burying an average of 869Å2 of solvent accessible surface area each in the process (59.6% non-polar). Molecules A and B also contact each other by forming a reciprocal hydrogen bond involving residues Asp63 and Arg66 of what is called “switch 2” in small GTPases. Molecules A and B also contact Cdc42 molecules D and C, respectively. The Lys107 sidechains of A and B appear to serve as hydrogen bond donors to His104 (Nε2 atom) of molecules D and C, while molecules D and C utilize their His103 sidechains to form hydrogen bonds with the backbone oxygen atoms of Lys107 of the A and B molecules.
Molecules C and D are bound primarily to GAPc residues (residues 935-1203) in GRD2 molecules E and F, respectively, burying an average of 2,888 Å2 of accessible surface of which 62% is non-polar. This “RasGAP-mode” binding, which we previously modeled (Kurella et al., 2009), is very similar to that seen in the Ras/RasGAP structure (Scheffzek et al., 1997).
With the exception of guanine nucleotide dissociation inhibitors (Garcia-Mata et al., 2011), a relevant Cdc42- effector interaction would allow for membrane insertion of the prenylated C-terminus. In this study, we used a version of Cdc42 lacking the 14 C-terminal residues. Modeling of the missing eleven residues (that would remain after CAAX box processing) in an extended conformation indicates that if either the “top” or the “bottom” of the complex was presented towards a membrane surface, each Cdc42 molecule would be able to insert its prenylated C-terminus into that membrane. Mapping electrostatic potential to the molecular surface of the complex does not reveal a predominantly positively-charged surface which might favorably interact with negatively-charged phospholipid head groups. However, an argument can be made that it is the top surface shown in Figure 2B that will likely be adjacent to the plasma membrane. Firstly, this surface is relatively flat whereas the bottom surface is concave and not shape-complimentary to an approximately planar membrane. Secondly, a completely extended conformation for the missing eleven residues of Cdc42 is required if the bottom surface is presented to the membrane whereas a more relaxed conformation of these residues can achieve the same result if the top surface is membrane proximal. Finally, IQGAP GRDs contain a stretch of thirty-one residues which do not have a structural equivalent in RasGAP, neurofibromin or SynGAP (Kurella et al., 2009). Five of these residues in each GRD are making some contact with Cdc42 molecules (RasGAP modes). Presumably, the other twenty-six residues are required for an unknown IQGAP-specific function. These twenty-six residues remain solvent exposed and accessible when the top surface is membrane proximal (Figure 2C).
Interactions between the two GRDs in the complex
There are twenty-two hydrogen bonds and two salt bridges between the Ex domains of the two GRD2 molecules. Twelve hydrogen bonds are common, meaning that each GRD2 uses the same residue as a hydrogen bond donor or a hydrogen bond acceptor. The remaining non-common polar interactions arise from conformational differences between the two GRD2 molecules (Figures S1, S3).
Residues 1208-1214 of each GRD2 participate in a short, antiparallel β-sheet. Within these β-strands, there is just one sequence difference between GRD2 and GRD1 (Glu1213 of GRD2 vs Gly1300 of GRD1). Although the isolated GRD1 structure is all helical, the secondary structure prediction server JPRED (http://www.compbio.dundee.ac.uk/www-jpred/) predicts that GRD1 residues equivalent to 1208 and 1209 should have strand conformation.
Cdc42 binding in the RasGAP-mode
Residues of the conformation-variable Cdc42 “switches” (residues 25-38 (switch 1) and 57-75 (switch 2)) are involved in most of the contacts between Cdc42 molecules and GRD2s (Figure 4, S4). Molecules C and D interact with their respective GRD2 molecules E and F very similarly to the Ras/RasGAP interaction (Scheffzek et al., 1997). Glu31 and Asp38 of switch 1 are involved in salt bridges with GRD2 residues Lys1098 and Lys1143, respectively. Leu61, Glu62, Asp63, Tyr64, Arg66, Leu67 and Leu70 of switch 2 lose over 545Å2 of accessible surface area upon binding to GRD2. Asp63 and Arg66 are involved in salt bridge interactions with Arg973 and Asp1117, respectively. Tyr1106, of the conserved YYR motif seen in IQGAPs (FLR in RasGAP), is sandwiched between Pro34 and Tyr64 of Cdc42 with the phenol rings of the two tyrosines nearly parallel to each other (Figure 5A). The hydroxyl of Tyr1106 is hydrogen-bonding to the backbone oxygen of Thr35 of switch 1 while the hydroxyl of Tyr64 of switch 2 is hydrogen-bonding to both the backbone oxygen of Tyr1106 and the sidechain nitrogen of Asn1110. There is also direct contact to the nucleotide sugar hydroxyl O3 atom made by the Gln955 sidechain nitrogen. Gln955 is also using its sidechain oxygen to hydrogen-bond to the Tyr32 amide nitrogen. Note, a glutamine equivalent to Gln955 in IQGAP2 is found in all three human IQGAPs (Figure S3). Surprisingly, the highly conserved Thr959, which in a structure-based alignment occupies the position equivalent to the catalytic arginine finger in Ras GAPs, does not appear to play an important role in binding Cdc42. However, the Thr959 sidechain has adopted a rotomer different to that seen in the isolated GRD1 structure (Kurella et al., 2009), presumably to accommodate Ala13 of the phosphate-binding loop (P-loop).
Figure 4.
Common contacts for Cdc42 molecules in RasGAP- and Ex-binding modes. A, RasGAP-mode Cdc42 molecule with GTP and magnesium (sticks and blue sphere) surrounded by the two switch regions (salmon). B, Ex-mode Cdc42 with switches, GTP, and magnesium rendered similarly. Nearly all Cdc42 residues in black lettering lose >20Å2 of solvent accessible surface area upon binding to the GRD2. GRD2 residues in red are van der Waals’ contacts, and residues in green and blue denote hydrogen-bonds and salt bridges, respectively. Green and black asterisks indicate that the GRD2 or the GTPase sidechain, respectively, is used in the hydrogen bond. Green residues with no asterisk are involved in mainchain-to-mainchain hydrogen bonds. Underlined GRD2 residues contacting the cyan molecules are from the “opposite” GRD2 (e.g. Cdc42 molecule A contacted by GRD2 molecule F). See also Supplemental Figures S3 and S4.
Figure 5.
Stereo view of important Cdc42 “switch” interactions in the RasGAP- and Ex-binding modes. A, RasGAP-mode switch 1 and switch 2 (salmon) hydrogen bonds and salt bridges (dashed lines) to the GRD2 (yellow) as defined by PDBePISA (see Methods). Gln61 has been modeled in the same conformation that is found in PDB ID 1GRN (Nassar et al., 1998). B, Key Ex-mode switch 1 interactions with the GRD2 are represented by dashed lines. The bound nucleotide is shown in stick representation with carbon atoms in light grey. Note the direct contacts to the nucleotide atoms O2 and O3 of the sugar and N2 of the base. See also Supplemental Figures S3 and S4.
Previously, we modeled the GRD1•Cdc42 RasGAP-mode interaction (Kurella et al., 2009). In the model, Tyr1193, like Tyr1106 in GRD2, also acts as a hydrogen bond donor to the carbonyl oxygen of Thr35 and competes with Gln61 for hydrogen bonding to the nucleophilic water. In the RasGAP mode, which is very similar the previous model (Figure S2), we do not see a water molecule in the vicinity of Leu61. However, when Gln61 is modeled in the same conformation as is seen in the 1GRN structure (Cdc42/Cdc42GAP structure (Nassar et al., 1998)), which is essentially identical to the conformation of Gln61 in our model, the Tyr1106 sidechain hydroxyl oxygen is ~3Å from the glutamine sidechain oxygen and ~3Å from the Thr35 backbone oxygen. Because both of these Cdc42 atoms are hydrogen bond acceptors, and a tyrosine hydroxyl can only donate one hydrogen bond, the arrangement appears unstable.
Understanding why IQGAPs do not accelerate GTP hydrolysis
It has been postulated that IQGAPs do not function as GAPs because they lack the arginine “finger” seen in RasGAP, neurofibromin and synGAP. In the Ras/RasGAP structure, Ras is bound to GDP•AlF3 to mimic the transition state and increase binding affinity for RasGAP (Scheffzek et al., 1997). Gln61of Ras, required for GTP hydrolysis, is stabilized by hydrogen bonding via its sidechain amide to the backbone oxygen of the “finger” Arg789, and the guanidinium group of that arginine is 2.6Å from a fluoride ion and 3.2Å from the oxygen atom that bridges the β and γ phosphates in GTP. Our crystal structure elucidates why IQGAPs do not accelerate GTP hydrolysis on Cdc42. After aligning the Ras/RasGAP structure onto one of our RasGAP-mode interactions (with GRD2 mutated to contain Arg959 and Cdc42 to contain Gln61), we see that the Cα atoms of Arg789 of RasGAP and Arg959 of the GRD2 are separated by ~4.3Å and this difference ultimately would prevent Arg959 from duplicating RasGAP’s interactions with the nucleotide. Other differences, including steric clashes and the intrusion of Tyr1106 into the active site as discussed above, make it unlikely that simple mutation of Thr959 to arginine would convert IQGAPs into true GAPs.
An arginine finger is not the only feature required for a GTPase-activating protein. The p85α subunit of phosphatidylinositol 3-kinase (PI3K) contains a domain with homology to the breakpoint cluster region gene product; a Cdc42 GAP (Diekmann et al., 1991). Despite binding to both Cdc42 and Rac1 and possessing the critical arginine residue, the breakpoint cluster homology (BH) domain of p85α does not accelerate hydrolysis of bound GTP. Mutational analysis reveals that this lack of GAP activity likely arises from improper stabilization of the switch regions due to sequence differences found between the p85α BH domain and Cdc42GAP, a BH domain with demonstrated GAP activity (Fidyk and Cerione, 2002). Presumably, correct positioning of the switch residues of Ras is also required for full GAP activity by RasGAP domains, however additional work is required to elucidate the lack of GAP activity due to improper switch positioning by IQGAP GRDs.
Previously, it was observed that the ground state and transition state complexes of GTPases bound to RhoGAP differed by a rotation of the GTPase by ~20 degrees with respect to RhoGAP (Rittinger et al., 1997a; Rittinger et al., 1997b). In the ground state complex, Arg85 of RhoGAP is hydrogen-bonding to the backbone oxygen of P-loop residue Gly12 of the GTPase. In the transition-state complex, due to the rotation of the GTPase, the relative position of Arg85 has been shifted several angstroms, allowing the guanidinium group to interact with the nucleotide – presumably stabilizing the transition state. Since RasGAPs share a degree of structural homology with RhoGAPs (Bax, 1998), significant differences between ground-state and transition-state complexes should be considered. Our RasGAP binding modes, which represent ground-state complexes, are very similar to the transition-state Ras/RasGAP structure (Scheffzek et al., 1997)(Figure S2). For Cdc42 bound in the RasGAP mode, the nearest GRD arginine residue is Arg1107 of the conserved YYR motif found in IQGAPs. The guanidinium of Arg1107 is > 9 Å distant from Gly12 and the P-loop. If Arg1107 were to directly interact with the nucleotide, an even larger structural re-arrangement would need to accompany the shift in Cdc42 from ground-state to transition-state.
Cdc42 contacts that stabilize GRD dimers
Cdc42 molecules A and B (Ex-mode) are tightly bound to GRD2 through a series of polar interactions involving residues in the two switch regions, Ala13 in the P-loop, Gln116 of the NKxD motif (Kjeldgaard et al., 1996) as well as residues within the helix α3 which is specific to Rho-family GTPases (Thapar et al., 2002) (Figure 4B). In total, switch 1 residues lose more than 660Å2 of solvent exposed surface area upon binding to GRD2. In addition, there are three direct polar contacts from GRD2 residues to the bound nucleotide. Tyr1197 hydrogen bonds to N2 of the base, while the O2 and O3 hydroxyl oxygens of the sugar are within hydrogen bonding distance of Glu931 and Arg930, respectively. Asn928, which has an equivalent in GRD1 (Asn1015), hydrogen bonds to both the Ala13 backbone oxygen and the Gln116 sidechain. In IQGAP3, there is a serine at the position equivalent to Asn928, and although serine may serve as an H-bond donor and acceptor, a significant rearrangement would be required to move the serine close enough to Cdc42 to duplicate these interactions.
There are three mainchain-mediated hydrogen bonds between switch 1 residues Phe28 and Ser30 and residues Val1201 and Val1203, respectively, of GRD2. This short intermolecular, parallel β-sheet is bolstered by hydrogen bonds between the sidechains of Ser30 and Ser1204, and the backbone oxygen of Pro29 with the Arg930 sidechain. Nearby, Asn924 hydrogen bonds to the Tyr32 hydroxyl using its sidechain amide. Major Ex-mode switch 1 interactions are depicted in Figure 5B. When Gln61 is modeled using the same conformer as seen in 1GRN (Nassar et al., 1998), the sidechain carbonyl oxygen of Asn924 is within hydrogen bonding distance of the Nε atom of the glutamine. An asparagine equivalent to Asn924 in GRD2 is found in all 3 human IQGAPs. Another notable switch 1 interaction is a salt bridge between Asp38 and Arg878of the opposite Ex domain (e.g. molecule F-to-molecule A). This “cross” salt bridge is strengthened by additional cross hydrophobic interactions which bury Val36, Phe37 of switch 1 and Leu70 and Pro73 of switch 2. Asp122 and Glu127 of Cdc42 α3 are involved in salt bridges with Lys1196 and Lys1191, respectively. Ser124 and Lys131, also of α3, are in hydrogen bonding interactions with Gln929 and the backbone oxygen of Leu1070, respectively.
Calorimetry confirms two-site binding
Previously, ITC experiments at 20°C using Cdc42(Q61L, C188S)•GTP indicated that just one Cdc42 binds to the GRD1 (Kurella et al., 2009). This result was expected considering the structural similarities between Cdc42 and Ras, and between GRD1 and other RasGAP domains, and motivated our use of a 1:1 stoichiometry for crystallization purposes. The 2:1 stoichiometry revealed by the crystal structure prompted additional ITC experiments using both the GRD1 and GRD2 proteins and the Cdc42 version used in the crystal structure. At 25°C, only one-site binding was observed for GRD1, while two-site binding was seen for the GRD2 (data not shown). At 30°C in the same buffer, two-site binding was observed for both GRD1 and GRD2 (Figure 6). We suspect that the GRD1 Ex domain may be slightly more stable than the GRD2 Ex domain, and the transition to 30°C is necessary to allow creation of Ex-mode binding sites similar to what we see in the structure. The argument that GRD1 binds two molecules of Cdc42 in a manner very similar to GRD2 is supported by the high degree of sequence conservation between the GRDs, which is especially high for residues contacting Cdc42 (Figure S3).
Figure 6.
Isothermal titration calorimetry indicates two-site binding by Cdc42 for both GRD1 and GRD2. Experiments were performed at 30°C and integrated heats were fitted to the “TwoSites” model using Origin 7 software. All experiments have Cdc42 (Q61L; residues 1-177) in the syringe and the wild-type GRD protein in the cell. A, GRD1 estimated dissociation constants are: Kd1 = 9.9 × 10−8 M, Kd2 = 2.6 × 10−6 M. B, GRD2 estimated dissociation constants are: Kd1 = 6.8 × 10−7 M, Kd2 = 6.0 × 10−6 M. See also Supplemental Figures S5, S6 and S7.
We next introduced alanine substitutions in both GRD1 and GRD2 at six positions where the sidechains of GRD2 contact Cdc42 in the Ex-binding mode (N924A, N928A, Q929A, R930A, E931A and S1204A in GRD2, and N1011A, N1015A, Q1016A, R1017A, E1018A and T1291A in GRD1). The mutant GRD proteins were well behaved and purified identically to their wild-type counterparts. As predicted from our crystal structure, removal of important Ex mode contacts converted binding to one-site for both GRDs. In fact, the one-site binding we see for the mutants is endothermic in nature and very similar to the endothermic one-site Cdc42 binding observed for either GRD at 20°C (Figure S5). This would indicate that the RasGAP mode calorimetry “signature” is entirely endothermic in these conditions whereas Ex mode binding is (net) exothermic (Figure 6). These same six mutations were made in a GST-IQGAP1 construct (IQGAP1-C6A) spanning the C-terminal half of the protein (residues 864-1657). In this much larger IQGAP1 construct, these mutations completely abolished Cdc42 binding whereas the wild-type fragment (IQGAP1-C) still binds to Cdc42 (Figure S6). Since we do see one-site binding for the GRDs containing these same Ex-mode mutations, we speculate that sequences in this larger fragment are occluding the RasGAP binding site. Since the wild-type, isolated GRDs do exhibit two-site binding, it appears that in the C-terminal half, binding is sequential, and the Ex mode binding event must precede RasGAP mode binding.
We next wanted to determine if Rac1 (Q61L; residues 1 – 177) would demonstrate two-site binding to our GRD constructs. Rac1 and Cdc42 share ~70% sequence identity and both are known interactors of IQGAP proteins (Smith et al., 2015). ITC experiments in buffer and temperature conditions identical to those for Cdc42 reveal that Rac1•GTP only binds in a one-site mode to all of our GRD constructs including the 6xAla mutants (Figure S7). Furthermore, the calorimetry heats are entirely endothermic, as was observed for Cdc42 binding to the 6xAla mutants. This binding signature, coupled with sequence information (below) and the fact that Rac1 still binds the 6xAla mutation GRDs, strongly suggesting that Rac1 is binding to the RasGAP site and that the Ex-mode is Cdc42-specific.
Potential to interact with other GTPases
Sequence alignments combined with knowledge of the Cdc42 contacting residues allows prediction of the likelihood of other GTPases binding to the GRD2 (Figures 4, S3, S4). There are twenty-two Cdc42 residues which lose >30Å2 of solvent accessible surface area in the RasGAP-mode. Seventeen of these are identical between Cdc42 and Rac1.With regard to the seven sidechain-specific polar contacts made in the RasGAP-mode, six of these sidechains are identical between Cdc42 and Rac1. The only difference is Thr3 (Ala3 in Rac1). However, since hydrogen bonding by Thr3 is seen by only one of the RasGAP Cdc42 molecules, this bond is not a critical requirement. This sequence information along with our ITC results strongly suggest that Rac1 binds to GRD2 using the RasGAP-mode.
Of the eleven polar interactions utilized by both Ex mode molecules A and B, there are four significant sequence differences between Cdc42 and Rac1. Ser30 is involved in a hydrogen bond (Figures 4B and 5B) and this switch 1 residue in Rac1 is a glycine. Other differences occur within or flanking helix α3. Gln116, Lys131 and Asn132 of Cdc42 are all involved in hydrogen-bonding interactions with GRD2. Gln116 is part of the NKxD motif which in Rac1 is occupied by a lysine, and in the context of our structure, Lys116 of Rac1 cannot duplicate the glutamine’s hydrogen-bonding interactions. In α3, Lys131 of Cdc42 is hydrogen bonding to the backbone oxygen of Leu1069 of GRD2 and this interaction cannot be duplicated by Glu131 in Rac1. Finally, Asn132 of molecules A and B are hydrogen-bonding via their OD1 atoms to the sidechain amide of Gln983, and Lys132 of Rac1 cannot duplicate this interaction. Because our 6xAla GRD mutants had no effect on Rac1 binding, these specific sequence differences likely preclude Rac1’s utilization of the Ex-mode binding sites.
IQGAP3 is the only family member reported to bind to H-Ras (Nojima et al., 2008), while IQGAP2 has been reported to bind to RhoG (Wennerberg et al., 2002), and IQGAP1 reportedly binds both RhoA and RhoC (Casteel et al., 2012; Wu et al., 2012). In each case, binding of the GTPases is either to full-length proteins or sizeable IQGAP fragments, and was not specifically mapped to the respective GRD. Figure S4 shows that key contacting residues are not always conserved among Cdc42, RhoG, RhoA, RhoC and H-Ras. Note, some sequence differences involve changes in residues at salt bridge positions that would clearly abrogate that salt bridge (e.g. glutamate for lysine).
Binding preferences for the three human IQGAPs
Residues that contact Cdc42 in the Ex and RasGAP modes (Figures 4 and S3) are more highly conserved between GRD2 and GRD1 than the overall sequence identity between the two GRDs (75.6%). Twenty-five of thirty-one (80.6%) RasGAP-mode contacting residues are identical in the two proteins, as are thirty-three of forty-one (80.5%) contacting Ex-mode residues. Twenty-eight GRD2 residues make polar contacts using their sidechains, and twenty-four (85.7%) are identical in GRD2 and GRD1. The differences for GRD2 vs GRD1 are Arg878 (Lys965), Glu1157 (Asp1244), Lys1196 (Glu1283) and Ser1204 (Thr1291). Unfortunately, all of our previous attempts to produce GRD3 protein have been unsuccessful, so we have not been able to perform the obvious ITC experiments. However, comparing GRD2 with GRD3 reveals much greater variation at contacting positions. Only 67.7% of RasGAP contacts are identical between GRD2 and GRD3 and this number falls to just over 56% identity in the Ex-mode contacts. Only seventeen of the twenty-eight polar contacts that are sidechain-dependent are identical between GRD2 and GRD3. However, many of these sequence differences are quite conservative changes (e.g., Glu vs. Asp, Arg vs. Lys), so the least complicated explanation for GTPase binding to GRD3 would involve a slight variation on what we see here for the GRD2 rather than invoking entirely new modes of binding.
Discussion
To our knowledge this is the first example of two different binding events for the same GTPase and a single effector. Ex-mode binding clearly stabilizes the dimer by simultaneously contacting both GRDs, and for the IQGAP-C fragment, it appears that Ex-mode binding must precede the RasGAP-mode binding by Cdc42. Both binding events likely stabilize distinct IQGAP conformations and the four bound Cdc42 molecules with their geranylgeranyl groups will securely anchor and orient the IQGAP dimer at membrane surfaces.
A previous study by Ren et al. determined that an important point of intermolecular contact for IQGAP1 dimers includes residues 763-863 which lie N-terminal to GRD1 (Ren et al., 2005). Ordinarily, over-expressing IQGAP1 in cells significantly increases levels of GTP-bound Cdc42. However, use of a peptide encompassing residues 763-863 blocks dimerization and reduces active Cdc42 levels in cell lysates (Ren et al., 2005). Since IQGAP1 is not a guanine nucleotide exchange factor for Cdc42 (Swart-Mataraza et al., 2002), a likely mechanism for increasing levels of active Cdc42 is through tight binding to Cdc42•GTP and the consequent reduction in GTP hydrolysis (Zhang et al., 1997). If residues 763-863 facilitate an association of Ex domains as seen in our structure, the reduced levels of active Cdc42 observed using the inhibitory peptide may be the result of the loss of Ex-mode binding sites and, given our results with IQGAP1-C6A, subsequent loss of RasGAP-mode binding. For crystallization and in our ITC experiments, we have used just the GRD domains in isolation. In the context of the large, full-length proteins (see Figure 1), dimerization by residues 763-863 (and their equivalents in IQGAP2) may be required to precede necessary conformational changes that allow for the subsequent Cdc42-mediated interactions that we see in the crystal structure. More information is required in order to fully understand the need for these separate dimerization determinants. Interestingly, residues 763-863 of IQGAP1 lie within the calmodulin-binding IQ motifs, suggesting that calmodulin may influence IQGAP1 dimer formation and therefore impact Cdc42 binding. This hypothesis is supported by the observation that calmodulin abrogates Cdc42 binding to IQGAP1 (Joyal et al., 1997).
In our structure, Ex domain residues are those mediating contacts between the two GRDs. Because specific mutations within the Ex domain reduce Cdc42 binding to ‘one-site’ for both GRD1 and GRD2, and because sequences from two adjacent GRDs comprise the Ex-mode binding sites, we believe that the GRD1 forms a dimer similar to that seen in the X-ray structure. Of the 55 residues making some contact at the E-F interface, 42 are identical between IQGAP1 and IQGAP2, and of the 10 residues making sidechain hydrogen bonds or salt bridges, 8 are identical between the two IQGAPs. Although we are not aware of any report supporting their existence, this high degree of sequence conservation at the GRD dimer interface hints at the possibility of IQGAP1/IQGAP2 heterodimers. How such a heterodimer might be advantageous to the cell is unclear.
In the complex, two GRD2 molecules are arranged in parallel or “head-to-head”. Parallel arrangement potentially allows like domains and motifs in the full-length protein to contact each other as we see here with the GRD2. Such an arrangement might be used to regulate activity of the domains and motifs within the dimer. For instance, other portions of the molecule might create bipartite binding surfaces as we see here for the Ex-mode. Another possible regulation mechanism in a parallel homodimer is intra-dimer auto-inhibition where juxtaposed domains regulate each other’s activity. For instance, the calponin homology domain might bind to its counterpart across the dimer interface and in the process, bury surfaces required for F-actin binding. Under the correct cellular conditions, the two CH domains release each other and crosslink F-actin. In fact, we do see a parallel dimer of the IQGAP1 calponin homology domain and the dimer interface does appear to participate in F-actin binding (Liu J, Kurella VB, LeCour Jr. L, Worthylake, DK -unpublished observations). Another possible advantage for the parallel arrangement monomers pertains to their role as protein scaffolds. IQGAP1 has been shown to bind to MAP kinase components B-Raf, Mek and Erk,(Ren et al., 2007; Roy et al., 2004, 2005). Modulating levels of IQGAP1 in cells has profound consequences on MAP kinase signaling (Roy et al., 2005). In a parallel dimer, efficient sequential activation (phosphorylation) of MAPK components might be accomplished across the dimer interface. In this scenario, the requirement for Cdc42 to promote stable dimer formation would link MAP kinase signaling to Cdc42 activation and a particular sub-cellular locale where that activation occurs.
EXPERIMENTAL PROCEDURES
Protein expression and purification
Human GRD2 (UniProtKB accession Q13576; residues 875-1257), human GRD1 (UniProtKB accession P46940; residues 962-1345) and human hydrolase deficient (Q61L) Cdc42 (UniProtKB accession P60953; residues 1 – 177), and human hydrolase deficient (Q61L) Rac1 (UniprotKB accession P63000; residues 1 – 177) were expressed in E. coli BL21(DE3) RIL cells (Novagen). The Cdc42 coding sequence was inserted into pProEX HTb (Invitrogen) which encodes a tobacco etch virus (TEV) protease-cleavable 6xHis Ni2+ affinity tag N-terminal to the Cdc42 coding sequence. After tag removal, there is a Gly-Ala artifact preceding the initiator methionine. The GRD2, GRD1, and Rac1 coding sequences were placed into pET Trx (a kind gift from Dr. Gunter Stier, Umeå University) which encodes E. coli thioredoxin and a TEV protease-cleavable 6xHis Ni+2 affinity tag N-terminal to the proteins. After protease cleavage, there is a Gly-Ala-Met artifact preceding Ser875 of GRD2 and Ser962 of GRD1, and a Gly-Ala artifact preceding Met1 of Rac1. Cells were grown in SM media (24g yeast extract, 12g tryptone, 1mL 1N NaOH per liter) in 2800mL baffled Fernbach flasks shaking at 150 RPM at 37°C until their OD600 ≈1.0 and then the temperatures of the incubators were reduced to 27°C for Cdc42, Rac1 and GRD1 cells, or 18°C for GRD2 cells, and protein expression was induced with 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG). Cdc42, Rac1 and GRD1 proteins were expressed for 8 h while GRD2 protein was expressed for ~18hrs. Cells were collected by centrifugation at 4°C and stored at −80°C. Cells were later resuspended in N1 buffer (300mM NaCl, 20mM tris(hydroxymethyl)aminomethane(Tris)-HCL pH 8.0, 15mM imidazole, 5% glycerol and 2mM MgCl2) and lysed using an EmulsiFlex C5 high pressure homogenizer (Avestin) operating at 15,000 p.s.i. Lysate was centrifuged for 45min at 260,000g at 4°C in a Ti45 rotor and an Optima L-80 XP ultracentrifuge (Beckman). The supernatant was loaded onto 2 × 5mL HisTrap HP columns (GE Healthcare) and then washed with approximately 120mL of N1 buffer. Proteins were then eluted with N1 buffer + 300mM imidazole. TEV protease was added and the proteins were dialyzed overnight in N1 buffer at 4°C. The next morning, the dialysate was loaded onto fresh HisTrap HP columns previously equilibrated to N1. TEV protease-cleaved proteins in the flow-through were collected and concentrated to <10mL volume and loaded onto either a 26/60 Sephacryl 300 gel filtration column (GRD proteins) or a 26/60 Sephacryl 100 column (GTPases) each equilibrated to 300mM NaCl, 10mM Tris-HCL (pH 8.0), 5% glycerol,2mM dithiothreitol (DTT), and 1mM MgCl2 (Columns from GE Healthcare). Gel filtration fractions were analyzed by SDS-PAGE and Coomassie staining and pooled. The 6xAla point mutations were introduced using the services of GenScript. These are N924A, N928A, Q929A, R930A, E931A and S1204A (GRD2) and N1011A, N1015A, Q1016A, R1017A, E1018A and T1291A (GRD1). Mutated coding sequences were placed into pET Trx. After 6xHis tag removal, each mutant GRD has a Gly-Ala-Met-Ala artifact at the N-terminus. GRD mutants were purified as described above. Creation of the GST-IQGAP1-C (amino acids 864-1657) has been described previously (Ho et al., 1999). IQGAP1-C with N1011A, N1015A, Q1016A, R1017A, E1018A and T1291A (GST-IQGAP1-C6A) was generated using site-directed mutagenesis. His-Cdc42-Q61L was generated from pGEX-Cdc42-Q61L (residues 1-184; generously donated by Darerca Owen, University of Cambridge, Cambridge, UK). DNA sequencing confirmed constructs were as intended. GST-IQGAP1-C and GST-IQGAP1-C-6A were expressed in E. coli and isolated using affinity chromatography (GE Healthcare) essentially as previously described (Ho et al., 1999). His-Cdc42-Q61L was purified with Ni-NTA Agarose (Qiagen), following the company’s protocol. The size and purity of the GST- and His-tagged proteins were evaluated by SDS-PAGE and Coomassie staining. All proteins were at least 90% pure.
Crystallization
Cdc42 was incubated in a 20-fold molar excess of GTP (Sigma) in the presence of 2mM EDTA (to chelate magnesium and promote nucleotide exchange) for 30 minutes at room temperature. After nucleotide exchange, 5mM MgCl2 was added to stop nucleotide exchange and excess nucleotide was removed via buffer exchange using an Amicon Ultra centrifugal concentrator (MWCO = 3,000). GRD2 protein was added to Cdc42 in the concentrator at a 1:1 stoichiometry and the mixture was buffer exchanged to 75mM NaCl, 10mM Tris-HCL, 5% glycerol, 1mM MgCl2 and 2mM DTT for initial vapor diffusion screening. Small crystals were obtained in the Qiagen PEGs I and PEGs II screens (drops 9 and 10, respectively). Use of Hampton Research's additive screen reagent #14 (praseodymium (III) acetate) yielded larger crystals, although there was considerable precipitate in the drops. A data set extending to 3.4Å was collected on one of these crystals using the home X-ray system (Microstar generator, Helios optics, Platinum 135 CCD detector (Bruker AXS)) and was used for molecular replacement using both AMoRe and MolRep(CCP4, 1994)and portions of GRD1 (PDB ID 3FAY) and Cdc42 (PDB ID 1GRN, chain A) as search molecules (Kurella et al., 2009; Nassar et al., 1998). The initial molecular replacement solution indicated that the correct protein stoichiometry was 2:1 (Cdc42:GRD2). We speculate that the praseodymium (III) acetate precipitated some of the GRD2 protein, thereby altering the protein stoichiometry in the drop and improving crystal growth. Once the proper 2:1 ratio was used, crystals grew much more rapidly and to a larger size without using praseodymium (III) acetate. The complex was at 13mg/mL concentration as estimated from theoretical absorption coefficients (λ=280nm) for both proteins and GTP. Final crystallization conditions were 28.5% (v/v) polyethylene glycol (PEG) MW 400 (Fluka), 100mM 2-(N-morpholino)ethanesulfonic acid (MES) pH 6.5 and 40mM MgCl2(Sigma) using a drop ratio of 1:1 (protein-to-well solution). Crystals were cryo-protected for 100K data collection by immersion in 32% PEG 400, 100mM MES pH 6.5, and 40mM MgCl2.
Data collection and structure refinement
(see Table 1 for statistics). Data were integrated with Mosfilm and scaled using SCALA(CCP4, 1994; Leslie AGW, 2007).CNS was used for refinement and the molecular graphics program O was used for model building (Brünger et al., 1998; Jones et al., 1991). Selective non-crystallographic symmetry restraints (≤ 30 kcal mol−1 Å−2) were used during refinement. The model includes residues 1-177 of the four Cdc42 molecules and residues 875-1246 of the two GRD2 molecules. C-terminal GRD residues 1247-1257 as well as the Gly-Ala-Met N-terminal cloning artifact are disordered and not modeled. The N-terminal Gly-Ala artifacts on the Cdc42 molecules are also not modeled. In a few locations, there is density consistent with partially ordered molecules of polyethylene glycol which have not been modeled. The coordinates have been deposited at the Protein Data Bank (5CJP)
Table 1.
| Data Collection Statistics | |
|---|---|
| Advanced Photon Source beamline | 31-ID |
| Detector | Rayonix MX225-HE |
| Spacegroup / Cell parameters (Å) | P212121 / a=101.63, b=120.17, c=157.08 |
| Wavelength (Å) | 0.97931 |
| Resolution (Å) | 19.97 - 2.57 |
| Observations | 452,319 |
| Unique | 61,742 |
| Completeness (%) Overall (Shell) | 99.7 (100) |
| Redundancy (fold) Overall (Shell) | 7.3 (7.2) |
| Rsymmb Overall (Shell) | 0.098 (0.81) |
| <I/δI>c Overall (Shell) | 10.5 (2.9) |
| Wilson Plot B (3.97Å-2.58Å) | 66.51Å2 |
| Refinement statistics | |
| Resolution (Å) | 19.97 - 2.6 |
| No. reflections | 56,575 (working)/2,993 (test) |
| Rcryst d (%) | 24.74 |
| Rfreee (%) | 28.73 |
| No. of atoms (protein/solvent/ligands) | 11,514/118/132 |
| <B> (Å2) overall/protein/solvent/ligands | 72.7/73.2/47.1/47.3 |
| r.m.s.d. bond length (Å) | 0.008 |
| r.m.s.d. bond angle (°) | 1.314 |
| φ/ψ (core / allowed / generous / disallowed)f | 84.9% / 12.8% / 2.2% / 0.2% |
(2.71Å - 2.57Å)
RSymm = 100 × ∑|I - <I>|/∑I, where I is the integrated intensity of a measured reflection.
<I/σI>, mean signal to noise, where I is the integrated intensity of a measured reflection and δI is the estimated error in the measurement.
, where Foh and Fch are the observed and calculated structure factor amplitudes for reflection h
R-factor calculate as for Rcryst for 2,993 reflections chosen randomly and not included in the refinement.
PROCHECK (CCP4, 1994)was used for Ramachandran statistics.
Isothermal titration calorimetry
ITC was performed with an ITC200 (Malvern Instruments). Proteins were dialyzed against a common buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.0, 100mM KCl, 100mM NaCl, 5% glycerol, 1mM MgCl2, 0.5 mM Tris(2-carboxyethyl)phosphine (TCEP)) at 4°C. Experiments were conducted at 20°C and 30°C. GRD proteins were in the calorimetry cell with Cdc42(Q61L; residues 1-177) in the syringe. A series of 2μL injections with 700 rpm stirring and 210 seconds between injections, provided the raw data. Cdc42 dilution heats were calculated using the same data collection parameters, but with only dialysis buffer in the cell. Dilution heats were subtracted from raw data and then data was fitted using Origin 7 software.
In vitro pull-down assay
2μg His-Cdc42-Q61L in 1 ml Buffer A (50mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100 and 1mM EGTA), containing protease and phosphatase inhibitors (Thermo Scientific) and 1mM PMSF, was pre-cleared with 40μl glutathione-Sepharose at 4 °C for 1 h. Then, 3μg GST-IQGAP1-C, GST-IQGAP1-C-6A or GST on glutathione-Sepharose beads was added and samples were rotated for 3 h at 4 °C. After five washes with Buffer A, samples were resolved by SDS-PAGE. The gel was cut at the 50 kDa region. The upper portion of the gel was stained with Coomassie Blue, while the lower portion was transferred to PVDF. The PVDF membrane was probed with anti-Cdc42 antibody (BD Transduction Lab) and imaged with the Odyssey Imaging System (LI-COR) essentially as previously(Erdemir et al., 2014).
Analysis and figures
PDBePISA (http://www.ebi.ac.uk/pdbe/pisa/) and NACCESS (Hubbard, 1993) were used to identify hydrogen bonds and salt bridges, and for calculation of buried surface areas. In structures that do not include hydrogen atoms, PDBePISA deems H-bonds to be present when the donor to acceptor (“heavy atoms”) is < 3.89Å. For salt bridges, the distance between oppositely-charged heavy atoms should be < 4.0Å. PyMOL (http://www.pymol.org) was used to make figures. Sequence alignments were made using ClustalX with default settings (Chenna et al., 2003). The webserver STRIDE and the program DSSP were used to assign secondary structure in the figures (Heinig and Frishman, 2004; Kabsch and Sander, 1983).
Supplementary Material
Highlights.
The GTPase-activating protein (GAP)-related domain of IQGAPs is used in dimerization
IQGAPs form head-to-head (parallel) dimers
The structure contains two IQGAP2 GAP-related domains and four Cdc42 molecules
Cdc42, but not Rac1, stabilizes IQGAP dimers
Acknowledgements
We thank Kent Rossman, John Sondek, Fareed Aboul-Ela, Francis Whitby and Arthur Haas for critical reading of the manuscript. We thank Verna Frasca for help with ITC data fitting. This research was supported by the National Institutes of Health RO1 GM084072 (to D.K.W.) and by the Intramural Research Program of the National Institutes of Health (to D.B.S). The authors declare that they have no financial or personal relationships with other people or organizations that might influence the content of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions. L.L Jr. and V. K. B. expressed and purified all protein samples, crystallized the complex, collected diffraction data, and aided in figure preparation. V.K.B. and J.L. performed the calorimetry experiments, Z. L. prepared the IQGAP1-C constructs and performed the binding studies, D.B.S. analysis of results and manuscript preparation, and D.K.W crystallography and manuscript preparation.
References
- Bax B. Domains of rasGAP and rhoGAP are related. Nature. 1998;392:447–448. doi: 10.1038/33040. [DOI] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore MG, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang J-S, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR System: A New Software Suite for Macromolecular Structure Determination. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Casteel DE, Turner S, Schwappacher R, Rangaswami H, Su-Yuo J, Zhuang S, Boss GR, Pilz RB. Rho isoform-specific interaction with IQGAP1 promotes breast cancer cell proliferation and migration. J. Biol. Chem. 2012;287:38367–38378. doi: 10.1074/jbc.M112.377499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCP4 CCP4. Acta Crystallogr Sec D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann D, Brill S, Garrett MD, Totty N, Hsuan J, Monfries C, Hall C, Lim L, Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991;351:400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Erdemir HH, Li Z, Sacks DB. IQGAP1 binds to estrogen receptor-alpha and modulates its function. J. Biol. Chem. 2014;289:9100–9112. doi: 10.1074/jbc.M114.553511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidyk NJ, Cerione RA. Understanding the catalytic mechanism of GTPase-activating proteins: demonstration of the importance of switch domain stabilization in the stimulation of GTP hydrolysis. Biochemistry. 2002;41:15644–15653. doi: 10.1021/bi026413p. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J. Biol. Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Boulter E, Burridge K. The 'invisible hand': regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitre L, Trapani E, Trabalzini L, Retta SF. The Ras superfamily of small GTPases: the unlocked secrets. Methods Mol. Biol. 2014;1120:1–18. doi: 10.1007/978-1-62703-791-4_1. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- Hedman AC, Smith JM, Sacks DB. The biology of IQGAP proteins: beyond the cytoskeleton. EMBO Rep. 2015;16:427–446. doi: 10.15252/embr.201439834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig M, Frishman D. STRIDE: a Web server for secondary structure assignment from known atomic coordinates of proteins. Nucl Acids Res. 2004;32:W500–W502. doi: 10.1093/nar/gkh429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YD, Joyal JL, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J. Biol. Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- Hubbard SJ, Thornton JM. 'NACCESS' Computer Program. Department of Biochemistry and Molecular Biology, University College; London: 1993. [Google Scholar]
- Jadeski L, Mataraza JM, Jeong HW, Li Z, Sacks DB. IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells. J. Biol. Chem. 2008;283:1008–1017. doi: 10.1074/jbc.M708466200. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Joyal JL, Annan RS, Ho YD, Huddleston ME, Carr SA, Hart MJ, Sacks DB. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J. Biol. Chem. 1997;272:15419–15425. doi: 10.1074/jbc.272.24.15419. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kjeldgaard M, Nyborg J, Clark BF. The GTP binding motif: variations on a theme. FASEB J. 1996;10:1347–1368. [PubMed] [Google Scholar]
- Kurella VB, Richard JM, Parke CL, Lecour LF, Jr., Bellamy HD, Worthylake DK. Crystal Structure of the GTPase-activating Protein-related Domain from IQGAP1. J. Biol. Chem. 2009;284:14857–14865. doi: 10.1074/jbc.M808974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AGW, Powell HR. Evolving Methods for Macromolecular Crystallography. 2007;245:41–51. ISBN 978-1-4020-6314-5. [Google Scholar]
- Mataraza JM, Briggs MW, Li Z, Entwistle A, Ridley AJ, Sacks DB. IQGAP1 promotes cell motility and invasion. J. Biol. Chem. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- Nassar N, Hoffman GR, Manor D, Clardy JC, Cerione RA. Structures of Cdc42 bound to the active and catalytically compromised forms of Cdc42GAP. Nature Struct. Biol. 1998;5:1047–1052. doi: 10.1038/4156. [DOI] [PubMed] [Google Scholar]
- Nojima H, Adachi M, Matsui T, Okawa K, Tsukita S, Tsukita S. IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nature Cell Biol. 2008;10:971–978. doi: 10.1038/ncb1757. [DOI] [PubMed] [Google Scholar]
- Pena V, Hothorn M, Eberth A, Kaschau N, Parret A, Gremer L, Bonneau F, Ahmadian MR, Scheffzek K. The C2 domain of SynGAP is essential for stimulation of the Rap GTPase reaction. EMBO Rep. 2008;9:350–355. doi: 10.1038/embor.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Frontiers Biosci. 2011;16:2561–2571. doi: 10.2741/3872. [DOI] [PubMed] [Google Scholar]
- Ren JG, Li Z, Crimmins DL, Sacks DB. Self-association of IQGAP1: characterization and functional sequelae. J. Biol. Chem. 2005;280:34548–34557. doi: 10.1074/jbc.M507321200. [DOI] [PubMed] [Google Scholar]
- Ren JG, Li Z, Sacks DB. IQGAP1 modulates activation of B-Raf. Proc. Natl. Acad. Sci. USA. 2007;104:10465–10469. doi: 10.1073/pnas.0611308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittinger K, Walker PA, Eccleston JF, Nurmahomed K, Owen D, Laue E, Gamblin SJ, Smerdon SJ. Crystal structure of a small G protein in complex with the GTPase-activating protein rhoGAP. Nature. 1997a;388:693–697. doi: 10.1038/41805. [DOI] [PubMed] [Google Scholar]
- Rittinger K, Walker PA, Eccleston JF, Smerdon SJ, Gamblin SJ. Structure at 1.65 A of RhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature. 1997b;389:758–762. doi: 10.1038/39651. [DOI] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB. IQGAP1 binds ERK2 and modulates its activity. J. Biol. Chem. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol. Cell Biol. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Wiesmuller L, Kabsch W, Stege P, Schmitz F, Wittinghofer A. Structural analysis of the GAP-related domain from neurofibromin and its implications. EMBO J. 1998a;17:4313–4327. doi: 10.1093/emboj/17.15.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Wittinghofer A. GTPase-activating proteins: helping hands to complement an active site. Trends Biochem. Sci. 1998b;23:257–262. doi: 10.1016/s0968-0004(98)01224-9. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Lautwein A, Kabsch W, Ahmadian MR, Wittinghofer A. Crystal structure of the GTPase-activating domain of human p120GAP and implications for the interaction with Ras. Nature. 1996;384:591–596. doi: 10.1038/384591a0. [DOI] [PubMed] [Google Scholar]
- Schmidt VA, Chiariello CS, Capilla E, Miller F, Bahou WF. Development of hepatocellular carcinoma in Iqgap2-deficient mice is IQGAP1 dependent. Mol. Cell Biol. 2008;28:1489–1502. doi: 10.1128/MCB.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Hedman AC, Sacks DB. IQGAPs choreograph cellular signaling from the membrane to the nucleus. Trends Cell Biol. 2015;25:171–184. doi: 10.1016/j.tcb.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart-Mataraza JM, Li Z, Sacks DB. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 2002;277:24753–24763. doi: 10.1074/jbc.M111165200. [DOI] [PubMed] [Google Scholar]
- Thapar R, Karnoub AE, Campbell SL. Structural and biophysical insights into the role of the insert region in Rac1 function. Biochemistry. 2002;41:3875–3883. doi: 10.1021/bi0120087. [DOI] [PubMed] [Google Scholar]
- Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J. Biol. Chem. 1994;269:20517–20521. [PubMed] [Google Scholar]
- Wennerberg K, Ellerbroek SM, Liu RY, Karnoub AE, Burridge K, Der CJ. RhoG signals in parallel with Rac1 and Cdc42. J. Biol. Chem. 2002;277:47810–47817. doi: 10.1074/jbc.M203816200. [DOI] [PubMed] [Google Scholar]
- White CD, Brown MD, Sacks DB. IQGAPs in cancer: A family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell. Signal. 2012;24:826–834. doi: 10.1016/j.cellsig.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Tao Y, Chen Y, Xu W. RhoC regulates the proliferation of gastric cancer cells through interaction with IQGAP1. PloS Biol. 2012;7:e48917. doi: 10.1371/journal.pone.0048917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang ZX, Zheng Y. Characterization of the interactions between the small GTPase Cdc42 and its GTPase-activating proteins and putative effectors. Comparison of kinetic properties of Cdc42 binding to the Cdc42-interactive domains. J. Biol. Chem. 1997;272:21999–22007. doi: 10.1074/jbc.272.35.21999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.