Abstract
Evolutionary biology has yet to reconcile the ubiquity of sex with its costs relative to asexual reproduction. Here, we test the hypothesis that coevolving parasites maintain sex in their hosts. Specifically, we examined the distributions of sexual reproduction and susceptibility to local parasites within a single population of freshwater snails (Potamopyrgus antipodarum). Susceptibility to local trematode parasites (Microphallus sp.) is a relative measure of the strength of coevolutionary selection in this system. Thus, if coevolving parasites maintain sex, sexual snails should be common where susceptibility is high. We tested this prediction in a mixed population of sexual and asexual snails by measuring the susceptibility of snails from multiple sites in a lake. Consistent with the prediction, the frequency of sexual snails was tightly and positively correlated with susceptibility to local parasites. Strikingly, in just two years, asexual females increased in frequency at sites where susceptibility declined. We also found that the frequency of sexual females covaries more strongly with susceptibility than with the prevalence of Microphallus infection in the field. In linking susceptibility to the frequency of sexual hosts, our results directly implicate spatial variation in coevolutionary selection in driving the geographic mosaic of sex.
Keywords: asexual reproduction, evolution of sex, geographic mosaic of coevolution, Red Queen hypothesis, sexual reproduction, susceptibility
Introduction
The paradox of sex remains one of evolutionary biology's most fascinating and troublesome questions. Sexual females produce sons, which reduces the per-capita birth rate of sexual lineages relative to asexual ones (two-fold cost of males: Maynard Smith 1971; Maynard Smith 1978). Given this substantial cost, a mutation to asexual reproduction should rapidly sweep to fixation. Asexuality, however, is rare in eukaryotes (Bell 1982; Suomalainen et al. 1987; Billiard et al. 2012). How can the prevalence of sex be reconciled with its inherent costs relative to asexual reproduction?
Coevolving parasites may confer a selective advantage to sexual females. Known as the Red Queen hypothesis (Hamilton 1975; Levin 1975; Jaenike 1978; Hamilton 1980; Bell 1982), this idea proposes that parasites exert negative frequency-dependent selection by rapidly adapting to infect common host genotypes. Sexual females benefit from the rare advantage, because recombination and segregation can produce offspring with rare genotypes. In contrast, asexual lineages are less able to evade coevolving parasites (Haldane 1949; Jaenike 1978; Hamilton 1980; Hamilton et al. 1990). Thus, if parasites are prevalent and sufficiently virulent, the fitness cost of infection can outweigh the reproductive advantage of asexual lineages, leading to the coexistence of sexual and asexual females, or to the exclusion of asexual females (e.g. Hamilton et al. 1990; Howard and Lively 1994).
The geographic mosaic theory of coevolution provides a powerful framework in which to test the Red Queen in natural systems. One of the core tenets of the geographic mosaic theory is that coevolution is spatially structured, varying in strength across interconnected landscapes (Thompson 1994; Thompson 1999, 2005). Using this framework, the Red Queen hypothesis makes a strong biogeographic prediction: for species that vary in reproductive mode, the frequency of sexual females should increase with the strength of selection by coevolving parasites (Glesener and Tilman 1978; Jaenike 1978; Lloyd 1980; Bell 1982). Although this prediction seems simple at first glance, the strength of coevolution is challenging to quantify. Most studies have attempted to quantify coevolutionary selection by measuring infection prevalence, which is simply the proportion of infected hosts (Lively 1987; Johnson 1994; Schrag et al. 1994; Kumpulainen et al. 2004; Ben-Ami and Heller 2005; Meirmans et al. 2006; Killick et al. 2008; Verhoeven and Biere 2013). Under the Red Queen hypothesis, a positive correlation between infection prevalence and the frequency of sexual females requires that variation in prevalence is correlated with the strength of selection resulting from coevolution (Jaenike 1978). This is likely true at large spatial scales, because variation in prevalence may capture the relatively wide variation in selection among isolated sites (e.g. Levin 1975; Glesener and Tilman 1978; Bell 1982).
Infection prevalence may, however, be too coarse a measure of coevolution at finer spatial scales. From a statistical standpoint, interconnectedness of sites can reduce the variation in selection among sites. Moreover, variation in prevalence can arise from both genetic and environmental factors (McNew 1960; Stevens 1960; Scholthof 2007). In fact, there is substantial evidence that environmental variables influence prevalence, while there is relatively little evidence that variation in prevalence arises from variation in the strength of a coevolutionary interaction (studies reviewed in Little 2002; Gibson et al. 2016). Environmental “noise” may therefore overwhelm any relationship that may exist between coevolution and prevalence. This is a problem, as one cannot conduct a fair test of the Red Queen hypothesis, or of other coevolutionary hypotheses, without a meaningful measure of coevolution.
We addressed this problem in a natural system that is ideal for studying the evolutionary maintenance of sex. The freshwater snail Potamopyrgus antipodarum has coexisting sexual and asexual forms (Winterbourn 1970), and the frequency of sexual forms is positively correlated with the prevalence of infection by the sterilizing trematode Microphallus (Lively 1987; Lively and Jokela 2002; Vergara et al. 2013; McKone et al. 2016). In this system, however, a more precise measure of coevolution is susceptibility, as measured by the proportion of hosts from a given site that become infected following controlled exposure to a fixed dose of Microphallus. In inoculations of hosts with parasites from different lakes, most variation in susceptibility (43-95%) arises from the interaction of coevolving host and parasite genotypes (Lively et al. 2004). Snails are more susceptible to their local Microphallus population than to foreign ones (Lively 1989; Lively and Dybdahl 2000; Jokela et al. 2009). This pattern of local adaptation arises from strong coevolution, with parasites rapidly adapting to evolutionary change in their local host population (Parker 1985; Lively 1989; Gandon 2002; Lively 2016). Hence susceptibility reflects the strength of coevolution between P. antipodarum and Microphallus.
If coevolving parasites are responsible for the maintenance of sexual reproduction in P. antipodarum, we predicted that 1) sexual females would be more common at sites where susceptibility to local parasites is high, and 2) the frequency of sexual females would covary more strongly with susceptibility than with infection prevalence. To test these predictions, we measured the covariance between susceptibility and sex for P. antipodarum. We found a geographic cline in the frequency of sexual females along the shoreline of a single small lake (6.4 km2), home to an interconnected population of snails (Fox et al. 1996; Paczesniak et al. 2014). Consistent with prediction (1), the geographic distribution of susceptibility to local parasites can explain the majority of variation in sex within this population, with the frequency of sexual females increasing with susceptibility. Consistent with prediction (2), susceptibility explains substantially more variation in the frequency of sexual females than does infection prevalence. Our findings specifically support the role of coevolution in the maintenance of sexual reproduction in P. antipodarum.
Natural history
The freshwater snail, Potamopyrgus antipodarum, is abundant in freshwater streams and lakes of its native New Zealand. Across its native range, a diverse array of asexual clones coexists with sexual males and females, with the frequency of sexual individuals varying from 0 to near 100% (Winterbourn 1970; Lively 1987; Dybdahl and Lively 1995). In lakes with a mixture of sexual and asexual snails, the asexual lineages are commonly derived from the local sexual lineages, consistent with recent and repeated origins of clones (Dybdahl and Lively 1995; Neiman et al. 2005). Reproductive modes are differentiated by ploidy: sexuals are diploid and asexuals primarily triploid (Dybdahl and Lively 1995; higher ploidy levels are occasionally found - Neiman et al. 2011). Sexual females and clones are otherwise indistinguishable, having equal fecundities and overlapping ecological niches (Jokela et al. 1997a; Jokela et al. 1997b).
Potamopyrgus antipodarum is the first intermediate host to at least 20 species of digenean trematodes (Hechinger 2012), of which Microphallus sp. is particularly well studied and prevalent. The parasite has severe fitness consequences: infected snails are sterilized as their gonads are replaced by larval trematodes (metacercariae) over the course of ∼three months. This is the primary fitness cost of infection, as there is no evidence that Microphallus increases the mortality rate of P. antipodarum in the field. To our knowledge, the infection process does not vary with snail reproductive mode, as infected male, sexual female, and asexual female snails do not vary inherently in the number and infectivity of metacercariae they produce. Metacercariae are infective to waterfowl, the definitive hosts of Microphallus. Ducks ingest infected snails, and Microphallus reproduces sexually in their intestines. Within 24-36 hours (pers. obs. CML), infected ducks begin to shed parasite eggs in their feces that are infective to snails (Hechinger 2012).
This study was conducted at Lake Alexandrina (Mackenzie Basin; South Island, New Zealand). Prior studies have used susceptibility assays to infer variation in coevolution at Lake Alexandrina. Notably, King et al. (2009, 2011) found that shoreline snails are far more susceptible to Alexandrina's Microphallus population than are snails from deep water. They suggested that Alexandrina's Microphallus population is able to coevolve with shoreline snails, because ducks forage in shallow water, thereby propagating successful parasite lineages. In contrast, ducks do not dive to deeper water (>4 m), preventing coevolution with deep-water snails. Thus Lake Alexandrina's Microphallus population is less able to infect snails from the deep (King et al. 2009, 2011). Here, we investigate variation in coevolution within the same depth zone along the shoreline of Alexandrina. The shoreline contains an interconnected population of P. antipodarum, as evidenced by a lack of structure at neutral loci among distant shoreline sites (Fox et al. 1996; Paczesniak et al. 2014).
Methods
Experimental surveys of susceptibility and the proportion of sexual females
Susceptibility and the proportion of sexual females were obtained from an artificial inoculation experiment replicated over three years. Some of the data from this experiment and the associated methods were initially reported in Gibson et al. (2016), which was focused on spatio-temporal variation in infection rather than coevolutionary interactions and sex. We summarize the methods here, and provide additional detail on the determination of snail reproductive mode.
In early February of each year, we collected snails from shoreline sites of Lake Alexandrina (12 sites in 2013; 13 in 2014/2015). We isolated juvenile snails (< 2.5 mm in length) for artificial inoculations because they have experienced relatively little exposure in the field (Levri and Lively 1996). We simultaneously collected duck feces, which contain parasite eggs infective to sympatric snails. In 2013 and 2015, duck feces were collected lake-wide and pooled to ensure that the inoculum represented Lake Alexandrina's Microphallus population as a whole. In 2014, we were only able to use duck feces from a single site, JMS. Lack of structure at neutral loci suggest that the Microphallus population is well mixed (Dybdahl and Lively 1996), but we recently found some evidence for divergence in the parasite subpopulations at different sites (Gibson et al. 2016). Use of a single site in 2014 is therefore a conservative measure of host susceptibility.
For each of the 12-13 sites, we divided juvenile snails into experimental replicates of 75 (2014-2015) or 100 snails (2013). In 2013 and 2015, susceptibility and the proportion of sexual females were determined from four experimental replicates per site. In 2014, susceptibility was determined from three replicates, and the proportion of sexual females was determined from six replicates. Snails were exposed to a high dose of parasite eggs to ensure that every host encountered enough parasites to become infected if susceptible (Osnas and Lively 2004). These high doses did not result in excess mortality (Gibson et al. 2016).
Following parasite development, we dissected each snail to determine gender and infection status. We froze the heads of dissected female snails for flow cytometry analysis, which can distinguish diploid (sexual) from triploid (asexual) females with high confidence (Osnas and Lively 2006) (Supporting Information, SI1). In 2013, we analyzed ∼20 female snails per replicate, for a total of 950. In 2014 and 2015, we analyzed up to 30 females per replicate, for totals of 1195 and 1348, respectively. Male snails were not analyzed because they are exclusively diploid at Alexandrina's shoreline (Neiman et al. 2011). Moreover, we restricted our analyses to females, because 1) This allowed comparison of individuals that are identical but for reproductive mode and 2) Males are highly variable in size, behavior, and frequency, making comparisons between sites difficult.
Field-based surveys for infection prevalence
We initially reported infection prevalence data in Gibson et al. (2016), and the full details of field sampling can be found there. Briefly, prevalence was estimated by sampling approximately 100 snails from shallow sites and dissecting them to determine length, gender, and infection status. In 2014 and 2015, we sieved each sample to obtain adult snails (> 2.5 mm) prior to dissection and ran flow cytometry on dissected females to determine reproductive mode. We analyzed ∼50 female snails per site, for totals of 664 and 662 in 2014 and 2015.
Statistical analyses
Does the proportion of sexual females vary in space?
To evaluate variation in the proportion of sexual females between sites, we used the function glm in R v3.2.1 (R Core Team 2013) to fit a logistic model with site, year (factor), and their interaction as predictors of the number of sexual and asexual females in a replicate (binomial response variable, logit link function). To test the significance of an effect, we performed likelihood ratio tests of models with and without the effect. We quantified the overall explanatory power of the model using the following likelihood ratio (McFadden's pseudo-R2):
which provides the proportional increase in log likelihood L of the full model (Lfull) over the intercept-only model (Lint) (McFadden 1974). We infer strong explanatory power for R2L values between 0.2 and 0.4 (McFadden 1979). Data were slightly overdispersed (ratio of the squared Pearson residuals to the residual degrees of freedom = 1.360) (Venables and Ripley 2002). Re-fitting the model with a quasi-binomial distribution (Crawley 2013) did not alter the results, so we report results from the original binomial model for ease of interpretation.
To evaluate the distribution of sex in space, we obtained GPS coordinates for each site in Google Earth. We fit a generalized estimating equation (GEE) in SPSS v21 (IBM; Armonk, NY, USA) with latitude and longitude as covariates of the annual mean proportion of sexual females (subject variable: site; within-subject variable: year) (annual mean = mean of experimental replicates in a single year). GEEs are ideal for the analysis of longitudinally clustered data (Liang and Zeger 1986; Zeger and Liang 1986), because the correlation between measurements taken from a single site at multiple time points can be specified using a first-order autoregressive variance-covariance matrix (Wang and Carey 2003; Ziegler and Vens 2010; Vens and Ziegler 2012). We also tested if nearby sites have similar proportions of sexual females by measuring the correlation of straight-line geographic distance and the absolute value of the difference in overall mean proportion of sexual females (equivalent of Euclidean and Manhattan distances for our data) using a Mantel test in the vegan package v2.3-0 (Dixon and Palmer 2003) in R (Pearson correlation, 999 permutations) (overall mean = mean of experimental replicates in all years). Proportions were arcsine transformed for spatial analyses. To verify the results obtained for experimental juveniles, we evaluated variation in the proportion of sexual females in field collections of adults. We found similar patterns for juveniles and adults (SI2).
Does variation in susceptibility explain variation in sex?
If coevolving parasites are responsible for the maintenance of sex, we predicted that sexual females should be more common at sites where susceptibility to parasites is high. Susceptibility varies dramatically between sites (Gibson et al. 2016) and is based strongly in genetics (Dybdahl and Krist 2004; Krist et al. 2004; Dybdahl et al. 2008; Koskella et al. 2011), particularly in the interaction of host and parasite genotype (Lively et al. 2004; Dybdahl et al. 2008).
We calculated susceptibility in two ways. First, we calculated overall susceptibility: the proportion of females infected in an experimental replicate (as reported in Gibson et al. 2016). This is an accurate measure of the susceptibility of hosts at a site, because the value from each experimental replicate is based upon a relatively large sample of snails (43.5 ± SD 15.3). However, the susceptibility of all females at a site is a function of the proportion of sexual and asexual females at that site. Any relationship between susceptibility and the proportion of sexual females could thus be confounded by non-independence. We therefore calculated susceptibility in a second way: the proportion of sexual females infected in a replicate. While statistically more sound, the value of sexual susceptibility obtained from each experimental replicate is based upon a smaller sample of snails (18.5 ± SD 6.5). Accordingly, when calculating mean susceptibility of sexual females at a site, we weighted each replicate by its total number of sexual females. Spatial variation in susceptibility of all females is reported in Gibson et al. (2016), and spatial variation in susceptibility of sexual females is reported in this article's Supporting Information (SI3).
We conducted all analyses using both calculations of susceptibility, with proportions arcsine transformed. First, we fit a GEE with annual mean susceptibility as a predictor of annual mean proportion of sexual females (subject variable: site; within-subject variable: year). We also conducted Pearson and Spearman rank correlations of overall and annual means of susceptibility and the proportion of sexual females. Finally, we tested for a positive correlation of temporal changes in susceptibility and the proportion of sexual females (Pearson and Spearman). We calculated temporal change by subtracting annual means in 2013 from those in 2015.
Does spatial autocorrelation explain the covariation of susceptibility and sex?
Any observed correlation between susceptibility and the proportion of sexual females could arise from correlations of both variables with underlying environmental variables. If so, we predicted that spatial variables would better explain the variation in sex than susceptibility. We used a partial linear regression technique to estimate the amount of variation in sex that can be attributed to susceptibility alone (x), the correlated effect of space and susceptibility (y), and space alone (z) (proportions arcsine transformed). We represented space by longitude, which was a far stronger predictor of spatial variation in the proportion of sexual females than latitude (see first section of Results). Consistent with this result, multiple regressions including susceptibility, longitude, and latitude as predictors were over-fit and did not improve upon models that excluded latitude (model selection in SI4, Table S3). We performed the following ordinary least squares regressions to obtain adjusted R2 values (unbiased estimator - Peres-Neto et al. 2006):
| [1] |
| [2] |
| [3] |
For [1], the R2 includes variation explained by x, y, and z. For [2], the R2 includes variation explained by x and y, and for [3], the R2 includes variation explained by y and z. We determined the variation attributable to the correlated effect (y) via subtraction: R2 [2] + R2 [3] - R2 [1] = (x+y) + (y+z) – [x+y+z]. We then calculated the variation explained by x and z (Legendre and Legendre 1998: pg. 528-536). To estimate the significance of each fraction, we used redundancy analysis ordination with the function rda in the package vegan (Dixon and Palmer 2003) followed by permutation testing with the function anova in R. The significance of fraction y cannot be estimated. We performed variation partitioning for overall and annual means of susceptibility and the proportion of sexual females.
To validate these results, we performed a partial Mantel test (R, package vegan) of the correlation between the absolute value of differences in overall mean proportion of sexual females and in susceptibility between sites, controlling for the straight-line geographic distance between sites. Lastly, we fit a GEE with susceptibility, latitude and longitude as covariates predicting variation in annual mean proportion of sexual females (subject variable: site; within-subject variable: year; arcsine transformations).
Is susceptibility a better predictor of variation in sex than infection prevalence?
The Red Queen hypothesis is founded upon the specific genetic interaction of coevolving host and parasite lineages. In the P. antipodarum-Microphallus system, variation in susceptibility is tightly linked to the interaction of host and parasite genetics (Lively et al. 2004; Dybdahl et al. 2008). In contrast, variation in infection prevalence arises from both susceptibility and environmental factors, with environmental variation explaining ∼2/3 of the variation in mean prevalence at the within-lake scale (Gibson et al. 2016). If coevolving parasites are responsible for the maintenance of sexual reproduction in P. antipodarum, we predicted that the proportion of sexual females would covary more strongly with susceptibility than with prevalence.
We first tested if sexual females are more common at sites where prevalence is high. Variation in Microphallus prevalence between sites from 2013-2015 is reported in Gibson et al. (2016) (all females). Prevalence values are the estimated marginal means produced by a generalized linear model with shell length of individual females as a covariate (Gibson et al. 2016). Shell length is positively correlated with snail age, so its inclusion as a covariate controls for age-dependent variation in cumulative infection risk among sites (Jokela and Lively 1995). Here, we test the relationship between sex and length-corrected infection prevalence of all females (2013-2015) and sexual females only (2014-2015) (proportions arcsine transformed). We conducted Pearson and Spearman rank correlations of overall and annual mean prevalence and proportion of sexual females. To analyze the relationship across all years, we fit a GEE with annual mean prevalence as a predictor of annual mean proportion of sexual females (subject variable: site; within-subject variable: year).
To compare susceptibility and infection prevalence as predictors of variation in sex, we added annual mean susceptibility as a covariate in this GEE. We also compared the variation in the proportion of sexual females explained in linear regressions with susceptibility vs. prevalence as predictors (overall and annual means). Comparisons were made using R2 values and the ratio of the likelihoods of susceptibility vs. prevalence models, with ratios exceeding 1 indicating a greater likelihood of the susceptibility model (models are equivalent in parameter number). Lastly, we conducted a partial correlation of overall mean proportion of sexual females and prevalence, controlling for overall mean susceptibility. In these analyses, susceptibility of all females was compared against prevalence of all females, and susceptibility of sexual females against prevalence of sexual females.
Results
Proportion of sexual females varies in space
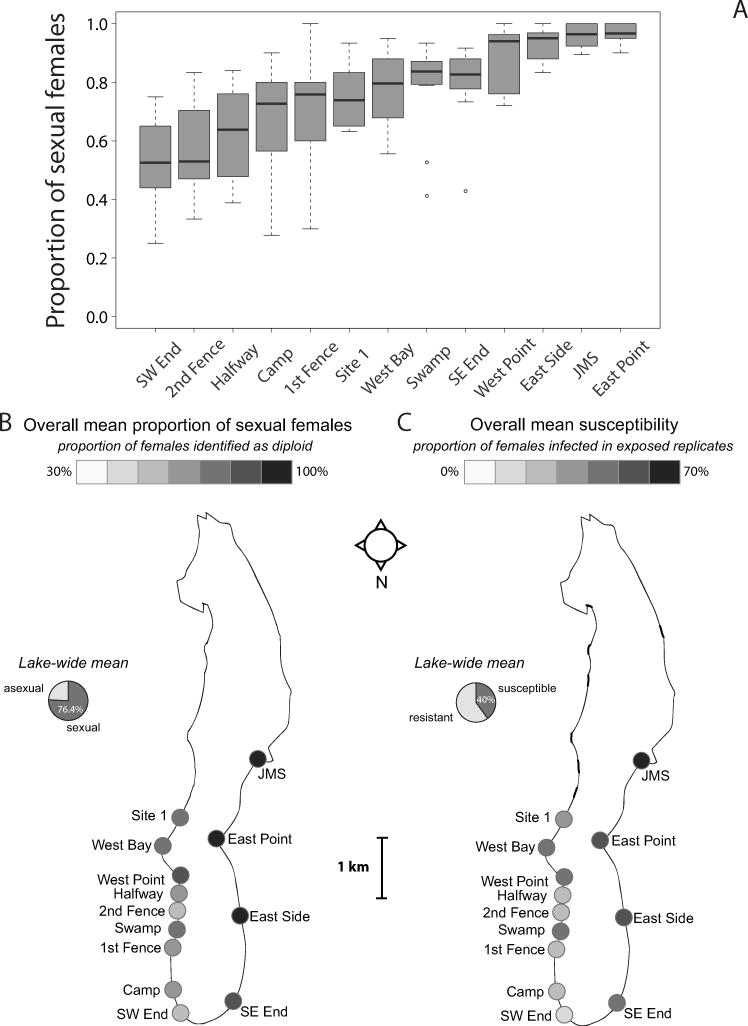
In 2013, 2014 and 2015, we surveyed 12-13 sites around Lake Alexandrina for the proportion of sexual females. The proportion of juvenile females identified as sexual (diploid) varied strongly among sites (Fig. 1A) and somewhat between years (minimum yearly mean = 0.711 ± 0.016 in 2013, maximum = 0.792 ± 0.012 in 2014) (Table 1). The ranking of sites with regards to the proportion of sexual females also changed among years (Table 1: interaction effect) (Fig. S1). We identified a geographic cline in sex (Fig. 1B) with the proportion of sexual females increasing strongly from west to east (GEE, longitude: Wald χ2 = 16.957, df = 1, p<0.001; coefficient = 18.633 ± 4.525 SEM) and, to a lesser degree, from south to north (latitude: Wald χ2 = 6.380, df = 1, p = 0.012; coefficient = 7.250 ± 2.870). Similarly, geographic distance between sites was correlated with the difference in overall mean proportion of sexual females (Mantel: n = 13, r = 0.396, p = 0.004).
Figure 1. Proportion of sexual females varies in space.
(A) The proportion of females identified as sexual (diploids) across all experimental replicates at 13 surveyed sites from 2013-2015. Each site is represented by 14 replicates total: 4 in 2013, 6 in 2014, and 4 in 2015 (exceptions: 13 for East Side and JMS – 3 replicates in 2015; 10 for Halfway – 0 replicates in 2013). Sites are arranged in order of increasing overall mean. Box plots show median (black bar), upper and lower quartiles (limits of box), minimum and maximum (whiskers, excluding outliers), and outliers (dots). (B) Sites around Lake Alexandrina are colored according to the overall mean proportion of sexual females from 2013-2015, demonstrating the north-easterly increase. Pie chart indicates lake-wide mean proportion of sexual females from 2013-2015. (C) The same sites colored according to the overall mean proportion of susceptible females from 2013-2015, demonstrating a similar geographic distribution of susceptibility. Pie chart indicates lake-wide mean susceptibility from 2013-2015. Susceptibility data from Gibson et al. (2016), where susceptibility was calculated as the proportion of females infected in experimental replicates after exposure to high doses of local parasites.
Table 1. The proportion of sexual females varies with site, year, and their interaction.
We fit a model with the number of sexual and asexual females in an experimental replicate as the binomial response variable. The results are shown in the form of likelihood ratio (D) tests of models with and without the effect of interest. The R2L value indicates very strong explanatory power of the full model.
| df | D | p-value | |
|---|---|---|---|
| site | 12 | 532.11 | <0.001 |
| year | 2 | 37.49 | <0.001 |
| site*year | 23 | 125.49 | <0.001 |
| residual | 138 | ||
|
| |||
| R2L = 0.497 | |||
Variation in susceptibility explains the observed cline in sex
We previously reported variation between Lake Alexandrina sites in susceptibility to local parasites (Gibson et al. 2016). Susceptibility was measured as the proportion of female juvenile snails that were infected following exposure to high doses of local parasites. As with the proportion of sexual females, we found that susceptibility increased to the north and east of Lake Alexandrina (Fig. 1C).
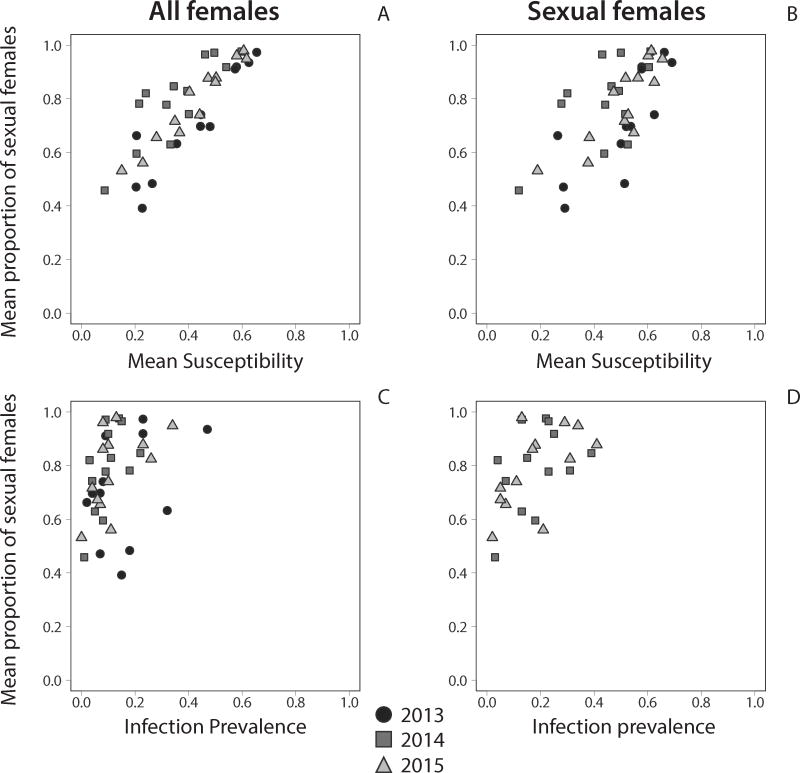
If coevolving parasites maintain sexual P. antipodarum, we predicted that sexual females would be more common at sites where susceptibility was high. Consistent with this prediction, we found that the proportion of sexual females increased with susceptibility at a site (annual means, GEE: all females - Wald χ2 = 144.971, df = 1, p<0.001, coefficient = 1.144 ± 0.095 SEM; sexual females only: Wald χ2 = 42.220, df = 1, p<0.001, coefficient = 0.822 ± 0.127 SEM). Similarly, the mean proportion of sexual females is positively correlated with mean susceptibility in each of the three years (Table 2A,B; Fig. 2A,B). Susceptibility of all females was consistently able to explain the majority of variation in the proportion of sexual females between sites (0.740 ≤ R2 ≤ 0.918). Susceptibility of sexual females was similarly able to explain a large portion of the variation between sites (0.342 ≤ R2 ≤ 0.654). These results are supported by more conservative Spearman rank tests (Table S4A,B), except that sexual susceptibility in 2014 was not correlated with the proportion of sexual females under the Spearman rank test (Table S4B).
Table 2. Results of Pearson correlations of the proportion of sexual females with susceptibility and infection prevalence.
We used susceptibility (A,B) and infection prevalence (C,D) for all females (A,C) and sexual females only (B,D) to quantify the strength of coevolutionary selection. Results are shown for annual means in each year and for overall means. Infection prevalence of sexual females was not obtained in 2013. There are 11 degrees of freedom for each correlation (n=13), excepting for analyses in 2012 (df = 10, n = 12).
| 2013 | 2014 | 2015 | Overall mean | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | r | p | r | p | r | p | r | p | ||
| A. | Susceptibility | All ♀ | 0.926 | <0.001 | 0.860 | <0.001 | 0.958 | <0.001 | 0.956 | <0.001 |
| B. | Sexual ♀ | 0.770 | 0.003 | 0.585 | 0.036 | 0.809 | 0.001 | 0.765 | 0.002 | |
|
| ||||||||||
| C. | Prevalence | All ♀ | 0.382 | 0.221 | 0.587 | 0.035 | 0.585 | 0.036 | 0.566 | 0.044 |
| D. | Sexual ♀ | 0.421 | 0.152 | 0.617 | 0.025 | 0.465 | 0.110 | |||
Figure 2. The proportion of sexual females is strongly correlated with susceptibility.
We used susceptibility to local Microphallus (A,B) and prevalence of infection with Microphallus (C,D) to predict variation in the proportion of sexual females. For susceptibility, annual means are shown for all females (A) and sexual females only (B) in 2013-2015, with each point representing one of 13 sites around Lake Alexandrina (12 sites in 2013). The proportion of sexual females was positively correlated with both measures of susceptibility in all years. For prevalence, annual means are shown for all females in 2013-2015 (C) and sexual females in 2014 and 2015 (D). Infection prevalence for sexual females was not measured in 2013. The proportion of sexual females was positively correlated with prevalence of all females in 2014 and 2015 and with prevalence of sexual females in 2015.
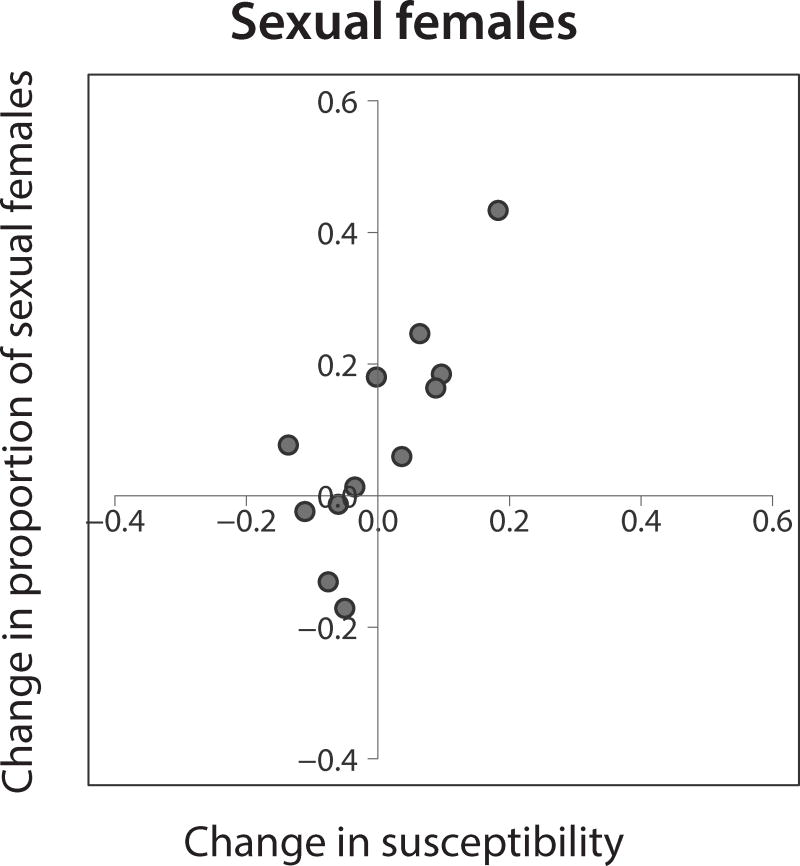
We next tested if temporal change in the proportion of sexual females at a site is related to change in susceptibility at that site. The change in the proportion of sexual females from 2013 to 2015 was positively correlated with the change in susceptibility of all females (difference in annual means, Pearson correlation, r = 0.91, df = 10, p<0.001) and sexual females only (r = 0.79, df = 10, p = 0.002) (Fig. 3). In other words, a decrease in the susceptibility of hosts to local parasites was accompanied by an increase in the proportion of asexual females at that site, while an increase in susceptibility was accompanied by an increase in the proportion of sexual females. These results are supported by Spearman rank tests (all females: ρ = 0.93, df = 10, p<0.001; sexual only: ρ = 0.75, df = 10, p = 0.005).
Figure 3. Change in susceptibility to local parasites is positively correlated with change in the proportion of sexual females.
Change in susceptibility at a site is shown for sexual females only. Changes are calculated as annual means in 2015 minus annual means in 2013. Each point represents one of 12 sites around Lake Alexandrina (Halfway was not evaluated in 2013).
Correlation of sex and susceptibility does not arise from spatial autocorrelation
Susceptibility and the proportion of sexual females both vary along the same spatial gradient. We therefore used a partial linear regression technique to test the hypothesis that the observed correlation of sex and susceptibility arises from spatial autocorrelation alone. We found no support for this hypothesis. We modeled space using longitude, thus capturing the strong increase in the proportion of sexual females from west to east (Fig. 1b, section 1 of Results). The proportion of sexual females did not vary as strongly from south to north, so we excluded latitude to avoid over-fitting our models (SI4, Table S3). Analyzing all females, the correlated effect of susceptibility and space (y) accounted for the largest portion of explained variation in sex (overall means). Susceptibility alone (x) also accounted for a substantial portion of the explained variation, while space alone (z, represented by longitude) explained a negligible portion (Table 3A). Analyzing only sexual females, we similarly found that the correlated effect (y) contributed the most to explained variation. Sexual susceptibility alone (x) and space alone (z) each accounted for smaller portions of the explained variation (Table 3B). Analyses of annual means gave similar results: the contribution of space alone (z) to the explained variation in sex was consistently outweighed by the contribution of the correlated effect (y) plus susceptibility alone (x).
Table 3. Results of partial linear regressions to account for variation in the proportion of sexual females explained by susceptibility alone (x), the correlated effect of susceptibility and space (y), and space alone (z).
Results are given for overall means, with susceptibility calculated using all females (A) and sexual females only (B). Adjusted coefficients of determination (R2) show the contributions of each component to the total variation explained by the full model. The significance of each component was calculated with redundancy analyses. The significance of y (correlated effect) cannot be calculated. Space is represented by longitude only (SI4).
| All females | Sexual females | ||||
|---|---|---|---|---|---|
| adj R2 | p | adj R2 | p | df | |
| Full model (x+y+z) | 0.909 | 0.001 | 0.705 | 0.002 | 2 |
| Unexplained | 0.091 | 0.295 | |||
|
| |||||
| Uniquely susceptibility (x) | 0.358 | 0.001 | 0.154 | 0.026 | 1 |
| Correlated effect (y) | 0.548 | 0.393 | 0 | ||
| Uniquely space (z) | 0.004 | 0.243 | 0.158 | 0.026 | 1 |
Additionally, differences between sites in susceptibility and the proportion of sexual females were strongly correlated when controlling for the geographic distances between sites (overall means, partial Mantel tests, all females: r=0.858, n = 13, p=0.001; sexual only: r = 0.394, n = 13, p = 0.009). Lastly, susceptibility predicted variation in the proportion of sexual females (annual means, GEE, all females: Wald χ2 = 82.725, df = 1, p<0.001, coefficient = 1.032 ± 0.113 SEM; sexual only: Wald χ2 = 10.251, df = 1, p = 0.001, coefficient = 0.461 ± 0.144) when accounting for latitude and longitude. We therefore rejected spatial autocorrelation as the explanation for the strong correlation of susceptibility and the proportion of sexual females.
Susceptibility is a better predictor of sex than is infection prevalence
The annual mean proportion of sexual females was positively correlated with annual mean infection prevalence of all females in two of three years (Table 2C: 2014 Table 2C: 2015; Fig. 2C) and with infection prevalence of sexual females in one of two years (Table 2D: 2015; Fig. 2D). However, in a full model incorporating all three years, infection prevalence did not predict variation in the proportion of sexual females (annual means, GEE: Wald χ2 = 2.238, df = 1, p = 0.135, coefficient = 0.210 ± 0.140 SEM), while infection prevalence of sexual females was a marginally significant predictor (Wald χ2 = 3.758, df = 1, p = 0.053, coefficient = 0.348 ± 0.180).
If coevolution maintains sex, we predicted that the proportion of sexual females would covary more strongly with susceptibility than with infection prevalence. Consistent with this prediction, susceptibility explained a greater proportion of variation in the proportion of sexual females than did infection prevalence (Table 2A,B vs. C,D; Table S6). Accordingly, a linear regression model with overall mean susceptibility as a predictor of the proportion of sexual females was 3.1-fold (all females) and 1.8-fold (sexual females) more likely than a model with mean infection prevalence as a predictor. Analysis of annual means gave similar results (Table S6). After accounting for susceptibility at a site, there was no correlation of overall mean prevalence and the proportion of sexual females (all females: r = -0.076, n = 13, p = 0.806; sexual: r = 0.084, n = 13, p = 0.785), indicating that prevalence can explain no additional variation. For all females, a model including both factors found that susceptibility predicted variation in the proportion of sexual females (annual means, GEE: Wald χ2= 49.496, df = 1, p<0.001, coefficient = 0.897 ± 0.128 SEM), but infection prevalence did not (Wald χ2 = 1.401, df = 1, p = 0.273, coefficient = 0.145 ± 0.123). For sexual females only, both factors predicted variation in the proportion of sexual females (annual means, GEE, susceptibility: Wald χ2 = 11.807, df = 1, p = 0.001, coefficient = 0.606 ± 0.176 SEM; prevalence: Wald χ2 = 4.754, df = 1, p = 0.029, coefficient = 0.338 ± 0.155).
Discussion
Here, we tested the prediction that sexual females are more common at sites where susceptibility to local parasites is high. Consistent with this prediction, three years of replication show that sexual reproduction is tightly and positively coupled with susceptibility to local parasites (Fig. 2, 3; Table 2). A striking geographic cline in the proportion of sexual females at our small study lake (Fig. 1B) is linked to the spatial distribution of susceptibility itself (Fig. 1C), and not to the distribution of any environmental factor correlated with sex and susceptibility (Table 3). We then tested the prediction that the proportion of sexual females covaries more strongly with susceptibility than with infection prevalence. We found consistent support for this prediction. Taken together, our results support the hypothesis that sex is maintained by the specific interaction between coevolving host and parasite genotypes.
For testing coevolutionary hypotheses in the P. antipodarum-Microphallus system, the distinction between susceptibility and the prevalence of infection is important. Artificial inoculations (Dybdahl and Krist 2004; Krist et al. 2004), hybrid crosses (Dybdahl et al. 2008), and selection experiments (Koskella et al. 2011) all demonstrate that genetic variation in the host and parasite underlies variation in host susceptibility. Susceptibility also consistently depends upon the combination of host and parasite genotype, at the within (Gibson et al. 2016) and between-lake scale (Lively et al. 2004). In contrast to susceptibility, prevalence of infection in the field has a large environmental component at Lake Alexandrina. In a previous study, we estimated that genetic factors explain approximately one-third of the variation in mean prevalence, while environmental factors explained most of the remaining two-thirds (Gibson et al. 2016). Susceptibility is therefore a stronger proxy for coevolution than prevalence, particularly at the small spatial scale that we studied here. If coevolving parasites maintain sex, susceptibility should thus covary more strongly with the proportion of sexual females. The opposite result – that prevalence better predicts variation in sex – would undermine the significance of coevolutionary interactions.
We found that the proportion of sexual females does indeed covary more strongly with susceptibility than does infection prevalence. Though there is consistent evidence for a positive correlation between the proportion of sexual P. antipodarum and the prevalence of Microphallus (Lively 1987; Lively and Jokela 2002; Vergara et al. 2013), we found that correlations with susceptibility tend to be stronger than those with prevalence (Fig. 2, Table 2). Moreover, our correlations with susceptibility are stronger than correlations with prevalence that included ∼50% more sites and were thus more powerful (compare to other within-habitat studies: Vergara et al. 2013; McKone et al. 2016). An open question in the field is the extent to which coevolving parasites alone are sufficient to explain the distribution of sex in nature. To tackle that question, our results highlight the importance of selecting a metric that specifically reflects variation in coevolution at the spatial scale of interest.
This problem applies to other host-parasite systems and coevolutionary hypotheses. In general, infection prevalence only coarsely reflects coevolution, because prevalence is commonly linked to environmental factors (e.g. Grosholz 1993; Johnson et al. 2007; Duffy et al. 2012; Altman and Byers 2014; Penczykowski et al. 2014). Even when genetic factors are found to predict prevalence, environmental factors likely also contribute (e.g. Jousimo et al. 2014). Hence, because prevalence is an imprecise measure of coevolution, the lack of a correlation between sex and prevalence cannot automatically falsify the Red Queen hypothesis. Nonetheless, laboratory assays of susceptibility to local parasites may not provide a valid or feasible alternative for quantifying coevolution in all systems.
Our results bring into sharp focus a curious, albeit logical, extension of the Red Queen's prediction. Sexual reproduction enables the production of offspring with rare, resistant genotypes (Haldane 1949; Jaenike 1978). Yet sites with a high proportion of sexual individuals are also those with the lowest proportion of individuals resistant to coevolving parasites (highest susceptibility). While puzzling at first glance, a crucial point is that the Red Queen does not predict that sexual lineages will escape their coevolving parasites altogether. The Red Queen predicts only that sexual lineages will, on average, perform as well or better than asexual lineages when coevolving parasites are present (Hamilton 1980; Vergara et al. 2014). This is quite evident in Figure 3: in a span of only two years, asexual individuals were excluded at sites where susceptibility to coevolving parasites increased. Asexual individuals increased at sites where susceptibility declined, consistent with the idea that common clones can arise in coevolutionary coldspots.
This variation in the strength of coevolution (as inferred from susceptibility) likely arises from the spatial distribution of foraging waterfowl, the definitive hosts of Microphallus. High densities of foraging waterfowl can create coevolutionary hotspots by propagating successful parasite lineages (King et al. 2009, 2011; Vergara et al. 2013). Various ecological factors may determine the suitability of a site for foraging ducks, such as water depth, wind speed and direction, productivity, and human activity. The patchy distribution of these factors could then explain the observed geographic mosaic of coevolution. Our results argue that this geographic mosaic of coevolution underlies spatial variation in the relative fitness of sexual and asexual reproduction within a single habitat of our small study lake.
Supplementary Material
Acknowledgments
We would like to thank Christiane Hassel and the IUB Flow Cytometry Core Facility for extensive support in flow cytometry, Spencer Hall for invaluable advice on analyses, Peyton Joachim, Samantha Klosak, and Alyssa Aungst for their contributions to the maintenance of experimental replicates and flow cytometry, Jukka Jokela for collecting snails from the field for estimates of infection prevalence in 2013, Kirsten Klappert for assistance in the field, Daniela Vergara and Lynda Delph for assistance in snail dissections, Alex Strauss and Jessica Hite for helpful discussion, Lynda Delph for comments on the manuscript, and the staff of the University of Canterbury's Edward Percival Field Station and the Mount John University Observatory. This study was supported by awards to AKG from the Society for the Study of Evolution (Rosemary Grant Award), the American Society of Naturalists (Student Research Award, Ruth Patrick Student Poster Award), the Indiana Academy of Science (Senior Research Grant), Indiana University (Provost's Travel Award for Women in Science), the National Science Foundation (DDIG-1401281; GRFP), and the US National Institutes of Health (Common Themes in Reproductive Diversity: T32 training grant at IU) and to JYX from Indiana University (Integrated Freshman Learning Experience; Science, Technology, and Research Scholars Summer Research Scholarship).
Literature Cited
- Altman I, Byers JE. Large-scale spatial variation in parasite communities influenced by anthropogenic factors. Ecology. 2014;95:1876–1887. doi: 10.1890/13-0509.1. [DOI] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. University of California Press; Berkeley: 1982. [Google Scholar]
- Ben-Ami F, Heller J. Spatial and temporal patterns of parthenogenesis and parasitism in the freshwater snail Melanoides tuberculata. J Evol Biol. 2005;18:138–146. doi: 10.1111/j.1420-9101.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- Billiard S, Lopez-Villavicencio M, Hood ME, Giraud T. Sex, outcrossing and mating types: unsolved questions in fungi and beyond. J Evol Biol. 2012;25:1020–1038. doi: 10.1111/j.1420-9101.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- Crawley MJ. The R Book. John Wiley & Sons, Ltd; West Sussex, UK: 2013. Chapter 16: Proportion Data. [Google Scholar]
- Dixon P, Palmer M. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14:927–930. [Google Scholar]
- Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR. Ecological context influences epidemic size and parasite-driven evolution. Science. 2012;335:1636–1638. doi: 10.1126/science.1215429. [DOI] [PubMed] [Google Scholar]
- Dybdahl MF, Jokela J, Delph LF, Koskella B, Lively CM. Hybrid fitness in a locally adapted parasite. Am Nat. 2008;172:772–782. doi: 10.1086/592866. [DOI] [PubMed] [Google Scholar]
- Dybdahl MF, Krist AC. Genotypic vs. condition effects on parasite-driven rare advantage. J Evol Biol. 2004;17:967–973. doi: 10.1111/j.1420-9101.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- Dybdahl MF, Lively CM. Diverse endemic and polyphyletic clones in mixed populations of the freshwater snail, Potamopyrgus antipodarum. J Evol Biol. 1995;8:385–398. [Google Scholar]
- Dybdahl MF, Lively CM. The geography of coevolution: comparative population structures for a snail and Its trematode parasite. Evolution. 1996;50:2264–2275. doi: 10.1111/j.1558-5646.1996.tb03615.x. [DOI] [PubMed] [Google Scholar]
- Fox J, Dybdahl MF, Jokela J, Lively CM. Genetic structure of coexisting sexual and clonal subpopulations in a freshwater snail (Potamopyrgus antipodarum) Evolution. 1996;50:1541–1548. doi: 10.1111/j.1558-5646.1996.tb03926.x. [DOI] [PubMed] [Google Scholar]
- Gandon S. Local adaptation and the geometry of host–parasite coevolution. Ecol Lett. 2002;5:246–256. [Google Scholar]
- Gibson AK, Jokela J, Lively CM. Fine-scale spatial covariation between infection prevalence and susceptibility in a natural population. Am Nat. 2016;188:000–000. doi: 10.1086/686767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glesener RR, Tilman D. Sexuality and the components of environmental uncertainty: clues from geographic parthenogenesis in terrestrial animals. Am Nat. 1978;112:659–673. [Google Scholar]
- Grosholz ED. The influence of habitat heterogeneity on host-pathogen population dynamics. Oecologia. 1993;96:347–353. doi: 10.1007/BF00317504. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. Disease and evolution. Ric Sci. 1949;19:68–76. [Google Scholar]
- Hamilton WD. Gamblers since life began: barnacles, aphids, elms. Quart. Rev Biol. 1975;50:175–180. [Google Scholar]
- Hamilton WD. Sex versus non-sex versus parasite. Oikos. 1980;35:282–290. [Google Scholar]
- Hamilton WD, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc Natl Acad Sci USA. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechinger RF. Faunal survey and identification key for the trematodes (Platyhelminthes: Digenea) infecting Potamopyrgus antipodarum (Gastropoda: Hydrobiidae) as first intermediate host. Zootaxa. 2012;3418:1–27. [Google Scholar]
- Howard RS, Lively CM. Parasitism, mutation accumulation and the maintenance of sex. Nature. 1994;367:554–557. doi: 10.1038/367554a0. [DOI] [PubMed] [Google Scholar]
- Jaenike J. An hypothesis to account for the maintenance of sex within populations. Evol Theor. 1978;3:191–194. [Google Scholar]
- Johnson PT, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, Sutherland DR, Carpenter SR. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci U S A. 2007;104:15781–15786. doi: 10.1073/pnas.0707763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SG. Parasitism, reproductive assurance and the evolution of reproductive mode in a freshwater snail. Proc Roy Soc B. 1994;255:209–213. [Google Scholar]
- Jokela J, Dybdahl MF, Lively CM. The maintenance of sex, clonal dynamics, and host-parasite coevolution in a mixed population of sexual and asexual snails. Am Nat. 2009;174:S43–S53. doi: 10.1086/599080. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively C, Fox J, Dybdahl M. Flat reaction norms and “frozen” phenotypic variation in clonal snails (Potamopyrgus antipodarum) Evolution. 1997a;51:1120–1129. doi: 10.1111/j.1558-5646.1997.tb03959.x. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively CM. Spatial variation in infection by digenetic trematodes in a population of freshwater snails (Potamopyrgus antipodarum) Oecologia. 1995;103:509–517. doi: 10.1007/BF00328690. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively CM, Dybdahl MF, Fox J. Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology. 1997b;78:452–460. [Google Scholar]
- Jousimo J, Tack AJ, Ovaskainen O, Mononen T, Susi H, Tollenaere C, Laine AL. Ecological and evolutionary effects of fragmentation on infectious disease dynamics. Science. 2014;344:1289–1293. doi: 10.1126/science.1253621. [DOI] [PubMed] [Google Scholar]
- Killick SC, Obbard DJ, West SA, Little TJ. Parasitism and breeding system variation in North American populations of Daphnia pulex. Ecol Res. 2008;23:235–240. [Google Scholar]
- King KC, Delph LF, Jokela J, Lively CM. The geographic mosaic of sex and the Red Queen. Curr Biol. 2009;19:1438–1441. doi: 10.1016/j.cub.2009.06.062. [DOI] [PubMed] [Google Scholar]
- King KC, Delph LF, Jokela J, Lively CM. Coevolutionary hotspots and coldspots for host sex and parasite local adaptation in a snail-trematode interaction. Oikos. 2011;120:1335–1340. [Google Scholar]
- Koskella B, Vergara D, Lively CM. Experimental evolution of sexual host populations in response to sterilizing parasites. Evol Ecol Res. 2011;13:315–322. [Google Scholar]
- Krist AC, Jokela J, Wiehn J, Lively CM. Effects of host condition on susceptibility to infection, parasite developmental rate, and parasite transmission in a snail–trematode interaction. J Evol Biol. 2004;17:33–40. doi: 10.1046/j.1420-9101.2003.00661.x. [DOI] [PubMed] [Google Scholar]
- Kumpulainen T, Grapputo A, Mappes J. Parasites and sexual reproduction in psychid moths. Evolution. 2004;58:1511–1520. doi: 10.1111/j.0014-3820.2004.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre L. Numerical Ecology. Elsevier Science; Amsterdam: 1998. [Google Scholar]
- Levin DA. Pest pressure and recombination systems in plants. Am Nat. 1975;109:437–451. [Google Scholar]
- Levri EP, Lively CM. The effects of size, reproductive condition, and parasitism on foraging behaviour in a freshwater snail, Potamopyrgus antipodarum. Anim Behav. 1996;51:891–901. [Google Scholar]
- Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Little TJ. The evolutionary significance of parasitism: do parasite-driven genetic dynamics occur ex silico? J Evol Biol. 2002;15:1–9. [Google Scholar]
- Lively CM. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature. 1987;328:519–521. [Google Scholar]
- Lively CM. Adaptation by a parasitic trematode to local populations of its snail host. Evolution. 1989;43:1663–1671. doi: 10.1111/j.1558-5646.1989.tb02616.x. [DOI] [PubMed] [Google Scholar]
- Lively CM. Coevolutionary epidemiology: disease spread, local adaptation, and sex. Am Nat. 2016;187:E77–E82. doi: 10.1086/684626. [DOI] [PubMed] [Google Scholar]
- Lively CM, Dybdahl MF. Parasite adaptation to locally common host genotypes. Nature. 2000;405:679–681. doi: 10.1038/35015069. [DOI] [PubMed] [Google Scholar]
- Lively CM, Dybdahl MF, Jokela J, Osnas EE, Delph LF. Host sex and local adaptation by parasites in a snail-trematode interaction. Am Nat. 2004;164:S6–S18. doi: 10.1086/424605. [DOI] [PubMed] [Google Scholar]
- Lively CM, Jokela J. Temporal and spatial distributions of parasites and sex in a freshwater snail. Evol Ecol Res. 2002;4:219–226. [Google Scholar]
- Lloyd DG. Evol Biol. Springer; 1980. Benefits and handicaps of sexual reproduction; pp. 69–111. [Google Scholar]
- Maynard Smith J. In: The origin and maintenance of sex. Williams GC, editor. Group Selection; Aldine Atherton, Chicago: 1971. pp. 163–175. [Google Scholar]
- Maynard Smith J. The Evolution of Sex. Cambridge University Press; Cambridge, UK: 1978. [Google Scholar]
- McFadden D. Conditional logit analysis of qualitative choice behavior. In: Zarembka P, editor. Frontiers in Econometrics. Academic Press; New York: 1974. pp. 105–142. [Google Scholar]
- McFadden D. Quantitative methods for analyzing travel behavior of individuals: some recent developments. In: Hensher D, Stopher P, editors. Behavioral Travel Modeling. Croom Helm; London: 1979. pp. 279–318. [Google Scholar]
- McKone M, Gibson AK, Cook D, Freymiller LA, Mishkind D, Quinlan A, York JM, Lively CM, Neiman M. Fine-scale association between parasites and sex in Potamopyrgus antipodarum within a New Zealand lake. New Zeal J Ecol. 2016;40:1. [Google Scholar]
- McNew G. The nature, origin, and evolution of parasitism. Academic Press; New York: 1960. [Google Scholar]
- Meirmans S, Skorping A, Løyning M, Kirkendall L. On the track of the Red Queen: bark beetles, their nematodes, local climate and geographic parthenogenesis. J Evol Biol. 2006;19:1939–1947. doi: 10.1111/j.1420-9101.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- Neiman M, Jokela J, Lively CM. Variation in asexual lineage age in Potamopyrgus antipodarum, a New Zealand snail. Evolution. 2005;59:1945–1952. [PubMed] [Google Scholar]
- Neiman M, Paczesniak D, Soper DM, Baldwin AT, Hehman G. Wide variation in ploidy level and genome size in a New Zealand freshwater snail with coexisting sexual and asexual lineages. Evolution. 2011;65:3202–3216. doi: 10.1111/j.1558-5646.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- Osnas EE, Lively CM. Parasite dose, prevalence of infection and local adaptation in a host-parasite system. Parasitology. 2004;128:223–228. doi: 10.1017/s0031182003004360. [DOI] [PubMed] [Google Scholar]
- Osnas EE, Lively CM. Host ploidy, parasitism and immune defence in a coevolutionary snail-trematode system. J Evol Biol. 2006;19:42–48. doi: 10.1111/j.1420-9101.2005.00994.x. [DOI] [PubMed] [Google Scholar]
- Paczesniak D, Adolfsson S, Liljeroos K, Klappert K, Lively CM, Jokela J. Faster clonal turnover in high-infection habitats provides evidence for parasite-mediated selection. J Evol Biol. 2014;27:417–428. doi: 10.1111/jeb.12310. [DOI] [PubMed] [Google Scholar]
- Parker MA. Local population differentiation for compatibility in an annual legume and its host-specific fungal pathogen. Evolution. 1985;39:713–723. doi: 10.1111/j.1558-5646.1985.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Penczykowski RM, Hall SR, Civitello DJ, Duffy MA. Habitat structure and ecological drivers of disease. Limnol Oceanogr. 2014;59:340–348. [Google Scholar]
- Peres-Neto PR, Legendre P, Dray S, Borcard D. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology. 2006;87:2614–2625. doi: 10.1890/0012-9658(2006)87[2614:vposdm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Scholthof KBG. The disease triangle: pathogens, the environment and society. Nat Rev Microbiol. 2007;5:152–156. doi: 10.1038/nrmicro1596. [DOI] [PubMed] [Google Scholar]
- Schrag SJ, Mooers AO, Ndifon GT, Read AF. Ecological correlates of male outcrossing ability in a simultaneous hermaphrodite snail. Am Nat. 1994:636–655. [Google Scholar]
- Stevens R. Cultural practices in disease control. In: Horsfall J, Dimond A, editors. Plant Pathology an Advanced Treatise. Academic Press; New York: 1960. pp. 357–429. [Google Scholar]
- Suomalainen E, Saura A, Lokki J. Cytology and Evolution in Parthenogenesis. CRC Press, Inc.; Boca Raton, Florida: 1987. [Google Scholar]
- Thompson J. The Coevolutionary Process. University of Chicago Press; Chicago: 1994. [Google Scholar]
- Thompson JN. Specific hypotheses on the geographic mosaic of coevolution. Am Nat. 1999;153:S1–S14. [Google Scholar]
- Thompson JN. The Geographic Mosaic of Coevolution. University of Chicago Press; Chicago, IL, USA: 2005. [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. Springer; New York City, NY: 2002. [Google Scholar]
- Vens M, Ziegler A. Generalized estimating equations and regression diagnostics for longitudinal controlled clinical trials: a case study Comput. Stat Data An. 2012;56:1232–1242. [Google Scholar]
- Vergara D, Jokela J, Lively CM. Infection dynamics in coexisting sexual and asexual host populations: support for the Red Queen Hypothesis. Am Nat. 2014;184:S22–S30. doi: 10.1086/676886. [DOI] [PubMed] [Google Scholar]
- Vergara D, Lively CM, King KC, Jokela J. The geographic mosaic of sex and infection in lake populations of a New Zealand snail at multiple spatial scales. Am Nat. 2013;182:484–493. doi: 10.1086/671996. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Biere A. Geographic parthenogenesis and plant-enemy interactions in the common dandelion. BMC Evol Biol. 2013;13:23. doi: 10.1186/1471-2148-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Carey V. Working correlation structure misspecification, estimation and covariate design: implications for generalised estimating equations performance. Biometrika. 2003;90:29–41. [Google Scholar]
- Winterbourn M. Population studies on the New Zealand freshwater gastropod, Potamopyrgus antipodarum. Proc Malacol Soc Lon. 1970;39:139–149. [Google Scholar]
- Zeger S, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Ziegler A, Vens M. Generalized estimating equations: notes on the choice of the working correlation matrix. Methods Inform Med. 2010;49:421–425. doi: 10.3414/ME10-01-0026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.