Abstract
Objective
Treatment interruption (TI) has been safe and durable in some pediatric studies but none have compared TI to continued antiretroviral treatment (ART) following ART initiation in early HIV. The objective of this study was to compare outcomes in TI versus continued ART among early-treated infants.
Design
Randomized trial (OPH-03; NCT00428116)
Methods
The trial included HIV-infected infants who initiated ART at <13 months of age, received ART for 24 months, and, if eligible (CD4>25%, normal growth), were randomized to TI vs. continued ART. Children in the TI group re-started ART if they met WHO ART-eligibility criteria. During 18-months post-randomization, primary outcomes were incidence of serious adverse events (SAEs) and growth. CD4, viral load, morbidity, and growth were compared.
Results
Of 140 infants enrolled, 121 started ART, of whom 75 completed ≥24 months ART and 42 were randomized (21 per arm). ART was initiated at median age 5 months and randomization at 30 months. Among 21 TI children, 14 met ART re-start criteria within 3 months. Randomization was discontinued by DSMB due to low TI durability. At 18 months post-randomization, growth and SAEs were comparable between arms; hypercholesteremia incidence was higher in the continued arm (p=0.03). CD4% and VL did not differ between arms (CD4% 35% and median VL undetectable (<150 c/ml) in both arms, p=0.9 for each comparison). No infants had post-treatment viral control.
Conclusion
Short TI did not compromise 18-month CD4%, viral control, growth, or morbidity compared to continued ART among infants who started ART in early HIV infection.
Keywords: HIV, pediatric, antiretroviral treatment (ART), interruption
BACKGROUND
Early antiretroviral treatment (ART) is recommended for HIV-infected infants based on significant benefits of early versus eligibility-deferred ART[1]. Prior to widespread ART use, some HIV-infected untreated children had long-term non-progression (LTNP)[2]. It is plausible that some children who would be LTNP without therapy, as well as others who control virus following early ART, could benefit from treatment interruption (TI) after immune recovery. This approach could reduce ART toxicity or resistance and preserve ART regimens for later in life.
There are several differences between pediatric and adult TI. In children it is frequently possible to diagnose HIV soon after infection, which makes treatment during primary HIV infection more feasible than in adults. Initiation of ART during primary HIV may enable sustained TI with post-treatment virologic control (PTC) as observed in the ‘Mississippi baby’ and in a teen from France[3, 4]. The ANRS VISCONTI study noted durable PTC among adults who started ART during primary HIV[5] and had TI, although PTC may be infrequent[6, 7]. Immune outcomes and safety of TI may also differ between children and adults. The presence of functional thymus in infants could enhance immune recovery following early ART[8]. While some adult TI studies, notably the SMART trial, observed increased cardiovascular mortality risk with TI, these findings have not been replicated in children[9–12]. In a large pediatric TI trial, PENTA-11, more than half of children randomized to TI completed a 48-week period of TI without meeting criteria to re-start ART[12]. Importantly, 2-year follow-up noted no differences in long-term outcomes, including cognitive or growth outcomes in children with TI versus continued ART in this trial[13]. In adults, ART plays a dual role in treatment and prevention and TIs could undermine HIV prevention, however, this Is not the case for children until adolescence [14, 15]. High rates of unplanned TIs, poor adherence and resistance are noted in children, leading to the possibility of exhausting regimens for children who start lifelong ART in infancy[16, 17],[18]. Thus, on balance, strategic TIs may permit a longer life period in which ARTs are effective for some children.
We hypothesized that infants treated early in HIV infection may safely sustain TI making this a viable approach to preserving limited ART options for infants. We conducted a randomized clinical trial (RCT), to compare outcomes of children who were treated in the first year of life, and randomized following 2-year ART to a single TI versus continued treatment.
METHODS
Study design, ethical approvals, and trial data and safety monitoring
The Optimizing Pediatric HIV-1 (OPH) Treatment study ((NCT00428116) was an RCT in which HIV-infected infants who completed ≥2 years ART and attained immune reconstitution and normalized growth were randomized to TI or continued ART. Written informed consent was obtained from caregivers of children. The study received ethical approval from the Institutional Review Boards at the University of Washington and the Ethical Review Committee at Kenyatta National Hospital (KNH). An independent Data and Safety Monitoring Board (DSMB) reviewed the study at 6-monthly intervals.
Recruitment of trial participants
HIV-infected infants were identified from prevention of mother-to-child HIV transmission (PMTCT) sites in Nairobi, KNH wards, and KNH HIV Treatment Clinic. Initial eligibility criteria restricted to infants <4 months of age were extended to <13 months to include infants who may have acquired postnatal HIV and infants already on ART who started at <13 months. For these children, pre-ART clinical, CD4, and growth data were abstracted from medical records.
Enrolment and pre-randomization follow-up
At enrollment, a questionnaire was administered and physical examination was performed; blood was collected for CD4, viral load, lipids, and liver function tests. Children were initiated on ART (PMTCT-exposed children received lopinavir/ritonavir, lamivudine, and zidovudine; children with no prior PMTCT exposure received nevirapine, lamivudine, and zidovudine; later enrollees received abacavir instead of zidovudine). Children were seen monthly for clinical assessment and anthropometry with 3-monthly blood collection for CD4, HIV RNA, complete blood count, lipids and liver transaminases.
Randomization
Block randomization was conducted with variable block sizes generated using STATA 8.0 ralloc.ado v2.2.1. Treatments were allocated in 1:1 ratio. All study investigators were blinded to block number, block size and sequence in the block. Treatments were assigned in pre-prepared sealed, opaque envelopes ordered in the sequence of treatment assignments. Once an infant’s eligibility was determined, the first available allocation envelope was assigned to the infant.
Treatment interruption (TI)
To prevent resistance, NRTIs were continued for 2 weeks after stopping NNRTI to allow for NNRTI decay.
Post-randomization follow-up
After randomization, children were reviewed after 2 weeks and monthly to 18 months with anthropometric measurements, WHO staging and adverse event determination. Blood was collected 3-monthly for CD4% and VL.
Restart of ART in the TI arm
Criteria for ART re-start were CD4% <20–25% (CD% threshold changed from 20% to 25% in November 2010 following a change in WHO guidelines for ART initiation) or a decrease greater than one-third of the peak CD4 count, more advanced WHO stage, or weight loss (drop in weight-for-age percentile to <5th percentile or weight crossing more than 2 major weight-for-age percentiles). Once a child restarted ART there was no further interruption.
Sample size
The targeted sample size was 150 enrolled children, of which 100 would be randomized. Using data from a previous perinatal HIV study in Nairobi, the average weight-for-height z-score (WHZ) among HIV-uninfected and HIV-infected children at ~20 months was −0.7002 with a standard deviation (SD) of 0.8529[19]. Assuming the mean WHZ among the continuous ART children was –0.7002, the same SD for both groups, a 2-sided test, significance level of 0.05, and 10% loss to follow-up: with 50 children in each trial arm (45 with complete follow-up), there was an estimated 80% power to exclude differences in WHZ of 0.50 or larger between RCT arms.
Laboratory Methods
CD4 counts were determined at the University of Nairobi using FACSCount (BD Biosciences (Franklin Lakes NJ) and CD4% using the Humalyser hematology analyzer. HIV RNA measurements were conducted at the Fred Hutchinson Cancer Research Center in Seattle, using the Gen-Probe HIV-1 RNA assay (Gen Probe, San Diego CA). Genotypic resistance testing was conducted in Nairobi using a population-based Sanger sequencing method described previously[20]. Interpretation of the sequences conferring resistance utilized the Stanford University HIV Drug resistance Database (http://hivdb.stanford.edu/).
Statistical Analysis
All analyses were conducted using Stata SE version 12 (StataCorp, College Station, Texas). To assess adequacy of randomization, baseline characteristics were compared between trial arms using Wilcoxon rank sum tests and Fisher’s exact tests. The primary outcome was comparison of growth and SAEs post-randomization between arms. Plasma HIV RNA, CD4 count and percentage, and morbidity were also compared. Growth was measured as weight-for-age (WAZ), weight-for-height (WHZ) and height-for-age (HAZ) z-scores. Primary outcomes were compared using Wilcoxon rank sum tests (continuous variables) and Cox regression (time-to-event variables) with Anderson-Gill methods for recurrent events, as appropriate. Time to death was compared using the log-rank statistic.
RESULTS
Baseline characteristics and pre-randomization follow-up
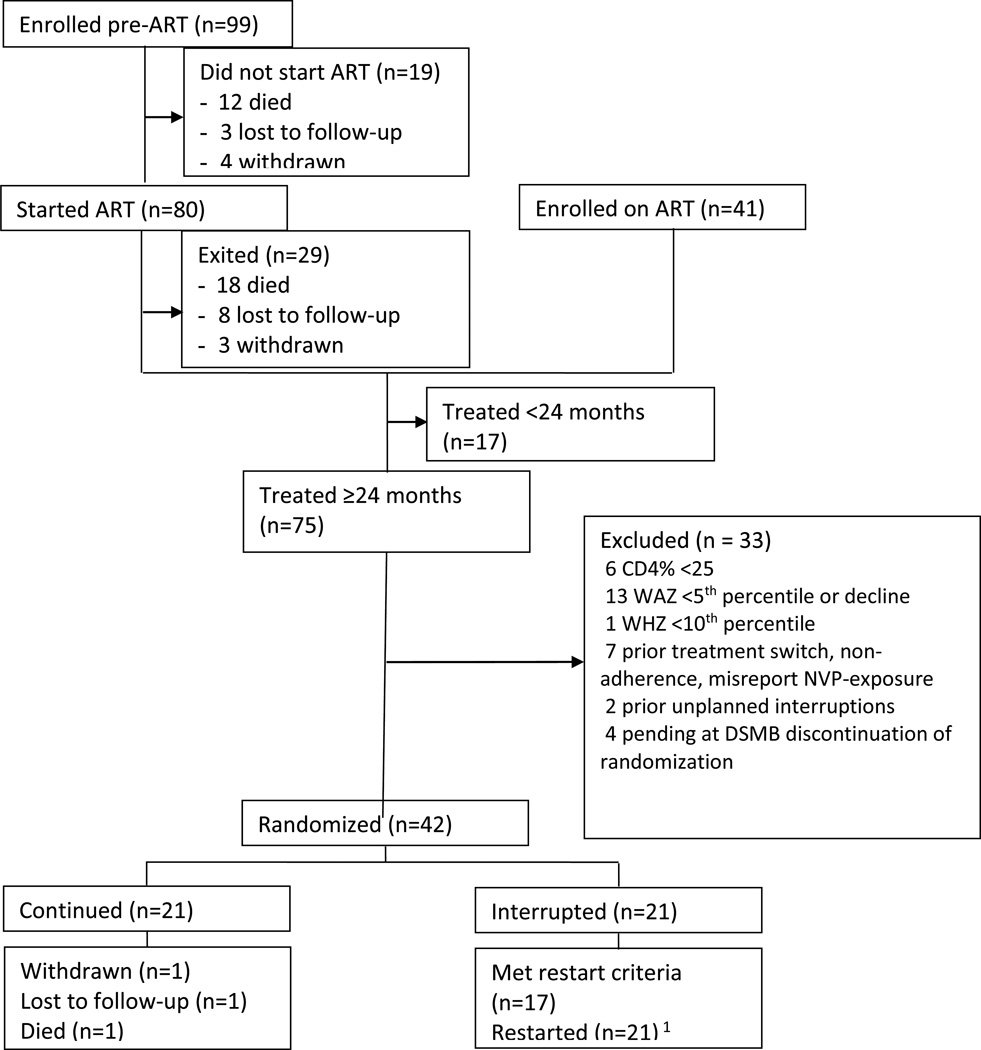
Between September 2007 and August 2010, 140 HIV-infected children were enrolled, and 121 enrolled children initiated ART (80 initiated ART in study, 41 previously on ART), of whom 18 died, 8 were lost to follow-up, 3 withdrew prior to randomization, 75 completed 2 years of ART, and 17 had not completed 2-year ART (Figure 1). The pre-randomization cohort has been described[21]. Among infants enrolled prior to ART, pre-ART mortality was high despite short median time to ART (2 weeks from diagnosis)[20]. Of 75 children who completed 2-year ART, 29 did not meet randomization eligibility criteria or had evidence of non-adherence, treatment switch or unplanned TI; 4 children were pending randomization when the DSMB recommended stopping the study. Forty-two children were randomized.
Figure 1. CONSORT Diagram of Trial.
1. 4 did not meet ART restart criteria but were started due to caregiver’s preference (2) or study team advice (2)
Characteristics of randomized children
Pre-ART (over 2 years before randomization), median age of the 42 infants was 5.0 months and the pre-ART CD4% was 23% and 19% in the continued and TI arms respectively (p=0.2) (Figure 1, Table 1). Initial ART regimens (38% received PI and the remainder NNRTI regimens) and anthropometric measures pre-ART were comparable between arms.
Table 1.
Baseline Characteristics by Randomization Arm
| Characteristics | Continued Median (IQR) or n (%) N=21 |
TI* Median (IQR) or N (%) N=21 |
|---|---|---|
| Pre-ART Characteristics | ||
| Age at ART initiation (months) | 5.0 (4.4, 7.5) | 5.0 (4.0, 7.1) |
| Male | 6 (29) | 13 (62) |
| Ever breastfed | 16 (76) | 19 (90) |
| Growth [N=20, 21] | ||
| Weight for age z-score (WAZ) | −2.31 (−3.02, −0.72) | −1.34 (−2.85, −0.79) |
| Height for age z-score (HAZ) | −2.23 (−3.02, −1.52) | −1.03 (−2.34, −0.35) |
| Weight for height z-score (WHZ) | −0.62 (−1.88, 0.39) | −0.96 (−1.65, −0.02) |
| WHO Stage [N=18, 16]** | ||
| I | 8 (44) | 8 (50) |
| II | 3 (17) | 1 (6) |
| III | 7 (39) | 5 (31) |
| IV | 0 (0) | 2 (13) |
| WHO Stage III/IV [N=18, 16]¶ | 7 (39) | 7 (44) |
| CD4% | 23 (14, 29) | 19 (15, 23) |
| CD4 count (cells/µL) | 1370 (830, 1939) | 1385 (738, 1941) |
| Plasma HIV RNA log10 copies/ml [N=17, 15] | 6.50 (6.12, 7.25) | 6.59 (5.98, 6.93) |
| ART Initial Regimen | ||
| LPV/r-based regimen | 8 (38) | 8 (38) |
| Characteristics at Randomization | ||
| Age at randomization (months) | 29.9 (28.9, 33.8) | 30.0 (29.3, 34.9) |
| Time on HAART (months) | 25.2 (24.9, 25.5) | 25.2 (24.9, 27.4) |
| Regimen at randomization | ||
| LPV/r;3TC;AZT | 10 (48) | 14 (67) |
| LPV/r;3TC;ABC | 6 (29) | 2 (10) |
| NVP;3TC;AZT | 4 (19) | 5 (24) |
| NVP;3TC;AZT | 1 (5) | 0 (0) |
| LPV/r-based regimen | 16 (76) | 16 (76) |
| Growth | ||
| Weight for age z-score (WAZ) | −0.47 (−0.87, −0.02) | −0.34 (−0.63, 0.47) |
| Height for age z-score (HAZ) | −1.33 (−2.04, −1.03) | −0.91 (−1.66, −0.16) |
| Weight for height z-score (WHZ) | 0.52 (0.19, 0.79) | 0.67 (0.15, 1.18) |
| WHO Stage 1 | 21 (100) | 21 (100) |
| CD4% | 33 (30, 40) | 34 (32, 38) |
| CD4 count (cells/µL) | 1750 (1547, 2299) | 1654 (1300, 1924) |
| Plasma HIV RNA copies/ml | 150 (150, 440) | 150 (150, 515) |
| Plasma HIV RNA log10 copies/ml | undetectable** (2.18, 2.64) | undetectable** (2.18, 2.71) |
TI: treatment interruption
Excludes infants already on ART at enrollment who did not have this information.
Below level of detection is 2.18 (<150 copies/ml)
At randomization, time on ART, clinical, immunologic, virologic, and growth characteristics were similar between trial arms. Median CD4% was 33% versus 34% in the continued versus TI arm, respectively (p=0.9). Plasma viral load (VL) was comparable with each arm median <150 copies/ml.
Data Safety Monitoring Board (DSMB) discontinuation of randomization
In July 2011 the DSMB determined that although randomization into the TI arm was safe, most children met ART restart criteria at 3-months post-TI. The DSMB recommended stopping randomization due to low TI durability and that randomized children complete 18-month follow-up with the option of restarting ART depending on caregiver preference.
Post-randomization follow-up
There were 30.6 person-years of follow-up in the continued arm and 32.1 person-years of follow-up in the TI arm. There was 1 loss to follow-up and 1 withdrawal, both in the continued arm. Among 21 children randomized to TI, 17 (81%) met criteria for ART restart. Fourteen (66%) experienced a CD4 drop to ≤25% within 3 months and one child after 6 months; one child met criteria of a greater than 1/3 drop from peak CD4% at 15 months, and one had a worsened WHO stage at 15 months post-TI. Four children (19%) never met ART restart criteria. Following the DSMB recommendation, 2 caregivers re-started ART at 9 and 12 months while 2 children remained on TI until 12 and 15 months until the team advised restart due to inability to monitor children following study completion. Median time off treatment for children in the TI arm was 4.3 months (IQR, 3.7, 9.7).
Immunologic responses following TI and restart
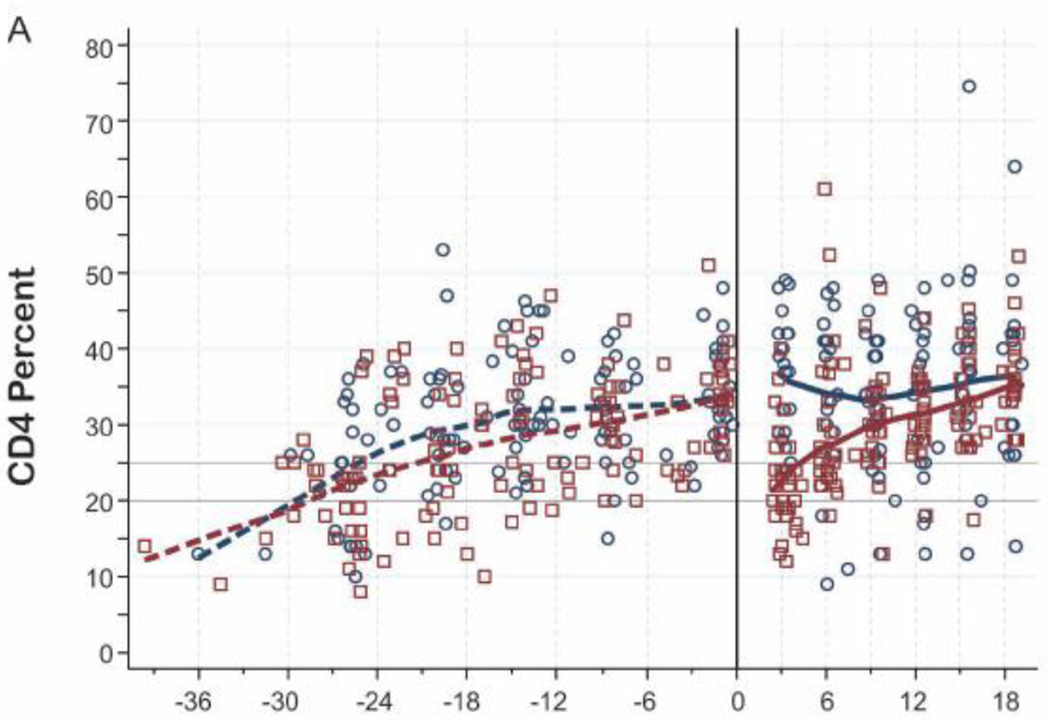
Following TI, median CD4% dropped from 34% to 23% at 3-months post-randomization (Table 2). Median CD4% was significantly lower in the TI arm at 3 months post-randomization (median 23% vs. 37% in TI vs. continued, p<0.001). Among the 17 children who met ART restart criteria, median increase in CD4% was 8% at 3 months following ART re-start (Figure 2A) CD4% was identical between trial arms at 18 months (CD4% 35% in each arm, p=0.92).
Table 2.
| A. Summary of Post-Randomization CD4%, CD4 Count, Viral Load, Growth, and Morbidity by Arm* | |||
|---|---|---|---|
| Characteristics | Continued Median (IQR) or n (%) N=21 |
TI Median (IQR) or n (%) N=21 |
P-value |
| CD4% | |||
| Randomization [N=21, 21] | 33 (30, 40) | 34 (32, 38) | 0.950 |
| 3-month [N=21, 21] | 37 (29, 42) | 23 (18, 27) | <0.001 |
| 6-month [N=21, 20] | 34 (26, 41) | 26 (24, 37) | 0.155 |
| 9-month [N=21, 21] | 34 (27, 41) | 29 (26, 34) | 0.170 |
| 12-month [N=19, 21] | 35 (25, 42) | 30 (28, 35) | 0.303 |
| 15-month [N=19, 20] | 37 (30, 44) | 32 (29, 38) | 0.172 |
| 18-month [N=18, 21] | 35 (27, 42) | 35 (33, 37) | 0.921 |
| Plasma log10 VL | |||
| Randomization [N=21, 21] | 2.18 (2.18, 2.64) | 2.18 (2.18, 2.71) | 0.655 |
| 03-month [N=20, 21] | 2.18 (2.18, 5.30) | 6.02 (5.42, 6.37) | <0.001 |
| 06-month [N=21, 19] | 2.28 (2.18, 5.29) | 3.21 (2.69, 5.27) | 0.174 |
| 09-month [N=21, 21] | 2.18 (2.18, 5.36) | 3.07 (2.18, 4.87) | 0.506 |
| 12-month [N=19, 21] | 2.18 (2.18, 4.24) | 2.76 (2.18, 3.82) | 0.398 |
| 15-month [N=17, 20] | 2.18 (2.18, 4.38) | 2.30 (2.18, 4.35) | 0.618 |
| 18-month [N=18, 21] | 2.18 (2.18, 4.03) | 2.18 (2.18, 2.96) | 0.920 |
| % with VL>1000 copies/ml | |||
| Randomization [N=21, 21] | 5 (24) | 5 (24) | 1.000 |
| 03-month [N=20, 21] | 7 (35) | 19 (90) | <0.001 |
| 06-month [N=21, 19] | 8 (38) | 12 (63) | 0.205 |
| 09-month [N=21, 21] | 8 (38) | 11 (52) | 0.536 |
| 12-month [N=19, 21] | 6 (32) | 9 (43) | 0.527 |
| 15-month [N=17, 20] | 5 (29) | 7 (35) | 1.000 |
| 18-month [N=18, 21] | 6 (33) | 5 (24) | 0.723 |
| Growth | |||
| Follow-up time since randomization, months | 18.3 (18.0, 18.4) | 18.4 (18.3, 18.5) | |
| Weight for Age z scores | |||
| Randomization [N=21, 21] | −0.47 (−0.87. −0.02) | −0.34 (−0.63, 0.47) | 0.155 |
| 3-month [N=21, 21] | −0.54 (−0.96, −0.07) | −0.31 (−0.53, 0.70) | 0.105 |
| 6-month [N=21, 21] | −0.42 (−0.93, −0.13) | −0.30 (−0.81, 0.47) | 0.297 |
| 12-month [N=20, 21] | −0.54 (−0.98, −0.19) | −0.29 (−0.71, 0.29) | 0.141 |
| 18-month [N=17, 20] | −0.57 (−0.88, −0.28) | −0.17 (−0.56, 0.37) | 0.148 |
| Height for Age z scores | |||
| Randomization [N=21, 21] | −1.33 (−2.04, −1.03) | −0.91 (−1.66, −0.16) | 0.155 |
| 3-month [N=21, 21] | −1.32 (−1.91, −0.93) | −0.94 (−1.67, −0.42) | 0.232 |
| 6-month [N=21, 21] | −1.31 (−1.75, −0.96) | −1.00 (−1.76, −0.21) | 0.237 |
| 12-month [N=20, 21] | −1.14 (−1.87, −0.73) | −0.92 (−1.67, −0.13) | 0.498 |
| 18-month [N=17, 20] | −1.04 (−1.58, −0.59) | −0.78 (−1.62, 0.00) | 0.446 |
|
Morbidity and adverse events |
Incidence per 100 child-years (number of cases) |
Incidence per 100 child-years (number of cases) |
|
| Clinical severe adverse events (per 100 child-years) | 3.3 (1) | 3.1 (1) | 0.972 |
| URTI | 324 (99) | 334 (107) | 0.875 |
| Rash | 68.7 (21) | 84.3 (27) | 0.569 |
| Anemia | 29.4 (9) | 31.2 (10) | 0.957 |
| High cholesterol | 58.9 (18) | 34.3 (11) | 0.032 |
| Diarrhea | 45.8 (14) | 40.6 (13) | 0.774 |
| Pneumonia | 13.1 (4) | 9.37 (3) | 0.603 |
| Lymphadenopathy | 22.9 (7) | 50.0 (16) | 0.087 |
| Death | 3.3 (1) | 0 (0) | 0.293 |
| B. Comparison of TI Children who met ART Re-start Criteria at 3 Months to Those with met ART Re-start Criteria Later | |||
|---|---|---|---|
| Characteristics | Median (IQR) or n (%) Met ART-restart criteria at 3 Months N= 14 |
Median (IQR) or n (%) Did not meet ART- restart criteria at 3 Months N= 7 |
p- value |
| Age at ART (months) | 4.8 (3.8, 7.1) | 5.3 (4.4, 7.3) | 0.654 |
| Growth | |||
| Pre-ART WAZ | −1.64 (−2.51, −0.79) | −1.34 (−2.98, −0.60) | 0.852 |
| Randomization WAZ | −0.29 (−0.63, 0.47) | −0.34 (−0.65, 0.62) | 1.000 |
| Pre-ART HAZ | −1.01 (−2.24, −0.19) | −1.14 (−3.73, −0.35) | 0.794 |
| Randomization HAZ | −0.76 (−1.18, −0.13) | −1.89 (−2.17, −0.76) | 0.073 |
| Pre-ART WHZ | −1.15 (−2.93, 0.21) | −0.54 (−1.62, 0.02) | 0.478 |
| Randomization WHZ | 0.58 (−0.09, 0.69) | 0.98 (0.45, 1.41) | 0.101 |
| CD4% | |||
| Pre-ART CD4% | 19 (15, 23) | 19 (14, 23) | 0.822 |
| CD4% Nadir | 16 (10, 19) | 18 (14, 23) | 0.525 |
| Randomization CD4% | 33 (29, 36) | 39 (33, 41) | 0.043 |
| CD4 Count | |||
| Pre-ART CD4 Count (cells/mL) | 1365 (738, 1760) | 1572 (596, 2140) | 0.654 |
| Randomization CD4 count (cells/µL) | 1390 (1177, 1887) | 1747 (1654, 2238) | 0.073 |
| HIV-1 Viral Load | |||
| Pre-ART viral load (log10) [N=9, 6] | 6.63 (6.30, 6.93) | 6.28 (5.52, 6.83) | 0.556 |
| Randomization viral load | 150 (150, 325) | 150 (150 − 343,305) | 0.328 |
| Enrollment WHO Stage 3/4 [N=10, 6]** | 4 (40) | 3 (50) | 1.000 |
| Randomization WHO Stage I | 14 (100) | 7 (100) | - |
| Randomization Regimen | |||
| PI-regimen | 12 (86) | 4 (57) | 0.280 |
| NNRTI-regimen | 2 (14) | 3 (43) | |
intent to treat analyses
Excludes infants already on ART at enrollment who did not have this information.
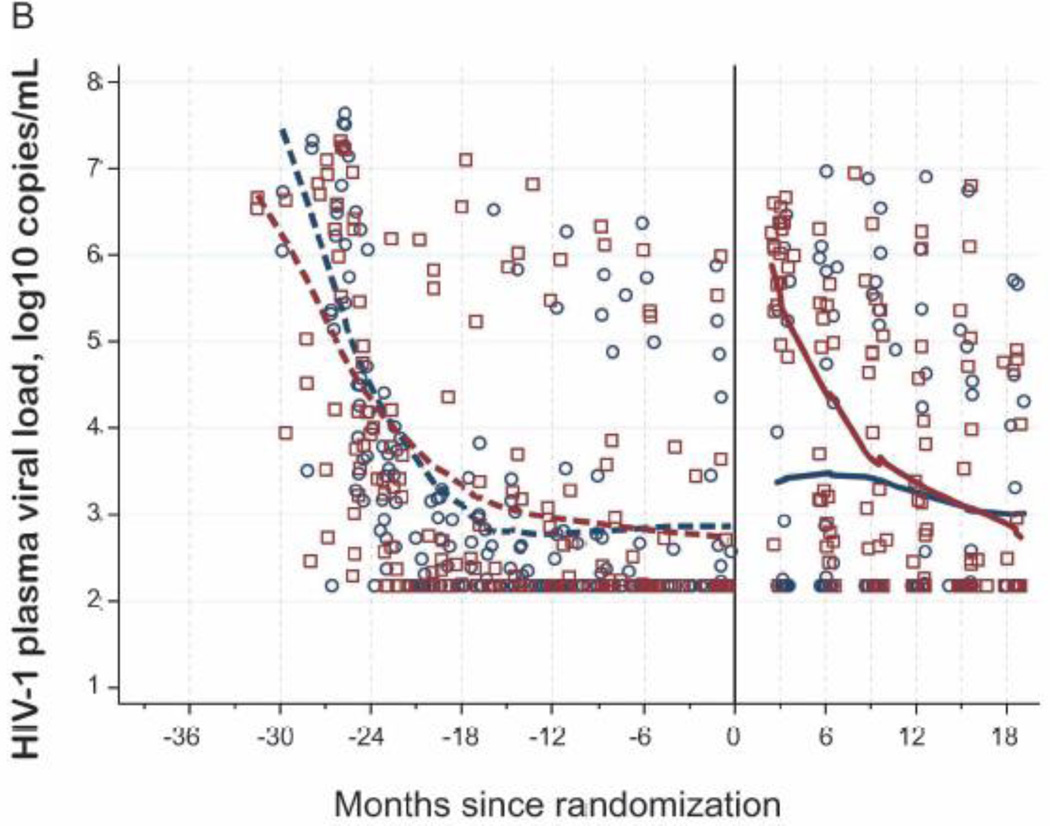
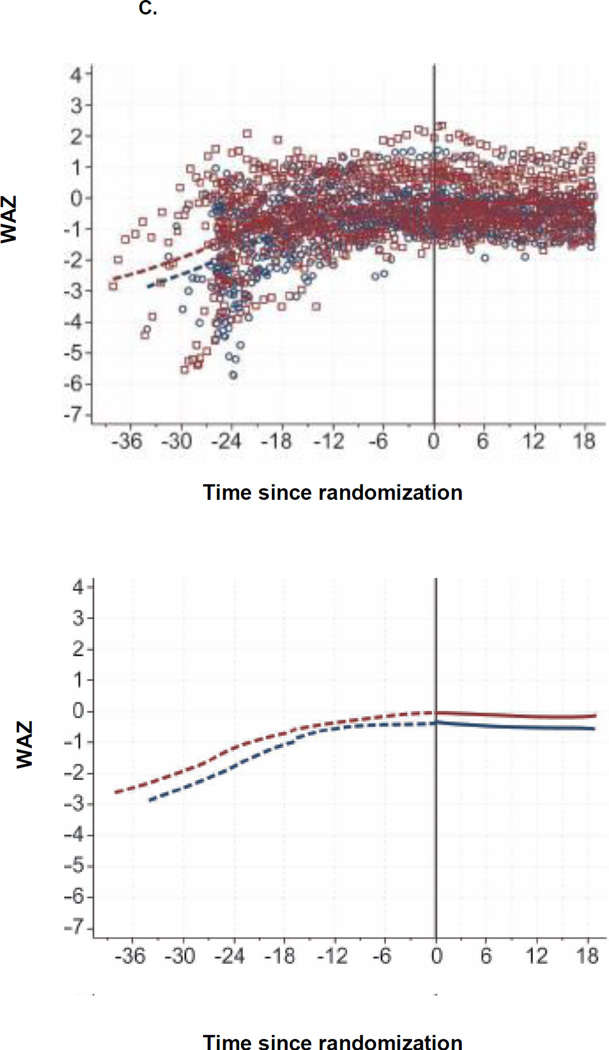
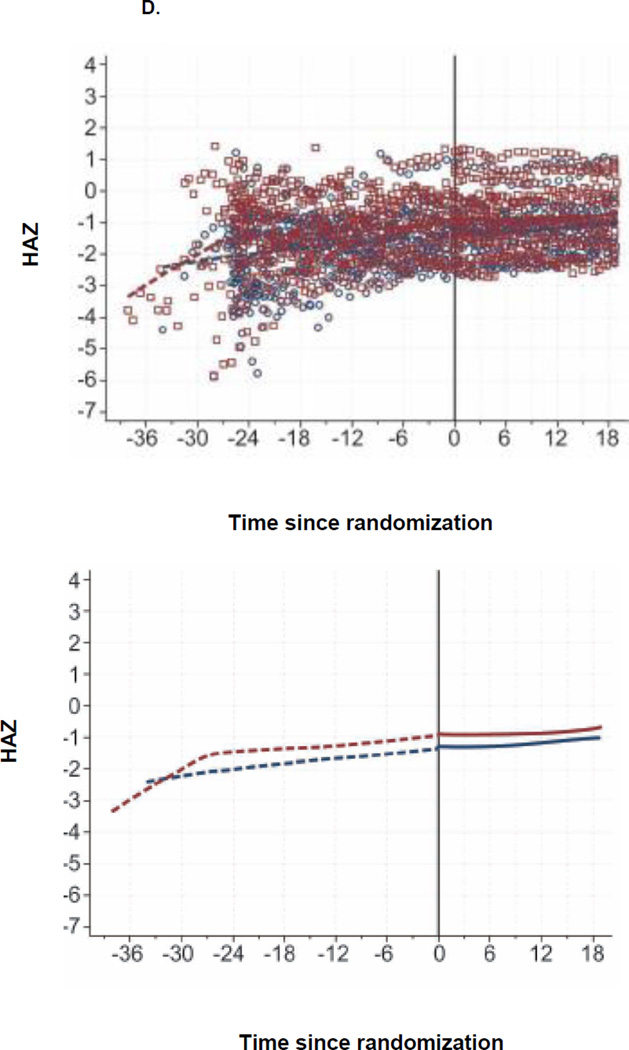
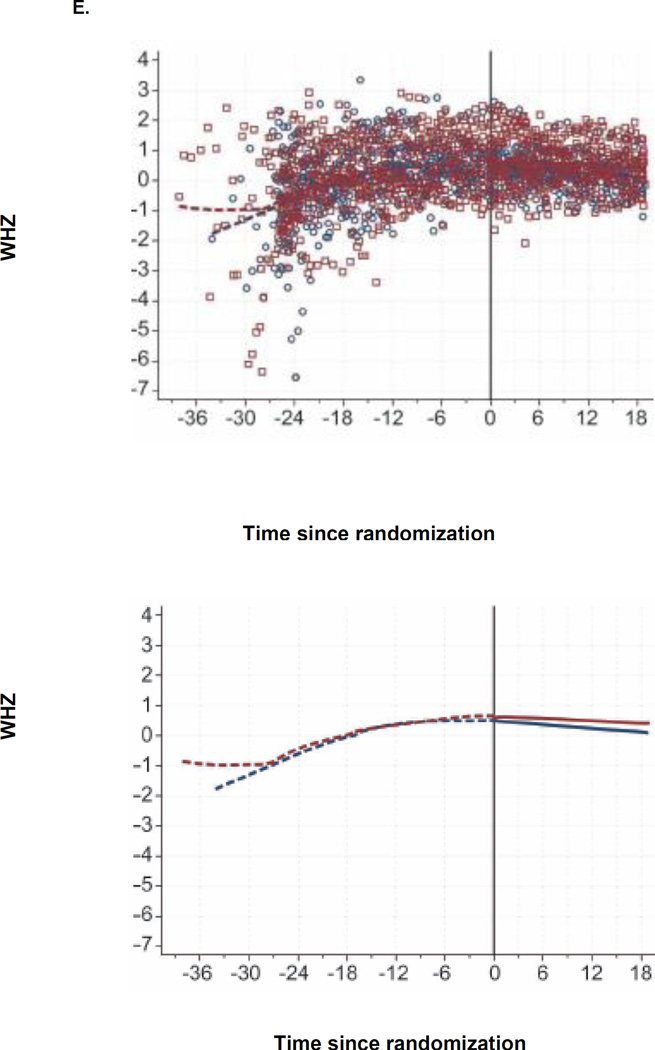
Figure 2. CD4, Viral Load, and Growth Changes by Randomization Arm.
A. CD4% by Randomization Arm
Note. Individual observations for Continued (circles) and Interrupted (squares) are plotted. Lowess curves of pre-randomization data (dashed lines) and of post-randomization (solid lines) for Continued (navy) and Interrupted (maroon) are included.
B. Viral Loads for Randomized Subjects by Arm
C. WAZ since Randomization, by Arm
D. HAZ since Randomization, by Arm
E. WHZ since Randomization, by Arm
Growth and morbidity
At 6, 12, and 18 months post-randomization, the median WAZ was −0.42, −0.54, and −0.57 in the continued arm, respectively and −0.30, −0.29, and −0.17 in the TI arm, respectively (Table 2, Figure 2C). There were no significant differences in WAZ, WHZ and HAZ between arms (Figure 2C–E).
Incidence of serious adverse events
Serious adverse events were infrequent and did not differ between arms. In the continued arm, one infant died (incidence 3.3 per 100 person-years). In the TI arm, one infant was hospitalized for cellulitis (Grade 3) (incidence 3.1 per 100 person-years) (Table 2). Children in the TI arm had a trend for higher incidence of lymphadenopathy, while children on continued ART had significantly higher incidence of hypercholesterolemia. During 6 months post-randomization, those with shorter TI had highest rate of lymphadenopathy (135.8 cases per 100-person years), while those with longer TI had less lymphadenopathy (31.2 cases per 100 person-years) (p=0.125), and those on the continued arm had a rate of lymphadenopathy of 20.2 cases per 100 person-years.
Virologic response and resistance compared post-randomization
Plasma viral loads rose sharply in the TI arm compared to continued arm to a median of 6.02 log10 c/ml vs. undetectable 2.18 log10 c/ml (<150 c/ml) at 3 months post-randomization (p<0.001), Figure 2B. Following ART restart, median plasma viral load dropped by 3.49 log10 copies/ml after 6 months post-restart. By 18 months, median viral levels were undetectable in continued and TI arms (p=0.92); however, 6 of 18 (33%) children in the continued arm had VL>1000 c/ml vs. 5 (24%) of 21 children in the TI arm (p=0.72) (Table 2). Pre-randomization, 5 children in the continued arm had VL>1000 c/ml, of whom 1 did not amplify, 2 tested without resistance, and 2 tested with resistance (M46I, I54V, V82F, Y188L, H221Y, M184V, D67N; and M184V, K103N); 5 children in the TI arm had VL>1000c/ml, of whom 2 were not tested, 1 tested without resistance, and 2 tested with resistance (Y181C, M184V; and A98AG, K101AE, G190A, M184V). Post-randomization, 10 in the continued arm had a VL>1000 c/ml, of whom 4 were tested and had no resistance; all 21 in the TI arm had at least one VL>1000 c/ml, of whom 11 tested without resistance and 2 tested with resistance (K103N; and A98AG, K101AEKT, G190AG, M184MV).
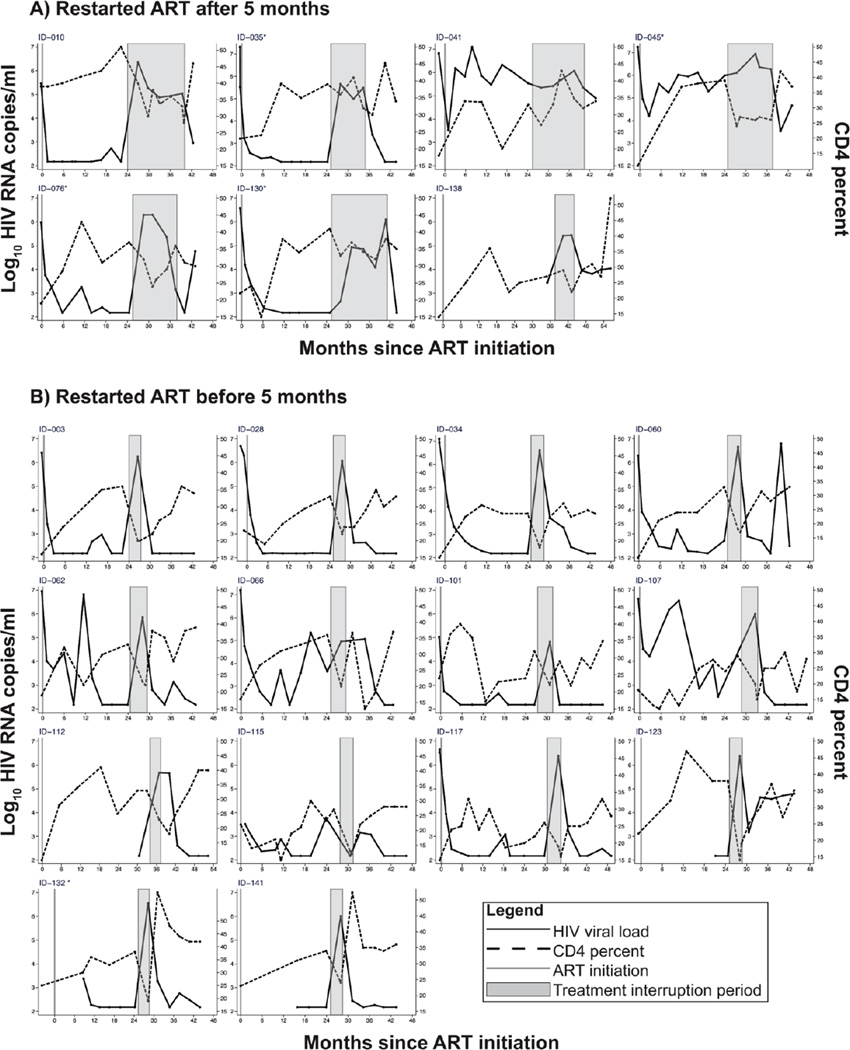
Characteristics of children who sustained longer TI
Although most children rapidly met ART restart criteria following TI, 7 (one-third) sustained longer TI (>5 months). Patterns of VL and CD4 before, during and after TI are summarized in Figure 3. Because VLs were measured retrospectively and were not part of eligibility criteria some children were not virally suppressed at randomization. Two children with viremia prior to TI had minimal changes in CD4% after TI (#041 and #045). In children with longer TI, ID #010 and #076 had VL decline after initial high levels. All children had rapid viral decline following re-starting ART.
Figure 3. CD4 and viral load changes among infants before, during and after treatment interruption.
Children in the TI arm who sustained TI longer than 3 months had similar nadir pre-ART CD4% to those who restarted ART by 3 months after TI but had a higher CD% at randomization (randomization CD4%: 39% versus 33% for TI >3 month vs. <3 months; p=0.04) (Table 2B).
DISCUSSION
In this study (OPH), which involved infants who started ART before 13 months of age, received at least 2 years of ART, and were randomized to TI versus continued ART, most children in the TI arm met criteria for restarting ART within 3 months of TI. During post-randomization follow-up, TI was safe and 18-month CD4%, VL, incidence of severe adverse events, and growth in children in the TI arm did not differ from those who continued ART. These findings complement those from the PENTA-11 trial, which evaluated TI in older children and the CHER trial, which evaluated TI among children who started ART in infancy, but lacked a continued ART comparison group. Collectively these studies suggest that monitored short-term TI does not increase adverse effects over 1–2 years following the TI and ART restart period.
We observed a lower proportion of children (<20%) sustaining TI (not meeting ART restart criteria for >3 months) compared to the CHER study (median TI duration 70 weeks in CHER at trial end among children with a similar duration of early ART prior to TI [96 weeks] as in our study) or the PENTA-11 study (60% had TI up to 48 weeks)[10, 12]. One reason for our observed shorter TI is that our CD4% criterion for ART restart was higher (25%) to align with later WHO recommendations[22], while PENTA-11 and CHER used CD4% of 20% eligibility for ART restart. Small pediatric TI studies have observed CD4% increase following initial decline as HIV-1 specific immunity increases in response to viremia emerging after TI [23]. Lower CD4% criteria for ART re-start may permit time to develop HIV-specific immunity to contain virus and regain immune reconstitution. Another potential reason for shorter TI in the OPH cohort is the more advanced HIV disease pre-ART. CHER infants started ART while asymptomatic with CD4% >25%[1]. In OPH, over half of children had CD4% <25% and WHO stage 3–4 at ART initiation. Children in OPH were older at ART initiation (median 5 months versus 1.9 months in CHER). We extended age-eligibility to broaden generalizability to postpartum-infected and later-diagnosed infants with the hypothesis that infants treated in the first year of life could restore immune function. Consistent with this hypothesis, we found that nadir pre-ART CD4% was not as predictive of TI duration as CD4% following 2-year ART, suggesting that some infants may safely sustain TI despite a low nadir CD4%.
In the PENTA-11 trial, 60% completed TI for 48 weeks with no serious adverse effects[12]. Children in PENTA-11 were older (median age 9 years) and received ART longer (median 6 years) than in our study. Although the period of TI in OPH was shorter, we also observed prompt CD4 recovery and viral suppression following re-initiation of ART with no discernable differences in viral control or CD4% in TI children at 18 months post-TI. The incidence of serious clinical events in OPH was not elevated in the TI arm, suggesting that TI can be safe in the short-term with close monitoring. A higher CD4% at TI rather than nadir CD4% predicted TI durability in OPH, in contrast to PENTA-11 and SPARTAC (an adult TI trial following ART in primary HIV) studies, which observed that pre-ART immune status predicted TI durability or PTC[12, 24]. This suggests that both pre- and post-ART immune function may influence TI durability and PTC[25, 26].
We did not identify post-treatment virologic controllers. This differs from the VISCONTI ANRS cohort[5], but is consistent with other adult studies[6, 7] and preliminary data from children in the CHER study[27]. Most studies have had limited statistical power to estimate frequency of PTC (estimated 0–15%)[7]. We used immune, growth, and clinical criteria rather than viral suppression to identify children for TI and our data underscore limitations of a clinically guided approach to pediatric TI. Given the timing of ART initiation and slow suppression on ART in our cohort, children likely had large viral reservoirs at TI, which correlate with rapid rebound. In contrast to adults, in whom PTC has been observed following ART initiation within months of acute HIV, rapid progression in infants suggests need for accelerated detection and treatment. While our study suggests clinical safety of short TI, children with limited viral reservoirs following very early ART could have more to lose from TI than children such as those in our cohort, with more established reservoirs, because of potential to expand their viral reservoir[29]. Studies to determine whether shorter treatment pauses can safely reveal PTC are ongoing.
Several issues are uniquely relevant to pediatric TI. Children appear not to have morbidity risks noted in adult TI trials, with comparable morbidity in TI versus continued ART. Poor viral control and unplanned TIs contribute to poor outcomes in infants on ART, lending conceptual appeal to strategic TI to preserve regimen efficacy. We observed prompt declines in viral load following ART restart. Long-term virologic failure post-randomization was not uncommon (33%) in the continued arm and actually more frequent than in the TI arm (24%), although underpowered for comparison. This illustrates the challenges of ART adherence in children. Our data suggests safety of short TI, however does not provide evidence to support longer TI in this population.
Limitations of our study include a small sample size and the short duration of TI. Thus, we may not have realized longer-term risks or benefits of TI. Despite limited numbers, prevalence of hypercholesterolemia was higher in children in the continued arm. Increased incidence of lymphadenopathy in children with TI is consistent with an acute infection-like syndrome and has been consistently observed in TI studies. Incidence of lymphadenopathy was highest among those with short TI, suggesting that lymphadenopathy occurred in a parallel with rapid CD4 decline and consequent ART restart in this group.
In summary, our study provides clinical trial evidence that children who initiate ART during early HIV do not have clinically discernable long-term adverse outcomes from brief monitored TI. Our study did not identify any post-treatment controllers among infants started on ART. Complementary strategies such as immune therapies may be necessary to enhance likelihood of PTC[30]. The study also underscores need for improved ART regimens for young children[31]
Acknowledgments
We thank the OPH03 study participants and their families, without whom this research would not be possible and the OPH03 administrative, clinical, and data teams for their dedication and support. We thank the Data Safety and Monitoring Board (DSMB) for their thoughtful oversight: Drs. Michael Hughes (Chair), Mary Glenn Fowler, Walter Jaoko, Shahin Lockman, James McIntyre, Philippa Musoke, and Sarah Walker. We thank Drs. Jennifer Slyker and Dara Lehman for their thoughtful review of the manuscript and UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) and Kenya Research Training Center (KRTC) for their support.
Funding: This study was supported by National Institute of Child Health and Development (R01 HD023412 and K24 HD054314) and National Institute of Health (P30 A1027757 University of Washington Center for AIDS Research)
References
- 1.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. The New England Jounral of Medicine. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander L, Cuchura L, Simpson BJ, Andiman WA. Virologic and host characteristics of human immunodeficiency virus type 1-infected pediatric long term survivors. The Pediatric Infectious Disease Journal. 2006;25:135–141. doi: 10.1097/01.inf.0000199299.00345.83. [DOI] [PubMed] [Google Scholar]
- 3.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. The New England Journal of Medicine. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frange P, Faye A, Avettand-Fenoel V, Bellaton E, Descamps D, Angin M, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3:e49–e54. doi: 10.1016/S2352-3018(15)00232-5. [DOI] [PubMed] [Google Scholar]
- 5.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathogens. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maenza J, Tapia K, Holte S, Stekler JD, Stevens CE, Mullins JI, et al. How often does treatment of primary HIV lead to post-treatment control? Antiviral Therapy. 2015 doi: 10.3851/IMP2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianella S, Anderson C, Richman D, Smith D, Little SJ. No evidence of post-treatment control after early initiation of antiretroviral therapy. AIDS. 2015;29:2093–2097. doi: 10.1097/QAD.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa R, Munoz-Fernandez MA. Production of new T cells by thymus in children: effect of HIV infection and antiretroviral therapy. Pediatric Research. 2002;52:207–212. doi: 10.1203/00006450-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 9.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England Journal of Medicine. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 10.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibb DM, Duong T, Leclezio VA, Walker AS, Verweel G, Dunn DT. Immunologic changes during unplanned treatment interruptions of highly active antiretroviral therapy in children with human immunodeficiency virus type 1 infection. The Pediatric Infectious Disease Journal. 2004;23:446–450. doi: 10.1097/01.inf.0000122601.62358.74. [DOI] [PubMed] [Google Scholar]
- 12.PENTA. Response to planned treatment interruptions in HIV infection varies across childhood. AIDS. 2010;24:231–241. doi: 10.1097/QAD.0b013e328333d343. [DOI] [PubMed] [Google Scholar]
- 13.Bunupuradah T, Duong T, Compagnucci A, McMaster P, Bernardi S, Kanjanavanit S, et al. Outcomes after reinitiating antiretroviral therapy in children randomized to planned treatment interruptions. AIDS. 2013;27:579–589. doi: 10.1097/QAD.0b013e32835c1181. [DOI] [PubMed] [Google Scholar]
- 14.Marzel A, Shilaih M, Yang WL, Boni J, Yerly S, Klimkait T, et al. HIV-1 Transmission During Recent Infection and During Treatment Interruptions as Major Drivers of New Infections in the Swiss HIV Cohort Study. Clinical Infectious Diseases : an official publication of the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/civ732. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aupiais C, Faye A, Le Chenadec J, Rouzioux C, Bouallag N, Laurent C, et al. Interruption of cART in Clinical Practice is Associated with an Increase in the Long-Term Risk of Subsequent Immunosuppression in HIV-1-Infected Children. The Pediatric Infectious Disease Journal. 2014 doi: 10.1097/INF.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 17.Siberry GK, Patel K, Van Dyke RB, Hazra R, Burchett SK, Spector SA, et al. CD4+ lymphocyte-based immunologic outcomes of perinatally HIV-infected children during antiretroviral therapy interruption. Journal of Acquired Immune Deficiency Syndromes. 2011;57:223–229. doi: 10.1097/QAI.0b013e318218e068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutwa PR, Boer KR, Rusine J, Muganga N, Tuyishimire D, Schuurman R, et al. Long-term effectiveness of combination antiretroviral therapy and prevalence of HIV drug resistance in HIV-1-infected children and adolescents in Rwanda. The Pediatric Infectious Disease journal. 2014;33:63–69. doi: 10.1097/INF.0b013e31829e6b9f. [DOI] [PubMed] [Google Scholar]
- 19.Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha A, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA. 2001;286:2413–2420. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehman DA, Wamalwa DC, McCoy CO, Matsen FA, Langat A, Chohan BH, et al. Low-frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. Journal of Acquired Immune Deficiency Syndromes. 2012;60:225–233. doi: 10.1097/QAI.0b013e3182515730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wamalwa DC, Obimbo EM, Farquhar C, Richardson BA, Mbori-Ngacha DA, Inwani I, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatrics. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Antiretroviral therapy for HIV infection in infants and children: towards universal access. 2010 http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf?ua=1. [PubMed]
- 23.Palacios GC, Sanchez LM, Briones E, Ramirez TJ, Castillo H, Rivera LG, et al. Structured interruptions of highly active antiretroviral therapy in cycles of 4 weeks off/12 weeks on therapy in children having a chronically undetectable viral load cause progressively smaller viral rebounds. International journal of Infectious Diseases : IJID : official publication of the International Society for Infectious Diseases. 2010;14:e34–e40. doi: 10.1016/j.ijid.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Hurst J, Hoffmann M, Pace M, Williams JP, Thornhill J, Hamlyn E, et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nature Communications. 2015;6:8495. doi: 10.1038/ncomms9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assoumou L, Weiss L, Piketty C, Burgard M, Melard A, Girard PM, et al. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS. 2015;29:2003–2007. doi: 10.1097/QAD.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 26.Pinkevych M, Cromer D, Tolstrup M, Grimm AJ, Cooper DA, Lewin SR, et al. HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days--Implications for HIV Remission. PLoS Pathogens. 2015;11:e1005000. doi: 10.1371/journal.ppat.1005000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne HWS, Hsaio M, et al. Early ART and sustained virologic suppression limits HIV proviral DNA reservoir: CHER evidence. Conference on Retroviruses and Opportunistic Infections (CROI) 2015 n. Abstract 35. [Google Scholar]
- 28.Persaud D, Luzuriaga K. Absence of HIV-1 after treatment cessation in an infant. The New England Journal of Medicine. 2014;370:678. doi: 10.1056/NEJMc1315498. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Bonet M, Puertas MC, Fortuny C, Ouchi D, Mellado J, Rojo P, et al. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clinical Infectious Diseases : an official publication of the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/civ456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein N, Palma P, Luzuriaga K, Pahwa S, Nastouli E, Gibb DM, et al. Early antiretroviral therapy in children perinatally infected with HIV: a unique opportunity to implement immunotherapeutic approaches to prolong viral remission. The Lancet. Infectious Diseases. 2015;15:1108–1114. doi: 10.1016/S1473-3099(15)00052-3. [DOI] [PubMed] [Google Scholar]
- 31.Prendergast AJ, Penazzato M, Cotton M, Musoke P, Mulenga V, Abrams EJ, et al. Treatment of young children with HIV infection: using evidence to inform policymakers. PLoS Medicine. 2012;9:e1001273. doi: 10.1371/journal.pmed.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]