Abstract
Intrauterine growth restriction (IUGR) caused by placental insufficiency is one of the most common and complex problems in perinatology, with no known cure. In pregnancies affected by placental insufficiency, a poorly functioning placenta restricts nutrient supply to the fetus and prevents normal fetal growth. Among other significant deficits in organ development, the IUGR fetus characteristically has less lean body and skeletal muscle mass than their appropriately-grown counterparts. Reduced skeletal muscle growth is not fully compensated after birth, as individuals who were born small for gestational age (SGA) from IUGR have persistent reductions in muscle mass and strength into adulthood. The consequences of restricted muscle growth and accelerated postnatal “catch-up” growth in the form of adiposity may contribute to the increased later life risk for visceral adiposity, peripheral insulin resistance, diabetes, and cardiovascular disease in individuals who were formerly IUGR. This review will discuss how an insufficient placenta results in impaired fetal skeletal muscle growth and how lifelong reductions in muscle mass might contribute to increased metabolic disease risk in this vulnerable population.
Keywords: amino acids, muscle protein synthesis, myofiber, myoblast, myogenesis, developmental programming
1. The effects of placental insufficiency on fetal skeletal muscle growth
1.1 Fetal adaptation to increased placental resistance
Placental insufficiency affects ∼8% of all pregnancies and commonly begins early in pregnancy for a multitude of reasons, including maternal chronic disease (chronic hypertension, pregnancy-induced hypertension, and other vascular disorders), placental disorders (preeclampsia, abruption, infarcts), and idiopathic causes (Rozance et al., 2016). Maternal/placental conditions that obliterate small muscular arteries result in increased placental vascular resistance and decreased diastolic flow in the umbilical artery (demonstrated by a high pulsatility index) (Berkley et al., 2012). In response to reduced umbilical blood flow and decreasing fetal oxygenation, the fetal ductus venosus dilates to shunt oxygenated blood from the umbilical vein away from the liver to ensure an adequate supply of oxygen and nutrients to the heart and brain (Tchirikov et al., 2006). The middle cerebral artery also dilates (demonstrated by a low pulsatility index) to maximize blood flow to the brain; providing nutrients and oxygen that spare brain growth restriction relative to overall fetal growth. Doppler velocimetry abnormalities in the umbilical artery are the gold standard for defining placental insufficiency-induced IUGR status (ACOG, 2013). This is opposed to either the population-based term small for gestational age (SGA), which refers to infants born <10% on standard intrauterine growth charts, or low birth weight (LBW) at term gestation, which refers to neonates born with a birthweight of <2500 grams. Most human epidemiological studies to date use these terms to represent IUGR status. However, it should be noted that these terms will include IUGR neonates as well as those normal neonates born constitutionally small.
Redistribution of blood flow to the vital organs such as the brain occurs at the expense of nutrient and oxygen delivery to the periphery (Tchirikov et al., 1998, Yajnik, 2004) (Figure 1). These selective reductions in blood flow and oxygen supply to the peripheral musculature likely contribute to 25-40% reductions in muscle mass observed in IUGR fetuses and neonates when compared to their appropriate for gestational age (AGA) counterparts (Baker et al., 2010, Beltrand et al., 2008, Larciprete et al., 2005, Padoan et al., 2004, Yau et al., 1993). However, even “brain sparing” is incomplete, as head circumference in IUGR infants is frequently below the 10th percentile for gestational age (Kramer et al., 1989) with reduced brain volume (Toft et al., 1995) and higher risk of poor neurodevelopmental outcome (Arcangeli et al., 2012, De Jesus et al., 2013, Guellec et al., 2015, von Beckerath et al., 2013).
Figure 1. Redistribution of blood flow in IUGR.

Dilation of the ductus venosus and middle cerebral artery shunts oxygen and nutrients from the umbilical vein directly to the heart and brain and away from skeletal muscle (diagram modified from Yainik, 2004).
1.2 Myogenesis
In severe cases of placental insufficiency, onset occurs as early as the second trimester of human pregnancy (De Jesus et al., 2013) with progressive reduction in nutrient and oxygen delivery to the fetus and subsequent development of growth factor deficiencies. Understanding fetal skeletal muscle development (myogenesis) across gestation is important in order to investigate how placental insufficiency and chronic nutrient restriction impact skeletal muscle growth.
Much of what we know about the timing and regulation of myogenesis comes from animal models, notably livestock (sheep, cows) in which the timing of myogenesis is similar to humans (Du et al., 2010, Romero et al., 2013) (Figure 2). The primary (embryonic) stage of muscle fiber formation occurs by the end to end fusion of myogenic precursor cells into primary myotubes and begins at 0.2 of gestation (Wilson et al., 1992). Primary myotubes form the scaffold for subsequent myotube formation. The secondary (fetal) stage of myogenesis involves rapid proliferation of myoblasts that differentiate and fuse to form multinucleated myotubes and secondary myofibers, beginning at 0.2 of gestation and peaking around 0.6 of gestation (Fahey et al., 2005). Based on environmental signals, such as insulin like-growth factors (Ren et al., 2010), myoblasts either proliferate for self-renewal or align to differentiate into myotubes (Yan et al., 2013). Pax transcription factors (Pax3, Pax7) in myogenic precursors regulate the progressive expression of highly conserved myogenic regulatory factors (MRFs), including Myf5 and MyoD, which are considered determination factors, and myogenin (MyoG), which signifies terminal differentiation (Bentzinger et al., 2012).
Figure 2. Schematic representation of fetal myogenesis during pregnancy.
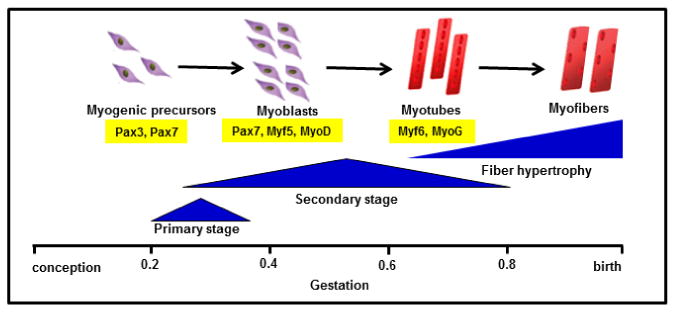
The fraction of gestation when primary (embryonic) and secondary (fetal) stages of myogenesis are based on studies from sheep, cow, and human (Du et al., 2010, Romero et al., 2013, Wilson et al., 1992). Pax transcription factors Pax3 and Pax7 define the progenitor cell population during fetal myogenesis (Wang et al., 2010). Pax7 is expressed in fetal myoblasts, in addition to Myf5 and MyoD which commit cells to the myogenic program. Expression of the terminal differentiation genes, Myf6 (also known as Mrf4) and MyoG, are expressed in myotubes(Bentzinger et al., 2012). Myofibers grow by hypertrophy late in gestation and in postnatal life.
Muscle grows primarily by myofibrillar protein synthesis and hypertrophy of myofibers during the final third of gestation, though this process includes continued proliferation and fusion of myoblasts into established myofibers (McCoard et al., 2001, Stickland, 1981). The postnatal (adult) stage of myogenesis involves maintenance of satellite cells that reside around the muscle fibers in a quiescent state and are activated during muscle growth, regeneration, and repair (Wang et al., 2014, Zammit et al., 2006). The stages of myogenesis are similar among vertebrates, though secondary myogenesis in humans, sheep, and cows occurs earlier in gestation than in rodents (Du et al., 2010) and is more complex with successive generations of myotubes (tertiary) adding to the scaffold (McLennan, 1994, Wilson et al., 1992).
1.3 Impact of placental insufficiency on myoblast proliferation
The concept that chronic fetal undernutrition in utero may disrupt normal myogenesis was introduced over 40 years ago by Elsie Widdowson, a pioneer of child nutrition and growth (Widdowson et al., 1972). Her statements were based on several studies which showed muscle fiber number to be set at birth (Rowe et al., 1969, Stickland et al., 1975, Widdowson et al., 1972, Wigmore et al., 1983). In a variety of species, maternal nutrient restriction during pregnancy limits fetal myoblast cell cycle activity, reduces myonuclei per myofiber, and reduces myofiber number in offspring (Bayol et al., 2004, Costello et al., 2008, Dwyer et al., 1995, Dwyer et al., 1992, Fahey et al., 2005, Greenwood et al., 2000, Greenwood et al., 1999, Osgerby et al., 2002, Prakash et al., 1993, Wilson et al., 1988). Though less well studied, placental insufficiency independent of maternal nutrient intake also results in decreased proliferative capacity of fetal myoblasts, as measured by decreased expression of proliferating cell nuclear antigen (PCNA) and reduced rates of replication in vitro (Yates et al., 2014).
The mechanisms that result in impaired proliferation and formation of myofibers are not known, though reduced availability of important fetal growth factors known to regulate myoblast proliferation, such as insulin and insulin-like growth factors (IGFs) (Fowden, 2003), are likely candidates. Igf1 heterozygous knockouts in mice demonstrate reduced muscle mass (Powell-Braxton et al., 1993), whereas homozygous knockouts have severe muscle hypoplasia from both decreased myocyte number and myofiber cross-sectional area (Liu et al., 1993, Mavalli et al., 2010). Similarly, mutations in the IGF1 and IGF1 receptor genes in humans cause both intrauterine and postnatal growth restriction (Abuzzahab et al., 2003, Woods et al., 1996), as do newly discovered mutations in the IGF2 gene (Begemann et al., 2015). Insulin also functions as a potent skeletal muscle growth factor. The absence of insulin signaling in insulin receptor knock-out mice contributes to reduced muscle mass and function though decreased protein synthesis (O'Neill et al., 2010). Pancreatectomy in fetal sheep results in growth restricted fetuses with decreased upper and lower extremity limb length (Fowden et al., 1989). These data are consistent with lack of insulin as the cause of growth restriction in cases of pancreatic agenesis in humans (Lemons et al., 1979). Interestingly, IGFs have been shown to paradoxically stimulate both myoblast proliferation and differentiation (Florini et al., 1993). The presence of hypoxia might be one of the factors that determine whether IGF1 promotes proliferation over differentiation (Ren et al., 2010). These interactions may be particularly important in the complex physiological milieu of an IUGR fetus, where concentrations of insulin, IGF1, and oxygen are lower than those of normally growing fetuses.
1.4 Impact of placental insufficiency on myofiber hypertrophy
Myofiber hypertrophy, or an increase in fiber diameter and length, occurs as a net increase in protein accretion based on balance between protein synthesis and degradation rates. Late gestation fetal and postnatal muscle growth occurs primarily by myofiber hypertrophy (White et al., 2010). Maternal nutrient restriction in sheep, especially towards the end of gestation, reduces myofiber hypertrophy and muscle weights in the fetus (Fahey et al., 2005), as do models of placental insufficiency in guinea pigs and sheep (Bauer et al., 2003, Yates et al., 2014). The AKT-mTORC1 signaling pathway is one of the primary regulators of muscle protein synthesis in response to anabolic stimuli such as amino acids, insulin, and IGF1 in fetal lambs (Anderson et al., 2005, Brown et al., 2009, Shen et al., 2002) as well as in neonatal piglets (O'Connor et al., 2003, O'Connor et al., 2003, Suryawan et al., 2008, Suryawan et al., 2012). This pathway also has been implicated in reducing fetal muscle protein synthesis under conditions of short term (5 day) maternal fasting in sheep (Shen et al., 2005), and in men who were SGA at birth (Jensen et al., 2008, Ozanne et al., 2005). However, it is not known whether the IUGR fetus slows myofiber hypertrophy via adaptation to reduced nutrients and growth factors, or whether it activates protein breakdown as a result of cellular stress. In fact, AKT is a regulator for both synthesis (4E-BP1, p70S6K) and breakdown pathways (FOXO3) in response to growth factor availability (Bonaldo et al., 2013). Likely, these processes are not mutually exclusive; the fetus might develop a slower growth rate in response to redistribution of blood flow away from the peripheral vasculature (including skeletal muscle) early in the course of placental insufficiency, but then might activate catabolic pathways in the setting of worsening hypoxia and increased catecholamine and cortisol production as placental insufficiency progresses. This is a fundamental area of future investigation, as treatments to improve muscle growth will vary based on whether growth is slowed because of decreased anabolism, or increased catabolism.
1.4 Postnatal growth restriction of muscle in preterm infants
Early life reductions in skeletal muscle growth extend beyond pregnancies affected by placental insufficiency. Extremely preterm infants who were born AGA experience postnatal growth restriction in the Neonatal Intensive Care Unit that includes deficits in muscle mass (Dusick et al., 2003, Ehrenkranz et al., 2006, Johnson et al., 2012). The principal cause of postnatal growth restriction in preterm infants is under-nutrition compared to the nutrition these infants would have received in utero, with contribution of adverse effects of their medical complications (hypoxia, anemia, stress hormone production, and medications that limit protein accretion) (Ehrenkranz, 2010, Ramel et al., 2014). Therefore, preterm birth and its subsequent problems of under-nutrition and unstable physiological conditions can disrupt normal muscle development independent of IUGR, as preterm infants who were not IUGR have decreased lean mass and increased fat mass at the time of discharge (Johnson et al., 2012). Thus, determining the mechanisms responsible for reduced muscle mass in the perinatal and neonatal period has even broader implications, as the rate of preterm birth in the United States remains unacceptably high (Blencowe et al., 2012).
2. Implications of reduced fetal muscle mass
2.1. Persistent reductions in muscle mass and strength
A large number of epidemiological studies have found associations between birthweight and body composition outcomes in later life and are summarized in Table 1. The overwhelming majority of studies show that birthweight, used as an index of the effects of the intrauterine environment on fetal growth, is positively correlated with lean mass and/or fat free mass, and, to a much lesser extent (and even sometimes inversely correlated), with fat mass. The correlation between birthweight and lean mass has been observed in large, diverse populations globally and applies to both males and females. In most studies, the positive correlation between birthweight and lean mass persists after adjusting for body size (weight, height, body mass index) as well as other factors that might influence body composition, such as socioeconomic status, alcohol consumption, and physical activity. Body composition in relation to birthweight has been evaluated during early childhood, prior to the onset of lifestyle factors, as well as during late adulthood, when the programming of body composition tends to amplify (Singhal et al., 2003). Table 1 includes three studies that evaluated both monozygotic (MZ) and dizygotic (DZ) twin pairs to further distinguish effects of the fetal environment from those of the postnatal environment on body composition (Loos et al., 2001, Loos et al., 2002, Skidmore et al., 2009). All three of these twin studies showed a positive correlation between birthweight and lean mass and an inverse relationship between birthweight and measures of body fat percentage (skinfold thickness and muscle to fat ratios). When pair-wise comparisons were made, the heavier twin at birth had more lean mass in adulthood compared to the lighter twin in males (Loos et al., 2001), with less of an effect observed in females (Loos et al., 2002). Both within-pair and between-pair effects were noted in MZ and DZ twins (Skidmore et al., 2009), implicating that both an adverse intrauterine environment as well as postnatal effects impact later life body composition.
Table 1. Studies that associate birthweight to muscle mass and strength later in life.
| Author | Place | Age | Measurements | Associations among birthweight, body composition, and muscle strength |
|---|---|---|---|---|
| Children and Adolescents | ||||
| (Lapillonne et al., 1997) | France | 2d | DXA | SGA (vs. AGA): less LM and FM at the same GA |
| (Hediger et al., 1998) | U.S. | 2-47m | ANTH | SGA (vs. LGA): less lower arm muscle area, % body fat less affected |
| (Singhal et al., 2003) | U.K. | 7-16y | ANTH; BIA, DXA | BW positively correlated with FFM, not FM or BMI; Adjusted for age, sex, puberty, SES, PA |
| (Murphy et al., 2006) | U.K. | ∼6y | ANTH; BIA | BW positively correlated with FFM and FM; Adjusted for BMI |
| (Joglekar et al., 2007) | India | 6-7y | ANTH; DXA | BW positively correlated with FFM and FM; Adjusted for GA, age |
| (Chomtho et al., 2008)* | U.K. | 4-20y | ANTH; DXA, air-plethysmography, 2H-dilution | BW positively correlated with FFM (males), not FM or % fat; Adjusted for Ht, puberty, PA, SES, ethnicity, parental BMI |
| (Ortega et al., 2009) | Spain | 13-18y | ANTH; grip strength | BW positively correlated with Ht, BMI, FFM, fat %, WC, and grip strength (grip strength attributed to FFM) |
| (Baker et al., 2010) | U.S. | 2m - 8y | ANTH | LBW (vs. NBW): less growth of arm muscle area; Adjusted for sex, ethnicity, SES, body size |
| (Moura-Dos-Santos et al., 2013) | Brazil | 7-10y | ANTH; grip strength | LBW (vs. HBW): lower grip strength and running speed; Adjusted for age, sex, Wt, PA, FFM |
| Young adulthood | ||||
| (Kahn et al., 2000) | U.S. | 17-22y M | ANTH | BW positively correlated with BMI and MTC+bone area, not % body fat |
| (Loos et al., 2001)‡ | Belgiu m | 18-34y M twins | ANTH; BIA | BW positively correlated with LM, inversely correlated with % body fat, W-H ratio; Heavier (vs. lighter) twin was taller, less FM, more LM; Adjusted for adult Wt |
| (Loos et al., 2002)‡ | Belgiu m | 18-34y F twins | ANTH; BIA | BW positively correlated with LM, inversely correlation with % body fat, W-H ratio; Heavier (vs. lighter) twin was taller; Adjusted for adult Wt |
| (Sachdev et al., 2005) | India | 26-32y | ANTH | BW positively correlated with Ht and LM (both sexes) and BMI and FM (women only); Adjusted for age, education, occupation, alcohol use, smoking, PA, parity, SES |
| (Brutsaert et al., 2011) | U.S. | ∼20y F | Hydro-densitometry; grip strength | Low PI (vs. high PI): lower grip strength, lower max isometric contraction, higher rate of fatigue, and diminished training response; adjusted for FFM |
| Older adulthood | ||||
| (Gale et al., 2001) | U.K. | 70-75y | Anthropometrics; DXA | BW positively correlated with LM and Ht, inversely correlated with FM; Adjusted for age, sex, Ht, Wt |
| (Eriksson et al., 2002) | Finland | 65-75y | ANTH; BIA | BW, PI, and length at birth positively correlated with FFM |
| (Sayer et al., 2004) | U.K. | ∼64y M | ANTH; grip strength | BW positively correlated with FFM, BMI, MUAC, MTC, and grip strength; not % body fat, W-H ratio, and grip strength; Adjusted for SES, PA, smoking, alcohol use |
| (Kensara et al., 2005) | U.K. | 64-72y M | ANTH; DXA, Air-plethysmography | LBW (vs. HBW): less FFM, less muscle, higher % body fat, lower MTF ratio, and central fat distribution; Adjusted for Wt plus Ht, SES, PA, smoking |
| (Yliharsila et al., 2007) | Finland | 56-70y | ANTH; BIA; grip strength | BW positively correlated with lean mass and grip strength, inversely correlated with % body fat; Adjusted for age/adult Ht, age/adult BMI, mat size, age, smoking, PA, SES |
| (Skidmore et al., 2009)*† | U.K. | 18-80y twins | ANTH; DXA | BW positively correlated with LM, FM, and MTF ratio; Both within-pair and between-pair effects noted; Adjusted for age and BMI |
| (Patel et al., 2012) | U.K. | 68-76y M | ANTH; muscle biopsy; DXA | LBW (vs. HBW): less FFM, higher % body fat, and lower fiber score (myofiber density × leg lean mass); fiber score attenuated when adjusted for age, Ht, PA |
Birthweight (BW) obtained from birth records or birth certificates.
indicates BW obtained by recall;
GA unknown;
GA >35 wks. Both sexes were included unless noted by M, males or F, females only.
Abbreviations: d, days; m, months; y, years; GA, gestational age; M, male; F, female; PI, ponderal index; LBW, low BW; NBW, normal BW; HBW, high BW; SGA, small for GA; AGA, appropriate for GA; LGA, large for GA; WC, waist circumference; W-H, waist to hip; MTF, muscle-to-fat; FFM, fat free mass; FM, fat mass; LM, lean mass; MUAC, midupper arm circumference; MTC, midthigh circumference; HC, hip circumference; SES, socioeconomic status; PA, physical activity; BIA, bioelectrical impedance; DXA, dual-energy X-ray absorptiometry; ANTH, anthropometrics
In some studies, birthweight is used categorically to compare low birth weight (LBW) or SGA neonates (represented as < 2500 grams or <10% on standard intrauterine growth charts at term gestation, respectively) to normal weight neonates, which might better identify specific effects of pathological fetal undergrowth on body composition (Table 1). For example, a study by Baker et al. used data from the Third National Health and Nutrition Examination Survey to demonstrate that while head circumference growth is normal between 2 months and 8 years of age in LBW children born at term, muscle growth remains restricted (Baker et al., 2010). These results indicate that the relationship between the developmental trajectories of brain and muscle tissues differ among those affected by IUGR. In addition, the relative impact of fetal growth on muscle mass later in life is not insignificant. The effect size of being born LBW on muscle mass in men ∼65 years of age was estimated as 4.4 kg less muscle and 25% lower muscle/fat ratios when compared to a higher birth weight group (Kensara et al., 2005).
Several studies also have found positive associations between birthweight with later life muscle strength (Table 1), which raises the question as to whether reductions in fetal muscle mass contribute to sarcopenia, or the age-related decline in muscle mass, strength, and functional ability. Grip strength is commonly used as an indicator of muscle strength and is a predictor of morbidity and mortality (Rantanen et al., 2000). Sayer et al. pooled 10 independent articles that described the relationship between birthweight and grip strength and found a 2 kg increase in grip strength per kg increase in birthweight (Sayer et al., 2008). Another study comprehensively evaluated muscle strength and force generation in college-aged women born with a low ponderal index (representing low muscle mass) and found not only an 11% lower grip strength, but also a 9-24% lower capacity for maximal isometric contraction, a higher rate of fatigue, and a diminished training response (Brutsaert et al., 2011). This study and others adjusted for body size and fat free mass, with the goal of teasing apart the effects of fetal growth on morphological changes during muscle development as opposed to simply reflecting muscle mass. In most cases, adjusting for muscle mass attenuated the association between birthweight and muscle strength but did not eliminate it (Sayer et al., 2008), and it actually increased the strength of association between a low ponderal index and measures of muscle strength (Brutsaert et al., 2011). The most important contributing factors to how fetal growth impacts muscle growth, whether they be muscle mass, morphology, capacity for conditioning, and/or neuromuscular factors, have yet to be definitively determined.
It is important to note that all of these studies are associative and do not prove causality. There are many postnatal factors, even when the youngest children are evaluated, that impact body composition and muscle strength. While fat free mass and lean mass assessment is a good proxy for skeletal muscle mass, they include tissues other than muscle. Finally, birthweight is used as a surrogate for fetal growth patterns, because it is easily obtained from birth records and/or birth certificates for large populations of individuals. Definitive associations between placental insufficiency-induced IUGR and later life muscle mass will require prospective studies that measure umbilical arterial and middle cerebral artery Doppler velocimetry and novel methods to measure muscle mass more specifically. Comprehensive analysis of these relationships, including interventional studies that aim to improve the life trajectory of muscle growth, will require the use of animal models (discussed in Section 3.1) in addition to human clinical studies.
2.2 Reduced muscle-to-fat ratios
Interactions between birthweight, muscle mass, and fat mass have been demonstrated, such that birthweight strongly correlates with lean mass, but either does not correlate or is inversely correlated with fat mass in later life. This, in turn, can produce a reduced muscle-to-fat ratio. Excess adiposity and central (visceral) obesity are well known metabolic disease risk factors (Amato et al., 2013). However, excess adiposity in combination with low muscle mass, termed “sarcopenic obesity”, has emerged as an even more concerning phenotype for cardiometabolic disease risk (Prado et al., 2012). Reduced muscle-to-fat ratios were found by DXA in low birth weight (<3.2 kg) vs. high birth weight infants (>3.9 kg) in a cohort of Englishmen at 56-70 years of age (Kensara et al., 2005). Additionally, other studies have associated low birthweight with reduced waist-to-hip ratios, which is an anthropometric measure of central obesity and also might reflect gluteal muscle mass with the hip circumference (Table 1). Reduced muscle-to-fat ratios also have been implicated as a possible reason for the current diabetes epidemic in India. The “thin-fat” phenotype of Indian diabetic patients is characterized by less muscle mass but greater body fat and central obesity (Yajnik 2004a). Infants born in India compared to infants born in the United Kingdom are lighter, shorter, and thinner, but have similar subscapular skin fold thicknesses, suggesting that intrauterine growth patterns contribute to lower muscle mass but preserved fat mass in this population (Yajnik et al. 2003).
It is possible that persistent limitations in muscle growth and/or reduced muscle-to-fat ratios after placental insufficiency and IUGR set the trajectory for rapid catch-up growth that favors adiposity over muscle. The concept of “catch-up growth” is well described in the literature as the growth pattern of a deprived intrauterine environment and poor infant growth followed by rapid weight gain (Claris et al., 2010). Barker et al. were some of the first to show the potential impact of accelerated postnatal growth on cardiovascular risk. In this landmark study, individuals who were born SGA who then underwent rapid weight gain between ages 2 and 11 were at highest risk for adverse cardiovascular outcomes as adults (Barker et al., 2005). Other systematic reviews have identified rapid weight gain during the first year of life, regardless of birthweight, as a risk factor for childhood obesity (Ong et al., 2006, Weng et al., 2012). When postnatal growth patterns and body composition have been specifically evaluated in SGA children between ages 2 and 4 years, those born SGA gained more abdominal fat and body adiposity and less lean mass than AGA children; by age 4 years, SGA children had greater adiposity and insulin resistance (Ibanez et al., 2006). In a similar cohort, SGA children with rapid catch up growth transitioned from insulin sensitive to an insulin resistant phenotype (Mericq et al., 2005). Animal studies also have demonstrated an imbalance in lean versus fat accumulation in the catch-up growth process. Growth restricted lambs have accelerated postnatal growth driven by increases in adiposity and limited muscle growth (De Blasio et al., 2007, Greenwood et al., 2003), leading to 25% lower muscle/fat ratios at 9 months of age (Ford et al., 2007). In LBW mice that were undernourished in utero, those given a control diet and demonstrated catch-up growth developed obesity and glucose intolerance at 6 months of age, whereas those given a calorie-restricted diet 50% of that of controls were leaner and had better glucose tolerance (Jimenez-Chillaron et al., 2006). Similarly, increased catch-up growth after prenatal (Bieswal et al., 2006) and postnatal (Jou et al., 2013) growth restriction in rats resulted in higher fat mass and decreased protein content compared to those that did not catch up. Animal studies support that catch-up growth in the form of increased adiposity over lean mass plays an important, if not independent, role in the development of later life obesity (Jimenez-Chillaron et al., 2007, Morrison et al., 2010).
Despite a large body of literature that associates catch-up growth with the development of obesity and metabolic disease, the exact mechanisms by which catch-up growth occurs, including the potential role of reduced muscle-to-fat ratios in the development of metabolic disease, are still unknown. Also, it is important to note that the vast majority of SGA infants will demonstrate some degree of accelerated growth. Catch-up growth is beneficial for optimization of brain growth and neurodevelopment (Dusick et al., 2003, Lundgren et al., 2001, Ong, 2007) as well as for avoiding short stature (Karlberg et al., 1995). Consideration for gain in fat mass separately from gain in lean mass in future clinical studies will facilitate a better understanding of the roles of compensatory adiposity vs. muscle growth for overall health, metabolism, and neurodevelopment, and will aid in the development of nutritional strategies that achieve “healthy” catch-up growth.
2.3 Impaired glucose disposal, insulin resistance, and diabetes
Clear associations have been found between birth weight, insulin resistance, and the development of type 2 diabetes, as evidenced by a large meta-analysis which found inverse associations between birth weight and type 2 diabetes in 23 populations (Whincup et al., 2008). Barker et al. found an inverse relationship between birthweight and the metabolic syndrome (which includes insulin resistance, obesity, type 2 diabetes, hypertension, and hyperlipidemia) and a 22% incidence of the metabolic syndrome in those men whose birthweights were < 2900 grams (Barker et al., 1993). Rat models of IUGR have reproduced these relationships by demonstrate striking phenotypes in offspring of hyperinsulinemia, hyperglycemia, central obesity, and diabetes (Fernandez-Twinn et al., 2005, Simmons et al., 2001). Skeletal muscle is a major determinant of regulates whole-body insulin sensitivity and glucose disposal, as it accounts for 80% of insulin-stimulated glucose uptake (Wolfe, 2006). A large epidemiological study showed that higher muscle mass was associated with better insulin sensitivity and lower risk of pre- or overt diabetes (Srikanthan et al., 2011). Similarly, in a study of over 1100 men, insulin resistance was found to be a central abnormality in the metabolic syndrome, as were lower muscle mass and strength (Atlantis et al., 2009). As proof of principle, increasing the number, size, and function of myofibers in mice specifically resulted in decreased fat mass and increase glucose uptake (Harrison et al., 2008). Undoubtedly, there are several mechanisms that contribute to the developmental origins of adult metabolic disease. A reduction in the functional capacity of muscle to respond to insulin and dispose of glucose might be responsible, in part, for the development of later life obesity and diabetes in formerly IUGR individuals.
3. Potential strategies to improve muscle growth
3.1 Prenatal therapies
Currently, there are limited means of improving either whole body or skeletal muscle-specific growth in the IUGR fetus. Several attempts at improving fetal growth have been made, including bed rest, maternal oxygen delivery, and increasing maternal nutrient delivery (ACOG, 2013). Balanced protein-calorie supplementation to high risk mothers was shown to increase birth weight by 41 grams (Rush et al., 1980); however, whether the additional fetal weight added was in the form of lean mass or adiposity was not determined. Prenatal treatments to improve fetal growth are still worth pursuing, as it was estimated from a cohort of over 13,000 men and women that increasing birth weight and minimizing accelerated postnatal growth could reduce diabetes risk by 57% and the incidence of hypertension and coronary heart disease by 25% and 40%, respectively (Barker et al., 2002). This analysis did not take into account body composition, however, when growth was assessed, as strategies that simply accelerate adipose tissue growth might be counter-productive.
Insights into how fetal skeletal muscle restriction occurs can be determined using a well characterized sheep model of chronic and progressive placental insufficiency (Bell et al., 1987, Brown, 2014) (Figure 3). This model has the same fundamental characteristics of human IUGR (Barry et al., 2008), including elevated resistive indices in the umbilical artery (Galan et al., 2005), brain and heart growth that are relatively preserved (Anthony et al., 2003), and skeletal muscle weights normalized to limb length and brain weight that are reduced (unpublished observations). Fetal hypoxemia progressively becomes severe over gestation in this model (Regnault et al., 2007), with increased production of counter regulatory hormones (Yates et al., 2011). This model affords the capability to use chronic catheterization techniques (Maliszewski et al., 2012) to manipulate substrate and growth factor concentrations for prolonged periods of time during critical periods in myogenesis, including myoblast proliferation and myofiber hypertrophy (Fahey et al., 2005). Coinciding with these critical time periods, the IUGR fetus has reduced circulating insulin and IGF-1 concentrations (Brown et al., 2012, Macko et al., 2013, Thorn et al., 2009) and receives a reduced amino acid supply from the placenta (Brown et al., 2012, Ross et al., 1996) (Figure 3). Effects on fetal skeletal muscle growth specifically can be made by measuring femoral arterial-venous concentration differences, external iliac blood flow (Wilkening et al., 1994), and muscle-specific protein metabolic rates. Promisingly, short term amino acid infusion given directly to the fetus in this model increased protein accretion rates by suppressing protein breakdown rates (Brown et al., 2012).
Figure 3. Proposed mechanisms for reduced fetal skeletal muscle growth during conditions of placental insufficiency.
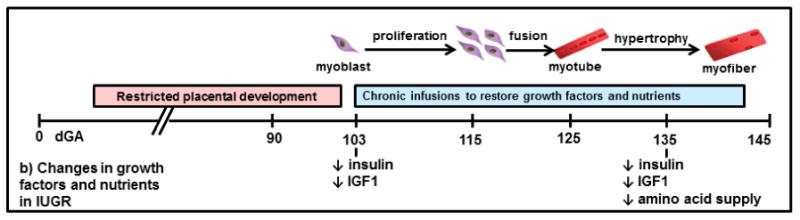
We propose that the combination of decreased growth factors and amino acids in the IUGR fetus leads to reduced rates of myoblast proliferation and myofiber hypertrophy, ultimately producing reductions in skeletal muscle mass. a) Normal fetal myogenesis and b) progressive decline in fetal insulin, IGF-1, and AA supply in a sheep model of placental insufficiency and IUGR. IUGR, intrauterine growth restriction; dGA, days gestation (term=145 dGA); ↔, no change.
3.2 Postnatal nutritional strategies
Until prenatal strategies to improve fetal growth are developed, those neonates who are born after IUGR, especially when born preterm, represent a nutritional challenge. Due to the lack of published data about the nutritional requirements of this population, recommendations for the nutritional management of the IUGR or SGA infant are not distinguished from AGA infants (Tudehope et al., 2013). However, current nutritional practices for the IUGR neonate do not target muscle growth but instead favor fat deposition (Gianni et al., 2009). Very limited data are available to guide the optimization of energy and protein balance in the IUGR infant, and studies are conflicting. Preterm SGA infants have higher metabolic rates and increased energy expenditure when compared to AGA infants in the first month of life (Bohler et al., 1999, Chessex et al., 1984, Davies et al., 1996). Some studies show that SGA infants are more efficient at protein gain (Cauderay et al., 1988, Van Goudoever et al., 1995), and others indicate that SGA infants have deficiencies in protein metabolism (Boehm et al., 1988, Boehm et al., 1998). We do not have a clear understanding of how to best provide nutrition to the IUGR neonate, especially when they are born preterm and require supplemental nutrition either by enteral or parenteral nutrition. It is reasonable to target nutritional strategies that optimize linear and skeletal muscle growth without inducing excess adiposity, such as by increasing protein delivery, and this is a critically important area of future investigation.
4. Conclusions
We have discussed several reasons for how reduced muscle mass in the context of placental insufficiency and IUGR might impact later life metabolic health. Fetal adaptations to placental insufficiency leave skeletal muscle vulnerable to reduced blood flow and nutrient delivery. Several phenotypic features of the IUGR fetus, including lower than normal plasma growth factor concentrations, adversely impact myogenesis. A large number of epidemiological studies consistently demonstrate strong correlations between birthweight and lean mass and muscle strength, which support the concept that reductions in muscle mass established by the intrauterine environment persist throughout the life span. Persistent restrictions in the capacity for muscle growth may contribute to postnatal growth patterns that produce a body composition with less than normal muscle mass and excessive adiposity, or at least a lower muscle-to-fat ratio. Furthermore, skeletal muscle itself facilitates glucose disposal and insulin sensitivity, and therefore reductions in muscle mass could predispose the IUGR individual to glucose intolerance, insulin resistance, and diabetes. Animal studies, in conjunction with human clinical studies, are needed for in depth examination of the mechanisms that restrict fetal and limit postnatal muscle growth, as well as to test nutritional strategies that might prevent the onset of later life metabolic disease.
Highlights.
Placental insufficiency results in redistribution of fetal cardiac output to vital organs at the expense of muscle.
Fetal myoblast proliferation and myotube hypertrophy are reduced in the IUGR fetus.
Birthweight is positively correlated with lean mass and grip strength.
Reduced fetal muscle mass in IUGR may set the trajectory for catch-up growth that favors adiposity.
Nutritional strategies are needed to improve muscle growth and body composition in the IUGR fetus and neonate.
Acknowledgments
Funding: Funding for LDB's work is provided by NIH R01 HD079404 and the University of Colorado Center for Women's Health Research. Funding for WWH Jr's work is provided by NIH T32 HD007186 (PI and PD), NIH K12 HD068372 (PD), NIH R01 DK088139 (Co-I; P. Rozance, PI), and UL1TR001082 (Co-I; R. Sokol, PI).
Footnotes
Declaration of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura D. Brown, Email: laura.brown@ucdenver.edu, Department of Pediatrics, University of Colorado School of Medicine, Anschutz Medical Campus F441, Perinatal Research Center, 13243 East 23rd Avenue, Aurora, CO 80045, Phone: 303-724-0106, Fax: 303-724-0898.
William W. Hay, Email: Bill.Hay@ucdenver.edu, Department of Pediatrics, University of Colorado School of Medicine, Anschutz Medical Campus F441, Perinatal Research Center, 13243 East 23rd Avenue, Aurora, CO 80045, Phone: 303-724-1600, Fax: 303-724-0898.
References
- ACOG. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121:1122–33. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfaffle R, Raile K, Seidel B, Smith RJ, Chernausek SD, Intrauterine Growth Retardation Study, G. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med. 2003;349:2211–22. doi: 10.1056/NEJMoa010107. [DOI] [PubMed] [Google Scholar]
- Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Invest. 2013;36:537–43. doi: 10.3275/8943. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Thamotharan M, Kao D, Devaskar SU, Qiao L, Friedman JE, Hay WW., Jr Effects of acute hyperinsulinemia on insulin signal transduction and glucose transporters in ovine fetal skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R473–81. doi: 10.1152/ajpregu.00405.2004. [DOI] [PubMed] [Google Scholar]
- Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction, Reprod Suppl. 2003;61:183–94. [PubMed] [Google Scholar]
- Arcangeli T, Thilaganathan B, Hooper R, Khan KS, Bhide A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol. 2012;40:267–75. doi: 10.1002/uog.11112. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58:1013–1022. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Baker J, Workman M, Bedrick E, Frey MA, Hurtado M, Pearson O. Brains versus brawn: an empirical test of Barker's brain sparing model. Am J Hum Biol. 2010;22:206–15. doi: 10.1002/ajhb.20979. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. New England Journal of Medicine. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–30. doi: 10.1053/j.semperi.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Bauer R, Walter B, Brust P, Fuchtner F, Zwiener U. Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S40–S49. doi: 10.1016/s0301-2115(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Bayol S, Jones D, Goldspink G, Stickland NC. The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. British Journal of Nutrition. 2004;91:331–339. doi: 10.1079/BJN20031070. [DOI] [PubMed] [Google Scholar]
- Begemann M, Zirn B, Santen G, Wirthgen E, Soellner L, Buttel HM, Schweizer R, van Workum W, Binder G, Eggermann T. Paternally Inherited IGF2 Mutation and Growth Restriction. N Engl J Med. 2015;373:349–56. doi: 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- Bell AW, Wilkening RB, Meschia G. Some Aspects of Placental Function in Chronically Heat-Stressed Ewes. Journal of Developmental Physiology. 1987;9:17–29. [PubMed] [Google Scholar]
- Beltrand J, Verkauskiene R, Nicolescu R, Sibony O, Gaucherand P, Chevenne D, Claris O, Levy-Marchal C. Adaptive changes in neonatal hormonal and metabolic profiles induced by fetal growth restriction. J Clin Endocrinol Metab. 2008;93:4027–32. doi: 10.1210/jc.2008-0562. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley E, Chauhan SP, Abuhamad A. Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol. 2012;206:300–308. doi: 10.1016/j.ajog.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Bieswal F, Ahn MT, Reusens B, Holvoet P, Raes M, Rees WD, Remacle C. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 2006;14:1330–1343. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Boehm G, Senger H, Braun W, Beyreiss K, Raiha NC. Metabolic differences between AGA- and SGA-infants of very low birthweight. I. Relationship to intrauterine growth retardation. Acta Paediatr Scand. 1988;77:19–23. doi: 10.1111/j.1651-2227.1988.tb10591.x. [DOI] [PubMed] [Google Scholar]
- Boehm G, Teichmann B, Jung K, Moro G. Postnatal development of urea synthesis capacity in preterm infants with intrauterine growth retardation. Biol Neonate. 1998;74:1–6. doi: 10.1159/000014004. [DOI] [PubMed] [Google Scholar]
- Bohler T, Kramer T, Janecke AR, Hoffmann GF, Linderkamp O. Increased energy expenditure and fecal fat excretion do not impair weight gain in small-for-gestational-age preterm infants. Early Hum Dev. 1999;54:223–34. doi: 10.1016/s0378-3782(98)00097-8. [DOI] [PubMed] [Google Scholar]
- Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J Endocrinol. 2014;221:R13–29. doi: 10.1530/JOE-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW., Jr Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab. 2009;296:E56–63. doi: 10.1152/ajpendo.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW., Jr Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–64. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert TD, Tamvada KH, Kiyamu M, White DD, Gage TB. Low ponderal index is associated with decreased muscle strength and fatigue resistance in college-aged women. Early Human Development. 2011;87:663–669. doi: 10.1016/j.earlhumdev.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauderay M, Schutz Y, Micheli JL, Calame A, Jequier E. Energy-nitrogen balances and protein turnover in small and appropriate for gestational age low birthweight infants. Eur J Clin Nutr. 1988;42:125–36. [PubMed] [Google Scholar]
- Chessex P, Reichman B, Verellen G, Putet G, Smith JM, Heim T, Swyer PR. Metabolic consequences of intrauterine growth retardation in very low birthweight infants. Pediatr Res. 1984;18:709–713. doi: 10.1203/00006450-198408000-00006. [DOI] [PubMed] [Google Scholar]
- Chomtho S, Wells JC, Williams JE, Lucas A, Fewtrell MS. Associations between birth weight and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;88:1040–8. doi: 10.1093/ajcn/88.4.1040. [DOI] [PubMed] [Google Scholar]
- Claris O, Beltrand J, Levy-Marchal C. Consequences of intrauterine growth and early neonatal catch-up growth. Semin Perinatol. 2010;34:207–10. doi: 10.1053/j.semperi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Costello PM, Rowlerson A, Astaman NA, Anthony FE, Sayer AA, Cooper C, Hanson MA, Green LR. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008;586:2371–9. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PS, Clough H, Bishop NJ, Lucas A, Cole JJ, Cole TJ. Total energy expenditure in small for gestational age infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F46–8. doi: 10.1136/fn.75.1.f46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol. 2007;292:R875–R886. doi: 10.1152/ajpregu.00430.2006. [DOI] [PubMed] [Google Scholar]
- De Jesus LC, Pappas A, Shankaran S, Li L, Das A, Bell EF, Stoll BJ, Laptook AR, Walsh MC, Hale EC, Newman NS, Bara R, Higgins RD. Outcomes of small for gestational age infants born at <27 weeks' gestation. J Pediatr. 2013;163:55–60. doi: 10.1016/j.jpeds.2012.12.097. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Tong J, Zhao J, Underwood KR, Zhu M, Ford SP, Nathanielsz PW. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 2010;88:E51–60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–10. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Dwyer CM, Madgwick AJ, Ward SS, Stickland NC. Effect of maternal undernutrition in early gestation on the development of fetal myofibres in the guinea-pig. Reprod Fertil Dev. 1995;7:1285–92. doi: 10.1071/rd9951285. [DOI] [PubMed] [Google Scholar]
- Dwyer CM, Stickland NC. Does the anatomical location of a muscle affect the influence of undernutrition on muscle fibre number? J Anat. 1992;181(Pt 2):373–6. [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz RA. Early nutritional support and outcomes in ELBW infants. Early Hum Dev. 2010;86(Suppl 1):21–25. doi: 10.1016/j.earlhumdev.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, fat-free mass and resting metabolic rate in adult life. Horm Metab Res. 2002;34:72–6. doi: 10.1055/s-2002-20518. [DOI] [PubMed] [Google Scholar]
- Fahey AJ, Brameld JM, Parr T, Buttery PJ. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J Anim Sci. 2005;83:2564–2571. doi: 10.2527/2005.83112564x. [DOI] [PubMed] [Google Scholar]
- Fahey AJ, Brameld JM, Parr T, Buttery PJ. Ontogeny of factors associated with proliferation and differentiation of muscle in the ovine fetus. J Anim Sci. 2005;83:2330–8. doi: 10.2527/2005.83102330x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–73. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA, Mangiacapra FJ. IGFs and muscle differentiation. Adv Exp Med Biol. 1993;343:319–26. doi: 10.1007/978-1-4615-2988-0_31. [DOI] [PubMed] [Google Scholar]
- Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85:1285–94. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Hughes P, Comline RS. The effects of insulin on the growth rate of the sheep fetus during late gestation. Q J Exp Physiol. 1989;74:703–14. doi: 10.1113/expphysiol.1989.sp003322. [DOI] [PubMed] [Google Scholar]
- Galan HL, Anthony RV, Rigano S, Parker TA, de Vrijer B, Ferrazzi E, Wilkening RB, Regnault TR. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol. 2005;192:272–9. doi: 10.1016/j.ajog.2004.05.088. [DOI] [PubMed] [Google Scholar]
- Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001;86:267–72. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- Gianni ML, Roggero P, Taroni F, Liotto N, Piemontese P, Mosca F. Adiposity in small for gestational age preterm infants assessed at term equivalent age. Arch Dis Child Fetal Neonatal Ed. 2009;94:F368–72. doi: 10.1136/adc.2008.153163. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Bell AW. Consequences of intra-uterine growth retardation for postnatal growth, metabolism and pathophysiology. Reprod Suppl. 2003;61:195–206. [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J Anim Sci. 2000;78:50–61. doi: 10.2527/2000.78150x. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Slepetis RM, Hermanson JW, Bell AW. Intrauterine growth retardation is associated with reduced cell cycle activity, but not myofibre number, in ovine fetal muscle. Reprod Fertil Dev. 1999;11:281–291. doi: 10.1071/rd99054. [DOI] [PubMed] [Google Scholar]
- Guellec I, Marret S, Baud O, Cambonie G, Lapillonne A, Roze JC, Fresson J, Flamant C, Charkaluk ML, Arnaud C, Ancel PY. Intrauterine Growth Restriction, Head Size at Birth, and Outcome in Very Preterm Infants. J Pediatr. 2015;167:975–981. doi: 10.1016/j.jpeds.2015.08.025. e2. [DOI] [PubMed] [Google Scholar]
- Harrison BC, Leinwand LA. Fighting fat with muscle: bulking up to slim down. Cell Metab. 2008;7:97–98. doi: 10.1016/j.cmet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics. 1998;102:E60. doi: 10.1542/peds.102.5.e60. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91:2153–8. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- Jensen CB, Martin-Gronert MS, Storgaard H, Madsbad S, Vaag A, Ozanne SE. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. PLoS One. 2008;3:e3738. doi: 10.1371/journal.pone.0003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, Ruest S, Barry K, Otis JP, Patti ME. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–84. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- Jimenez-Chillaron JC, Patti ME. To catch up or not to catch up: is this the question? Lessons from animal models. Curr Opin Endocrinol Diabetes Obes. 2007;14:23–29. doi: 10.1097/MED.0b013e328013da8e. [DOI] [PubMed] [Google Scholar]
- Joglekar CV, Fall CH, Deshpande VU, Joshi N, Bhalerao A, Solat V, Deokar TM, Chougule SD, Leary SD, Osmond C, Yajnik CS. Newborn size, infant and childhood growth, and body composition and cardiovascular disease risk factors at the age of 6 years: the Pune Maternal Nutrition Study. Int J Obes (Lond) 2007;31:1534–44. doi: 10.1038/sj.ijo.0803679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm Birth and Body Composition at Term Equivalent Age: A Systematic Review and Meta-analysis. Pediatrics. 2012;130:E640–E649. doi: 10.1542/peds.2011-3379. [DOI] [PubMed] [Google Scholar]
- Jou MY, Lonnerdal B, Griffin IJ. Effects of early postnatal growth restriction and subsequent catch-up growth on body composition, insulin sensitivity, and behavior in neonatal rats. Pediatr Res. 2013;73:596–601. doi: 10.1038/pr.2013.27. [DOI] [PubMed] [Google Scholar]
- Kahn HS, Narayan KM, Williamson DF, Valdez R. Relation of birth weight to lean and fat thigh tissue in young men. Int J Obes Relat Metab Disord. 2000;24:667–72. doi: 10.1038/sj.ijo.0801211. [DOI] [PubMed] [Google Scholar]
- Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38:733–9. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82:980–987. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- Kramer MS, McLean FH, Olivier M, Willis DM, Usher RH. Body proportionality and head and length ‘sparing' in growth-retarded neonates: a critical reappraisal. Pediatrics. 1989;84:717–23. [PubMed] [Google Scholar]
- Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997;86:196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Larciprete G, Valensise H, Di Pierro G, Vasapollo B, Casalino B, Arduini D, Jarvis S, Cirese E. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet Gynecol. 2005;26:258–62. doi: 10.1002/uog.1980. [DOI] [PubMed] [Google Scholar]
- Lemons JA, Ridenour R, Orsini EN. Congenital absence of the pancreas and intrauterine growth retardation. Pediatrics. 1979;64:255–257. [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young adult men--a prospective twin study. Int J Obes Relat Metab Disord. 2001;25:1537–45. doi: 10.1038/sj.ijo.0801743. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young women: a prospective twin study. Am J Clin Nutr. 2002;75:676–82. doi: 10.1093/ajcn/75.4.676. [DOI] [PubMed] [Google Scholar]
- Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Intellectual and psychological performance in males born small for gestational age with and without catch-up growth. Pediatr Res. 2001;50:91–6. doi: 10.1203/00006450-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Macko AR, Yates DT, Chen X, Green AS, Kelly AC, Brown LD, Limesand SW. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced beta-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. Journal of Developmental Origins of Health and Disease. 2013;4:402–410. doi: 10.1017/S2040174413000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliszewski AM, Gadhia MM, O'Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab. 2012;302:E1483–92. doi: 10.1152/ajpendo.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM, Clemens TL. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–20. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoard SA, McNabb WC, Birtles MJ, Harris PM, McCutcheon SN, Peterson SW. Immunohistochemical detection of myogenic cells in muscles of fetal and neonatal lambs. Cells Tissues Organs. 2001;169:21–33. doi: 10.1159/000047857. [DOI] [PubMed] [Google Scholar]
- McLennan IS. Neurogenic and myogenic regulation of skeletal muscle formation: a critical re-evaluation. Prog Neurobiol. 1994;44:119–40. doi: 10.1016/0301-0082(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25:669–77. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- Moura-Dos-Santos M, Wellington-Barros J, Brito-Almeida M, Manhaes-de-Castro R, Maia J, Gois Leandro C. Permanent deficits in handgrip strength and running speed performance in low birth weight children. Am J Hum Biol. 2013;25:58–62. doi: 10.1002/ajhb.22341. [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Does lean rather than fat mass provide the link between birth weight, BMI, and metabolic risk? EarlyBird 23. Pediatr Diabetes. 2006;7:211–4. doi: 10.1111/j.1399-5448.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- O'Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–9. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- O'Neill ED, Wilding JP, Kahn CR, Van Remmen H, McArdle A, Jackson MJ, Close GL. Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: evidence for decreased protein synthesis and not increased degradation. Age (Dordr) 2010;32:209–22. doi: 10.1007/s11357-009-9125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK. Catch-up growth in small for gestational age babies: good or bad? Curr Opin Endocrinol Diabetes Obes. 2007;14:30–4. doi: 10.1097/MED.0b013e328013da6c. [DOI] [PubMed] [Google Scholar]
- Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–8. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- Ortega FB, Labayen I, Ruiz JR, Martin-Matillas M, Vicente-Rodriguez G, Redondo C, Warnberg J, Gutierrez A, Sjostrom M, Castillo MJ, Moreno LA. Are muscular and cardiovascular fitness partially programmed at birth? Role of body composition. J Pediatr. 2009;154:61–66. doi: 10.1016/j.jpeds.2008.07.041. e1. [DOI] [PubMed] [Google Scholar]
- Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on ovine fetal growth. J Endocrinol. 2002;173:131–41. doi: 10.1677/joe.0.1730131. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005;48:547–552. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- Padoan A, Rigano S, Ferrazzi E, Beaty BL, Battaglia FC, Galan HL. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am J Obstet Gynecol. 2004;191:1459–64. doi: 10.1016/j.ajog.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Patel HP, Jameson KA, Syddall HE, Martin HJ, Stewart CE, Cooper C, Sayer AA. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J Gerontol A Biol Sci Med Sci. 2012;67:82–7. doi: 10.1093/gerona/glr020. [DOI] [PubMed] [Google Scholar]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–17. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol. 1993;1985;75:1044–52. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- Ramel SE, Brown LD, Georgieff MK. The Impact of Neonatal Illness on Nutritional Requirements-One Size Does Not Fit All. Curr Pediatr Rep. 2014;2:248–254. doi: 10.1007/s40124-014-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men, Journals of Gerontology Series a- Biological Sciences and Medical Sciences. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta. 2007;28:714–723. doi: 10.1016/j.placenta.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ren HX, Accili D, Duan CM. Hypoxia converts the myogenic action of insulin-like growth factors into mitogenic action by differentially regulating multiple signaling pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5857–5862. doi: 10.1073/pnas.0909570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero NB, Mezmezian M, Fidzianska A. Main steps of skeletal muscle development in the human: morphological analysis and ultrastructural characteristics of developing human muscle. Handb Clin Neurol. 2013;113:1299–310. doi: 10.1016/B978-0-444-59565-2.00002-2. [DOI] [PubMed] [Google Scholar]
- Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol. 1996;270:E491–503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- Rowe RW, Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. J Anat. 1969;104:519–30. [PMC free article] [PubMed] [Google Scholar]
- Rozance PJ, Brown LD, Thorn SR, Anderson MS, Hay WW., Jr . Avery's Neonatology: Pathophysiology and Management of the Newborn 7th ed. Wolters Kluwer; 2016. Intrauterine Growth Restriction and Small-For-Gestational-Age Infant. [Google Scholar]
- Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics. 1980;65:683–697. [PubMed] [Google Scholar]
- Sachdev HS, Fall CH, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, Reddy KS, Barker DJ, Bhargava SK. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–32. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer AA, Syddall HE, Dennison EM, Gilbody HJ, Duggleby SL, Cooper C, Barker DJ, Phillips DI. Birth weight, weight at 1 y of age, and body composition in older men: findings from the Hertfordshire Cohort Study. Am J Clin Nutr. 2004;80:199–203. doi: 10.1093/ajcn/80.1.199. [DOI] [PubMed] [Google Scholar]
- Shen W, Boyle DW, Liechty EA. Changes in 4E-BP1 and p70S6K phosphorylation in skeletal muscle of the ovine fetus after prolonged maternal fasting: effects of insulin and IGF-I. Pediatr Res. 2005;58:833–9. doi: 10.1203/01.PDR.0000182588.20368.12. [DOI] [PubMed] [Google Scholar]
- Shen W, Mallon D, Boyle DW, Liechty EA. IGF-I and insulin regulate eIF4F formation by different mechanisms in muscle and liver in the ovine fetus. Am J Physiol Endocrinol Metab. 2002;283:E593–603. doi: 10.1152/ajpendo.00570.2001. [DOI] [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–30. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- Skidmore PM, Cassidy A, Swaminathan R, Richards JB, Mangino M, Spector TD, MacGregor AJ. An obesogenic postnatal environment is more important than the fetal environment for the development of adult adiposity: a study of female twins. Am J Clin Nutr. 2009;90:401–6. doi: 10.3945/ajcn.2008.27269. [DOI] [PubMed] [Google Scholar]
- Srikanthan P, Karlamangla AS. Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. Journal of Clinical Endocrinology & Metabolism. 2011;96:2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- Stickland NC. Muscle development in the human fetus as exemplified by m. sartorius: a quantitative study. J Anat. 1981;132:557–79. [PMC free article] [PubMed] [Google Scholar]
- Stickland NC, Widdowson EM, Goldspink G. Effects of severe energy and protein deficiencies on the fibres and nuclei in skeletal muscle of pigs. Br J Nutr. 1975;34:421–8. doi: 10.1017/s0007114575000487. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatric Research. 2012;71:324–331. doi: 10.1038/pr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchirikov M, Rybakowski C, Huneke B, Schroder HJ. Blood flow through the ductus venosus in singleton and multifetal pregnancies and in fetuses with intrauterine growth retardation. American Journal of Obstetrics and Gynecology. 1998;178:943–949. doi: 10.1016/s0002-9378(98)70528-9. [DOI] [PubMed] [Google Scholar]
- Tchirikov M, Schroder HJ, Hecher K. Ductus venosus shunting in the fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet Gynecol. 2006;27:452–61. doi: 10.1002/uog.2747. [DOI] [PubMed] [Google Scholar]
- Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–3030. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft PB, Leth H, Ring PB, Peitersen B, Lou HC, Henriksen O. Volumetric analysis of the normal infant brain and in intrauterine growth retardation. Early Hum Dev. 1995;43:15–29. doi: 10.1016/0378-3782(95)01657-o. [DOI] [PubMed] [Google Scholar]
- Tudehope D, Vento M, Bhutta Z, Pachi P. Nutritional requirements and feeding recommendations for small for gestational age infants. J Pediatr. 2013;162:S81–9. doi: 10.1016/j.jpeds.2012.11.057. [DOI] [PubMed] [Google Scholar]
- Van Goudoever JB, Sulkers EJ, Halliday D, Degenhart HJ, Carnielli VP, Wattimena JL, Sauer PJ. Whole-body protein turnover in preterm appropriate for gestational age and small for gestational age infants: comparison of [15N]glycine and [1-(13)C]leucine administered simultaneously. Pediatr Res. 1995;37:381–8. doi: 10.1203/00006450-199504000-00001. [DOI] [PubMed] [Google Scholar]
- von Beckerath AK, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol. 2013;208:130, e1–6. doi: 10.1016/j.ajog.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Wang H, Noulet F, Edom-Vovard F, Tozer S, Le GF, Duprez D. Bmp signaling at the tips of skeletal muscles regulates the number of fetal muscle progenitors and satellite cells during development. Dev Cell. 2010;18:643–654. doi: 10.1016/j.devcel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Wang YX, Dumont NA, Rudnicki MA. Muscle stem cells at a glance. J Cell Sci. 2014;127:4543–8. doi: 10.1242/jcs.151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–26. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de R, Sr, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsen T, Grill V, Gudnason V, Hulman S, Hypponen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- White RB, Bierinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol. 2010;10:21. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson EM, Crabb DE, Milner RD. Cellular development of some human organs before birth. Arch Dis Child. 1972;47:652–5. doi: 10.1136/adc.47.254.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat. 1983;137(Pt 2):235–45. [PMC free article] [PubMed] [Google Scholar]
- Wilkening RB, Boyle DW, Teng C, Meschia G, Battaglia FC. Amino acid uptake by the fetal ovine hindlimb under normal and euglycemic hyperinsulinemic states. Am J Physiol. 1994;266:E72–8. doi: 10.1152/ajpendo.1994.266.1.E72. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, McEwan JC, Sheard PW, Harris AJ. Early stages of myogenesis in a large mammal: formation of successive generations of myotubes in sheep tibialis cranialis muscle. J Muscle Res Cell Motil. 1992;13:534–50. doi: 10.1007/BF01737996. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Ross JJ, Harris AJ. A critical period for formation of secondary myotubes defined by prenatal undernourishment in rats. Development. 1988;102:815–821. doi: 10.1242/dev.102.4.815. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–7. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc. 2004;63:387–96. doi: 10.1079/pns2004365. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhu MJ, Dodson MV, Du M. Developmental programming of fetal skeletal muscle and adipose tissue development. J Genomics. 2013;1:29–38. doi: 10.7150/jgen.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates DT, Clarke DS, Macko AR, Anderson MJ, Shelton LA, Nearing M, Allen RE, Rhoads RP, Limesand SW. Myoblasts from intrauterine growth-restricted sheep fetuses exhibit intrinsic deficiencies in proliferation that contribute to smaller semitendinosus myofibres. J Physiol. 2014;592:3113–25. doi: 10.1113/jphysiol.2014.272591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates DT, Green AS, Limesand SW. Catecholamines Mediate Multiple Fetal Adaptations during Placental Insufficiency That Contribute to Intrauterine Growth Restriction: Lessons from Hyperthermic Sheep. J Pregnancy. 2011;2011:740408. doi: 10.1155/2011/740408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KI, Chang MH. Growth and body composition of preterm, small-for-gestational-age infants at a postmenstrual age of 37-40 weeks. Early Hum Dev. 1993;33:117–131. doi: 10.1016/0378-3782(93)90207-b. [DOI] [PubMed] [Google Scholar]
- Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond) 2007;31:1392–9. doi: 10.1038/sj.ijo.0803612. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold, Journal of Histochemistry &. Cytochemistry. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]


