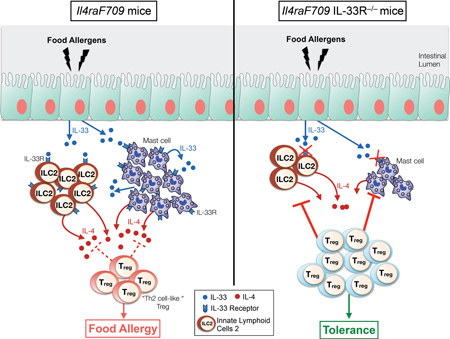
Abstract
Background
Food allergy is a major health issue, but its pathogenesis remains obscure. Group 2 innate lymphoid cells (ILC2) promote allergic inflammation. However their role in food allergy is largely unknown.
Objective
We sought to investigate the role of ILC2 in food allergy.
Methods
Food allergy-prone mice with a gain of function mutation in the IL-4 receptor a chain (Il4raF709) were orally sensitized with food allergens and the ILC2 compartment was analyzed. The requirement for ILC2 in food allergy was investigated using Il4raF709, IL-33 receptor deficient (Il1rl1−/−), IL-13 (Il13−/−) and IL-4 (Il4−/−) deficient mice and by adoptive transfers of in vitro-expanded ILC2. Direct effects of ILC2 on regulatory T (Treg) cells and mast cells were analyzed in co-culture experiments. Treg cell control of ILC2 was assessed in vitro and in vivo.
Results
Food allergic Il4raF709 mice exhibit increased numbers of ILC2. IL-4 secretion by ILC2 contributes to the allergic response by reducing allergen-specific Treg cells and activating mast cells. IL-33 receptor deficiency in Il4raF709Il1rl1−/− mice protects against allergen sensitization and anaphylaxis while reducing ILC2 induction. Adoptive transfer of WT and Il13−/− but not Il4−/− ILC2 restored sensitization in Il4raF709 Il1rl1−/− mice. Treg cells suppress ILC2 in vitro and in vivo.
Conclusion
IL-4 production by IL-33-stimulated ILC2 blocks the generation of allergen-specific Treg cells and favors food allergy. Strategies to block ILC2 activation or the IL-33:IL-33R pathway may lead to innovative therapies in food allergy.
Keywords: Anaphylaxis, Food Allergy, IL-4, IL-13, IL-33, ILC2, Innate Lymphoid Cells, Nuocytes, Mast Cells, Regulatory T Cells, Oral Tolerance
Graphical Abstract
Capsule summary
IL-4 production by ILC2 plays a crucial role in enabling sensitization to food allergens.
Introduction
Food allergy is a major health issue in westernized countries with increasing prevalence over the last decades and a lack of curative treatment 1. Several major food staples, including milk, soy and wheat can induce food allergic reactions 2. Food allergies reflect a failure of oral tolerance of innocuous food allergens, resulting in the development of a dysregulated Th2-immune response, the secretion of allergen specific-IgE and the recruitment of effector cells to the gastrointestinal (GI) tract. By their production of Th2 cytokines, mostly IL-4 and IL-13, mast cells, basophils and Type 2 helper CD4+ T (Th2) cells are key effector cells of the allergic reaction. Their Th2 cytokine production amplifies the allergic immune response by blocking the induction of allergen-specific regulatory T cells (Treg), directing further Th2 cell differentiation and IgE-driven mast cell activation 3,4. The sequence of early events in food allergen sensitization and the involvement of innate immune cells in triggering disease in susceptible individuals remain to be fully elucidated.
A population of innate mucosal Th2 cytokine-secreting cells, called group 2 innate lymphoid cells (ILC2), has emerged as central to a broad collection of allergic disorders such as asthma, chronic rhinosinusitis, and atopic dermatitis 5–11. ILC2 are characterized by the expression of CD90 (Thy1), CD25 (IL-2Rα), IL-25R (IL-17RB), IL-33R (ST2) and CD127 (IL-7Rα) in the absence of specific markers that define other leukocytes lineages (Lin−; non-T/non-B cells) 12. They share morphological characteristics with lymphocytes, but do not express the rearranged antigen receptors characteristic of adaptive immune cells 13,14. ILC2 emerge from a common lymphoid progenitor present in the bone marrow and their development is critically dependent on the transcription factors Id2 12,15, RORα 16,17 and the Th2-specific transcription factor GATA-3 8,18. ILC2 are mostly found at the barrier surfaces, including skin, lung and the gastrointestinal tract 13. In response to cytokines produced by innate immune sources, such as TSLP, IL-25 and IL-33, ILC2 rapidly expand at the mucosal surfaces in an innate, antigen-independent manner and produce large quantities of Th2 cytokines especially IL-5 and IL-13 but also IL-4 and IL-98,19–22.
One of the key issues in food allergy is the impaired induction of allergen-specific Treg cell and their subversion into non-functional Th2 cell-like Treg cells that further promote disease 3. The cellular and molecular processes linking Th2 effector cells to the defective regulatory immune response during food allergy are still not well understood. The role of ILC2 in disrupting tolerance in food allergy remains similarly obscure, with conflicting reports on the requirement for ILC2 in food allergen sensitization 23,24.
In this study, we have made use of a well characterized murine transgenic model of food allergy involving a gain of function in the IL-4Rα chain to investigate the role of ILC2 during food allergy 3,4,25,26. We found that ILC2 play a critical role in the induction of food allergy by virtue of their secretion of IL-4, which acts to enhance mucosal mast cell activation and disrupt allergen-specific Treg cell induction and suppressive functions.
Methods
Sensitization and challenge protocol
Mice were treated intragastrically once a week over 4 weeks with either PBS or with 23 mg of Peanut (PE) butter (Skippy, Hormel Foods), corresponding to 5 mg of peanut protein, suspended in 250 µl of 0.1 M sodium bicarbonate (pH 8.0), as previously described 4. For ovalbumin (OVA) sensitization, OVA (250µg) together with 10 µg of staphylococcal enterotoxin B (SEB, Toxin Technology) was gavaged once weekly for 8 weeks. On the ninth week, mice were challenged intragastrically with either 450 mg peanut butter (~ 100 mg of protein) or 150 mg of OVA as previously described 3,26. WT and DO11.10+ Rag2−/− Il4raF709 Foxp3EGFP mice were fed with 1% OVA in drinking water for 7 days followed by oral gavage every 2 days with 250 µg OVA the following week. Anaphylaxis was assessed by measuring changes in total body core temperature with transponders placed subcutaneously 2 days before challenge (IPTT-300; Bio Medic Data Systems) and a DAS-6001 console (Bio Medic Data Systems).
In vitro ILC2 and ILC3 culture and cell purification
Lamina propria lymphocytes from IL-13YFPCre mice were isolated as previously described and incubated at 37°C in complete RPMI medium supplemented with IL-33 (10ng/ml, Biolegend), IL-25 (10ng/ml, Shenandoah Biotechnology) and IL-2 (5ng/ml, Shenandoah Biotechnology) for a week 27. The ILC were then sorted on a BD FACSAria II based on IL-13YFP expression and Lin− Thy1+ Sca-1+ c-Kitlow for ILC2 and the lack of IL-13YFP expression and Lin− Thy1+ Sca-1− c-Kit+ for ILC3. Cells were subsequently maintained in cultured with IL-2, IL-25 and IL-33 for 3–4 weeks.
ILC adoptive transfer
ILC were expanded in vitro from the lamina propria of WT, Il13−/− or Il4−/− mice and cell-sorted as mentioned above. Il4raF709 Il1rl1−/− mice were injected intravenously through the retro-orbital sinus with 2×105 ILC each week the day prior to sensitization.
Co-culture experiments
Bone marrow mast cells (BMMC) were prepared as described and cultured overnight at 105 cells/well with ILC2 at 20:1 ratio in conical 96 well plates in the presence of IL-2 (500 pg/ml), IL-3 (2 ng/ml), and IgE anti-DNP (5 ng/ml, SPE-7, Sigma) 4. IL-4 (10 ng/ml, Shenandoah Biotech) or anti-IL-4 (40 µg/ml, 11B11) was supplemented in some cases. BMMC were stimulated for 10 min with 50 ng/ml DNP-albumin (Sigma Aldrich) in the presence of anti-LAMP-1-eFluor 660 (eBio1D4B, eBioscience), anti-c-Kit-PE-Cy7 and fixable viability dye eFluor 780 to detect degranulation. CD4+ T cells (105) from WT DO11.10+ Rag2−/− Foxp3EGFP mice were sorted to >98% purity using Miltenyi CD4 microbeads and labeled with the CellTrace Violet dye (Life Technologies). Naïve T cells were then cultured with ILC2 at a 20:1 ratio in round bottom 96 well plates coated with 1 µg/ml anti-CD3 and 5 µg/ml anti-CD28. Recombinant human TGF-β1 (2 ng/ml) and IL-2 (1 ng/ml) were added to promote Foxp3 induction.
Statistical analysis
Anaphylaxis temperature curves were analyzed using 2-way ANOVA. Student’s unpaired two-tailed t tests were used for 2 groups comparisons. For more than 2 groups, 1-way ANOVA with Bonferroni post-test analysis was used. Results are presented as means and SEM where each point represents one sample. In cases where values were spread across multiple orders of magnitude, data were log-transformed for analysis with parametric tests.
Study approval
All animal studies were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee.
Other Methods
Information on mouse strains employed, flow cytometry and related antibodies, reagents and methods, real time PCR analysis, IgE and cytokine ELISAs, tissue histology and TGF-β-mediated in vitro induced Treg (iTreg) cell induction is provided in the Methods section in this article’s Online Repository at www.jacionline.org.
Results
ILC2 enrichment in orally sensitized Il4raF709 mice
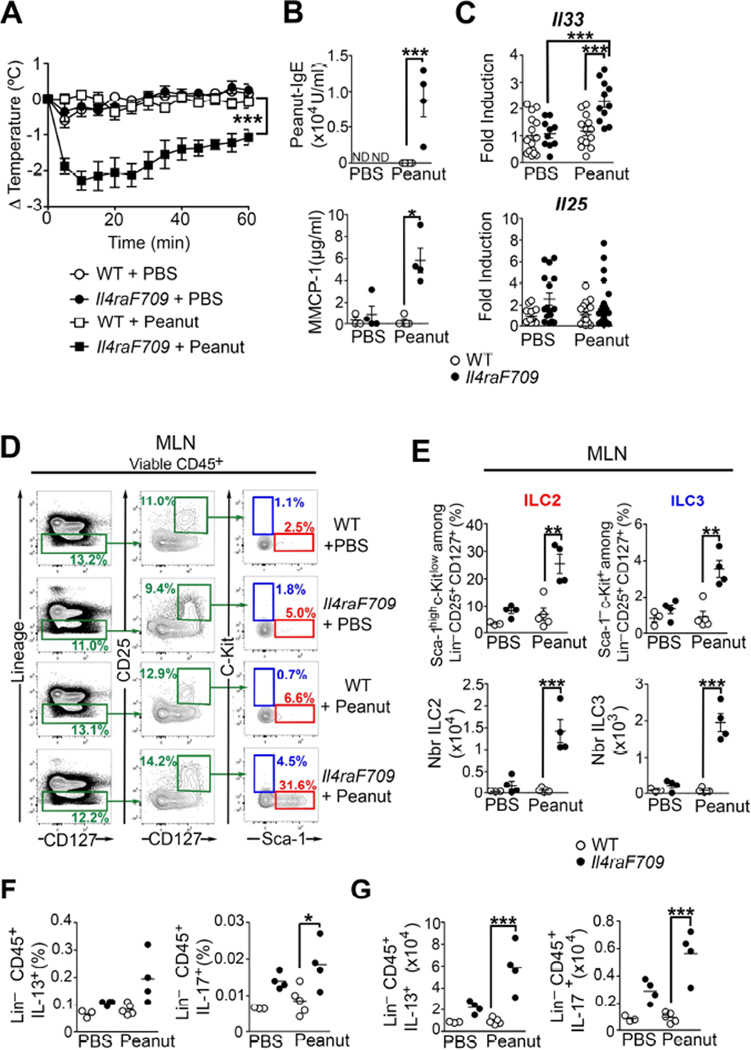
Il4raF709 mice carry an IL-4Rα chain mutation that inactivates the receptor’s immunotyrosine inhibitory motif. This mutation results in augmented signal transducer and activator of transcription 6 (STAT6) activation by IL-4 and IL-13, and renders the mice prone to oral allergic sensitization 25,26. Il4raF709, but not WT mice, are particularly susceptible to oral sensitization with food allergens, such as peanut or chicken egg ovalbumin (OVA) and respond to oral challenge by a mast cell- and IgE-dependent anaphylactic response 3,4,28. Peanut or OVA-sensitized Il4raF709 mice, but not WT controls, developed an anaphylactic reaction after oral challenge as evidenced by a significant drop in their core body temperature (Fig 1, A and Fig E1, A in this article’s Online Repository). Food allergic Il4raF709 mice produced peanut-and OVA-specific IgE antibodies, and they released mouse mast cell protease-1 (MMCP-1), a marker of mast cell degranulation, upon allergen-sensitization and challenge (Fig 1, B and Fig E1, B and C in this article’s Online Repository). mRNA transcripts of Il33, whose production by epithelial cells promotes ILC2 differentiation, were significantly higher in the small intestine (SI) of Il4raF709 mice following allergen sensitization and challenge (Fig 1, C and Fig E1, D in this article’s Online Repository). Accordingly, we analyzed the presence of ILC2 and ILC3 by flow cytometry in allergen-sensitized WT and Il4raF709 mice. ILC2 and ILC3 identification in the draining mesenteric lymph nodes (MLN) of orally sensitized mice was performed by analysis of their specific patterns of cell-surface markers: Lin− CD45+ CD25+ CD127+ and either Sca-1high c-Kitlow (ILC2) or Sca-1− c-Kit+ (ILC3) as previously described (Fig 1, D and Fig E1, E in this article’s Online Repository) 16,29. Compared to PBS-treated mice, the frequencies and absolute numbers of ILC2 and ILC3 populations were higher in the MLN of allergen-sensitized Il4raF709 mice (Fig 1, E and Fig E1, F in this article’s Online Repository). Compared to OVA-sensitized WT mice, increased ILC2 frequencies and absolute numbers were also observed in the SI lamina propria of OVA-sensitized Il4raF709 mice (Fig E1, G and H in this article’s Online Repository). Similar data were obtained using intracellular cytokine staining to define ILC2 as CD45+Lin−IL-13+ and ILC3 as CD45+ Lin− IL-17+ (Fig 1, E).
Figure 1. ILC2 are enriched in peanut-sensitized Il4raF709 mice.
(A) Core body temperature changes in PBS or peanut-sensitized WT and Il4raF709 mice after oral peanut challenge. (B) Serum concentrations of peanut-specific IgE and MMCP-1 after anaphylaxis. (C) Il33 and Il25 mRNA levels in the SI of PBS and Peanut-sensitized WT and Il4raF709 mice. (D – E) Flow cytometric analysis (D), frequencies and absolute numbers (E) of ILC2 (Lin− CD25+ CD127+ Sca-1high c-Kitlow) and ILC3 (Lin− CD25+ CD127+ Sca-1− c-Kit+) in the MLN. (F and G), Frequencies and absolute numbers of IL-13+ ILC2 and IL-17+ ILC3. Results represent data on 3 to 15 mice per group derived from two independent experiments. *P < .05, **P < 0.01 and ***P < 0.001 by two-way ANOVA and one-way ANOVA with post-test analysis.
IL-33 receptor (IL-33R) signaling regulates ILC2 expansion and allergic sensitization
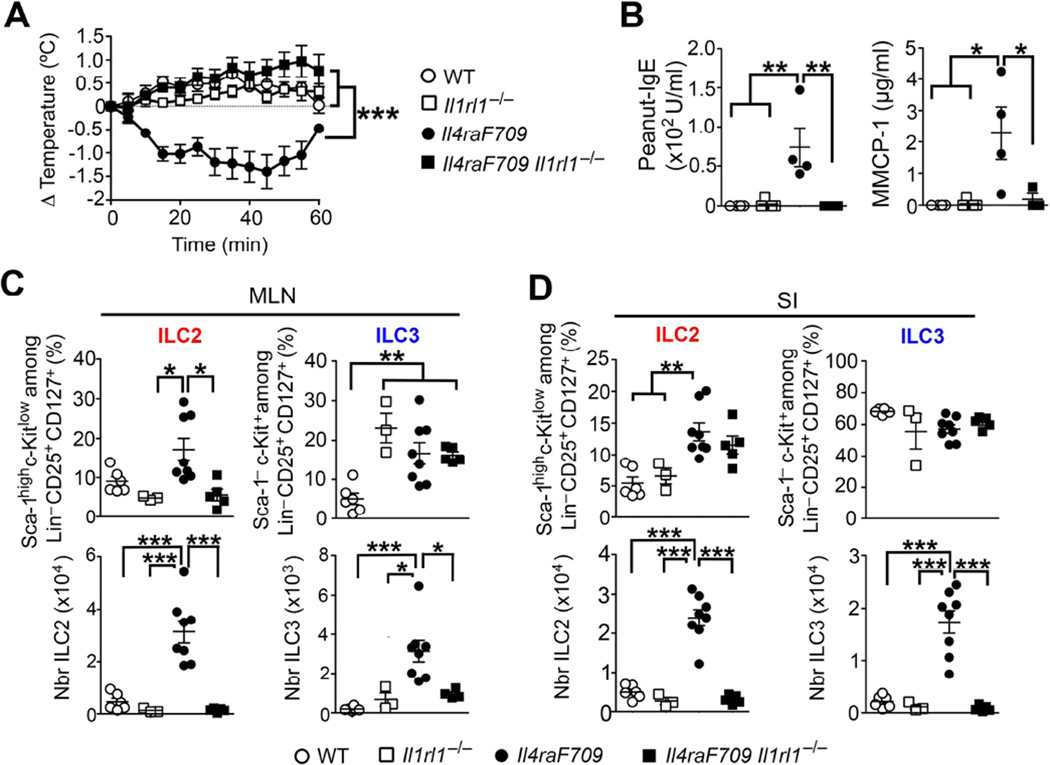
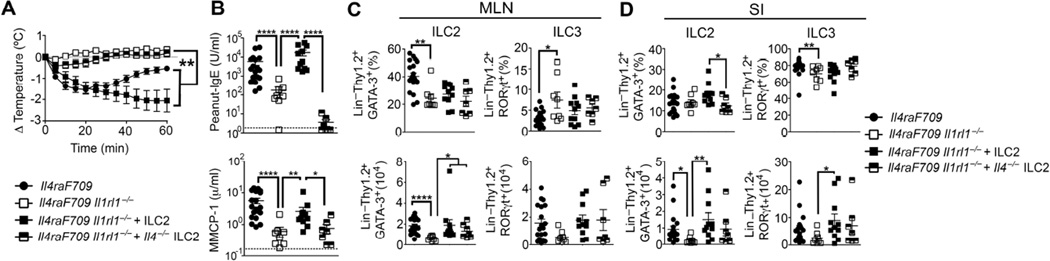
IL-33 is a key cytokine that promotes the growth and maturation of ILC2 and their consequent Th2 cytokine production 29. IL-33 signaling through IL-33R, encoded by Il1rl1, and its associated IL-1 receptor accessory protein is implicated in airway inflammation after influenza virus infection and in several murine models of asthma 7,30,31. Because Il33 mRNA levels are increased in the SI of allergen-sensitized Il4raF709 (Fig 1, C and Fig E1, D in this article’s Online Repository), we investigated the dependence of allergic-sensitization driven by ILC2 expansion on IL-33R signaling. Surprisingly, Il4raF709 Il1rl1−/− mice failed to sensitize to peanut, exhibiting no decrease in core body temperature, a lack of peanut-IgE production and minimal release of MMCP-1 after oral challenge (Fig 2, A and B). Flow cytometric analysis of ILC populations by means of the cellular markers Sca-1 and c-Kit revealed reduced ILC2 frequencies and absolute numbers in the MLN and SI lamina propria of allergen-sensitized Il4raF709 Il1rl1−/− mice (Fig 2, C and D).
Figure 2. Il1rl1 signaling drives ILC2 expansion and peanut allergy.
(A) Core body temperature change in peanut-sensitized WT, Il1rl1−/−, Il4raF709 and Il4raF709 Il1rl1−/− mice after oral challenge. (B) Serum concentrations of peanut-specific IgE and MMCP-1 after anaphylaxis. (C –D) Frequencies and absolute numbers of ILC2 and ILC3 in the MLN (C) and SI (D) from the mouse groups in (A). Results represent 3 to 8 mice per group derived from two independent experiments; *P < .05, **P < .01 and ***P < .001 by two-way ANOVA and one-way ANOVA with post-test analysis.
GATA-3 is an essential transcription factor for ILC2 and its expression can be used to identify those cells by flow cytometry, as previously published 8,32. By using the following markers, Lin− CD45+ Thy1.2+ GATA-3+ and Lin− CD45+ Thy1.2+ RORγt+ to identify ILC2 and ILC3 respectively, we confirmed that ILC2 frequencies and absolute numbers are increased in the MLN and SI lamina propria of allergen-sensitized Il4raF709 mice and that Il1rl1 deficiency significantly reduced those numbers (Fig E2, A to C in this article’s Online Repository).
ILC2-derived IL-4 is indispensible for allergic sensitization
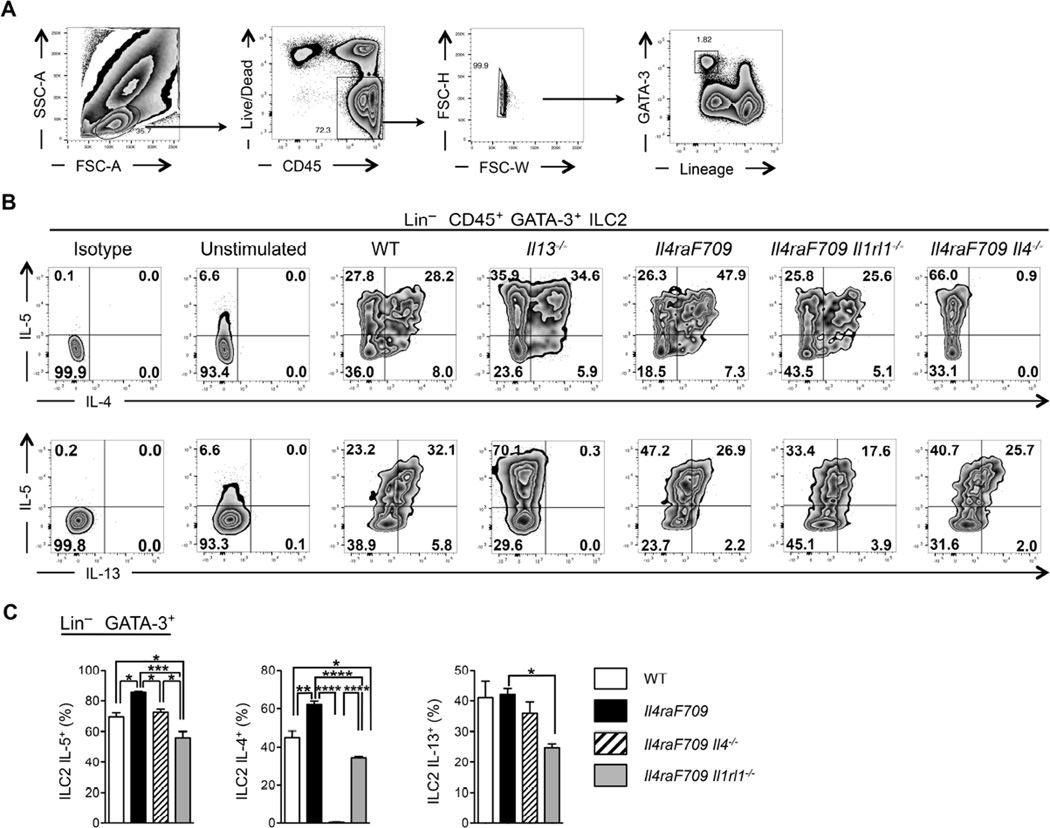
Following activation with IL-2, IL-25 or IL-33, ILC2 secrete large amounts of Th2 cytokines, such as IL-13 and IL-5, and it has become dogma that ILC2 produce minimal quantities of IL-4 15,33,34. Flow cytometric analysis of in vitro expanded ILC2 derived from SI lamina propria of unsensitized WT, Il13−/−, Il4raF709, Il4raF709 Il4−/− and Il4raF709 Il1rl1−/− mice demonstrates that ILC2 identified by the expression of the transcription factor GATA-3 (Lin− Thy1.2+ CD45+ GATA-3+) express IL-5, IL-13 and copious amounts of IL-4 (Fig 3, A to C). In contrast, IL-9 expression by ILC2 was minimal (data not shown). IL-4 expression by MLN and SI ILC2 was further confirmed by using reporter mice expressing the enhanced green fluorescent protein under control of the Il4 locus (4Get mice) (Fig E3, A and B in this article’s Online Repository) 35. Il1rl1 deficiency strongly reduced the production of IL-4, IL-13 and IL-5 by intestinal ILC2, even after peanut oral sensitization (Fig E4, A to C in this article’s Online Repository). Il4 deletion in Il4raF709 mice did not affect IL-13 production by intestinal ILC2 whereas it completely abolished their IL-4 production (Fig 3, B and C). IL-13Rα1 was not expressed by CD4+ T cells, mast cells (FcεR1α+ c-Kit+), Treg (CD4+ Foxp3+) or B cells (CD19+ B220+), ruling out an effect by ILC2-derived IL-13 on these cell types (Fig E3, C in this article’s Online Repository).
Figure 3. Th2 cytokine production by in vitro expanded SI ILC2.
(A) Flow cytometric gating strategy used to identify in vitro-expanded ILC2 from the SI of naïve mice. (B) Flow cytometric analysis of IL-5, IL-4 and IL-13 expression by Lin− CD45+ GATA-3+ ILC2 in vitro-expanded from the SI of naïve WT, Il13−/−, Il4raF709, Il4raF709 Il1rl1−/− and Il4raF709 Il4−/− mice. (C) Frequencies of IL-5+, IL-4+ and IL-13+ Lin− CD45+ GATA-3+ ILC2 isolated from the SI of the mouse groups from (B). Results represent 3 to 5 mice per group derived from 2 independent experiments. *P < .05, **P < .01, ***P < .001 and ****P < 0.0001 by one-way ANOVA with post-test analysis.
We reasoned that enforced restoration of the ILC2 compartment by adoptive transfer of in vitro-derived ILC2 might rescue allergic sensitization in Il4raF709 Il1rl1−/− mice by acting as an early source of IL-4. Il4raF709 Il1rl1−/− mice reconstituted with either in vitro expanded WT or IL-13 deficient ILC2 (Il13−/− ILC2), and orally-sensitized with peanut developed food allergy as evidenced by a decrease in their core body temperature, higher levels of peanut-specific IgE and the release of MMCP-1 in the serum after oral challenge with peanut (Fig 4, A and B and Fig E5, A and B in this article’s Online Repository). The recovery from the drop in core body temperature in mice reconstituted with Il13−/− ILC2 was faster, suggestive of a role for ILC2-derived IL-13 in potentiating the food allergic response (Fig E5, A in this article’s Online Repository). In contrast, adoptive transfer of in vitro generated ILC3 into Il4raF709 Il1rl1−/− mice subsequently orally sensitized and challenged with peanut did not restore the allergic reaction, thereby demonstrating the specific requirement for ILC2 in food allergy (Fig E5, A to C in this article’s Online Repository). Critically, adoptive transfer of IL-4 deficient ILC2 (Il4−/− ILC2) failed to restore oral allergic sensitization in Il4raF709 Il1rl1−/− mice (Fig 4, A and B). ILC2 frequencies and absolute numbers were similarly restored in the MLN and SI of mice receiving either WT, Il4−/− ILC2 or Il13−/− ILC2 (Fig 4, C and D and Fig E5, A to C in this article’s Online Repository). These results revealed the importance of ILC2-secreted IL-4 in favoring allergic oral sensitization.
Figure 4. Oral allergic sensitization and anaphylaxis are obligately dependent on IL-4 production by ILC2.
(A) Change in core body temperature after peanut oral challenge of peanut-sensitized Il4raF709 and Il4raF709 Il1rl1−/− mice reconstituted with either WT or Il-4−/− ILC2. (B) Peanut-specific IgE concentration and MMCP-1 release after anaphylaxis. (C – D) Frequencies and absolute numbers of ILC2 (Lin− Thy1.2+ GATA-3+) and ILC3 (Lin− Thy1.2+ RORγt+) in the MLN (C) and SI (D) from the mouse group in (A). Results represent data of minimum 7 to 18 mice per group derived from two independent experiments. *P < .05, **P < .01, ***P < .001, ****P < .0001 by two-way and one-way ANOVA with post-tests analysis.
Treg cells suppress ILC2 expansion and activation
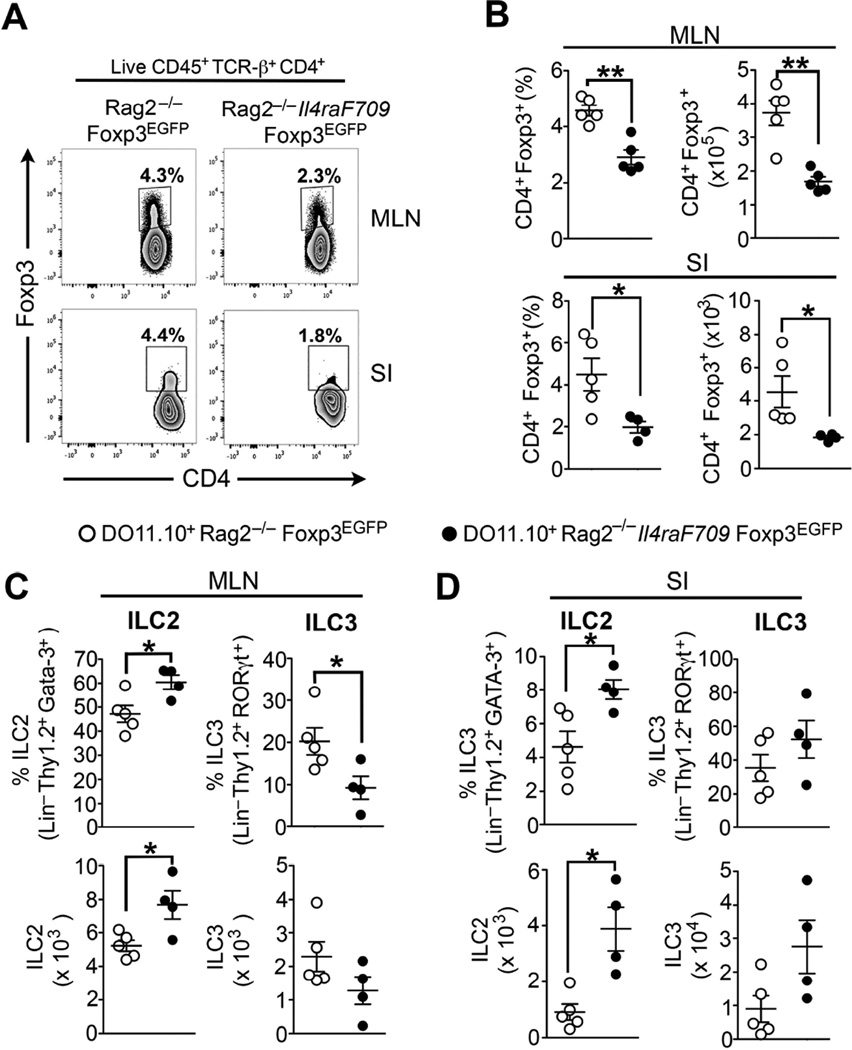
Establishment of oral tolerance and Treg cells suppressive functions are critical to prevent the development of oral sensitization and are involved in the resolution process of food allergy 3,36,37. Failure of Treg cell-mediated oral tolerance or Treg cells absence exacerbates food allergy 3,38,39. To demonstrate that Treg cells can directly suppress ILC2 expansion during food allergy we used transgenic DO11.10+ Rag2−/− Foxp3EGFP mice, which express the DO11.10+ TCR specific to OVA323–339 peptide on a RAG2-deficient genetic background. Those mice do not carry DO11.10+ CD4+ Foxp3+ T cells at steady-state, however supplying them with OVA in the drinking water triggers the conversion of naïve DO11.10+ CD4+ Foxp3− T cells into induced DO11.10+ CD4+ Foxp3EGFP iTreg cells 3. As previously established, the presence of the Il4raF709 allele markedly impaired iTreg cell conversion in the MLN and SI of DO11.10+ Rag2−/− Il4raF709 Foxp3EGFP mice (Fig 5, A and B) 3. Impaired allergen-specific iTreg cell formation was associated with a marked increase of the Lin− CD45+ CD4− TCR-β− Thy1.2+ GATA-3+ ILC2 but not RORgt+ ILC3 population in the MLN and SI of DO11.10+ Rag2−/− Il4raF709 Foxp3EGFP mice (Fig 5, C and D and Fig E6, A to B in this article’s Online Repository). These results established that defective iTreg cell induction and impaired oral tolerance are correlated with a significant expansion of the intestinal ILC2 population.
Figure 5. Defective allergen-specific Treg cell induction is associated with ILC2 expansion.
(A) Flow cytometric analysis of iTreg cells (CD4+ Foxp3EGFP+) in the MLN and SI of OVA-fed WT DO11.10+ Rag2−/− Foxp3EGFP and DO11.10+ Rag2−/− Il4raF709 Foxp3EGFP mice. (B) Frequencies and absolute numbers of CD4+ Foxp3+ iTreg cells in the MLN and SI of the mouse groups from (A). (C – D) Frequencies and absolute numbers of ILC2 (Lin− Thy1.2+ GATA-3+) and ILC3 (Lin− Thy1.2+ RORγt+) in the MLN (C) and SI (D) from the mouse groups in (A). Results represent data from 4 to 5 mice per group derived from two independent experiments. *P < .05, **P < 0.01, unpaired two-tailed Student’s t test.
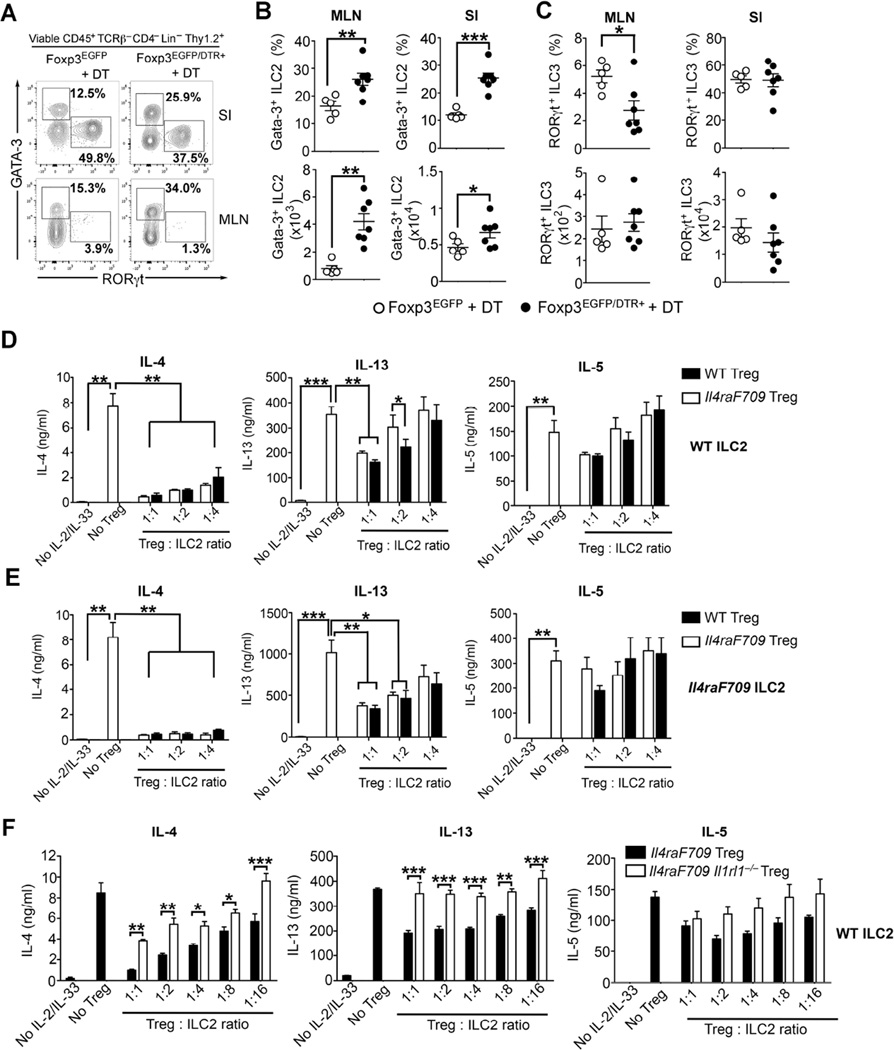
To further demonstrate the regulation of the ILC2 compartment by Treg cells, we employed Foxp3-deficient weanling mice (Foxp3K276x), which lack functional Treg cells because of a nonsense mutation in Foxp3 40,41. Compared to age-matched WT mice, 20 days old Foxp3K276X exhibited marked increase in the frequency and absolute numbers of ILC2 in the SI and MLN (Figure E7, A to D in this article’s Online Repository). ILC3 were also increased, but to a lesser extent. Since Foxp3K276x mice succumb to early death due to an uncontrolled lymphoproliferative disease, we examined the impact of acute transient ablation of Treg cells on ILC2 expansion by using adult Balb/c Foxp3EGFP/DTR+ mice 41. Those mice express the diphtheria toxin receptor (DTR) under the control of the Foxp3 promoter. Compared to age-matched WT Foxp3EGFP mice, two weeks of diphtheria toxin (DT) treatment resulted in significant weight loss and a 2-fold decrease of MLN and SI Treg cell population in Foxp3EGFP/DTR+ (Fig E8, A to C in this article’s Online Repository). Treg cell depletion with DT treatment resulted in increased ILC2 frequencies and absolute numbers in the SI and draining MLN of Foxp3EGFP/DTR+ (Fig 6, A to C). These results indicate that Treg cell deficiency, either congenital or inducible, results in dysregulated ILC2 expansion.
Figure 6. Treg cell inhibit ILC2 expansion and their Th2 cytokine production.
(A) Flow cytometric analysis of ILC2 (Lin− Thy1.2+ GATA-3+) and ILC3 (Lin− Thy1.2+ RORγt+) in the SI and MLN of Foxp3EGFP and Foxp3EGFP/DTR+ mice following DT administration. (B – C) Frequencies and absolute numbers of Lin− Thy1.2+ GATA-3+ ILC2 (B) and Lin− Thy1.2+ RORγt+ ILC3 (C) in the MLN and SI from the mouse groups in (A). (D – E) IL-4, IL-13 and IL-5 production by an increased gradient of cell-sorted WT ILC2 (D) or Il4raF709 ILC2 (E) cultured in the presence of either WT or Il4raF709 iTreg cells. (F) IL-4, IL-13 and IL-5 production by WT ILC2 cells co-cultured in the presence of Il4raF709 or IL4raF709 Il1rl1−/− iTreg cells. Results represent data from 5 to 7 mice per group. *P < .05, **P < .01, and ***P < .001 by one-way ANOVA with post-tests analysis or unpaired two-tailed Student’s t test.
We further analyzed the capacity of Treg cells to regulate ILC2 Th2 cytokines production using an in vitro co-culture system. Cell-sorted iTreg cells in vitro derived from either WT or Il4raF709 naïve CD4+ T cells were used in a suppressive assay and cultured in the presence of an increased gradient of cell-sorted WT or Il4raF709 ILC2 (Fig 6, D and E). In the absence of iTreg cells and under IL-2 and IL-33 stimulation, purified ILC2 derived from either WT or Il4raF709 mice secrete substantial quantities of IL-4, IL-13 and IL-5 (Fig 6, D and E). Addition of iTreg cells resulted in a dose-dependent reduction in the production of IL-4 and IL-13, with IL-4 being more amenable to suppression (Fig 6, D and E). IL-33R expression on Treg cells plays a critical role in the suppression of intestinal inflammation 42. When co-cultured with in vitro derived ILC2, Il4raF709 Il1rl1−/− iTreg cells displayed significantly less efficient suppressive function than their Il4raF709 counterparts, being only half as potent in reducing IL-4 and completely failing to reduce IL-13 secretion by ILC2 (Fig 6, F). In summary, Treg cells restrain ILC2 expansion in the gastrointestinal mucosa, and directly suppress ILC2 activation in an IL-33R-dependent manner.
IL-4 secretion by ILC2 antagonizes allergen-specific Treg cells induction and impairs their suppressive function
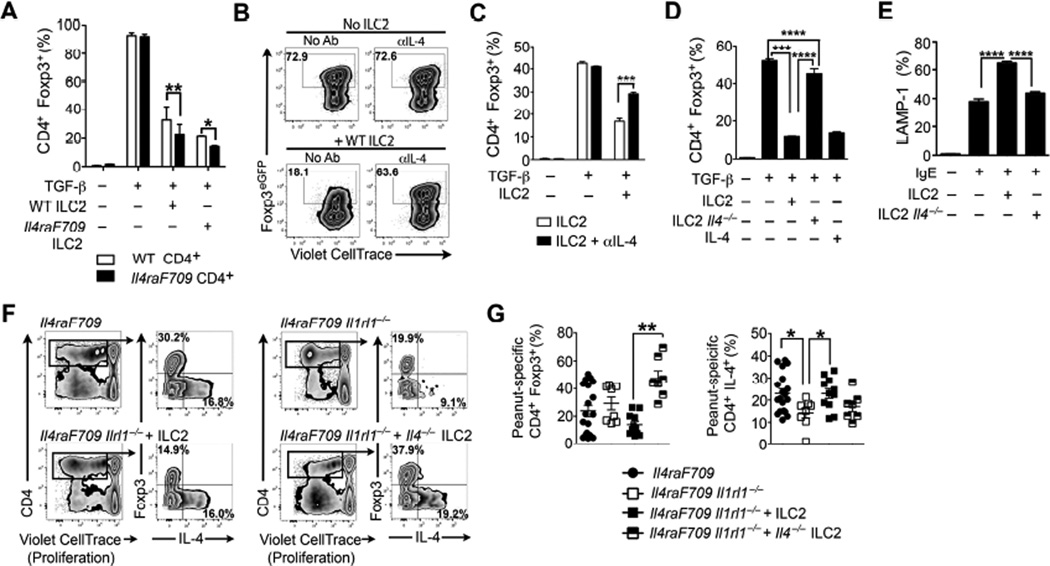
In vitro differentiation of naïve CD4+ T cells into CD4+ Foxp3+ iTreg cells is disrupted by the presence of IL-4 3,43. Accordingly, we examined the capacity of ILC2-derived IL-4 to disrupt oral tolerance in food allergy by impairing allergen-specific Treg cell formation and function. When naïve CD4+ Foxp3EGFP– T cells are cultured in Treg cell-inducing conditions, addition of ILC2 to the culture resulted in a significant reduction of CD4+ Foxp3EGFP+ iTreg cell conversion in an IL-4-dependent fashion (Fig 7, A to D). Proliferation was not markedly altered, indicating a specific effect on Treg cell lineage commitment rather than outgrowth of the Foxp3− compartment. IL-4 is a potent modulator of mast cell activation 44. Co-culture of IgE-sensitized mast cells in the presence of ILC2 resulted by upwards of a 50% increase of degranulating Lysosomal-associated membrane protein 1 (LAMP-1)+ mast cells which was largely abrogated when ILC2 were derived from IL-4-deficient mice (Fig 7, E).
Figure 7. IL-4 secretion by ILC2 promotes food allergy by decreasing allergen-specific Treg cell induction.
(A) TGF-β induction of iTreg cells from naïve CD4+ WT or Il4raF709 T cells co-cultured with cell-sorted WT or Il4raF709 ILC2. (B) Flow cytometric analysis of the effect of ILC2 and anti(α)-IL-4 treatment on TGF-β-driven in vitro iTreg cell induction. (C) Frequency of TGF-β-induced iTreg cells co-cultured in the presence of WT ILC2 without or with αIL4. (D) Frequency of TGFβ-induced iTreg cells co-cultured with either WT ILC2, Il4−/− ILC2 or recombinant IL-4. (E) IgE-mediated mast cell degranulation following co-culture with ILC2, ILC2 supernatants or IL-4 in the presence or absence of αIL-4. (F) IgE-mediated degranulation of mast co-cultured with WT ILC2 or Il4−/− ILC2. (G) Flow cytometric analysis of peanut extract-induced in vitro proliferation of CD4+ Foxp3+ Treg cells and CD4+ Foxp3− T conventional cells isolated from the MLN of peanut-sensitized Il4raF709 and Il4raF709 Il1rl1−/− mice reconstituted with either WT or Il-4−/− ILC2 (F) Frequency of peanut-specific CD4+ Foxp3+ Treg cells and CD4+ T cells producing IL-4. Results represent data of 5 mice per group (A to F) and 7 to 18 mice (G and H) derived from two independent experiments. *P < .05, **P < .01, ***P < .001, ****P < .0001 by two-way and one-way ANOVA with post-tests analysis.
We further examined the impact of ILC2 on allergen-specific Treg cells in vivo using the ILC2 reconstitution approach described in Figure 4. MLN cells isolated from peanut-sensitized Il4raF709 Il1rl1−/− that received either WT or Il4−/− ILC2 were labeled with a proliferative cell dye and cultured in the presence of peanut. Analysis revealed significantly higher frequencies of peanut-specific Treg cells in recipients treated with Il4−/− ILC2 (Fig 7 F and G). Together, these results established that ILC2-derived IL-4 may act to promote food allergy by antagonizing the induction of allergen-specific Treg cells and inhibiting their regulatory function, which feeds into dysregulated mast cell activation.
Discussion
In this report, we demonstrate that ILC2 play a requisite role in mediating oral sensitization to food allergens and delineate the mechanisms involved in such action. Il4raF709 mice, which are genetically prone to develop food allergy, fail to do so when their ILC2 differentiation is compromised by concurrent IL-33R deficiency. Furthermore, we have established for the first time, using several genetic approaches, that Treg cells regulate ILC2 expansion and suppress their Th2 cell cytokine secretion. In turn, ILC2 activation and expansion promote food allergy by secreting IL-4, which compromises Treg cell function and augments mast cell activation. Our results define a cellular network involving ILC2, Treg cells and mast cells that is normally poised towards tolerance to food allergens but whose perturbation upon ILC2 activation may tip the balance towards food allergy.
A key finding in our studies relevant to food allergy is that Treg cells and ILC2 are here shown to engage in reciprocal regulation centered on ILC2-derived IL-4. While some previous reports have documented IL-4 secretion by ILC2, most have focused on the production by ILC2 of IL-5 and IL-13 8,15,33,45. Our present investigations reveal that ILC2 produce copious amounts of IL-4 that are sufficient to suppress allergen-specific iTreg cell differentiation and promote food allergy. Dysregulated ILC2 expansion and activation may well be an initiating step in the pathogenesis of food allergy. The ensuing impairment in the mucosal regulatory compartment contributes to a Th2-skewed, food allergen-specific adaptive immune response and disease persistence.
The function of ILC2 in the mucosal innate immune response and their localization at the environmental interface indicates that they may play a critical role in fueling food allergy in response to alarmin signals emanating from the intestinal epithelium, such as IL-33 46. Of note, production of the alarmin IL-33 is augmented in the intestinal tract of food allergic mice. Increased expression of alarmins by intestinal epithelial cells could be induced by an altered intestinal microbial flora 47,48. We have demonstrated that food allergy is associated with intestinal microbial dysbiosis, which in turn further promotes oral allergic sensitization 26. A dysbiotic flora may upregulate the expression of alarmins in the intestinal epithelium, creating an environment conducive to ILC2 activation and expansion following oral exposure to allergen. Some allergens, including peanut, have been shown to activate alarmins secretion by epithelial cells and provide one trigger leading to Th2 cytokines production and food allergy induction 49.
In addition to disabling Treg cells, IL-4 production by ILC2 amplified mast cell activation. We have previously shown that that Treg cell inhibition of mast cell degranulation is critical to forestalling food allergy 3,4. In turn, dysregulated IgE-mast cell interaction profoundly inhibits iTreg cells induction and mediates the latter Th2 cell-like reprogramming by an IL-4-dependent mechanism, providing a requisite step in food allergy pathogenesis 3. Allergen re-exposure of intestinal IgE-stimulated mast cells could also lead to robust IL-33 production and consequently create an ILC2 activation and expansion loop that positively feeds back on mast cell activation and negatively regulates allergen-specific iTreg cells induction 50,51.Thus ILC2 may also sustain the immune dysregulation during the adaptive phase of the allergic immune response to food allergens.
While oral immunotherapy has been effective in inducing a desensitized state with short-lived unresponsiveness to food allergens, it has had more limited success in conferring true long-term tolerance following cessation of therapy 52. Our previous results and those reported herein emphasize the importance of interrupting the ILC2-Treg cell-mast cell circuit of immune dysregulation that acts to suppress oral tolerance by blocking effective allergen-specific iTreg cell responses in food allergy. Resetting this circuit either pharmacological and/or by the use of immunomodulators, such as anti-IL-4R antibody, therapy may be essential in conferring long-term tolerance to food allergens.
Supplementary Material
Key Messages.
Food allergy is accompanied by ILC2 expansion and activation in the gastrointestinal tract.
Treg cells control ILC2 expansion and activation.
IL-4 secretion by ILC2 blocks allergen-specific Treg cell induction.
Acknowledgments
We thank Drs. Calvin B Williams and Dipica Haribhai for provision of Foxp3EGFP/DTR+ mice on BALB/c background, and Dale Umetsu and Andrew McKenzie for the gift of Il1rl1−/− mice. This work was supported by NIH NIAID grants 1R56AI117983-01 (T.A.C.), 1R01AI119918-01 (H.C.O.), 5T32AI007512-28 (O.T.B.) and 1K01DK106303-01 and (O.T.B.), by the Rao Chakravorti Family Fund (H.C.O.), and by the Bunning Foundation (H.C.O., T.A.C.).
Abbreviations
- GI
Gastrointestinal
- ILC2
innate lymphoid cell type 2
- iTreg
induced regulatory T
- ILC3
innate lymphoid cell type 3
- MLN
mesenteric lymph nodes
- SI
small intestine
- Treg
regulatory T
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–S125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–295. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Noval Rivas M, Burton OT, Wise P, Charbonnier L-M, Georgiev P, Oettgen HC, et al. Regulatory T Cell Reprogramming toward a Th2-Cell- like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E Signal Inhibition during Allergen Ingestion Leads to Reversal of Established Food Allergy and Induction of Regulatory T Cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HY, Chang Y-J, Subramanian S, Lee H-H, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. e1–e6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y-J, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013 doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005374. 170ra16–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker JA, Barlow JL, McKenzie ANJ. Innate lymphoid cells - how did we miss them? Nat Rev Immunol. 2013 doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 13.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 14.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 15.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 16.Halim TYF, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-Acid-Receptor-Related Orphan Nuclear Receptor Alpha Is Required for Natural Helper Cell Development and Allergic Inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyler T, Klose CSN, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CGK, Kim BS, Brestoff JR, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner J-E, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld J-C, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e1–e8. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee J-B, Chen C-Y, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, et al. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tachdjian R, Khatib Al S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol. 2010;125:1128–1128. doi: 10.1016/j.jaci.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noval Rivas M, Burton OT, Wise P, Zhang Y-Q, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noval Rivas M, Koh YT, Chen A, Nguyen A, Lee YH, Lawson G, et al. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122:1933–1947. doi: 10.1172/JCI40591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathias CB, Hobson SA, Garcia Lloret M, Lawson G, Poddighe D, Freyschmidt E-J, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127:795–805. e1–e6. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halim TYF, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Oczypok EA, Milutinovic PS, Alcorn JF, Khare A, Crum LT, Manni ML, et al. Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, et al. Adaptation of Innate Lymphoid Cells to a Micronutrient Deficiency Promotes Type 2 Barrier Immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 35.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syed A, Garcia MA, Lyu S-C, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, et al. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 39.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 41.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veldhoen M, Uyttenhove C, Van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 44.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, et al. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013;6:740–750. doi: 10.1038/mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 47.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 48.Mosconi I, Geuking MB, Zaiss MM, Massacand JC, Aschwanden C, Kwong Chung CKC, et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol. 2013;6:1157–1167. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Wang Y, Tang L, de Villiers WJS, Cohen D, Woodward J, et al. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J Allergy Clin Immunol. 2013;131:442–450. doi: 10.1016/j.jaci.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu C-L, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS ONE. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–323. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.