Abstract
Paternal exposure to constituents of cigarette smoke (CS) is reportedly associated with infertility, birth defects and childhood cancers even though the mechanism behind this relationship is still unclear. Chronic cigarette smoking by men leads to poor sperm quality and quantity mainly through oxidative stress and also direct assault by CS metabolites. Among several carcinogenic and teratogenic components of cigarette smoke condensate (CSC), polycyclic aromatic hydrocarbons (PAHs) display a preeminent role in accelerating germ cell death via the cytoplasmic transcription factor, aryl hydrocarbon receptor (AHR) that is present across all stages of spermatogenesis. Activation of AHR by growth factors though benefits normal cellular functions, its mediation by CSC in a spermatocyte cell line [Gc2(spd)ts] adversely affects the expression of a battery of genes associated with antioxidant mechanisms, cell proliferation and apoptosis, and cell cycle progress. Besides, the CSC-mediated cross talk either between AHR and NRF2 or AHR-NRF2 and MAPKs pathways inhibits normal proliferation of the spermatogenic GC-2spd(ts) cells in vitro and cell death of spermatocytes in vivo. Pharmacological inactivation of CSC-induced AHR but not its genetic manipulation seems preventing DNA and cell membrane damage in Gc2(spd)ts. Data from recent reports suggest that the cigarette smoke affects both the genomic and epigenomic components of the sperm and attributes any associated changes to developmental defects in the offspring. Thus, the studies discussed here in this review shed light on possible mechanistic factors that could probably be responsible for the paternally mediated birth defects in the offspring following exposure to the toxic constituents of cigarette smoke.
Introduction
Tobacco use is the single largest preventable cause of death and disease for both men and women. Tobacco causes nearly six million deaths per year worldwide. In the US, smoking and second-hand smoke cause one in every five deaths and incur almost $300 billion annually in total economic costs (USDHHS, 2014). Approximately 30% of women and 35% men of reproductive age smoke cigarettes, affecting not just themselves but also the environment and their progeny (ASRM, 2012). Cigarette smoke (CS) contains more than 7000 chemicals, including at least 539 polycyclic aromatic hydrocarbons (PAHs), of which 69 are proven carcinogens (IARC, 2004; Rodgman and Perfetti, 2006) and mutagens (DeMarini, 2004). Additionally, CS is comprised of the entire top ten hazardous substances listed in section 204 of the Comprehensive Environmental Response, Compensation, and Liability Act. Cigarette smoke condensate (CSC), the particulate, or tar, phase of CS, consists mainly of dioxins (TCDD) and halogenated and nonhalogenated PAHs including benzo(a)pyrene (B[a]P) and pro-oxidants such as lipophilic semiquinones (Smith and Hansch, 2000; Ding et al. 2007). This review on paternal smoking and its impacts on offspring will summarize the current state of research in this area and describe possible mechanisms by which paternal smoking causes poor reproductive outcomes and developmental defects. Moreover, the molecular mechanisms we tried to understand by using a spermatogenic cell line [GC-2spd(ts)] may or may not reflect upon the actual events happening in the spermatocytes in vivo.
Paternal smoking and developmental defects
Maternal smoking and in utero exposure during pregnancy has so far been believed to be associated with reduced sperm quality, count, and testis size in adults (Jensen et al. 2004; Virtanen et al. 2010). However, several epidemiological and case control studies in humans have reported that children born to male smokers are at increased risk of childhood cancers (Ji et al. 1997; Chang et al. 2006; Vine, 1996) and the existence of a significant correlation between paternal smoking and childhood cancer (Liu et al. 2011) with the emphasis on the need to focus on underlying toxicological mechanisms, such as genotoxic, transcriptomic, or epigenomic effects on sperm or cord blood. Similarly, several other reports have highlighted close connection between paternal smoking and childhood leukemia (Pang et al. 2003; Lee et al. 2009). Birth defects such as anorectal malformations (Zwink et al. 2011), cardiovascular anomalies, congenital heart disease (Cresci et al. 2011), cleft palate, hydrocephalus, urethral stenosis (Savitz et al. 1991), spina bifida (Zhang et al. 1992), and reduced kidney volume (Kooijman et al. 2015) were some of the developmental defects observed in the offspring of paternal exposure. Additionally, paternal smoking has been reported to cause implantation failure (Janny & Menezo, 1994; Sofikitis et al. 1995; Ubaldi et al. 1999). However, only a few studies have correlated these outcomes to the intratesticular levels of harmful and potentially harmful constituents of cigarette smoke. Godschalk et al. (2015) recently showed that B[a]P is able to induce hypomethylation in testicular DNA, that leads to heritable mutations in the offspring. However, the data are not very conclusive on the effects of male smoking on in vitro fertilization (IVF) outcomes. (Pattinson et al. 1991; Hughes et al. 1994; Joesbury et al. 1998) even though the sperm numbers were found decreased in young men prenatally exposed to paternal smoking (Axelsson et al. 2013). So far in humans, the studies on the association between paternal smoking and congenital anomalies among offspring have yielded mixed results. They are either poorly understood mechanistically, and or very limited data are available on the germ cell and reproductive effects of paternal exposure even though maternal exposure to second-hand smoke during pregnancy is known to cause adverse fetal outcomes (Olshan AF, Faustman EM, 1993; Leonardi-Bee, and Britton, 2011). In case of rodents, CS-induced mutations in sperm DNA (Yauk et al. 2007) cause lack of pregnancy after fertilization, disrupted blastocyst implantation, impaired embryonic development, and IVF failures (Kapawa et al. 2004).
Cigarette smoking and male infertility
Even though several studies have indicated the harmful effects of in utero exposure to CS on male fertility (Jensen et al. 2004; Mackenzie and Angevine, 1981), chronic cigarette smoking by men also leads to male infertility; the available biologic, experimental, and epidemiological data indicate that 13% of male infertility is attributed to cigarette smoking (ASRM, 2012), and the time to pregnancy is extended in cases in which the man smokes more than 15 cigarettes per day (Ford et al. 2000). A comprehensive review by Ramlau-Hansen et al. (2007) and Mostafa (2010) have provided a thorough overview on cigarette smoking by men and associated abnormalities in sperm count, motility, and morphology, as well as other qualitative and quantitative measures of sperm characteristics. Male smokers exhibit several seminal anomalies including increased levels of oxidative DNA damage (Fraga et al. 1996; Shen et al. 1997), sperm DNA strand breaks (Potts et al. 1999), DNA adducts (Horak et al. 2003), chromosomal abnormalities (Robbins et al. 1997; Rubes et al. 1998), and decreased viability, and fertility (Kunzle et al. 2003). Exposure to cigarette smoke (CS) results in decreased sperm membrane permeability and activity of acrosin (Sofikitis et al. 2000). The testicular endocrine and spermatogenic functions, and epididymal functions are also reportedly reduced in rats upon exposure to B[a]P (Ramesh et al. 2008). Nicotine causes testicular toxicity by degenerating germ cells (Jana et al. 2010) and cigarette smoke metabolites such as cotinine drastically affect seminal parameters such as sperm membrane damage, reduced motility, capacitation and hyperactivation (Pacifici et al. 1993; 1995) that correlates to low sperm count (Chia et al. 1994; Vine et al. 1996). Vine et al. (1994, 1996) reported that sperm concentration is 13% lower in smokers than non-smokers. Meanwhile, there is a modest reduction (10–17%) in sperm counts reported in adult men who smoke heavily and these reductions in sperm quality and quantity are directly proportional to the number of cigarettes smoked daily (Ramlau-Hansen et al. 2007). Such adverse effects of male smoking are thought to be due to absorption of constituents of CS and its metabolites into the systemic circulation and accumulation, either by diffusion or active transport, into seminal plasma (Zavos and Zarmakoupis-Zavos, 1999).
Several environmental and food contaminants are known to reach the testis in significant concentrations (Gaspari, et al. 2003; Bjorge et al. 1996). However, little is known about the molecular mechanisms by which the components of CS damage male germ cells during spermatogenesis. One likely candidate is the oxidative stress caused by the generation of excess reactive oxygen species (ROS) or free oxygen radicals by the toxic constituents of CS (Saleh et al. 2002; Aitken and Baker, 2004). Additionally, the sperm of smokers have increased levels of oxidized unsaturated fatty acids (Jones et al. 1979). Although ROS are required for sperm maturation, capacitation, and the acrosome reaction (de Lamirande et al. 1993), mature male gametes are highly susceptible to oxidative damage because they express low levels of antioxidant enzymes and have high concentrations of polyunsaturated fatty acids in their plasma membrane (Aitken and Roman, 2008). Cigarette smokers with high levels of ROS in their seminal plasma capable of causing DNA damage mediate oxidative male infertility (Potts et al. 1999; Tremellen, 2008). Meanwhile, infertile men who smoke cigarettes have higher levels of seminal OS than infertile nonsmokers and significantly low sperm count (Saleh et al. 2002; Collodel et al. 2010; Zenses, 2000). Therefore, the oxidative imbalance could be, in part, responsible for CS-mediated male infertility. Genetically, smoking has been known to be associated with sperm disomy in teenage men (Rubes et al. 1998). Smoking also affects morphology and ultrastructure of the flagellum and, more specifically, the axoneme of the human spermatozoon (Evans et al. 1981; Hoidas et al. 1985; Zavos et al. 1998). In addition, the change mediated by CS in sperm mRNA profile can serve as the marker of gene–environmental toxicants interactions in human germ cells (Linschooten et al. 2009). In contrast, studies by Mocarelli et al. (2011) showed that only in utero and lactational exposure of children to low doses of TCDD could permanently reduce sperm quality. Meanwhile, a meta-analysis by Li et al. (2011) have highlighted that the smoking seems to degrade semen volume and total sperm count in heavy smokers.
Polycyclic aromatic hydrocarbons (PAHs) act as testicular toxicants through aryl hydrocarbon receptor (AHR)
Treating adult rodents with PAHs such as TCDD, B[a]P, and 3-Methylchloranthrene increases the number of abnormal sperm and immature germ cells (Viczian, 1968; Wyrobeck and Bruce, 1975), blocks spermatogenesis, and causes testicular atrophy (Mattison, 1982), decreased testis weight, and increased apoptosis in seminiferous tubules (Denison and Heath-Pagliuso, 1998; Revel et al. 2001; Coutts et al. 2007). PAHs have a strong effect on germ cells because the pre-and post-meiotic germ cells, particularly the spermatocytes (Georgellis et al. 1990; Essenberg et al. 1951) in the seminiferous epithelium (Schultz et al. 2003; Coutts et al. 2007), highly express the aryl hydrocarbon receptor (AHR). AHR is a ligand-activated transcription factor that, upon activation by a natural or synthetic compound, translocates to the nucleus, binds to the AHR nuclear transporter, and regulates several downstream targets (Thackaberry et al 2005). AHR is best known for induction of the cytochrome P450 superfamily of genes (Cyp1a1 and Cyp1a2) in response to detoxification of endogenous and exogenous ligands (Barouki et al. 2007). AHR binds with high affinity and specificity to dioxin-like compounds (Grassman et al. 1998). Given that various environmental toxicants including TCDD have been found in human seminal fluid (Schecter et al. 1996), the presence of AHR may make the sperm highly vulnerable to PAHs of CSC. For example, adult exposure to TCDD decreased daily sperm production in rat and the critical period for TCDD effects on testis weight suggested to occur before puberty (Simanainen et al. 2004a; 2004b). However, in utero exposure of male rats on different days of gestation adversely affected the ejaculated sperm counts with lesser effect on testicular sperm production (Rider et al. 2010). A cross species comparative study further revealed that the prenatal maternal exposure to TCDD at low dose causes functional alterations rather structural malformations (Peterson et al. 1993).
AHR is essential for spermatogenesis and post-testicular sperm maturation
Despite its capacity to mediate the damaging effects of PAHs in sperm and the controversies surrounding the direct impact of AHR activation, several lines of evidence indicated that AHR is essential for germ cell development, differentiation, and maturation during its epididymal transit. First, earlier findings collectively suggested that either the absence of AHR or its activation would lead to inflammation, apoptosis, and oxidative stress-mediated DNA damage in sperm (Aitken and Roman, 2008; Matsumura, 2009). Expression of AHR is tissue-, and developmental stage-specific and regulates normal cellular processes, such as cell cycle, stem cell proliferation, and tissue differentiation (Puga et al. 2002; Gasiewicz et al. 2012). In rat, AHR expression in the seminiferous tubule is restricted to the primary pachytene spermatocytes, whereas in humans, AHR is expressed across all stages of spermatogenesis (Roman et al. 1998; Schultz et al. 2003). In mice, male fertility, sperm count, seminal vesicle weight, and dorsolateral prostate weights are all decreased in Ahr–deficient mice (Karman et al. 2012; Baba et al. 2008; Lin et al. 2001). On the other hand, in utero and lactational exposure of male rats to TCDD alone significantly decreased the weight of testis, epididymis and daily sperm production (Mably et al. 1992). Likewise, even though AHR appears to be critical for post-testicular sperm survival and maturation in the epididymis, the testicular production of germ cells remain unaffected due to TCDD treatment in adult mice (Foster et al. 2010). Using an AHR knockout mouse model, we recently showed that AHR is required for the structure and function of the seminiferous tubules (Hansen et al. 2014). Histologically, the testes from Ahr−/− mice have significant structural disorientations in the seminiferous epithelium suggestive of germ cell degeneration and compromise in Sertoli cell function (Boekelheide, 2005). Additionally, we noted drastic down regulation of several germ cell-associated marker genes such as Magea4, HspA2, Prm1, and Prm2 in Ahr−/− mice. Finally, we found that AHR protein was highly expressed across different germ cell stages and on the acrosome and principal piece of the sperm tail. This expression was reflected in AHR function of the mature sperm, as we noticed that Ahr−/− sperm were less efficient than wild-type sperm in fertilizing oocytes. Therefore, the role of AHR in spermatogenesis though appears promising remains inconclusive and warrants further attention.
CSC deregulates gene expression in testis
Only a few studies have investigated the influence of cigarette smoking on the development and function of testis. In other tissue types, CS affects the expression of genes involved in antioxidant mechanisms, metabolism of PAHs, and cell cycle progress (Georgellis et al. 1990; Narayan et al. 2004). For example, the expression of several genes that are associated with PAH metabolism were up regulated in oral cancer cells upon exposure to CSC (Nagaraj et al. 2006). Bosio et al. (2002) and others have attributed the strong oxidative potential of CSC as a likely mechanism by which CSC influences gene expression in various cell and tissue types (Fields et al. 2005; Van Leeuwen et al. 2005; Han et al. 2008). Therefore, determining the mechanisms by which CS impairs spermatogenesis requires evaluation of the gene expression changes that occur in response to CS-induced oxidative stress.
In a study using a mouse spermatogenic GC-2spd(ts) cell line, we explored the effects of CSC on the expression of several antioxidants both in vitro and in vivo (Esakky et al. 2012). We reported that exposure to CSC leads to oxidative stress, which in turn affects normal cellular functions by modifying the expression of several antioxidant genes such as Hsp90, Nrf2, Sod1, Sod2, Ahr, Arnt, Cyp1a1 Gpx4, and Ucp2 through both AHR-dependent and -independent manners. The in vivo data further indicated that the constituents of CSC mediate cell death in the testis and suggested that this may occur both directly, by genotoxicity of CSC constituents, and indirectly through generation of ROS. Notably, we observed profound AHR-dependent up-regulation of Cyp1a1 even with low concentrations of CSC. CYP1A1 contributes to detoxification of PAHs, but its induction may be harmful due to the generation of mutagenic metabolites like benzopyrene diol epoxide (BPDE). Consistent with this, we observed germ cell death in the testis and BPDE-intercalated DNA in the apoptotic spermatocytes. This study also revealed that exposure to CSC induced Ahr gene expression but did not increase the levels of AHR protein similar to an earlier outcome (Song and Pollenz, 2003). Thus, this report was an illustration of how CSC could cause germ cell death by inducing AHR mediated oxidative stress and thereby altering gene expression in the testis.
CSC modulates cell cycle in spermatogenic GC-2spd(ts) cells via AHR-NRF2 pathway
The constituents of CSC such as TCDD and other PAHs exert their growth-modifying effects primarily through AHR even though it exhibited direct detrimental impact (Gu et al. 2000). Similarly, ROS activate Nrf2, which regulates genes that possess antioxidant response elements (AREs) in their promoters (Moi et al. 1994; Venugopal and Jaiswal, 1996). The regulation of Ahr target genes by Nrf2 in liver in response to TCDD indicated the convergence of Ahr and Nrf2 pathways (Yeager et al. 2009). Though the precise mechanism still remains obscure, a large body of evidence implicated Ahr in cell cycle control including the TCDD-induced thymic atrophy (Kremer et al. 1994; Puga et al. 2000; Marlowe et al. 2004). For instance, Ma and Whitlock (1996) showed that Ahr-defective Hepa-1 cells exhibit growth arrest at G1 phase, while mouse embryonic fibroblasts from Ahr-null mice show delayed S-phase progress (Tohkin et al. 2000) and accumulation at the G2/M phase (Elizondo et al. 2000). Abdelrahim et al. (2003) showed that Ahr silencing accelerates MCF-7 cells to S phase, whereas it works opposite in HepG2 cells. Therefore, a comprehensive model that illustrates the contradictory roles of Ahr in cell cycle control particularly in germ cells is still evolving.
To understand the molecular conundrum behind the CSC mediated AHR role in cell cycle, we examined the effect of CSC on the Ahr-Nrf2 pathway by using the spermatogenic GC-2spd(ts) cells (Esakky et al. 2014). This study provided evidence that, as it does in other cell types (Jeffy et al. 2000; Khan and Dipple, 2000; Hamouchene et al. 2011), CSC blocks GC-2spd(ts) cell cycle progress at the S-G2/M phase and deregulates expression of cell cycle regulators such as cyclin D1, P21, and Gadd45a. We found that cyclin D1 expression requires Ahr and growth factors for its basal expression under normal condition, but is down regulated by CSC either in presence or absence of AHR. Our finding that CSC caused AHR-dependent upregulation of NRF2 suggested that NRF2 might protect spermatogenic GC-2spd(ts) cells from oxidative stress (Rangasamy et al. 2004; Nakamura et al. 2010) based on its nuclear translocation, which was found both complementary (Niture et al. 2010) and contradictory to earlier findings (Nguyen et al. 2005). As has been reviewed in other cellular systems (Hayes et al. 2009), there exists an autoregulatory loop between Ahr and Nrf2 in spermatogenic GC-2spd(ts) cells. Moreover, the absence of CSC-induced Cyp1a1 expression in absence of Ahr or Nrf2 in spermatogenic GC-2spd(ts) cells corroborates TCDD action on NAD(P)H:quinone oxidoreductase 1 (Yeager et al. 2009). Furthermore, the CSC induced growth arrest in spermatogenic GC-2spd(ts) cells at G2-M checkpoint parallels the induction of DNA-damage-inducible Gadd45a since it has been shown earlier as a molecular sensor of DNA damage and adducts formation in response to genotoxic agents such as benzo[a]pyrene (Schackelford et al. 1999; Wan et al. 2000; Akerman et al. 2004). Thus, this body of evidence supported the fact that CSC mediates the formation of an autoregulatory loop between AHR and NRF2 in the spermatogenic GC-2spd(ts) cells during S-/G2-M phase.
CSC facilitates crosstalk between AHR-NRF2 and MAPK in spermatogenic GC-2spd(ts) cells
AHR agonists in CSC such as TCDD and B[a]P activate multiple cell signaling including MAPKs (Henklová et al. 2008), implicating MAPKs in connecting AHR with various physiological processes (Tan et al. 2002; Long et al. 1998). However, the mechanistic link between these toxicants to their effects on a particular signaling pathway have not been sufficiently established. AHR ligands activate MAP kinases in a cell or tissue specific manner, and that the kinase in turn mediates AHR activation, facilitating the transactivation of target genes (Weiss et al. 2005). We demonstrated that growth arrest of the spermatogenic GC-2spd(ts) cells by CSC involves a bidirectional crosstalk between AHR and MAPKs and regulation of a cascade of downstream targets (Esakky et al. 2015a). As demonstrated in this study, CSC induced accumulation of spermatogenic GC-2spd(ts) cells at S-phase and downregulation of cyclins. We further showed that CSC activates p38 and ERK MAPKs through AHR, and pharmacological inhibition of these pathways prevented CSC-mediated cell cycle arrest. When examined for the influence of MAPKs-mediated Ahr-Nrf2 role in cell cycle progress, the accumulation of the spermatogenic GC-2spd(ts) cells at G2-M implicated Ahr and Nrf2 in the activation of G2/M kinases (Elizondo et al. 2000), and DNA lesions (Reddy et al. 2008), respectively. Taking into account the cell type-specific functional dependency of MAPKs on AHR, the MAPKs activation in the spermatogenic GC-2spd(ts) cells suggested that the CSC constituents that activate p38- and ERK-MAPKs might also be the ligands of AHR. This was later confirmed by using the MAPK specific inhibitors.
Activating transcription factor 3 (Atf3) has been shown earlier to be induced by the CSC constituent, benzo(a)pyrene diolepoxide (Hai et al. 1999) and its regulation by MAPK here corroborated previous studies (Inoue et al. 2004; Lu et al. 2007). Corresponding to the Ahr-Nrf2 pathway, CSC facilitated cross talk between Nrf2 and Atf3 through AREs (Kim et al. 2010) even though the primary regulation of Atf3 by Nrf2 indicated the interplay of other upstream mediators of oxidative stress. Several lines of evidence suggested that CSC ligands like TCDD (Puga et al. 2002; Marlowe and Puga, 2005) cause cell cycle arrest (Ge and Elferink, 1998; Puga et al. 2000) by catalyzing the interaction between the transformed AHR and the retinoblastoma (RB)/E2F complex. In conjunction with earlier reports, this study has proposed a model for E2F4 action that the CSC-induced triad of AHR-RB-E2F4 inhibitory complex could be responsible for inhibiting E2F4 target. Thus, this complex CSC elicited intracellular in vitro signaling in the spermatogenic GC-2spd(ts) cells added greater strength by complementing in vivo TCDD-mediated MAPK activation (Jin et al. 2008).
AHR inhibition is anti-apoptotic in GC-2spd(ts) cells
Germ cell apoptosis is an indispensable evil to maintain cellular homeostasis during normal spermatogenesis. Since germ cells are highly sensitive to environmental toxicants such as the various harmful constituents of CS, it is indeed necessary to develop a protective mechanism that would help safeguarding the process of spermatogenesis by preventing unwanted germ cell death. With this objective, we have showed in a recent study, that treating spermatogenic GC-2spd(ts) cells with an AHR-specific pharmacological inhibitor, CH223191, (Kim et al. 2006) significantly reduced the proapoptotic actions of CSC by reducing DNA and membrane damage and caspase activation (Esakky et al. 2015b). Apoptosis is regulated by interaction between pro- and anti-apoptotic genes, and our work revealed that AHR coordinates the two. We found that CSC treatment of spermatogenic GC-2spd(ts) cells elevated the levels of both BCL2L1 and BCL2 prosurvival proteins and increased the numbers of apoptotic BAX- and BAD-positive cells. On the other hand, the activation of this intrinsic mitochondrial apoptotic signaling underlined the adaptive ability of the cells against growth-inhibitory CSC, which generates excessive oxidative stress in AHR-deficient cells. One of the key findings of this report was the inhibition of CSC induced caspase-3/7 by CH223191 while its elevation in Ahr silenced Spermatogenic GC-2spd(ts) cells and AHR-KO MEF. This divergence in caspase-3 response under both basal and CSC-induced conditions has been attributed to its heightened sensitivity to ROS.
Cigarette smoking destroys sperm ultrastructure and plasma membrane integrity (Belcheva et al. 2004). We found here that the spermatogenic GC-2spd(ts) cells treated with CSC exhibited externalization of phosphatidylserine on the plasma membrane, a hallmark feature of apoptosis. However, pretreatment with CH223191 prevented this membrane damage phenomenon. Meanwhile, the significant rise in apoptotic spermatogenic GC-2spd(ts) cells in the Ahr-deficient state while reiterating the cytoprotective role of AHR (Esakky et al. 2015a), appeared increasingly sensitive to oxidative stress due to reduced expression of super oxide dismutase. Moreover, the apoptosis regulating ability of AHR seems not uncommon as AHR-suppressed spermatogenic GC-2spd(ts) cells also displayed greater sensitivity to smoke-induced apoptosis. Therefore, the outcome of this particular study suggested that the hyperinducibility of AHR by environmental toxicants such as CSC is an undesirable event and the activation of such pathways need to be blocked / prevented to avoid the accumulation of unwanted metabolites such as free oxygen radicals that may lead to complete germ cell loss. Specific AHR antagonists such as CH223191 can be employed under these circumstances to prevent undesirable excess germ cell death. Others have adapted similar approach to prevent unwanted AHR activation by using natural antagonists such as resveratrol (Revel et al. 2001; Ciolino and Yeh, 1999). Thus, this study inferred that AHR could be a viable therapeutic target to prevent germ cell death induced by environmental toxicants such as CSC.
The need for improved animal models of paternal cigarette smoking
Our data indicate that mouse pups sired by males subjected to long-term CSC exposure have phenotypic defects (unpublished observation) at the early days of development. One challenge with such studies is that mice are obligate nose breathers that do not realistically model human exposure to CS, and the contribution of components of CS to overall cytotoxicity remains ambiguous. However, the animal data cannot be ignored as it provides an incontrovertible link between DNA damage in spermatozoa and defects in embryonic development. Therefore, there is a continuing need to develop an in vivo rodent model first to mimic human exposure to cigarette smoke and second, to reliably establish the reproductive toxicity thresholds of constituents of cigarette smoke on male fertility and the development of offspring.
Timing of paternal exposure and transfer of effects to progeny
Fabia and Thuy (1974) reported that paternal exposure to chemical substances could affect the integrity of spermatogenesis and result in the transmission of carcinogenic effects to children. Later, Wilkins and Sinks (1984) demonstrated that children born to painters are six times more likely to develop Wilms’ tumor than children from other fathers. Paternal exposures to PAHs of cigarette smoke are related to poor sperm quality and childhood leukemia (Castro-Jimenez and Orozco-Vargas, 2011; Jeng et al. 2013; Ji et al. 2013). An overview by Friedler in the year 1996 suggested that the paternal exposures to a variety of toxicants could induce a broad spectrum of deleterious effects on the normal course of offspring development. The Avon Longitudinal Study of Parents and Children (ALSPAC), led by Pembrey (2014), showed that adolescent sons of fathers who were smoking before puberty are at greater risk of becoming obese and further suggested that cigarette smoke metabolites may induce epigenetic changes in the spermatogonial stem cells during prepubertal stage. Several recent reviews on epidemiological and experimental studies suggest that paternal nutritional, and toxicological exposures can lead to several male-mediated developmental toxicity in the following generations (Curley et al. 2010; Nanassy and Carrell, 2008).
In rodents, males do not interact with their offspring and thus transmit information only via germ cells. If the offspring are programmed differently as a result of paternal exposures to cigarette smoke, then the father’s sperm must carry that information. Both genomic and epigenetic pathways can be evoked to explain the transmissible effects of environmental toxicants. For example, plastic derived endocrine disruptors can promote epigenetic transgenerational inheritance of adult onset disease and sperm DNA methylation regions can serve as potential epigenetic biomarkers for transgenerational disease and/or ancestral environmental exposures (Manikkam et al. 2013). Its known from animal models that high ROS in testis is related to epigenetic changes in sperm (Tremellen, 2008; Kumar et al. 2013). Therefore, paternal exposure to cigarette smoke might affect both genetic and epigenetic characteristics of the sperm through altered ROS, which ultimately increases the risk for disorders in the offspring. For example, males exposed to chronic stress either during puberty or adult age may reprogram the sperm to generate male and female offspring with a hypo functioning HPA stress axis (Rodgers et al. 2013). The increasing number of reports on associations between paternal environmental exposures and risk of disease in the next generation evokes the serious question of how and when the effects of CS exposures are transferred to the male gamete, and whether these effects are sustained through developmental processes.
Possible means of transferring paternal CSC exposure from germ cells to developing embryos may include DNA methylation, histone modifications, mRNAs, and non-coding RNAs. Several periods during the life span of a male may be “windows of susceptibility” when these epigenetic marks are programmed. These include spermatogenesis in the testis and sperm maturation in the epididymis (Nixon et al. 2015). Therefore, studies are needed to determine the point in spermatogenesis at which the germ line is susceptible to constituents of cigarette smoke and the types of epigenetic marks, if any, are established (Bale, 2014). Several small RNAs have been detected in sperm, suggesting that any change in non-coding RNAs due to paternal smoking would be inheritable (Peng et al. 2012; Kiani and Rassoulzadegan, 2013), and their delivery into the oocyte may alter essential functions during early embryogenesis (Sendler et al. 2013). Sperm RNAs reportedly have the ability to direct histone modifications and DNA methylation, for instance in response to paternal smoking (Marczylo et al. 2012), whereas chromatin structure and DNA modifications in turn affect transcription of RNAs. Therefore, exposure to cigarette smoke toxicants might influence this epigenetic crosstalk (Rando, 2012). Male gametes are consistently at enormous risk of epigenetic damage during epigenetic reprogramming, and paternal smoking could change the fidelity of this process. Therefore, research on human sperm is necessary to obtain better insights into the epigenetic mechanisms underlying transmission of environmental effects through the paternal lineage (Soubry et al. 2014). Such work will also lay the foundation for identification of potential biomarkers in predicting disease risk.
Conclusions and future perspectives
As summarized here, the paternal smoking causes generation of ROS, alteration of gene expression, activation of xenobiotic metabolism and MAPK pathways, and apoptosis of germ cells. These mechanisms, which have mainly been identified in vitro, accompanied by other unknown in vivo changes, could cause deleterious outcomes in smokers and their offspring. However, the development of a more suitable animal model with the potential to truly express the effects of paternal exposure to CS and transgenerational transmission of such impact would be an ideal milestone in the field of research. Studies on humans can be improved by establishment of a biomarker of exposure, such as cotinine, B[a]P, and determination of its pharmacokinetics in the offspring would be of added value. As suggested by others, the change in protamine 1 / protamine 2 could serve as an accurate predictor and important marker in better understanding the key regulatory signaling during spermatogenesis (Carrell et al. 2007). In addition to the current most plausible Mendelian concept of disease etiology normally involves DNA sequence mutations, environmentally induced epigenetic inheritance of disease should likely be an equally important consideration. Work such as is described in this review is likely to have impacts beyond cigarette smoking, as PAHs are also released as the unintentional byproducts of industrial processes such as incineration, burning treated wood, and the incomplete combustion of fossil fuels such as diesel truck exhaust (Evans et al. 1993; Douben et al. 2003). Men exposed to these environmental toxicants in occupational, industrial, and military settings experience several negative reproductive effects. For example, the children of firefighters (Olshan et al. 1990) and veterans of Gulf War I (Araneta et al. 2003) and the Vietnam war (IOM, 2000) have higher rates of cardiac defects, cleft palate, renal agenesis, and neural tube defects than the children of men in other professions. Thus, the mechanistic lessons learned in studies of the effects of paternal smoking will apply to many other environmental exposures that affect offspring health.
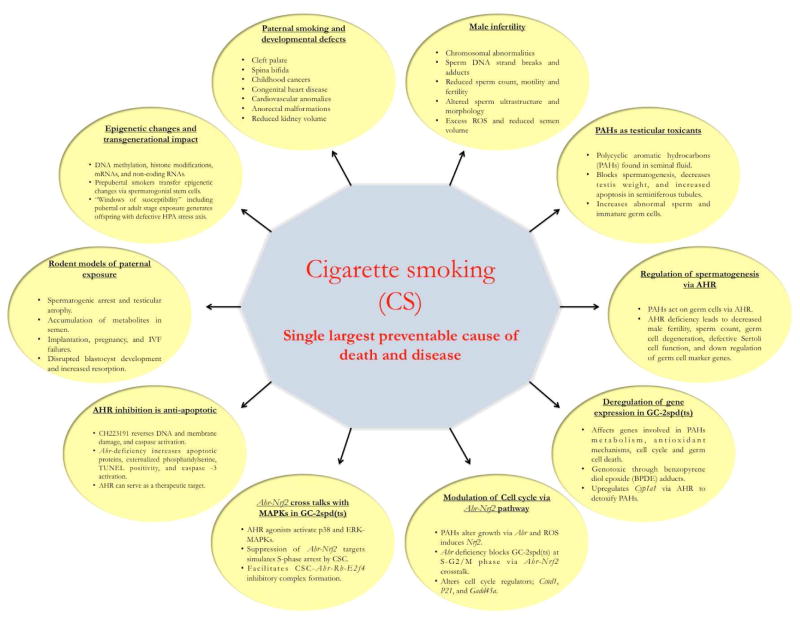
Figure 1.
Highlights.
Paternal smoking is both directly and indirectly associated with poor sperm quality and quantity.
Constituents of cigarette smoke appear to block spermatogenesis and cause testicular atrophy in animal models.
Cigarette smoke condensate (CSC) is mutagenic, carcinogenic, and teratogenic.
Polycyclic aromatic hydrocarbons (PAHs) of CSC act as germ cell toxicants via AHR.
CSC generates oxidative stress and modulates gene expression in testis.
CSC mediates AHR-NRF2 crosstalk in spermatogenic GC-2spd(ts) cells.
CSC mediates interaction between AHR-NRF2 and MAPK pathways during cell cycle arrest in spermatogenic GC-2spd(ts) cells.
AHR in germ cells can be targeted for therapeutic purposes by pharmacological inhibition.
Paternal smoking may mediate epigenetic changes in the offspring through spermatogonial stem cells.
Acknowledgments
The authors thank Dr. Deborah Frank for her suggestions and scientific editing expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USDHHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta GA: USDHHS; 2014. [Google Scholar]
- 2.Smoking and infertility: a committee opinion. The Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98:1400–1406. doi: 10.1016/j.fertnstert.2012.07.1146. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking. Lyon (France): International Agency for Research on Cancer; 2004. p. 83. [PMC free article] [PubMed] [Google Scholar]
- 4.Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton (FL): CRC Press, Taylor & Francis Group; 2009. [Google Scholar]
- 5.DeMarini D. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567:447–474. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Smith CJ, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol. 2000;38:637–646. doi: 10.1016/s0278-6915(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 7.Ding YS, Ashley DL, Watson CH. Determination of 10 Carcinogenic Polycyclic Aromatic Hydrocarbons in Mainstream Cigarette Smoke. J Agric Food Chem. 2007;55:5966–5973. doi: 10.1021/jf070649o. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TK, Jørgensen N, Punab M, Haugen TB, Suominen J, Zilaitiene B, Horte A, Andersen AG, Carlsen E, Magnus Ø, Matulevicius V, Nermoen I, Vierula M, Keiding N, Toppari J, Skakkebaek NE. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol. 2004;59:49–58. doi: 10.1093/aje/kwh002. [DOI] [PubMed] [Google Scholar]
- 9.Virtanen HE, Sadov S, Toppari J. Prenatal exposure to smoking and male reproductive health. Curr Opin Endocrinol Diabetes Obes. 2012;19:228–232. doi: 10.1097/MED.0b013e3283537cb8. [DOI] [PubMed] [Google Scholar]
- 10.Ji BT, Shu XO, Zheng W, Ying DM. Paternal Cigarette Smoking and the Risk of Childhood Cancer among Offspring of Nonsmoking Mothers. J Natl Cancer Inst. 1997;89:238–243. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 11.Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental Smoking and the Risk of Childhood Leukemia. Am J Epidemiol. 2006;163:1091–1100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 12.Vine MF. Smoking and male reproduction: a review. Int J Androl. 1996;19:323–337. doi: 10.1111/j.1365-2605.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Zhang L, McHale CM, Hammond SK. Paternal Smoking and Risk of Childhood Acute Lymphoblastic Leukemia: Systematic Review and Meta-Analysis. J Oncol. 2011:1–16. doi: 10.1155/2011/854584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. British J Cancer. 2003;88:373–381. doi: 10.1038/sj.bjc.6600774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KM, Ward MH, Han S. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leukemia Res. 2009;33:250–258. doi: 10.1016/j.leukres.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwink N, Jenetzky E, Brenner H. Parental risk factors and anorectal malformations: systematic review and meta-analysis. Orphanet J Rare Dis. 2011;6:25. doi: 10.1186/1750-1172-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cresci M, Foffa I, Ait-Ali L, Pulignani S, Gianicolo EA, Botto N, Picano E, Andreassi MG. Maternal and paternal environmental risk factors, metabolizing GSTM1 and GSTT1 polymorphisms, and congenital heart disease. Am J Cardiol. 2011;108:1625–1631. doi: 10.1016/j.amjcard.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Savitz DA, Schwing PJ, Keels MA. Influence of paternal age, smoking, and alcohol consumption on congenital anomalies. Teratol. 1991;44:429–440. doi: 10.1002/tera.1420440409. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Savitz DA, Schwingl PJ, Cai WW. A case control study of paternal smoking and birth defects. Int J Epidemiol. 1992;21:273–278. doi: 10.1093/ije/21.2.273. [DOI] [PubMed] [Google Scholar]
- 20.Kooijman MN, Bakker H, Franco OH, Hofman A, Taal HR, Jaddoe VWV. Fetal smoke exposure and kidney outcomes in school-aged children. Am J Kidney Dis. 2015;66:412–420. doi: 10.1053/j.ajkd.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Janny L, Menezo YJ. Evidence for a strong paternal effect on human preimplantation embryo development and blastocyst formation. Mol Reprod Dev. 38:36–42. doi: 10.1002/mrd.1080380107. [DOI] [PubMed] [Google Scholar]
- 22.Sofikitis N, Miyagawa I, Dimitriadis D, Zavos P, Sikka S, Hellstrom W. Effects of smoking on testicular function, semen quality and sperm fertilizing capacity. J Urol. 1995;154:1030–1034. [PubMed] [Google Scholar]
- 23.Ubaldi F, Nagy Z, Rienzi L, Tesarik J, Anniballo R, Franco G, Fabris F, Greko E. Reproductive capacity of spermatozoa from men with testicular failure. Hum Reprod. 1999;14:2796–2800. doi: 10.1093/humrep/14.11.2796. [DOI] [PubMed] [Google Scholar]
- 24.Godschalk RWL, Verhofstad N, Verheijea M, Yauk CL, Linschooten JO, van Steeg H, van Oostrom CT, van Benthem J, van Schooten FJ. Effects of benzo[a]pyrene on mouse germ cells: heritable DNA mutation, testicular cell hypomethylation and their interaction with nucleotide excision repair. Toxicol Res. 2015;4:718–724. [Google Scholar]
- 25.Pattinson HA, Taylor PJ, Pattinson MH. The effect of cigarette smoking on ovarian function and early pregnancy outcome of in vitro fertilization treatment. Fertil Steril. 1991;55:730–733. doi: 10.1016/s0015-0282(16)54248-4. [DOI] [PubMed] [Google Scholar]
- 26.Hughes EG, Yeo J, Claman P, et al. Cigarette smoking and the outcomes of in vitro fertilization: measurement of effect size and levels of action. Fertil Steril. 1994;62:807–814. doi: 10.1016/s0015-0282(16)57009-5. [DOI] [PubMed] [Google Scholar]
- 27.Joesbury KA, Edirisinghe WR, Phillips MR, Yovich JL. Evidence that male smoking affects the likelihood of a pregnancy following IVF tteatment: application of the modified cumulative embryo score. Hum Reprod. 1998;13:1506–1513. doi: 10.1093/humrep/13.6.1506. [DOI] [PubMed] [Google Scholar]
- 28.Axelsson J, Rylander L, Rignell-Hydbom A, Silfver KA, Stenqvist A, Giwercmanet A. The Impact of Paternal and Maternal Smoking on Semen Quality of Adolescent Men. PLoS ONE. 2013;8(6):e66766. doi: 10.1371/journal.pone.0066766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olshan AF, Faustman EM. Male-mediated developmental toxicity. Annu Rev Public Health. 1993;14:159–181. doi: 10.1146/annurev.pu.14.050193.001111. [DOI] [PubMed] [Google Scholar]
- 30.Leonardi-Bee J, Britton J. Secondhand Smoke and Adverse Fetal Outcomes in Nonsmoking Pregnant Women: A Meta-analysis. Pediatrics. 2011;127:734–741. doi: 10.1542/peds.2010-3041. [DOI] [PubMed] [Google Scholar]
- 31.Yauk CL, Berndt ML, Williams A, Rowan-Carroll A, Douglas GR, Stämpfli MR. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res. 2007;67:5103–5106. doi: 10.1158/0008-5472.CAN-07-0279. [DOI] [PubMed] [Google Scholar]
- 32.Kapawa A, Giannakis D, Tsoukanelis K, Kanakas N, Baltogiannis D, Agapitos E, Loutradis D, Miyagawa I, Sofikitis N. Effects of paternal cigarette smoking on testicular function, sperm fertilizing capacity, embryonic development, and blastocyst capacity for implantation in rats. Andrologia. 2004;36:57–68. doi: 10.1111/j.1439-0272.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 33.MacKenzie KM, Angevine DM. Infertility in mice exposed in utero to benzo(a)pyrene. Biol Reprod. 1981;24:183–191. doi: 10.1095/biolreprod24.1.183. [DOI] [PubMed] [Google Scholar]
- 34.Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood) Hum Reprod. 2000;5:1703–1708. doi: 10.1093/humrep/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 35.Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS, Jensen MS, Toft G, Bonde JP. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum Reprod. 2007;22:188–196. doi: 10.1093/humrep/del364. [DOI] [PubMed] [Google Scholar]
- 36.Mostafa M. Cigarette smoking and male infertility. J Adv Res. 2010;1:179–186. [Google Scholar]
- 37.Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res. 1996;351:199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 38.Shen HM, Chia SE, Ni ZY, New AL, Lee BL, Ong CN. Detection of oxidative DNA damage in human sperm and the association with cigarette smoking. Reprod Toxicol. 1997;11:675–680. doi: 10.1016/s0890-6238(97)00032-4. [DOI] [PubMed] [Google Scholar]
- 39.Potts RJ, Newbury CJ, Smith G, Notarianni LJ, Jefferies TM. Sperm chromatin damage associated with male smoking. Mutat Res. 1999;423:103–111. doi: 10.1016/s0027-5107(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 40.Horak S, Polanska J, Widlak P. Bulky DNA adducts in human sperm: relationship with fertility, semen quality, smoking, and environmental factors. Mutat Res. 2003;537:53–65. doi: 10.1016/s1383-5718(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 41.Robbins WA, Vine MF, Truong KY, Everson RB. Use of fluorescence in situ hybridization (FISH) to assess effects of smoking, caffeine, and alcohol on aneuploidy load in sperm of healthy men. Environ Mol Mutagen. 1997;30:175–183. doi: 10.1002/(sici)1098-2280(1997)30:2<175::aid-em10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Rubes J, Lowe X, Moore D, 2nd, Perreault S, Slott V, Evenson D, Selevan SG, Wyrobek AJ. Smoking cigarettes is associated with increased sperm disomy in teenage men. Fertil Steril. 1998;70:715–723. doi: 10.1016/s0015-0282(98)00261-1. [DOI] [PubMed] [Google Scholar]
- 43.Kunzle R, Mueller MD, Hanggi W, Birkhauser MH, Drescher H, Bersinger NA. Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003;79:287–291. doi: 10.1016/s0015-0282(02)04664-2. [DOI] [PubMed] [Google Scholar]
- 44.Sofikitis N, Takenaka M, Kanakas N, Papadopoulos H, Yamamoto Y, Drakakis P, Miyagawa I. Effects of cotinine on sperm motility, membrane function, and fertilizing capacity in vitro. Urol Res. 2000;28:370–375. doi: 10.1007/s002400000138. [DOI] [PubMed] [Google Scholar]
- 45.Ramesh A, Inyang F, Lunstra DD, Niaz MS, Kopsombut PM, Jones KM, Hood DB, Hills ER, Archibong AE. Alteration of Fertility Endpoints in Adult Male F-344 Rats by Subchronic Exposure to Inhaled Benzo(a)pyrene. Exp Toxicol Pathol. 2008;60:269–280. doi: 10.1016/j.etp.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jana K, Samanta PK, De DK. Nicotine Diminishes Testicular Gametogenesis, Steroidogenesis, and Steroidogenic Acute Regulatory Protein Expression in Adult Albino Rats: Possible Influence on Pituitary Gonadotropins and Alteration of Testicular Antioxidant Status. Toxicol Sci. 2010;116:647–659. doi: 10.1093/toxsci/kfq149. [DOI] [PubMed] [Google Scholar]
- 47.Pacifici R, Altieri I, Gandini L, Lenzi A, Rosa M. Nicotine, cotinine, and trans-3 hydroxycotinine levels in seminal plasma of smokers: effects on sperm parameters. Ther Drug Monit. 1993;15:358–363. doi: 10.1097/00007691-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Pacifici R, Altieri I, Gandini L, Lenzi A, Passa AR, Pichini S, Rosa M, Zuccaro P, Dondero F. Environmental tobacco smoke: nicotine and cotinine concentration in semen. Environ Res. 1995;68:69–72. doi: 10.1006/enrs.1995.1009. [DOI] [PubMed] [Google Scholar]
- 49.Chia SE, Ong CN, Tsakok FM. Effects of cigarette smoking on human semen quality. Arch Androl. 1994;33:163–168. doi: 10.3109/01485019408987820. [DOI] [PubMed] [Google Scholar]
- 50.Vine MF, Tse CKJ, Hu PC, Truong YK. Cigarette smoking and semen quality. Fertil Steril. 1996;65:835– 842. doi: 10.1016/s0015-0282(16)58223-5. [DOI] [PubMed] [Google Scholar]
- 51.Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994;61:35–43. [PubMed] [Google Scholar]
- 52.Zavos PM, Zarmakoupis-Zavos PN. Impact of cigarette smoking on human reproduction: its effects on male and female fecundity. Technol. 1999;6:9–16. [Google Scholar]
- 53.Gaspari L, Chang SS, Santella RM, Garte S, Pedotti P, Taioli E. Polycyclic aromatichydrocarbon-DNAadducts inhumansperm as amarker of DNA damage and infertility. Mutat Res. 2003;535:155–160. doi: 10.1016/s1383-5718(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 54.Bjorge C, Brunborg G, Wiger R, Holme JA, Scholz T, Dybing E, Søderlund EJ. A comparative study of chemically induced DNA damage in isolated human and rat testicular cells, Reprod. Toxicol. 1996;10:509–519. doi: 10.1016/s0890-6238(96)00138-4. [DOI] [PubMed] [Google Scholar]
- 55.Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ., Jr Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002;78:491–499. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 56.Aitken RJ, Baker MA. Oxidative stress and male reproductive biology. Reprod Fertil Dev. 2004;16:581–588. doi: 10.10371/RD03089. [DOI] [PubMed] [Google Scholar]
- 57.Jones R, Mann Sherins RJ. Peroxidative breakdown of phospholipids in human spermatozoa: spermicidal effects of fatty acid peroxides and protective action of seminal plasma Fertil. Steril. 1979;31:531–537. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 58.de Lamirande E, Gagnon C. A positive role for the superoxide anion in the triggering of human sperm hyperactivation and capacitation. Int J Androl. 1993;16:21–25. doi: 10.1111/j.1365-2605.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 59.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol. 2008;636:154–171. doi: 10.1007/978-0-387-09597-4_9. [DOI] [PubMed] [Google Scholar]
- 60.Potts R, Newbury C, Smith G, Notarianni L, Jefferies T. Sperm chromatin damage associated with male smoking. Mutation Res. 1999;423:103–111. doi: 10.1016/s0027-5107(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 61.Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 62.Collodel G, Capitani S, Pammolli A, Giannerinin V, Geminiani M, Moretti E. Semen Quality of Male Idiopathic Infertile Smokers and Nonsmokers: An Ultrastructural Study. J Androl. 2010;31:108–113. doi: 10.2164/jandrol.109.007773. [DOI] [PubMed] [Google Scholar]
- 63.Zenses MT. Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod update. 2000;6:122–131. doi: 10.1093/humupd/6.2.122. [DOI] [PubMed] [Google Scholar]
- 64.Evans HJ, Fletcher J, Torrance M, Hargreave TB. Sperm abnormalities and cigarette smoking. Lancet. 1981;1:627–629. doi: 10.1016/s0140-6736(81)91550-6. [DOI] [PubMed] [Google Scholar]
- 65.Hoidas S, Williams AE, Tocher JL, Hargreave TB. Scoring sperm morphology from fertile and infertile cigarette smokers using the scanning electron microscope and image analysis. Fertil Steril. 1985;43:595–598. doi: 10.1016/s0015-0282(16)48503-1. [DOI] [PubMed] [Google Scholar]
- 66.Zavos PM, Correa JR, Karagounis CS, Ahparaki A, Phoroglou C, Hicks CL, Zarmakoupis-Zavos PN. An electron microscope study of the axonemal ultrastructure in human spermatozoa from male smokers and nonsmokers. Fertil Steril. 1998;69:430–434. doi: 10.1016/s0015-0282(97)00563-3. [DOI] [PubMed] [Google Scholar]
- 67.Linschooten JO, Van Schooten FJ, Baumgartner A, Cemeli E, van Delft J, Anderson D, Godschalk RWL. Use of spermatozoal mRNA profiles to study gene–environment interactions in human germ cells. Mut Research. 2009;667:70–76. doi: 10.1016/j.mrfmmm.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 68.Mocarelli P, Gerthoux PM, Needham LL, Patterson DG, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P. Perinatal Exposure to Low Doses of Dioxin Can Permanently Impair Human Semen Quality. Environ Health Perspect. 2011;119:713–718. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 70.Viczian M. The effect of cigarette smoke inhalation on spermatogenesis in rats. Experientia. 1968;24:511–513. doi: 10.1007/BF02144424. [DOI] [PubMed] [Google Scholar]
- 71.Wyrobeck AJ, Bruce WR. Chemical induction of sperm abnormalities in mice. Proc Nat Acad Sci USA. 1975;72:4425–4429. doi: 10.1073/pnas.72.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattison DR. The effects of smoking on fertility from gametogenesis to implantation. Environ Res. 1982;28:410–433. doi: 10.1016/0013-9351(82)90139-6. [DOI] [PubMed] [Google Scholar]
- 73.Denison MS, Heath-Pagliuso S. The ahr receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- 74.Revel A, Raanani H, Younglai E, Xu J, Han R, Savouret JF, Casper RF. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod Toxicol. 2001;15:479–486. doi: 10.1016/s0890-6238(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 75.Coutts SM, Fulton N, Anderson RA. Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum Reprod. 2007;22:2912–2918. doi: 10.1093/humrep/dem300. [DOI] [PubMed] [Google Scholar]
- 76.Georgellis A, Toppari J, Veromaa T, Rydström J, Parvinen M. Inhibition of meiotic divisions of rat spermatocytes in vitro by polycyclic aromatic hydrocarbons. Mutat Res. 1990;231:125–135. doi: 10.1016/0027-5107(90)90019-z. [DOI] [PubMed] [Google Scholar]
- 77.Essenberg JM, Fagan L, Mallerstein AJ. Chronic poisoning of the ovaries and testis of albino rats by nicotine and cigarette smoke. West J Surg Obstet Gynecol. 1951;59:27–32. [PubMed] [Google Scholar]
- 78.Schultz R, Suominen J, Varre T, Hakovirta H, Parvinen M, Toppari J, Pelto-Huikko M. Expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator messenger ribonucleic acids and proteins in rat and human testis. Endocrinol. 2003;144:767–776. doi: 10.1210/en.2002-220642. [DOI] [PubMed] [Google Scholar]
- 79.Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: modulation of cell cycle and extracellular matrix genes. Toxicol Sci. 2005;88:231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- 80.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 81.Grassman JA, Masten SA, Walker NJ, Lucier GW. Animal models of human response to dioxins. Environ Health Perspect. 1998;106:761–775. doi: 10.1289/ehp.98106761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schecter A, McGee H, Stanley JS, Boggess K, Brandt-Rauf P. Dioxins and dioxin-like chemicals in blood and semen of American Vietnam veterans from the state of Michigan. Am J Ind Med. 1996;30:647–654. doi: 10.1002/(SICI)1097-0274(199612)30:6<647::AID-AJIM1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 83.Simanainen U, Haavisto T, Tuomisto JT, Paranko J, Toppari J, Tuomisto J, Peterson RP, Viluksela M. Pattern of Male Reproductive System Effects After in Utero and Lactational 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Exposure in Three Differentially TCDD-Sensitive Rat Lines. Toxicol Sci. 2004a;80:101–108. doi: 10.1093/toxsci/kfh142. [DOI] [PubMed] [Google Scholar]
- 84.Simanainen U, Adamsson A, Tuomisto JT, Miettinen HM, Toppari J, Tuomisto J, Viluksela M. Adult 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure and effects on male reproductive organs in three differentially TCDD-susceptible rat lines. Toxicol Sci. 2004b;81:401–407. doi: 10.1093/toxsci/kfh212. [DOI] [PubMed] [Google Scholar]
- 85.Rider CV, Furr JR, Wilson VS, Gray EL. Cumulative Effects of In Utero Administration of Mixtures of Reproductive Toxicants that Disrupt Common Target Tissues via Diverse Mechanisms of Toxicity. Int J Androl. 2010;33:443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 2008;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- 87.Matsumura F. The significance of the non-genomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem Pharmacol. 2009;77:608–626. doi: 10.1016/j.bcp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Puga A, Marlowe J, Barnes S, Chang CY, Maier A, Tan Z, Kerzee JK, Chang X, Strobeck M, Knudsen ES. Role of the aryl hydrocarbon receptor in cell cycle regulation. Toxicol. 2002;181–182:171–177. doi: 10.1016/s0300-483x(02)00276-7. [DOI] [PubMed] [Google Scholar]
- 89.Gasiewicz T, Henry E. History of research in AHR. In: Pohjanvirta R, editor. The AH Receptor in Biology and Toxicology. Hoboken, NJ: Wiley; 2012. pp. 3–32. [Google Scholar]
- 90.Roman BL, Pollenz RS, Peterson RE. Responsiveness of the adult male rat reproductive tract to 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: Ah receptor and ARNT expression, CYP1A1 induction, and Ah receptor down-regulation. Toxicol Appl Pharmacol. 1998;150:228–239. doi: 10.1006/taap.1998.8388. [DOI] [PubMed] [Google Scholar]
- 91.Karman B, Hernandez-Ochoa I, Ziv-Gal A, Flaws J. Involvement of the AHR in development and functioning of the female and male reproductive systems. In: Pohjanvirta R, editor. The AH Receptor in Biology and Toxicology. Hoboken, NJ: Wiley; 2012. pp. 437–466. [Google Scholar]
- 92.Baba T, Shima Y, Owaki A, Mimura J, Oshima M, Fujii-Kuriyama Y, Morohashi KI. Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice. Sex Dev. 2008;2:1–11. doi: 10.1159/000117714. [DOI] [PubMed] [Google Scholar]
- 93.Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, Peterson RE. Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed male mice. J Toxicol Environ Health. 2001;64:327–342. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- 94.Mably TA, Bierke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: 3. Effects on spermatogenesis and reproductive capability. Toxicol Appl Pharmacol. 1992;114:118–126. doi: 10.1016/0041-008x(92)90103-y. [DOI] [PubMed] [Google Scholar]
- 95.Foster WG, Maharaj-Briceño S, Cyr DG. Dioxin-induced changes in epididymal sperm count and spermatogenesis. Environ Health Perspect. 2010;118:458–464. doi: 10.1289/ehp.0901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hansen DA, Esakky P, Drury AM, Lamb L, Moley KH. The Aryl Hydrocarbon Receptor Is Important for Proper Seminiferous Tubule Architecture and Sperm Development in Mice. Biol Reprod. 2014;90:1–12. doi: 10.1095/biolreprod.113.108845. [DOI] [PubMed] [Google Scholar]
- 97.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005:6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- 98.Narayan S, Jaiswal AS, Kang D, Srivastava P, Das GM, Gairola CG. Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene. 2004;23:5880–5889. doi: 10.1038/sj.onc.1207792. [DOI] [PubMed] [Google Scholar]
- 99.Nagaraj NS, Beckersa S, Mensaha JK, Waigela S, Vigneswaranc N, Zacharias W. Cigarette smoke condensate induces cytochromes P450 and aldo-keto reductases in oral cancer cells. Toxicol Letters. 2006;165:182–194. doi: 10.1016/j.toxlet.2006.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bosio A, Knorr C, Janssen U, Gebel S, Haussmann HJ, Muller T. Kinetics of gene expression profiling in Swiss 3T3 cells exposed to aqueous extracts of cigarette smoke. Carcinogenesis. 2002;23:741–748. doi: 10.1093/carcin/23.5.741. [DOI] [PubMed] [Google Scholar]
- 101.Fields WR, Leonard RM, Odom PS, Nordskog BK, Ogden MW, Doolittle DJ. Gene Expression in Normal Human Bronchial Epithelial (NHBE) Cells Following In Vitro Exposure to Cigarette Smoke Condensate. Toxicol Sci. 2005;86:84–91. doi: 10.1093/toxsci/kfi179. [DOI] [PubMed] [Google Scholar]
- 102.van Leeuwen DM, Gottschalk RWH, van Herwijnen MH, Moonen EJ, Kleinjans JCS, van Delft JHM. Differential Gene Expression in Human Peripheral Blood Mononuclear Cells Induced by Cigarette Smoke and Its Constituents. Toxicol Sci. 2005;86:200–210. doi: 10.1093/toxsci/kfi168. [DOI] [PubMed] [Google Scholar]
- 103.Han ES, Muller FL, Perez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, Epstein CJ, Roberts J, Remmen HV, Richardson A. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Esakky P, Hansen DA, Drury AM, Moley KH. Cigarette smoke condensate induces aryl hydrocarbon receptor-dependent changes in gene expression in spermatocytes. Reprod Toxicol. 2012;34:665–676. doi: 10.1016/j.reprotox.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Song Z, Pollenz RS. Functional Analysis of murine aryl hydrocarbon (AH) receptors defective in nuclear import: impact of receptor degradation and gene activation. Mol Pharmacol. 2003;63:597–606. doi: 10.1124/mol.63.3.597. [DOI] [PubMed] [Google Scholar]
- 106.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: Sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 107.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicol Sci. 2009;111:238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kremer J, Gleichmann E, Esser C. Thymic stroma exposed to arylhydrocarbon receptor-binding xenobiotics fails to support proliferation of early thymocytes but induces differentiation. J Immunol. 1994;153:2778–2786. [PubMed] [Google Scholar]
- 111.Puga A, Barnes SJ, Dalton TP, Chang C, Knudsen ES, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275:2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 112.Marlowe JL, Knudsen ES, Schwemberger S, Puga A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J Biol Chem. 2004;279:29013–29022. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- 113.Ma Q, Whitlock JP., Jr The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol. 1996;16:2144–2150. doi: 10.1128/mcb.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tohkin M, Fukuhara M, Elizondo G, Tomita S, Gonzalez FJ. Aryl hydrocarbon receptor is required for p300-mediated induction of DNA synthesis by adenovirus E1A. Mol Pharmacol. 2000;58:845–851. doi: 10.1124/mol.58.4.845. [DOI] [PubMed] [Google Scholar]
- 115.Elizondo G, Fernandez-Salguero P, Sheikh MS, Kim GY, Fornace AJ, Lee KS, Gonzalez FJ. Altered cell cycle control at the G2/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Mol Pharmacol. 2000;57:1056–1063. [PubMed] [Google Scholar]
- 116.Abdelrahim M, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol Pharmacol. 2003;63:1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 117.Esakky P, Hansen DA, Drury A, Moley KH. Modulation of cell cycle progression in the spermatocyte cell line [GC-2spd(ts) Cell-Line] by cigarette smoke condensate (CSC) via aryl hydrocarbon receptor-nuclear factor erythroid 2-related factor 2 (Ahr-Nrf2) pathway. Biol Reprod. 2014;90:1–12. doi: 10.1095/biolreprod.113.113225. [DOI] [PubMed] [Google Scholar]
- 118.Jeffy BD, Chen EJ, Gudas JM, Romagnolo DF. Disruption of cell cycle kinetics by benzo[a]pyrene: inverse expression patterns of BRCA-1 and p53 in MCF-7 cells arrested in S and G2. Neoplasia. 2000;2:460–470. doi: 10.1038/sj.neo.7900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khan QA, Dipple A. Diverse chemical carcinogens fail to induce G(1) arrest in MCF-7 cells. Carcinogenesis. 2000;21:1611–1618. [PubMed] [Google Scholar]
- 120.Hamouchene H, Arlt VM, Giddings I, Phillips DH. Influence of cell cycle on responses of MCF-7 cells to benzo[a]pyrene. BMC Genomics. 2011;12:333. doi: 10.1186/1471-2164-12-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD, Ortiz L, Rau BA, Luderer U. Knockout of the transcription factor Nrf2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49:1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Applied Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280:32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 125.Hayes JD, Dinkova-Kostova AT, McMahon M. Cross talk between Transcription Factors Ahr and Nrf2: Lessons for Cancer Chemoprevention from Dioxin. Toxicol Sci. 2009;111:199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 126.Schackelford RE, Kaufmann WK, Paules RS. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect. 1999;107:5–24. doi: 10.1289/ehp.99107s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wan Y, Wang Z, Shao Y, Xu Y, Voorhees J, Fisher G. UV-induced expression of GADD45 is mediated by an oxidant sensitive pathway in cultured human keratinocytes and in human skin in vivo. Int J Mol Med. 6:683–688. doi: 10.3892/ijmm.6.6.683. [DOI] [PubMed] [Google Scholar]
- 128.Akerman GS, Rosenzweig BA, Domon OE, McGarrity LJ, Blankenship LR, Tsai CA, Culp SJ, MacGregor JT, Sistare FD, Chen JJ, Morris SM. Gene expression profiles and genetic damage in benzo(a)pyrene diol epoxide-exposed TK6 cells. Mutat Res. 2004;18:43–64. doi: 10.1016/j.mrfmmm.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 129.Henklová P, Vrzal R, Ulrichová J, et al. Role of mitogen-activated protein kinases in aryl hydrocarbon receptor signaling. Chem Biol Interact. 2008;172:93–104. doi: 10.1016/j.cbi.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 130.Tan Z, Chang X, Puga A, et al. Activation of mitogen-activated protein kinases (MAPKs) by aromatic hydrocarbons: role in the regulation of aryl hydrocarbon receptor (AHR) function. Biochem Pharmacol. 2002;64:771–780. doi: 10.1016/s0006-2952(02)01138-3. [DOI] [PubMed] [Google Scholar]
- 131.Long WP, Pray-Grant M, Tsai JC, et al. Protein kinase C activity is required for aryl hydrocarbon receptor pathway-mediated signal transduction. Mol Pharmacol. 1998;53:691–700. doi: 10.1124/mol.53.4.691. [DOI] [PubMed] [Google Scholar]
- 132.Weiss C, Faust D, Durk H, et al. TCDD induces c-jun expression via a novel Ah (dioxin) receptor-mediated p38-MAPK-dependent pathway. Oncogene. 2005;24:4975–4983. doi: 10.1038/sj.onc.1208679. [DOI] [PubMed] [Google Scholar]
- 133.Esakky P, Hansen DA, Drury A, Moley KH. Cigarette smoke-induced cell cycle arrest in spermatocytes [GC-2spd(ts)] is mediated through crosstalk between Ahr-Nrf2 pathway and MAPK signaling. J Mol Cell Biol. 2015a;7:73–87. doi: 10.1093/jmcb/mju049. [DOI] [PubMed] [Google Scholar]
- 134.Reddy NM, Kleeberger SR, Bream JH. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene. 2008;27:5821–5832. doi: 10.1038/onc.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hai T, Wolfgang CD, Marsee AK, et al. ATF3 and stress responses. Gene Exp. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- 136.Inoue K, Zama T, Kamimoto T, et al. TNFα-induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes To Cells. 2004;9:59–70. doi: 10.1111/j.1356-9597.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 137.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim K, Jeong J, Surh Y, et al. Expression of stress-response ATF3 is mediated by Nrf2 in Astrocytes. Nucleic Acids Res. 2010;38:48–59. doi: 10.1093/nar/gkp865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marlowe JL, Puga A. Arylhydrocarbon receptor, cell cycle regulation, toxicity, and tumorignesis. J cell Biochem. 2005;96:1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- 140.Ge NL, Elferink CJ. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J Biol Chem. 1998;28:22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 141.Jin MH, Hong CH, Lee HY, et al. Enhanced TGF-b1 is involved in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induced oxidative stress in C57BL/6 mouse testis. Toxicol Letters. 2008;178:202–209. doi: 10.1016/j.toxlet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 142.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 143.Esakky P, Hansen DA, Drury A, Cusumano A, Moley KH. Cigarette smoke-induced cell death of a spermatocyte cell line can be prevented by inactivating the Aryl hydrocarbon receptor. Cell Death Discover. 2015b;1:15050. doi: 10.1038/cddiscovery.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Belcheva A, Ivanova-Kicheva M, Tzvetkova P, Marinov M. Effects of cigarette smoking on sperm plasma membrane integrity and DNA fragmentation. Int J Androl. 2004;5:296–300. doi: 10.1111/j.1365-2605.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 145.Ciolino HP, Yeh GC. Inhibition of Aryl Hydrocarbon-Induced Cytochrome P-450 1A1 Enzyme Activity and CYP1A1 Expression by Resveratrol. Mol Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- 146.Fabia J, Thuy TD. Occupation of father at time of birth of children dying of malignant diseases. Br J Prev Soc Med. 1974;28:98–100. doi: 10.1136/jech.28.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wilkins JR, III, Sinks TH., Jr Paternal occupation and Wilms’ tumour in offspring. J Epidemiol Commun Health. 1984;38:7–11. doi: 10.1136/jech.38.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Castro-Jimenez MA, Orozco-Vargas LC. Parental exposure to carcinogens and risk for childhood acute lymphoblastic leukemia, Colombia, 2000–2005. Prev Chronic Dis. 2011;8:A106. [PMC free article] [PubMed] [Google Scholar]
- 149.Jeng HA, Pan CH, Lin WY, Wu MT, et al. Biomonitoring of polycyclic aromatic hydrocarbons from coke oven emissions and reproductive toxicity in nonsmoking workers. J Hazard Mater. 2013;245:436–43. doi: 10.1016/j.jhazmat.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 150.Ji G, Yan L, Wu S, Liu J, et al. Bulky DNA adducts in human sperm associated with semen parameters and sperm DNA fragmentation in infertile men: a cross-sectional study. Environ Health. 2013;12:82. doi: 10.1186/1476-069X-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Friedler G. Paternal Exposures: Impact on Reproductive and Developmental Outcome. An Overview. Pharmacol Biochem Behavior. 1996;55:691–700. doi: 10.1016/s0091-3057(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 152.Northstone K, Davey GJ, Smith G, Miller LL, Pembrey M. Prepubertal start of father’s smoking and increased body fat in his sons: further characterization of paternal transgenerational responses. Eur J Hum Genet. 2014;22:1382–1386. doi: 10.1038/ejhg.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Hormones and Behavior. 2011;59:306–314. doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nanassy L, Carrell DT. Paternal effects on early embryogenesis. J Exp Clinic Assist Reprod. 2008;5:1–9. doi: 10.1186/1743-1050-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner NK. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS One. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kumar M, Kumar K, Jain S, Hassan T, Dada R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics (Sao Paulo) 2013;68:5–14. doi: 10.6061/clinics/2013(Sup01)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm miRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, Holt JE, McLaughlin EA. The MicroRNA Signature of Mouse Spermatozoa Is Substantially Modified During Epididymal Maturation. Biol Reprod. 2015;93:1–20. doi: 10.1095/biolreprod.115.132209. [DOI] [PubMed] [Google Scholar]
- 159.Bale TL. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci. 2014;16:297–305. doi: 10.31887/DCNS.2014.16.3/tbale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peng H, Shi J, Zhang Y, Zhang H, et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012;22:1609–1612. doi: 10.1038/cr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kiani J, Rassoulzadegan M. A load of small RNAs in the sperm – how many bits of hereditary information? Cell Res. 2013;23:18–9. doi: 10.1038/cr.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sendler E, Johnson GD, Mao S, Goodrich RJ, et al. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res. 2013;41:4104–4117. doi: 10.1093/nar/gkt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7:432–439. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- 164.Rando OJ. Daddy issues: paternal effects on phenotype. Cell. 2012;151:702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays. 2014;36:359–371. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the linl? Hum Reprod Update. 2007;13:313–327. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 167.Evans WH, Thomas NC, Boardman MC, Nash SJ. Relationships of polycyclic aromatic hydrocarbon yield with particulate matter (water and nicotine free) yields in mainstream and sidestream cigarette smoke. Sci Total Environ. 1993;136:101–109. [Google Scholar]
- 168.Douben PET, editor. PAHs: An Ecotoxicological Perspective. Hoboken (NJ): John Wiley & Sons; 2003. [Google Scholar]
- 169.Olshan AF, Teschke K, Baird PA. Birth defects among offspring of firemen. Am J Epidemiol. 1990;131:312–321. doi: 10.1093/oxfordjournals.aje.a115500. [DOI] [PubMed] [Google Scholar]
- 170.Araneta MR, Schlangen KM, Edmonds LD, Destiche DA, Merz RD, et al. Prevalence of birth defects among infants of Gulf War veterans in Arkansas, Arizona, California, Georgia, Hawaii, and Iowa, 1989–1993. Birth Defects Res A Clin Mol Teratol. 2003;67:246–260. doi: 10.1002/bdra.10033. [DOI] [PubMed] [Google Scholar]
- 171.IOM; IOM, editor. Veterans and Agent Orange Update 2000: Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Institute of Medicine DoHPaDP; Washington DC: The National Academy Press; 2000. [Google Scholar]