Abstract
The projected number of people who will develop age-related macular degeneration in estimated at 2020 is 196 million and is expected to reach 288 million in 2040. Also, the number of people with Diabetic retinopathy will grow from 126.6 million in 2010 to 191.0 million by 2030. In addition, it is estimated that there are 2.3 million people suffering from uveitis worldwide. Because of the anti-inflammatory properties of glucocorticoids (GCs), they are often used topically and/or intravitreally to treat ocular inflammation conditions or edema associated with macular degeneration and diabetic retinopathy. Unfortunately, ocular GC therapy can lead to severe side effects. Serious and sometimes irreversible eye damage can occur as a result of the development of GC-induced ocular hypertension causing secondary open-angle glaucoma. According to the world health organization, glaucoma is the second leading cause of blindness in the world and it is estimated that 80 million will suffer from glaucoma by 2020. In the current review, mechanisms of GC-induced damage in ocular tissue, GC-resistance, and enhancing GC therapy will be discussed.
Keywords: glaucoma, glucocorticoids, steroid receptors, trabecular meshwork
1. Introduction
Glaucoma is a group of heterogeneous diseases resulting in optic nerve degeneration characterized by functional and structural impairment of ocular tissues (Dibas and Yorio 2004). Particularly affected are the trabecular meshwork (TM), the optic nerve head and retinal ganglion cells, the loss of the latter results in blindness. It is estimated that more than 2 million Americans have glaucoma and additional individuals have the disease but it is undiagnosed since there are no symptoms until peripheral vision deteriorates. Primary open angle glaucoma (POAG) is a late-onset disease with elevated IOP as the greatest risk factor associated with this disease. One to two percent of humans over 40 are diagnosed with POAG and the incidence among African American and Hispanic populations is especially high (Quigley and Brown 2006).
Although a number of the risk factors have been identified (i.e., family history, elevated intraocular pressure (IOP), age, race, and sensitivity to glucocorticoids (GCs)), elevated IOP remains the key risk factor for the development and progression of primary open-angle glaucoma (POAG). Therefore, IOP lowering drugs have been the first line of defense in delaying the progression of the disease. The elevation of IOP in POAG results from increased aqueous humor outflow resistance and is associated with changes in TM cells accompanied by increased deposition of extracellular matrix material (ECM) (Tamm and Fuchshofer 2007; and Rönkkö et al., 2007) as well as differences in the ECM profile (see review by Vranka et al., 2015). GCs can induce similar changes in the TM ECM (Johnson and Gottanka 1997; and Zhou and Li 1998) that results in elevated IOP.
It is estimated that 1.2% of the US population is currently being treated with oral GCs (Overman et al., 2013). Two other studies estimated that glucocorticoids are still taken by around 2% of adults in the US at any given time (Ettinger et al., 2001; and van Staa et al., 2000).
Although anti-VEGF represent the first line of defense in the treatment of macular edema in macular degeneration and diabetic retinopathy, GCs, due to their powerful anti-inflammatory properties, are often used topically and/or intravitreally to treat ocular inflammation conditions or edema associated with macular degeneration and diabetic retinopathy (Haeck et al., 2011; and Noble and Goa 1998). More recently, GCs has been used in combination with anti-VEGF (Vakalis et al., 2015; Calvo et al.; and 2015, Lim et al. 2015) or photodynamic monotherapy (Selım et al., 2014). These combinations have been shown to have superior results than either treatment alone.
Over 2 million people worldwide are thought to have uveitis and in the United States uveitis with an incidence of 15 cases per 100,000 population (Foster and Vitale 2002) and 43,000 newly diagnosed cases a year (Vadot 1992). Women have a higher prevalence of uveitis than men, and the largest differences occur in older age groups (Gritz and Wong 2004). The use of steroid implants for noninfectious uveitis has been reviewed in Brady et al., 2016. Glucocorticoids are a mainstay of therapy of ocular inflammation and are either administered topically, regionally, or systemically. Regional application is done either by periocular injection or intravitreal injections. Periocular injections of GCs are effective in treating ocular inflammation (Sen et al., 2014). Approximately 20% of uveitis patients in the United States develop glaucoma that is not race, sex, or age-dependent. Glaucoma is much more common in chronic uveitis with incidence of 11% after 5 years. This differs from acute uveitis with, 7.6% of patients diagnosed with glaucoma after only 12 months (Herbert et al., 2004; and Neri et al., 2004).
Unfortunately, chronic use of GCs is associated with several deleterious side effects including: hepatic steatosis, osteoporosis, muscle wasting, growth retardation, infertility, cognitive dysfunction, glaucoma, cataracts, and topical skin thinning (Patel et al., 2014). In addition to humans, GCs have been shown to induce IOP elevation in rabbits (Lorenzetti 1970), cats (Zhan et al., 1992), dogs (Gelatt and Mackay 1998), cows (Gerometta et al., 2004), sheep (Gerometta et al., 2009), monkeys (Fingert et al., 2001), rats (Sawaguchi et al., 2005), and mouse (Whitlock et al., 2010).
The current review covers mechanisms controlling endogenous glucocorticoids regulation as well as steroid-induced changes in cells mediated by different glucocorticoid receptors. The review will also describe the cellular signaling mediating steroid-induced ocular hypertension and the damage inflicted on the trabecular meshwork with steroid use. In addition, we report on mechanisms of steroid-resistance in patients in order to help predict not only steroid responsiveness but also possible ways to minimize side effects.
2. GC-induced IOP elevation and age-dependence
There are differences in steroid responsiveness among the population, where topical ocular administration of GCs elevates IOP in approximately 30%–40% of the general population (also known as “steroid-responders”). In contrast, nearly all of POAG patients are steroid responders (Paterson 1965; Davies 1968; Schwartz et al., 1973; Meredig and Pulhorn 1980; and Bartlett et al., 1993).
Steroid responsiveness is defined differently. An elevation of 5 mmHg following steroid therapy has been defined as a steroid responder (Yamamoto et al., 2008). Others considered an IOP above 21 mmHg (Herbert et al., 2004) or 24 mmHg (Heinz et al. 2009) following steroid treatment as a steroid responder. However, another group considered the combination of both an increase of 5 mmHg and IOP above 24 as a steroid responder (Levine et al 2002). In this context, we shall consider all these referenced IOP elevations as a characteristic of steroid responder.
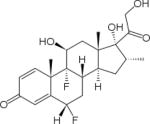
Surprisingly, children are especially susceptible to an IOP increase secondary to steroids (Tripathi et al., 1992). Although older patients are at increased risk, children have been shown to be greater steroid-responders as compared with adults with >60% of children being steroid-responders (Lam et al., 2005; Kwok et al., 1997; and Ng et al., 2000). For example fluorometholone (FML), which is a hydrophilic synthetic fluorinated corticosteroid with minimal corneal penetration, has been shown to induce steroid-induced glaucoma in children (Stewart and Kimbrough 1979; and Akingbehin 1983). Current prescribed steroid are shown in Table 1 for comparison.
Table 1.
Relative potencies of commonly used corticosteroids (Chrousos 2012).
| Agent | aRelative glucocorticoid activity (Anti-inflammatory) | Duration of action (hours) | Transcortin binding | Structure |
|---|---|---|---|---|
| Cortisol (hydrocortisone) | 1 | S | ++++ |
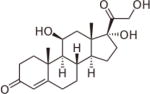
|
| Cortisone | 0.8 | S | − |
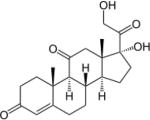
|
| Prednisone | 4–5 | S | +++ |
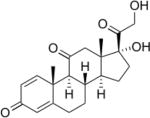
|
| Prednisolone | 5 | S | ++ |
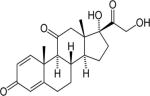
|
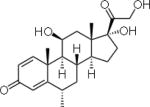
| Methylprednisolone | 5 | S | − |
|
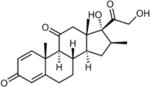
| Meprednisone | 5 | S | − |
|
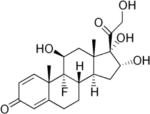
| Triamcinolone | 5 | I | − |
|
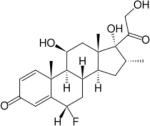
| Paramethasone | 10 | I | − |
|
| Flupredinsolone | 15 | I | − |
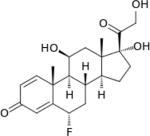
|
| Betamethasone | 25–40 | L | − |
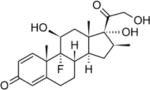
|
| Triamcinolone acetonide | 30 | I | − |
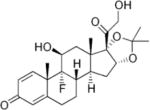
|
| Dexamethasone | 30 | L | − |
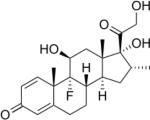
|
| Flumethasone | 120 | L | − |
|
S= short (~12 hr.), I=intermediate (~24 hr.), L=Long (~ 48–72 hr.).
Relative to cortisol.
3. Mechanisms that control levels of GCs
3.1. Physiological Fluctuations in cortisol levels
Glucocorticoids are secreted in a diurnal rhythm where levels of cortisol are the highest in the morning (~51 nM at 8 AM), but gradually decrease during the day and reaching the lowest levels at night (~14 nM at 8 PM). Such oscillations in cortisol secretion are synchronized with individuals activity level (Charmandari et al., 2011). Interestingly, nocturnal rodents (e.g., mice and rats) exhibit an inverse rhythm of endogenous glucocorticoid profile to humans, where peak plasma levels of corticosterone (rodent version of glucocorticoid) are at night and lowest concentrations occur during the day. In addition, it has been shown that IOP fluctuates diurnally and may be linked fluctuations to in to cortisol levels, where the greatest IOP occurs during the early morning mirroring high levels of plasma cortisol levels (Smith 1966). Patients with POAG have elevated blood levels of cortisol compared to age-matched subjects (Ray and Mehra 1977; Meredig and Pulhorn 1980; Rozsival et al., 1981; Schwartz and McCarty 1987; and McCarty and Schwartz 1991). Aqueous humor cortisol levels are also higher than plasma levels in both cataract and glaucoma patients (Rozsival et al., 1981).
3.2. Mechanisms controlling concentrations of cortisol extracellularly and intracellularly
A key mechanism involved in GC-induced actions occurs via feedback inhibition. Stress induces the release of corticotropin-releasing hormone (CRH) from the hypothalamus which stimulates the release of adrenocorticotropic hormone (ACTH) and cortisol from the pituitary and adrenal cortex, respectively. Cortisol acts via feedback inhibition of its own secretion by attenuating CRH and ACTH release (Sharma et al., 2013).
The plasma half-life of glucocorticoids ranges between 80 mins for cortisol and 270 min for dexamethasone, where min approximately 90% of endogenous circulating cortisol is bound with high affinity to the plasma protein corticosteroid-binding globulin (Schäcke et al., 2002), and the remaining 6% binds to albumin and 4% exists as free and active (Siiteri et al., 1982; and Meulenberg and Hofman 1990). Corticosteroid-binding globulin (CBG), or transcortin, is a 50–60 kDa high affinity plasma transport glycoprotein that binds cortisol in humans and corticosterone in rodents (Westphal 1986; and Hammond 1990). CBG also binds progesterone with relatively high affinity (Westphal 1986). However, many synthetic steroids, with the exception of prednisolone, have low affinity for the corticosteroid-binding globulin. Interestingly, while dexamethasone failed to bind mammalian CBG, it binds chicken CBG with equal affinity to corticosterone (Gould and Seigel 1978). CBG is secreted principally from hepatocytes with circulating concentrations ranging from 30–52 pg/ml (Torpy and Ho 2007). CBG is a substrate for neutrophil elastin enriched at sites of inflammation where it is cleaved releasing cortisol (Hammond et al., 1991). CBG’s affinity for cortisol is diminished by elevated temperature (typically seen in fever and inflamed sites) such changes in temperature results in the release of cortisol (Cameron et al., 2010; and Lin et al., 2010).
While CBG-unbound cortisol passively diffuses across the plasma membrane, its intracellular bioavailability within the cell is controlled by two enzymes functioning in an opposing manner. 11β-Hydroxysteroid dehydrogenase type 2 (11β-HSD2) oxidizes cortisol into the inactive metabolite cortisone. In contrast, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1, 292 amino acids) converts cortisone to cortisol (Gathercole et al., 2013). The biological activity of any glucocorticoid appears to depend on the presence of a hydroxyl group at position C11 of the steroid structure, and the inactivation of this group to a C-11 oxo group inactivates the steroid (Cope and Black 1958). Such a mechanism is crucial for the kidney where cortisol is known to activate mineralocorticoid receptor and its inactivation by 11β-HSD2 is of great importance. Polymorphisms (Cys468Ala and Gly534Ala) in the gene that encodes 11βHSD2 were found associated with hypertension (Melander et al., 2000). Such a regulation of GCs can be named as the “pre-receptor” mechanism, i.e., prior to activation of the glucocorticoid GRα receptor and its translocation to the nucleus for either activation of repression of genes. 11β-HSD isozyme expression has recently been described in the human and rodent eye (Stokes et al., 2000, Rauz et al., 2001; and Suzuki et al., 2001), especially 11β-HSD1 in ciliary epithelial cells, suggesting a role in aqueous humor production and the regulation of intraocular pressure.
In the aqueous humor, the cortisol concentration is 14-fold higher than cortisone, suggesting a predominantly 11β-HSD1 reductase activity (Rauz et al., 2001). 11β-HSD2 appears to be restricted to the corneal endothelium. Carbenoxolone (CBX), an 11β-HSD1 and 2 inhibitor, decreased IOP significantly (Rauz et al., 2001). Although the study may suggest that the use of topical 11β-HSD1 inhibitors may be a novel therapeutic for glaucoma, CBX is a non-selective drug that blocks gap junctions and GABA receptors (Connors 2012). Nevertheless, while Anderson et al. 2009 have shown that three different 11β-HSD1 selective blockers (PF-904955, PF-3319913, and PF-3859622) completely blocked cortisone conversion in rabbits and inhibited pLuc-GRE luciferase activity, the group did not show any IOP changes in rabbit following their topical application (Anderson et al., 2009). Assays of cortisol-metabolizing enzymes in homogenates of human trabecular meshwork cells also indicated a marked increase in delta 4-reductase and the decrease in 3-oxidoreductase (Weinstein et al., 1985), indicating a possible imbalance in the endogenous cortisol pathways.
Studies on steroid metabolites led to the discovery of the IOP lowering cortisenes, including anecortave acetate. Anecortave acetate (AA) lowered IOP of glaucoma patients (5 of 6 patients showed significant reduction in IOP (Robin et al., 2008). Cortisenes lack GC activity and antagonistic effects due to the lack of 11β-hydroxyl group and oxidation of the C9–C11 carbons to form a double bond preventing rehydroxylation of the C11 position (lack of anti-inflammatory effects and GRα nuclear translocation (Clark 2007). Although the exact IOP lowering mechanism of action for anecortave acetate is currently not known, binding studies have identified phosphodiesterase 6-delta (PDE6D) as the target of anecortave. Overexpression of PDE6D in mouse eyes caused elevated IOP, and this elevation was reversed by topical ocular application of either AA or anecortave desacetate (AdesA) (Shepard et al., 2013).
3.3. Glaucoma is an age disease and glucocorticoid levels increase with aging
Van Cauter et al., have reported increases in plasma cortisol levels up to 50% between 20 and 80 years of age in both male and female subjects (Van Cauter et al., 1996). Growth hormone which inhibits 11β-HSD1 declines with aging (Iranmanesh et al., 1991; and Landin-Wilkelmsen et al., 1994). Whereas, circulating inflammatory cytokines IL-6 and TNF-α increase with age and they are known to stimulate 11β-HSD1 (Payette et al., 2003). Not surprisingly, both cytokines are elevated in POAG donor retinas (Gramlich et al., 2013; and Yang et al., 2011). However, while aqueous humor IL-6 levels were lower in POAG patients (Takai et al., 2012), TNF-α levels were higher (Sawada et al., 2010). In another study both cytokines were found to have increased in plasma of glaucomatous patients (Huang et al., 2010). Interestingly, increased systemic circulating 11β- HSD1mRNA is observed in blood with increasing age (Al Bakir et al., 2008). Hypertensive patients however, were reported to have reduced 11β- HSD2 activities (Henschkowski et al., 2008). Transgenic mice overexpressing TNF-α have both decreased mRNA levels and activity of 11beta-HSD2 in kidney tissue (Kostadinova et al., 2005), which may explain elevated cortisol levels. Overall, the above studies may explain in part prevalence of glaucoma in the aged population and the link to elevated cortisol levels, however they do not explain why only ~ 30% of the population develops steroid-induced glaucoma. In contrast, 60% of children receiving steroids end up suffering from elevated IOP and glaucomatous onset. The exact mechanisms to explain such differences are still not clear but may involve epigenetics. Growing evidence suggests that epigenetics such as DNA methylation, histone modification, and microRNA induce changes in gene function without changes in nucleotide sequence. Epigenetic mechanisms therefore can switch the genome into active and inactive domains based on endogenous and exogenous environmental changes and developmental stages, which may help explain steroid inter-individual and population variability. For example, an increase in GRα methylation was associated with distressed children (Tyrka et al., 2012). Furthermore, De Rooij et al., 2012 reported decreases in GRα promoter methylation correlated with reduced stress levels in the human adult population (de Rooij et al., 2012). However, the same hypermethylation mechanism has also been shown to augment GC effects. For example, increased methylation of 11β-HSD2 was detected in rats prenatally exposed to stress (Jensen Peña et al., 2012) and patients who developed hypertension following prednisone treatment had hypermethylated 11β-HSD2 promoter (Friso et al., 2008). Also, diet can affect GRα methylation as high intake of folic acid during pregnancy enhanced methylation of GRα in rats (Burdge et al., 2009). It is speculated that epigenetic changes induced by factors such diet, stress, and environment, which may be initiated early during fetal development, can appear during early childhood or adulthood producing long-lasting effects (Newnham 2001). The only encouraging factor is that such epigenetic alterations can be reversible (Tyrka et al., 2012).
3.4. Role of Intracranial pressure in glaucoma development and link to cortisol levels
Intracranial hypertension has also been linked to elevated cortisol levels. Obese patients with reduced 11β-HSD1 activities have the biggest drop in intracranial hypertension (Sinclair et al., 2010). In contrast, brain injured patients with elevated intracranial pressure had elevated cortisol concentrations (Feibel et al., 1983). Interestingly, IOP-elevated patients have higher intracranial pressure (Wostyn et al., 2015). While higher intracranial pressure was reported to be protective in glaucoma subjects and animal models of glaucoma (Zhao et al., 2015; and Zhang et al., 2015), Nusbaum et al., 2015 have shown detrimental effects of intracranial pressure elevation on survival of mice retinal ganglion cells. Although Rauz et al., 2003 have shown that carbenoxolone, a nonspecific inhibitor of 11β-HSD1 and 2, lowered IOP in glaucoma patients, the authors did not investigate its effects on intracranial pressure. Also, the effect of selective 11b-HSD1 on intracranial pressure has not been investigated (Anderson et al., 2009).
3.5. Mechanism of GC activity
3.5.1. GRα
The clinical and cellular actions of GCs are mediated via a 95 kDa cytosolic α–isoform of the glucocorticoid receptor (GRα), a member of the nuclear receptor family of ligand-dependent transcription factors. Upon GC binding, GRα undergoes conformational change that exposes a nuclear import signal and translocates to the nucleus. The translocation mechanism is ATP-dependent and heat shock protein 90-dependent (as it is inhibited by geldanamycin) (Dibas et al., 2012).
Multiple translational sites can generate up to 8 different subtypes of GRα (i.e. GRα-A, GRα-B, GRα-C1, GRα-C2, GRα-C3, GRα-D1, GRα-D2, and GRα-D3, Oakley and Cidlowski 2011; and Chrousos and Kino 2005). Although the GRα-A and GRα-B isoforms are the most abundant GRα proteins in many cell-types, trabecular meshwork cells from the human eye preferentially express the GRα-C and GRα-D isoforms (Nehme et al., 2009). Unfortunately there are no published studies on the expression of the different GR subtypes in normal vs. glaucomatous TM tissues nor studies of such subtypes in steroid-responders vs. non-responders TM tissues. Such studies are essential in improving steroid ocular therapy and worth immediate attention. In addition, immature dendritic cells predominantly express the GRα-D isoforms, whereas mature dendritic cells predominantly express the GRα-A subtype (Cao et al., 2013). In rodents, GRα-C isoforms are highest in pancreas and colon and the GRα-D subtypes are highest in spleen and lung (Lu and Cidlowski 2005). Individual GR isoforms were stably transfected into Jurkat cells. Cells were treated with DEX for 48 h and it was found that the treatment induced apoptosis in ~ 30% of the cells expressing the GRα, -A, or -B isoforms and in 50% of the cells expressing the GR-C3 isoform (Wu et al., 2013). Therefore, the GRα-C isoforms have been suggested to be the most biologically active, while the GRα-D isoforms are the most deficient in glucocorticoid-mediated functions (Wu et al., 2013).
The GRα-D isoform is constitutively present in the nucleus and bound to certain GRE-containing target genes (Oakley and Cidlowski 2011). The GRα-D receptors are basic proteins. While the isoelectric point (IP) for GRαA–C ranges between 5.88 and 6.32, the IP for GRαa-D1-3 is 8.66–8.74 (unpublished observations, Dibas et al). The GRα-D isoforms are basic and could explain their nuclear presence even in the absence of GCs.
3.5.2. Post-translational modification of GRα
Typically, GRα basal phosphorylation is low, however, following addition of an agonist, GRα becomes hyperphosphorylated. The phosphorylation of the S211 residue is important in the transactivation mechanism of GRα (Blind and Garabedian 2008; and Chen et al., 2008). Other phosphorylation sites also appear important (Galliher-Beckley et al., 2011; and Avenant et al., 2010). In contrast, CpdA (a GRα modulator) does not cause an increase in S211 and S226 phosphorylation (De Bosscher et al., 2005; and Avenant et al., 2010). Furthermore, GCs can induce downregulation of the GRα via proteasomal degradation following K419 ubiquitination (Deroo et al., 2002). Interestingly, a triple GRα mutant with 3 Ser phosphorylation sites mutated to Ala (S212, S220, S234), failed to undergo proteasomal degradation, suggesting that hyperphosphorylation drives the onset of the ubiquitination-mediated proteasomal degradation of GRα.
The GRα is also a substrate for sumoylation, in which SUMO (small ubiquitin-related modifier) peptides are covalently attached to the receptor at specific lysine residues (Lys-277, Lys-293, and Lys-703). Sumoylation of GRα dramatically promotes its degradation and inhibits the transcriptional activity of the receptor in a promoter-dependent fashion through the recruitment of corepressors (Davies et al., 2008; Holmstrom et al., 2003, 2008; Le Drean et al., 2002; and Lin et al., 2006). Overexpression of SUMO-1 destabilizes the GRα and this can be prevented with a proteasome inhibitor (Le Drean et al., 2002). In HEK293 cells expressing wild-type GRα (wtGRα) or a sumoylation-defective GRα (GRα3KR), the gene expression profiles revealed that dexamethasone regulated genes are affected by the GRα sumoylation (Paakinaho et al., 2014). Furthermore, recent reports have demonstrated that GRα is acetylated at Lys-494 and Lys-495 in response to glucocorticoids, and this modification impairs its antagonism of NF-κB (Ito et al., 2006; Nader et al., 2009; Charmandari et al., 2011; and Oakley and Cidlowski 2013). The same study reported that deacetylation of the GRα by HDAC2 was necessary for binding of the receptor to NF-κB and repression of downstream target genes. Knockdown of HDAC2 correlated with decreased sensitivity to corticosteroid treatment in primary alveolar macrophages, whereas overexpression of HDAC2 in alveolar macrophages from chronic obstructive pulmonary disease (COPD) patients exhibiting glucocorticoid resistance, restored their sensitivity to glucocorticoid therapy (Kassel and Herrlich 2007). In summary, posttranslational modification of GRα may play an important role in GC-resistance.
3.5.3 GRβ
The GR gene NR3C1 consists of 9 exons where exon-2 is the starting translating exon. However, the pre-mRNA has 2 exons at its end known as 9α and 9β, separated by an intron. Alternative splicing can generate either GRα or GRβ. The GRα contains 777 amino acids whereas the GRβ contains 742 amino acids (Oakley et al., 1996). The human GRβ shares with GRα the first 727 amino acids but has a unique 15-amino acid carboxyl terminus (Goecke and Guerrero 2006). However, in other species such as zebrafish and mice, alternative splicing occurs at exon 7 and intron 8, respectively (Schaaf et al., 2008; and Hinds et al., 2010). A similar intron inclusion also was reported in rat GRβ (DuBois et al., 2013). Alternative splicing is carried by a unique complex known as the spliceosome. The spliceosome has many components including serine-arginine (SR) proteins (SRps, Will and Luhrmann R 2011). Whereas the overexpression of SRP20 increased GRα levels, overexpression of SRP30c and SRP40 increased GRβ levels (Jain et al., 2012). The human GRβ also has 8 different subtypes due to alternative translational sites (termed; GRβ-A, GRβ-B, GRβ-C1, GRβ-C2, GRβ-C3, GRβ-D1, GRβ-D2, and GRβ-D3) (Oakley et al., 1996). This means that there may be 256 different combinations of GRα/GRβ hetero-dimer receptors depending on different subtypes expressed. This alternative splicing of the GRβ receptor eliminates the GC binding domain. GRβ has been shown to act as a dominant negative regulator of GC-induced activation of GRα-mediated gene regulation (Goecke and Guerrero 2006; and Lewis-Tuffin and Cidlowski 2006). Lewis-Tuffin et al., 2007 reported that RU 486 bound GRβ weakly and slowly induced its nuclear translocation. However, Kino et al., 2009 failed to observe any RU486-induced GRβ nuclear translocation. Three groups have recently shown that overexpression of GRβ affected mRNA expression positively and negatively in a gene-specific fashion, suggesting that that GRβ has its own intrinsic transcriptional activities independent of GRα (Kino et al., 2009; Lewis-Tuffin et al.; 2007, and Nagy et al., 2016). Nevertheless, this could be due to dimerization with other receptors or transcription factors. Whereas FKBP51 is involved in GC-independent transport of both GRα and GRβ from cytoplasm to nucleus, FKBP52 is only involved in nuclear transport of the GC-bound activated GRα (Zhang et al., 2008).
3.5.4 Other GR isoforms
At least three other unique GRα isoforms exist; GRγ, GR-A, and GR-P. GRγ is produced from splicing of the GR primary transcript from the intron separating exons 3 and 4, resulting in the insertion of a single arginine between the DNA-binding zinc fingers of the DBD (Rivers et al., 1999; and Ray et al., 1996). GRγ exhibited half of the activity of GRα for target genes. However, a recent study revealed that GRγ binds degenerate glucocorticoid response elements (GREs) in genes that are not targets of GRα and therefore expands the genes susceptible to regulation by glucocorticoids (Thomas-Chollier et al., 2013). GR-A transcripts lack exons 5–7, which encode the amino-terminal half of the LBD, as a result of mRNA splicing from exon 4 to 8 has no known biological functions (Moalli et al., 1993). Failed splicing at the exon 7/8 boundary produces GR-P that lacks the carboxy-terminal half of the LBD (Moalli et al., 1993). The expression of GRγ, GR-A, and GR-P receptor has yet to be analyzed in normal vs. glaucomatous TM tissues and whether their levels vary in steroid-responders vs. non-responders TM tissues. Such studies could provide useful information in enhancing steroid ocular therapy.
4 Role of importins and exportins in GRα nuclear trafficking
The subcellular location of GRα is controlled by both the import and export of the receptor through the nuclear pore complexes. Import of GRα via importinα/β-based mechanisms is controlled via the nuclear localization (NL1) and (NL2) domains (Picard and Yamamoto 1987). Hakim et al have shown importin-7 siRNA attenuated fluticasone propionate-induced GRα nuclear translocation in U937 cells, a human macrophage cell-line (Hakim et al., 2013). Deletion studies showed that NL1 supports rapid and hormone-independent translocation of GRα, whereas NL2 facilitates slower and hormone-dependent nuclear import (Savory et al., 1999; Tao et al., 2006; and Echeverria et al., 2009). Interestingly, importin alpha selectively bound NL1 and both importin 7 and the importin alpha-importin beta heterodimer could import a GRα NL1 fragment (Freedman and Yamamoto 2004). In addition, importin 7, importin 8, and importin alpha bind GRα even in the absence of hormone (Freedman and Yamamoto 2004). Tanaka et al., 2003 have shown that fluorescently-tagged GRα translocated to the nucleus along with fluorescently-tagged importin-α but not fluorescently-tagged importin β (Tanaka et al., 2003). While there was evidence for GRα-GFP interacting with GFP-importin-α, no such binding was observed between GFP-GRα and GFP-importin-β (Tanaka et al., 2003).
Echeverria et al., 2009 found that importin β and the integral nuclear pore glycoprotein Nup62 interact with hsp90-GRa complex (Echeverria et al., 2009). Tao et al., 2006 have shown that importin-13 siRNA inhibited steroid-induced GRα nuclear translocation in A549 cells (Tao et al., 2006). GRα export appears to be more complex. Pharmacological blockade of exportin1/CRM1-mediated export abrogates the translocation of GRα to the cytosol (Savory et al., 1999; and Beck et al., 2008). A nuclear export sequence (NES) was identified in the GRα LBD on the basis of sequence homology (Carrigan et al., 2007), but so far no experimental confirmation via mutation analysis has been performed. Carrigan et al 2007 identified a nuclear-retention signal (NRS) in GRα. This NRS, which overlaps closely with the NL1 in the hinge region of GRα, can actively counteract the redistribution of GRα to the cytoplasm after steroid withdrawal (Carrigan et al., 2007). In fact, following DEX removal only 50% of RFPGRα was exported back to cytosol 12hr post DEX withdrawal in transformed TM5 cells, a human trabecular meshwork cell-line (Dibas et al., 2012). Such persistent presence of GRα may therefore predict augmented gene regulation by steroids as the receptor is slow to be exported back to the cytosol. Such a mechanism may explain some of the side effects of steroids including sustained IOP elevation. A proposed model for GR trafficking is shown in Fig. 1.
Fig. 1. Proposed model for the nuclear import and the glucocorticoid receptors α and β.
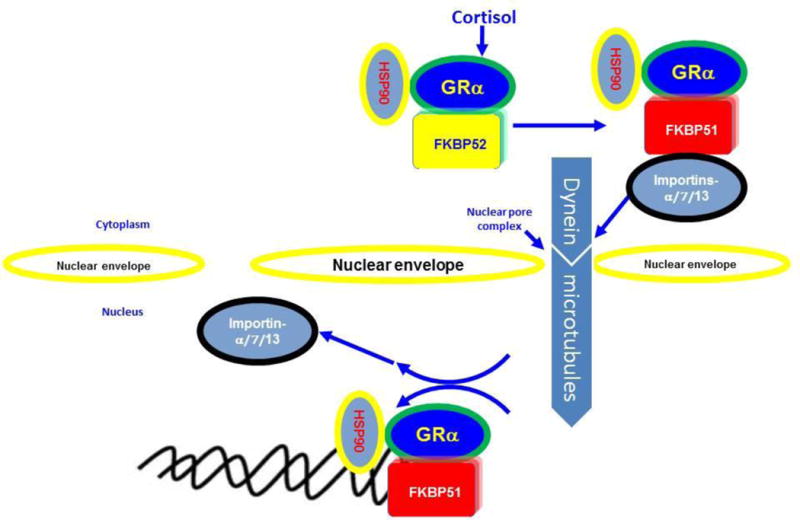
FKBP51 is involved in hormone-independent transport of both GRα and GRβ. However, FKBP52 is hormone-dependent and transports only GRα. Importins a, 7, and 13 are involved in GRα-nuclear import.
5. GC-induced regulation of gene expression
Following glucocorticoid binding, GRα induces or represses the transcription of target genes which can comprise up to 10–20% of the human genome (Galon et al., 2002). Surprisingly, glucocorticoid binding sequences are not located in the promoter region in close proximity to the transcription starting regions but are present in intragenic regions with > 11 kb from the transcription starting regions (Kuo et al., 2012) that either induce gene activation or repression. Reddy et al., have shown that while the GRE sequence of DEX-induced genes is located at a median of 11kb from the transcription start site, the nearest GR binding for genes repressed by DEX is at a distance of 146 kb from the transcription site (Reddy et al., 2009). Genes induced by DEX in TM include; serine protease inhibitor (alpha1-antichymotrypsin), pigment epithelium-derived factor (PEDF), cornea-derived transcript 6, prostaglandin D-2 synthase (Lo et al., 2003), secretory leukocyte protease inhibitor (SLP1), serum amyloid A2 (SAA2), Angiopoietin-Like 7 protein (ANGPTL7), serum amyloid A1 (SAA1), Serpin Peptidase Inhibitor, Clade A3 (SERPINA3), Zinc finger and BTB domain containing 16 protein (ZBTB16) (Rozsa et al., 2006), myocilin (MYOC) and Growth arrest-specific protein 1 (GAS1) (Fan et al., 2008). Known genes repressed by DEX include Sentrin-specific protease 1 (SENP1), Zinc finger protein 343 (ZNF343), and sex Determining Region Y)-Box 30 (SOX30) (Fan et al., 2008).
GC-induced GRα activation occurs via at least 3 different mechanisms (Beck et al., 2009; and Sundahl et al., 2015). GRα forms a dimer, binds to the glucocorticoid response element (GRE), and activates the promoter (Chalepakis et al., 1990). Another mechanism that has been suggested is that the GRα monomer binds to DNA with a transcription factor to cooperatively enhance gene expression (Morin et al., 2006). Alternatively, GRα can interact with transcription factors directly without interacting with DNA (also known as tethering, Johansson-Haque et al., 2008). GC-induced GRα-gene repression occurs via 4 potential mechanisms (Beck et al., 2009; and Sundahl et al., 2015). GRα can bind a transcription factor and thereby inhibit it from activation. This appears to be the predominant transrepression mechanism and is called tethering. A well-known example for such sequestration of factors is the inhibition of NF-kβ and activator protein 1 (AP-1) (De Bosscher et al., 2006; and Beck et al., 2009). A second repression method involves a transcription factor overlapping with the GRE where its subsequent binding to GRE blocks the transcription factor from binding (a form of competitive binding, Meyer et al., 1997). A third mechanism involves “Composite” GREs that consist of DNA binding sites (DBS) for GRα which, in association with binding sites for other factors, can act synergistically to mediate transrepression (Surjit et al., 2011). Finally, GRα binds to a negative GRE (nGRE), resulting in transcription repression (Stahn and Buttgereit 2008; and Beck et al., 2009).
GCs have been hypothesized to exert their anti-inflammatory effects due to transrepression via the GRα monomer formation, while GR-induced side effects are caused by transactivation due to dimerization of GRα. For example, while GC-induced hyperglycemia and muscle wasting are caused by transactivation, hypothalamic-pituitary adrenal axis suppression is caused by transrepression (Patel et al., 2014). Osteoporosis is caused by both transactivation and transrepression (Schacke et al., 2002; and Carballo-Jane et al., 2004). In addition, GRα-induced transactivation is involved in anti-inflammatory factors such as annexin-1 (Parente and Solito 1994) and the leucine zipper (Cannarile et al., 2001).
The hypothesis that the GRα monomer mediates transrepression was postulated based on the GRα mutant harboring GRA458T mutation in the DBD (Heck et al., 1994; and Reichardt et al., 1998). This mutant cannot form dimers and has impaired GRα transactivation, yet it exhibited transrepression (Heck et al., 1994; and Reichardt et al., 1998). However, this simple explanation of transrepression vs. transactivation mechanisms can be easily challenged. First, GRα can still dimerize not only via the DBD, but also via an LBD (Bledsoe et al., 2002). Second, using immunoprecipitation and the “Number and Brightness” technology, the GRdim variants still showed GRα dimer formation in solution but significantly less than wild type (Jewell et al., 2012; and Presman et al., 2014). Nevertheless, GRdim mutant showed weakened GRE binding (Presman et al., 2014) and prednisolone-induced gene expression was greatly reduced compared to wild type (Frijters et al., 2010). The ability of prednisolone in GRdim mice to still induce genes raised speculation on the existence of alternate GRα dimers involving NTD-LBD contacts, GRα:MR heterodimers, or GRα multimeric complexes (Nixon et al., 2013). GRdim/dim mice still express normal mRNA levels of the GRα-dependent phenylethanolamine-N-methyltransferase (Adams et al., 2003). Therefore, the GRα’s ability to repress or activate genes is not simply dependent on its monomer or dimer status but rather the GRα-binding sequences and cofactors involved. There are no studies examining effects of steroids in the GRdim mice and whether fewer side effects would be anticipated. It would be very interesting to examine if DEX-induced IOP elevation would be attenuated in GRdim mice or not. That may indicate the involvement of non GRs in mediating side effect.
6. Mechanism of steroid-induced secondary ocular hypertension
Clinical trials have shown that GC-induced IOP elevation can occur within hours (Weinreb et al., 1985) or weeks when used topically (Armaly 1963), and years if used systemically (Bernstein et al., 1977). It appears that GC-induced IOP is both GRα-dependent and independent. For example, the rise in steroid-induced IOP is formulation dependent. For example, steroid acetates are lipophilic and tend to penetrate the cornea better than other derivatives such phosphates (hydrophilic) and not surprisingly, while dexamethasone acetate 0.1% produces ~ 22 mmHg rise, medrysone 1.0% caused a modest 1.0 mmHg rise in IOP (Jones and Rhee 2006). Also, sudden and acute increases of vitreous volume have been shown to induce a sharp increase in IOP. For example, injection of 0.1 ml of TA, 0.09 ml pegaptanib, and 0.05 ml bevacizumab produced increases IOP with the TA group having the highest IOP elevation followed by the pegaptanib and bevacizumab treated group (Bakri et al., 2003). In addition, fine white crystalline opacities following TA injection have been observed and such particulate material is suggested to occlude the trabecular meshwork (Singh et al., 2004). Therefore, some of the GRα-independent factors that can elevate IOP include formulation, intravitreal injection volume, particulate size and appearance. However, the previous effects tend to be short-term.
6.1. Mechanism of glucocorticoid (GC)-induced damage in trabecular meshwork
Similar to POAG, GC exposure increases aqueous humor (AH) outflow resistance. This is mediated in part by increased deposition of extracellular matrix (ECM) proteins due to inhibition of metalloproteases (MMPs) while increasing their natural blockers known as tissue inhibitors of metalloprotease (TIMP) (Li et al., 2011). GCs induce build-up of fine fibrillar-like material in the JCT and deposits of type IV collagen, heparin sulfate proteoglycan, and fibronectin (Tawara et al., 2008). Similar changes have been observed in human perfused donor eyes (Clark et al., 1995). DEX increases synthesis of unbreakable glycosaminoglycans (GAGs) (Johnson et al., 1990). Considerable amounts of GAGs in DEX-treated samples (48hrs) remained undigested after sequential treatment with hyaluronidase, chondroitinase AC, chondroitinase ABC, keratinase, and heparatinase (Johnson et al., 1990). DEX also increases β3 integrin via the Nuclear factor of activated T-cells 1c (NFAT1c) transcription factor (Faralli et al., 2013). DEX treatment induces a 2-fold and ~ 4-fold increase in HTM and rabbit TM cell stiffness and elevates the expression of matrix proteins (decorin, myocilin, and fibrillin, secreted frizzle-related protein (SFRP1) (Raghunathan et al., 2015), and increases protein levels of ZO-1 and Cx43 in both NTM and GTM cells (Zhuo et al., 2010). Tektas and Lutjen-Drecoll, 2009 have shown differences between POAG and steroid-induced glaucomatous TM tissues. Steroid-induced glaucomatous TM showed increased deposits of collagen type IV throughout the layers of the TM (Tektas and Lütjen-Drecoll 2009).
GC-induced trabecular meshwork dysfunction includes inhibition of phagocytosis (Zhang et al., 2007) and a major reorganization of the cytoskeletal cellular network and production of CLANS (Clark et al., 1994). CLANS are cytoskeletal tangles of actin filaments that influence stiffness and contractility of TM. Cdc42 is a Rho GTPase and an important modulator of the cytoskeleton and is induced by DEX (Qiu et al., 2015). However, its role in CLAN formation is not known. DEX also induces the upregulation of noncanonical Wnt ligand Wnt5a that can induce CLAN formation through the noncanonical Wnt receptor Tyrosine-protein kinase transmembrane receptor ROR2/Ras homolog gene family, including A/Rho-associated protein kinase 1 (ROR2/RhoA/ROCK) signaling axis (Yuan et al., 2013). DEX and transforming growth factor β 2 (TGFbeta2) significantly induce the upregulation of alkaline phosphatase (ALP) activity in trabecular meshwork primary cell lines. In glaucomatous human trabecular meshwork there is a significantly higher level of ALP activity than normal eyes (Xue et al., 2007).
6.2. Role of myocilin in GC-induced TM damage
MYOC is expressed in multiple ocular and nonocular tissues and despite intense investigation the function of this protein is largely unknown. It spans approximately 17 kb and contains 3 exons transcripting a 2.3 kb gene product. It contains 504 amino acids (Kubota et al., 1998; and Fingert et al., 1998) and has a predicted molecular weight of approximately 57 kDa. The MYOC protein has 2 major domains with a N-terminal myosinlike domain and a C-terminal olfactomedin-like domain. Although myocilin is expressed in many tissues, its abnormal or mutant isoforms has only been shown to ocular diseases. Mutation in the C-terminal of myocilin is linked to >10% of childhood glaucoma and ~ 3–5% of adult glaucoma (Stone et al., 1997; Wiggs et al., 1998; and Adam et al., 1997). To date, 101 out of 266 mutations have been associated with glaucoma pathology (www.myocilin.com). Interestingly, while one mutation in exon-1 and 9 mutations in exon-2 were reported, the remaining 91 mutations occur in exon-3. Although GC-induced production of myocilin, when studying 70 human steroid responders and 114 control subjects no link between MYOC mutations and steroid-induced ocular hypertension has been detected (Fingert et al., 2001).
7. Mechanisms of GC-resistance
At least 10 different mechanisms have been discovered that explains GC-resistance. For example, polymorphisms of GRα gene has been implicated in GC-insensitivity therapy. First, the ER22/23EK polymorphism found in exon 2 was associated with decreased GRα transcriptional activity in reporter assays and decreased expression of endogenous genes compared to wild-type GRα (Russcher et al., 2005). Second, a mutation in the DNA-binding domain of the glucocorticoid receptor has been described in a patient with glucocorticoid resistance syndrome (Ruiz et al., 2001). The third mechanism that explains attenuated GC response has been proposed to be due to the elevated ratios of GRβ:GRα. GRβ acts as a dominant negative receptor and blocks steroid-induced gene expression. Overexpression of RFPGRβ inhibited DEX-induction of plasminogen activator inhibitor-1 (PA1) (Fig. 2). A higher ratio of GRβ to GRα appears to make TM cells more resistant to GCs (Zhang et al., 2005; Zhang et al., 2007; and Zhang et al., 2008). Interestingly, it has been shown that overexpression of SRP20 increases GRα levels and converts normal TM GC-resistant cells into CG-sensitive cells. In contrast, overexpression of SRP30c and SRP40 increases GRβ levels and converts GC-sensitive TM cells into GC-resistant ones (Jain et al., 2012).
Fig. 2. DEX-induced expression of plasminogen activator inhibitor-1 (PAI-1) is inhibited in RFP-GRβ overexpressing TM5 cells.

TM5, a normal transformed human trabecular meshwork cell-line, was treated with DEX (100 nM) for 24 h. Conditioned medium was collected for western blot. Experiments were performed in triplicates (n = 5).
Overexpression of hGRβ in Hela S3 cells can inhibit GC-induced reporter induction (Webster et al., 2001). Moreover, the viral transduction of mouse hybridoma GRβ-deficient cells with GRβ made the cell GC-resistant (Hauk, et al., 2002). While mRNA of hGRβ was detected in most tissues, hGRβ protein appears restricted and is only enriched in immune cells (T-lymphocytes, macrophages, peripheral blood mononuclear cells (PBMCs), and eosinophils, Oakley et al., 1996). In fact, in TM cells, the only way to detect hGRβ is by immunoprecipitation studies followed by western blot on immunoprecipitated fractions (unpublished observations, Dibas et al). The inability to detect hGRβ protein in many tissues and the lower expression mRNA levels compared to hGRα raised questions about the importance of hGRβ in tissues. However, numerous studies have shown that the difference in the ratio of GRβ:GRα is involved in the pathology of many diseases. Patient PBMCs and CD3+T GC-resistant cells had high GRβ levels (Hamid et al., 1999; and Leung et al., 1997). Similar findings were reported in lungs of fatal asthma subjects compared to normal (Christodoulopoulos et al., 2000). In addition, T cells and macrophages showed higher GRβ in GC-resistant asthma individuals (Sousa et al., 2000). PBMCs from GC-resistant ulcerative colitis showed higher hGRβ expression and no change in hGRα (Honda et al., 2000; and Orii et al., 2002). Finally, GC-resistant leukemia patients have higher GRβ:GRα ratio (Shahidi et al., 1999; and Longui et al., 2000). Elevated levels of GRβ also result from a naturally occurring polymorphism (A3669G) in the 3′-untranslated region of the GRβ mRNA that disrupts an mRNA destabilization motif (AUUUA) (Derijk et al., 2001). However, two cytokines IL5 and IL13 which contribute to the asthmatic phenotype and suppressed by GRα are also GRβ repressed. GRβ mimicked GRα-induced suppression of IL5 and IL13 by recruiting HDAC1 to their promoters (Kelly et al., 2008). Another study has shown that the inhibition of HDAC using trichostatin-A in rodents exacerbated the inflammatory response in an LPS-induced myocarditis model (Zhang et al., 2012). Interestingly, Knockdown of HDAC2 correlated with decreased sensitivity to corticosteroid treatment in primary alveolar macrophages, and overexpression of HDAC2 in alveolar macrophages from COPD patients exhibiting glucocorticoid resistance restored their sensitivity to glucocorticoid therapy (Kassel and Herrlich 2007).
An additional mechanism of GC-resistance is caused by GRγ overexpression. GRγ expression in childhood acute lymphoblastic leukemia has been shown to correlate with resistance to dexamethasone treatment (Beger et al., 2003). Also, GR-P is linked to GC-resistance as it is the predominant splice variant in many glucocorticoid-resistant cancers (de Lange et al., 2001; and Krett et al., 1995). A mechanism in GC resistance in primates involves the FK-506 binding immunophilin FKBP51 (Reynolds 1999; and Scammell et al., 2001). Whereas FKBP51 is involved in GC-independent transport of both GRα and GRβ from cytoplasm to nucleus, FKBP52 is only involved in nuclear transport of the GC-bound activated GRα (Zhang et al., 2008). Not surprisingly, FKBP51 overexpression induced GC-resistance in human (Holownia et al., 2009) and animals (Westberry et al., 2006; and Scammell et al., 2001). It is likely that FKBP51 transports AP-1 and GRβ which both compete with GRα (Westberry et al., 2006; and Scammell et al., 2001).
GC-resistance may be induced by different factors that may be related to mutations in either the GR gene itself affecting its transcription or DNA binding. Other important regulations of resistance are mediated by competing GR receptors such as GRβ, GRγ, and GR-P, or transporting immunophilins (FKBP51). Stress also appears to be a dominant player in GC-resistance as is posttranslational modifications of GRα by phosphorylation, ubiquitination, acetylation, and sumoylation.
8. Prediction of glucocorticoid therapy outcome
Achieving successful GC therapy for uveitis, AMD, and diabetic retinopathy is dependent on steroid-responsiveness, GC-sensitivity, and reducing ocular side effects. Numerous attempts have been made to develop assays assessing glucocorticoid sensitivity in inflammatory disorders. Glucocorticoid sensitivity can be evaluated in vivo by assessment of the cortisol response during a low-dose oral or intravenous dexamethasone suppression test (DST) assuming that the dose will cause inhibition of ACTH secretion which in turn reduces cortisol levels. However, healthy populations have variable cortisol levels after a DST, which indicates individual differences in glucocorticoid sensitivity. It has been shown that serum cortisol levels were higher in the glaucomatous group compared to normal subjects. Systemic administration of cortisone reduced cortisol levels in the glaucomatous patients compared with the control group (Freedman et al., 1976). Although dexamethasone’s half-life is almost four times that of cortisol (270 min vs. 80 min) and does not bind CBG protein as cortisol does, cortisol levels were not suppressed by orally administered dexamethasone in glaucoma patients (Schwartz and Levene 1972; and Rosenberg and Levene 1974). However, measuring the negative feedback with the low-dose DST gives only an indication of glucocorticoid sensitivity in the pituitary gland and may not reflect what occurs in other tissues. Also, glucocorticoids can downregulate the expression of the glucocorticoid receptor in various cell types to compensate for glucocorticoid overexposure (Silva et al., 1994). In addition, Kass et al., 1976 and Klemetti 1990 followed 174 GC-responder patients and concluded that the plasma cortisol suppression testing may not actually predict the development of primary open-angle glaucoma in GG responders. However, POAG patients had a greater cutaneous vasoconstrictor response to glucocorticoids than patients with elevated IOP and normal subjects (Stokes et al., 2003). More work is needed in order to predict GC-responsiveness.
9. Selective glucocorticoid receptor agonists (SEGRAS)
Based on the questionable hypothesis that GC-induced anti-inflammatory effects are due to transrepression while side effects are due to gene transactivation, researchers have been looking for a selective dissociated GRα modulator where such an “ideal” drug induces transrepression (i.e., anti-inflammatory effects) without gene transactivation (i.e., side effects). Such dissociated compounds could be either selective glucocorticoid receptor agonists (SEGRAs) or modulators (SEGRMs) (Rosen and Miner 2005; and Beck et al., 2009). The first and most widely studied drug was (4-acetoxyphenyl)-2-chloro-N-methyl-ethylammonium chloride (compound A, CpdA) (De Bosscher et al., 2005). Unlike GCs, CpdA does not cause GRα dimerization nor did it allow binding of GRα to GREs and failed to induce GRα transactivation of reporter genes (De Bosscher et al., 2005; Dewint et al., 2008; Robertson et al., 2010; and 2013; and Presman et al., 2014). CpdA and another SERGA known as “ZK 216346” elicit partial or full nuclear translocation (De Bosscher et al., 2005; Dewint et al., 2008; Robertson et al., 2010 and 2013; Presman et al., 2014; and Drebert et al., 2015). In contrast, CpdA failed to induce RFPGRα nuclear translocation in TM5 cells (Dibas et al unpublished observations). In addition, CpdA functions as an AR antagonist (Zheng et al., 2015). CpdA promoted the interactions between GRα and NF-κB (Zheng et al., 2015) and attenuated nuclear factor (NF)-κB, indicating induction of GRα-mediated transrepression and the expression of NF-κB-related molecules. Matrix metalloproteinase-2, matrix metalloproteinase-9, interleukin-6, and vascular endothelial growth factor, were significantly down-regulated by CpdA. However, CpdA is unstable due to alkylating potential and its clinical usefulness is limited (Rauner et al., 2013). A new SEGRA termed “GW870086X” like DEX exhibits full trans-repressive activity, however, its transactivation is dramatically diminished compared to DEX (Uings et al., 2013). Nevertheless, GW870086X exerted potent anti-inflammatory effects in rodent models of both lung and delayed-type hypersensitivity induced inflammation (Uings et al., 2013). Similar to DEX, GW870086X increased myocilin secretion. However, GW870086X failed to increase fibronectin or decrease MMP2, effects exerted by DEX. Interestingly, while DEX decreased tissue plasminogen activator (tPA), GW870086X increased it (Stamer et al., 2013). Yet, there is no data on GW870086-induced IOP modulation.
Interestingly, another SERGA “BOL-303242-XC” moderately increased IOP in normotensive rabbits compared to dexamethasone and induced minimal induction of mRNA and protein myocilin compared to DEX or prednisolone in monkey TM cells (Budzynski et al., 2009). A Phase III trial has been initiated to evaluate an ophthalmic suspension of BOL-303242-X for the treatment of inflammation following cataract surgery (sponsored by Bausch & Lomb). BOL-303242-X is also in clinical trials for the topical treatment of allergic conjunctivitis. However, the results of these clinical trials have not yet been disclosed and no SEGRA has yet been approved for clinical use.
10. Conclusion
Glucocorticoids are potent drugs with unsurpassed anti-inflammatory effects. However, glucocorticoid-based therapies are still a challenge for clinicians due the variability in glucocorticoid responsiveness among individuals and side effects. This is especially complicated as there are many factors that cause GC-resistance including altered expression of GRα isoforms. The expression of GRγ, GR-A, GR-P, and GRα subtype receptor have yet to be analyzed in normal vs. glaucomatous TM tissues and whether their levels vary in steroid-responders vs. non-responders TM tissues. Such studies are essential in improving steroid ocular therapy and worth immediate attention. It is also as important to follow whether epigenetic changes induced by environmental/diet can be inherited. As such epigenetic modifications are reversible, it would be interesting to utilize epigenetics in the prediction of GC-therapeutic outcome which may attenuate steroid-unwanted side effects.
Nevertheless, there are promising advancements. The ongoing clinical testing of SEGRAs or selective GRα dissociated ligands is underway which may help reduce adverse side effects commonly seen in patients receiving GC-therapy. Finally, based on clinical observations on IOP elevation in patients following intravitreal steroids (injections or implants), it has been recommended to follow IOP in patients immediately (30 min), at 1-week and 2-weeks, and monthly for up to 6 months following intravitreal injection of TA (Kiddee et al., 2013). The same report also recommended IOP measurement in patients receiving steroid implants at 2-weeks, 4-weeks, and monthly for up to 6 months following DEX implants and 9 months after fluocinolone implants. While glucocorticoids are a mainstay drug for inflammatory conditions, the side effect of increasing intraocular pressure remains a great risk to develop glaucoma. Understanding the mechanisms responsible for this action continues to remain an important area for study.
Acknowledgments
This work was supported by a grant from the National Eye Institute (EY016242).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams M, Meijer OC, Wang J, Bhargava A, Pearce D. Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine N-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol. 2003;17:2583–2592. doi: 10.1210/me.2002-0305. [DOI] [PubMed] [Google Scholar]
- Adam MF, Belmouden A, Binisti P, Brézin AP, Valtot F, Béchetoille A, Dascotte JC, Copin B, Gomez L, Chaventré A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Human molecular genetics. 1997;6:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- Akingbehin AO. Comparative study of the intraocular pressure effects of fluorometholone 0.1% versus dexamethasone 0.1% Br J Ophthalmol. 1983;67:661–663. doi: 10.1136/bjo.67.10.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Bakir M, Butt AN, Swaminathan R. Circulating 11beta-hydroxysteroid dehydrogenase type 1 mRNA and cardiovascular risk factors. Ann N Y Acad Sci. 2008;1137:283–289. doi: 10.1196/annals.1448.007. [DOI] [PubMed] [Google Scholar]
- Anderson S, Carreiro S, Quenzer T, Gale D, Xiang C, Gukasyan H, Lafontaine J, Cheng H, Krauss A, Prasanna G. In vivo evaluation of 11beta-hydroxysteroid dehydrogenase activity in the rabbit eye. J Ocul Pharmacol Ther. 2009;25(3):215–22. doi: 10.1089/jop.2008.0120. [DOI] [PubMed] [Google Scholar]
- Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics: II. The effect of dexamethasone on the glaucomatous eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- Avenant C, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Role of ligand dependent GR phosphorylation and half-life in determination of ligand-specific transcriptional activity. Mol Cell Endocrinol. 2010;327:72–88. doi: 10.1016/j.mce.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Pulido JS, McCannel CA, Hodge DO, Diehl N, Hillemeier J. Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond) 2009;23(1):181–185. doi: 10.1038/sj.eye.6702938. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Woolley TW, Adams CM. Identification of high intraocular pressure responders to topical ophthalmic corticosteroids. J Ocul Pharmacol. 1993;9(1):35–45. doi: 10.1089/jop.1993.9.35. [DOI] [PubMed] [Google Scholar]
- Beck IM, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30:830–882. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, De Bosscher K. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. EMBO J. 2008;27:1682–1693. doi: 10.1038/emboj.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger C, Gerdes K, Lauten M. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukemia cells using an isoform-specific real-time polymerase chain reaction approach. Br J Haematol. 2003;122:245–252. doi: 10.1046/j.1365-2141.2003.04426.x. [DOI] [PubMed] [Google Scholar]
- Bernstein HN, Mills DW, Becker B. Steroid-induced elevation of intraocular pressure. Arch Ophthalmol. 1977;9:1075–1080. doi: 10.1001/archopht.1963.00960050017005. [DOI] [PubMed] [Google Scholar]
- Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110:93–105. doi: 10.1016/s0092-8674(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady CJ, Villanti AC, Law HA, Rahimy E, Reddy R, Sieving PC, Garg SJ, Tang J. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst Rev. 2016;12(2):CD010469. doi: 10.1002/14651858.CD010469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynski ESA, López FJ, Ward KW. BOL-303242-X, a selective glucocorticoid receptor agonist (SEGRA), offers a better in vivo side effect profile than dexamethasone on intraocular pressure elevation in normotensive rabbits. ARVO Annual Meeting; Fort Lauderdale (FL). 2009. [Google Scholar]
- Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr. 2009;139(6):1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- Calvo P, Ferreras A, Al Adel F, Wang Y, Brent MH. Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. Br J Ophthalmol. 2015;99(6):723–6. doi: 10.1136/bjophthalmol-2014-305684. [DOI] [PubMed] [Google Scholar]
- Cameron A, Henley D, Carrell R, Zhou A, Clarke A, Lightman S. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab. 2010;95:4689–4695. doi: 10.1210/jc.2010-0942. [DOI] [PubMed] [Google Scholar]
- Cannarile L, Zollo O, D’Adamio F, Ayroldi E, Marchetti C, Tabilio A, Bruscoli S, Riccardi C. Cloning, chromosomal assignment and tissue distribution of human GILZ, a glucocorticoid hormone-induced gene. Cell Death Differ. 2001;8:201–203. doi: 10.1038/sj.cdd.4400798. [DOI] [PubMed] [Google Scholar]
- Cao Y, Bender IK, Konstantinidis AK, Shin SC, Jewell CM, Cidlowski JA, Schleimer RP, Lu NZ. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013;121(9):1553–1562. doi: 10.1182/blood-2012-05-432336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Jane E, Pandit S, Santoro JC, Freund C, Luell S, Harris G, Forrest MJ, Sitlani A. Skeletal muscle: a dual system to measure glucocorticoid-dependent transactivation and transrepression of gene regulation. J Steroid Biochem Mol Biol. 2004;88:191–201. doi: 10.1016/j.jsbmb.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Carrigan A, Walther RF, Salem HA, Wu D, Atlas E, Lefebvre YA, Haché RJ. An active nuclear retention signal in the glucocorticoid receptor functions as a strong inducer of transcriptional activation. J Biol Chem. 2007;282:10963–10971. doi: 10.1074/jbc.M602931200. [DOI] [PubMed] [Google Scholar]
- Chalepakis G, Schauer M, Cao XA, Beato M. Efficient binding of glucocorticoid receptor to its responsive element requires a dimer and DNA flanking sequences. DNA Cell Biol. 1990;9(5):355–368. doi: 10.1089/dna.1990.9.355. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Gchrousos GP, Lambrou GI, Pavlaki A, Koide H, Man Ng SS, Kino T. Peripheral CLOCK Regulates Target-Tissue Glucocorticoid Receptor Transcriptional Activity in a Circadian Fashion in Man. Plos One. 2011;6:e25612. doi: 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, Rogatsky I, Logan SK, Garabedian MJ. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol. 2008;22:1754–1766. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulopoulos P, Leung DY, Elliott MW, Hogg JC, Muro S, Toda M, Laberge S, Hamid QA. Increased number of glucocorticoid receptor-β-expressing cells in the airways in fatal asthma. J Allergy Clin Immunol. 2000;106:479–484. doi: 10.1067/mai.2000.109054. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Adrenocorticosteroids and adrenocortical antagonists. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. McGraw Hill Publishing; New York: 2012. pp. 697–714. [Google Scholar]
- Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formly simple system turns stochastic. Sci STKE. 2005;48 doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- Clark AF. Mechanism of action of the angiostatic cortisene anecortave acetate. Surv Ophthalmol. 2007;52(Suppl. 1):S26–S34. doi: 10.1016/j.survophthal.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- Clark AF, Wilson K, De Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest Ophthalmol Vis Sci. 1995;36:478–489. [PubMed] [Google Scholar]
- Clinical trial number NCT00905450 for “Evaluation of BOL-303242-X Versus Vehicle for the Treatment of Inflammation Following Cataract Surgery” at ClinicalTrials.gov.
- Clinical trial number Ophthalmic Formulation in Subjects With Allergic Conjunctivitis NCT01289431Mapracorat Ophthalmic Formulation in Subjects With Allergic Conjunctivitis at Clinical.Trials.gov.
- Connors BW. Tales of a dirty drug: carbenoxolone, gap junctions, and seizures. Epilepsy Curr. 2012;12(2):66–68. doi: 10.5698/1535-7511-12.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope CL, Black E. The production rate of cortisol in man. Lancet. 1958;14:1020–1024. doi: 10.1136/bmj.1.5078.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L, Karthikeyan N, Lynch JT, Sial EA, Gkourtsa A, Demonacos C, Krstic-Demonacos M. Mol Endocrinol. 2008;22:1331–1344. doi: 10.1210/me.2007-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TG. Tonographic survey of the close relatives of patients with chronic simple glaucoma. Br J Ophthalmol. 1968;52(1):32–39. doi: 10.1136/bjo.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25:6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Beck IM, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA. 2005;102:15827–15832. doi: 10.1073/pnas.0505554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange, Segeren CM, Koper JW. Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res. 2001;61:3937–3941. [PubMed] [Google Scholar]
- de Rooij SR, Costello PM, Veenendaal MV, Lillycrop KA, Gluckman PD, Hanson MA, Painter RC, Roseboom TJ. Associations between DNA methylation of a glucocorticoid receptor promoter and acute stress responses in a large healthy adult population are largely explained by lifestyle and educational differences. Psychoneuroendocrinology. 2012;37(6):782–788. doi: 10.1016/j.psyneuen.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Dejager L, Vandevyver S, Petta I, Libert C. Dominance of the strongest: inflammatory cytokines versus glucocorticoids. Cytokine Growth Factor Rev. 2014;25:21–33. doi: 10.1016/j.cytogfr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Derijk RH, Schaaf MJ, Turner G, Datson NA, Vreugdenhil E, Cidlowski J, de Kloet ER, Emery P, Sternberg EM, Detera-Wadleigh SD. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28:2383–2388. [PubMed] [Google Scholar]
- Deroo BJ, Rentsch C, Sampath S, Young J, DeFranco DB, Archer TK. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol Cell Biol. 2002;22:4113–4123. doi: 10.1128/MCB.22.12.4113-4123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewint P, Gossye V, De Bosscher K, Vanden Berghe W, Van Beneden K, Deforce D, Van Calenbergh S, Müller-Ladner U, Vander Cruyssen B, Verbruggen G. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol. 2008;180:2608–2615. doi: 10.4049/jimmunol.180.4.2608. [DOI] [PubMed] [Google Scholar]
- Dibas A, Yorio T. Review of patent application on anti-glaucoma drugs. Expert Opinion on Therapeutic Patents. 2004;14:1743–1762. [Google Scholar]
- Dibas A, Jiang M, Fudala R, Gryczynski I, Gryczynski Z, Clark AF, Yorio T. Fluorescent protein-labeled glucocorticoid receptor alpha isoform trafficking in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53(6):2938–2950. doi: 10.1167/iovs.11-8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois DC, Sukumaran S, Jusko WJ, Almon RR. Evidence for a glucocorticoid receptor beta splice variant in the rat and its physiological regulation in liver. Steroids. 2013;78:312–320. doi: 10.1016/j.steroids.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebert Z, Bracke M, Beck IM. Glucocorticoids and the non-steroidal., selective glucocorticoid receptor modulator, compound A, differentially affect colon cancer-derived myofibroblasts. J Steroid Biochem Mol Biol. 2015;149:92–105. doi: 10.1016/j.jsbmb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- DuBois DC, Sukumaran S, Jusko WJ, Almon RR. Evidence for a glucocorticoid receptor beta splice variant in the rat and its physiological regulation in liver. Steroids. 2013;78:312–320. doi: 10.1016/j.steroids.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien Pilipuk G, Galigniana MD. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol Cell Biol. 2009;29(17):4788–4797. doi: 10.1128/MCB.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger B, Pressman A, Shah HA. Who bears responsibility for glucocorticoid-exposed patients in a large health maintenance organization. J Manag Care Pharm. 2001;7:228–232. [Google Scholar]
- Fan BJ, Wang DY, Tham CC, Lam DS, Pang CP. Gene expression profiles of human trabecular meshwork cells induced by triamcinolone and dexamethasone. Invest Ophthalmol Vis Sci. 2008;49(5):1886–1897. doi: 10.1167/iovs.07-0414. [DOI] [PubMed] [Google Scholar]
- Faralli JA, Gagen D, Filla MS, Crotti TN, Peters DM. Dexamethasone increases αvβ3 integrin expression and affinity through a calcineurin/NFAT pathway. Biochim Biophys Acta. 2013;1833(12):3306–3313. doi: 10.1016/j.bbamcr.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feibel J, Kelly M, Lee L, Woolf P. Loss of adrenocortical suppression after acute brain injury: role of increased intracranial pressure and brain stem function. J Clin Endocrinol Metab. 1983;57(6):1245–50. doi: 10.1210/jcem-57-6-1245. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Clark AF, Craig JE, Alward WL, Snibson GR, McLaughlin M, Tuttle L, Mackey DA, Sheffield VC, Stone EM. Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42:145–152. [PubMed] [Google Scholar]
- Fingert JH, Ying L, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res. 1998;8:377–384. doi: 10.1101/gr.8.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CS, Vitale AT. In: Diagnosis and treatment of Uveitis. 1st. Foster CS, Vitale AT, editors. WB Saunders; 2002. pp. 17–23. [Google Scholar]
- Freedman J, David R, van der Walt LA, Luntz MH. Plasma cortisol suppression response in the South African black population with glaucoma. Br J Ophthalmol. 1976;60(11):786–788. doi: 10.1136/bjo.60.11.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ND, Yamamoto KR. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell. 2004;15(5):2276–2286. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijters R, Fleuren W, Toonen EJ, Tuckermann JP, Reichardt HM, van der Maaden H, van Elsas A, van Lierop MJ, Dokter W, de Vlieg J, Alkema W. Prednisolone-induced differential gene expression in mouse liver carrying wild type or a dimerization-defective glucocorticoid receptor. BMC Genomics. 2010;11:359. doi: 10.1186/1471-2164-11-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, Carletto A, Pattini P, Corrocher R, Olivieri O. Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199(2):32332–7. doi: 10.1016/j.atherosclerosis.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011;31:4663–4675. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O’Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16(1):61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Gathercole LL, Lavery GG, Morgan SA, Cooper MS, Sinclair AJ, Tomlinson JW, Stewart PM. 11b-hydroxysteroid dehydrogenase 1: translational and therapeutic targets. Endocrine reviews. 2013;34:525–555. doi: 10.1210/er.2012-1050. [DOI] [PubMed] [Google Scholar]
- Gelatt KN, Mackay EO. The ocular hypertensive effects of topical 0.1% dexamethasone in beagles with inherited glaucoma. J Ocul Pharmacol Ther. 1998;14:57–66. doi: 10.1089/jop.1998.14.57. [DOI] [PubMed] [Google Scholar]
- Gerometta R, Podos SM, Candia OA, Wu B, Malgor LA, Mittag T, Danias J. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol. 2004;122:1492–1497. doi: 10.1001/archopht.122.10.1492. [DOI] [PubMed] [Google Scholar]
- Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Invest Ophthalmol Vis Sci. 2009;50:669–673. doi: 10.1167/iovs.08-2410. [DOI] [PubMed] [Google Scholar]
- Goecke A, Guerrero J. Glucocorticoid receptor beta in acute and chronic inflammatory conditions: clinical implications. Immunobiol. 2006;211(1–2):85–96. doi: 10.1016/j.imbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gould NR, Siegel HS. Partial purification and characeterization of chicken corticosteroid-binding globulin. Poult Sci. 1978;57:1733–1739. doi: 10.3382/ps.0571733. [DOI] [PubMed] [Google Scholar]
- Gramlich OW, Beck S, von Thun Und Hohenstein-Blaul N, Boehm N, Ziegler A, Vetter JM, Pfeiffer N, Grus FH. Enhanced Insight into the Autoimmune Component of Glaucoma: IgG Autoantibody Accumulation and Pro-Inflammatory Conditions in Human Glaucomatous Retina. PLos One. 2013;8(2):e57557. doi: 10.1371/journal.pone.0057557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmol. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Haeck IM, Rouwen TJ, Timmer-de Mik L, de Bruin-Weller MS, Bruijnzeel-Koomen CA. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64(2):275–281. doi: 10.1016/j.jaad.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Hakim A, Barnes PJ, Adcock IM, Usmani OS. Importin-7 mediates glucocorticoid receptor nuclear import and is impaired by oxidative stress, leading to glucocorticoid insensitivity. FASEB J. 2013;27(11):4510–4519. doi: 10.1096/fj.12-222604. [DOI] [PubMed] [Google Scholar]
- Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY. Increased glucocorticoid receptor β in airway cells of glucocorticoid-insensitive asthma. Am J Resp Crit Care Med. 1999;159:1600–1604. doi: 10.1164/ajrccm.159.5.9804131. [DOI] [PubMed] [Google Scholar]
- Hammond GL. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr Rev. 1990;11:65–79. doi: 10.1210/edrv-11-1-65. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Smith CL, Underhill DA. Molecular studies of corticosteroid binding globulin structure, biosynthesis and function. J Steroid Biochem Mol Biol. 1991;40:755–762. doi: 10.1016/0960-0760(91)90300-t. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Smith CL, Paterson NP, Sibbald WJ. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990;71:34–39. doi: 10.1210/jcem-71-1-34. [DOI] [PubMed] [Google Scholar]
- Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999;2(3):201–205. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- Hauk PJ, Goleva E, Strickland I, Vottero A, Chrousos GP, Kisich KO, Leung DY. Increased glucocorticoid receptor β expression converts mouse hybridoma cells to a corticosteroid-insensitive phenotype. Am J Resp Cell Mol Biol. 2002;27:361–367. doi: 10.1165/rcmb.4861. [DOI] [PubMed] [Google Scholar]
- Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz C, Koch JM, Zurek-Imhoff B, Heiligenhaus A. Prevalence of uveitic secondary glaucoma and success of nonsurgical treatment in adults and children in a tertiary referral center. Ocul Immunol Inflamm. 2009;17(4):243–248. doi: 10.1080/09273940902913035. [DOI] [PubMed] [Google Scholar]
- Henschkowski J, Stuck AE, Frey BM, Gillmann G, Dick B, Frey FJ, Mohaupt MG. Age-dependent decrease in 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) activity in hypertensive patients. Am J Hypertens. 2008;21(6):644–649. doi: 10.1038/ajh.2008.152. [DOI] [PubMed] [Google Scholar]
- Herbert HM, Viswanathan A, Jackson H, Lightman SL. Risk factors for elevated intraocular pressure in uveitis. J Glaucoma. 2004;13(2):96–99. doi: 10.1097/00061198-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Hinds TD, Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol Endocrinol. 2010;24:1715–1727. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom S, Van Antwerp ME, Iñiguez-Lluhi JA. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc Natl Acad Sci USA. 2003;100:15758–15763. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom SR, Chupreta S, So AY, Iniguez-Lluhi JA. SUMO-mediated inhibition of glucocorticoid receptor synergistic activity depends on stable assembly at the promoter but not on DAXX. Mol Endocrinol. 2008;22:2061–2075. doi: 10.1210/me.2007-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holownia A, Mroz RM, Kolodziejczyk A, Chyczewska E, Braszko JJ. Increased FKBP51 in induced sputum cells of chronic obstructive pulmonary disease patients after therapy. Eur J Med Res Suppl. 2009;4:108–111. doi: 10.1186/2047-783X-14-S4-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Orii F, Ayabe T, Imai S, Ashida T, Obara T, Kohgo Y. Expression of glucocorticoid receptor β in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000;11:859–866. doi: 10.1016/s0016-5085(00)70172-7. [DOI] [PubMed] [Google Scholar]
- Huang P, Qi Y, Xu YS, Liu J, Liao D, Zhang SS, Zhang C. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J Glaucoma. 2010;19(5):324–330. doi: 10.1097/IJG.0b013e3181b4cac7. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Lizarral de G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73:1081–1088. doi: 10.1210/jcem-73-5-1081. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Wordinger RJ, Yorio T, Clark AF. Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53(2):857–866. doi: 10.1167/iovs.11-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7(6):e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell CM, Scoltock AB, Hamel BL, Yudt MR, Cidlowski JA. Complex human glucocorticoid receptor dim mutations define glucocorticoid induced apoptotic resistance in bone cells. Mol Endocrinol. 2012;26:244–256. doi: 10.1210/me.2011-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Haque K, Palanichamy E, Okret S. Stimulation of MAPK-phosphatase 1 gene expression by glucocorticoids occurs through a tethering mechanism involving C/EBP. J Mol Endocrinol. 2008;41(4):239–49. doi: 10.1677/JME-08-0015. [DOI] [PubMed] [Google Scholar]
- Johnson D, Gottanka J. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch Ophthalmol. 1997;115(3):375–383. doi: 10.1001/archopht.1997.01100150377011. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Invest Ophthalmol Vis Sci. 1990;31:2568–2571. [PubMed] [Google Scholar]
- Jones R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kass MA, Krupin T, Becker B. Plasma cortisol suppression test used to predict the development of primary open-angle glaucoma. Am J Ophthalmol. 1976;82(3):496–497. doi: 10.1016/0002-9394(76)90501-8. [DOI] [PubMed] [Google Scholar]
- Kelly A, Bowen H, Jee YK, Mahfiche N, Soh C, Lee T, Hawrylowicz C, Lavender P. The glucocorticoid receptor beta isoform can mediate transcriptional repression by recruiting histone deacetylases. J Allergy Clin Immunol. 2008;121:203–208. doi: 10.1016/j.jaci.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, Baath J, Buys YM. Intraocular Pressure Monitoring Post Intravitreal Steroids: A Systematic Review. Surv Ophthalmol. 2013;58(4):291–310. doi: 10.1016/j.survophthal.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem Biophys Res Commun. 2009;381:671–675. doi: 10.1016/j.bbrc.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemetti A. The dexamethasone provocative test: a predictive tool for glaucoma? Acta Ophthalmol (Copenh) 1990;68(1):29–33. doi: 10.1111/j.1755-3768.1990.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Kostadinova RM, Nawrocki AR, Frey FJ, Frey BM. Tumor necrosis factor alpha and phorbol 12-myristate-13-acetate down-regulate human 11beta-hydroxysteroiddehydrogenase type 2 through p50/p50 NF-kappaB homodimers and Egr-1. FASEB J. 2005;19(6):650–652. doi: 10.1096/fj.04-2820fje. [DOI] [PubMed] [Google Scholar]
- Kozlov G, Määttänen P, Thomas DY, Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277:3924–3936. doi: 10.1111/j.1742-4658.2010.07793.x. [DOI] [PubMed] [Google Scholar]
- Kwok AK, Lam DS, Ng JS, Fan DS, Chew SJ, Tso MO. Ocular hypertensive response to topical steroids in children. Ophthalmol. 1997;104:2112–2116. doi: 10.1016/s0161-6420(97)30052-9. [DOI] [PubMed] [Google Scholar]
- Krett NL, Pillay S, Moalli PA. A variant glucocorticoid receptor messenger RNA is expressed in multiple myeloma patients. Cancer Res. 1995;55:2727–2729. [PubMed] [Google Scholar]
- Kubota R, Kudoh J, Mashima Y, Asakawa S, Minoshima S, Hejtmancik JF, Oguchi Y, Shimizu N. Genomic organization of the human myocilin gene (MYOC) responsible for primary open angle glaucoma (GLC1A) Biochem Biophys Res Commun. 1998;242:396–400. doi: 10.1006/bbrc.1997.7972. [DOI] [PubMed] [Google Scholar]
- Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci USA. 2012;109(28):11160–11165. doi: 10.1073/pnas.1111334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DS, Fan DS, Ng JS, Yu CB, Wong CY, Cheung AY. Ocular hypertensive and anti-inflammatory responses to different dosages of topical dexamethasone in children: a randomized trial. Clin Exp Ophthalmol. 2005;33:252–258. doi: 10.1111/j.1442-9071.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Wilhelmsen L, Lappas G. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- Le Drean Y, Mincheneau N, Le Goff P, Michel D. Potentiation of glucocorticoid receptor transcriptional activity by sumoylation. Endocrinol. 2002;143:3482–3489. doi: 10.1210/en.2002-220135. [DOI] [PubMed] [Google Scholar]
- Leung DYM, Hamid Q, Vottero A, Szefler SJ, Surs W, Minshall E, Chrousos GP, Klemm DJ. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor β. J Exp Med. 1997;186:1567–1574. doi: 10.1084/jem.186.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DS, Han DP, Dev S, Wirostko WJ, Mieler WF, Connor TB, George V, Eastwood D. Subtenon’s depot corticosteroid injections in patients with a history of corticosteroid-induced intraocular pressure elevation. Am J Ophthalmol. 2002;133(2):196–202. doi: 10.1016/s0002-9394(01)01372-1. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann NY Acad Sci. 2006;1069:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor β binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(11):7996–8005. doi: 10.1167/iovs.11-8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JI, Niec M, Wong V. One year results of a phase 1 study of the safety and tolerability of combination therapy using sustained release intravitreal triamcinolone acetonide and ranibizumab for subfoveal neovascular AMD. Br J Ophthalmol. 2015;99(5):618–23. doi: 10.1136/bjophthalmol-2014-306002. [DOI] [PubMed] [Google Scholar]
- Lin DY, Huang YS, Jeng JC, Kuo HY, Chang CC, Chao TT, Ho CC, Chen YC, Lin TP, Fang HI, Hung CC, Suen CS, Hwang MJ, Chang KS, Maul GG, Shih HM. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol Cell. 2006;24:341–354. doi: 10.1016/j.molcel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol Cell Endocrinol. 2010;316:3–12. doi: 10.1016/j.mce.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Lo WR, Rowlette LL, Caballero M, Yang P, Hernandez MR, Borrás T. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003;44(2):473–85. doi: 10.1167/iovs.02-0444. [DOI] [PubMed] [Google Scholar]
- Longui CA, Vottero A, Adamson PC, Cole DE, Kino T, Monte O, Chrousos GP. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res. 2000;32:401–406. doi: 10.1055/s-2007-978661. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Lorenzetti OJ. Effects of corticosteroids on ocular dynamics in rabbits. J Pharmacol Exp Ther. 1970;175:763–772. [PubMed] [Google Scholar]
- McCarty GR, Schwartz B. Increased plasma noncortisol glucocorticoid activity in open-angle glaucoma. Invest Ophthalmol Vis Sci. 1991;32:1600–1608. [PubMed] [Google Scholar]
- Melander O, Orho-Melander M, Bengtsson K, Lindblad U, Råstam L, Groop L, Hulthén UL. Association between a variant in the 11 β-hydroxysteroid dehydrogenase type 2 gene and primary hypertension. J Hum Hypertens. 2000;14:819–823. doi: 10.1038/sj.jhh.1001116. [DOI] [PubMed] [Google Scholar]
- Meredig WE, Pulhorn G. Plasma cortisol level in patients suffering from glaucoma. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;213:215–218. doi: 10.1007/BF00410991. [DOI] [PubMed] [Google Scholar]
- Meulenberg PM, Hofman JA. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clin Chem. 1990;36:70–75. [PubMed] [Google Scholar]
- Meyer T, Gustafsson JA, Carlstedt-Duke J. Glucocorticoid-dependent transcriptional repression of the osteocalcin gene by competitive binding at the TATA box. DNA Cell Biol. 1997;16(8):919–927. doi: 10.1089/dna.1997.16.919. [DOI] [PubMed] [Google Scholar]
- Moalli PA, Pillay S, Krett NL. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells. Cancer Res. 1993;53:3877–3879. [PubMed] [Google Scholar]
- Morin B, Nichols LA, Holland LJ. Flanking sequence composition differentially affects the binding and functional characteristics of glucocorticoid receptor homo- and heterodimers. Biochemistry. 2006;45(23):7299–306. doi: 10.1021/bi060314k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional., activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential., physiological., implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Acs B, Butz H, Feldman K, Marta A, Szabo PM, Baghy K, Pazmany T, Racz K, Liko I, Patocs A. Overexpression of GRβ in colonic mucosal cell line partly reflects altered gene expression in colonic mucosa of patients with inflammatory bowel disease. J Steroid Biochem Mol Biol. 2016;155(Pt A):76–84. doi: 10.1016/j.jsbmb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Nehme A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009;2:58. doi: 10.1186/1755-8794-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri P, Azuara-Blanco A, Forrester JV. Incidence of glaucoma in patients with uveitis. J Glaucoma. 2004;13:461–465. doi: 10.1097/01.ijg.0000146391.77618.d0. [DOI] [PubMed] [Google Scholar]
- Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol. 2001;28(11):957–961. doi: 10.1046/j.1440-1681.2001.03559.x. [DOI] [PubMed] [Google Scholar]
- Ng JS, Fan DS, Young AL, Yip NK, Tam K, Kwok AK, Lam DS. Ocular hypertensive response to topical dexamethasone in children: a dose-dependent phenomenon. Ophthalmol. 2000;107:2097–2100. doi: 10.1016/s0161-6420(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Nixon M, Andrew R, Chapman KE. It takes two to tango: dimerization of glucocorticoid receptor and its anti-inflammatory functions. Steroids. 2013;78:59–68. doi: 10.1016/j.steroids.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Noble S, Goa KL. Loteprednol etabonate: clinical potential in the management of ocular inflammation. BioDrugs. 1998;10(4):329–339. doi: 10.2165/00063030-199810040-00007. [DOI] [PubMed] [Google Scholar]
- Nusbaum DM, Wu SM, Frankfort BJ. Elevated intracranial pressure causes optic nerve and retinal ganglion cell degeneration in mice. Exp Eye Res. 2015;136:38–44. doi: 10.1016/j.exer.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271(16):9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. Cellular processing of glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;268:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii F, Ashida T, Nomura M, Maemoto A, Fujiki T, Ayabe T, Imai S, Saitoh Y, Kohgo Y. Quantitative analysis for human glucocorticoid receptor α/β mRNA in IBD. Biochem Biophys Res Commun. 2002;296:1286–1294. doi: 10.1016/s0006-291x(02)02030-2. [DOI] [PubMed] [Google Scholar]
- Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken) 2013;65(2):294–298. doi: 10.1002/acr.21796. [DOI] [PubMed] [Google Scholar]
- Paakinaho V, Kaikkonen S, Makkonen H, Benes V, Palvimo JJ. Sumoylation regulates the chromatin occupancy and anti-proliferative gene programs of glucocorticoid receptor. Nucleic Acids Res. 2014;42(3):1575–1592. doi: 10.1093/nar/gkt1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente L, Solito E. Association between glucocorticosteroids and lipocortin 1. Trends Pharmacol Sci. 1994;15:362. doi: 10.1016/0165-6147(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Patel R, Williams-Dautovich J, Cummins CL. Minireview: new molecular mediators of glucocorticoid receptor activity in metabolic tissues. Mol Endocrinol. 2014;28(7):999–1011. doi: 10.1210/me.2014-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson G. Studies of the response to topical dexamethasone of glaucoma relatives. Trans Ophthalmol Soc U K. 1965;85:295–305. [PubMed] [Google Scholar]
- Payette H, Roubenoff R, Jacques PF. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- Presman DM, Ogara MF, Stortz M, Alvarez LD, Pooley JR, Schiltz RL, Grøntved L, Johnson TA, Mittelstadt PR, Ashwell JD, Ganesan S, Burton G, Levi V, Hager GL, Pecci A. Live cell imaging unveils multiple domain requirements for in vivo dimerization of the glucocorticoid receptor. PLos Biol. 2014;12:e1001813. doi: 10.1371/journal.pbio.1001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D, Yamamoto KR. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987;6:3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Wu K, Lin X, Liu Q, Ye Y, Yu M. Dexamethasone increases Cdc42 expression in human TM-1 cells. Curr Eye Res. 2015;40(3):290–299. doi: 10.3109/02713683.2014.922191. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Brown AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells and Matrix. Invest Ophthalmol Vis Sci. 2015;56(8):4447–59. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauz S, Walker EA, Shackleton CH, Hewison M, Murray PI, Stewart PM. Expression and putative role of 11 beta-hydroxysteroid dehydrogenase isozymes within the human eye. Invest Ophthalmol Vis Sci. 2001;42:2037–2042. [PubMed] [Google Scholar]
- Ray S, Mehra KS. Plasma cortisol in glaucoma. Ann Ophthalmol. 1977;9:1151–1154. [PubMed] [Google Scholar]
- Ray DW, Davis JR, White A. Glucocorticoid receptor structure and function in glucocorticoid-resistant small cell lung carcinoma cells. Cancer Res. 1996;56:3276–3280. [PubMed] [Google Scholar]
- Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19(12):2163–71. doi: 10.1101/gr.097022.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozsa FW, Reed DM, Scott KM, Pawar H, Moroi SE, Kijek TG, Krafchak CM, Othman MI, Vollrath D, Elner VM, Richards JE. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006;12:125–41. [PubMed] [Google Scholar]
- Rozsíval P, Hampl R, Obenberger J, Stárka L, Rehák S. Aqueous humor and plasma cortisol levels in glaucoma and cataract patients. Curr Eye Res. 1981;1(7):391–396. doi: 10.3109/02713688109019976. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Rivers C, Levy A, Hancock J, Lightman S, Norman M. Insertion of an amino acid in the DNA-binding domain of the glucocorticoid receptor as a result of alternative splicing. J Clin Endocrinol Metab. 1999;84(11):4283–4286. doi: 10.1210/jcem.84.11.6235. [DOI] [PubMed] [Google Scholar]
- Robertson S, Allie-Reid F, Vanden Berghe W, Visser K, Binder A, Africander D, Vismer M, De Bosscher K, Hapgood J, Haegeman G, Louw A. Abrogation of glucocorticoid receptor dimerization correlates with dissociated glucocorticoid behavior of compound A. J Biol Chem. 2010;285:8061–8075. doi: 10.1074/jbc.M109.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S, Hapgood JP, Louw A. Glucocorticoid receptor concentration and the ability to dimerize influence nuclear translocation and distribution. Steroids. 2013a;78:182–194. doi: 10.1016/j.steroids.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Robertson S, Rohwer JM, Hapgood JP, Louw A. Impact of glucocorticoid receptor density on ligand-independent dimerization, cooperative ligand-binding and basal priming of transactivation: a cell culture model. PLoS One. 2013b;8:e64831. doi: 10.1371/journal.pone.0064831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AL, Clark AF, Covert DW, Krueger S, Bergamini MV, Landry TA, Dickerson JE, Jr, Scheib SA, Realini T, Defaller JM, Cagle GD. Anterior juxtascleral delivery of anecortave acetate in eyes with primary open-angle glaucoma: a pilot investigation. Am J Ophthalmol. 2008;147(1):45–50. doi: 10.1016/j.ajo.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Rönkkö S, Rekonen P, Kaarniranta K, Puustjärvi T, Teräsvirta M, Uusitalo H. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):697–704. doi: 10.1007/s00417-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005;26:452–464. doi: 10.1210/er.2005-0002. [DOI] [PubMed] [Google Scholar]
- Rosenberg S, Levene R. Suppression of plasma cortisol in normal. Arch Ophthalmol. 1974;92:6–9. doi: 10.1001/archopht.1974.01010010010003. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Lind U, Gåfvels M, Eggertsen G, Carlstedt-Duke J, Nilsson L, Holtmann M, Stierna P, Wikström AC, Werner S. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf) 2001;55:363–371. doi: 10.1046/j.1365-2265.2001.01323.x. [DOI] [PubMed] [Google Scholar]
- Russcher H, Smit P, van den Akker EL, van Rossum EF, Brinkmann AO, de Jong FH, Lamberts SW, Koper JW. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab. 2005;90:5804–5810. doi: 10.1210/jc.2005-0646. [DOI] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Haché RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci. 2010;51(2):903–906. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- Sawaguchi K, Nakamura Y, Nakamura Y, Sakai H, Sawaguchi S. Myocilin gene expression in the trabecular meshwork of rats in a steroid-induced ocular hypertension model. Ophthalmic Res. 2005;37:235–242. doi: 10.1159/000086946. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2011;124(2):152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Champagne D, van Laanen IH, van Wijk DC, Meijer AH, Meijer OC, Spaink HP, Richardson MK. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinol. 2008;149:1591–1599. doi: 10.1210/en.2007-1364. [DOI] [PubMed] [Google Scholar]
- Selım A, Koçak N, Aslankara H, Kaynak S. Comparative study of photodynamic therapy monotherapy versus triple management in age-related macular degeneration. Turk J Med Sci. 2014;44(5):889–95. [PubMed] [Google Scholar]
- Sharma A, Aoun P, Wigham J, Weist S, Veldhuis JD. Gender determines ACTH recovery from hypercortisolemia in healthy older humans. Metabolism. 2013;62(12):1819–1829. doi: 10.1016/j.metabol.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B, Levene RZ. Plasma cortisol differences between normal and glaucomatous patients. Arch Ophthalmol. 1972;87:369–377. doi: 10.1001/archopht.1972.01000020371001. [DOI] [PubMed] [Google Scholar]
- Schwartz JT, Reuling FH, Jr, Feinleib M, Garrison RJ, Collie DJ. Twin heritability study of the corticosteroid response. Trans Am Acad Ophthalmol Otolaryngol. 1973;77(2):126–136. [PubMed] [Google Scholar]
- Schwartz B, McCarty G. Increased plasma free cortisol in ocular hypertension and open angle glaucoma. Arch Ophthalmol. 1987;105:1060–1065. doi: 10.1001/archopht.1987.01060080062029. [DOI] [PubMed] [Google Scholar]
- Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Kempen JH. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmol. 2014;121(11):2275–2286. doi: 10.1016/j.ophtha.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi H, Vottero A, Stratakis CA, Taymans SE, Karl M, Longui CA, Chrousos GP, Daughaday WH, Gregory SA, Plate JM. Imbalanced expression of the glucocorticoid receptor isoforms in cultured lymphocytes from a patient with systemic glucocorticoid resistance and chronic lymphocytic leukemia. Biochem Biophys Res Commun. 1999;254:559–565. doi: 10.1006/bbrc.1998.9980. [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21–q31. Nature Genetics. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Conrow RE, Pang IH, Jacobson N, Rezwan M, Rutschmann K, Auerbach D, Sriramaratnam R, Cornish VW. Identification of PDE6D as a molecular target of anecortave acetate via a methotrexate-anchored yeast three-hybrid screen. ACS Chem Biol. 2013;8(3):549–558. doi: 10.1021/cb300296m. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids. 1994;59:436–442. doi: 10.1016/0039-128x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Walker EA, Burdon MA, van Beek AP, Kema IP, Hughes BA, Murray PI, Nightingale PG, Stewart PM, Rauz S, Tomlinson JW. Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: a link between 11beta-HSD1 and intracranial pressure regulation? J Clin Endocrinol Metab. 2010;95(12):5348–5356. doi: 10.1210/jc.2010-0729. [DOI] [PubMed] [Google Scholar]
- Singh IP, Ahmad SI, Yeh D, Challa P, Herndon LW, Allingham RR, Lee PP. Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2004;138:286–287. doi: 10.1016/j.ajo.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Smith CL. Corticosteroid glaucoma: A summary and review of the literature. Am J Med Sci. 1966;252:239–244. doi: 10.1097/00000441-196608000-00017. [DOI] [PubMed] [Google Scholar]
- Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor β-isoform. J Allergy Clin Immunol. 2000;105:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Hoffman EA, Kurali E, Krauss AH. Unique response profile of trabecular meshwork cells to the novel selective glucocorticoid receptor agonist, GW870086X. Invest Ophthalmol Vis Sci. 2013;54(3):2100–2107. doi: 10.1167/iovs.12-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RH, Kimbrough RL. Intraocular pressure response to topically administered fluorometholone. Arch Ophthalmol. 1979;97:2139–2140. doi: 10.1001/archopht.1979.01020020457010. [DOI] [PubMed] [Google Scholar]
- Stokes J, Noble J, Brett L, Phillips C, Seckl JR, O’Brien C, Andrew R. Distribution of glucocorticoid and mineralocorticoid receptors and 11 beta-hydroxysteroid dehydrogenases in human and rat ocular tissues. Invest Ophthalmol Vis Sci. 2000;41:1629–1638. [PubMed] [Google Scholar]
- Stokes J, Walker BR, Campbell JC, Seckl JR, O’Brien C, Andrew R. Altered peripheral sensitivity to glucocorticoids in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003;44(12):5163–5167. doi: 10.1167/iovs.02-1318. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Sundahl N, Bridelance J, Libert C, De Bosscher K, Beck IM. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacol Ther. 2015;152:28–41. doi: 10.1016/j.pharmthera.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, Li M, Chambon P. Widespread Negative Response Elements Mediate Direct Repression by Agonist- Liganded Glucocorticoid Receptor. Cell. 2011;145(2):224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Kaneko C, Ogawa S, Darnel AD, Krozowski ZS. Immunohistochemical distribution of 11 beta-hydroxysteroid dehydrogenase in human eye. Mol Cell Endocrinol. 2001;173:121–125. doi: 10.1016/s0303-7207(00)00403-2. [DOI] [PubMed] [Google Scholar]
- Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis Sci. 2012;53(1):241–247. doi: 10.1167/iovs.11-8434. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S101–104. doi: 10.1016/j.survophthal.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nishi M, Morimoto M, Sugimoto T, Kawata M. Yellow fluorescent protein-tagged and cyan fluorescent protein-tagged imaging analysis of glucocorticoid receptor and importins in single living cells. Endocrinol. 2003;144(9):4070–4079. doi: 10.1210/en.2003-0282. [DOI] [PubMed] [Google Scholar]
- Tao T, Lan J, Lukacs GL, Haché RJ, Kaplan F. Importin 13 regulates nuclear import of the glucocorticoid receptor in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;35(6):668–680. doi: 10.1165/rcmb.2006-0073OC. [DOI] [PubMed] [Google Scholar]
- Tawara A, Tou N, Kubota T, Harada Y, Yokota K. Immunohistochemical evaluation of the extracellular matrix in trabecular meshwork in steroid-induced glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1021–1028. doi: 10.1007/s00417-008-0800-0. [DOI] [PubMed] [Google Scholar]
- Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Thomas-Chollier M, Watson LC, Cooper SB. A naturally occurring insertion of a single amino acid rewires transcriptional regulation by glucocorticoid receptor isoforms. Proc Natl Acad Sci USA. 2013;110:17826–17831. doi: 10.1073/pnas.1316235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpy DJ, Ho JT. Corticosteroid-binding globulin gene polymorphisms: clinical implications and links to idiopathic chronic fatigue disorders. Clin Endocrinol (Oxf) 2007;67:161–167. doi: 10.1111/j.1365-2265.2007.02890.x. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Kirschner BS, Kipp M. Corticosteroid treatment for inflammatory bowel disease in pediatric patients increases intraocular pressure. Gastroenterol. 1992;102:1957–1961. doi: 10.1016/0016-5085(92)90319-t. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uings IJ, Needham D, Matthews J, Haase M, Austin R, Angell D, Leavens K, Holt J, Biggadike K, Farrow SN. Discovery of GW870086: a potent anti-inflammatory steroid with a unique pharmacological profile. Br J Pharmacol. 2013;169(6):1389–1403. doi: 10.1111/bph.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadot E. Epidemiology of intermediate uveitis: a prospective study in Savoy. Dev Ophthalmol. 1992;23:33–34. doi: 10.1159/000429625. [DOI] [PubMed] [Google Scholar]
- Vakalis N, Echiadis G, Pervena A, Deligiannis I, Kavalarakis E, Giannikakis S, Papaefthymiou Intravitreal combination of dexamethasone sodium phosphate and bevacizumab in the treatment of exudative AMD. Sci Rep. 2015;5:8627. doi: 10.1038/srep08627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Polansky JR, Kramer SG, Baxter JD. Acute effects of dexamethasone on intraocular pressure in glaucoma. Invest Ophthalmol Vis Sci. 1985;26:170–175. [PubMed] [Google Scholar]
- Weinstein BI, Munnangi P, Gordon GG, Southren AL. Defects in cortisol-metabolizing enzymes in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1985;26(6):890–893. [PubMed] [Google Scholar]
- Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100(1–3):34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Westphal U. Steroid-protein interactions II. Monogr Endocrinol. 1986;27:1–603. [PubMed] [Google Scholar]
- Whitlock NA, McKnight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with dexamethasone. Invest Ophthalmol Vis Sci. 2010;51:6496–6503. doi: 10.1167/iovs.10-5430. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J human genetics. 1998;63:1549–1552. doi: 10.1086/302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CL, Luhrmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3(7) doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP. Fast circulation of cerebrospinal fluid: an alternative perspective on the protective role of high intracranial pressure in ocular hypertension. Clin Exp Optom. 2015 doi: 10.1111/cxo.12332. [DOI] [PubMed] [Google Scholar]
- Wu I, Shin SC, Cao Y, Bender IK, Jafari N, Feng G, Lin S, Cidlowski JA, Schleimer RP, Lu NZ. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4:e453. doi: 10.1038/cddis.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Comes N, Borrás T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest Ophthalmol Vis Sci. 2007;48(7):3184–3194. doi: 10.1167/iovs.06-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Komatsu T, Koura Y, Nishino K, Fukushima A, Ueno H. Intraocular pressure elevation after intravitreal or posterior sub-tenon triamcinolone acetonide injection. Can J Ophthalmol. 2008;43(1):42–47. doi: 10.3129/i07-186. [DOI] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G. Neurodegenerative and inflammatory pathway components linked to TNF-α/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci. 2011;52(11):8442–8454. doi: 10.1167/iovs.11-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Call MK, Yuan Y, Zhang Y, Fischesser K, Liu CY, Kao WW. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Invest Ophthalmol Vis Sci. 2013;54(10):6502–6509. doi: 10.1167/iovs.13-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan GL, Miranda OC, Bito LZ. Steroid glaucoma: corticosteroid-induced ocular hypertension in cats. Exp Eye Res. 1992;54:211–218. doi: 10.1016/s0014-4835(05)80210-6. [DOI] [PubMed] [Google Scholar]
- Zhang HN, He YH, Zhang GS, Luo MS, Huang Y, Wu XQ, Liu SM, Luo JD, Chen MS. Endogenous glucocorticoids inhibit myocardial inflammation induced by lipopolysaccharide: involvement of regulation of histone deacetylation. J Cardiovasc Pharmacol. 2012;60:33–41. doi: 10.1097/FJC.0b013e3182567fef. [DOI] [PubMed] [Google Scholar]
- Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005;46:4607–4616. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor β. Exp Eye Res. 2007;84:275–284. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Clark AF, Yorio T. FK506-binding protein 51 regulates nuclear transport of the glucocorticoid receptor beta and glucocorticoid responsiveness. Invest Ophthalmol Vis Sci. 2008;49:1037–1047. doi: 10.1167/iovs.07-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu S, Jonas JB, Zhang J, Liu K, Lu Q, Wang N. Dynein, kinesin and morphological changes in optic nerve axons in a rat model with cerebrospinal fluid pressure reduction: the Beijing Intracranial and Intraocular Pressure (iCOP) study. Acta Ophthalmol. 2015 doi: 10.1111/aos.12768. [DOI] [PubMed] [Google Scholar]
- Zhao D, He Z, Vingrys AJ, Bui BV, Nguyen CT. The effect of intraocular and intracranial pressure on retinal structure and function in rats. Physiol Rep. 2015;3(8):e12507. doi: 10.14814/phy2.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ishiguro H, Ide H, Inoue S, Kashiwagi E, Kawahara T, Jalalizadeh M, Reis LO, Miyamoto H. Compound A Inhibits Bladder Cancer Growth Predominantly via Glucocorticoid Receptor Transrepression. Mol Endocrinol. 2015;29(10):1486–1497. doi: 10.1210/me.2015-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo YH, He Y, Leung KW, Hou F, Li YQ, Chai F, Ge J. Dexamethasone disrupts intercellular junction formation and cytoskeleton organization in human trabecular meshwork cells. Mol Vis. 2010;16:61–71. [PMC free article] [PubMed] [Google Scholar]


