Abstract
Mechanisms by which regulatory T (Treg) cells fail to control inflammation in asthma remain poorly understood. We show that a severe asthma-associated polymorphism in the interleukin-4 receptor alpha chain (IL4RA R576) promotes conversion of induced Treg (iTreg) cells towards a T helper 17 (TH17) cell fate. This skewing is mediated by the recruitment by IL-4Rα-R576 of the growth factor receptor-bound protein 2 (GRB2) adaptor protein, which drives IL-17 expression by activating a pathway involving extracellular signal-regulated kinase, IL-6 and STAT3. Treg cell-specific deletion of Il6ra or Rorc, but not Il4 or Il13, prevented exacerbated airway inflammation in Il4raR576 mice. Furthermore, treatment of Il4raR576 mice with a neutralizing anti-IL-6 antibody prevented iTreg cell reprogramming into TH17-like cells and protected against severe airway inflammation. These findings identify a novel mechanism for the development of mixed TH2-TH17 cell inflammation in genetically prone individuals, and point to interventions that stabilize iTreg cells as potentially effective therapeutic strategies.
Asthma is characterized by airway hyperresponsiveness (AHR), chronic inflammation and tissue remodeling 1. Evidence points to reduced frequency and/or suppressive activity of FOXP3+ Treg cells in asthma and atopic conditions 2-4. Intense inflammation in experimental allergic asthma as well as other conditions, including rheumatoid arthritis and multiple sclerosis, may lessen Treg cell suppression, or redirect Treg cells to pro-inflammatory phenotypes 5-9. However, the molecular basis for impaired Treg cell function in asthma, and the impact of Treg cell dysfunction on asthma severity, remain to be fully elucidated.
The cytokines IL-4 and IL-13, acting via two distinct heterodimeric IL-4 receptor complexes, play a pivotal role in asthma pathogenesis. IL-4, which binds to both the IL-4R type I (composed of the IL-4Rα and the common cytokine receptor γc chains) and II (composed of IL-4Rα and IL-13Rα1 chains) directs TH2 cell differentiation. In contrast, IL-13, which exclusively binds to the IL-4R type II, promtes airway inflammation and remodeling (IL-13) 10. Increased signaling via IL-4rα and the downstream transcription factor STAT6 exacerbates allergic airway inflammation and promotes food allergy, the latter shown to involve suppressed differentiation of allergen-specific Treg cells and their re-programming into TH2 cell-like cells 9,11.
Polymorphisms in IL-4Rα are associated with atopy and asthma 12. In particular, a glutamine- (Q) to arginine (R) substitution at position 576 of IL-4Rα (IL-4Rα-Q576R) has been linked to asthma exacerbation and severity 13-16. The Q576 residue is conserved in the murine IL-4Rα chain. Its replacement by R576 in ll4ratm2Tch mice (also known as Il4raR576 mice) resulted in augmented allergic airway inflammation, severe eosinophilic infiltration and increased TH2 responses 17,18. Q576 lies immediately downstream of a STAT6 binding site at Y575, one of three present in IL-4Rα 19. Yet no discernable effect of the R576 mutation on STAT6 activation has been noted, indicating that it mobilizes alternative signaling pathways 17,20. We here show the IL-4Rα-R576 mutation enables recruitment of the adaptor GRB2 to promote airway inflammation by a novel mechanism involving IL-4-directed iTreg cell differentiation towards the TH17 cell lineage.
Results
Il4raR576 Treg cell phenotype in allergic airway inflammation
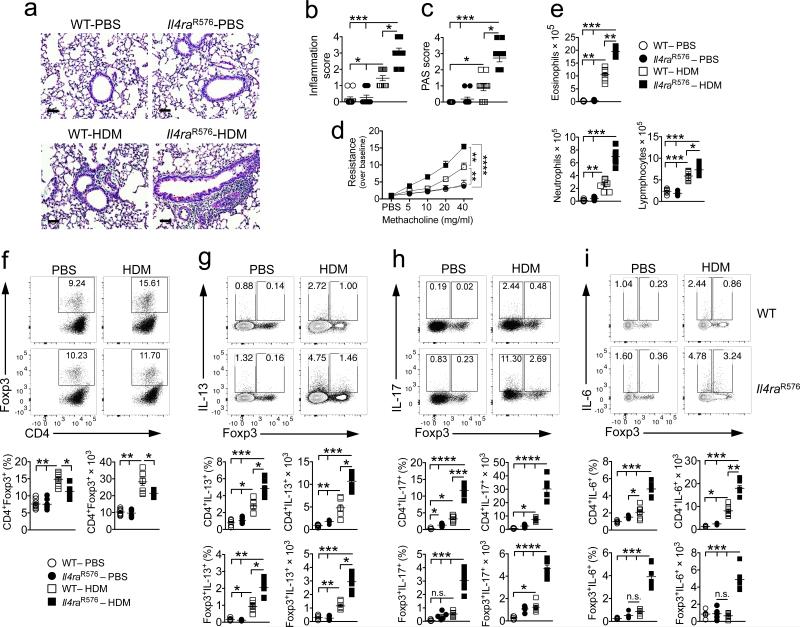
Whereas allergic asthma has classically been associated with a skewed TH2 cell response, a subgroup of patients with severe disease manifest a mixture of TH2 and TH17 cell responses in their airways 21-24. To determine whether the IL-4Rα-R576 mutation elicits TH17 airway responses in vivo, we employed a house dust mite (HDM)-induced model of airway inflammation 18. Il4raR576 mice, homozygous for the IL-4Rα-R576 mutation, exhibited severe airway inflammation as compared to control mice homozygous for the wild-type (WT) IL-4Rα-Q576 residue. The Il4raR576 mice had increased inflammatory infiltrates and mucus production, accentuated methacholine–induced AHR and robust lung tissue eosinophilia accompanied by neutrophilia, the latter masked in earlier studies by the use of alum as an adjuvant (data not shown) (Fig. 1a–e) 17. HDM treatment led to increased frequency and number of Treg cells in lung tissues of WT mice, but this increase was tempered in Il4raR576 mice (Fig. 1f). In contrast, HDM-treated Il4raR576 mice exhibited substantially increased frequencies and numbers of IL-17, IL-13 and IL-6 expressing CD4+Foxp3− conventional T (Tconv) and CD4+Foxp3+ Treg cells as compared to WT controls (Fig. 1g–i). Expression of IL-17 and IL-6, but not IL-13, in Il4raR576 Tconv and Treg cells was largely overlapping (Supplementary Fig. 1a,b). Expression of IL-17, IL-13 and IL-5 was increased, and that of IFN-γ was decreased, in the BAL fluid of HDM-treated Il4raR576 mice as compared to WT controls (Supplementary Fig. 1c). Furthermore, HDM exposure was associated with increased expression of the TH17 and TH2-promoting transcription factors RORγt and GATA3, respectively, in lung Tconv and Treg cells (Supplementary Fig. 1d–g). The frequencies and numbers of IL-4 producing Tconv and Treg cells in lung tissues of HDM-treated Il4raR576 mice trended to increase as compared to WT controls, while those of IFN-γ cells were unchanged (Supplementary Fig. 1h,i).
Figure 1.
Il4raR576 polymorphism promotes enhanced lung inflammation and AHR, associated with increased IL-17, IL-6 and IL-13 expression. (a) Representative PAS staining of lung sections isolated from WT and Il4raR576 mice either sham treated with PBS or immunized and challenged with HDM and analyzed 24 hr after the last challenge (20×, scale bar 50 μm) (n = 11 counts per field-of-view). (b,c) Inflammation and PAS scores from the group of mice shown in a. (d) Methacholine–induced AHR for the same groups shown in a (n = 8 mice per group). (e) Absolute numbers of lung tissue eosinophils, neutrophils and lymphocytes, in the respective mouse groups (n = 5 mice for PBS and 7 mice for HDM groups). (f) Flow cytometric analysis, frequencies and absolute numbers of CD4+Foxp3+ Treg cells within lung tissue (n = 8 mice per group). (g–i) Flow cytometric analysis of IL-13 (g), IL-17 (h) and IL-6 (i) expression by CD4+Foxp3− Tconv or CD4+Foxp3+ Treg cells within CD90.2+ gated cells (representing all T lymphocytes) in lung tissues of WT and Il4raR576 mice treated with PBS or HDM (n = 5 mice for PBS and 7 mice for HDM groups). Results represent means ± s.e.m. from two independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 by one-way ANOVA with Bonferroni posttest analysis. For AHR analysis, *P < 0.05 and **P < 0.01 by two-way repeated measures ANOVA.
Expression of the transcription factor Helios differentiates between natural Treg (nTreg) cells, which develop in the thymus and are biased towards recognition of self-antigens, from iTreg cells that arise de novo in the peripheral tissues and are biased towards foreign antigens 25. Analysis of lung tissue Treg cells revealed decreased Foxp3+Helioslow Treg cells in HDM-treated Il4raR576 as compared to WT mice, indicative of existence of a reduced iTreg cell population (Supplementary Fig. 2a,b). Expression of IL-17 and the TH17 cell-associated chemokine receptor CCR6 was largely overlapping and highly enriched in Treg and Tconv cells of HDM-treated Il4raR576 as compared to WT mice (Supplementary Fig. 2c,d). In Il4raR576 Treg cells, IL-17 and CCR6 were almost exclusively restricted to the Foxp3+Helioslow iTreg cell population (Supplementary Fig. 2e,f) 26,27.
Il4raR576 iTreg cells express IL-13, IL-17 and IL-6
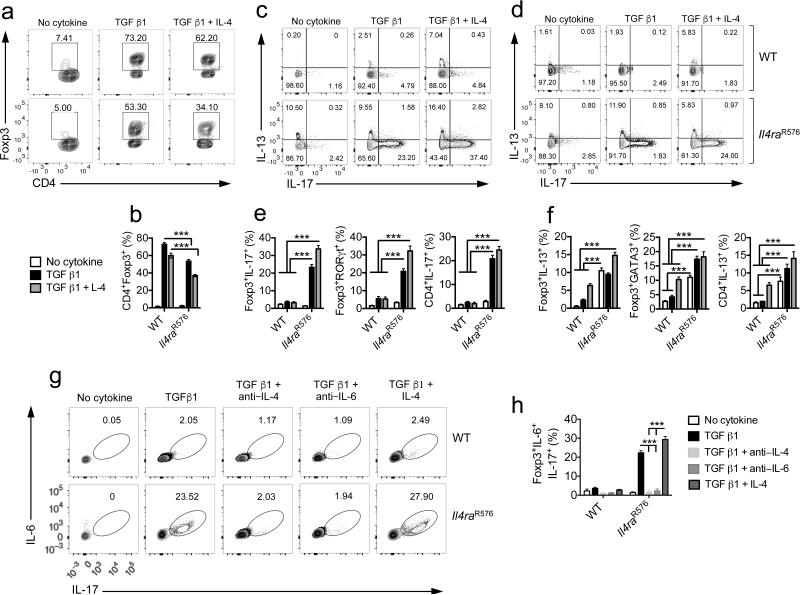
TGFβ and T cell receptor (TCR) stimulation converts naïve CD4+Foxp3− T cells into Foxp3+ Treg cells 28. Given the increased IL-17 and IL-13 production in lung Treg cells of allergen-exposed Il4raR576 mice, we examined in vitro-generated Il4raR576 iTreg cells for aberrant cytokine expression and functional competency to suppress allergic airway inflammation. WT and Il4raR576 naïve CD4+DO10.11+Rag2−/−Foxp3EGFP T cells were purified by cell sorting and differentiated into Treg cells in culture by stimulation with anti-CD3 and anti-CD28 mAbs and TGF β1. Il4raR576 CD4+ T cells exhibited decreased conversion to Foxp3+ Treg cells as compared to WT controls, a deficit exacerbated by the addition of IL-4 to the cell cultures (Fig. 2a,b). Importantly, a sizeable fraction of Il4raR576, but not WT, CD4+Foxp3+ iTreg cells and CD4+Foxp3− Tconv cells expressed IL-17, which further increased by IL-4 (Fig. 2c,d). Anti-CD3 and anti-CD28 stimulation of Il4raR576 T cells induced elevated expression of IL-13 as compared to WT controls, independent of added TGF β1. IL-13 and IL-17 expression was largely non-overlapping. Addition of IL-4 induced increased IL-17 and IL-13 production in both CD4+Foxp3+ and CD4+Foxp3− populations (Fig. 2e,f). In line with the above studies, expression of RORγt and GATA3 was upregulated in Il4raR576 iTreg cells relative to WT controls (Fig. 2e,f and Supplementary Fig. 3a,b). IL-6 was co-expressed with IL-17 in Il4raR576 Treg cells (Fig. 2g,h); both cytokines were IL-4-dependent, and IL-17 was also IL-6-dependent, consistent with autocrine and paracrine IL-4 production driving IL-6-dependent IL-17 expression (Fig. 2g,h). In contrast, IL-13 and IL-4 were increased in Il4raR576 Treg cells in an IL-4 but not IL-6 dependent manner (Supplementary Fig. 3c–e). IL-4 + TGF β1 treatment induced a modest, equivalent increase in IL-9 expression in WT and Il4raR576 iTreg cells (Supplementary Fig. 3f–h).
Figure 2.
Defective formation and impaired suppressive function of Il4raR576 induced-Treg cells. (a,b) Flow cytometric plot (a) and bar graph (b) demonstrating in vitro generation of iTreg cells form DO11.10+Rag2−/−Foxp3EGFP and DO11.10+Il4raR576Rag2−/−Foxp3EGFP naïve CD4+ Tconv cells in the presence of anti-CD3 and anti–CD28 mAbs and TGF β1 in the absence or presence of IL-4 for 5 d (n = 6 replicates per group). (c,d) Flow cytometric analysis of IL-17 and IL-13 expression by converted Foxp3+ iTreg cells (c) and CD4+Foxp3− Tconv cells (d) in culture. (e,f) Bar graphs demonstrating the frequencies of converted Foxp3+ iTreg and CD4+Foxp3− Tconv cells IL-17 and RORγt (e) and IL-13 and GATA3 expression (f) (n = 6 replicates for IL-17 and IL-13 and 6 replicates for RORγt and GATA3 expression). (g) Flow cytometric analysis of dual IL-6 and IL-17 expression by converted iTreg cells. (h) Bar graph demonstrating the frequencies of double IL-6 and IL-17 expression within converted iTreg cells (n = 6 replicates per group). Each dot represents one replicate. Data represent means ± s.e.m. from two independent experiments. ***P < 0.001 by one-way ANOVA with Bonferroni posttest analysis.
The cell surface protein neuropillin1 (Nrp1) is highly expressed on nTreg cells but not iTreg cells 29,30. To determine the impact of IL-4 signaling on Il4raR576 nTreg cells, splenic Nrp1high WT and Il4raR576 Treg cells, which are overwhelmingly nTreg cells 29,30, were isolated to high purity by cell sorting (Supplementary Fig. 4a), and were examined for their suppressive capacity in an in vitro T cell proliferation assay. IL-4 treatment did not impact the suppressive function of either WT or Il4raR576 nTreg cells (Supplementary Fig. 4b,c). Treatment with anti-CD3 and anti-CD28 mAbs in the presence of IL-4 induced IL-17, but not IL-13, expression in a minor population of Il4raR576 but not WT nTreg cells that was on average 10 fold less in frequency than that noted for IL-4-treated Il4raR576 iTreg cells (Supplementary Fig. 4d,e). Also, treatment of naïve Il4raR576 Tconv cells with IL-4 failed to induce IL-17 expression in the absence of TGF β1 (Supplementary Fig. 4f,g). These results indicated that the capacity of Il4raR576 Treg cells to express IL-17 in response to IL-4 treatment is largely restricted to the iTreg cell population.
Impaired function of Il4raR576 Treg cells
To assess suppressive capacities of Il4raR576 vs. WT iTreg cells, we employed an adoptive transfer model. iTreg cells were derived from naïve splenic CD4+ T cells of WT and Il4raR576Thy1.1+DO10.11+Rag2−/−Foxp3EGFP mice and adoptively transferred to OVA-sensitized Il4raR576Thy1.2+ mice, which were then challenged with aerosolized OVA and analyzed (Supplementary Fig. 5a). WT iTreg cells almost completely abrogated OVA–induced tissue inflammation, goblet cell hyperplasia, AHR, eosinophilia neutrophilia and lymphocytosis in lungs of recipient Il4raR576 mice. They also suppressed IL-17 and IL-13 expression in the recipient lung Tconv and Treg cells. In contrast, Il4raR576 iTreg cells failed to do so (Supplementary Fig. 5b–f). Unlike the transferred WT iTreg cells, up to a third of donor Il4raR576 iTreg cells lost their EGFP expression to become exiTreg cells that expressed either IL-17 or, less frequently, IL-13, consistent with heightened instability (Supplementary Fig. 5g–j).
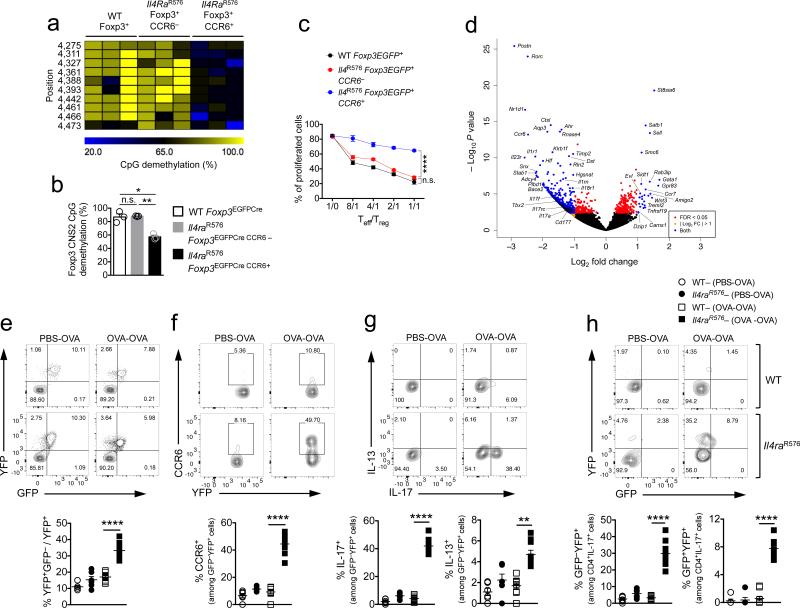
We utilized CCR6 as a marker of Treg cells committed towards the TH17 cell lineage to examine their functional, epigenetic and transcriptional profiles. CCR6+ Il4raR576 Treg cells isolated from OVA-sensitized and challenged mice exhibited decreased methylation of the Foxp3 CNS2 locus, indicative of decreased Treg cell phenotypic stability (Fig. 3a,b). They also exhibited profoundly decreased suppressive function in an in vitro T cell proliferation assay as compared to CCR6− WT and CCR6− Il4raR576 counterparts (Fig. 3c). Transcriptional profiling revealed increased expression in CCR6+ Il4raR576 Treg cells of genes associated with a TH17 cell signature, including Rorc, Ccr6, Il23r, Il17a, Il17f, Il1r1, Nr1d1, Cstl, and Ahr (Fig. 3d and Supplementary Data Set 1) 26,31-33. To determine whether the TH17 cell-like Treg cells in the lungs of allergen treated Il4raR576 mice gave rise to Foxp3− TH17 cells, we employed a lineage tracing approach using a Rosa26 Stop-flox YFP reporter (R26YFP) crossed with a Foxp3-directed Cre recombinase (Foxp3EGFPCre) on WT and Il4raR576 background. Il4raR576Foxp3EGFPCreR26YFP and control Foxp3EGFPCreR26YFP mice were either sham or OVA sensitized than challenged with aerosolized OVA. CCR6 and cytokine expression was examined in Treg (EGFP+YFP+), exTreg (EGFP−YFP+) and CD4+ Tconv cells (EGFP− YFP−). We found markedly increased frequencies of EGFP−YFP+ exTreg cells in the lungs of OVA-sensitized and challenged Il4raR576Foxp3EGFPCreR26YFP relative to controls, indicative of heightened Treg cell instability (Fig. 3e). Furthermore, about half of those exTreg cells expressed IL-17 and CCR6, consistent of their differentiation into TH17 cells. In contrast, expression of IL-13 by exTreg cells was modest (Fig. 3f,g). Together, IL-17 producing Treg and exTreg cells accounted for 35-40% of the total CD4+IL-17+ and CD4+CCR6+ T cells in the lungs of OVA-treated Il4raR576Foxp3EGFPCreR26YFP mice but less than 3% in similarly treated WT controls (Fig. 3h). These results indicated that Il4raR576–driven Treg cell instability and TH17-cell-like programming in the context of allergic airway inflammation ultimately leads to Treg cell transformation into TH17 cells.
Figure 3.
CCR6+ IL-17+ Il4R576 Treg cells exhibit instability and compromised suppressive activity (a) Methylation status of CpG motifs in Treg cells isolated from lung tissue of OVA–sensitized and challenged mice. Numbers on the left side indicate the position of the respective motifs. (b) Global methylation status of Foxp3 CNS2 in the respective Treg cell populations (n = 3 mice per group with 7-12 clones per mouse). (c) In vitro suppression of the proliferation of WT responder CD4+ T cells (Teff) by the respective Treg cell populations (n = 3 replicates per group) (d) Gene expression profiles (volcano plot) of EGFP+CCR6− versus EGFP+CCR6+ Treg cells isolated by FACS from lung digests of OVA-sensitized and challenged Foxp3EGFP Il4R576 mice (n = 3–4 mice). FDR: false discovery rate; Log2FC: Log2 fold change. (e) Flow cytometric analysis and frequencies of exTreg (GFP−YFP+) cells, plotted as a fraction of exTreg to total Treg cells in lung tissue. (f,g) Flow cytometric analysis and frequencies of CCR6 producing (f) and IL-17 and IL-13 producing (g) exTreg cells in lung tissues. (h) Flow cytometric analysis and frequencies of exTreg and Treg cells among CD4+IL-17+ Tconv cells in lung tissues of the respective mouse groups (n = 6 mice for PBS- and 9 mice for OVA-treated groups for e–h). Data represent means ± s.e.m. from two independent experiments. *P < 0.05, **P < 0.005 and ****P < 0.0001 by one-way ANOVA with Bonferroni posttest analysis. For suppression assay ****P < 0.0001 by repeated measures two-way ANOVA.
Recruitment of GRB2 to IL-4Rα-pY575 activates MAPK
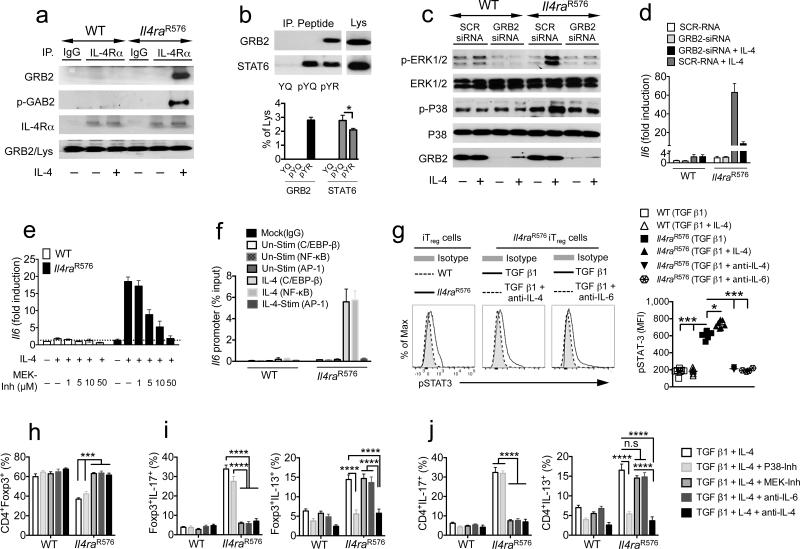
We noted that the R576 substitution rendered the sequence at Y575 (574-GpYREF-578) homologous to a previously reported consensus sequence for high specificity binding of the src homology 2 (SH2) domain of the adaptor protein GRB2 (pY-K/R-N-I/L) 34. Consistent with this prediction, GRB2 and the GRB2-associated binding protein 2 (GAB2) were detected by immunoblotting in IL-4Rα immunoprecipitates derived from IL-4–treated Il4raR576 but not WT T cells (Fig. 4a). Furthermore, GRB2 was recovered from mouse splenocyte and human PBMC lysates using biotinylated 15 amino acid murine and human IL-4Ra peptides containing phosphorylated Y575 residue and R576 (pY575R576) but not the Q576 residue (pY575Q576) (Fig. 4b and Supplementary Fig. 6a). Both murine and human pY575R576 peptides demonstrated a modest decrease in STAT6 binding compared to pY575Q576. Together, these data indicated that the R576 substitution altered the specificity of pY575 towards binding GRB2 versus STAT6.
Figure 4.
IL-4Rα-R576 activates GRB2–coupled MAPK. (a) Immunoprecipitates (IP) from medium and IL-4-treated WT and Il4raR576 splenocytes using IgG or anti–IL4Rα mAb were probed with anti-GRB2, -GAB2, and -IL-4Rα mAbs. Lys: lysates (b) Binding of GRB2 and STAT6 in splenocyte lysates to biotinylated murine IL-4Rα Y575Q576 (YQ), pY575Q576 (pYQ) and pY575R576 (pYR) peptides. Immunoblotted proteins (upper panel) were quantified by densitometry (lower panel). (c) Immunoblots of phospho- and total ERK1/2 and p38, and of GRB2 in medium or IL-4–treated WT and Il4raR576 BMDM transfected with scrambled or GRB2–specific siRNA. (d) RT-PCR analysis of Il6 transcripts in the same groups as c (e) Il6 transcripts in splenocytes treated with medium or IL-4 and the indicated concentrations of MEK-Inh. (f) ChIP analysis of C/EBP-β, NF-κB and AP-1 binding at the Il6 promoter in medium (Un-Stim) or IL-4–treated WT and Il4raR576 splenocytes. (g) Flow cytomeric analysis (left) and mean fluorescence intensity (MFI, right) of pSTAT3 in WT and Il4raR576 iTreg cells differentiated with anti–CD3, anti–C28 mAbs and TGF β1 without or with IL-4, anti-IL-4 or anti–IL-6 mAbs (n = 3–6 replicates per group for b–g). (h–j) Frequencies of iTreg cells (h) IL-17 and IL-13 expressing Foxp3+ iTreg cells (i) and CD4+Foxp3− Tconv cells (j) treated with the indicated combinations of cytokines, inhibitors and mAbs (n = 3–6 replicates per group). Data represent means ± s.e.m. from 2–3 independent experiments. *P < 0.05, ***P < 0.001 and ****P < 0.0001 by one-way ANOVA with Bonferroni posttest analysis.
Receptor-bound GRB2 activates the guanine nucleotide exchange factor Son of Sevenless (SOS), which in turn activates the mitogen-activated protein kinase (MAPK) kinase cascade 35. Consistent with GRB2 recruitment and activation, IL-4 stimulation of Il4raR576 but not WT splenocytes increased the phosphorylation of MAPKs, including the c-Raf, MKK3/6, ERK1/2 and p38 MAPK (Fig. 4c and Supplementary Fig. 6b,c). Activation of these kinases was inhibited by GRB2-specific but not scrambled small interfering RNA (siRNA) (Fig. 4c). ERK1/2 but not p38 MAPK activation was suppressed by treatment with a c-Raf inhibitor, pointing to two downstream cascades branching out from GRB2, one involving c-Raf–ERK1/2 and the other P38 MAPK and its activator MKK3/6 (Supplementary Fig. 6d). IL-4 activated Il6 transcription in several cell types, including bone marrow derived macrophages (BMDM), splenocytes and purified T cells in a GRB2-ERK1/2 cascade-dependent manner, evidenced by inhibition of Il6 transcripts by Grb2 siRNA and a MAPK kinase (MEK) inhibitor, which inhibits ERK1/2 activation, but not by a P38 MAPK inhibitor (Fig. 4d,e and Supplementary Fig. 6e). IL-4 induced ERK1/2-dependent phosphorylation of NF-κB p65, known transcriptional activators of Il6, and mobilized p65 and C/EBPβ to the Il6 promoter 36,37 (Fig. 4f and Supplementary Fig. 6f). The activation of NF-κB was inhibited by MEK inhibitor treatment (Supplementary Fig. 6g).
IL-6-dependent STAT3 activation in Il4raR576 cells
IL-6 skews iTreg cell differentiation towards the TH17 cell lineage in a STAT3-dependent manner 38,39. Consequent to IL-4Rα-R576-mediated IL-6 production, differentiating Il4raR576 iTreg cells exhibited increased STAT3 activation as compared to WT controls, and this STAT3 activation that was IL-4 and IL-6 dependent (Fig. 4g). Analysis of IL-4 induced STAT3 activation in Il4raR576 naïve CD4+ T cells revealed delayed phosphorylation of STAT3 that started at 30 min post stimulation and progressively increased over time. It was completely abrogated by treatment with an anti–IL-6 mAb, indicating that IL-4–induced STAT3 phosphorylation proceeded by IL-4-mediated IL-6 production. In contrast, activation by IL-4 of STAT6, and by IL-6 of STAT3, was indistinguishable between WT and Il4raR576 T cells (Supplementary Fig. 7a–c).
We next analyzed the role of GRB2-activated MAPK cascades in iTreg cell skewing towards a TH17 versus TH2 cell phenotype. Treatment of differentiating Il4raR576 iTreg cells with MEK inhibitor or anti–IL-6 mAb, but not p38 MAPK inhibitor, rescued the decreased iTreg cell differentiation, decreased IL-6 levels in culture, and blocked IL-17 expression in differentiating iTreg cells and in CD4+ Tconv cells present in the same co-cultures while sparing IL-13 (Fig. 4h–j and Supplementary Fig. 6h–j). Treatment with p38 MAPK inhibitor blocked IL-13 but not IL-17 expression, consistent with the role of p38 in the upregulation of Il13 gene transcription by a GATA3-dependent mechanism 40, while treatment with anti–IL-4 mAb blocked both (Fig. 4i,j and Supplementary Fig. 6i,j). Thus the two branches of MAPK signaling downstream of IL-4Rα–R576 mediate activation of distinct TH cell cytokine programs.
Whereas T cells express only the type I IL-4R, which responds to IL-4, bone marrow derived macrophages (BMDM) also express the type II receptor, which responds to IL-4 and IL-13. Stimulation of Il4raR576 but not WT BMDM with IL-4 or IL-13 induced a similar profile of delayed, sustained STAT3 activation. In contrast, STAT3 was similarly activated in IL-6–treated WT and Il4raR576 BMDM (Supplementary Fig. 7d,e).
Expression of transcripts encoding CCL11, which mediates tissue eosinophil recruitment, is markedly upregulated in the airways of allergen-challenged Il4raR576 mice 17. Ccl11 transcripts were greatly increased in IL-4-treated Il4raR576 BMDM as compared to WT controls (Supplementary Fig. 7f). Ccl11 transcriptional activation by IL-4/IL-13 is normally mediated by STAT6, while that by IL-17 and IL-9 is mediated by STAT3 41-43. Chromatin immunoprecipitation (ChIP) assays revealed that whereas STAT6 bound equally well to the Ccl11 promoter in IL-4-treated WT and Il4raR576 BMDM, STAT-3 was selectively recruited to the Ccl11 promoter in Il4raR576 BMDM in an IL-6-dependent manner (Supplementary Fig. 7g). Knockdown of STAT3 by siRNA treatment abrogated the increase in Ccl11 transcription in IL-4-treated Il4raR576 BMDM, reducing it to levels similar to those of WT controls (Supplementary Fig. 7h,i). Thus dual STAT6-STAT3 activation by IL-4Rα-R576 may promote tissue eosinophilia by super-inducing Ccl11 transcription.
iTreg cells of humans expressing IL4RR576 produce IL-17
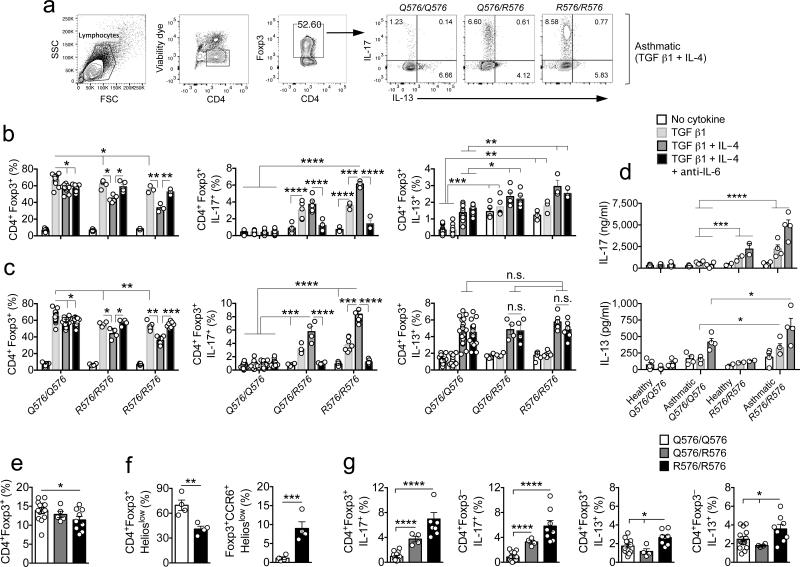
We examined in vitro iTreg cell differentiation in prospectively recruited healthy and asthmatic subjects who were either WT (IL4RQ576/Q576), heterozygous (IL4RQ576/R576) or homozygous for the mutant allele (IL4RR576/R576). The gating strategy in these studies is outlined in Figure 5a. In healthy subjects, whose demographics are detailed in Supplementary Table 1, naïve heterozygous and homozygous mutant Tconv cells differentiated to iTreg cells at lower frequencies as compared to WT T cells, especially in the presence of IL-4 (Fig. 5b). The deficit in iTreg conversion was rescued by treatment with an anti–IL-6 mAb. The differentiated iTreg cells and CD4+ Tconv cells present in the same co-cultures produced increased amounts of IL-17 as assessed by flow cytometric analysis and by ELISA, which was further upregulated by IL-4 treatment but abrogated by anti–IL-6 mAb (Fig. 5b,d). IL-13 expression was significantly increased in activated T cells of subjects carrying the mutant allele independent of their differentiation into iTreg cells, mirroring the findings in murine Il4raR576 T cells (Fig. 5b,d).
Figure 5.
Naïve CD4+ Tconv cells from asthmatic subjects bearing R576 mutation show defective induction of iTreg cells and their skewing towards a TH17-like phenotype. (a) Flow cytometric analysis of CD4+Foxp3+ iTreg cells induced from purified naïve CD4+ Tconv in the presence of anti-CD3 + anti-CD28 mAbs and TGF β1 + IL-4 in the absence or presence of anti-IL-6 mAb. (b,c) Cumulative frequencies of CD4+Foxp3+ iTreg cells and IL-17 and IL-13 producing Foxp3+ iTreg cells within converted iTreg cells of non-asthmatic (n = 3–13) (b) and asthmatic (n = 4–19) (c) subjects with respective genotypes differentiated in the absence or presence of TGF β1 or TGF β1 + IL-4 or TGF β1 + IL-4 + anti-IL-6 mAb. (d) IL-17 and IL-13 cytokine levels in the supernatant of differentiated iTreg cells in the treated groups mentioned in b (n = 2–5). (e-g) Frequencies of total CD4+Foxp3+ Treg cells (n = 5–14) (e) CD4+Foxp3+Helioslow and Foxp3+CCR6+Helioslow Treg cells (n = 4) (f) and frequencies of IL-17 and IL-13 producing Foxp3+ Treg and CD4+ Tconv cells in respective groups within PBMC of asthmatic subjects (n = 4–14). Each dot represents one subject. Data represent means ± s.e.m. For subjects see Supplementary Tables 2 and 3. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P <0.001 by one-way ANOVA with Bonferroni posttest analysis.
We also examined the differentiation and cytokine expression of iTreg cells in prospectively recruited asthmatics as a function of IL4R genotype. The demographics, IL4R genotype and disease severity classification of asthmatic subjects are described in Supplementary Table 2. Whereas there were no differences in age and sex distribution between asthmatics bearing the WT allele versus those heterozygous and homozygous for the mutant allele, the latter subjects exhibited increased asthma severity, in agreement with previously published reports (Supplementary Table 2) 13-16. Heterozygous and homozygous mutant, but not WT, iTreg cells of asthmatics also exhibited IL-6–dependent decreased differentiation and increased IL-17 production (Fig. 5c,d). IL-13 expression was upregulated in activated Tconv and in iTreg cells of asthmatics regardless of their genotype, although ELISA assays revealed its increased production by cells carrying the mutant allele (Fig. 5c,d). Analysis of peripheral blood mononuclear cells (PBMC) revealed a modest but significant decrease in circulating total Treg cells in asthmatics homozygous for the IL4RR576 allele. This decrease was restricted to the Foxp3+Helioslow (iTreg) cell population, which also manifested a selective increase in CCR6 expression, echoing the results found in Il4rR576 mice (Fig.5 e,f; Supplementary Fig. 8). Importantly, the frequency of IL-17–expressing CD4+ Tconv and Treg cells was selectively increased in subjects either heterozygous or homozygous for IL4RR576. IL-13 expression was modestly increased in CD4+ Tconv and Treg cells of PBMC of subjects homozygous for IL4RR576 consistent with heightened TH17 and TH2 responses (Fig. 5g). These results established that IL-4–driven acquisition by iTreg cells of a TH17 cell-like phenotype is an attribute that segregates with the IL4-Rα-R576 mutation.
Il4raR576 Treg cell phenotype change impacts airway inflammation
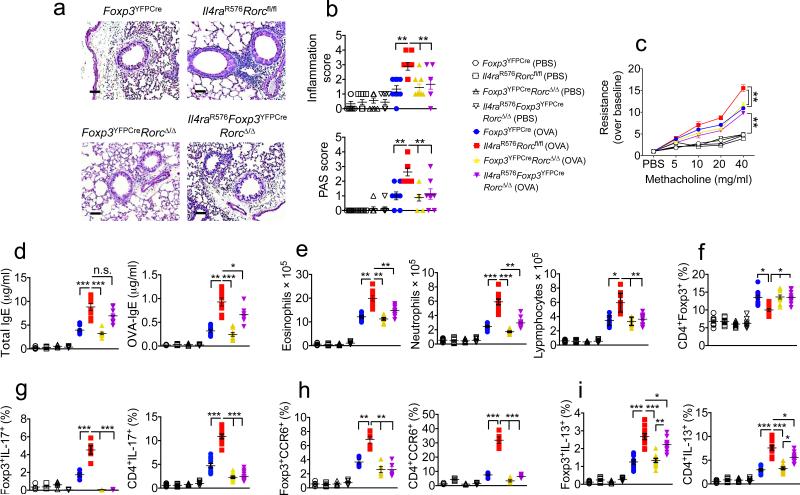
To determine whether the acquisition by lung Il4raR576 Treg cells of a TH17 cell-like phenotype contributed to exaggerated airway inflammation, we examined the consequences of Treg cell-specific deletion of Rorc, which encodes the TH17-promoting transcription factor RORγt, on airway inflammation 44. Treg cell specific deletion was effected by crossing mice expressing a floxed Rorc allele with mice expressing Foxp3YFPCre, a Cre recombinase under control of the Foxp3 locus (Supplementary Fig. 9a,b). Treg cell-specific deletion of Rorc in Il4raR576 mice (Il4raR576Foxp3YFPCreRorcΔ/Δ) decreased airway inflammation, mucus production and AHR upon OVA sensitization and challenge to an extent sufficient to resemble WT controls that were either sufficient or deficient in Rorc (Foxp3YFPCre and Foxp3YFPCreRorcΔ/Δ, respectively), whose aforementioned responses were not significantly different (Fig. 6a–c). It also led to a sharp decrease in lung tissue neutrophilia, lymphocytosis and to a lesser extent eosinophilia. In contrast, total and OVA-specific serum IgE concentrations were marginally decreased (Fig. 6d,e). Rorc deletion rescued the decrease in lung tissue Treg cells in OVA-sensitized and challenged Il4raR576 mice (Supplementary Fig. 9c). It abolished IL-17 expression and normalized CCR6 expression in Il4raR576 Treg cells, and normalized IL-17 and CCR6 expression in CD4+ Tconv cells. In contrast, IL-13 expression was unaffected in Il4raR576Foxp3YFPCreRorcΔ/Δ Treg cells and marginally so in CD4+ Tconv cells (Fig. 6f–i and Supplementary Fig. 9d–h). Virtually identical results were obtained upon Il4raR576 Treg cell-specific deletion of Il6ra, encoding IL-6Rα chain, consistent with the requirement for IL-4-induced IL-6 production in programming TH17 cell-like Treg cells (Supplementary Fig. 10). Similarly, treatment with anti–IL-6 mAb suppressed airway inflammation and AHR in HDM-treated Il4raR576 mice, abrogated airway neutrophilia and profoundly inhibited the airway eosinophilia. It normalized lung tissue Treg cell numbers and prevented iTreg cell reprograming into TH17-like cells, and it inhibited TH2 and TH17 cytokine production and the increased IgE response (Supplementary Fig. 11). In contrast, treatment with an anti–IL-17 mAb was less effective in suppressing HDM-induced airway inflammation in Il4raR576 mice. It partially decreased tissue inflammation and AHR, normalized airway neutrophilia and inhibited IL-17 production. However, it only marginally decreased airway eosinophilia. It failed to suppress IgE or TH2 cytokine production or to rescue reduced Treg cell numbers in lung tissues (Supplementary Fig. 12). Treatment with an anti-CCL11 mAb, while inhibiting dysregulated airway eosinophilia and CCL11 production in HDM-treated Il4raR576 mice, did not suppress tissue inflammation, airway neutrophilia, TH2 and TH17 cytokine or IgE production, nor did it rescue ineffective Treg cell generation in lung tissues (Supplementary Fig. 13). Unlike the case of Rorc and Il6ra, combined Treg cell-specific deletion of Il4 and Il13 had no significant effect on airway inflammation, AHR, or lung tissue neutrophilia in OVA-sensitized and challenged Il4raR576 mice despite decreased total eosinophilia and total and OVA-specific IgE responses. It failed to rescue the decrease in lung tissue Treg cells in inflamed Il4raR576 mice. While it completely suppressed IL-13 expression in Treg cells, and decreased it in CD4+ Tconv cells, it left unaltered IL-17 expression in either cell population (Supplementary Fig. 14).
Figure 6.
Treg cell lineage-specific deletion of Rorc reverses the aggravated airway inflammation in Il4raR576 mice. (a) PAS staining of lung sections isolated from the mouse groups shown and immunized and challenged with OVA (20×, scale bar 50 μm). (b) Inflammation and PAS scores in the mouse groups shown (n = 8–9 counts per field of view). (c) Methacholine–induced AHR in the respective mouse groups (n = 4 mice for PBS- and 6 mice for OVA–treated mouse groups). (d) Total and OVA–specific serum IgE concentrations in the mouse groups shown in b (n = 8). (e) Frequencies of lung tissue eosinophils, neutrophils and lymphocytes (n = 8 mice for PBS- and 9 mice for OVA-treated mouse groups). (f–i) Frequencies of lung tissue Foxp3+ Treg cells (f), and IL-17 (g), CCR6 (h) and IL-13 (i) producing CD4+ Tconv and Foxp3+ Treg and CD4+ Tconv cells (n = 5–9 mice per group). Each dots represents one animal. Data represent means ± s.e.m. from two to three independent experiments. *P <0.05, **P <0.01, ***P <0.001 and ****P <0.0001 by one-way ANOVA with Bonferroni posttest analysis. For AHR studies, *P < 0.05 and **P < 0.01 by repeated measures two-way ANOVA.
For comparison, we employed Il4raF709 mice, homozygous for a Y709F substitution that inactivates the immunotyrosine inhibitory motif of the IL-4Rα chain and consequently augments STAT6 activation 11. This mutation, which models human IL-4Rα polymorphisms that promote STAT6 activation, results in exaggerated allergic airway inflammation due to dysregulated TH2 immunity 11,45,46. Il4raF709 Treg cells undergo TH2 cell-like reprogramming that plays a fundamental role in mediating the exaggerated allergic phenotype 9. Unlike Il4raR576 mice, Treg cell-specific deletion of Il4/Il13 in Il4raF709 mice normalized their airway inflammation, AHR, and tissue eosinophilia and decreased their total and OVA-specific IgE responses. It reversed Treg cell TH2 cell-like reprogramming, evidenced by abolishment of their heightened IL-13 expression, and reduced IL-13 production by CD4+ Tconv cells, without affecting the low IL-17 expression in CD4+ Tconv and Treg cells (Supplementary Fig. 15). Thus, the Il4raR576 and Il4raF709 augment airway inflammation by two distinct mechanisms: one eliciting a TH17 and the other a TH2 cell-like reprograming of Treg cells.
Discussion
Our studies elucidate a novel mechanism for the development of mixed TH2 and TH17 cell responses relevant to asthma and other allergic inflammatory diseases. By activating an additional branch of IL-4R signaling initiated by the adaptor protein GRB2, an IL-4Rα polymorphism associated with asthma exacerbations and severity potentiates TH17 and to a lesser extent TH2 responses. Importantly, IL-4-induced GRB2 recruitment mediates MAPK-dependent autocrine IL-6 signaling in T cells, which redirects TGF β-dependent iTreg cell differentiation towards the TH17 cell lineage. Deletion of Il6ra or Rorc in Treg cells or treatment with an anti–IL-6 mAb protected against the deleterious effects of IL-4Rα-R576 on airway inflammation, prevented iTreg cell reprogramming towards the TH17 lineage and normalized the TH17 cell response, confirming the centrality of iTreg cell acquisition of a TH17 cell-like phenotype in mediating disease exacerbation by this polymorphism.
In mouse models, IL-17 induces steroid-resistant airway inflammation and neutrophilic infiltration 27,47,48. However, while anti–IL-17 mAb treatment suppressed lung tissue neutrophilia and neutralized IL-17 in the airways of Il4raR576 mice, it only partially ameliorated the augmented AHR and tissue inflammation and failed to normalize the Treg cell response or suppress TH17/TH2 polarization. Thus, the efficacy of interventions such as Treg cell-specific deletion of Rorc or Il6ra or treatment with anti–IL-6 mAb relates not simply to their suppression of IL-17 production but more fundamentally to their stabilization of the iTreg cell response, resulting in better control of airway inflammation. Studies on Il4raR576 and Il4raF709 mice revealed two distinct modes of pathogenic iTreg cell programming relevant to airway inflammation. The former involved GRB2-dependent iTreg cell programming towards a TH17-cell like phenotype, while the latter involved STAT6-dependent programming towards a TH2 cell-like phenotype 9. Treg cell-specific deletion of Il6ra or Rorc in Il4raR576 mice and Il4-Il13 in Il4raF709 mice reduced airway inflammation in the respective strain back to levels associated with the WT Il4ra allele. Il4raR576 and Il4raF709 mice model mixed TH2-TH17 and TH2 cell-high asthma inflammatory mechanisms, or endotypes, respectively 49-51. Both models would indicate that acquisition of iTreg cells of a TH17 or a TH2 cell-like program plays a fundamental role in the pathogenicity of the respective allele in airway inflammation. Accordingly, pro-asthmatic IL-4R polymorphisms may promote distinct asthma endotypes (TH2 cell-high versus mixed TH2-TH17 cell inflammation) by directing iTreg cells differentiation towards the respective TH cell program.
Our results indicate that IL-4-directed and IL-6-dependent subversion of IL4RR576 iTreg cells into a TH17 like cells may play a critical role in asthma severity associated with this allele. Blocking the IL-4R or IL-6R pathways may prevent the subversion of allergen-specific iTreg cells into TH2 and/or TH17 like cells, respectively, and provide personalized therapeutic approaches to tolerance re-establishment in asthma 52,53.
Online Methods
Animals
BALB/cByJ (WT) and all the following strains, except where indicated, were obtained from or rederived at the JAX lab. C.129X1-Il4ratm2.1Tch (Il4raR576), C.129X1-Il4ratm3.1Tch (Il4raF709), and C.Cg-Foxp3tm2Tch/J (Foxp3EGFP) have been previously described 11,17,54. NOD/ShiLt-Tg(Foxp3EGFP-cre)1cJbs/J (Foxp3EGFPCre) were backcrossed 12 generations on BALB/cBYJ 55. C.129P2(Cg)-Il4-Il13tm1.1Lky (Il4-Il13fl/fl) mice were crossed with Il4raR576 and Il4raR576Foxp3EGFPCre mice 56. DO11.10+Rag2−/− mice were obtained from Taconic Farms (Hudson, NY) and were crossed with Thy1.1+Foxp3EGFP and Thy1.1+Il4raR576 mice to generate Thy1.1+DO11.10+Rag2−/−Foxp3EGFP and Thy1.1+Il4raR576DO11.10+Rag2−/−Foxp3EGFP mice. ROR-gtf/fB6(Cg)-Rorctm3Litt/J (Rorcfl/fl), B6;SJL-Il6ratm1.1Drew/J (Il6rafl/fl), B6.129(Cg) Foxp3tm4(YFP/cre)Ayr/J) (Foxp3YFPCre) and Il4raR576 C57BL/6 congenic were crossed to generate Il4raQ576Foxp3YFPCre, Foxp3YFPCreRorcΔ/Δ and Il4raR576Foxp3YFPCreRorcΔ/Δ. BALB/c Il4raR576 mice were crossed to congenic Foxp3EGFPCreRosa26YFPCre mice to generate Il4raR576 Foxp3EGFPCreRosa26YFPCre 57,58. Male and female mice, age 5-7 weeks, were employed in experiments in equal proportions. Mice were bred and maintained under specific pathogen-free conditions and used under protocols approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital.
Flow cytometry
Antibodies against the following murine antigens were used for flow cytometric analyses: IL-4 (clone 11B11, 1:300 dilution), Siglec-F (E50-2440, 1:300), Phospho-Stat3 (pY705, 1:50), Phospho-Stat6 (pY641, 1:50) (BD Biosciences), Thy1.2 (30-H12, 1:500), Foxp3 (FJK-16S, 1:300), DO11.10 (KJ1-26, 1:500), IFN-γ (XMG1.2, 1:300), IL-13 (eBio13a, 1:300), GATA3 (TWAJ, 1:200), RORγt (AFKJS-9, 1:200), Helios (22F6, 1:200), CD25 (PC61.5, 1:500), CTLA4 (UC10-4B9, 1:200), CD11c (30-f11, 1:500), CD11b (M1/70, 1:500) (eBioscience), CD4 (RM4-5, 1:500), CD3 (145-2C11, 1:500), IL-17 (TC11-18H10.1, 1:200), IL-6 (MP5-20F3, 1:200), GR-1 (RB6-8C5, 1:500), CCR6 (29-2L17, 1:300) and CD45 (30-F11, 1:300) and CD304 (Neuropilin-1; 3E12, 1:200) (Biolegend). Antibodies against the following human antigens were used: CD4 (RPA-T4, 1:300), Foxp3 (236A/E7, 1:200), IL-13 (JES10-5A2, 1:200), IL4 (MP4-25D2, 1:400) (eBioscience), IL-17A (BL168, 1:200), Helios (22F6, 1:200), CCR6 (G034E3, 1:200) (Biolegend). The specificity and optimal dilution of each antibody was validated by testing on appropriate negative and positive controls or otherwise provided on the manufacturer's website. Intracellular cytokine staining was performed as previously described 9. Cells were routinely excluded from the analysis based on the staining of eFluor 780 Fixable Viability Dye (eBioscience). Stained cells were analyzed on a BD LSRFortessa cell analyzer (BD Biosciences) and data were processed using Flowjo (Tree Star Inc.).
Cell purification
Cell suspensions were enriched in CD4+ T cells by Magnetic-activated cell sorting (MACS) negative selection (Miltenyi Biotec) and further sorted by a fluorescence-activated cell sorting (FACS) on a FACSAria II (BD Biosciences) based on CD4 and EGFP (Foxp3) expression.
Mouse allergic sensitization and antibody treatment
Mice were sensitized to ovalbumin (OVA) (Grade V; Sigma Aldrich) by intraperitoneal injection of 100 μg of OVA in 100 μL of PBS and then boosted 2 weeks later with a second intraperitoneal injection of OVA in PBS. Control mice were sham sensitized and boosted with PBS alone. Starting on day 29, both OVA- and sham-sensitized mice were challenged with aerosolized OVA at 1% delivered through a Schuco 2000 nebulizer (Allied Health Care Products) for 30 minutes daily for 3 days. Mice were euthanized on day 32 post sensitization and analyzed 18. For House Dust mite (HDM)– induced allergic airway inflammation, mice received 20 μg of lyophilized Dermatophagoides pteronyssinus (DP) extract in 100 μl of PBS intranasally for 3 days at the start of the protocol and then challenged with the same dose of DP extract on days 15 to 17. Mice were euthanized on day 18 and analyzed for measures of airway inflammation 18. In some experiments at day 15 to 17 monoclonal anti-IL-6 (MP5-20F3, BioXCell) or anti-IL-17 (17F3, BioXCell) antibodies or the isotype-matched control antibodies (BioXCell) were administered intra-tracheally (100 μg/day), 30 min prior to allergen challenge.
Preparation of cell suspensions from lung tissues
Lungs were removed, minced, and incubated for 45 minutes at 37’C in collagenase (Sigma-Aldrich) (0.5 mM in PBS) and passed through a 40-mm strainer. Single-cell suspensions were resuspended in RPMI medium (Invitrogen)
Measurement of airway tissue and functional responses
Paraffin-embedded lungs sections were stained with periodic acid–Schiff (PAS) as previously described 59. Lung inflammation was scored for cellular infiltration around the airways: 0, no infiltrates; 1, few inflammatory cells; 2, a ring of inflammatory cells 1 cell layer deep; 3, a ring of inflammatory cells 2 to 4 cells deep; and 4, a ring of inflammatory cells greater than 4 cells deep 11. The number and distribution of goblet cells was assessed by using PAS staining of mucin granules. Individual airways (bronchi/bronchioles) were scored for goblet cell hyperplasia according to the following scale: 0, no PAS-positive cells; 1, less than 5% PAS-positive cells; 2, 5% to 10% PAS-positive cells; 3, 10% to 25% PAS-positive cells; and 4, greater than 25% PAS-positive cells 11. Allergen-induced airway hyperreactivity (AHR) was measured, as previously described 18. Anesthetized mice were exposed to doubling concentrations of aerosolized acetyl-β-methacholine (Sigma-Aldrich) by using a Buxco small-animal ventilator (Data Sciences International). The relative peak airway resistance for each methacholine dose, normalized to the saline baseline, was calculated.
In Vitro iTreg cell differentiation
Sorted naive CD4+CD62L+Foxp3EGFP− T cells (1 × 106/ml) were cultured with plate-bound anti-CD28 (5 μg/ml, Biolegend), anti-CD3 (5 μg/ml, Biolegend), recombinant TGF β1 (5 ng/ml, R&D Systems), with or without murine recombinant IL-4 (10 ng/ml) (Perpotech), anti-IL-4 (11B11, 10 μg/ml) (Biolegend) or anti-IL-6 mAb (MP5-20F3, 10 μg/ml) (Biolegend), mitogen activated protein kinase kinase (MEK) inhibitor PD98059 (50 μM, Sigma-Aldrich) or P38 inhibitor IV (10 μM, Sigma-Aldrich). After 4 days, the induced iTreg cells were analyzed by flow cytometry for Foxp3 expression and intracellular cytokines production and/or re-sorted on the basis of EGFP fluorescence.
Differentiation of Bone marrow derived macrophages (BMDM)
BMDM were generated from bone marrow cells collected from femurs were cultured in complete medium supplemented with 10 ng/mL recombinant murine M-CSF (PeproTech) for 10 days. The medium was changed every other day. Adherent BMDM were harvested at day 10. Purity of BMDM was verified by using flow cytometry and was greater than 90%, as determined by using CD11c and F/480 staining.
Quantitative real-time PCR
RNA was extracted from treated cells, as mentioned in the figure legends, using Quick-RNA MiniPrep kit (Zymo Research) according to the manufacturer protocol. Reverse transcription was performed with the SuperScript III RTPCR system (Invitrogen) and quantitative real-time reverse transcription (RT)-PCR with Taqman® Fast Universal PCR master mix, internal house keeping gene mouse (GAPDH VIC-MGB dye) and specific target gene primers for murine Il6, Ccl11, Rorc or Il4 genes, as indicated (FAM Dye) (Applied Biosystems) on Step-One-Plus machine. Relative expression was normalized to GAPDH and calculated as fold change compared to un-stimulated WT cells.
Immunoblotting and immunoprecipitation
Cells were treated with medium alone or medium with IL-4 (10 ng/ml) for the indicated times, lysed with the lysis buffer (25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA (pH 8), 1 mM Na3VO4, 0.5% NP-40, 1 × diluted protease inhibitor cocktail (Sigma-Aldrich). Whole cell lysates were resolved by SDS/PAGE and transferred to PVDF membrane (Thermo Scientific) for immunoblotting analysis 59. Immunoprecipitates were derived from cell lysates using the indicated antibodies and Protein G superparamagnetic beads (Life Technologies). Following overnight incubation, the immunoprecipitates were eluted from the beads by boiling in gel loading buffer and subjected to immunoblotting as previously described 59. The following antibodies were used: GRB2 (clone 4C6-H6, 1:1000 dilution), p-GAB2 (Ser159, EP369Y, 1:1000) p-ERK1/2 (Thr202/Tyr204, D13.14.4E, 1:1000), ERK1/2 (p44/42 MAP Kinase, 137F5, 1:1000), STAT6 (D3H4, 1:1000), STAT3 (D3Z2G, 1:1000) p-c-Raf (Ser338, 56A6, 1:1000), c-Raf (D5X6R, 1:1000), p-P38 (Thr180/Tyr182, D3F9, 1:1000), P38 (D13E1, 1:1000), p-MKK3/6 (Ser189/Ser207, 22A8, 1:1000), MKK3 (D4C3, 1:1000), NF-κB p-p65 (Ser536, 93H1, 1:1000), NF-κB p65 (D14E12, 1:1000) (all obtained from Cell Signaling Technology), C/EBPβ (C-190, 1:1000) (Santa Cruz Biotechnology) and anti-IL-4Rα (CD124) mAb (mIL-4R-M1, 1:1000) (BD Pharmingen). The specificity and optimal dilution of each antibody was validated by testing on appropriate negative and positive controls or otherwise provided on the manufacturer's website.
Chromatin immunoprecipitation (ChIP)
ChIP was performed with Agarose ChIP Kit (Pierce) and antibodies toward STAT3 (D3Z2G, 1:100), STAT6 (D3H4, 1:100), c-Jun (AP-1, 1:100) and NF-κB p65 (D14E12, 1:100) (all obtained from Cell Signaling Technology), C/EBPβ (C-190, 1:100) (Santa Cruz Biotechnology) and the respective isotype control antibodies on 2 × 106 cells starved for 3 hr and treated with medium alone or with IL-4 (10 ng/ml, 2 hrs). The DNA from sample and input was amplified by RT-PCR (40 cycles), using the following primers specific for the Il6 promoter; 5’-AGTGGTGAAGAGACTCAGTG-3’ (forward) and 5’-GGCAGAATGAGCCTCAGA-3’ (reverse) and specific primers for the Ccl11 promoter; 5’-CTTCATGTTGGAGGCTGAAG-3’ (forward) and 5’-GGATCTGGAATCTGGTCAGC-3’ (reverse). Results presented as the ratio of the cycling threshold value of immunoprecipitated DNA to that of input DNA.
siRNA–mediated knockdown of GRB-2
Cells were transfected for 72 hr with scrambled small interfering RNA (siRNA) or Grb2 siRNA I (Cell Signaling Technology) for inhibition of GRB2 expression (50 nM /106 cells). Stat3 siRNA I (Cell Signaling Technology) was used for STAT3 inhibition. Lipofectamine 3000 reagent (Life Technologies) was used to increase the efficiency of transfection (0.5 nmol/L).
IgE and cytokine ELISA assays
Total mouse serum IgE was measured by ELISA using Mouse IgE ELISA Ready-SET-Go kit (eBioscience). OVA-specific IgE was measured by a modified assay such that the plates were coated overnight with 200 μg/ml OVA rather than anti-IgE capture antibody and the rest of the test was similar to the total serum IgE ELISA assay. Mouse IL-4, IL-5, IL-13, IL-17 IFN-γ and Eotaxin cytokines were analyzed in BAL fluids collected from mice using eBioscience ELISA kits, according to the manufacturer's protocol. Human IL-13 and IL-17 were analyzed in culture supernatants of human peripheral blood cells activated with anti-CD3 + anti-CD28 mAbs in the absence or added presence of TGF β or TGF β + IL-4 using eBioscience ELISA kits.
Peptide affinity pull-down experiment
The following biotinylated peptides, corresponding to amino acids 569-583 and 569-584 of the human and mouse IL-4Ra, respectively, and encompassing either WT (Q) or mutant (R) at position 576 were used: Y575Q576: Biotin-SAPTSGYQEFVHAVE (human), Biotin-PAPAGGYQEFVQAVKQ; pY575Q576 (mouse); pY575R576: Biotin-SAPTSGpYQEFVHAVE (human), PAPAGGpYQEFVQAVKQ (mouse); pY575R576: Biotin-SAPTSGpYREFVHAVE (human), PAPAGGpYREFVQAVKQ (mouse). The peptides were incubated with whole splenocyte lysates and avidin-conjugated magnetic beads overnight. The beads were then separated by magnetic sorting and washed. The bound proteins were eluted and analyzed using SDS-PAGE and immunoblotting 11,60.
Methylation analysis
The methylation status of the Foxp3 Treg cell–specific demethylation region (CNS2) of WT Foxp3+, Il4raR576 Foxp3+CCR6− and Il4raR576 Foxp3+CCR6+ lung Treg cells of OVA-sensitized and challenged mice was assessed by bisulfite sequence analysis, as described 61. The Treg cell-specific demethylation region of converted DNA was amplified with methylation-specific primer sequences: Foxp3 CNS2, 5’-TATTTTTTTGGGTTTTGGGATATTA-3’ (forward) and Foxp3 CNS2 5’-AACCAACCAACTTCCTACACTATCTAT-3’ (Reverse). The PCR product was subcloned and sequenced. Blast analyses were done by comparison of the resulting sequences with converted Foxp3 sequences.
In vitro suppression assays
Total CD4+ T cells were isolated using a CD4 negative isolation kit (Miltenyi Biotec) followed by cell sorting on FACSAria. Isolated cells were labeled with CellTrace Violet Cell Proliferation dye according to the manufacturer's instructions (Life Technologies) and were used as responder cells. Treg cells were isolated on a FACSAria on the basis of CD4, EGFP and/or CCR6 expression and were used as suppressor cells. Responder cells were co-cultured with Treg cells, at the indicated ratios, and stimulated for 3 days with 2 μg/ml of soluble anti-CD3 and 5 μg/ml of soluble anti-CD28 in 96-well, round-bottomed plates in triplicates. The cells were then analyzed for CellTrace dye dilution by flow cytometry.
Transcriptome profiling
CCR6− or CCR6+ CD4+EGFP+Treg cells were isolated by FACS from total lung digests of OVA-sensitized and challenged Il4raR576 mice. Total RNA was extracted using TRIzol reagent (Invitrogen) and converted into Double-stranded DNA (dsDNA), using SMART-Seq v4 Ultra Low Input RNA kit (Clontech). dsDNA was then fragmented to 200-300 bp size, using M220 Focused-ultrasonicator (Covaris), and utilized for construction of libraries for Illumina sequencing using KAPA Hyper Prep Kit (Kapa Biosystems). Libraries were then quantified using Qubit dsDNA HS (High Sensitivity) Assay Kit on Agilent High Sensitivity DNA Bioanalyzer.
RNA sequencing data was demultiplexed using perfect matches to indices and quality inspected using FastQC. The sequencing data was aligned to the mm10 build (Gencode annotation) of the mouse genome using STAR 62, and counts were quantified using HTSeq 63. Raw counts were filtered for non-mitochondrial protein-coding genes with at least 3 counts in one sample, and were normalized using the DESeq2 package in R 64. Pairwise comparisons of differential gene expression were computed using DESeq2.
Human cell studies
Asthmatic subjects and healthy controls were recruited under protocols approved by the Institutional Review Board at Boston Children's Hospital. The demographic details of the respective groups are described in Supplemental Tables 2 and 3. Asthmatic subjects, age range 6-47 years, were identified as having intermittent asthma or persistent mild, moderate to severe asthma following the classification of The National Asthma Education and Prevention Program: Expert Panel Report 3 65. Inclusion Criteria for Asthma included current doctor's diagnosis of asthma, and ≥ 12 % FEV1 bronchodialtor reversibility or airway hyperresponsiveness reflected by a methacholine PC20 ≤16 mg/ml. Healthy control subjects were recruited with no medical history of asthma. Informed consent was obtained from all participants prior to blood collection. Blood samples were used for isolation of DNA using DNeasy Blood & Tissue Kit (Qiagen) for genotyping. Mutation detection was carried out using amplification resistance mutation screen (ARMS) PCR method with the following primers: Common Oligo: 5'-CCAGAGTCCAGACAACCTGACTTGCAAGAGAC-3'; Q576-specific: 5'-TGGGTGCCACCCTGCTCCACCGCATGTACAAACTCAT-3'; R576-specific: 5'-TGGGTGCCACCCTGCTCCACCGCATGTACAAACTCAC-3' 66. For iTreg cell differentiation, peripheral blood mononuclear cells (PBMC) were isolated and naïve CD4+CDRA+CDRO−T cells were derived by magnetic separation (Miltenyi biotech). The Isolated T cells were differentiated into iTreg differentiation as described for murine study and directly used for intracellular cytokine and FOXP3 expression analysis, using flow cytometry.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad), and employing the statistical tests indicated in the individual figure legends. Samples size was selected based on previous experiments. No samples were excluded. The investigators were blinded as to scoring the lung histopathology in individual experiments. P values of <0.05 were considered significant; n.s.: not significant. All error bars represent s.e.m. as noted in the individual figure legends. Unless otherwise stated, ≥2 independent experiments were carried out for all assays, and the displayed figures are representative of the obtained results. Comparisons between the average severity scores and ages between asthmatics subjects homozygous for IL4RA Q576 versus those heterozygous and homozygous for the IL4RA R576 were carried out using Student's unpaired two-tailed t-test. The sex distribution between those two groups was compared using Fisher's exact test.
Supplementary Material
Acknowledgements
This work was supported by a National Institutes of Health grants 2 R01 AI065617 (to T.A.C.), and by U10HL098102 and U10HL109172 (to W.P.). We thank Dr. Elena Crestani, Doris Schierembergg and Amparito Cunningham for help with patient recruitment, and Drs. Hans C. Oettgen and Louis-Marie Charbonnier for their critical review of the manuscript.
Footnotes
Accession codes
RNA-seq data are available under accession number GSE80804 at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=qxcnscaolpcrfeb&acc=GSE80804
Author Contributions
T.A.C. conceived of the project and directed the research. A.H.M. and T.A.C. designed the experiments and evaluated the data; W.P. provided blood samples of asthmatic subjects and discussed results; A.M. performed the experiments and prepared the figures. L.-M.C. performed epigenetic studies, and D.L. and M. P. performed gene expression profiling studies. A.M. and T.A.C. wrote the manuscript.
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 2.Mamessier E, et al. T-cell activation during exacerbations: a longitudinal study in refractory asthma. Allergy. 2008;63:1202–1210. doi: 10.1111/j.1398-9995.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 3.Hartl D, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamoorthy N, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsu N, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 8.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noval Rivas M, et al. Regulatory T Cell Reprogramming toward a Th2-Cell-like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015 doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachdjian R, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol. 2010;125:1128–1136. e1128. doi: 10.1016/j.jaci.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–499. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–1725. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel SE, et al. IL4R alpha mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med. 2007;175:570–576. doi: 10.1164/rccm.200607-909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104:1008–1014. doi: 10.1016/s0091-6749(99)70082-5. [DOI] [PubMed] [Google Scholar]
- 16.Al-Muhsen S, et al. IL-4 receptor alpha single-nucleotide polymorphisms rs1805010 and rs1801275 are associated with increased risk of asthma in a Saudi Arabian population. Annals of thoracic medicine. 2014;9:81–86. doi: 10.4103/1817-1737.128849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachdjian R, et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009;206:2191–2204. doi: 10.1084/jem.20091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia M, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan JJ, McReynolds LJ, Huang H, Nelms K, Paul WE. Characterization of a mobile Stat6 activation motif in the human IL-4 receptor. J Immunol. 1998;161:1811–1821. [PubMed] [Google Scholar]
- 20.Wang HY, et al. Cutting edge: effects of an allergy-associated mutation in the human IL- 4R alpha (Q576R) on human IL-4-induced signal transduction. J Immunol. 1999;162:4385–4389. [PubMed] [Google Scholar]
- 21.Al-Ramli W, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosmi L, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–230. e221–224. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Irvin C, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–1186. e1177. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss JM, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742. S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav M, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yosef N, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou L, et al. The protease cathepsin L regulates Th17 cell differentiation. Journal of autoimmunity. 2015;65:56–63. doi: 10.1016/j.jaut.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessels HW, Ward AC, Schumacher TN. Specificity and affinity motifs for Grb2 SH2-ligand interactions. Proc Natl Acad Sci U S A. 2002;99:8524–8529. doi: 10.1073/pnas.142224499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas JM, Oliva JL, Santos E. Mammalian son of sevenless Guanine nucleotide exchange factors: old concepts and new perspectives. Genes & cancer. 2011;2:298–305. doi: 10.1177/1947601911408078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanden Berghe W, et al. Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochemical pharmacology. 2000;60:1185–1195. doi: 10.1016/s0006-2952(00)00412-3. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 39.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 40.Maneechotesuwan K, et al. Regulation of Th2 cytokine genes by p38 MAPK- mediated phosphorylation of GATA-3. J Immunol. 2007;178:2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- 41.Saleh A, Shan L, Halayko AJ, Kung S, Gounni AS. Critical role for STAT3 in IL-17A-mediated CCL11 expression in human airway smooth muscle cells. J Immunol. 2009;182:3357–3365. doi: 10.4049/jimmunol.0801882. [DOI] [PubMed] [Google Scholar]
- 42.Mathew A, et al. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med. 2001;193:1087–1096. doi: 10.1084/jem.193.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshino A, et al. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int Immunol. 2004;16:1497–1505. doi: 10.1093/intimm/dxh151. [DOI] [PubMed] [Google Scholar]
- 44.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 45.Ford AQ, Heller NM, Stephenson L, Boothby MR, Keegan AD. An atopy-associated polymorphism in the ectodomain of the IL-4R(alpha) chain (V50) regulates the persistence of STAT6 phosphorylation. J Immunol. 2009;183:1607–1616. doi: 10.4049/jimmunol.0803266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephenson L, Johns MH, Woodward E, Mora AL, Boothby M. An IL-4R alpha allelic variant, I50, acts as a gain-of-function variant relative to V50 for Stat6, but not Th2 differentiation. J Immunol. 2004;173:4523–4528. doi: 10.4049/jimmunol.173.7.4523. [DOI] [PubMed] [Google Scholar]
- 47.Kudo M, et al. IL-17A produced by alphabeta T cells drives airway hyper- responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhakta NR, Erle DJ. IL-17 and “TH2-high” asthma: Adding fuel to the fire? J Allergy Clin Immunol. 2014;134:1187–1188. doi: 10.1016/j.jaci.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 51.Muraro A, et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137:1347–1358. doi: 10.1016/j.jaci.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Wenzel S, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 53.Samson M, et al. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
Methods-only References
- 54.Haribhai D, et al. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voehringer D, Wu D, Liang HE, Locksley RM. Efficient generation of long- distance conditional alleles using recombineering and a dual selection strategy in replicate plates. BMC biotechnology. 2009;9:69. doi: 10.1186/1472-6750-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 58.McFarland-Mancini MM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 59.Blaeser F, et al. Targeted Inactivation of the IL-4 Receptor Chain I4R Motif Promotes Allergic Airway Inflammation. J Exp Med. 2003;198:1189–1200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrera P, Righetti PG, Gelfi C, Ferrari M. Amplification refractory mutation system analysis of point mutations by capillary electrophoresis. Methods in molecular biology. 2001;163:95–108. doi: 10.1385/1-59259-116-7:95. [DOI] [PubMed] [Google Scholar]
- 61.Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol. 2015;16:1162–1173. doi: 10.1038/ni.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high- throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 66.Little S. Amplification-refractory mutation system (ARMS) analysis of point mutations. Current protocols in human genetics / editorial board, Jonathan L. Haines ... [et al. 2001 doi: 10.1002/0471142905.hg0908s07. Chapter 9, Unit 9 8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.