Abstract
Objective
To determine whether maraviroc (MVC) has unique neurocognitive benefits in the context of initial antiretroviral therapy (ART).
Design
Randomized, double-blind, placebo-controlled, 48-week trial
Setting
Participants were enrolled in US domestic ACTG clinical trial sites.
Participants
262 ART naïve, CCR5 tropic HIV, and HIV RNA < 1000 cps/ml participants were randomized, 230 participants completed the study.
Intervention
Participants received MVC 150mg or tenofovir disoproxil fumarate (TDF) 300mg on a background of ritonavir-boosted darunavir and emtricitabine.
Main outcome measure(s)
The neuropsychological (NP) battery of 15 tests done at baseline, week 24 and week 48 assessed 7 domains, and were standardized into z scores then converted into deficit scores (DS) and a global deficit score (GDS). The 48-week changes from baseline in the NP scores and the GDS were compared by Wilcoxon or Kruskal-Wallis test between arms, and among baseline impairment groups (classified as normal, mild (2 DS ≥1) and moderate (2 DS ≥2)). It was hypothesized that the MVC arm would have improved NP performance over TDF.
Results
In this double blind randomized placebo controlled trial, there were no differences in NP between MVC and TDF. Those with moderate NP impairment at baseline experienced greater ART-mediated NP improvement than those with mild or no NP impairment.
Conclusions
Improvement in neurocognitive functioning was greater with more baseline impairment but was comparable with MVC or TDF.
Keywords: Neurocognitive, Maraviroc, Tenofovir, Central Nervous System (CNS), Neuropathogenesis, HIV Associated Neurocognitive Disorders (HAND)
Introduction
The central nervous system (CNS) is a privileged compartment protected by the blood-brain barrier, which reduces influx of potentially toxic and therapeutic substances including antiretroviral drugs. Despite this protective barrier, HIV enters the CNS within days of infection, trafficking into the CNS through infected T-cells and monocytes [1]. In a minority of individuals, HIV establishes an autonomous, infection in the CNS [2]. Genetically distinct, or compartmentalized, HIV in the CNS has been correlated with HIV Associated Neurocognitive Disorders (HAND) [3]. In the current era of combination antiretroviral treatment (cART), subtle or mild neurocognitive deficits are prevalent in almost 40% of those who have effective treatment and despite having successfully suppressed systemic viral replication [4].
Although the mechanism of neuronal injury underlying HAND remains to be completely elucidated, HIV replication within the CNS is thought to drive an inflammatory process by inducing cytokine production that impairs neuronal functioning and eventually leads to neuronal cell death [1, 5]. Up to 10% of individuals receiving ART experience ongoing HIV replication and its consequences in the CNS despite having undetectable HIV RNA in plasma, a phenomenon known as CNS escape [6-8]. HIV may also establish a quiescent, non-replicating infection within the CNS, possibly contributing to inflammation, neuronal dysfunction and ultimately HAND [9]. Controlling HIV replication and viral load in the CNS and associated inflammatory processes could lead to improved neurocognitive outcomes and reduce the current prevalence of mild HAND.
The main co-receptor for HIV entry into target cells is the chemokine coreceptor 5 (CCR5). The antiretroviral maraviroc (MVC) is an effective inhibitor of CCR5. MVC is also thought to have anti-inflammatory effects, since CCR5 is a primary ligand of macrophage inflammatory protein -1 alpha (MIP-1α) which is pro-inflammatory [10]. In a rat model, CCR5 inhibition downregulated proinflammatory matrix metalloproteinase-9 [11], and in a macaque model MVC reduced replicating and latent Simian Immunodeficiency Virus (SIV) as well as monocyte and macrophage activation in the brain [12]. Small clinical studies in HIV-1 infected individuals have also suggested that intensifying ART with MVC may improve neuronal integrity [13] or neurocognitive performance [14]. On the other hand, CCR5 deficiency has been associated with worse outcomes during CNS viral infections [15, 16], and an animal study reported increased microglial activation with MVC, suggesting the possibility of exacerbating neuronal pathology with chronic MVC use [17]. To ascertain the effects of MVC on HAND, we investigated changes in neuropsychological performance in AIDS Clinical Trials Group (ACTG) study A5303 [ClinicalTrials.gov. Identifier: NCT 01400412], a randomized, double blind, clinical trial of MVC-versus tenofovir disoproxil fumarate (TDF)-containing ART in treatment naïve HIV-1-infected participants. Our hypothesis was that MVC would be associated with greater improvement in neuropsychological performance compared to TDF since MVC has potential anti-inflammatory effects in addition to antiviral effects.
Methods
Study design
As detailed in the primary publication [18], A5303 was a phase 2, prospective, double-blind, placebo-controlled, randomized multicenter, 48-week clinical trial conducted between January 2012 and June 2014 at 33 ACTG and 4 Adolescent Trials Network research sites in the US. Individuals whom the site investigator felt could not complete the neurocognitive protocol due to HIV or other illness, and those with HIV associated neurological disease as documented by their clinical provider were excluded. The study enrolled 262 ART-naïve HIV-1-infected participants (18 years or older) with plasma viral load (VL) greater than 1000 copies/mL and R5 tropism on the Trofile® phenotypic assay [Monogram Biosciences, South San Francisco, California]. The Institutional Review Board of each study site approved the protocol. Each subject provided a written informed consent (Clinicaltrials.gov identifier NCT01400412).
Study Procedures
Participants received MVC 150 mg or TDF 300 mg (1:1 ratio), each combined with darunavir (DRV) 800 mg, ritonavir (RTV) 100 mg and emtricitabine (FTC) 200 mg once daily. Randomization was stratified by screening VL < or ≥ 100,000 copies/mL and age <30 or ≥30 years.
Neurocognitive Assessment
Neuropsychological performance was assessed at study entry, week 24, and week 48. The neuropsychological battery consisted of 15 tests assessing the following 7 domains (measures): Language/Premorbid skills (WRAT-4 Reading [19]), Verbal Learning (Hopkins Verbal Learning Test Revised [20] learning trials), Attention/Working Memory (WAIS-III Symbol Search [21], Stroop Word [23,24]), Speed of Information Processing (Digit Symbol [21], Trailmaking A [22], Stroop color naming [24]), Executive Function (Trailmaking B [22], Stroop Interference [23,24], Letter Fluency, Semantic Verbal Fluency [25,26]), Fine Motor Skills (Grooved Pegboard bilateral [27], [28]), and Verbal Memory (Delayed Recall – HVLT-R [20], Recognition – HVLT-R [20]). Participants also completed an assessment of Activities of Daily Living (ADL) to assess functional ability [30].
The tests were averaged into two summary scores: total z score and global deficit score (GDS)[31]. Total z score was computed through the average of the 15 individual test z scores. Individual test z scores were computed by subtracting the test raw score from the demographically corrected normative score adjusted for age, education, gender, and race where appropriate, then dividing by the normative standard deviation [32]. Resulting z scores vary around zero which reflects average performance, positive scores denote better than average performance, and negative scores reflect impaired performance. GDS was computed through the average of the individual test deficit scores (DS). Increasing positive scores from zero reflect increasing deficits or impairment; 1 reflects mild impairment while 5 reflects severe impairment. Deficit scores set to 0 any normal or above normal performances, and thus avoid issues of summing positive and negative performances [29]. Deficit scores were computed as follows: 0 (z >-1); 1 (-1.5 ≤ z ≤-1); 2 (-2.0 ≤ z <-1.5); 3 (-2.5 ≤ z <-2.0); 4 (-3.0 ≤ z <-2.5); and 5 (z ≤ -3.0); missing if z-score is missing. For domains, z score and deficit scores were computed. The total ADL score is the sum of 16 ADL scores (excluding score 8, not applicable). Question 5 of the ADL questionnaire has scores 1, 2, and 3 so that it was changed to 0, 1, and 2.
To ensure that the neuropsychological tests were done consistently across the study sites, all staff assigned to administer the tests received appropriate training and certification under the supervision of a neuropsychologist (KR). Staff training was supported by several mechanisms: in-person training at the annual ACTG meetings, video training films, and PowerPoint presentations. After the initial training and completion of a web-based certification test, subsequent review of the training materials and re-certification of the research staff occurred at least annually.
Neurocognitive Impairment
Mild neurocognitive impairment was defined as having at least 2 neurocognitive domains with the mean domain DS of 1 or more. Moderate neurocognitive impairment was defined as having at least 2 neurocognitive domains with the mean domain DS of 2 or more. We also defined impairment according to conventional HAND categorization [31]: Normal (DS < 1 for all domains, ADL = 0); Asymptomatic Neurocognitive Impairment (ANI, DS ≥ 1 for at least 2 domains, ADL = 0); Mild Neurocognitive Disorder (MND, DS ≥ 1 for at least 2 domains and ADL ≥ 1, or DS ≥ 2 for 2 domains and ADL 0-3); HAD (DS ≥ 2 for 2 domains, ADL ≥ 4).
Other Study Procedures
Routine study visits for safety, virologic, and immunologic assessments occurred at week 4 (±7 days), and weeks 16, 24, 36, and 48, all ±14 days. Adherence to study medications was assessed by self-report at all study visits post-entry except week 36.
Statistical analyses
The 24 and 48-week changes in the individual test z scores, the total z score, and the GDS were compared by Wilcoxon rank sum tests between study treatment arms, and by Kruskal-Wallis test between the baseline impairment groups. The changes from baseline, week 24 and week 48 total z scores were assessed by Wilcoxon signed rank tests. All analyses were as-treated and included only participants who remained on their randomized MVC or TDF component by week 48 without an interruption in treatment of more than 10 weeks with available data for both baseline and week 48. If participants were unable to perform any individual test because of a reason unrelated to HIV associated neurological disease their individual test z scores were treated as missing. All statistical tests were two-sided and interpreted at the 5% nominal level of significance without adjustment for multiple comparisons. Analyses were conducted using SAS statistical software 9.4.
Results
Baseline Characteristics
Two hundred and thirty participants were randomized to the MVC (N= 119) versus TDF arm (N=111) (Table 1). The arms were comparable by sex (total 91% male), and age (median age 33 years; IQR, 26-42). Eighty-two percent were English speakers, 9% were Spanish speakers, and 70% had at least some college education. Baseline characteristics were similar between the study arms, except for a chance racial imbalance with more non-Hispanic blacks in the MVC arm (total 44% Non-Hispanic White, and 31% Non-Hispanic Black, p<.05). Both arms were also comparable by HIV viral load (total median plasma HIV RNA 4.5 log10 copies/mL; IQR 4.0, 5.0) and current immune functioning (total CD4 count 389 cells/mm3; IQR 293, 508).
Table 1. Baseline demographic characteristics by treatment arm.
| Characteristic | MVC (n=119) | TDF (n=111) | All Participants (n=230) |
|---|---|---|---|
| Male – no. (%) | 105 (88%) | 104 (94%) | 209 (91%) |
| Race – no. (%) | |||
| White Non-Hispanic | 51 (43%) | 51 (46%) | 102 (44%) |
| Black Non-Hispanic | 43 (36%) | 29 (26%) | 72 (31%) |
| Hispanic (Regardless of Race) – no. (%) | 21 (18%) | 29 (26%) | 50 (22%) |
| Asian, Pacific Islander | 2 (2%) | 1 (1%) | 3 (1%) |
| American Indian, Alaskan Native | 0 (0%) | 1 (1%) | 1 (0%) |
| More than one race | 2 (2%) | 0 (0%) | 2 (1%) |
| Age – year | |||
| Median (IQR) | 33 (27, 43) | 33 (26, 42) | 33 (26, 42) |
| Primary Language – no. (%) | |||
| English | 97 (82%) | 91 (82%) | 188 (82%) |
| Spanish | 12 (10%) | 9 (8%) | 21 (9%) |
| Unknown | 8 (7%) | 10 (9%) | 18 (8%) |
| Educational Status – no. (%) | |||
| < 12 years | 9 (8%) | 9 (9%) | 18 (9%) |
| HAND diagnosis – no. (%) | |||
| Normal | 66 (55%) | 60 (54%) | 126 (55%) |
| Asymptomatic Neurocognitive Impairment | 13 (11%) | 15 (14%) | 28 (12%) |
| Mild Neurocognitive Disorder | 37 (31%) | 33 (30%) | 40 (17%) |
| HIV Associated Dementia | 3 (3%) | 2 (2%) | 35 (15%) |
| Missing* | 0 | 1 | 1 (1%) |
HIV Associated Neurocognitive Disorder (HAND) diagnosis could not be computed due to missing ADL score.
At baseline, individual z scores, total z scores, GDS scores and well as domain z scores (fine motor, speed of processing, executive functioning, verbal learning, verbal memory, and attention score) were similar between the MVC and TDF arms. For example, there were no significant differences in GDS between the arms at baseline (median MVC 0.33; IQR 0.07, 0.73 vs. median TDF 0.33; IQR 0.13, 0.64). At baseline, 55% of participants were normal and 45% had a HAND diagnosis. ANI was found in 12.2%, MND in 30.6% and 2.2% HAD. None of the participants were unable to perform a test because of a reason related to HIV associated neurological disease.
Neurocognitive Change by Treatment Arm
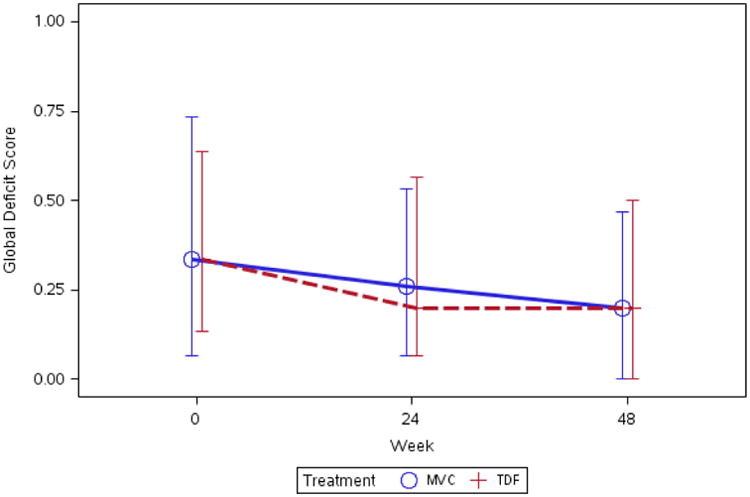
The primary analysis was to compare changes in neurocognitive performance (total z score and GDS) by arm from baseline to weeks 24 and 48. Most neurocognitive test performances improved through week 48 and were significantly better than the baseline performance. The median (IQR) GDS was 0.3 (0.1, 0.7) at baseline and 0.2 (0.0, 0.5) at week 48. The median (IQR) 48-week change in the GDS was -0.08 (-0.27, 0) (p-value < 0.001). There were no significant differences in neurocognitive performance for z score or GDS between the MVC and TDF arms at 24 weeks or 48 weeks (see Appendix and Figure 1).
Figure 1. Median (IQR) 48-week change in GDS by arm.
Neurocognitive Change by Baseline Neurocognitive Status
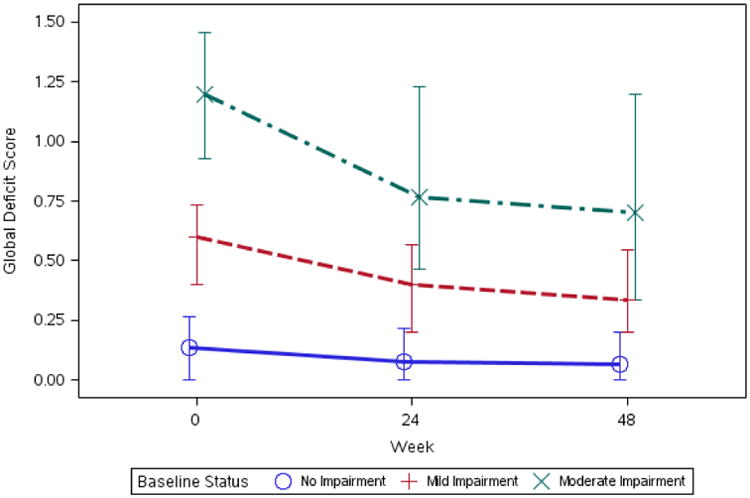
Those with GDS normal baseline functioning had very little changes, while those with GDS mild and moderate impairment improved from baseline to 48 weeks (p-value < 0.001; Figure 2). The median (IQR) 48 week change in GDS was 0.0 (-0.1, 0.1) for unimpaired (n=126), -0.2 (-0.3, -0.1) for mildly impaired (n=69), and -0.4 (-0.7, -0.2) for moderately impaired (n=35). For HAND diagnoses, among participants with ANI, MND, or HAD at baseline, there was greater improvement in those with more severe HAND at baseline (p < 0.001). The median (IQR) 48 week change in GDS was 0.0 (-0.1, 0.1) for unimpaired (n=126), -0.2 (-0.3, 0.1) for ANI (n=28), -0.3 (-0.6, -0.1) for MND (n=70), and -0.7 (-0.7, -0.3) for HAD (n=5). Fifteen ANI participants (53.6%) at baseline, 33 MND participants (48.5%), and 3 HAD participants (60.0%) became unimpaired at week 48.
Figure 2. Median (IQR) 48-week change in GDS by baseline impairment group.
Discussion
ART initiation has been shown to improve overall neurocognitive performance [34], [35]. We found that ART initiation with either MVC-containing or TDF-containing regimens significantly improved neurocognitive functioning. The neurocognitive improvement is a combination of antiretroviral effects due to viral suppression, as well as learning and practice effects [34] Those with worse baseline functioning had greater improvement. Since neurocognitively impaired individuals generally have poorer learning abilities and less practice effects, our findings suggest that improvements in this study were driven more by ART as opposed to learning/practice effects.
We found no apparent advantage for neurocognitive performance with MVC over TDF containing regimens. The primary results of A5303 also showed similar efficacy in suppressing plasma HIV-1 viremia with both the MVC-containing and TDF-containing antiretroviral regimens [18].
The neuropathogenesis of HAND is likely related to neuronal dysfunction and death due to neuroinflammation induced in response to HIV [37, 38]. Thus, anti-inflammatory adjunctive therapies may be effective for treating HAND. While several potential neuroprotective pathways for MVC have been proposed [12], our results suggest that at least in the context of ART initiation, MVC does not produce unique modulation of the inflammatory pathways underlying HAND beyond what is attributable to control of viral replication or that modulation of these pathways does not lead to a measureable change in neurocognitive function over a short period following ART initiation. MVC may have independent anti-inflammatory effects that were not detected because their magnitude was modest relative to the effects produced by controlling viral replication. Of note, a small open label single arm study found neurocognitive improvement among impaired participants whose ART was intensified with MVC [14]. Another study [39] found that in those with diagnosed HAND on ART, intensification with MVC improved neurocognition over those with no change in ART. A study investigating switching to maraviroc in participants with HIV-associated neurocognitive impairment found a trend towards neurocognitive improvement [40]. These pilot studies suggest that MVC may confer neurocognitive benefits when used to intensify ART in contrast to our results in ART naïve participants. Notably, studies of the immune effects of MVC have also produced mixed results with a recent open label MVC intensification study reporting decreased immune activation [41], while a randomized MVC intensification study in those with incomplete CD4 restoration found contradictory immune activation effects [42] One possibility is that MVC has several effects, including some that are potentially beneficial to the CNS (such as good CNS penetration and reduction in trafficking of activated T cells and monocytes) counterbalanced by other effects that may not be beneficial Neurocognitive impairment remains a relatively prevalent problem in those who are treatment naïve and immunosuppressed, and to some extent in those who are virally suppressed on ART. We found substantial overall neurocognitive improvement on ART regardless of regimen, with 51 of the 104 (49%) impaired participants returning to normal functioning. However, neurocognitive impairment was persistent in some of our participants, 23% of all participants, and 51% of those impaired at baseline, similar to the results of other studies such as CHARTER [43]. Based on the findings of this study, the largest US randomized and double blind trial in HAND to date, inclusion of MVC in initial ART regimens is unlikely to provide unique neurocognitive benefits compared to other potent ART combinations in treatment naïve individuals. A large double blind randomized placebo controlled study (ACTG A5324) is ongoing to determine whether MVC has a beneficial effect when used to intensify ART in virologically suppressed HIV-1 patients with neurocognitive impairment.
Acknowledgments
The authors would like to acknowledge the support of NIMH, NINDS to the ACTG and the ACTG grant number AI-68636.
Support: The project described was supported by the National Institute of Mental Health, the National Institute of Neurological Diseases and Stroke, and the AIDS Clinical Trials Group (ACTG) funded by The National Institute of Allergy and Infectious Diseases (NIAID) Award Number U01AI068636, and Statistical and Data Analysis Center (SDAC) Grant Number AI-068634. The content is solely the responsibility of the above authors and does not necessarily represent the official views of the NIAID, NIMH, NINDS or the National Institutes of Health (NIH), or the institutions with which the authors are affiliated.
Source of Funding: Dr. Kevin Robertson has consulted for GlaskoSmithKline (GSK) in 2015. Dr. Babafemi Taiwo has served as a consultant to ViiV, Pfizer, Janssen, GSK, and Gilead Sciences, and has received research support to Northwestern University from ViiV Healthcare and Pfizer. Dr. Joseph Eron has served as consultant to Gilead, Merck, BristolMyers Squibb (BMS), ViiV, and Janssen. The University of North Carolina School of Medicine has received research support for projects led by Dr. Eron from ViiV, Janssen, and Gilead. Dr. Todd Brown has served as a consultant for Gilead Sciences, Merck, EMD-Serono, AbbVie, and Theratechnologies.
Appendix: 24- and 48-week changes in GDS, total z-score, and domain z-scores by arm
| 24 week changes | 48 week changes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MVC (n=119) |
TDF (n=111) |
p-value | MVC (n=119) |
TDF (n=111) |
p-value | |||||
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | |||
| Global deficit score | 116 | -0.1 (-0.2, 0.1) | 108 | -0.1 (-0.3, 0.1) | 0.732 | 116 | -0.1 (-0.3, 0.0) | 109 | -0.1 (-0.3, 0.0) | 0.356 |
| Total z-score | 116 | 0.2 (-0.1, 0.4) | 108 | 0.3 (0.0, 0.4) | 0.335 | 116 | 0.3 (0.0, 0.5) | 109 | 0.3 (0.1, 0.5) | 0.233 |
| Domain z-scores | ||||||||||
| Fine motor | 115 | 0.2 (-0.3, 0.8) | 108 | 0.4 (-0.2, 0.8) | 0.436 | 115 | 0.4 (-0.2, 0.8) | 109 | 0.5 (0.0, 0.8) | 0.485 |
| Speed of processing | 116 | 0.2 (-0.2, 0.6) | 108 | 0.2 (-0.2, 0.6) | 0.670 | 116 | 0.3 (-0.2, 0.7) | 109 | 0.4 (-0.0, 0.8) | 0.241 |
| Executive functioning | 116 | 0.1 (-0.1, 0.5) | 108 | 0.2 (-0.1, 0.5) | 0.414 | 116 | 0.3 (-0.1, 0.6) | 109 | 0.3 (0.0, 0.6) | 0.476 |
| Verbal learning | 115 | 0.3 (-0.4, 1.0) | 106 | 0.3 (-0.3, 1.0) | 0.618 | 115 | 0.4 (-0.2, 1.0) | 107 | 0.4 (-0.2, 1.0 | 0.869 |
| Verbal memory | 114 | 0.1 (-0.4, 0.7) | 108 | 0.0 (-0.3, 0.6) | 0.705 | 114 | 0.1 (-0.3, 0.7) | 109 | 0.0 (-0.2, 0.6) | 0.797 |
| Attention domain | 116 | 0.1 (-0.3, 0.4) | 108 | 0.2 (-0.2, 0.6) | 0.303 | 116 | 0.1 (-0.2, 0.5) | 109 | 0.3 (-0.1, 0.7) | 0.077 |
Footnotes
Contributions: Drs. Taiwo, Brown, Eron and Robertson conceived of and led the study. Drs. Miyahara, Lee and Chan analyzed the data, Mr. Rusin was the data manager, and Ms. Berzins was the field representative for the study. All authors contributed to the writing and editing of the manuscript.
Conflicts of Interest: For the remaining authors no conflicts were declared.
References
- 1.Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013 Jun 1;207(11):1703–12. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neurovirol. 2015;21(3):276–89. doi: 10.1007/s13365-014-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritola K, Robertson K, Fiscus SA, Hall C, Swanstrom R. Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. J Virol. 2005;79(16):10830–4. doi: 10.1128/JVI.79.16.10830-10834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 5.Airoldi M, Bandera A, Trabattoni D, Tagliabue B, Arosio B, Soria A, et al. Neurocognitive impairment in HIV-infected naive patients with advanced disease: the role of virus and intrathecal immune activation. Clin Dev Immunol. 2012;2012:467154. doi: 10.1155/2012/467154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep. 2015;12(2):280–8. doi: 10.1007/s11904-015-0267-7. [DOI] [PubMed] [Google Scholar]
- 7.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 8.Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bednar MM, Sturdevant CB, Tompkins LA, Arrildt KT, Dukhovlinova E, Kincer LP, et al. Compartmentalization, Viral Evolution, and Viral Latency of HIV in the CNS. Curr HIV/AIDS Rep. 2015 Jun;12(2):262–71. doi: 10.1007/s11904-015-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menten P, Wuyts A, van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 11.Gramegna P, Latronico T, Branà MT, Di Bari G, Mengoni F, Belvisi V, et al. In vitro downregulation of matrix metalloproteinase-9 in rat glial cells by CCR5 antagonist maraviroc: therapeutic implication for HIV brain infection. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly KM, Beck SE, Metcalf Pate KA, Queen SE, Dorsey JL, Adams RJ, et al. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS. 2013;27(18):F21–8. doi: 10.1097/QAD.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvey L, Nelson M, Latch N, Erlwein OW, Allsop JM, Mitchell A, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother. 2012;67(1):206–12. doi: 10.1093/jac/dkr427. [DOI] [PubMed] [Google Scholar]
- 14.Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND) J Neurovirol. 2014;20(6):571–82. doi: 10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindberg E, Mickiene A, Ax C, Akerlind B, Vene S, Lindquist L, et al. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tick-borne encephalitis. J Infect Dis. 2008;197:266–32. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]
- 16.Lim JK, Louie CY, Glaser C, Jean C, Johnson B, Johnson H, et al. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis. 2008;197:262-. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- 17.Lisi L, Tramutola A, De Luca A, Navarra P, Dello Russo C. Modulatory effects of the CCR5 antagonist maraviroc on microglial pro-inflammatory activation elicited by gp120. J Neurochem. 2012 Jan;120(1):106–14. doi: 10.1111/j.1471-4159.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- 18.Taiwo BO, Chan ES, Fichtenbaum CJ, Ribaudo H, Tsibris A, Klingman KL, et al. Less Bone Loss With Maraviroc- Versus Tenofovir-Containing Antiretroviral Therapy in the AIDS Clinical Trials Group A5303 Study. Clin Infect Dis. 2015;61(7):1179–88. doi: 10.1093/cid/civ455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson GS, Robertson GJ. Wide range achievement test-fourth edition: Professional manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 20.Brandt J, Benedict RHB. The Hopkins Verbal Learning Test–Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 21.Wechsler D. WMS-III: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation Harcourt Brace & Co; 1997. [Google Scholar]
- 22.Reitan RM, Davison LA. Clinical Neuropsychology: Current status and applications. New York: Winston/Wiley; 1974. [Google Scholar]; Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. Army Individual Test Battery. [Google Scholar]
- 23.Stroop JR. Studies of interference in serial verbal reactions. J ExperPsychol. 1935;18:643–662. [Google Scholar]
- 24.Comalli PE, Jr, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962 Mar;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- 25.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 26.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 27.Klove H. Clinical neuropsychology. In: Forster EM, editor. The Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- 28.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the Finger Tapping and Grooved Pegboard tests. Perceptual and Motor Skills. 1993;76(3c):1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 29.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. HNRC Group. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004 May;26(3):307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969 Autumn;9(3):179–86. [PubMed] [Google Scholar]
- 31.Blackstone K, 1, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894–908. doi: 10.1080/13854046.2012.694479. Epub 2012 Jun 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasion adults scoring program. Lutz, FL: American Psychological Assessment Resourcse, Inc.; 2004. [Google Scholar]
- 33.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson K, Jiang H, Kumwenda J, Supparatpinyo K, Evans S, Campbell TB, et al. AIDS Clinical Trials Group. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis. 2012 Sep;55(6):868–76. doi: 10.1093/cid/cis507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson KR, Robertson WT, Ford S, Watson D, Fiscus S, Harp AG, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr. 2004 May 1;36(1):562–6. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Grund B, Wright EJ, Brew BJ, Price RW, Roediger MP, Bain MP, et al. INSIGHT SMART Study Group. Improved neurocognitive test performance in both arms of the SMART study: impact of practice effect. J Neurovirol. 2013 Aug;19(4):383–92. doi: 10.1007/s13365-013-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zayyad Z, 1, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND) Curr HIV/AIDS Rep. 2015 Mar;12(1):16–24. doi: 10.1007/s11904-014-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MF, Gill AJ, Kolson DL. Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS. 2014 Nov;9(6):559–64. doi: 10.1097/COH.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016 Feb 20;30(4):591–600. doi: 10.1097/QAD.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 40.Tiraboschi JM, Muñoz-Moreno JA, Puertas MC, Alonso-Villaverde C, Prats A, Ferrer E, et al. Viral and inflammatory markers in cerebrospinal fluid of patients with HIV-1-associated neurocognitive impairment during antiretroviral treatment switch. HIV Med. 2015 Jul;16(6):388–92. doi: 10.1111/hiv.12243. [DOI] [PubMed] [Google Scholar]
- 41.Cillo A, Hilldorfer B, Lalama C, McKinnon J, Coombs R, Tenorio A, et al. Virologic and immunologic effects of adding maraviroc to suppressive antiretroviral therapy in individuals with suboptimal CD4+ T-cell recovery. AIDS. 2015 Oct 23;29(16):2121–2129. doi: 10.1097/QAD.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013 Jun 6;121(23):4635–46. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]


