Review on the properties of LPSs and how in vivo interactions with host molecules influence both the bioactivity of circulating endotoxin and quantitation ex vivo.
Keywords: LPS, lipopolysaccharide, translocation, endotoxin, Limulus
Abstract
Endotoxemia is in its scientific ascendancy. Never has blood-borne, Gram-negative bacterial endotoxin (LPS) been invoked in the pathogenesis of so many diseases—not only as a trigger for septic shock, once its most cited role, but also as a contributor to atherosclerosis, obesity, chronic fatigue, metabolic syndrome, and many other conditions. Finding elevated plasma endotoxin levels has been essential supporting evidence for each of these links, yet the assays used to detect and quantitate endotoxin have important limitations. This article describes several assays for endotoxin in plasma, reviews what they do and do not measure, and discusses why LPS heterogeneity, LPS trafficking pathways, and host LPS inactivation mechanisms should be considered when interpreting endotoxin assay results.
Introduction
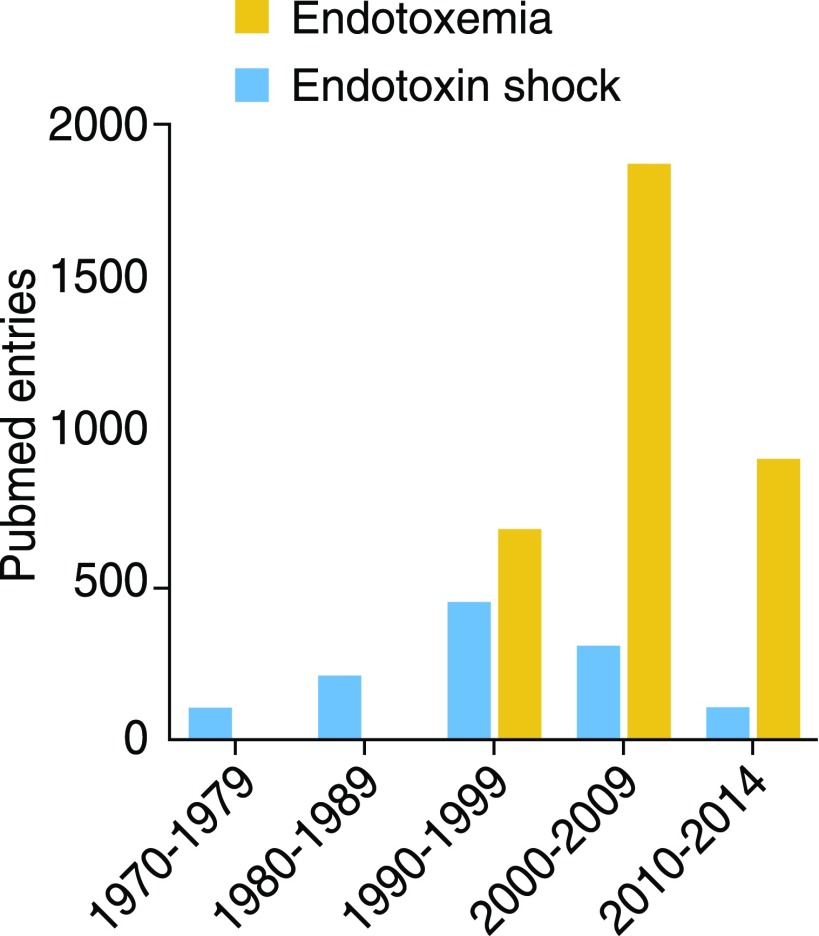
It wasn’t so long ago that endotoxemia was the most often-cited cause of septic shock. Interest in the “endotoxic shock” idea gradually waned as various endotoxin antagonists were tested in septic patients and failed to improve survival, yet investigators’ fascination with blood-borne endotoxin steadily increased during the same period (Fig. 1). Circulating endotoxin, usually attributed to translocation of LPS from the GI tract into the bloodstream (“intestinal endotoxemia”), has been invoked to cause or exacerbate human pathologies as diverse as atherosclerosis, alcoholic liver disease, autoimmunity, metabolic syndrome, renal injury, transplant rejection, traumatic brain injury, obesity, multiple organ failure, depression, chronic fatigue, HIV disease, and type 2 diabetes.
Figure 1. Publications with “endotoxemia” or “endotoxin shock” as keywords.
Data tabulated from PubMed entries with these Medical Subject Headings, by decade. Note that the columns for 2010–2014 include entries for only 5 yr. There were 345 entries for endotoxemia in 2015.
To be sure, LPS is a very potent stimulus. Human cells that express the LPS receptor complex, MD-2–TLR4, can often sense picograms of purified LPS (<10−9 mol) per milliliter of culture medium and respond within minutes. Intravenous injection of 4 ng LPS/kg body weight reduces blood pressure in many volunteers [1]. The Gram-negative bacteria in the human GI tract produce large amounts of LPS, some of which may make its way into the bloodstream, and it is a pretty safe bet that such potent agonists would influence host physiology. The endotoxin produced by commensal bacteria has even been likened to an exogenous hormone [2].
Every proposed endotoxin-disease association has been supported by measuring endotoxin levels in the plasma, serum, or tissues of affected individuals. Different assays for endotoxin have been used, sometimes with disparate results, and their interpretation has been controversial for decades. Plasma endotoxin levels have correlated with cytokine levels in some clinical series but not others [3]; plasma LPS has been detected in patients with Gram-positive bacteremia but not consistently in patients with Gram-negative bacteremia [4]; and the association of plasma endotoxin levels with inflammatory markers or metabolic changes has been inconsistent. How, many have asked, can high plasma endotoxin levels be found in asymptomatic humans when much lower levels can elicit fever and hypotension after a very small dose of LPS has been given intravenously? Is the endotoxin that can be detected in plasma of asymptomatic individuals truly bioactive in vivo?
This review approaches these issues by taking a critical look at assays for endotoxin in plasma. It begins with the factors that may influence the structure, physical state, and detection of LPS in vivo. How LPSs enter the blood will then be discussed, followed by a summary of several endotoxin assays and their uses. The review concludes by considering influences that determine whether the endotoxin found in plasma is bioactive in vivo.
ENDOTOXIN: STRUCTURE, PHYSICAL STATE, AND BIOACTIVITY
Heterogeneity in LPS structure, solubility, physical state, and bioactivity places important limitations on the interpretation of plasma endotoxin assays.
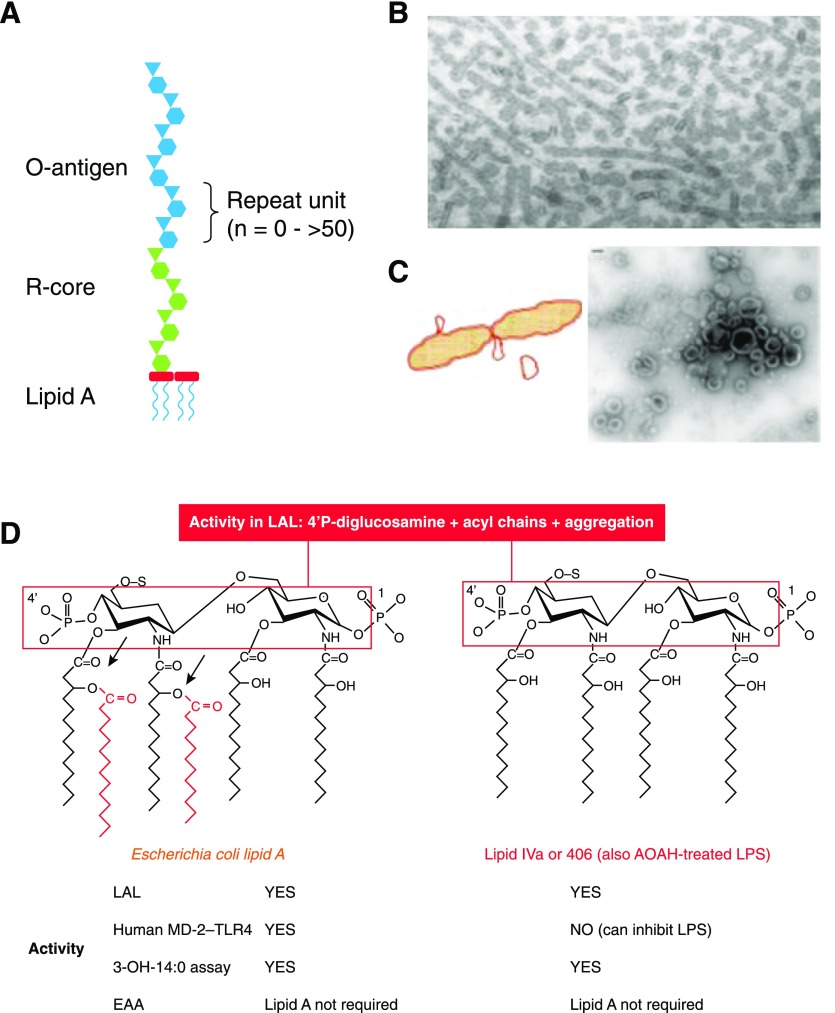
LPSs—even the LPS molecules isolated from a pure culture of a Gram-negative bacterium—are very heterogeneous molecules. They differ in polysaccharide chain length, acylation of the lipid A backbone, and the extent to which phosphates, ethanolamine, arabinose, and other “decorations” are attached to lipid A or the core oligosaccharide (Fig. 2A). Purified LPS is a mixture of water-insoluble aggregates that often form ribbons or amorphous blobs [5] (Fig. 2B). The molecules can usually be dispersed by sonication or agitation or the addition of triethylamine or EDTA, but solubility is rarely achieved. Few, if any, Gram-negative bacteria found in nature make lipid A without an attached saccharide moiety, so assays for lipid A in human plasma are assays for LPS or for bacterial lipooligosaccharide, which lacks an O-antigen.
Figure 2. LPS structure, lipid A structure-activity relationships.
(A) Diagram showing the structure of a typical LPS, with lipid A, R-core oligosaccharide, and O-antigen moieties. (B) Salmonella typhimurium LPS stained with uranyl acetate, ×98,000. Note tubular and globular forms. Originally published in Shands and Chun [5], © American Society for Biochemistry and Molecular Biology. (C) Diagram showing release of outer membrane vesicles (blebs) by growing Escherichia coli. Some blebs appear at the time of septum closure during cell division. Negatively stained electron micrograph of an S. typhimurium culture supernatant showing blebs. Original bar (upper left), 100 nm. Originally published in Vesy et al. [43], © 2000, American Society for Microbiology. (D) Maximal lipid A sensing by human MD-2–TLR4 requires phosphates at both 1 and 4′ on the diglucosamine backbone, 4 glucosamine-linked acyl chains, and 2 secondary (acyloxyacyl) chains (red). Both E. coli lipid A and lipid IVa (also known as compound 406) are active in the LAL assay, which requires the 4′ phosphoryl diglucosamine backbone and aggregated acyl chains. In contrast, aggregation is not necessary for lipid A with an attached O-antigen to activate cells via MD-2–TLR4. Both lipid IVa and AOAH-treated LPS have a tetraacyl lipid A structure (right) and can inhibit LPS signaling, but AOAH-treated LPS is much less active than lipid IVa in the LAL. Note in both structures that the 4 3-OH-14:0 chains are all attached to the backbone; releasing them, using hot acid hydrolysis, has been used to discriminate LPS-linked and non-LPS 3-OH-14:0 in plasma (see text). S, Attachment site of polysaccharide chain (missing in lipid IVa but not in AOAH-treated LPS); arrows, sites of cleavage by AOAH.
The natural LPS that can be found in blood is also a heterogenous mixture of molecules that may differ in structure, as described above, and in addition, may be found in bacterial cell walls or fragments of outer membrane (outer membrane vesicles [6], blebs; Fig. 2C), bound to bacterial [7] or host proteins, or associated with blood cells.
Optimal LPS recognition by MD-2–TLR4, the host LPS receptor complex, occurs when the lipid A moiety of LPS has 6 fatty acyl chains and 2 phosphates (Fig. 2D, left). This structure is found in the LPSs produced by most Enterobacteriaceae and certain other Gram-negative aerobes, whereas the LPSs produced by Pseudomonas aeruginosa, Burkholderia sp., and many others often have 5 acyl chains and are usually less stimulatory to human cells. The tetraacyl LPSs of Yersinia pestis and Francisella tularensis are even less stimulatory. Unlike enterobacterial LPS, the LPSs made by most Bacteroides sp. and many other Gram-negative anaerobes lack the 4′-phosphate, have 5 acyl chains, and are very weak agonists; some may even inhibit the ability of E. coli LPS to stimulate human cells in vitro [8–10]. There can also be great variability in LPS structure, even within the same bacterial species: Porphyromonas gingivalis bacteria, for example, produce LPSs that are TLR4 agonists, TLR4 antagonists, or nonstimulatory [11]. As will be discussed below, the lipid A structures that can be sensed by animal cells are not necessarily ones that are detected by assays for endotoxin in plasma.
HOW DOES ENDOTOXIN GET INTO THE BLOODSTREAM?
Plasma endotoxin may be derived from bacteria in an infected local tissue, the blood, the GI or respiratory tract, or food or other ingested matter.
Movement from infected extravascular sites to blood
In their landmark review of bacteremia, Bennett and Beeson [12] concluded that bacteria almost always make their way from a local site of infection to the bloodstream via lymphatics. This topic has received little attention in recent years, but there is evidence that LPS also reaches the blood from tissues largely through lymphatic channels [13, 14]. Traffic via lymphatics may allow LPS to bind inhibitory proteins (see below) before reaching the blood.
Release from blood-borne bacteria
Convincing evidence that bacteria can release LPS directly into the human bloodstream was reported by Brandtzaeg and colleagues [15], who obtained images of Neisseria meningitidis blebs in the plasma of children with meningococcemia and provided biochemical confirmation by demonstrating the presence of 3-OH-12:0, a component of Neisseria lipid A, in the same samples. Meningococcemia is an exceptional case, however. N. meningitidis bacteria can colonize and invade vascular endothelial cells [16] and reach very high titers in blood (>108 DNA copies/ml [17]), whereas the Gram-negative bacteria that are most often isolated from blood cultures (such as E. coli) typically cause low-level bacteremia (<100 cfu/ml) and produce much less endotoxin. Antibiotic therapy may sometimes increase plasma endotoxin levels in patients whose blood cultures grow Gram-negative bacteria [18].
Movement across mucosal epithelia
Rich sources of LPS live in the upper respiratory and GI tracts, especially in the mouth and colon. LPS and/or other TLR4 agonists may also be found in foodstuffs [19]. The LPS in oral secretions and food seems to translocate into the blood most frequently via small intestine lymphatics, often along with triglyceride-rich lipids (chylomicrons [20] and others), reaching the circulation through the thoracic duct [21]; plasma endotoxin levels have often peaked within 1 or 2 h after a fatty meal [22]. It is also possible that LPS crosses from the GI mucosa into the portal circulation, particularly when intestinal permeability increases [23, 24], although surgeons have had more success growing bacteria from mesenteric lymph nodes than from portal blood [25, 26]. LPS concentrations are much higher in the colon than in the small intestine, but Erridge [24] and Deitch [27] have argued cogently that most of the LPS that is detected in peripheral venous blood translocates from the small intestine. If this is correct, then the differences observed in the prevalence of intestinal endotoxemia in different patient groups (for example, in HIV patients [28, 29]) may, in part, reflect differences in the patients’ diets [30]. The recent description of a gut-vascular barrier [31] should stimulate studies to find out how barrier permeability influences the translocation of LPS in intact bacteria, in outer membrane vesicles, and attached to lipids or proteins.
The kind and amount of LPS that enters the blood during intestinal endotoxemia should be influenced by the relative abundance of the different LPS-producing bacteria in the patient’s food and mucosal microbiota and the ability of these bacteria or their LPS to cross into the blood. The LPS measured in an individual’s plasma may include molecules produced by many different bacteria, even in subjects whose blood cultures grow a single Gram-negative bacterial isolate. Not only do the LPS molecules produced by various mucosal Gram-negative bacteria differ in their ability to be detected in endotoxin assays [32], but their relative abundance and tendency to translocate may also be influenced by diet [30, 33], antibiotics, and other variables [24]. Anaerobic Gram-negative bacteria greatly outnumber Gram-negative aerobes in the gut, yet it is not known with certainty that the LPSs produced by the different anaerobes translocate into the circulation. LPS translocation is poorly mimicked by intraperitoneal LPS injection, and the in vivo interactions between stimulatory and nonstimulatory/inhibitory LPSs, if they exist, cannot be predicted accurately from in vitro results [34].
How does LPS not get into blood? By parenteral injection! Much has been learned from the “volunteer endotoxemia” model [35, 36], but intravenous LPS injection resembles no natural condition other than, perhaps, the infusion of contaminated intravenous fluids [37]. It is not surprising that a small dose of E. coli LPS, injected directly into the bloodstream, can elicit fever and other responses in volunteers. Most of the natural routes to the blood are via lymphatic channels, not veins; they deliver smaller amounts of endotoxin into the blood gradually (and intermittently) over time, and they provide more opportunities for the body’s LPS-neutralizing mechanisms (see below) to do their job.
WHAT HAPPENS TO LPS THAT ENTERS THE BLOODSTREAM?
Takeshita et al. [38] used flow cytometry to detect binding of the E5 anti-LPS antibody (see below) to blood monocytes. They reported that LPS was found on almost half of the CD14+ monocytes obtained from 5 children with Gram-negative bacteremia and on none of the monocytes from 4 children with Gram-positive bacteremia. The amount of cell-bound LPS could not be quantitated, but other evidence suggests that only a small fraction of bloodborne LPS is bound to cells. When Roth et al. [39] incubated LPS in citrated human blood for 15 min at room temperature, they found <5% of the LPS bound to cells (largely platelets), whereas >2/3 was bound to HDL. LPS may also be found on erythrocytes [40–42].
Plasma proteins can transfer LPSs from bacterial membranes to lipoproteins and other acceptors [43]. LBP and phospholipid transfer protein pass LPS to HDL and other plasma lipoproteins, many of which bind LPS in a way that prevents lipid A from interacting with MD-2–TLR4. These transfers can occur very quickly: when bacterial outer-membrane blebs were injected intravenously into rats, ∼50% of the LPS in the blebs had bound to lipoproteins within 1 min [44]. Rapid sequestration of lipid A also likely occurs when LPS translocates from the intestine via lymphatics. LPS that initially binds 1 lipoprotein class (usually HDL) can move to a different lipoprotein class, albumin, or other plasma proteins over time [45, 46]. LPS–lipoprotein complexes may be cleared from the circulation very slowly [47, 48]—an important point when considering the likely physical state of LPS that remains in plasma for more than a few minutes. LPS binding to other proteins will be discussed below.
It is important to note that endotoxin that enters the portal venous circulation passes through the microcirculations of the liver, lung, and an extremity before reaching a peripheral vein, the source of plasma for most endotoxin assays. Peripheral venous LPS that entered the blood via the thoracic duct has passed through lymph node(s), lymphatic vessels, the lungs, and an extremity. It is likely that most LPS molecules bind plasma proteins along the way and that certain organs, particularly the liver [49], remove a significant fraction of the LPS that passes through them. The LPS that is detectable in peripheral venous blood may be the fraction that, because it was bound to lipoproteins or otherwise altered, resisted clearance; it may not be a representative sample of the LPS that initially entered the bloodstream or that was removed by tissues.
ASSAYS FOR ENDOTOXIN IN PLASMA
Although there is a World Health Organization standard endotoxin [50], most providers of endotoxin assay kits include their own preparation of an E. coli LPS as the assay standard. In practice, 10–15 endotoxin units = 1 ng/ml LPS (∼0.25 pM, assuming average LPS Mr = ∼4000). As different pure LPS preparations can produce different dose-response curves [22], the assayed potency of the LPS that is produced by a given Gram-negative bacterium may not match that of the LPS used to standardize the assay or the LPS found in a different subject’s plasma. This point should be kept in mind when comparing endotoxin levels from individuals whose illnesses have been caused by different bacterial strains, even when the same assay method is used. As stressed by Boutagy et al. [51], measuring a change in an individual’s plasma endotoxin level should be more reliable than is comparing levels measured in different individuals or obtained using different assays.
Early studies found that endotoxin can be trapped in clots, so plasma is preferred to serum [52]; low concentrations of heparin are often recommended. Contamination by environmental LPS and non-LPS agonists is an issue for all of the assays. For discussions of these and other technical concerns, readers are referred to the informative reviews by Tsan and Gao [53], Erridge [54], and Gnauck et al. [55].
LAL assay
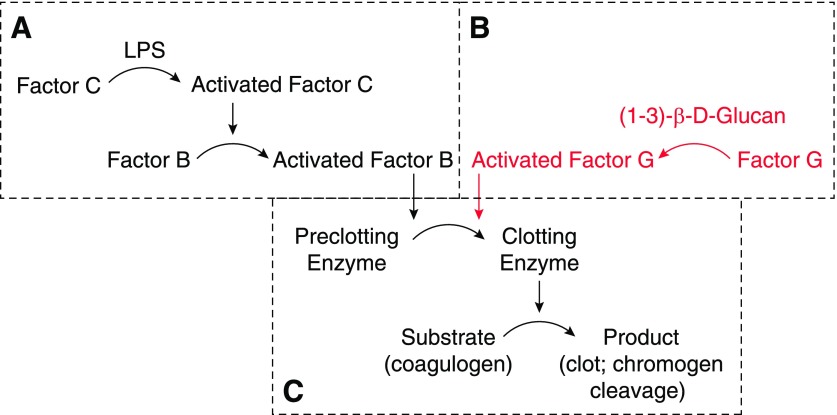
This test measures the activation of the horseshoe crab (Limulus polyphemus or Tachypleus species) amebocyte clotting cascade by the lipid A moiety of LPS (Fig. 3A and C). Introduced to clinical medicine by Levin et al. in 1970 [52, 56], the assay has since been widely used to detect endotoxin in fluids and is now the favored technique for screening parenteral therapeutics. The Limulus clotting system may also be activated by the β-glucans produced by Candida and certain other fungi (Fig. 3B and C); some commercially available LAL assays have been modified to inhibit the glucan-responsive proteins [57], whereas others include only recombinant LBP (factor C) and a chromogenic substrate [58]. Most kits now use a peptide substrate that produces a quantitative kinetic or end-point read-out after a short period of incubation.
Figure 3. The LAL assay.
The Limulus amebocyte contains a clotting enzyme cascade that can be initiated by (A) LPS binding factor C or (B) (1–3)-β-d-glucan binding factor G. Both pathways initiate cleavage of the proclotting enzyme to form the clotting enzyme (C). LPS may also be detected using recombinant factor C with its chromogenic substrate. A widely used clinical assay for β-glucan is based on the enzymes in B.
Before performing the LAL assay, plasma must be treated to remove inhibitors [52]. These are largely, if not entirely, plasma proteins [59]. Plasma is usually diluted 1:10 with pyrogen-free water and then heated at 70–80°C for 10 min. Some labs have successfully used protease digestion of plasma proteins to improve LPS recovery. With the use of sensitive LAL assays, investigators have found 0–200 pg/ml endotoxin in the plasma of healthy human subjects (see references in Erridge [24]). For a detailed discussion of LPS quantitation using LAL and its problems, see Gnauck et al. [55, 59].
LAL activation and lipid A structure
Studies using synthetic lipid A part-structures found that lipid A acylation is a strong determinant of LAL activity [60] (Fig. 2D). Whereas LPS stimulation of human monocytes is greatest when the lipid A moiety has 6 acyl chains (chains of 12–14 carbons are most stimulatory), lipid A analogs with 4 or 5 acyl chains can be highly active in the LAL [61]. Lipid A structures that can activate the Limulus clotting cascade thus may not be sensed by human cells. In other studies, the LAL-activating lipid A structure was shown to have a diglucosamine-4′-phosphate backbone [60, 62, 63]. The absence of the 4′-phosphate in Bacteroides LPS may account for the weak LAL activity observed in LPS from some species [8, 10]. Differences in the exposure of the 4′-phosphate may also explain the ability of serum albumin to decrease the activity of other LPSs in the LAL [61], whereas lactoferrin, which inhibits LPS-induced cytokine production by human cells, did not diminish LAL activity [60].
Do lipid A part-structures and natural LPSs have the same structure-activity relationships for LAL activation? LPSs are larger, more amphipathic, easier to disaggregate, and more often decorated with charged residues than are synthetic lipid A analogs. Two kinds of observation suggest that lipid A analog-activity relationships may not always apply to LPSs. First, some LPS preparations have been highly active in the LAL assay yet weak inducers of cytokine production by human monocytes [10, 64]. Other preparations of purified LPS have had low LAL reactivity yet been able to elicit IL-1β production by human monocytes [65]; the authors attributed LAL reactivity to large LPS aggregate size. Second, whereas several studies found that the tetraacyl lipid A part-structure 406 (also known as lipid IVa) was as potent as hexaacyl lipid A in the LAL assay, tetraacyl LPSs, produced by treating LPS with AOAH (see Fig. 2D), had greatly reduced LAL activity [66, 67]. The tetraacyl LPS produced by Y. pestis at 37°C was also 10-fold less potent than hexaacyl E. coli LPS in this assay [68].
In general, the LAL sensitively detects the LPSs produced by Enterobacteriaceae and many other proteobacteria. The observation that endotoxemia has been detected more frequently in patients whose blood cultures have grown N. meningitidis, Burkholderia pseudomallei, or Y. pestis [69] than in those with E. coli bacteremia may reflect the much higher levels of bacteria (measured as cfu or DNA copies/ml) often found in blood during meningococcemia, septicemic melioidosis, and plague. In the blood of patients with meningococcemia, the most intensively studied of these diseases, Ovstebo et al. [17] found a strong correlation between levels of LAL-positive endotoxin and N. meningitidis DNA. The low endotoxin levels detected using the LAL assay on plasma from typhoid fever patients [70] may occur because the bacteria in the blood are largely intracellular, and the poor correlation of LAL positivity with E. coli bacteremia [3] may reflect, in part, the small numbers of E. coli bacteria that circulate in most patients whose blood cultures grow E. coli. For a useful summary of the use of LAL to detect bacteremia, readers may consult J. C. Hurley and coworkers’ recent review [3].
An important unknown is the ability of the LAL assay to detect LPS molecules which, because they are abundantly produced in the GI tract, would be expected to constitute a substantial fraction of the LPS that translocates to the blood. Although LPSs from Bacteroides fragilis and P. gingivalis have been significantly less potent than E. coli LPS in the LAL [32, 71], the extent to which the LPSs of other anaerobes can activate the LAL has received less study. It is possible that the host-stimulatory LPS detected in the LAL is greatly outnumbered by weakly stimulatory or even inhibitory LPS molecules—perhaps these interfere with the LAL assay in some instances or stimulate host cells via TLR2 [72] or other receptors.
O-Antigen and core oligosaccharide assays
Anti-O-antigen polyclonal antibodies and mAb have been used to detect LPS in body fluids, usually in a sandwich ELISA [73] or competitive radioimmunoassay [74] format. Assays for different features of the LPS molecule (O-antigen ELISA vs. LAL detection of lipid A) in bacterial outer-membrane blebs yielded remarkably different quantitative results, even when LPS purified from the same E. coli strain was used to standardize both assays [75, 76]. The discrepancy probably was caused by differences in the extent to which lipid A and O-antigen were exposed on the bleb surface and in the purified LPS standard. Gram-negative bacteria also may release O-polysaccharides that are not attached to lipid A yet are detectable in O-antigen immunoassays.
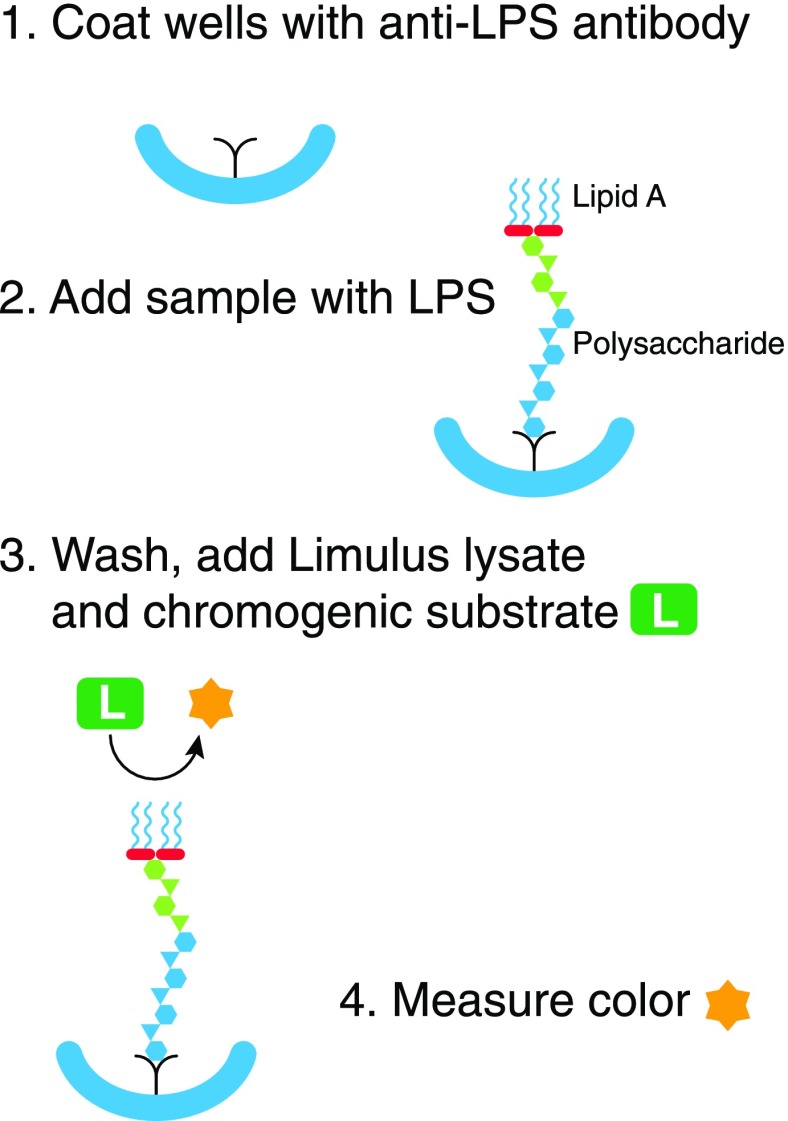
Specific LPSs can be detected by coating microtiter wells with a “capture” antibody to the target LPS, adding a sample containing that LPS, washing thoroughly, and measuring the active LPS that remains in the wells using the LAL (Fig. 4). This “immunolimulus” approach improves specificity, as the LAL should only be activated by the antibody-bound LPS. Proof of principle was first shown in a model of Haemophilus influenzae bacteremia in rats [77]. With the use of a cross-reactive capture antibody that recognizes core LPS epitope(s) [78, 79], Saxen et al. [80] were also able to detect LPS in the (diluted, heat-treated) plasma from rats infected with different Enterobacteriaceae. The lower level of detection was somewhat below that found with LAL alone but still much above the usual bacterial counts reported in bacteremic humans [37]. Polysaccharides that lack lipid A are not detectable with this assay.
Figure 4. The “immunolimulus” assay.
Microtiter wells are first coated with an antibody to the target LPS (1). After washing, plasma is added. LPS in the plasma binds to the antibody (2). After another thorough wash, Limulus lysate and a chromogenic peptide substrate are added (3). The color produced is measured over time (4). The approach makes the LAL assay more specific for LPS.
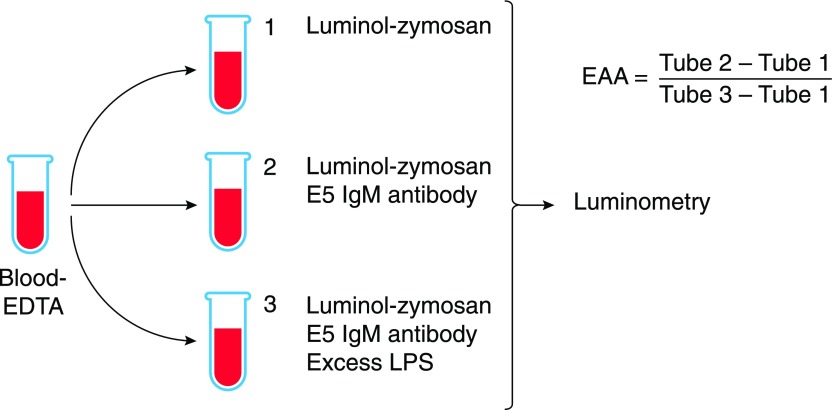
EAA
This assay measures the ability of LPS–antibody complexes to enhance the production of reactive oxygen species by neutrophils in a subject’s blood [81] (Fig. 5). E5, a monoclonal IgM that binds core LPS epitope(s), is first added to the blood sample. After incubation at 37°C to allow LPS–IgM immune complexes to form, be opsonized by complement, and prime the NADPH oxidase complex, opsonized zymosan (to trigger the respiratory burst) and luminol are added, and the chemiluminescence produced by the blood neutrophils is measured over time. The read-out is the difference in chemiluminescence in tubes containing the anti-LPS antibody versus tubes with no antibody; a tube that contains both the antibody and a large amount of purified LPS gives the maximum value. The chemiluminescent signal is proportional to the logarithm of the LPS concentration in the sample [82].
Figure 5. The EAA.
Anticoagulated blood is incubated with an excess of LPS in tube 3 before E5 IgM antibody is added to tubes 2 and 3 and luminol-zymosan is added to all tubes. After incubation at 37°C with shaking, photons are counted using a luminometer. EAA is calculated using the formula shown. Graphic adapted from Matsumoto et al. [85].
The assay uses a small amount of whole blood (1 ml or less), can be done rapidly (∼30 min), and does not require treating the blood to remove inhibitors. Assay performance was maintained in blood that contained as few as 500 neutrophils/ml. Like the LAL assay, the EAA detects different LPSs with different degrees of sensitivity, with different dose-response relationships [82].
Although the assay was cleared for human diagnostic use by the U.S. Food and Drug Administration in 2003, it has significant shortcomings. Despite its name, it does not measure “endotoxin activity.” Positivity in the EAA requires the formation of immune complexes between LPS (or another antigen) and the E5 IgM antibody. A bioactive lipid A moiety is not needed. The E5 antibody’s binding specificity and affinity for LPS have been challenged [83], and a historical precedent may be important: other IgM antibodies raised to core LPS structures have been polyreactive, binding host molecules as well as LPS [84]. Glucocorticoids (and presumably other molecules) that influence neutrophil responsiveness may reduce the read-out [81], whereas IL-8 and TNF may increase it; Matsumoto et al. [85] suggested that the EAA actually measures neutrophil priming, not LPS.
Activity assays using reporter cell lines
Erridge [24, 54] measured TLR ligands (LPS, lipopeptides, peptidoglycan, flagella) in foodstuffs and intestinal microbiota using TLR-deficient human embryonic kidney 293 cells that had been transfected to express individual TLRs and a NF-κB reporter. He found dramatic differences in the ability of gut bacteria to shed “LPS equivalents” (TLR4 agonists), confirming that enterobacterial species are more likely than Bacteroides or Firmicutes to release molecules that can be recognized by human MD-2–TLR4 [24, 86]. Strong TLR4 stimulation was also observed in heat-treated human feces and sterile stool filtrates [86], suggesting that the inhibitory LPSs in the intestinal microbiota are unable to block TLR4 activation by the stimulatory LPSs in stool. Others have used a similar approach to quantitate TLR4 agonists in portal venous serum [87] and stool [88]. The use of this strategy to detect endotoxin in peripheral blood plasma has not been reported; there may be ways to amplify the intracellular signal and increase sensitivity [89]. Although this assay should only detect LPS that can be sensed by host cells, plasma must still be treated to remove inhibitors before sensitive detection is possible.
Measurement of 3-hydroxy fatty acids
3-OH-14:0 is a key component of the lipid A produced by most enteric Gram-negative aerobes (Fig. 2D). Two molecules of 3-OH-14:0 are esterified to the glucosamine backbone, and 2 are attached in amide linkage; all of the 3:OH-14:0 can be released by hot acid hydrolysis. Attempts to measure LPS levels by using gas chromatography/MS to quantitate plasma 3-OH-14:0 were successful despite poor sensitivity and significant background [90, 91]. Another study detected 3-OH-12:0, the distinctive fatty acid in Neisseria LPS, in the plasma of children with meningococcal disease, with good correlation between its levels and those of plasma endotoxin detected by LAL [15].
Pais de Barros and colleagues [92] used HPLC, followed by tandem MS, to identify 3-OH-14:0 in plasma. They measured the difference between the amounts of free (i.e., non-LPS) 3-OH-14:0 and the 3-OH-14:0 that was released following hydrolysis with hot acid. The lower limit of detection in mouse plasma was 12 ng 3-OH-14:0/ml, and the lower limit for quantitation was 40 ng 3-OH-14:0/ml ; the assay was thus less sensitive than LAL. They studied a small sample of LAL-negative plasma samples from patients who had documented infections and found higher LPS levels in the plasma of those with more severe illness.
Finding 3-OH-14:0 in plasma does not mean that bioactive LPS is present. It can indicate the presence of lipid A, particularly when there is a significant excess of acid-hydrolyzable 3-OH-14:0 over baseline (without hydrolysis) 3-OH-14:0, but lipid A signaling via MD-2–TLR4 requires the acyloxyacyl structures and lipid A phosphates, not 3-OH-14:0 alone. The posthydrolysis 3-OH-14:0 content is unchanged after AOAH removes the secondary acyl chains or alkaline phosphatase partially dephosphorylates lipid A (see below).
Indirect assays for endotoxins
Plasma endotoxin levels can fluctuate, even over short time periods [93]. The EndoCab assay assumes that these fluctuations are, in part, a result of the ability of plasma antibodies to bind LPS and clear it from the circulation. A second indirect test, the ENA, measures the ability of plasma to neutralize purified LPS that is added to it ex vivo. Changes in levels of soluble CD14, LBP, or leukocyte molecules have often been used to confirm the presence of endotoxemia.
Anti-endotoxin antibody levels—the EndoCab assay
Normal human serum contains polyreactive “natural” antibodies that bind LPS antigens [94]. Noting that the enterobacterial LPS core structure is relatively well conserved and that anti-core LPS antibody titers are relatively stable over time, researchers at the Scottish National Blood Transfusion Service (Edinburgh, Scotland) developed an ELISA assay for IgG and IgM antibodies to LPS core epitopes and used it to infer the presence of LPS in plasma; a drop in anti-endotoxin antibody titer was taken as evidence that endotoxin was, or had been, present in the blood [95–97]. With the use of LPS from individual Gram-negative species as the capture antigen, antibodies that bound that particular LPS could also be measured; in one example, IgG antibodies to B. fragilis LPS decreased during surgical trauma but were high in nonsurvivors of severe sepsis [98, 99]. Low or high plasma endotoxin levels may accompany low EndoCab levels [100], however, and Bennett-Guerrero and colleagues [101] found that neither IgM nor IgG antibodies completely predicted the endotoxin neutralization capacity of plasma.
Endotoxin Neutralization Assay (ENA)
This assay measures the overall endotoxin-neutralizing capacity of plasma [102, 103]; a decrease in ENA is regarded as evidence that endotoxin is or was present. A known quantity of LPS is incubated with plasma for a period of time. Then, without further treatment of the plasma, the LAL is used to measure the LPS that has not been “neutralized” by plasma inhibitors. Bölke et al. [103] reported decreased ENA in the plasma of patients undergoing colonoscopy, and Erridge et al. [22] found both higher levels of endotoxin and decreased ENA in the plasma of volunteers who had consumed a high-fat diet. Champion et al. [104] found plasma ENA to be greater in women than in men and in patients with inflammatory bowel disease than in controls.
Changes in blood levels of host response molecules
Many authors have tried to support the validity of plasma endotoxin detection by measuring changes in the blood levels of molecules that can be produced by endotoxin-stimulated host cells. One of the most popular such “reactants” has been soluble CD14 [105, 106], a key molecule in LPS signaling that cells also may release in response to LPS stimulation. Non-LPS agonists can also promote CD14 release from monocyte–macrophages, however, and CD14 is also an acute-phase protein [107] that is produced in the liver [108] and secreted into the blood. High soluble CD14 levels have been found in patients with rheumatological diseases [107], following total hip replacement [109], and with other conditions. The measurement of soluble CD14, other acute-phase reactants (including LBP), or leukocyte activation markers [30, 110] may provide evidence for a host response, but these assays do not establish the presence or absence of endotoxemia per se.
CAN THE ENDOTOXIN DETECTED IN PERIPHERAL BLOOD PLASMA STIMULATE HOST CELLS?
A diverse repertoire of inactivation mechanisms modulates the ability of extracellular LPS to signal cells via MD-2–TLR4 [111]. The inactivation mechanisms operate on mucosal surfaces and in tissues, lymph, and blood, and they may profoundly influence LPS bioactivity, LPS detection in vivo, and the interpretation of plasma LPS assay results. They make it difficult to know with certainty that the endotoxin detected in plasma is bioactive in vivo.
Enzymatic inactivation
Host enzymes can remove the 2 decisive signaling structures from lipid A: the phosphates on the diglucosamine backbone and the secondary acyl chains (Fig. 2D). Alkaline phosphatase(s) produced in the small intestine and liver may have dephosphorylated LPS in several model systems [112]. In addition to a macrophage acid phosphatase that dephosphorylates lipid A [113] and a lipid A phosphatase in monocytes [114], most vertebrates produce a lipase, AOAH, that removes the secondary acyl chains from lipid A (Fig. 2D, arrows). Made by neutrophils, monocyte-macrophages (including Kupffer cells [115]), dendritic cells, NK cells, and renal proximal tubule cells, AOAH acts both intracellularly and in inflammatory fluids [116, 117] to inactivate LPS. When a few micrograms of LPS were injected intraperitoneally in mice that lacked AOAH, the LPS remained bioactive for many weeks; the inability of the animals’ alkaline phosphatases to inactivate the LPS is unexplained [118].
AOAH treatment produces LPS with a tetraacyl lipid A moiety (Fig. 2D) that is ∼100-fold less stimulatory to human cells than is fully acylated LPS. In addition, the partially deacylated LPS can compete with LPS for binding LBP, soluble CD14, and/or MD-2–TLR4 [119]; in substoichiometric concentrations, it inhibited LPS activation of human macrophages in vitro [120]. AOAH-treated LPS was also much less stimulatory than untreated LPS in the LAL (see above), so LPS that has been inactivated by AOAH in vivo should not contribute to LAL reactivity in plasma. The 3-OH-14:0 content of the partially deacylated molecules is not reduced, however, and the molecules may still form antibody–LPS complexes. AOAH- or phosphatase-inactivated LPS may thus be detectable in both the EAA and 3-OH-14:0 assays.
Inhibition in lymph
Lemaire et al. [121] found that thoracic duct lymph inhibits responses to LPS by normal PBMCs. Plasma and lymph from patients with systemic signs of inflammation were more inhibitory than were plasma and lymph from control patients. LPS injected into a subcutaneous site can remain there for many weeks [122]; the slower its movement to draining lymph nodes, the more likely that the LPS that arrives in the nodes will be partially deacylated by AOAH, before or after it leaves the site of inoculation [13].
Inhibition in plasma
By far, the most important mechanism for inactivating blood-borne LPS, at least transiently, is binding to circulating lipoproteins. Whereas binding to HDL and LDL prevents LPS from signaling, the LPS bound to chylomicrons and VLDL (both triglyceride-rich) may inhibit proinflammatory responses in hepatocytes and vascular endothelial cells [123, 124].
Although LPS can bind to monocytes in vitro and stimulate them more rapidly than it can bind to HDL or LDL [39, 125, 126], Kitchens et al. [126, 127] found that plasma CD14 can quickly remove LPS from the surfaces of monocytes and transfer it to HDL, in keeping with the strong association of CD14 with ENA (see above) in the postoperative patients studied by Hiki and colleagues [128]. The role of soluble CD14 in limiting LPS signaling may not be limited to its ability to strip LPS from cell surfaces: Gioannini et al. [129] found that it and LBP can also bind LPS in a way that increases LPS deacylation by AOAH. High levels of LBP can also strip LPS from cells and prevent signaling, and other plasma proteins may also bind LPS and modulate its bioactivity [54]. Bactericidal-permeability protein binds the LPS produced by many Gram-negative bacteria and blocks lipid A bioactivity [130, 131], including its ability to activate LAL [132]. Gelsolin, a highly conserved plasma protein, binds LPS and neutralizes its stimulatory potency [133], as do lactoferrin [134], albumin, and other plasma proteins. Antiendotoxin antibodies may bind O-antigen, core, or lipid A moieties, and the resulting immune complexes are probably cleared rapidly from the blood; the antibodies can neutralize LPS [135], and as noted above, plasma anti-core LPS antibody levels have been used as an indirect assay for endotoxemia (EndoCab). There are also proteins (most notably, high-mobility group box 1) [136] that can enhance cellular responsiveness to LPS [54]; the extent to which these proteins influence the stimulatory potency of LPS in plasma is not known.
Stress-induced inhibition
Plasma endotoxin levels are often measured in infected or otherwise sick patients whose stress responses may influence the outcome and interpretation of the assays.
When Brandtzaeg and coworkers [137] exposed naïve human monocytes to patient plasma that contained very large amounts of meningococcal LPS, they found that the cells responded very weakly, even when additional LPS was added. Adsorbing IL-10 and IL-4 from the plasma partially restored responsiveness, and in a follow-up study they found that IL-10 could account for much of the inhibition [138]. Another modulator is innate immune (“endotoxin”) tolerance, the reprogramming of monocytes and other cells to reduce most proinflammatory responses to LPS while maintaining or increasing certain modulatory ones [139]. Sepsis-induced modulation of neutrophil function [140, 141] might reduce the burst of chemiluminescence used to monitor the response in the EAA.
HDL levels typically decrease in the plasma and lymph of septic individuals [142], but LPS–lipoprotein binding increases nonetheless; in hypertriglyceridemic serum from septic patients, LPS bound mainly to LDL and also to VLDL [143]. At the high plasma concentrations reached in sick patients [144], LBP may inhibit cellular responses to LPS [145], even after the LPS has bound host cells [146] (LBP can also prevent LPS from activating LAL [132]). Soluble CD14 levels may also increase, potentially stripping LPS from cell surfaces and transferring it to lipoproteins [126]. Both LPS removal from the cell surface and LPS transfer to lipoproteins occurred more rapidly when monocytes were incubated with serum from septic patients than with normal human serum [126], and serum from septic patients reduced the stimulatory LPS–monocyte interaction by as much as 10-fold. The 2 plasma proteins that figure most prominently in LPS sensing—CD14 and LBP—thus may help prevent or reduce responses to LPS in ill individuals.
Given that there are numerous host mechanisms for enzymatically inactivating lipid A, sequestering lipid A so that it is unable to signal and inhibiting signaling downstream of TLR4—it seems quite possible that most of the LPS detected in peripheral blood plasma is not stimulatory. Is blood-borne LPS bioactive when it leaves the blood? Harris and colleagues [124] noted that LPS bound to triglyceride-rich lipoproteins can modulate endothelial cell and hepatocyte responses to cytokines, and Navab et al. [147] found that LPS bound to LDL could transit an endothelial cell monolayer and stimulate rabbit aortic smooth muscle cells. It is important to find out more about how LPSs interact with cells when they leave the blood—both during the “first pass” after entering the circulation and after remaining in the blood long enough to be found in a peripheral vein.
CONCLUSIONS
An ideal assay for plasma endotoxin should only detect LPS that can stimulate host cells, and it should be sufficiently sensitive to detect small amounts of LPS in plasma, quantitative and well standardized, reproducible, easy to perform, inexpensive, and rapid. None of the available assays meets all of these criteria, but each of them may be useful in certain circumstances.
If LPS is found in plasma, it is likely that other bacterial molecules are also present. It is impossible to know with certainty which of these, if any, is responsible for inducing metabolic abnormalities, organ hypofunction, or other systemic phenomena. Investigators have measured blood levels of bacterial DNA [17], 16S rRNA DNA [148, 149], peptidoglycan [150–152], Braun’s lipoprotein [7], and other molecules. As part of a battery of such tests, the LAL assay or measurement of 3-OH-14:0 may help confirm bacterial molecule translocation. More convincing evidence for host-stimulatory LPS would be the detection of LPS equivalents (TLR4 agonists) using transfected reporter cells. The LPS produced by a particular intestinal bacterium might be detected in plasma using the immunolimulus assay.
The EAA seems better suited for assessing the activation state of the patient’s neutrophils than for diagnosing endotoxemia. Nonspecific binding of the E5 IgM to host antigens and stress- or sepsis-induced neutrophil priming or tolerance are very difficult to exclude. Assays that quantitate the presence of 3-OH-14:0 are less sensitive than the others and are mainly useful for documenting or confirming the presence of the lipid A backbone; the detection of 3-OH-14:0 does not reveal the status of the lipid A phosphates and secondary acyl chains that are required to stimulate human cells via TLR4.
The LPS molecules produced by bacteria, the LPSs used for assay standards, and the LPSs in plasma are all heterogeneous. This heterogeneity greatly limits the interpretation of quantitative endotoxin assays and often precludes accurate comparisons of endotoxin levels among individuals [51]. An even more vexing problem is the possibility that stimulatory and nonstimulatory (or even inhibitory) LPS molecules coexist in plasma [8, 11]. None of the available assays can distinguish these LPSs in blood or indicate their relative abundance and stimulatory potency.
Although it is reasonable to think that most of the LPS detected in peripheral blood plasma does not stimulate blood cells, stimulatory LPS molecules may have already been cleared from the circulation. In addition, LPS molecules that are bound to lipoproteins or other inhibitors in the blood may be bioactive after they leave the circulation and encounter cells in tissues. This is a ripe area for further research.
Finding endotoxin in plasma is not always a mistake (concordant results using independent assays make this unlikely), but knowing its significance is often difficult. Plasma LPS can be a marker for the presence of Gram-negative bacteria in tissue or blood or for bacterial translocation, but none of the available assays shows conclusively that the LPS molecules detected in plasma are able to stimulate host cells in vivo. Despite decades of study, how this endotoxin contributes to clinical phenomena remains for future workers to discover.
AUTHORSHIP
R.S.M. did the literature search and wrote the paper.
ACKNOWLEDGMENTS
This work was supported by the Division of Intramural Research, National Institute for Allergy and Infectious Diseases, U.S. National Institutes of Health. The author thanks Klaus Brandenburg, Anthony Suffredini, Jack Levin, Jason Brenchley, Clett Erridge, and Alan Cross for helpful suggestions.
Glossary
- 3-OH-12:0
3-hydroxylaurate
- 3-OH-14:0
3-hydroxymyristate
- AOAH
acyloxyacyl hydrolase
- EAA
endotoxin activity assay
- ENA
endotoxin neutralization assay
- GI
gastrointestinal
- LAL
Limulus amebocyte lysate
- LBP
LPS-binding protein
- MD-2
myeloid differentiation 2 (lymphocyte antigen 96)
- MS
mass spectrometry
DISCLOSURES
There are no conflicts of interest.
REFERENCES
- 1.Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. (1989) The cardiovascular response of normal humans to the administration of endotoxin. N. Engl. J. Med. 321, 280–287. [DOI] [PubMed] [Google Scholar]
- 2.Marshall J. C. (2005) Lipopolysaccharide: an endotoxin or an exogenous hormone? Clin. Infect. Dis. 41 (Suppl 7), S470–S480. [DOI] [PubMed] [Google Scholar]
- 3.Hurley J. C., Nowak P., Öhrmalm L., Gogos C., Armaganidis A., Giamarellos-Bourboulis E. J. (2015) Endotoxemia as a diagnostic tool for patients with suspected bacteremia caused by gram-negative organisms: a meta-analysis of 4 decades of studies. J. Clin. Microbiol. 53, 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opal S. M., Scannon P. J., Vincent J. L., White M., Carroll S. F., Palardy J. E., Parejo N. A., Pribble J. P., Lemke J. H. (1999) Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 180, 1584–1589. [DOI] [PubMed] [Google Scholar]
- 5.Shands J. W. Jr.,Chun P. W. (1980) The dispersion of gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J. Biol. Chem. 255, 1221–1226. [PubMed] [Google Scholar]
- 6.Schwechheimer C., Kuehn M. J. (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellman J., Roberts J. D. Jr.,Tehan M. M., Allaire J. E., Warren H. S. (2002) Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J. Biol. Chem. 277, 14274–14280. [DOI] [PubMed] [Google Scholar]
- 8.Magnuson D. K., Weintraub A., Pohlman T. H., Maier R. V. (1989) Human endothelial cell adhesiveness for neutrophils, induced by Escherichia coli lipopolysaccharide in vitro, is inhibited by Bacteroides fragilis lipopolysaccharide. J. Immunol. 143, 3025–3030. [PubMed] [Google Scholar]
- 9.Yoshimura A., Kaneko T., Kato Y., Golenbock D. T., Hara Y. (2002) Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human Toll-like receptor 4. Infect. Immun. 70, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poxton I. R., Edmond D. M. (1995) Biological activity of Bacteroides lipopolysaccharide–reappraisal. Clin. Infect. Dis. 20 (Suppl 2), S149–S153. [DOI] [PubMed] [Google Scholar]
- 11.Slocum C., Coats S. R., Hua N., Kramer C., Papadopoulos G., Weinberg E. O., Gudino C. V., Hamilton J. A., Darveau R. P., Genco C. A. (2014) Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 10, e1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett I. L. Jr.,Beeson P. B. (1954) Bacteremia: a consideration of some experimental and clinical aspects. Yale J. Biol. Med. 26, 241–262. [PMC free article] [PubMed] [Google Scholar]
- 13.Lu M., Munford R. S. (2011) The transport and inactivation kinetics of bacterial lipopolysaccharide influence its immunological potency in vivo. J. Immunol. 187, 3314–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olofsson P., Nylander G., Olsson P. (1986) Endotoxin: routes of transport in experimental peritonitis. Am. J. Surg. 151, 443–446. [DOI] [PubMed] [Google Scholar]
- 15.Brandtzaeg P., Bryn K., Kierulf P., Ovstebø R., Namork E., Aase B., Jantzen E. (1992) Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Invest. 89, 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coureuil M., Bourdoulous S., Marullo S., Nassif X. (2014) Invasive meningococcal disease: a disease of the endothelial cells. Trends Mol. Med. 20, 571–578. [DOI] [PubMed] [Google Scholar]
- 17.Øvstebø R., Brandtzaeg P., Brusletto B., Haug K. B., Lande K., Høiby E. A., Kierulf P. (2004) Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J. Clin. Microbiol. 42, 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson A. J., Opal S. M., Angus B. J., Prins J. M., Palardy J. E., Parejo N. A., Chaowagul W., White N. J. (2000) Differential antibiotic-induced endotoxin release in severe melioidosis. J. Infect. Dis. 181, 1014–1019. [DOI] [PubMed] [Google Scholar]
- 19.Erridge C. (2011) The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll-like receptors 2 and 4. Br. J. Nutr. 105, 15–23. [DOI] [PubMed] [Google Scholar]
- 20.Ghoshal S., Witta J., Zhong J., de Villiers W., Eckhardt E. (2009) Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50, 90–97. [DOI] [PubMed] [Google Scholar]
- 21.Van Deventer S. J., ten Cate J. W., Tytgat G. N. (1988) Intestinal endotoxemia. Clinical significance. Gastroenterology 94, 825–831. [DOI] [PubMed] [Google Scholar]
- 22.Erridge C., Attina T., Spickett C. M., Webb D. J. (2007) A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 86, 1286–1292. [DOI] [PubMed] [Google Scholar]
- 23.Van Deventer S. J., Knepper A., Landsman J., Lawson J., ten Cate J. W., Buller H. R., Sturk A., Pauw W. (1988) Endotoxins in portal blood. Hepatogastroenterology 35, 223–225. [PubMed] [Google Scholar]
- 24.Erridge C. (2011) Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis 216, 1–6. [DOI] [PubMed] [Google Scholar]
- 25.Moore F. A., Moore E. E., Poggetti R., McAnena O. J., Peterson V. M., Abernathy C. M., Parsons P. E. (1991) Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J. Trauma 31, 629–636, discussion 636–638. [DOI] [PubMed] [Google Scholar]
- 26.MacFie J., O’Boyle C., Mitchell C. J., Buckley P. M., Johnstone D., Sudworth P. (1999) Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 45, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deitch E. A. (2012) Gut-origin sepsis: evolution of a concept. Surgeon 10, 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenchley J. M., Price D. A., Schacker T. W., Asher T. E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B. R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J. N., Hecht F. M., Picker L. J., Lederman M. M., Deeks S. G., Douek D. C. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371. [DOI] [PubMed] [Google Scholar]
- 29.Redd A. D., Dabitao D., Bream J. H., Charvat B., Laeyendecker O., Kiwanuka N., Lutalo T., Kigozi G., Tobian A. A., Gamiel J., Neal J. D., Oliver A. E., Margolick J. B., Sewankambo N., Reynolds S. J., Wawer M. J., Serwadda D., Gray R. H., Quinn T. C. (2009) Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc. Natl. Acad. Sci. USA 106, 6718–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herieka M., Erridge C. (2014) High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 58, 136–146. [DOI] [PubMed] [Google Scholar]
- 31.Spadoni I., Zagato E., Bertocchi A., Paolinelli R., Hot E., Di Sabatino A., Caprioli F., Bottiglieri L., Oldani A., Viale G., Penna G., Dejana E., Rescigno M. (2015) A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834. [DOI] [PubMed] [Google Scholar]
- 32.Erridge C., Spickett C. M., Webb D. J. (2007) Non-enterobacterial endotoxins stimulate human coronary artery but not venous endothelial cell activation via Toll-like receptor 2. Cardiovasc. Res. 73, 181–189. [DOI] [PubMed] [Google Scholar]
- 33.Erridge C. (2011) Accumulation of stimulants of Toll-like receptor (TLR)-2 and TLR4 in meat products stored at 5 °C. J. Food Sci. 76, H72–H79. [DOI] [PubMed] [Google Scholar]
- 34.DIABIMMUNE Study Group (2016) Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 1551. [DOI] [PubMed] [Google Scholar]
- 35.Suffredini A. F., Noveck R. J. (2014) Human endotoxin administration as an experimental model in drug development. Clin. Pharmacol. Ther. 96, 418–422. [DOI] [PubMed] [Google Scholar]
- 36.Bahador M., Cross A. S. (2007) From therapy to experimental model: a hundred years of endotoxin administration to human subjects. J. Endotoxin Res. 13, 251–279. [DOI] [PubMed] [Google Scholar]
- 37.Munford R. S. (2006) Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu. Rev. Pathol. 1, 467–496. [DOI] [PubMed] [Google Scholar]
- 38.Takeshita S., Nakatani K., Tsujimoto H., Kawamura Y., Sekine I. (2000) Detection of circulating lipopolysaccharide-bound monocytes in children with gram-negative sepsis. J. Infect. Dis. 182, 1549–1552. [DOI] [PubMed] [Google Scholar]
- 39.Roth R. I., Levin F. C., Levin J. (1993) Distribution of bacterial endotoxin in human and rabbit blood and effects of stroma-free hemoglobin. Infect. Immun. 61, 3209–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salden H. J., Bas B. M. (1994) Endotoxin binding to platelets in blood from patients with a sepsis syndrome. Clin. Chem. 40, 1575–1579. [PubMed] [Google Scholar]
- 41.Ståhl A. L., Svensson M., Mörgelin M., Svanborg C., Tarr P. I., Mooney J. C., Watkins S. L., Johnson R., Karpman D. (2006) Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood 108, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pöschl J. M. B., Leray C., Ruef P., Cazenave J. P., Linderkamp O. (2003) Endotoxin binding to erythrocyte membrane and erythrocyte deformability in human sepsis and in vitro. Crit. Care Med. 31, 924–928. [DOI] [PubMed] [Google Scholar]
- 43.Vesy C. J., Kitchens R. L., Wolfbauer G., Albers J. J., Munford R. S. (2000) Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from gram-negative bacterial membranes. Infect. Immun. 68, 2410–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. (1982) Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J. Clin. Invest. 70, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levels J. H., Abraham P. R., van den Ende A., van Deventer S. J. (2001) Distribution and kinetics of lipoprotein-bound endotoxin. Infect. Immun. 69, 2821–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida M., Roth R. I., Levin J. (1995) The effect of cell-free hemoglobin on intravascular clearance and cellular, plasma, and organ distribution of bacterial endotoxin in rabbits. J. Lab. Clin. Med. 126, 151–160. [PubMed] [Google Scholar]
- 47.Munford R. S., Andersen J. M., Dietschy J. M. (1981) Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J. Clin. Invest. 68, 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao B., Munford R. S., Kitchens R., Varley A. W. (2012) Hepatic uptake and deacylation of the LPS in bloodborne LPS-lipoprotein complexes. Innate Immun. 18, 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triger D. R., Boyer T. D., Levin J. (1978) Portal and systemic bacteraemia and endotoxaemia in liver disease. Gut 19, 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Findlay L., Desai T., Heath A., Poole S., Crivellone M., Hauck W, Ambrose M., Morris T., Daas A., Rautmann G., Buchheit K. H., Spieser J. M., Terao E. (2015) Collaborative study for the establishment of the WHO 3(rd) International Standard for Endotoxin, the Ph. Eur. endotoxin biological reference preparation batch 5 and the USP Reference Standard for Endotoxin Lot H0K354. Pharmeur. Bio. Sci. Notes 2015, 73–98. [PubMed] [Google Scholar]
- 51.Boutagy N. E., McMillan R. P., Frisard M. I., Hulver M. W. (2016) Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 124, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin J., Tomasulo P. A., Oser R. S. (1970) Detection of endotoxin in human blood and demonstration of an inhibitor. J. Lab. Clin. Med. 75, 903–911. [PubMed] [Google Scholar]
- 53.Tsan M. F., Gao B. (2007) Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J. Endotoxin Res. 13, 6–14. [DOI] [PubMed] [Google Scholar]
- 54.Erridge C. (2010) Endogenous ligands of TLR2 and TLR4: agonists or assistants? J. Leukoc. Biol. 87, 989–999. [DOI] [PubMed] [Google Scholar]
- 55.Gnauck A., Lentle R. G., Kruger M. C. (2016) Chasing a ghost?—Issues with the determination of circulating levels of endotoxin in human blood. Crit. Rev. Clin. Lab. Sci. 53, 197–215. [DOI] [PubMed] [Google Scholar]
- 56.Levin J., Poore T. E., Zauber N. P., Oser R. S. (1970) Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N. Engl. J. Med. 283, 1313–1316. [DOI] [PubMed] [Google Scholar]
- 57.Kambayashi J., Yokota M., Sakon M., Shiba E., Kawasaki T., Mori T., Tsuchiya M., Oishi H., Matsuura S. (1991) A novel endotoxin-specific assay by turbidimetry with Limulus amoebocyte lysate containing beta-glucan. J. Biochem. Biophys. Methods 22, 93–100. [DOI] [PubMed] [Google Scholar]
- 58.Ding J. L., Ho B. (2010) Endotoxin detection—from Limulus amebocyte lysate to recombinant factor C. Subcell. Biochem. 53, 187–208. [DOI] [PubMed] [Google Scholar]
- 59.Gnauck A., Lentle R. G., Kruger M. C. (2015) The Limulus amebocyte lysate assay may be unsuitable for detecting endotoxin in blood of healthy female subjects. J. Immunol. Methods 416, 146–156. [DOI] [PubMed] [Google Scholar]
- 60.Gutsmann T., Howe J., Zähringer U., Garidel P., Schromm A. B., Koch M. H., Fujimoto Y., Fukase K., Moriyon I., Martínez-de-Tejada G., Brandenburg K. (2010) Structural prerequisites for endotoxic activity in the Limulus test as compared to cytokine production in mononuclear cells. Innate Immun. 16, 39–47. [DOI] [PubMed] [Google Scholar]
- 61.Brandenburg K., Howe J., Gutsman T., Garidel P. (2009) The expression of endotoxic activity in the Limulus test as compared to cytokine production in immune cells. Curr. Med. Chem. 16, 2653–2660. [DOI] [PubMed] [Google Scholar]
- 62.Schromm A. B., Brandenburg K., Loppnow H., Moran A. P., Koch M. H. J., Rietschel E. T., Seydel U. (2000) Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267, 2008–2013. [DOI] [PubMed] [Google Scholar]
- 63.Takada H., Kotani S., Tanaka S., Ogawa T., Takahashi I., Tsujimoto M., Komuro T., Shiba T., Kusumoto S., Kusunose N., Hasegawa A., Kiso M. (1988) Structural requirements of lipid A species in activation of clotting enzymes from the horseshoe crab, and the human complement cascade. Eur. J. Biochem. 175, 573–580. [DOI] [PubMed] [Google Scholar]
- 64.Dehus O., Hartung T., Hermann C. (2006) Endotoxin evaluation of eleven lipopolysaccharides by whole blood assay does not always correlate with Limulus amebocyte lysate assay. J. Endotoxin Res. 12, 171–180. [DOI] [PubMed] [Google Scholar]
- 65.Laude-Sharp M., Haeffner-Cavaillon N., Caroff M., Lantreibecq F., Pusineri C., Kazatchkine M. D. (1990) Dissociation between the interleukin 1-inducing capacity and Limulus reactivity of lipopolysaccharides from gram-negative bacteria. Cytokine 2, 253–258. [DOI] [PubMed] [Google Scholar]
- 66.Erwin A. L., Mandrell R. E., Munford R. S. (1991) Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect. Immun. 59, 1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erwin A. L. (1990) Studies of bacterial lipopolysaccharides and of a leukocyte lipopolysaccharide-deacylating enzyme, acyloxyacyl hydrolase. Ph.D. dissertation, University of Texas Southwestern Medical Center at Dallas. [Google Scholar]
- 68.Butler T., Möller G. (1977) Mitogenic response of mouse spleen cells and gelation of limulus lysate by lipopolysaccharide of Yersinia pestis and evidence for neutralization of lipopolysaccharide by polymyxin B. Infect. Immun. 18, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butler T., Levin J., Linh N. N., Chau D. M., Adickman M., Arnold K. (1976) Yersinia pestis infection in Vietnam. II. Quantiative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J. Infect. Dis. 133, 493–499. [DOI] [PubMed] [Google Scholar]
- 70.Butler T., Bell W. R., Levin J., Linh N. N., Arnold K. (1978) Typhoid fever. Studies of blood coagulation, bacteremia, and endotoxemia. Arch. Intern. Med. 138, 407–410. [DOI] [PubMed] [Google Scholar]
- 71.Trautmann M., Scheibe C., Wellinghausen N., Holst O., Lepper P. M. (2010) Low endotoxin release from Escherichia coli and Bacteroides fragilis during exposure to moxifloxacin. Chemotherapy 56, 364–370. [DOI] [PubMed] [Google Scholar]
- 72.Katz J., Zhang P., Martin M., Vogel S. N., Michalek S. M. (2006) Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 74, 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munford R. S., Hall C. L. (1979) Radioimmunoassay for Gram-negative bacterial lipopolysaccharide O antigens: influence of antigen solubility. Infect. Immun. 26, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs D. M., Gutowski J. A. (1982) Solid-phase radioimmunoassay for bacterial lipopolysaccharide. Methods Enzymol. 84, 264–272. [DOI] [PubMed] [Google Scholar]
- 75.Munford R. S., Hall C. L. (1987) A comparison of two quantitative assays for bacterial lipopolysaccharides. Prog. Clin. Biol. Res. 231, 93–102. [PubMed] [Google Scholar]
- 76.Munford R. S., Hall C. L., Grimm L. (1984) Detection of free endotoxin in cerebrospinal fluid by the Limulus lysate test. Infect. Immun. 45, 531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mertsola J., Cope L. D., Munford R. S., McCracken G. H. Jr.,Hansen E. J. (1991) Detection of experimental Haemophilus influenzae type b bacteremia and endotoxemia by means of an immunolimulus assay. J. Infect. Dis. 164, 353–358. [DOI] [PubMed] [Google Scholar]
- 78.Müller-Loennies S., Brade L., MacKenzie C. R., Di Padova F. E., Brade H. (2003) Identification of a cross-reactive epitope widely present in lipopolysaccharide from enterobacteria and recognized by the cross-protective monoclonal antibody WN1 222-5. J. Biol. Chem. 278, 25618–25627. [DOI] [PubMed] [Google Scholar]
- 79.Gomery K., Müller-Loennies S., Brooks C. L., Brade L., Kosma P., Di Padova F., Brade H., Evans S. V. (2012) Antibody WN1 222-5 mimics Toll-like receptor 4 binding in the recognition of LPS. Proc. Natl. Acad. Sci. USA 109, 20877–20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saxen H., Vuopio-Varkila J., Luk J., Lindberg A., Lang A., Di Padova F., Cryz S. J. Jr, Mertsola J., McCracken G. H. Jr.,Hansen E. J. (1993) Detection of enterobacterial lipopolysaccharides and experimental endotoxemia by means of an immunolimulus assay using both serotype-specific and cross-reactive antibodies. J. Infect. Dis. 168, 393–399. [DOI] [PubMed] [Google Scholar]
- 81.Romaschin A. D., Harris D. M., Ribeiro M. B., Paice J., Foster D. M., Walker P. M., Marshall J. C. (1998) A rapid assay of endotoxin in whole blood using autologous neutrophil dependent chemiluminescence. J. Immunol. Methods 212, 169–185. [DOI] [PubMed] [Google Scholar]
- 82.Romaschin A. D., Klein D. J., Marshall J. C. (2012) Bench-to-bedside review: clinical experience with the endotoxin activity assay. Crit. Care 16, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warren H. S., Amato S. F., Fitting C., Black K. M., Loiselle P. M., Pasternack M. S., Cavaillon J.-M. (1993) Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J. Exp. Med. 177, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhat N. M., Bieber M. M., Chapman C. J., Stevenson F. K., Teng N. N. H. (1993) Human antilipid A monoclonal antibodies bind to human B cells and the i antigen on cord red blood cells. J. Immunol. 151, 5011–5021. [PubMed] [Google Scholar]
- 85.Matsumoto N., Takahashi G., Kojika M., Suzuki Y., Inoue Y., Inada K., Endo S. (2013) Interleukin-8 induces an elevation in the endotoxin activity assay (EAA) level: does the EAA truly measure the endotoxin level? J. Infect. Chemother. 19, 825–832. [DOI] [PubMed] [Google Scholar]
- 86.Erridge C., Duncan S. H., Bereswill S., Heimesaat M. M. (2010) The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS One 5, e9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W. Z., Strowig T., Thaiss C. A., Kau A. L., Eisenbarth S. C., Jurczak M. J., Camporez J. P., Shulman G. I., Gordon J. I., Hoffman H. M., Flavell R. A. (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chassaing B., Koren O., Goodrich J. K., Poole A. C., Srinivasan S., Ley R. E., Gewirtz A. T. (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varley A. W., Geiszler S. M., Gaynor R. B., Munford R. S. (1997) A two-component expression system that responds to inflammatory stimuli in vivo. Nat. Biotechnol. 15, 1002–1006. [DOI] [PubMed] [Google Scholar]
- 90.Maitra S. K., Schotz M. C., Yoshikawa T. T., Guze L. B. (1978) Determination of lipid A and endotoxin in serum by mass spectroscopy. Proc. Natl. Acad. Sci. USA 75, 3993–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szponar B., Kraśnik L., Hryniewiecki T., Gamian A., Larsson L. (2003) Distribution of 3-hydroxy fatty acids in tissues after intraperitoneal injection of endotoxin. Clin. Chem. 49, 1149–1153. [DOI] [PubMed] [Google Scholar]
- 92.Pais de Barros J. P., Gautier T., Sali W., Adrie C., Choubley H., Charron E., Lalande C., Le Guern N., Deckert V., Monchi M., Quenot J. P., Lagrost L. (2015) Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the Limulus amebocyte lysate assay. J. Lipid Res. 56, 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Danner R. L., Elin R. J., Hosseini J. M., Wesley R. A., Reilly J. M., Parillo J. E. (1991) Endotoxemia in human septic shock. Chest 99, 169–175. [DOI] [PubMed] [Google Scholar]
- 94.Gunti S., Notkins A. L. (2015) Polyreactive antibodies: function and quantification. J. Infect. Dis. 212 (Suppl 1), S42–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.The Sepsis Intervention Group (1995) Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. JAMA 274, 172–177. [PubMed] [Google Scholar]
- 96.Al-Bahrani A. Z., Darwish A., Hamza N., Benson J., Eddleston J. M., Snider R. H., Nylén E. S., Becker K. L., Barclay G. R., Ammori B. J. (2010) Gut barrier dysfunction in critically ill surgical patients with abdominal compartment syndrome. Pancreas 39, 1064–1069. [DOI] [PubMed] [Google Scholar]
- 97.Barclay G. R. (1995) Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog. Clin. Biol. Res. 392, 263–272. [PubMed] [Google Scholar]
- 98.Bennett-Guerrero E., Barclay G. R., Youssef M. E., Hossain S., Vela-Cantos F., Andres L. A., Poxton I. R. (2000) Exposure to Bacteroides fragilis endotoxin during cardiac surgery. Anesth. Analg. 90, 819–823. [DOI] [PubMed] [Google Scholar]
- 99.Allan E., Poxton I. R., Barclay G. R. (1995) Anti-Bacteroides lipopolysaccharide IgG levels in healthy adults and sepsis patients. FEMS Immunol. Med. Microbiol. 11, 5–12. [DOI] [PubMed] [Google Scholar]
- 100.Hawkesworth S., Moore S. E., Fulford A. J., Barclay G. R., Darboe A. A., Mark H., Nyan O. A., Prentice A. M. (2013) Evidence for metabolic endotoxemia in obese and diabetic Gambian women. Nutr. Diabetes 3, e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennett-Guerrero E., Barclay G. R., Weng P. L., Bodian C. A., Feierman D. E., Vela-Cantos F., Mythen M. G. (2001) Endotoxin-neutralizing capacity of serum from cardiac surgical patients. J. Cardiothorac. Vasc. Anesth. 15, 451–454. [DOI] [PubMed] [Google Scholar]
- 102.Warren H. S., Novitsky T. J., Ketchum P. A., Roslansky P. F., Kania S., Siber G. R. (1985) Neutralization of bacterial lipopolysaccharides by human plasma. J. Clin. Microbiol. 22, 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bölke E., Jehle P. M., Storck M., Nothnagel B., Stanescu A., Orth K. (2001) Endotoxin release and endotoxin neutralizing capacity during colonoscopy. Clin. Chim. Acta 303, 49–53. [DOI] [PubMed] [Google Scholar]
- 104.Champion K., Chiu L., Ferbas J., Pepe M. (2013) Endotoxin neutralization as a biomonitor for inflammatory bowel disease. PLoS One 8, e67736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marchetti G., Tincati C., Silvestri G. (2013) Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 26, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandler N. G., Koh C., Roque A., Eccleston J. L., Siegel R. B., Demino M., Kleiner D. E., Deeks S. G., Liang T. J., Heller T., Douek D. C. (2011) Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 141, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bas S., Gauthier B. R., Spenato U., Stingelin S., Gabay C. (2004) CD14 is an acute-phase protein. J. Immunol. 172, 4470–4479. [DOI] [PubMed] [Google Scholar]
- 108.Meuleman P., Steyaert S., Libbrecht L., Couvent S., Van Houtte F., Clinckspoor F., de Hemptinne B., Roskams T., Vanlandschoot P., Leroux-Roels G. (2006) Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin. Chim. Acta 366, 156–162. [DOI] [PubMed] [Google Scholar]
- 109.Bastian D., Tamburstuen M. V., Lyngstadaas S. P., Reikerås O. (2011) LBP and sCD14 patterns in total hip replacement surgery performed during combined spinal/epidural anaesthesia. Scand. J. Clin. Lab. Invest. 71, 486–491. [DOI] [PubMed] [Google Scholar]
- 110.Møller A. S., Bjerre A., Brusletto B., Joø G. B., Brandtzaeg P., Kierulf P. (2005) Chemokine patterns in meningococcal disease. J. Infect. Dis. 191, 768–775. [DOI] [PubMed] [Google Scholar]
- 111.Munford R. S. (2005) Detoxifying endotoxin: time, place and person. J. Endotoxin Res. 11, 69–84. [DOI] [PubMed] [Google Scholar]
- 112.Chen K. T., Malo M. S., Beasley-Topliffe L. K., Poelstra K., Millan J. L., Mostafa G., Alam S. N., Ramasamy S., Warren H. S., Hohmann E. L., Hodin R. A. (2011) A role for intestinal alkaline phosphatase in the maintenance of local gut immunity. Dig. Dis. Sci. 56, 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peterson A. A., Munford R. S. (1987) Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect. Immun. 55, 974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hampton R. Y., Raetz C. R. H. (1991) Macrophage catabolism of lipid A is regulated by endotoxin stimulation. J. Biol. Chem. 266, 19499–19509. [PubMed] [Google Scholar]
- 115.Shao B., Lu M., Katz S. C., Varley A. W., Hardwick J., Rogers T. E., Ojogun N., Rockey D. C., Dematteo R. P., Munford R. S. (2007) A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen. J. Biol. Chem. 282, 13726–13735. [DOI] [PubMed] [Google Scholar]
- 116.Katz S. S., Weinrauch Y., Munford R. S., Elsbach P., Weiss J. (1999) Deacylation of lipopolysaccharide in whole Escherichia coli during destruction by cellular and extracellular components of a rabbit inflammatory peritoneal exudate. J. Biol. Chem. 274, 36579–36584. [DOI] [PubMed] [Google Scholar]
- 117.Weinrauch Y., Katz S. S., Munford R. S., Elsbach P., Weiss J. (1999) Deacylation of purified lipopolysaccharides by cellular and extracellular components of a sterile rabbit peritoneal inflammatory exudate. Infect. Immun. 67, 3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu M., Varley A. W., Munford R. S. (2013) Persistently active microbial molecules prolong innate immune tolerance in vivo. PLoS Pathog. 9, e1003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kitchens R. L., Munford R. S. (1995) Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J. Biol. Chem. 270, 9904–9910. [DOI] [PubMed] [Google Scholar]
- 120.Kitchens R. L., Ulevitch R. J., Munford R. S. (1992) Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J. Exp. Med. 176, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lemaire L. C. J. M., van Lanschot J. J., van der Poll T., Buurman W. A., van Deventer S. J. H., Gouma D. J. (1998) Lymph of patients with a systemic inflammatory response syndrome inhibits lipopolysaccharide-induced cytokine production. J. Infect. Dis. 178, 883–886. [DOI] [PubMed] [Google Scholar]
- 122.Yokochi T., Inoue Y., Yokoo J., Kimura Y., Kato N. (1989) Retention of bacterial lipopolysaccharide at the site of subcutaneous injection. Infect. Immun. 57, 1786–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harris H. W., Grunfeld C., Feingold K. R., Read T. E., Kane J. P., Jones A. L., Eichbaum E. B., Bland G. F., Rapp J. H. (1993) Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. J. Clin. Invest. 91, 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spitzer A. L., Chuang K. I., Victorino G. P., Kasravi B., Curran B., Lee D., Harris H. W. (2011) Chylomicrons combined with endotoxin moderate microvascular permeability. Innate Immun. 17, 283–292. [DOI] [PubMed] [Google Scholar]
- 125.Netea M. G., Demacker P. N., Kullberg B. J., Jacobs L. E., Verver-Jansen T. J., Boerman O. C., Stalenhoef A. F., Van der Meer J. W. (1998) Bacterial lipopolysaccharide binds and stimulates cytokine-producing cells before neutralization by endogenous lipoproteins can occur. Cytokine 10, 766–772. [DOI] [PubMed] [Google Scholar]
- 126.Kitchens R. L., Thompson P. A., Viriyakosol S., O’Keefe G. E., Munford R. S. (2001) Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J. Clin. Invest. 108, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitchens R. L., Wolfbauer G., Albers J. J., Munford R. S. (1999) Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J. Biol. Chem. 274, 34116–34122. [DOI] [PubMed] [Google Scholar]
- 128.Hiki N., Berger D., Dentener M. A., Mimura Y., Buurman W. A., Prigl C., Seidelmann M., Tsuji E., Kaminishi M., Beger H. G. (1999) Changes in endotoxin-binding proteins during major elective surgery: important role for soluble CD14 in regulation of biological activity of systemic endotoxin. Clin. Diagn. Lab. Immunol. 6, 844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gioannini T. L., Teghanemt A., Zhang D., Prohinar P., Levis E. N., Munford R. S., Weiss J. P. (2007) Endotoxin-binding proteins modulate the susceptibility of bacterial endotoxin to deacylation by acyloxyacyl hydrolase. J. Biol. Chem. 282, 7877–7884. [DOI] [PubMed] [Google Scholar]
- 130.Elsbach P. (2000) Mechanisms of disposal of bacterial lipopolysaccharides by animal hosts. Microbes Infect. 2, 1171–1180. [DOI] [PubMed] [Google Scholar]
- 131.Elsbach P., Weiss J. (1993) The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology 187, 417–429. [DOI] [PubMed] [Google Scholar]
- 132.Dentener M. A., Von Asmuth E. J. U., Francot G. J. M., Marra M. N., Buurman W. A. (1993) Antagonistic effects of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein on lipopolysaccharide-induced cytokine release by mononuclear phagocytes. Competition for binding to lipopolysaccharide. J. Immunol. 151, 4258–4265. [PubMed] [Google Scholar]
- 133.Bucki R., Georges P. C., Espinassous Q., Funaki M., Pastore J. J., Chaby R., Janmey P. A. (2005) Inactivation of endotoxin by human plasma gelsolin. Biochemistry 44, 9590–9597. [DOI] [PubMed] [Google Scholar]
- 134.Drago-Serrano M. E., de la Garza-Amaya M., Luna J. S., Campos-Rodríguez R. (2012) Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 12, 1–9. [DOI] [PubMed] [Google Scholar]
- 135.Braun J. M., Blackwell C. C., Poxton I. R., El Ahmer O., Gordon A. E., Madani O. M., Weir D. M., Giersen S., Beuth J. (2002) Proinflammatory responses to lipo-oligosaccharide of Neisseria meningitidis immunotype strains in relation to virulence and disease. J. Infect. Dis. 185, 1431–1438. [DOI] [PubMed] [Google Scholar]
- 136.Youn J. H., Oh Y. J., Kim E. S., Choi J. E., Shin J. S. (2008) High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J. Immunol. 180, 5067–5074. [DOI] [PubMed] [Google Scholar]
- 137.Brandtzaeg P., Osnes L., Ovstebø R., Joø G. B., Westvik A.-B., Kierulf P. (1996) Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J. Exp. Med. 184, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gopinathan U., Brusletto B. S., Olstad O. K., Kierulf P., Berg J. P., Brandtzaeg P., Øvstebø R. (2015) IL-10 immunodepletion from meningococcal sepsis plasma induces extensive changes in gene expression and cytokine release in stimulated human monocytes. Innate Immun. 21, 429–449. [DOI] [PubMed] [Google Scholar]
- 139.Collins P. E., Carmody R. J. (2015) The regulation of endotoxin tolerance and its impact on macrophage activation. Crit. Rev. Immunol. 35, 293–323. [DOI] [PubMed] [Google Scholar]
- 140.Marie C., Muret J., Fitting C., Losser M. R., Payen D., Cavaillon J. M. (1998) Reduced ex vivo interleukin-8 production by neutrophils in septic and nonseptic systemic inflammatory response syndrome. Blood 91, 3439–3446. [PubMed] [Google Scholar]
- 141.Demaret J., Venet F., Friggeri A., Cazalis M.-A., Plassais J., Jallades L., Malcus C., Poitevin-Later F., Textoris J., Lepape A., Monneret G. (2015) Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J. Leukoc. Biol. 98, 1081–1090. [DOI] [PubMed] [Google Scholar]
- 142.Levels J. H., Lemaire L. C., van den Ende A. E., van Deventer S. J., van Lanschot J. J. (2003) Lipid composition and lipopolysaccharide binding capacity of lipoproteins in plasma and lymph of patients with systemic inflammatory response syndrome and multiple organ failure. Crit. Care Med. 31, 1647–1653. [DOI] [PubMed] [Google Scholar]
- 143.Kitchens R. L., Thompson P. A., Munford R. S., O’Keefe G. E. (2003) Acute inflammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J. Lipid Res. 44, 2339–2348. [DOI] [PubMed] [Google Scholar]
- 144.Froon A. H. M., Dentener M. A., Greve J. W. M., Ramsay G., Buurman W. A. (1995) Lipopolysaccharide toxicity-regulating proteins in bacteremia. J. Infect. Dis. 171, 1250–1257. [DOI] [PubMed] [Google Scholar]
- 145.Hamann L., Alexander C., Stamme C., Zähringer U., Schumann R. R. (2005) Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect. Immun. 73, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thompson P. A., Tobias P. S., Viriyakosol S., Kirkland T. N., Kitchens R. L. (2003) Lipopolysaccharide (LPS)-binding protein inhibits responses to cell-bound LPS. J. Biol. Chem. 278, 28367–28371. [DOI] [PubMed] [Google Scholar]
- 147.Navab M., Hough G. P., Van Lenten B. J., Berliner J. A., Fogelman A. M. (1988) Low density lipoproteins transfer bacterial lipopolysaccharides across endothelial monolayers in a biologically active form. J. Clin. Invest. 81, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermúdez-Humarán L. G., Smirnova N., Bergé M., Sulpice T., Lahtinen S., Ouwehand A., Langella P., Rautonen N., Sansonetti P. J., Burcelin R. (2011) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 3, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang W., Lederman M. M., Hunt P., Sieg S. F., Haley K., Rodriguez B., Landay A., Martin J., Sinclair E., Asher A. I., Deeks S. G., Douek D. C., Brenchley J. M. (2009) Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 199, 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shimizu T., Tani T., Endo Y., Hanasawa K., Tsuchiya M., Kodama M. (2002) Elevation of plasma peptidoglycan and peripheral blood neutrophil activation during hemorrhagic shock: plasma peptidoglycan reflects bacterial translocation and may affect neutrophil activation. Crit. Care Med. 30, 77–82. [DOI] [PubMed] [Google Scholar]
- 151.Clarke T. B., Davis K. M., Lysenko E. S., Zhou A. Y., Yu Y., Weiser J. N. (2010) Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim O. Y., Monsel A., Bertrand M., Cavaillon J. M., Coriat P., Adib-Conquy M. (2009) Translocation of bacterial NOD2 agonist and its link with inflammation. Crit. Care 13, R124. [DOI] [PMC free article] [PubMed] [Google Scholar]